Abstract
STUDY QUESTION
What are the associations between a history of cancer and outcomes after ART?
SUMMARY ANSWER
Compared to women without cancer, on average, women with cancer had a lower return for embryo transfer and a lower likelihood of clinical pregnancy and live birth after ART.
WHAT IS KNOWN ALREADY
Small, single-institution studies have suggested that cancer and its treatment may negatively affect ART outcomes.
STUDY DESIGN, SIZE, DURATION
We conducted a systematic review with meta-analysis of studies comparing ART outcomes between women with and without cancer. PubMed, Embase and Scopus were searched for original, English-language studies published up to June 2021.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Inclusion criteria required reporting of ART outcomes after controlled ovarian stimulation (COS) among women with a history of cancer compared to women without cancer who used ART for any indication. Outcomes of interest ranged from duration of COS to likelihood of live birth after embryo transfer. Random-effects meta-analysis was used to calculate mean differences and odds ratios (ORs) with 95% CIs and 95% prediction intervals (PIs). We assessed heterogeneity by age-adjustment, referent group indication for ART, study location and among women with breast cancer and women who initiated ART before cancer treatment. We used visual inspection, Egger’s test and the trim-and-fill method to assess funnel plot asymmetry.
MAIN RESULTS AND THE ROLE OF CHANCE
Of 6094 unique records identified, 42 studies met inclusion criteria, representing a median per study of 58 women with cancer (interquartile range (IQR) = 159) and 114 women without cancer (IQR = 348). Compared to women without cancer, on average, women with cancer had a lower return for embryo transfer (OR: 0.22; 95% CI: 0.07, 0.74; 95% PI: 0.00, 64.98); lower likelihood of clinical pregnancy (OR: 0.51; 95% CI: 0.35, 0.73; 95% PI: 0.19, 1.35); and lower likelihood of live birth (OR: 0.56; 95% CI: 0.38, 0.83; 95% PI: 0.19, 1.69). Substantial among-study heterogeneity was observed for COS duration, gonadotropin dose, cycle cancellation, total oocytes and mature oocytes. Fertilization percentage showed less heterogeneity, but study-specific estimates were imprecise. Similarly, number of embryos showed less heterogeneity, and most studies estimated minimal differences by cancer history. Funnel plot asymmetry was observed for estradiol peak and oocyte maturation percentage.
LIMITATIONS, REASONS FOR CAUTION
Appreciable confounding is possible in 11 studies that lacked adequate control for group differences in age, and among-study heterogeneity was observed for most outcomes. Lack of data limited our ability to assess how cancer clinical factors (e.g. cancers other than breast, cancer stage and treatment) and ART cycle characteristics (e.g. fresh versus frozen embryo transfers and use of gestational carriers) may affect outcomes.
WIDER IMPLICATIONS OF THE FINDINGS
Women with cancer may be less likely to achieve pregnancy and live birth after embryo transfer. Further examination of reproductive outcomes and sources of heterogeneity among studies is warranted to improve evidence of the expected success of ART after a cancer diagnosis.
STUDY FUNDING/COMPETING INTEREST(S)
This research was supported in part by R01 CA211093 and P30 ES010126. C.M. was supported by the University of North Carolina Lineberger Cancer Control Education Program (T32 CA057726) and the National Cancer Institute (F31 CA260787). J.A.R.-H. was supported by the National Cancer Institute (K08 CA234333, P30 CA016672). J.A.R.-H. reports receiving consulting fees from Schlesinger Group and Guidepoint. The remaining authors declare no competing interests.
REGISTRATION NUMBER
N/A.
Keywords: assisted reproduction, cancer survivors, female infertility, IVF/ICSI outcome, oocyte maturation, ovarian stimulation, pregnancy
Introduction
Cancer treatment can increase the risk of infertility for many of the roughly 1.3 million reproductive-age women diagnosed with cancer each year (Lee et al., 2006; Levine et al., 2015; Poorvu et al., 2019; International Agency for Research on Cancer, 2020), which can lead to increased psychosocial distress, poorer mental health and lower quality of life (Lee et al., 2006; Deshpande et al., 2015; Anazodo et al., 2019; Logan et al., 2019). ART is clinically recommended for women with cancer who want to preserve their fertility before cancer treatment, or who are not able to naturally conceive after treatment (Ethics Committee of the American Society for Reproductive Medicine, 2018; Oktay et al., 2018; Practice Committee of the American Society for Reproductive Medicine, 2019; Lambertini et al., 2020). ART procedures include oocyte or embryo cryopreservation for fertility preservation, and embryo transfer to attempt pregnancy using fresh (non-cryopreserved) embryos or previously cryopreserved embryos that have been thawed (Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology, 2017). However, evidence of reproductive success after ART in cancer populations has been limited (Dolmans et al., 2019; Lambertini et al., 2020). Women with cancer are often counseled using ART data from the general population, though it is unclear how prior exposure to cancer or its treatments may affect outcomes (Levine et al., 2015; Lambertini et al., 2016; Practice Committee of the American Society for Reproductive Medicine, 2019; Mulder et al., 2021).
Two meta-analyses comparing ART outcomes by cancer history have been previously published (Friedler et al., 2012; Turan et al., 2018). However, neither assessed study characteristics contributing to among-study heterogeneity, outcomes among women who initiated ART after cancer treatment, nor pregnancy or birth outcomes. Our systematic review and meta-analysis sought to fill these evidence gaps and contribute novel data to the comparison of ART outcomes between women with and without cancer.
Methods
Search strategy
The guidelines recommended by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) were followed (Page et al., 2021). A literature search in PubMed, Embase and Scopus was conducted in August 2020; a second search was conducted in June 2021. Additional studies were identified by other methods (e.g. reviewing references cited in included articles). Only studies that could be obtained in English language were eligible for inclusion. There were no restrictions on study years. The search string for each database is provided in Supplementary Table SI. The review protocol was not registered.
Study selection
Inclusion criteria required reporting of ART outcomes after controlled ovarian stimulation (COS) among women with a history of any type of cancer compared to women without cancer who used ART for any indication. Outcomes of interest included: duration of COS; total gonadotropin stimulation dose; peak estradiol level on the day of triggering oocyte maturation; cycle cancellation; total and mature oocytes retrieved; oocyte maturation percentage; fertilization percentage; embryos obtained; return for embryo transfer; oocyte/embryo survival after freeze and thaw; implantation percentage; clinical pregnancy after embryo transfer; and live birth after embryo transfer. Studies were excluded if they did not report any outcomes of interest for an exclusive cancer group and a non-cancer referent group. Non-original research and abstracts only were excluded. One reviewer (C.M.) screened titles, abstracts, and full-text articles for final inclusion.
Data extraction
Study characteristics
One author (C.M.) extracted data from the included articles, including study setting and years, inclusion and exclusion criteria, sample size, cancer types, referent group indications for ART, analytic methods and outcomes. Table I details the main characteristics of included studies and Supplementary Table SII details study-specific inclusion and exclusion criteria.
Table I.
Characteristics of studies comparing ART outcomes among women with versus without a history of cancer.
| First author (year) | Study period; country | Sample size |
Cancer typesa | ART timing relative to cancer treatment | Referent indication for ART | Age at ART initiation mean (SD) |
Comments regarding internal validity concerns | ||
|---|---|---|---|---|---|---|---|---|---|
| Cancer | Referent | Cancer | Referent | ||||||
| Pal et al. (1998) | 1995–1997; USA | 5 | 12 | gyn (3), other (2) | Prior | Tubal | 31 (4.5)b | 31 (3.5) | None |
| Oktay et al. (2006) | 2003–2005; USA | 47 | 56 | breast (47) | Prior | Tubal | 36.4 (3.6)b | 36.9 (3.9) | None |
| Knopman et al. (2009) | 2001–2006; USA | 28 | 135 | breast (10), gyn (9), heme (6), other (3) | Prior | Male | 34 (5.1)b | 35.4 (3.5) | None |
| Klock et al. (2010) | 2005–2008; USA | 28 | 57 | breast (11), gyn (1), heme (7), other (9) | Prior | Multiple | 31.0 (5.3)b | 31.2 (4.6) | None |
| Michaan et al. (2010) | 2002–2007; Israel | 22 | 22 | breast (12), heme (2), other (7) | Prior | Tubal | 32.8 (5.7)b | 34 (4.2) | Groups additionally matched on period of treatment and COS protocol. |
| *n = 1 non-cancer | |||||||||
| Noyes et al. (2010) | 2004–2009; USA | 50 | 32 | breast (12), gyn (22), heme (8), other (8) | Prior | Other | 31 (7.1) | 32 (5.7) | No age adjustment but groups were comparable. |
| Quintero et al. (2010) | 1999–2007; USA | 50 | 50 | breast (28), heme (11), other (11) | Prior | Multiple | 32.3 (5.0)b | 32.3 (5.0) | None |
| Werner et al. (2010) | 2006–2010; USA | 49 | 81 | breast (10), gyn (21), heme (9), other (9) | Prior | Elective | 30 (7.0) | 36 (3.2) | Cancer group younger and no age adjustment. |
| Das et al. (2011) | 2003–2010; Canada | 39 | 48 | gyn (7), heme (19), other (13) | Prior | Male | 28.4 (5.5)b | 30.7 (2.1) | None |
| Robertson et al. (2011) | 2001–2007; USA | 26 | 921 | breast (16), gyn (5), other (5) | Prior | Male | 34.8 (5) | 35 (4) | No age matching, but groups were comparable and total oocyte and embryo analysis adjusted for age, gonadotropin dose, peak estradiol, ICSI use and length of COS—no covariates were statistically significant, so only unadjusted summary statistics were presented. |
| Sabatini et al. (2011) | 1997–2007; USA | 28 | 393 | breast (17), gyn (6), other (5) | Prior | Multiple | 33.9 (3.4) | 36.1 (3.9) | Cancer group younger, and no age adjustment reported except for birth analysis (report conducting age-adjusted analysis for pregnancy, but outcomes not reported). Birth analysis additionally adjusted for infertility diagnosis, prior IVF, and fertilization method. |
| Almog et al. (2012) | 2000–2011; Israel | 81 | 81 | breast (42), heme (12), other (27) | Prior | Male | 31.8 (4.8)b | 31.7 (4.7) | Groups additionally matched on date of COS. |
| Barton et al. (2012) | 1998–2009; USA | 53 | 7030 | breast (17), gyn (8), heme (22), other (6) | After | Multiple | 34.2 (19.3–43.9)b,c | 35.8 (19.3–43.9)c | Additional adjustment for COS protocol and fertilization method, but did not change effect estimates by >10% so were not included in final models. |
| Das et al. (2012) | 2003–2010; Canada | 14 | 42 | breast (1), gyn (3), heme (7), other (3) | After | Male | 28.4 (4.5)b | 30.2 (2.6) | None |
| Domingo et al. (2012) | 2007–2011; Spain | 208 | 97 | breast (143), heme (37), other (28) | Prior | Male | 32.4 (4.9)b | 31.9 (5.3) | None |
| Garcia-Velasco et al. (2013) | 2007–2012; Spain | 340 | 560 | breast, heme, other | Prior | Multiple | 31.9 (5.1) | 36.7 (4.2) | Cancer group younger and no age adjustment. |
| Fujimoto et al. (2014) | 1999–2012; Japan | 21 | 42 | gyn (21) | After | Multiple | 34 (30–39)b,c | 34 (29–41)c | Groups additionally matched on date of ART. |
| Cardozo et al. (2015) | 1997–2014; USA | 63 | 122 | breast (41), gyn (8), heme (5), other (9) | Prior | Tubal | 33.7 (4.1)b | 34.5 (3.5) | Groups additionally matched on date of ART. |
| Goldrat et al. (2015) | 2012–2014; Belgium | 21 | 21 | breast (21) | Prior | Multiple | 31.7 (6.4) | 32.3 (4.8) | No age adjustment but groups were comparable. |
| Lekovich et al. (2016) | 2010–2013; USA | 192 | 365 | breast (99), gyn (15), heme (69), other (9) | Prior | Elective | 31.8 (4.8) | 36.5 (3.2) | Cancer group younger and no outcomes are reported after age adjustment (only P-values after age-adjustment reported). |
| Luke et al. (2016a) | 2004–2009; USA | 441 | 52985 | breast (152), gyn (56), other (233) | Unknown | Multiple | 34.9 (5.8)b | 35.3 (5.3) | Analysis of reproductive outcomes adjusted for age, parity, cumulative gonadotropin dose, infertility diagnosis and number of diagnoses, number of ART cycles, state of residency, and year of ART. |
| Luke et al. (2016b) | 2004–2009; USA | 270 | 68 | breast (131), gyn (37), heme (64), other (37) | Unknown | Male | 32.5 (5.5) | 32.2 (4.2) | No age adjustment but groups were comparable. |
| Nurudeen et al. (2016) | 2005–2012; USA | 49 | 49 | breast (35), gyn (1), heme (4), other (9) | Prior | Multiple | 33.6 (4.8)b | 34.3 (4.6) | None |
| Pereira et al. (2016) | 2005–2014; USA | 220 | 439 | breast (220) | Prior | Elective | 35.7 (3.7) | 36.7 (3.7) | No age adjustment but groups were comparable. |
| Quinn et al. (2017) | 2009–2015; USA | 191 | 398 | breast (191) | Prior | Elective | 34.9 (4.6)b | 36.4 (3.0) | COS length and peak estradiol adjusted for age and BMI; total oocytes, mature oocytes and maturation percentage analysis additionally adjusted for total gonadotropin dose and letrozole use. |
| Cobo et al. (2018) | 2007–2018; Spain | 1073 | 5289 | breast (694), gyn (44), heme (191), other (144) | Prior | Elective | 32.3 (3.5) | 37.2 (4.9) | Cancer group younger and no age adjustment, though pregnancy and live birth outcomes were stratified by broad age categories; age-adjusted ORs were calculated, though residual confounding may be present. |
| Decanter et al. (2018) | 2011–2014; France | 90 | 180 | breast (49), heme (25), other (16) | Prior | Male | 29 (5)b | 29 (5) | Groups additionally matched on date of COS. |
| Dolinko et al. (2018) | 2007–2014; USA | 147 | 664 | breast (79), gyn (8), heme (38), other (22) | Both | Male | 31.7 (6.0) | 34.6 (4.2) | No age matching but age-adjusted outcomes are reported. |
| Tsampras et al. (2018) | 2009–2016; UK | 157 | 2128 | breast (80), gyn (8), other (69) | Prior | Male | 30.3 (6.0)b | 32.0 (4.5) | None |
| Ben-Haroush et al. (2019) | 2007–2017; Israel | 313 | 105 | breast (145), other (168) | Prior | Elective | 29.8 (7.2) | 36.0 (3.5) | Cancer group younger and no age adjustment. |
| de Moraes et al. (2019) | 2010–2017; Brazil | 23 | 164 | breast (13), gyn, heme, other | Prior | Elective | 31.0 (5.1) | 35.7 (3.1) | Cancer group younger and no age adjustment. |
| Goldrat et al. (2019) | 2012–2017; Belgium | 23 | 24 | breast (23) | Prior | Multiple | 30.4 (3.8) | 30.8 (3.9) | No age adjustment but groups were comparable. |
| Gunnala et al. (2019) | 2010–2015; USA | 176 | 600 | breast (91), gyn (7), heme (42), other (36) | Prior | Elective | 31.4 (5.5) | 36.6 (3.0) | Cancer group younger and no age adjustment comparing cancer group vs. non-cancer group. |
| Rodriguez-Wallberg et al. (2019) | 1998–2018; Sweden | 382 | 180 | breast, gyn, heme, other | Both | Multiple | 30.9 (5.5) | 27.1 (7.2) | Cancer group older; only outcome adjusted for age was return for embryo transfer. |
| Bercaire et al. (2020) | 2015–2016; Brazil | 69 | 92 | breast (69) | Prior | Male | 31.5 (4.1)b | 33.1 (7.1) | None |
| Kawwass et al. (2020) | 2012–2016; USA | 2715 | 26916 | breast, heme, other | Unknown | Multiple | <35: 63%b | <35: 25% | Cancer group younger; age-adjusted outcomes are reported though residual confounding may result from broad age categorization. |
| Nordan et al. (2020) | 2007–2018; USA | 10 | 30 | other (10) | Prior | Male | 30.4 (4.5)b | 30.5 (3.9) | Groups additionally matched on COS protocol. |
| Porcu et al. (2020) | 2014–2019; Italy | 46 | 181 | breast (46) | Prior | Male | 32.4 (4.1) | 32.4 (2.8) | No age adjustment but groups were comparable. |
| Huang et al. (2021) | 2010–2018; China | 64 | 320 | other (64) | After | Other | 33.8 (4.3)b | 33.6 (3.9) | Groups additionally matched on BMI, infertility diagnosis, date of ART, COS protocol and type of embryo transfer. Reproductive outcomes further adjusted for free T4, TSH and fertilization method. Pregnancy outcomes were self-reported. |
| Hussein et al. (2021) | 2009–2018; USA | 96 | 75 | breast (30), gyn (20), heme (24), other (22) | Prior | Elective | 28.1 (7.0) | 34.9 (5.5) | Cancer group younger and no age adjustment. |
| Tamauchi et al. (2021) | 2009–2020; Japan | 14 | 30 | gyn (14) | After | Multiple | 34.8 (29.0–40.4)c | 36.5 (22.9–42.8)c | No age adjustment but groups were comparable. |
| Fabiani et al. (2022) | 2016–2019; Italy | 82 | 180 | breast (52), gyn (9), heme (15), other (6) | Prior | Multiple | 32.2 (4.3)b | 33.2 (5.1) | Groups additionally matched on date of ART. |
Cancer types without a sample size indicates that this data were not reported in the study.
Cancer and non-cancer referent groups were age-matched or the study reported age-adjusted outcomes.
Median (range) reported.
COS, controlled ovarian stimulation; gyn, gynecologic cancer; heme, hematologic malignancy; TSH, thyroid-stimulating hormone.
Outcome measures
For continuous outcomes, the mean and SD per group were extracted; standard errors were converted into SDs (Higgins et al., 2021), and the median and interquartile range (IQR) was used to approximate the mean and SD when necessary (Wan et al., 2014). For binary outcomes, the number of events and the total sample size per group were extracted. Adjusted odds ratios (ORs) were directly extracted when available. Oocyte maturation percentage and fertilization percentage were analyzed as continuous because the mean or median percentage was reported across studies (rather than data on the number of mature oocytes among all oocytes retrieved in order to analyze as binary). For all outcomes, multiple groups among either the cancer or referent group were combined (e.g. outcomes reported separately by cancer type were combined into one cancer group) (Higgins et al., 2021).
Most studies included a woman’s first or only cycle of COS and reported outcomes per woman. Some studies included all cycles of COS per woman and reported certain outcomes per cycle; in those instances, number of cycles was used as the sample size. Reproductive outcomes are reported as the cumulative proportion of clinical pregnancy or live birth per woman, except for two studies that reported outcomes as per vitrification and warming cycle (Cobo et al., 2018), or per frozen embryo transfer cycle (Cardozo et al., 2015). Study authors were contacted for additional data when needed for inclusion in meta-analysis (e.g. reporting of only a median and range for a continuous outcome).
Risk of bias assessment
Risk of bias was assessed by one author (C.M.) using the U.S. Preventive Services Task Force criteria for assessing internal validity (U.S. Preventive Services Task Force, 2021). All studies included in the review were cohort studies and were assessed based on comparability of groups, loss to follow-up, measurement of variables and appropriate adjustment for confounders.
Statistical analysis
Stata 16.1 was used to calculate summary mean differences (MDs) for continuous outcomes and ORs for binary outcomes using random-effects meta-analysis. ORs rather than risk ratios were calculated because of the availability of adjusted ORs and 95% CIs in some studies that could be directly extracted rather than unadjusted summary statistics. Among-populations variance (τ2) was estimated by restricted maximum likelihood. Both 95% CIs and 95% prediction intervals (PIs) are presented. The CI conveys probable values for the average treatment effect, but this range may not apply to all settings given among-study heterogeneity (Riley et al., 2011; IntHout et al., 2016). The PI accounts for this heterogeneity and presents the variation in effect on the same scale as the outcome, enhancing its interpretability relative to other measures of heterogeneity commonly presented such as I2 (IntHout et al., 2016). The PI can be interpreted as the expected range of possible effects in similar future study populations (Riley et al., 2011; IntHout et al., 2016). Summary effect estimates with 95% CIs and 95% PIs were calculated for outcomes that were reported in at least three studies. R 3.5.1 was used to create forest plots.
Heterogeneity assessment
Heterogeneity was assessed using Cochran’s Q statistic and PIs. Other measures of heterogeneity (I2 and τ2) are also presented within forest plots or summary tables. Subgroup analysis was used to investigate a priori covariates as possible sources of heterogeneity, including: age-matching or age-adjustment, referent group indication for ART, and study location (USA or non-USA). Study characteristics with at least three studies per stratum were eligible for inclusion in subgroup analysis. Most outcomes were also examined comparing women with breast cancer to a non-cancer referent group. Analyses across other cancer types were not possible due to lack of data.
Funnel plot asymmetry
Funnel plots were visually examined for asymmetry for outcomes with at least 10 studies; a scatter plot of the MD or OR (log scale) versus inverse-variance was examined (Sterne et al., 2011). The regression-based test of Egger was used to test the null hypothesis of funnel plot symmetry (Egger et al., 1997; Sterne et al., 2000, 2011). The trim-and fill-method to adjust for publication bias was used as a sensitivity analysis only, given that it tends to under-impute in cases of substantial among-study heterogeneity and is therefore anti-conservative (Peters et al., 2007). In cases of substantial visual funnel plot asymmetry, overall summary aggregation is not presented. R 3.5.1 was used to create funnel plots.
Sensitivity analyses
Continuous outcomes included data from some studies for which the mean and SD were approximated from the median and IQR. Given the potential limitations in this approach in the presence of highly skewed data (Wan et al., 2014), studies for which this approximation was done were excluded. We also had an a priori interest in assessing heterogeneity of effects by timing of ART initiation among women with cancer (i.e. ART initiated for cryopreservation of oocytes or embryos before cancer treatment or ART initiated after cancer treatment). However, only five studies exclusively included women who initiated ART after cancer treatment (Barton et al., 2012; Das et al., 2012; Fujimoto et al., 2014; Huang et al., 2021; Tamauchi et al., 2021). Instead, as a sensitivity analysis, we excluded studies which included women who initiated ART after cancer treatment, or which timing of ART initiation relative to cancer treatment was unknown. To make comparable to the two previous meta-analyses on this topic (Friedler et al., 2012; Turan et al., 2018), we present results restricted to studies which used age-matching or age-adjustment and only included women who initiated ART prior to cancer treatment.
Results
Study selection
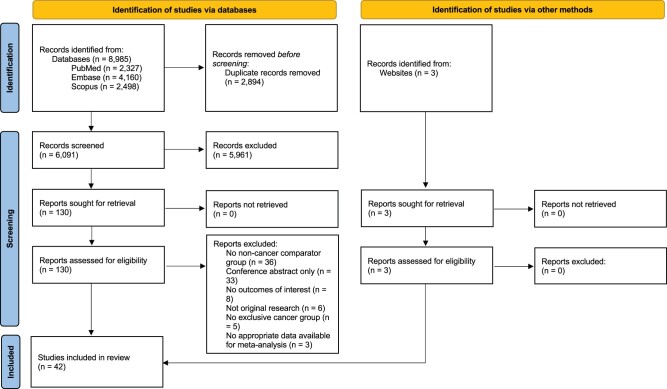
Records were identified from two database searches (n = 8985) and PubMed search alerts (n = 3), which yielded a total of 6094 unique records for screening. After title and abstract screening, 133 full-text articles were assessed for eligibility and 42 studies met inclusion criteria. A PRISMA flow diagram of study identification and selection is presented in Fig. 1. Studies that examined outcomes after ovarian tissue cryopreservation (Dolmans et al., 2014) or in vitro maturation (Moria et al., 2011) were not included, nor were studies that included non-malignancies in the case group (Johnson et al., 2013). Three studies that initially met inclusion criteria were subsequently excluded because additional data were needed in order to be included in meta-analysis and study authors did not respond to data requests (Pavone et al., 2014; Kim et al., 2015; Brun et al., 2021).
Figure 1.
PRISMA flow diagram of study identification and selection.
Study characteristics
Table I details the main characteristics of the 42 included studies, including concerns regarding internal validity. Studies had a median of 58 women with cancer (IQR = 159) and 114 women without cancer (IQR = 348). Roughly half of studies (k = 22) were conducted in the USA; the other studies were conducted in Canada, Europe, Asia or South America. Study periods spanned from 1995 to 2020, with a median study duration of 8 years (IQR = 5). Among studies that reported sample sizes by cancer type (k = 39), studies were a majority women with breast cancer (median = 42 women, IQR = 74), followed by hematologic malignancies (median = 15 women, IQR = 30), gynecologic cancers (median = 8 women, IQR = 14) and other cancers (median = 10 women, IQR = 2). Most studies included a mix of cancer types, though 10 were limited to one cancer type (k = 7 breast, k = 2 gynecologic and k = 1 glioma). The large majority of studies (k = 32) examined women with cancer who initiated ART prior to cancer treatment; five studies included women who initiated ART after cancer treatment; two studies included women who initiated ART before or after cancer treatment; and three studies reported unknown timing of ART initiation relative to cancer treatment. Many studies (k = 16) included women with multiple indications for ART in the non-cancer referent group, followed by women with male factor infertility (k = 13), elective cryopreservation (k = 9) or tubal factor infertility (k = 4). Three studies excluded women with ovarian insufficiency based on levels of FSH or anti-Müllerian hormone (AMH) among the cancer group only (Quintero et al., 2010) or among both the cancer and non-cancer groups (Goldrat et al., 2015, 2019).
Duration of COS
Twenty-five studies reported duration of COS (Table II and Supplementary Fig. S1; MD: 0.05; 95% CI: −0.21, 0.31; 95% PI: −1.16, 1.26). Twelve studies controlled for age, with one study additionally adjusting for BMI (Quinn et al., 2017). Among-study heterogeneity was high, but women with cancer can expect COS duration to be within roughly 1 day of the duration among women without cancer. Studies conducted in the USA tended to observe a longer COS duration among women with cancer relative to studies outside the USA (Cochran’s Q: 18.4, P < 0.0001), though the magnitude of difference was small (difference in MDs of <1 day) (Supplementary Table SIII). No large differences in summary estimates were observed for other study characteristics. The estimate among age-adjusted studies limited to women with cancer who initiated ART before cancer treatment (k = 11) was similar to the overall summary estimate (MD: 0.03; 95% CI: −0.38, 0.45; 95% PI: −1.45, 1.52). No funnel plot asymmetry was observed (Supplementary Fig. S2).
Table II.
Summary of ART outcomes comparing women with versus without a history of cancer.a
| Outcome | # of studies | Sample sizeb median (IQR) |
Cochran’s Q (P-value) | Random-effects variance (τ2) | MD (95% CI)c | (95% prediction interval) | |
|---|---|---|---|---|---|---|---|
| Cancer | Non-cancer | ||||||
| Stimulation duration (days) | 25 | 81 (158) | 105 (375) | 130.3 (<0.0001) | 0.33 | 0.05 (−0.21, 0.31) | (−1.16, 1.26) |
| Total gonadotropin dose (IU) | 29 | 50 (121) | 97 (133) | 369.7 (<0.0001) | 378856.04 | 228.20 (−11.36, 467.77) | (−1059.38, 1515.79) |
| Peak estradiol (pg/ml) | 17 | 40 (55) | 97 (132) | n/a | n/a | n/a | n/a |
| Total oocytes | 36 | 50 (135) | 105 (291) | 190.6 (<0.0001) | 4.44 | 0.47 (−0.34, 1.29) | (−3.89, 4.84) |
| Mature oocytes | 22 | 86 (141) | 172 (288) | 163.1 (<0.0001) | 3.61 | 0.27 (−0.64, 1.18) | (−3.81, 4.35) |
| Maturation percentage | 14 | 93 (1612) | 89 (130) | n/a | n/a | n/a | n/a |
| Fertilization percentage | 9 | 39 (37) | 55 (74) | 13.3 (0.10) | 13.30 | −1.81 (−5.73, 2.11) | (−11.65, 8.02) |
| Total embryos | 10 | 46 (38) | 151 (319) | 11.3 (0.25) | 0.09 | −0.30 (−0.68, 0.08) | (−1.11, 0.51) |
| Outcome | # of studies | Sample sizea median (IQR) |
Cochran’s Q (P-value) | Random-effects variance (τ2) | OR (95% CI)c | (95% prediction interval) | |
|---|---|---|---|---|---|---|---|
| Cancer | Non-cancer | ||||||
| Cycle cancellation | 11 | 76 (181) | 664 (3924) | 77.9 (<0.0001) | 0.83 | 1.85 (0.93, 3.67) | (0.20, 16.93) |
| Return for embryo transfer | 4 | 466 (390) | 423 (1512) | 55.2 (<0.0001) | 1.37 | 0.22 (0.07, 0.74) | (0.00, 64.98) |
| Clinical pregnancy | 9 | 53 (54) | 53 (645) | 20.4 (0.009) | 0.14 | 0.51 (0.35, 0.73) | (0.19, 1.35) |
| Live birth | 10 | 42 (48) | 50 (401) | 23.7 (0.005) | 0.19 | 0.56 (0.38, 0.83) | (0.19, 1.69) |
Summary aggregation is not reported for outcomes demonstrating funnel plot asymmetry.
In most studies, sample size represents the number of women, but in studies that reported outcomes at the cycle level, sample size represents the number of cycles.
Referent is women without a history of cancer.
IQR, interquartile range; OR, odds ratio.
Total gonadotropin stimulation dose
Twenty-nine studies reported total gonadotropin dose administered during COS (Table II and Supplementary Fig. S3; MD: 228.20; 95% CI: −11.36, 467.77; 95% PI: −1059.38, 1515.79). Among-study heterogeneity was high and study-specific estimates were imprecise. Variability was most evident by referent indication for ART: in most settings, women with cancer received a higher gonadotropin dose than women with male factor infertility or multiple/other indications, while women with cancer received a lower dose than women who used ART for elective or donor indications (Cochran’s Q: 19.9, P < 0.0001) (Supplementary Table SIII). Studies in the USA showed a more positive association between cancer history and gonadotropin dose (higher gonadotropin among women with cancer), while analysis among women with breast cancer only showed a less positive association compared to the overall summary estimate, though these subgroup estimates were imprecise. The estimate among age-adjusted studies limited to women with cancer who initiated ART before cancer treatment (k = 12) was similar to the overall summary estimate, though less precise (MD: 221.64; 95% CI: −188.77, 632.06; 95% PI: −1356.19, 1799.48). No funnel plot asymmetry was observed (Supplementary Fig. S4).
Peak estradiol level on day of triggering oocyte maturation
Seventeen studies reported peak estradiol levels during COS (Table II and Supplementary Fig. S5). Eleven studies controlled for age, with one study additionally adjusting for BMI (Quinn et al., 2017). Studies or subgroups within studies in which aromatase inhibitors (e.g. letrozole) or tamoxifen were used during COS to suppress estradiol were excluded from analysis (Bonardi et al., 2020).
Visual funnel plot asymmetry was observed and detected via Egger’s test, and trim-and-fill imputed five hypothetically missing estimates on the left side (estimates showing lower peak estradiol among women with cancer) (Supplementary Fig. S6). An overall summary estimate is thus not provided, though study characteristics were examined as potential sources of asymmetry due to true heterogeneity or methodologic quality—as such characteristics, in addition to publication bias, can contribute to funnel plot asymmetry (Sterne et al., 2011). The association varied by age-adjustment (lower estradiol among women with cancer in studies not age-adjusted); referent indication for ART (lower estradiol among women with cancer when compared with women who used ART for elective or donor indications); study location (lower estradiol among women with cancer in studies outside of the USA); and among women with breast cancer only (lower estradiol) (Supplementary Table SIII). Studies conducted outside the USA produced estimates that were generally more negative (lower estradiol among women with cancer) and less precise than estimates produced in the USA, and this heterogeneity appears to have driven the observed asymmetry (Supplementary Fig. S6).
Cycle cancellation
Eleven studies reported cycle cancellation (Table II and Supplementary Fig. S7; OR: 1.85; 95% CI: 0.93, 3.67; 95% PI: 0.20, 16.93). Among-study heterogeneity was high; studies tended to report point estimates on the right side of the null (positive association between a history of cancer and cycle cancellation), though study-specific estimates were imprecise. No funnel plot asymmetry was evident through visual assessment or Egger’s test, but trim-and-fill imputed one hypothetically missing estimate, which moved the summary estimate downward and slightly closer to the null (OR from 1.85 to 1.73) (Supplementary Fig. S8). Higher cancellation among women with cancer was observed in studies that were age-adjusted; when compared with women with male factor infertility (versus multiple/other indications); and in non-USA studies (Supplementary Table SIV). The estimate among age-adjusted studies limited to women with cancer who initiated ART before cancer treatment (k = 5) was similar to the overall summary estimate, though less precise (OR: 2.12; 95% CI: 0.57, 7.90; 95% PI: 0.03, 150.80).
Number of oocytes
Thirty-six studies reported the total number of oocytes retrieved after COS (Table II and Supplementary Fig. S9; MD: 0.47; 95% CI: −0.34, 1.29; 95% PI: −3.89, 4.84). Nineteen studies controlled for age, with one study additionally adjusting for BMI, total gonadotropin dose and letrozole use (Quinn et al., 2017). Among-study heterogeneity was high and showed variability by study characteristics. More oocytes were retrieved in women with cancer in studies that were not age-matched or when compared with women who used ART for elective/donor or tubal factor indications—though only one of the elective/donor studies controlled for age (Quinn et al., 2017), and the referent group was, on average, 1–7 years older than women with cancer across those studies (Supplementary Table SIII). Retrieval of more oocytes in women with cancer were also reported in studies conducted in the USA; when ART was initiated before cancer treatment; or when excluding studies which reported a median and IQR. The estimate among age-adjusted studies limited to women with cancer who initiated ART before cancer treatment (k = 17) was similar to the overall summary estimate (MD: 0.73; 95% CI: −0.44, 1.90; 95% PI: −3.79, 5.25).
No funnel plot asymmetry was evident through visual assessment or Egger’s test, though trim-and-fill imputed six hypothetically missing estimates, which moved the summary estimate upward and further from the null (MD from 0.47 to 1.03) (Supplementary Fig. S10).
Number of mature oocytes
Twenty-two studies reported the total number of mature oocytes retrieved after COS (Table II and Supplementary Fig. S11; MD: 0.27; 95% CI: −0.64, 1.18; 95% PI: −3.81, 4.35). Eight studies controlled for age, with one study additionally adjusting for BMI, total gonadotropin dose, and letrozole use (Quinn et al., 2017). Among-study heterogeneity was high and showed variability by study characteristics. More mature oocytes were retrieved in women with cancer when compared to women who used ART for elective/donor indications (though similar to the total oocytes analysis, only one study controlled for age (Quinn et al., 2017)); and in studies conducted in the USA (Supplementary Table SIII). The estimate among age-adjusted studies limited to women with cancer who initiated ART before cancer treatment (k = 8) was similar to the overall summary estimate (MD: 0.08; 95% CI: −1.40, 1.55; 95% PI: −5.01, 5.16).
No funnel plot asymmetry was evident through visual assessment or Egger’s test, though trim-and-fill imputed one hypothetically missing estimate, which moved the summary estimate upward and slightly further from the null (MD from 0.27 to 0.44) (Supplementary Fig. S12).
Oocyte maturation percentage
Fourteen studies reported oocyte maturation percentage after COS (Table II and Supplementary Fig. S13). Nine studies controlled for age, with one study additionally adjusting for BMI, total gonadotropin dose and letrozole use (Quinn et al., 2017). Visual funnel plot asymmetry was observed, and trim-and-fill imputed three hypothetically missing estimates on the left side (estimates showing lower oocyte maturation among women with cancer) (Supplementary Fig. S14). An overall summary estimate is thus not provided, though study characteristics were examined as potential sources of asymmetry. The association between cancer history and oocyte maturation percentage varied by age-adjustment (lower maturation among women with cancer in studies that were age-adjusted); study location (lower maturation among women with cancer in US studies); among women with breast cancer only (lower maturation); and in studies that were age-adjusted and only included women who initiated ART before cancer treatment (lower maturation) (Supplementary Table SIII). Studies conducted outside the USA produced estimates that were generally more positive (higher maturation among women with cancer) and slightly less precise than estimates produced in the USA, and this heterogeneity appears to have driven the observed asymmetry (Supplementary Fig. S14).
Fertilization percentage
Nine studies reported oocyte fertilization percentage (Table II and Supplementary Fig. S15; MD: −1.81; 95% CI: −5.73, 2.11; 95% PI: −11.65, 8.02). Study-specific estimates showed less heterogeneity, though they were imprecise. No clear patterns of association were observed by study characteristics in subgroup analysis (Supplementary Table SIII). The estimate among age-adjusted studies limited to women with cancer who initiated ART before cancer treatment (k = 5) was similar to the overall summary estimate (MD: −1.48; 95% CI: −5.62, 2.67; 95% PI: −8.21, 5.25).
Number of embryos
Ten studies reported the number of embryos obtained (Table II and Supplementary Fig. S16; MD: −0.30; 95% CI: −0.68, 0.08; 95% PI: −1.11, 0.51). Study-specific estimates showed less heterogeneity, with most studies estimating minimal differences by cancer history. Variability was observed by age-adjustment and cancer type: fewer embryos were obtained among women with cancer in studies that were age-adjusted (Cochran’s Q: 6.6, P = 0.01), and in studies that included women with breast cancer only (Supplementary Table SIII). The estimate among age-adjusted studies limited to women with cancer who initiated ART before cancer treatment (k = 5) was similar to the overall summary estimate (MD: −0.50; 95% CI: −0.66, −0.34; 95% PI: −0.75, −0.25).
No funnel plot asymmetry was evident through visual assessment or Egger’s test, though trim-and-fill imputed two hypothetically missing estimates, which moved the summary estimate downward and slightly further from the null (MD from −0.30 to −0.44) (Supplementary Fig. S17).
Proportion of return for embryo transfer
Four studies reported return for embryo transfer (Table II and Supplementary Fig. S18; OR: 0.22; 95% CI: 0.07, 0.74; 95% PI: 0.00, 64.98), of which one reported an age-adjusted estimate (Rodriguez-Wallberg et al., 2019). All studies observed a lower likelihood of return among women with cancer compared to women without cancer (summary OR: 0.22; 95% CI: 0.07, 0.74; 95% PI: 0.00, 64.98) (Table II), though follow-up times between groups were not necessarily comparable (e.g. Rodriguez-Wallberg et al., 2019 reported a mean follow-up time of 6.2 years in women with cancer compared to 7.8 years in women without cancer). Given the small number of studies, no subgroup analyses were conducted. Referent groups in these studies included male factor (k = 1), elective or donor indications (k = 1), and multiple/other indications (k = 2); three of the studies were conducted outside the USA; and two studies only included women who initiated ART before cancer treatment.
Oocyte or embryo survival percentage after freeze and thaw
Two studies reported oocyte or embryo survival after freeze and thaw. In one study that used vitrification, 81.8% of oocytes survived among women with cancer versus 83.9% among women undergoing elective fertility preservation, though among women who were aged ≤35 years at cryopreservation, women with cancer had lower oocyte survival (81.2% versus 91.4%) (Cobo et al., 2018). A second study that used slow-freezing reported that 62.8% of embryos survived over all transfer cycles among women with cancer versus 72.0% among women who froze embryos to reduce the risk of ovarian hyperstimulation syndrome (Sabatini et al., 2011).
Implantation percentage
Four studies reported data on implantation, though none reported the denominator of number of embryos transferred and thus could not be meta-analyzed. Over all transfer cycles, implantation ranged from 12.9% to 32.5% among women with cancer and 29.9–43.8% among women without cancer (Sabatini et al., 2011; Cardozo et al., 2015; Cobo et al., 2018). Per embryo transferred, women with cancer had 4.9% of embryos implant versus 21.0% among women without cancer (Fujimoto et al., 2014).
Clinical pregnancy after embryo transfer
Nine studies reported clinical pregnancy after embryo transfer (Table II and Supplementary Fig. S19; OR: 0.51; 95% CI: 0.35, 0.73; 95% PI: 0.19, 1.35). Six studies controlled for age, including one study that additionally adjusted for parity, cumulative gonadotropin dose, infertility diagnosis and number of diagnoses, number of ART cycles, state of residency and year of ART treatment (Luke et al., 2016a); and one study among women with thyroid cancer that additionally adjusted for free T4, thyroid-stimulating hormone and fertilization method (Huang et al., 2021). On average, women with cancer had a lower likelihood of clinical pregnancy compared to women without cancer. Subgroup differences were observed by study location, with studies conducted in the USA observing a stronger negative association of cancer on pregnancy (Cochran’s Q: 17.0, P < 0.0001) (Supplementary Table SIV). Only two studies were age-adjusted and limited to women with cancer who initiated ART before cancer treatment (OR: 0.63; 95% CI: 0.41, 0.98).
The use of gestational carriers is an additional clinically relevant factor to consider in these studies, but could not be assessed given the lack of data. Two studies reported that 48–67% of women with cancer used a gestational carrier (ART initiated before cancer treatment in both studies), while no gestational carriers were reported in the non-cancer group, though pregnancy rates by gestational carrier use were not reported (Sabatini et al., 2011; Cardozo et al., 2015). All other studies either excluded outcomes using gestational carriers, or were conducted in countries where surrogacy is not legal or regulated.
Live birth after embryo transfer
Ten studies reported live birth after embryo transfer (Table II and Supplementary Fig. S20; OR: 0.56; 95% CI: 0.38, 0.83; 95% PI: 0.19, 1.69). Seven studies controlled for age, including one study that additionally adjusted for parity, cumulative gonadotropin dose, infertility diagnosis and number of diagnoses, number of ART cycles, state of residency and year of ART treatment (Luke et al., 2016a); one study that additionally adjusted for infertility diagnosis, prior IVF and fertilization method (Sabatini et al., 2011); and one study among women with thyroid cancer that additionally adjusted for free T4, thyroid stimulating hormone, and fertilization method (Huang et al., 2021). On average, women with cancer had a lower likelihood of live birth compared to women without cancer. No meaningful differences in the summary estimate were observed by study characteristics (Supplementary Table SIV). The estimate among age-adjusted studies limited to women with cancer who initiated ART before cancer treatment (k = 3) was similar to the overall summary estimate (OR: 0.58; 95% CI: 0.36, 0.94; 95% PI: 0.03, 13.15). Adverse birth outcomes (e.g. preterm birth or low birth weight) were only reported in two studies (Garcia-Velasco et al., 2013; Huang et al., 2021) and could not be examined.
No funnel plot asymmetry was evident through visual assessment or Egger’s test, though trim-and-fill imputed one hypothetically missing estimate, which did not substantively change the summary estimate (Supplementary Fig. S21).
Risk of bias assessment
Eleven of 42 studies (26%) had no or inadequate control for age differences between cancer and non-cancer groups for some or all outcomes (Table I). In 10 of those studies, the cancer group was, on average, 2.2–6.8 years younger than the non-cancer group at ART initiation.
Discussion
This systematic review and meta-analysis of 42 studies provides an updated summary of ART outcomes comparing women with and without a history of cancer, including the first synthesis, to our knowledge, of reproductive outcomes after embryo transfer. Substantial among-study heterogeneity was observed for COS duration, total gonadotropin dose, cycle cancellation, total oocytes and mature oocytes; and evidence of appreciable funnel plot asymmetry was observed for peak estradiol and oocyte maturation percentage. However, our meta-analysis found that, on average, women with cancer had lower odds of return for embryo transfer, clinical pregnancy, and live birth, suggesting potentially adverse effects of cancer and its treatment on reproductive success using ART.
Two systematic reviews with meta-analyses on this topic have been previously published (Friedler et al., 2012; Turan et al., 2018). In a 2012 review of 7 studies, the authors concluded that, compared to women without cancer who used ART, women with cancer received a lower total gonadotropin dose, had lower peak estradiol levels, and had fewer total and mature oocytes retrieved (Friedler et al., 2012). In contrast, in a 2018 review of 10 studies (including the same seven studies as the 2012 review), the authors concluded that cancer was not associated with a differential response to ART for any outcome examined (Turan et al., 2018). However, neither previous review assessed study characteristics contributing to among-study heterogeneity nor examined likelihood of pregnancy or live birth. Importantly, our review used broader search criteria (including the search of two additional databases) and included three additional years of data, capturing 14 studies published after the end date of the 2018 review.
Notably, we found, on average, a lower likelihood of clinical pregnancy and live birth after ART among women with cancer, even among women who had initiated ART before cancer treatment for fertility preservation. It is well-documented that cancer treatments including chemotherapy and abdominal-pelvic radiation can rapidly accelerate the decline of a woman’s primordial follicles, or ovarian reserve, and result in immediate or premature ovarian failure (Lee et al., 2006; Knopman et al., 2010; Levine et al., 2015; Poorvu et al., 2019; Spears et al., 2019). But even among women who cryopreserve oocytes or embryos before cancer treatment, reproductive outcomes after ART may still be affected by radiation or surgical treatments that damage reproductive organs, impair cardiovascular or pulmonary function, or cause dysfunction of the hypothalamic-pituitary-gonadal axis (Critchley and Wallace, 2005; Lee et al., 2006; Wo and Viswanathan, 2009; Knopman et al., 2010; Poorvu et al., 2019; Griffiths et al., 2020). Given our findings, further exploration of how specific cancer treatments may affect these associations is warranted.
Though we observed lower reproductive success among women with cancer, evidence was not conclusive regarding differences in the number of total oocytes, mature oocytes or embryos obtained—all of which are clinically significant predictors of ART success (van Loendersloot et al., 2010; McLernon et al., 2016; Practice Committee of the American Society for Reproductive Medicine, 2019; Lambertini et al., 2020). Heterogeneity across these outcomes was observed by age-adjustment, referent indication for ART, study location and among women with breast cancer. Further examination of heterogeneity related to these study characteristics and other cancer- and ART-related factors is needed within larger studies that control for age, including the influence of institutional- or country-level variation in COS protocols and differential response by cancer type.
We additionally found that women with cancer, on average, were less likely to return after oocyte or embryo cryopreservation to attempt pregnancy, an outcome that had not been synthesized in prior meta-analyses. However, this result should be interpreted in the context of the small number of studies analyzed (four), the duration of follow-up after ART initiation across studies, and the potential for selection bias. Regarding follow-up, one study reported shorter follow-up among women with cancer compared to the referent group (6.2 versus 7.8 years) (Rodriguez-Wallberg et al., 2019); one pilot study reported a mean follow-up of all women of 2 years (Luke et al., 2016b); and two studies reported 5–11.5 years of ART data but did not report average follow-up per woman (Garcia-Velasco et al., 2013; Cobo et al., 2018). Additional studies with longer follow-up (at least 10 years) are needed to assess whether lower return among women with cancer persists, and to identify the barriers to return. If and when women return after cryopreservation may be influenced by recommendations to wait to attempt pregnancy for at least 1 year after completion of cancer treatment to decrease the likelihood of pregnancy complications (Hartnett et al., 2018; ESHRE Guideline Group on Female Fertility Preservation et al., 2020); return may also be influenced by physician beliefs that pregnancy after cancer (particularly for hormone-sensitive cancers) may increase the risk of recurrence (Lambertini et al., 2018), though the safety of both fertility preservation and pregnancy for these patients has been demonstrated (Lambertini et al., 2021; Arecco et al., 2022). Additionally, women with hormone-sensitive cancers may receive up to 10 years of endocrine therapy and may choose to attempt pregnancy after (rather than interrupting) such treatment (Lambertini et al., 2020).
Regarding potential selection bias, returning for transfer after cryopreservation is conditional on being alive and also on not being able to conceive naturally. Though cancer survival among reproductive-age women is high, 10–20% will die within 5 years (Close et al., 2019), higher than would be expected in the general infertile population without cancer. Additionally, women with a history of cancer could be more likely to conceive naturally compared to the general infertile population because the infertile population, by definition, has been unsuccessful in conceiving for at least 12 months; in contrast, in certain circumstances, women with cancer may maintain some fertility after cancer treatment and may not require use of their previously frozen oocytes or embryos. Thus, cancer history is associated with two factors that are being selected on for inclusion into the sample for this analysis (i.e. women with cancer are more likely to die and may be more likely to naturally conceive), which may contribute to the lower likelihood of return among women with cancer.
Appreciable funnel plot asymmetry was observed for estradiol peak and oocyte maturation percentage, which could result from publication bias, poor methodological quality, true heterogeneity or chance (Sterne et al., 2011). In this literature, we hypothesized that publication bias would result in the lower likelihood of publishing small studies that find more favorable ART outcomes among women with cancer. What we observed were gaps in the funnel plots for small studies that showed lower estradiol among women with cancer (unclear clinical favorability (Kosmas et al., 2004; Karatasiou et al., 2020)) and lower oocyte maturation percentage among women with cancer (less favorable). Given this, publication bias is not likely to be appreciable in this literature. The observed asymmetry may have been produced, instead, by true heterogeneity. For both estradiol peak and oocyte maturation percentage, we observed heterogeneity by study location that appears to have produced the asymmetry (generally less precise estimates produced in studies conducted outside the USA that drove estimates a certain direction).
Our results are limited by the potential for confounding by age; age at ART initiation is one of the strongest predictors of reproductive success (van Loendersloot et al., 2010; McLernon et al., 2016), but more than one in four studies reported baseline differences in age between cancer and non-cancer groups and lacked adequate control for these differences in study design or analysis. Results should also be interpreted in the context of substantial among-study heterogeneity, which did not appear to be fully explained by age-adjustment, referent group indication for ART, or study location.
Further, we lacked sufficient data to explore other relevant cancer- and ART-related factors that could influence our results. Such factors need further examination in larger studies, including outcomes by cancer type (particularly cancers other than breast), cancer treatments received, and other prognostic factors (e.g. BRCA mutation status); COS protocol and oocyte maturation trigger; type of embryo transfer (fresh versus frozen/thawed); and use of gestational carriers. We were also not able to examine differences by race and ethnicity, as most studies did not report such distributions, even though prior research demonstrates that racial and ethnic groups who are minoritized, particularly Black women, experience lower pregnancy and birth rates after ART (Huddleston et al., 2010; Seifer et al., 2020; Makhijani et al., 2021). The lack of reporting of race and ethnicity within this field precludes documentation of disparities in fertility treatment and outcomes (Krieger, 2021) and should be a focus for future research. Outcomes after ovarian tissue transplantation and in vitro maturation are also important to assess as these procedures become more common in clinical practice, particularly among prepubertal patients or those who need to start cancer treatment urgently (Practice Committee of the American Society for Reproductive Medicine, 2019).
Nevertheless, our review contributes new perspectives to this growing field of research, including the first synthesis, to our knowledge, of reproductive outcomes after ART. We were able to assess heterogeneity in observed associations across multiple a priori factors, including referent group indication for ART and study location, and examined outcomes subset to women with breast cancer and women who initiated ART before cancer treatment. Unfortunately, women with cancer continue to face a multitude of barriers in accessing ART for fertility preservation at the time of diagnosis (Jones et al., 2017; Logan et al., 2018; Covelli et al., 2019) and may not access fertility services until after cancer treatment, indicating a need for further study of how previous exposure to gonadotoxic therapies (e.g. chemotherapy) affects predictors of ART success (i.e. quantity and quality of oocytes and embryos) and subsequent pregnancy and birth rates (Practice Committee of the American Society for Reproductive Medicine, 2019).
In summary, we observed substantial among-study heterogeneity or funnel plot asymmetry that was not fully explained by the examined study characteristics for most outcomes. However, women with cancer, on average, were less likely to return for embryo transfer and less likely to have a clinical pregnancy or live birth after embryo transfer. Larger-scale studies with longer follow-up (from cancer diagnosis, to ART initiation, to pregnancy attempt and live birth) and comprehensive assessment of potentially influential patient-, cancer- and ART-related factors are needed to improve data on ART outcomes for cancer populations. Future studies should account for differences by age, BRCA status, AMH levels and COS protocol between cancer and comparator groups through study design or analysis and should consider improving data in areas with existing gaps, including: understanding the barriers to return after cryopreservation and how outcomes may differ based on time since last cancer treatment; examining the effect of specific cancer treatments (e.g. chemotherapeutic agents or pelvic radiation dosage) on ART outcomes (including pregnancy complications and adverse birth outcomes); assessing outcomes by race and ethnicity; and studying outcomes after ovarian tissue transplantation and in vitro maturation.
Though questions remain, these data add to the body of evidence available to clinicians who provide fertility counseling to women with cancer—a body of evidence acknowledged to be severely lacking by leading oncology and reproductive societies (Practice Committee of the American Society for Reproductive Medicine, 2019; Lambertini et al., 2020). Researchers, clinicians and policymakers should continue to increase access to fertility treatment at the time of cancer diagnosis and into survivorship; refine ovarian stimulation and embryo transfer protocols for individuals with cancer to maximize safety, efficiency, and effectiveness; and reduce the gonadotoxicity of cancer treatments to improve fertility-related outcomes after cancer diagnosis.
Supplementary Material
Contributor Information
Clare Meernik, Department of Population Health Sciences, Duke University School of Medicine, Durham, NC, USA.
Charles Poole, Department of Epidemiology, University of North Carolina at Chapel Hill Gillings School of Global Public Health, Chapel Hill, NC, USA.
Stephanie M Engel, Department of Epidemiology, University of North Carolina at Chapel Hill Gillings School of Global Public Health, Chapel Hill, NC, USA.
J Alejandro Rauh-Hain, Department of Gynecologic Oncology and Reproductive Medicine, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Barbara Luke, Department of Obstetrics, Gynecology, and Reproductive Biology, College of Human Medicine, Michigan State University, East Lansing, MI, USA.
Hazel B Nichols, Department of Epidemiology, University of North Carolina at Chapel Hill Gillings School of Global Public Health, Chapel Hill, NC, USA.
Data Availability
The data underlying this article are available in the article and its supplementary material.
Authors’ roles
C.M.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review & editing; C.P.: methodology, supervision, writing—review & editing; S.M.E.: writing—review & editing; J.A.R.-H.: writing—review & editing; B.L.: writing—review & editing; and H.B.N.: supervision, writing—review & editing.
Funding
This research was supported in part by R01 CA211093 and P30 ES010126. C.M. was supported by the University of North Carolina Lineberger Cancer Control Education Program (T32 CA057726) and the National Cancer Institute (F31 CA260787). J.A.R.-H. was supported by the National Cancer Institute (K08 CA234333, P30 CA016672). The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication.
Conflict of interest
J.A.R.-H. reports receiving consulting fees from Schlesinger Group and Guidepoint. The remaining authors declare no conflicts of interest.
References
- Anazodo A, Laws P, Logan S, Saunders C, Travaglia J, Gerstl B, Bradford N, Cohn R, Birdsall M, Barr R, et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum Reprod Update 2019;25:159–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arecco L, Blondeaux E, Bruzzone M, Ceppi M, Latocca MM, Marrocco C, Boutros A, Spagnolo F, Razeti MG, Favero D, et al. Safety of fertility preservation techniques before and after anticancer treatments in young women with breast cancer: a systematic review and meta-analysis. Hum Reprod 2022;37:954–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almog B, Azem F, Gordon D, Pauzner D, Amit A, Barkan G, Levin I.. Effects of cancer on ovarian response in controlled ovarian stimulation for fertility preservation. Fertil Steril 2012;98:957–960. [DOI] [PubMed] [Google Scholar]
- Barton SE, Missmer SA, Berry KF, Ginsburg ES.. Female cancer survivors are low responders and have reduced success compared with other patients undergoing assisted reproductive technologies. Fertil Steril 2012;97:381–386. [DOI] [PubMed] [Google Scholar]
- Ben-Haroush A, Wertheimer A, Klochendler E, Sapir O, Shufaro Y, Oron G.. Effect of letrozole added to gonadotropins in controlled ovarianstimulation protocols on the yield and maturity of retrieved oocytes. Gynecol Endocrinol 2019;35:324–327. [DOI] [PubMed] [Google Scholar]
- Bercaire LMN, Cavagna M, Donadio NF, Rocha AR, Portela R, Alves VR, Santos TBB, Cavagna F, Dzik A, Gebrim LH, et al. The impact of letrozole administration on oocyte morphology in breast cancer patients undergoing fertility preservation. JBRA Assist Reprod 2020;24:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonardi B, Massarotti C, Bruzzone M, Goldrat O, Mangili G, Anserini P, Spinaci S, Arecco L, Del Mastro L, Ceppi M. et al. Efficacy and safety of controlled ovarian stimulation with or without letrozole co-administration for fertility preservation: a systematic review and meta-analysis. Front Oncol 2020;10:574669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun T, Dion L, Jaillard S, Bales D, Domin M, Lavoué V, Levêque J, Houot R, Duros S.. Ovarian response to stimulation for fertility preservation in women with hematologic cancer. J Gynecol Obstet Hum Reprod 2021;50:101925. [DOI] [PubMed] [Google Scholar]
- Cardozo ER, Thomson AP, Karmon AE, Dickinson KA, Wright DL, Sabatini ME.. Ovarian stimulation and in-vitro fertilization outcomes of cancer patients undergoing fertility preservation compared to age matched controls: a 17-year experience. J Assist Reprod Genet 2015;32:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2017 Assisted Reproductive Technology Fertility Clinic Success Rates Report. Atlanta, GA: US Dept of Health and Human Services, 2017. [Google Scholar]
- Close AG, Dreyzin A, Miller KD, Seynnaeve BKN, Rapkin LB.. Adolescent and young adult oncology-past, present, and future. CA Cancer J Clin 2019;69:485–496. [DOI] [PubMed] [Google Scholar]
- Cobo A, García-Velasco J, Domingo J, Pellicer A, Remohí J.. Elective and onco-fertility preservation: factors related to IVF outcomes. Hum Reprod 2018;33:2222–2231. [DOI] [PubMed] [Google Scholar]
- Covelli A, Facey M, Kennedy E, Brezden-Masley C, Gupta AA, Greenblatt E, Baxter NN.. Clinicians’ perspectives on barriers to discussing infertility and fertility preservation with young women with cancer. JAMA Netw Open 2019;2:e1914511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HOD, Wallace WHB.. Impact of cancer treatment on uterine function. J Natl Cancer Inst Monogr 2005;2005:64–68. [DOI] [PubMed] [Google Scholar]
- Das M, , ShehataF, , MoriaA, , HolzerH, , SonW-Y, , Tulandi T.. Ovarian reserve, response to gonadotropins, and oocyte maturity in women with malignancy. Fertil Steril 2011;96:122–125. [DOI] [PubMed] [Google Scholar]
- Das M, Shehata F, Son W-Y, Tulandi T, Holzer H.. Ovarian reserve and response to IVF and in vitro maturation treatment following chemotherapy. Hum Reprod 2012;27:2509–2514. [DOI] [PubMed] [Google Scholar]
- Decanter C, Robin G, Mailliez A, Sigala J, Morschhauser F, Ramdane N, Devos P, Dewailly D, Leroy-Martin B, Keller L.. Prospective assessment of follicular growth and the oocyte cohort after ovarian stimulation for fertility preservation in 90 cancer patients versus 180 matched controls. Reprod Biomed Online 2018;36:543–551. [DOI] [PubMed] [Google Scholar]
- Deshpande NA, Braun IM, Meyer FL.. Impact of fertility preservation counseling and treatment on psychological outcomes among women with cancer: a systematic review. Cancer 2015;121:3938–3947. [DOI] [PubMed] [Google Scholar]
- Dolinko AV, Farland LV, Missmer SA, Srouji SS, Racowsky C, Ginsburg ES.. Responses to fertility treatment among patients with cancer: a retrospective cohort study. Fertil Res Pract 2018;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmans M-M, Lambertini M, Macklon KT, Almeida Santos T, Ruiz-Casado A, Borini A, Bordes V, Frith L, Van Moer E, Germeyer A.. EUropean REcommendations for female FERtility preservation (EU-REFER): a joint collaboration between oncologists and fertility specialists. Crit Rev Oncol Hematol 2019;138:233–240. [DOI] [PubMed] [Google Scholar]
- Dolmans M-M, Marotta M-L, Pirard C, Donnez J, Donnez O.. Ovarian tissue cryopreservation followed by controlled ovarian stimulation and pick-up of mature oocytes does not impair the number or quality of retrieved oocytes. J Ovarian Res 2014;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J, Guillén V, Ayllón Y, Martínez M, Muñoz E, Pellicer A, Garcia-Velasco JA.. Ovarian response to controlled ovarian hyperstimulation in cancer patients is diminished even before oncological treatment. Fertil Steril 2012;97:930–934. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith G, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESHRE Guideline Group on Female Fertility Preservation, Anderson RA, Amant F, Braat D, D'Angelo A, Chuva de Sousa Lopes SM, Demeestere I, Dwek S, Frith L, Lambertini M et al. ESHRE guideline: female fertility preservation. Hum Reprod Open 2020;2020:hoaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: an Ethics Committee opinion. Fertil Steril 2018;110:380–386. [DOI] [PubMed] [Google Scholar]
- Fabiani C, Ferrante MG, Meneghini C, Licata E, Paciotti G, Gallo M, Schiavi M, Spina V, Guarino A, Caserta D, et al. Female fertility preservation: Impact of cancer on ovarian function and oocyte quality. Int J Gynaecol Obstet 2022;156:166–171. [DOI] [PubMed] [Google Scholar]
- Friedler S, Koc O, Gidoni Y, Raziel A, Ron-El R.. Ovarian response to stimulation for fertility preservation in women with malignant disease: a systematic review and meta-analysis. Fertil Steril 2012;97:125–133. [DOI] [PubMed] [Google Scholar]
- Fujimoto A, Ichinose M, Harada M, Hirata T, Osuga Y, Fujii T.. The outcome of infertility treatment in patients undergoing assisted reproductive technology after conservative therapy for endometrial cancer. J Assist Reprod Genet 2014;31:1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Velasco JA, Domingo J, Cobo A, Martínez M, Carmona L, Pellicer A.. Five years’ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil Steril 2013;99:1994–1999. [DOI] [PubMed] [Google Scholar]
- Goldrat O, Kroman N, Peccatori FA, Cordoba O, Pistilli B, Lidegaard O, Demeestere I, Azim HA.. Pregnancy following breast cancer using assisted reproduction and its effect on long-term outcome. Eur J Cancer 2015;51:1490–1496. [DOI] [PubMed] [Google Scholar]
- Goldrat O, Van Den Steen G, Gonzalez-Merino E, Dechène J, Gervy C, Delbaere A, Devreker F, De Maertelaer V, Demeestere I.. Letrozole-associated controlled ovarian hyperstimulation in breast cancer patients versus conventional controlled ovarian hyperstimulation in infertile patients: assessment of oocyte quality related biomarkers. Reprod Biol Endocrinol 2019;17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths MJ, Winship AL, Hutt KJ.. Do cancer therapies damage the uterus and compromise fertility? Hum Reprod Update 2020;26:161–173. [DOI] [PubMed] [Google Scholar]
- Gunnala V, Fields J, Irani M, D’Angelo D, Xu K, Schattman G, Rosenwaks Z.. BRCA carriers have similar reproductive potential at baseline to noncarriers: comparisons in cancer and cancer-free cohorts undergoing fertility preservation. Fertil Steril 2019;111:363–371. [DOI] [PubMed] [Google Scholar]
- Hartnett KP, Mertens AC, Kramer MR, Lash TL, Spencer JB, Ward KC, Howards PP.. Pregnancy after cancer: does timing of conception affect infant health? Cancer 2018;124:4401–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions. Cochrane, 2021. http://www.training.cochrane.org/handbook (21 October 2022, date last accessed). [Google Scholar]
- Huang N, Zeng L, Yan J, Chi H, Qiao J.. Analysis of in vitro fertilization/intracytoplasmic sperm injection outcomes in infertile women with a history of thyroid cancer: a retrospective study. Reprod Biol Endocrinol 2021;19:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddleston HG, Cedars MI, Sohn SH, Giudice LC, Fujimoto VY.. Racial and ethnic disparities in reproductive endocrinology and infertility. Am J Obstet Gynecol 2010;202:413–419. [DOI] [PubMed] [Google Scholar]
- Hussein RS, Zhao Y, Khan Z.. Does type of cancer affect ovarian response in oncofertility patients? J Gynecol Obstet Hum Reprod 2021;50:101944. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Global Cancer Observatory. Cancer Today2020. https://gco.iarc.fr/today/home (21 October 2022, date last accessed).
- IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ.. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;6:e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LNC, Dillon KE, Sammel MD, Efymow BL, Mainigi MA, Dokras A, Gracia CR.. Response to ovarian stimulation in patients facing gonadotoxic therapy. Reprod Biomed Online 2013;26:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Hughes J, Mahmoodi N, Smith E, Skull J, Ledger W.. What factors hinder the decision-making process for women with cancer and contemplating fertility preservation treatment? Hum Reprod Update 2017;23:433–457. [DOI] [PubMed] [Google Scholar]
- Karatasiou GI, Bosdou JK, Venetis CA, Zepiridis L, Chatzimeletiou K, Tarlatzi TB, Lainas G, Tarlatzis BC, Grimbizis G, Kolibianakis EM.. Is the probability of pregnancy after ovarian stimulation for IVF associated with serum estradiol levels on the day of triggering final oocyte maturation with hCG? A systematic review and meta-analysis. J Assist Reprod Genet 2020;37:1531–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawwass JF, Shandley LM, Boulet SL, Hipp HS.. Oncologic oocyte cryopreservation: national comparison of fertility preservation between women with and without cancer. J Assist Reprod Genet 2020;37:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim SK, Lee HJ, Lee JR, Jee BC, Suh CS, Kim SH.. Efficacy of random-start controlled ovarian stimulation in cancer patients. J Korean Med Sci 2015;30:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klock SC, , ZhangJX, , Kazer RR.. Fertility preservation for female cancer patients: early clinical experience. Fertil Steril 2010;94:149–155. [DOI] [PubMed] [Google Scholar]
- Knopman JM, , NoyesN, , TalebianS, , KreyLC, , GrifoJA, , Licciardi F.. Women with cancer undergoing ART for fertility preservation: a cohort study of their response to exogenous gonadotropins. Fertil Steril 2009;91:1476–1478. [DOI] [PubMed] [Google Scholar]
- Knopman JM, Papadopoulos EB, Grifo JA, Fino ME, Noyes N.. Surviving childhood and reproductive-age malignancy: effects on fertility and future parenthood. Lancet Oncol 2010;11:490–498. [DOI] [PubMed] [Google Scholar]
- Kosmas IP, Kolibianakis EM, Devroey P.. Association of estradiol levels on the day of hCG administration and pregnancy achievement in IVF: a systematic review. Hum Reprod 2004;19:2446–2453. [DOI] [PubMed] [Google Scholar]
- Krieger N. Structural racism, health inequities, and the two-edged sword of data: structural problems require structural solutions. Front Public Health 2021;9:655447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini M, Blondeaux E, Bruzzone M, Perachino M, Anderson RA, de Azambuja E, Poorvu PD, Kim HJ, Villarreal-Garza C, Pistilli B. et al. Pregnancy after breast cancer: a systematic review and meta-analysis. J Clin Oncol 2021;39:3293–3305. [DOI] [PubMed] [Google Scholar]
- Lambertini M, Del Mastro L, Pescio MC, Andersen CY, Azim HA, Peccatori FA, Costa M, Revelli A, Salvagno F, Gennari A. et al. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med 2016;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini M, Di Maio M, Pagani O, Curigliano G, Poggio F, Del Mastro L, Paluch-Shimon S, Loibl S, Partridge AH, Demeestere I. et al. The BCY3/BCC 2017 survey on physicians’ knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast 2018;42:41–49. [DOI] [PubMed] [Google Scholar]
- Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg JB, Paluch-Shimon S, Halaska MJ, Uzan C, Meissner J. et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2020;31:1664–1678. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006;24:2917–2931. [DOI] [PubMed] [Google Scholar]
- Lekovich J, Lobel ALS, Stewart JD, Pereira N, Kligman I, Rosenwaks Z.. Female patients with lymphoma demonstrate diminished ovarian reserve even before initiation of chemotherapy when compared with healthy controls and patients with other malignancies. J Assist Reprod Genet 2016;33:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Kelvin JF, Quinn GP, Gracia CR.. Infertility in reproductive-age female cancer survivors. Cancer 2015;121:1532–1539. [DOI] [PubMed] [Google Scholar]
- Logan S, Perz J, Ussher J, Peate M, Anazodo A.. Clinician provision of oncofertility support in cancer patients of a reproductive age: a systematic review. Psychooncology 2018;27:748–756. [DOI] [PubMed] [Google Scholar]
- Logan S, Perz J, Ussher JM, Peate M, Anazodo A.. Systematic review of fertility-related psychological distress in cancer patients: informing on an improved model of care. Psychooncology 2019;28:22–30. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Missmer SA, Spector LG, Leach RE, Williams M, Koch L, Smith YR, Stern JE, Ball GD. et al. Assisted reproductive technology use and outcomes among women with a history of cancer. Hum Reprod 2016a;31:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke B, Brown MB, Spector LG, Stern JE, Smith YR, Williams M, Koch L, Schymura MJ.. Embryo banking among women diagnosed with cancer: a pilot population-based study in New York, Texas, and Illinois. J Assist Reprod Genet 2016b;33:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhijani R, Godiwala P, Grady J, Christy A, Thornton K, Grow D, Engmann L.. Black race associated with lower live birth rate in frozen-thawed blastocyst transfer cycles: an analysis of 7,002 Society for Assisted Reproductive Technology frozen-thawed blastocyst transfer cycles. Fertil Steril 2021;117:360–367. [DOI] [PubMed] [Google Scholar]
- McLernon DJ, Steyerberg EW, Te Velde ER, Lee AJ, Bhattacharya S.. Predicting the chances of a live birth after one or more complete cycles of in vitro fertilisation: population based study of linked cycle data from 113 873 women. BMJ 2016;355:i5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaan N, , Ben-DavidG, , Ben-YosefD, , AlmogB, , ManyA, , PauznerD, , LessingJB, , AmitA, , Azem F.. Ovarian stimulation and emergency in vitro fertilization for fertility preservation in cancer patients. Eur J Obstet Gynecol Reprod Biol 2010;149:175–177. [DOI] [PubMed] [Google Scholar]
- Moria A, Das M, Shehata F, Holzer H, Son W-Y, Tulandi T.. Ovarian reserve and oocyte maturity in women with malignancy undergoing in vitro maturation treatment. Fertil Steril 2011;95:1621–1623. [DOI] [PubMed] [Google Scholar]
- de Moraes CC, Marinho VF, Campos AL, de Souza Guedes J, de Sousa Xavier ÉB, Caetano JP, Marinho RM.. Oocyte cryopreservation for future fertility: comparison of ovarian response between cancer and noncancer patients. JBRA Assist Reprod 2019;23:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder RL, Font-Gonzalez A, Hudson MM, van Santen HM, Loeffen EAH, Burns KC, Quinn GP, van Dulmen-den Broeder E, Byrne J, Haupt R. et al. Fertility preservation for female patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2021;22:e45–e56. [DOI] [PubMed] [Google Scholar]
- Nordan T, Thomas AM, Ginsburg ES, Wen PY, Dolinko AV, Bortoletto P.. Fertility preservation outcomes in women with gliomas: a retrospective case-control study. J Neurooncol 2020;147:371–376. [DOI] [PubMed] [Google Scholar]
- Noyes N, , LabellaPA, , GrifoJ, , Knopman JM.. Oocyte cryopreservation: a feasible fertility preservation option for reproductive age cancer survivors. J Assist Reprod Genet 2010;27:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurudeen SK, Douglas NC, Mahany EL, Sauer MV, Choi JM.. Fertility Preservation Decisions Among Newly Diagnosed Oncology Patients: A Single-Center Experience. Am J Clin Oncol 2016;39:154–159. [DOI] [PubMed] [Google Scholar]
- Oktay K, , HourvitzA, , SahinG, , OktemO, , SafroB, , CilA, , Bang H.. Letrozole Reduces Estrogen and Gonadotropin Exposure in Women with Breast Cancer Undergoing Ovarian Stimulation before Chemotherapy. J Clin Endocrinol Metab 2006;91:3885–3890. [DOI] [PubMed] [Google Scholar]
- Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, Wallace WH, Wang ET, Loren AW.. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2018;36:1994–2001. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal L, , LeykinL, , SchifrenJL, , IsaacsonKB, , ChangYC, , NikruilN, , ChenZ, , Toth TL.. Malignancy may adversely influence the quality and behaviour of oocytes. Hum Reprod 1998;13:1837–1840. [DOI] [PubMed] [Google Scholar]
- Pavone ME, Hirshfeld-Cytron J, Lawson AK, Smith K, Kazer R, Klock S.. Fertility preservation outcomes may differ by cancer diagnosis. J Hum Reprod Sci 2014;7:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L.. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med 2007;26:4544–4562. [DOI] [PubMed] [Google Scholar]
- Pereira N, Hancock K, Cordeiro CN, Lekovich JP, Schattman GL, Rosenwaks Z.. Comparison of ovarian stimulation response in patients with breast cancer undergoing ovarian stimulation with letrozole and gonadotropins to patients undergoing ovarian stimulation with gonadotropins alone for elective cryopreservation of oocytes†. Gynecol Endocrinol 2016;32:823–826. [DOI] [PubMed] [Google Scholar]
- Poorvu PD, Frazier AL, Feraco AM, Manley PE, Ginsburg ES, Laufer MR, LaCasce AS, Diller LR, Partridge AH.. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectr 2019;3:pkz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu E, Cillo GM, Cipriani L, Sacilotto F, Notarangelo L, Damiano G, Dirodi M, Roncarati I.. Impact of BRCA1 and BRCA2 mutations on ovarian reserve and fertility preservation outcomes in young women with breast cancer. J Assist Reprod Genet 2020;37:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril 2019;112:1022–1033. [DOI] [PubMed] [Google Scholar]
- Quinn MM, Cakmak H, Letourneau JM, Cedars MI, Rosen MP.. Response to ovarian stimulation is not impacted by a breast cancer diagnosis. Hum Reprod 2017;32:568–574. [DOI] [PubMed] [Google Scholar]
- Quintero RB, Helmer A, Huang JQ, Westphal LM.. Ovarian stimulation for fertility preservation in patients with cancer. Fertil Steril 2010;93:865–868. [DOI] [PubMed] [Google Scholar]
- Riley RD, Higgins JPT, Deeks JJ.. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- Robertson AD, , MissmerSA, , Ginsburg ES.. Embryo yield after in vitro fertilization in women undergoing embryo banking for fertility preservation before chemotherapy. Fertil Steril 2011;95:588–591. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Marklund A, Lundberg F, Wikander I, Milenkovic M, Anastacio A, Sergouniotis F, Wånggren K, Ekengren J, Lind T. et al. A prospective study of women and girls undergoing fertility preservation due to oncologic and non-oncologic indications in Sweden-Trends in patients’ choices and benefit of the chosen methods after long-term follow up. Acta Obstet Gynecol Scand 2019;98:604–615. [DOI] [PubMed] [Google Scholar]
- Sabatini ME, Wolkovich AM, Macklin EA, Wright DL, Souter I, Toth TL.. Pronuclear embryo cryopreservation experience: outcomes for reducing the risk of ovarian hyperstimulation syndrome and for fertility preservation in cancer patients. J Assist Reprod Genet 2011;28:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifer DB, Simsek B, Wantman E, Kotlyar AM.. Status of racial disparities between black and white women undergoing assisted reproductive technology in the US. Reprod Biol Endocrinol 2020;18:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, Klinger FG.. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 2019;25:673–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Gavaghan D, Egger M.. Publication and related bias in meta-analysis. J Clin Epidemiol 2000;53:1119–1129. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- Tamauchi S, Kajiyama H, Osuka S, Moriyama Y, Yoshihara M, Kikkawa F.. Reduced response to controlled ovarian stimulation after radical trachelectomy: a pitfall of fertility-sparing surgery for cervical cancer. Int J Gynaecol Obstet 2021;154:162–168. [DOI] [PubMed] [Google Scholar]
- Tsampras N, Roberts SA, Gould D, Fitzgerald CT.. Ovarian response to controlled ovarian stimulation for fertility preservation before oncology treatment: A retrospective cohort of 157 patients. Eur J Cancer Care (Engl) 2018;27:e12797. [DOI] [PubMed] [Google Scholar]
- Turan V, Quinn MM, Dayioglu N, Rosen MP, Oktay K.. The impact of malignancy on response to ovarian stimulation for fertility preservation: a meta-analysis. Fertil Steril 2018;110:1347–1355. [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. U.S. Preventive Services Task Force Procedure Manual. 2021. https://www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/procedure-manual (16 May 2022, date last accessed).
- van Loendersloot LL, van Wely M, Limpens J, Bossuyt PMM, Repping S, van der Veen F.. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update 2010;16:577–589. [DOI] [PubMed] [Google Scholar]
- Wan X, Wang W, Liu J, Tong T.. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M, , RehA, , LabellaPA, , Noyes N.. Laboratory evaluation in oocyte cryopreservation suggests retrieved oocytes are comparable whether frozen for medical indications, deferred reproduction or oocyte donation. J Assist Reprod Genet 2010;27:613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wo JY, Viswanathan AN.. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys 2009;73:1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and its supplementary material.