Abstract
BACKGROUND
Reproductive tract infection is an important factor leading to male and female infertility. Among female infertility factors, microbial and viral infections are the main factors affecting female reproductive health and causing tubal infertility, ectopic tubal pregnancy and premature delivery. Among male infertility factors, 13–15% of male infertility is related to infection. Defensins are cationic antibacterial and antiviral peptides, classified into α-defensins, β-defensins and θ-defensins. Humans only have α-defensins and β-defensins. Apart from their direct antimicrobial functions, defensins have an immunomodulatory function and are involved in many physiological processes. Studies have shown that defensins are widely distributed in the female reproductive tract (FRT) and male reproductive tract (MRT), playing a dual role of host defence and fertility protection. However, to our knowledge, the distribution, regulation and function of defensins in the reproductive tract and their relation to reproduction have not been reviewed.
OBJECTIVE AND RATIONALE
This review summarizes the expression, distribution and regulation of defensins in the reproductive tracts to reveal the updated research on the dual role of defensins in host defence and the protection of fertility.
SEARCH METHODS
A systematic search was conducted in PubMed using the related keywords through April 2022. Related data from original researches and reviews were integrated to comprehensively review the current findings and understanding of defensins in the human reproductive system. Meanwhile, female and male transcriptome data in the GEO database were screened to analyze defensins in the human reproductive tracts.
OUTCOMES
Two transcriptome databases from the GEO database (GSE7307 and GSE150852) combined with existing researches reveal the expression levels and role of the defensins in the reproductive tracts. In the FRT, a high expression level of α-defensin is found, and the expression levels of defensins in the vulva and vagina are higher than those in other organs. The expression of defensins in the endometrium varies with menstrual cycle stages and with microbial invasion. Defensins also participate in the local immune response to regulate the risk of spontaneous preterm birth. In the MRT, a high expression level of β-defensins is also found. It is mainly highly expressed in the epididymal caput and corpus, indicating that defensins play an important role in sperm maturation. The expression of defensins in the MRT varies with androgen levels, age and the status of microbial invasion. They protect the male reproductive system from bacterial infections by neutralizing lipopolysaccharide and downregulating pro-inflammatory cytokines. In addition, animal and clinical studies have shown that defensins play an important role in sperm maturation, motility and fertilization.
WIDER IMPLICATIONS
As a broad-spectrum antimicrobial peptide without drug resistance, defensin has great potential for developing new natural antimicrobial treatments for reproductive tract infections. However, increasing evidence has shown that defensins can not only inhibit microbial invasion but can also promote the invasion and adhesion of some microorganisms in certain biological environments, such as human immunodeficiency virus. Therefore, the safety of defensins as reproductive tract anti-infective drugs needs more in-depth research. In addition, the modulatory role of defensins in fertility requires more in-depth research since the current conclusions are based on small-size samples. At present, scientists have made many attempts at the clinical transformation of defensins. However, defensins have problems such as poor stability, low bioavailability and difficulties in their synthesis. Therefore, the production of safe, effective and low-cost drugs remains a challenge.
Keywords: defensin, reproductive tract, fertility, infertility, maternal–fetal interface, sperm, premature delivery, antibacterial, antivirus, immunomodulation
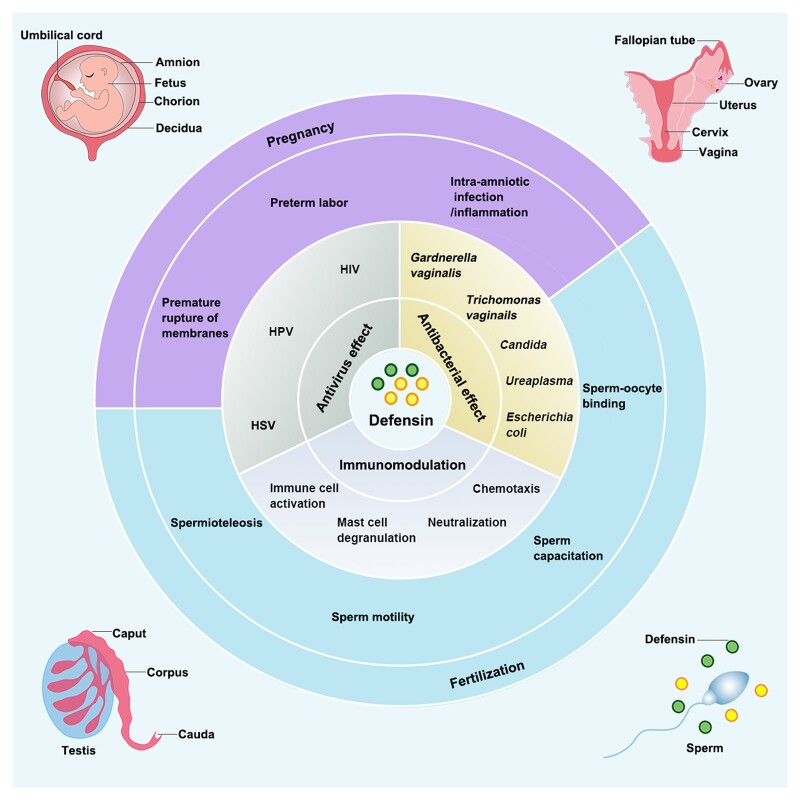
Graphical Abstract
Graphical Abstract.
The dual roles of defensins in the human reproductive system: host defence and protection of fertility.
Introduction
The mucosal innate immunity provides rapid and nonselective protection for the mucosa of the digestive, respiratory and urogenital system via the uppermost mucus lining, a cellular junctional complex-linked epithelial barrier, along with antimicrobial peptides and cytokines secreted by epithelial and innate immune cells (Wira et al., 2005). Among various antimicrobial peptides expressed in the human body, defensins are one of the most abundantly expressed classes. They are a class of cationic peptides expressed predominantly by neutrophils and epithelial cells and they act as an essential component in the mucosal innate immune response against infectious pathogens (Lehrer and Lu, 2012). Many comprehensive reviews have discussed the complex mechanism of the antimicrobial activity of defensins and their roles in the mucosal immunity of the digestive and respiratory systems (Schutte and Mccray, 2002; Ganz, 2003; Klotman and Chang, 2006; Ouellette, 2011; Xu and Lu, 2020).
Like the digestive and respiratory mucosa, reproductive tract mucosa is constantly exposed to endogenous and exogenous microbial invasion pressure. Different sources of pathogen invasion can lead to sexually transmitted diseases (STDs), endogenous infections or iatrogenic infections, which are classified as reproductive tract infections. They affect not only the patients’ health but also cause adverse reproductive outcomes, such as infertility, premature delivery and miscarriage (Brunham and Paavonen, 2020; Sharma et al., 2022). In the reproductive system, defensins are widely expressed, and their expression levels are modulated by hormone levels, age and microbial invasion (Quayle et al., 1998; Fleming et al., 2003; Cao et al., 2010; Yu et al., 2013). They are an important part of the innate immune defence system of the reproductive tract mucosa, which can protect the reproductive tract from microorganisms. In addition, defensins expressed in the reproductive systems are also involved in various reproductive events, such as sperm maturation, sperm transportation, sperm–oocyte binding, implantation, pregnancy process, fetal development and childbirth (Tollner et al., 2011, 2012; Burris et al., 2020). This review summarizes the expression, distribution and regulation of defensins in the female reproductive tract (FRT) and male reproductive tract (MRT) and shows the progress of research on the dual roles of defensins in host defence and the protection of reproductive tract fertility.
Methods
A comprehensive search of the scientific literature on PubMed was conducted through to April 2022 using the following keywords and phrases in combination with each other; ‘defensins’, ‘defensins general structure’, ‘defensins classification and expression’, ‘defensins and sterility’, ‘defensins and pregnancy’, ‘defensins and fertilization’, ‘defensins and preterm birth’, ‘defensins and reproductive tract’, ‘defensins and sperm’, ‘defensins and epididymis’, ‘defensins and testis’, ‘defensins and prostate’, ‘defensins and estrogen’, ‘defensins and androgen’, ‘defensins and vaginal microbiota’, ‘defensins and female microbiota’, ‘defensins and male microbiota’, ‘defensins and microbial infections’, ‘defensins and viral infections’, ‘defensins and sexually transmitted diseases’, ‘defensins and HIV’, ‘defensins and HPV’, ‘defensins and bacterial vaginosis’, ‘defensins and Candida’, ‘defensins and Mycoplasma’, ‘defensins and Neisseria gonorrhoeae’, ‘defensins and G. vaginalis’, ‘defensins and Escherichia coli’, ‘defensins and Ureaplasma’, ‘defensins and Trichomonas vaginalis’. Related data and conclusions from original research and reviews were integrated to comprehensively review the current findings and understanding of defensins in the human reproductive system. In certain parts where human research was limited, data from animal studies were used for supplementation.
Meanwhile, transcriptome data were searched in the GEO database using the keywords: ‘female reproductive tract’ and ‘male reproductive tract’. GeneChip array data GSE7307 and high-throughput sequencing data GSE150852 were screened to analyze defensins in the human reproductive tracts.
Defensins
Classification of defensins
Defensins are a class of cationic and Cys-rich peptides with 18–45 amino acids (2–5 kDa) with conserved three-dimensional structures: prominent β-sheets stabilized by three disulfide bonds (Pazgier et al., 2006; Lehrer and Lu, 2012; Shafee et al., 2017). According to the position of the disulfide bond, they are grouped into α-defensins, β-defensins and θ-defensins (Selsted et al., 1993; Tang et al., 1999; Lehrer, 2004; Lehrer et al., 2012). Alpha-defensins and β-defensins are conserved in humans and other animals, while θ-defensins are cyclic peptides found only in the white blood cells of non-human primates (Cole et al., 2002; Lehrer et al., 2012).
α-defensins
Six α-defensins have been identified in humans so far; based on their expression pattern and genetic organization, they are subdivided into myeloid and enteric peptides (Selsted and Ouellette, 2005). Lehrer’s group first identified three abundantly expressed human myeloid α-defensins in the azurophilic granules of neutrophils and named these peptides human neutrophil peptides 1, 2 and 3 (HNP1, HNP2, HNP3) in 1985 (Ganz et al., 1985). Although encoded by different genes (DEFA1 and DEFA3), HNP1 and HNP3 only differ at the first residue at the N-terminus, and HNP2 is the proteolytic product of either HNP1 or HNP3 (Sparkes et al., 1989; Linzmeier et al., 1993; Mars et al., 1995; Holly et al., 2017). HNP4, the last identified myeloid α-defensin, exhibits the same cysteine framework and structural similarity to HNP1–3 (Gabay et al., 1989; Wilde et al., 1989). However, the amino acid sequence of HNP4 is significantly different from that of HNP1–3 and this results in a notable functional difference between them (Hu et al., 2019). HNP1–4 are synthesized in the promyelocytic bone marrow precursors, and up to 30–50% of the proteins in the azurophilic granules are HNP1–3, but there is a hundred times lower amount of HNP4 (Gabay et al., 1989). They can also be found in monocytes/macrophages, natural killer cells, T cells, B cells and immature dendritic cells (DCs) (Selsted and Ouellette, 2005; Pazgier et al., 2007). The other two enteric α-defensins, human defensin 5 and 6 (HD5 and HD6), share almost the same three-dimensional structure as the myeloid α-defensins but have distinct amino acid compositions. The two peptides are mainly expressed in Paneth cells at the bottom of the intestinal glands (or the crypt of Lieberkühn) (Jones and Bevins, 1992; 1993). Moreover, HD5 is also expressed in the epithelial cells of the male and female urogenital tracts (Quayle et al., 1998; Com et al., 2003; Ouellette, 2011; Spencer et al., 2012). A more detailed structure and sequence information of human α-defensins can be found in the reviews written by Chang, Lu and Lehrer ( Klotman and Chang, 2006; Pazgier et al., 2007; Lehrer and Lu, 2012).
β-defensins
From the first human β-defensin, HBD1, identified in the plasma of a kidney disease patient in 1995, 34 β-defensin genes (including three pseudogenes: DEFB109A, DEFB117 and DEFB122) are now listed in the NCBI gene database (Bensch et al., 1995). However, not all have been studied at the protein level (Table I) and only four have been studied in detail (HBD1–4). The amino acid composition of β-defensins is significantly different from that of the α-defensins, resulting in a three-stranded β-sheet structure that can be further divided into hydrophobic and charged domains (Pazgier et al., 2006). Chromosome 8p21-p23 region was the first identified gene cluster to encode the β-defensin proteins HBD1–3 (corresponding genes: DEFB1, DEFB4 and DEFB103), and this region also contains all α-defensin protein genes. This finding indicates that there is a common ancestral defensin gene. Subsequently, three other DEFB gene clusters were found on chromosomes 6p12, 20q11.1 and 20p13 (Bevins et al., 1996; Liu et al., 1997; Schutte et al., 2002). Compared with the restricted expression of α-defensins, the expression of β-defensins is more widely distributed in various tissues in the human body. The major expression sites include skin keratinocyte and epithelial cells of the reproductive, urinary, digestive and respiratory tracts (O'Neil, 2003; Pazgier et al., 2006).
Table I.
The expression of defensins in the human reproductive tracts.
| Gene symbols | Female reproductive tract | Male reproductive tract | ||||
|---|---|---|---|---|---|---|
| Expression site | Detected molecule | Reference | Expression site | Detected molecule | Reference | |
| DEFA1–3 (HNP1–3) | Vaginal fluid | Protein | (Svinarich et al., 1997; Hein et al., 2002; Akinbi et al., 2004; Das et al., 2007, 2008; Lucovnik et al., 2011) | Testis | mRNA | (Com et al., 2003) |
| Cervical mucus | Protein | |||||
| Endometrium | Protein | |||||
| Follicular fluid | Protein | |||||
| Amniotic fluid | Protein | Spermatozoa | mRNA | |||
| Amnion | mRNA | |||||
| Chorion | mRNA | |||||
| Decidua | mRNA | |||||
| DEFA4 (HNP4) | Not reported | Not reported | ||||
| DEFA5 (HD5) | Vagina | Protein and mRNA | (Quayle et al., 1998) | Testis | mRNA | (Com et al., 2003) |
| Cervix | Protein and mRNA | |||||
| Endometrium | Protein and mRNA | |||||
| Fallopian tube | Protein and mRNA | Spermatozoa | mRNA | |||
| Cervicovaginal lavages | Protein | |||||
| DEFA6 (HD6) | Vagina | mRNA | (Fichorova et al., 1997; Klotman et al., 2008) | Testis | mRNA | (Com et al., 2003) |
| Ectocervix | mRNA | |||||
| Endocervix | mRNA | |||||
| Gene symbols | Female reproductive tract | Male reproductive tract | ||||
|---|---|---|---|---|---|---|
| Expression site | Detected molecule | Reference | Expression site | Detected molecule | Reference | |
| DEFB1 (HBD1) | Amnion epithelium | Protein | (Zhao et al., 1996; Hein et al., 2002; King et al., 2002, 2007a,b; Erhart et al., 2011; Noda-Nicolau et al., 2017) | Semen | Protein | (Zhao et al., 1996; Com et al., 2003; Zupin et al., 2019a) |
| Chorion trophoblast | Protein | |||||
| Decidua | Protein | Testis | Protein and mRNA | |||
| Placental syncytiotrophoblast | Protein | |||||
| Vagina | Protein and mRNA | Spermatozoa | Protein and mRNA | |||
| Cervicovaginal fluid | Protein | |||||
| DEFB1 (HBD1) | Amniotic fluid | Protein | (King et al., 2002; Varrey et al., 2018) | Prostate glands | Protein and mRNA | (Hong et al., 2017) |
| Endometrium | mRNA | |||||
| Placenta | mRNA | |||||
| DEFB2 (HBD2) | Amnion epithelium | Protein | (King et al., 2002; Soto et al., 2007; King et al., 2007a,b; Erhart et al., 2011; Noda-Nicolau et al., 2017) | Not reported | ||
| Chorion trophoblast | Protein | |||||
| Decidua | Protein | |||||
| Placental syncytiotrophoblast | Protein | |||||
| Amniotic fluid | Protein | |||||
| Vagina | Protein and mRNA | |||||
| Cervicovaginal fluid | Protein | |||||
| Endometrium | mRNA | |||||
| Genes (and proteins) | Female reproductive tract | Male reproductive tract | ||||
|---|---|---|---|---|---|---|
| Expression site | Detected molecule | Reference | Expression site | Detected molecule | Reference | |
| DEFB3 (HBD3,DEFB103A) | Amnion epithelium and mesenchyme | Protein | (King et al., 2002, 2003a,b, 2007a,b; Erhart et al., 2011; Noda-Nicolau et al., 2017; Para et al., 2020) | Not reported | ||
| Amniotic fluid | Protein | |||||
| Chorion trophoblast | Protein | |||||
| Decidua | Protein | |||||
| Placental syncytiotrophoblast | Protein | |||||
| Vagina | Protein and mRNA | |||||
| Cervicovaginal fluid | Protein | |||||
| Endometrium | mRNA | |||||
| DEFB4 (HBD4,DEFB104A) | Chorioamniotic membranes | mRNA | (King et al., 2003a,b; Polettini et al., 2011) | Epididymis | mRNA | (Lehrer and Ganz, 2002; Yamaguchi et al., 2002) |
| Endometrium | mRNA | Testis | mRNA | |||
| DEFB5 (DEFB105A, DEFB105B) | Not reported | Testis | mRNA | (Yamaguchi et al., 2002; Semple et al., 2003) | ||
| Epididymis | mRNA | |||||
| DEFB106 (DEFB106A, DEFB-6) | Not reported | Testis | mRNA | (Yamaguchi et al., 2002; Semple et al., 2003; Xin et al., 2014) | ||
| Epididymis | Protein and mRNA | |||||
| DEFB107 (DEFB107A) | Not reported | Testis | mRNA | (Semple et al., 2003) | ||
| Gene symbols | Female reproductive tract | Male reproductive tract | ||||
|---|---|---|---|---|---|---|
| Expression site | Detected molecule | Reference | Expression site | Detected molecule | Reference | |
| DEFB108 (DEFB108A/B/C) | Not reported | Testis | mRNA | (Semple et al., 2003) | ||
| DEFB109 (DEFB109B/C) | Not reported | Testis | mRNA | (Schutte et al., 2002; Patil et al., 2005) | ||
| Prostate | mRNA | |||||
| DEFB114 | Not reported | Epididymis | Protein and mRNA | (Yu et al., 2013) | ||
| DEFB118 | Not reported | Sperm | Protein | (Yenugu et al., 2004) | ||
| DEFB119 (DEFB120) | Not reported | Testis | mRNA | (Radhakrishnan et al., 2005) | ||
| Epididymis | mRNA | |||||
| DEFB121 | Not reported | Testis | mRNA | (Radhakrishnan et al., 2005) | ||
| Epididymis | mRNA | |||||
| DEFB123 | Not reported | Testis | mRNA | (Radhakrishnan et al., 2005) | ||
| Epididymis | mRNA | |||||
| DEFB124 | Not reported | Prostate epithelia | Protein and mRNA | (Kim et al., 2014) | ||
| Epididymis | mRNA | |||||
| DEFB125–128 (DEFB25–28) | Not reported | Testis | mRNA | (Rodríguez-Jiménez et al., 2003) | ||
| Epididymis | mRNA | |||||
| DEFB129 | Not reported | Testis | mRNA | (Rodríguez-Jiménez et al., 2003; Dubé et al., 2008) | ||
| Not reported | Epididymis | Protein and mRNA | ||||
| DEFB131 | Not reported | Prostate epithelia | Protein and mRNA | (Kim et al., 2015) | ||
| DEFB132 | Not reported | Epididymis | Protein | (Lin et al., 2008) | ||
| Not reported | Sperm | Protein | ||||
| Gene symbols | β-defensin mRNAs detected in the transcriptome analysis but not reported in the published articles on reproductive tracts | ||
|---|---|---|---|
| Expression site | Detected molecule | Reference | |
| DEFB110 | Not reported | (Schutte et al., 2002) | |
| DEFB111-112 | Gingival tissues and keratinocytes | mRNA | (Schutte et al., 2002; Premratanachai et al., 2004) |
| DEFB113 | Not reported | (Schutte et al., 2002) | |
| DEFB115-117 | Not reported | (Schutte et al., 2002) | |
| DEFB122 | Not reported | (Schutte et al., 2002) | |
| DEFB130 | Not reported | (Schutte et al., 2002) | |
| DEFB133 | Not reported | (Schutte et al., 2002) | |
θ-defensins
Naturally synthesized θ-defensins are only found in certain non-human primates, such as rhesus macaques (Tang et al., 1999) and olive baboons (Garcia et al., 2008). Different from the structure of α-defensin and β-defensin, the connecting positions of disulfide bonds in the molecular chain of θ-defensin are Cys1-Cys4, Cys2-Cys5 and Cys3-Cys6, forming a ring structure (Lehrer et al., 2012). Human bone marrow contains mRNA very similar to the rhesus θ-defensin precursor mRNA, while a stop codon exists in its signal sequence, resulting in the failure to produce human endogenous θ-defensin peptides (Lehrer et al., 2012). However, the pseudogene sequences provided the sequence foundation for in vitro synthesis of the functional peptides. Lehrer and colleagues hence reconstructed humanized θ-defensins based on these sequences via cloning, peptide synthesis and molecular archaeology and named them ‘retrocyclins’ (Cole et al., 2002). The unique cyclic structure allows θ-defensins to show more resistance to high salts and proteases, which are advantages in antimicrobial activities.
General function of defensins
Firstly discovered as a class of antibacterial peptides, human defensins also exhibit antiviral, antifungal and immunomodulatory effects in inflammation, development and cancer (Lai and Gallo, 2009; Semple and Dorin, 2012; Holly et al., 2017; Ordonez et al., 2017; Jin and Weinberg, 2019) (Fig. 1). Among the various functions, the antibacterial function of defensins is the most well-studied. Based on their positively charged character, defensins can kill bacteria or inhibit bacterial growth through different mechanisms, including directly punching holes in the negatively charged bacterial membrane (Lehrer and Lu, 2012) and inhibiting bacterial cell wall synthesis via interaction with the cell wall precursor lipid II (De Leeuw et al., 2010). They can also neutralize bacterial secreted toxins to reduce bacterial infection (Kim et al., 2005). The antibacterial mechanism of defensins mainly includes three steps: (i) the positively charged defensins bind with the negatively charged bacterial membrane by electrostatic attraction; (ii) defensin molecules or their polymers interact with phospholipid heads and water molecules on the bacterial plasma membrane to form a channel, which significantly increases the permeability of the biological membrane; and (iii) bacterial inner cell content leakage occurs (Lehrer et al., 1989; Zasloff, 2002; Selsted and Ouellette, 2005; Shafee et al., 2017). The detailed mechanisms of bacterial killing by human α-defensins and β-defensins can be different because α-defensins are more hydrophobic (Lehrer and Lu, 2012). Different β-defensins show distinct preferences for various bacteria: HBD1 and HBD2 are more active against Gram-negative bacteria (Harder et al., 1997), while HBD3 is active against both Gram-positive and -negative strains (Harder et al., 2001).
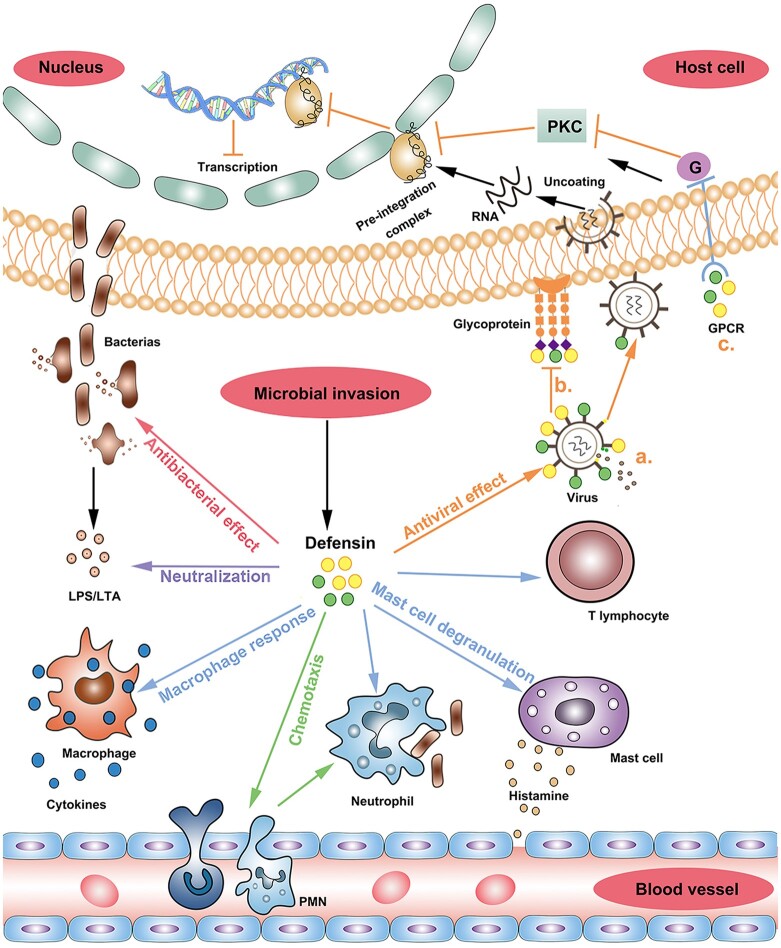
Figure 1.
Host defence functions of defensins. Different arrow colors represent different functions of defensins. Red arrow: antibacterial effect. Purple arrow: neutralization effect. Blue arrow: immune cell activation (including activating the phagocytosis of macrophages and neutrophils, promoting histamine release from mast cells, increasing vascular permeability and recruiting T cells to regulate specific immunity). Green arrow: chemotaxis. (Defensins stimulate the chemotaxis of peripheral blood monocyte and neutrophils to reach the infection site quickly.) Orange arrow: antiviral effect. a. The defensins bind to glycoprotein receptors of the virus and the cell surface, restricting the virus from entering the cell; b. The defensin pierces the virus surface, causing its contents to leak out and death; c. When a virus infects host cells, defensins inhibit the transcription of the virus by inhibiting the PKA signaling pathway. LPS/LTA, lipopolysaccharide/lipoteichoic acid; PKC, protein kinase C; GPCR, G-protein coupled receptor; PMN, polymorphonuclear neutrophil.
Defensins have a direct inhibitory effect on viral infections. The degree of inhibition of viruses depends on the concentration of defensins and the tightness of intramolecular disulfide bonds. Its antiviral efficacy is also impacted by time, pH, ionic strength and temperature. Defensins have strong antiviral activity under neutral and low ionic strength conditions (Holly et al., 2017). The α-defensins and β-defensins are sensitive to enveloped viruses. Furthermore, the antiviral activity is executed by inhibiting the interaction between viral glycoproteins and the receptors on the plasma membrane of target cells, which leads to the interruption of the fusion between viral particles and the host cell. Alternatively, they can be absorbed by the negatively charged glycoprotein on the surface of the enveloped virus, therefore perforating the virus particles and forming a breach on their surface, consequently leading to a leak of viral contents and death of the virus (Holly et al., 2017). For non-enveloped viruses, α-defensins prevent infection through direct interaction with the viral capsid, preventing uncoating and leading to internalized viruses that mislead lysosomal degradation. Unlike enveloped viruses, non-enveloped viruses have not been reported to be significantly affected by β-defensins (Wiens and Smith, 2017). In addition, HNP1–3 also execute their antiviral activity by inhibiting the protein kinase C (PKC) signaling pathway involved in virus fusion, transcription, integration and assembly (Yu et al., 1995; Chang et al., 2005).
Defensins also show immunomodulatory effects in both innate and adaptive immune responses. They promote the release of chemokines and activate or mobilize macrophages and mast cells to reach tissues infected by pathogens (Chertov et al., 1996). In addition, defensins can induce the maturation of DCs, mobilize DCs and enhance their antigen uptake ability (Yang et al., 2007). Studies have demonstrated that chemotactic characteristics of defensins on various immune cells: HNP1–3 and HBD3-4 for monocytes/macrophages (Territo et al., 1989; Verbanac et al., 1993; Yang et al., 2002), HBD2 for neutrophils activated by tumor necrosis factor (TNF)-alpha (Niyonsaba et al., 2004), HNP1, HBD1–3 for immature DCs (Yang et al., 2000; Conejo-Garcia et al., 2004), HNP1-2 for peripheral blood T cells (Chertov et al., 1996; Yang et al., 2000) and HBD1 and HBD2 for memory T cells expressing CCR6 (Yang et al., 1999). The chemoattractive effect of defensins on T cells suggests that they can help the recruitment of CD4+ and CD8+ effector T cells to the site of microbial infection (Yang et al., 2000).
Direct regulation of defensins expression
The production of defensins is induced by stimuli sensed by receptors called pattern recognition receptors (PRRs) on the surface of defensin-expressing cells. The stimuli include various microbial products, such as proteins, lipids, lipoproteins and nucleic acids (Tang et al., 2012). There are four types of PRR: the toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors, retinoic acid-inducible gene-I-like receptors and C-type lectin receptors (Takeuchi and Akira, 2010). Corresponding ligand binding of PRRs triggers the subsequent signaling cascade, resulting in the activation of transcription factors that target the gene transcription of defensins or other antimicrobial peptides in response to microbial stimuli or other physical, physiological or oxidative stressors (Oeckinghaus et al., 2011). The release of HNP1–3 by neutrophils can be triggered in response to chemokines, FCγ receptor cross-linking, phorbol myristate acetate and bacterial products binding TLRs (Fruitwala et al., 2019). NOD2 and Wnt signaling transcription factor TCF-4 (T cell factor 4) proteins regulate the expression of HD5 in the small intestine (Wehkamp et al., 2004, 2007). HD6 gene expression is influenced by the TCF-4 binding site and the E-box site in the proximal promoter region (Hayashi et al., 2016). The expression of HBD1–3 is mainly induced by TLRs (Fruitwala et al., 2019).
Defensins expression and hormonal regulation in the human reproductive tracts
Defensins expression and hormonal regulation in the female reproductive tract
The FRT is constantly challenged by exogenous pathogens; however, during pregnancy, the FRT immune response must distinguish between the developing fetus and evading pathogens to protect the former and eliminate the latter. In accordance with these double roles, the FRT is divided into the upper FRT (including the endocervix, uterus and fallopian tubes) and the lower FRT (including the vagina and ectocervix) (Wira et al., 2015). The upper FRT is lined by simple columnar epithelium, and the main immune cell population in the lamina propria is composed of T cells, macrophages and innate lymphoid cells (ILCs, including natural killer cells, ILC1, ILC2, ILC3 and lymphoid tissue-inducer (LTi) cells) (Vivier et al., 2018). In contrast, the lower FRT is lined by non-keratinized stratified squamous epithelium, and the immune cell population in the lamina propria is more diverse, including T cells, DCs, granulocytes, macrophages, ILCs and B cells (Iwasaki, 2010; Monin et al., 2020). While the composition of immune cells changes under the regulation of hormones during the menstrual cycle in the upper FRT, it remains relatively stable in the lower FRT. However, their function may differ during the menstrual cycle (Wira et al., 2015). On the inner surface of the FRT epithelium, a layer of mucus made up of mucins and containing many antimicrobial factors, like complement components and antimicrobial peptides, forms a chemical barrier for the FRT (Iwasaki, 2010). As important members of the antimicrobial peptides, α and β-defensins are produced by leucocytes or epithelial cells distributed along the FRT, with different preferences.
α-defensins
Transcriptome data (GEO accession No.: GSE7307) show that the levels of mRNA (DEFA1–3) encoding HNP1–3 in the lower FRT (vagina) are higher than in the other parts of the FRT (Fig. 2A, Table I). This is due to the ability of most of the immune cells in the vaginal lamina propria to produce and secrete HNP1–3, including neutrophils, eosinophils, natural killer cells, monocytes, γδT lymphocytes, B lymphocytes and immature DCs (Agerberth et al., 2000; Nathan, 2006; Rodríguez-García et al., 2007; Driss et al., 2009). HNP1–3 have also been tested via ELISA in cervicovaginal secretions and are regarded as an indicator of FRT viral and bacterial infection (Shust et al., 2010; Levinson et al., 2012; Guthrie et al., 2015). Moreover, HNP1–3 are expressed in the endometrium and play a role in maternal antibacterial process (as discussed below) (Das et al., 2007; King et al., 2007a,b). Although the mRNA encoding HNP4 was detected at relatively high levels in the vulva, vagina, cervix and uterus, and at low levels in the endometrium, myometrium, and ovary via the GeneChip array, the protein has not been tested in any research so far. Both the mRNA and protein for HD5 have been verified in FRT. It is constantly expressed in the upper half of the stratified squamous epithelium of the vagina and ectocervix with an increased amount toward the lumen and is also present in the endocervix, endometrium and fallopian tube with an individual differences (Quayle et al., 1998). The transcriptome data of endometrium and myometrium and in vitro studies using immortalized epithelial cells from normal human vagina, ectocervix and endocervix have reported the presence of mRNA encoding HD6 (Fichorova et al., 1997; Klotman et al., 2008). However, the HD6 protein has not been verified in the FRT so far. Also, in contrast with the transcriptome data where HNP4, HD5 and HD6 had relatively high mRNA levels in the vulva, Erhart et al. (2011) demonstrated scarcely detectable HNP1–3, HD5 and HD6 in normal or infected vulval tissue using QPCR (Quantitative real-time polymerase chain reaction) and immunohistochemical analysis.
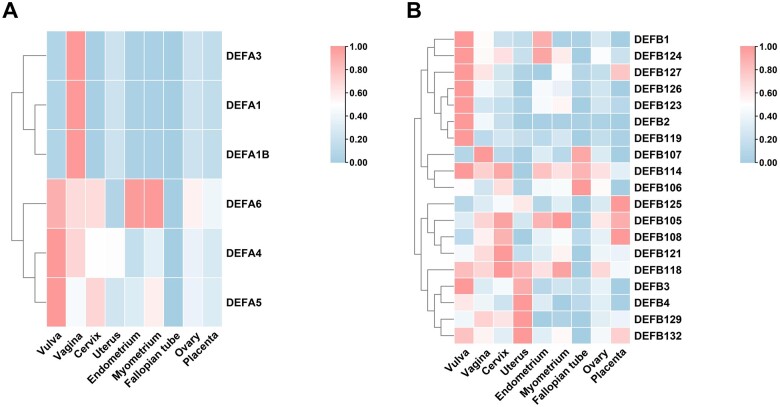
Figure 2.
Transcriptome data of defensins in the female reproductive tract. (A) α-defensins. (B) β-defensins. The mean mRNA levels of 6 α-defensins and 18 β-defensins in the MRT (including vulva, vagina, cervix, uterus, endometrium, myometrium, fallopian tube, ovary and placenta) were summarized from transcriptome data GSE7307 in the GEO database. Number of samples: 4 vulvae, 4 vaginae, 4 cervices, 1 uterus, 18 endometria, 19 myometria, 1 fallopian tube, 5 ovaries and 1 placenta.
β-defensins
Among the 11 β-defensins studied at the protein level (see Table I), only HBD1–4 have been exclusively studied in the FRT (Fig. 2B). All four proteins are mainly expressed in the corresponding epithelial cells. HBD1 and HBD2 are expressed along the upper and lower FRT and in the cervicovaginal secretions, and the expression can be upregulated upon infection status (Valore et al., 1998; King et al., 2003a; Ghosh et al., 2008; Meng et al., 2013). HBD3 mRNA or protein has been reported in the vagina, cervix and endometrium in large amounts; however, they are not present in the fallopian tube (King et al., 2003a; Meng et al., 2013; Mitchell et al., 2013). In contrast, HBD4 is only expressed in the endometrium (King et al., 2003b).
Hormonal regulation of defensins expression during the menstrual cycle
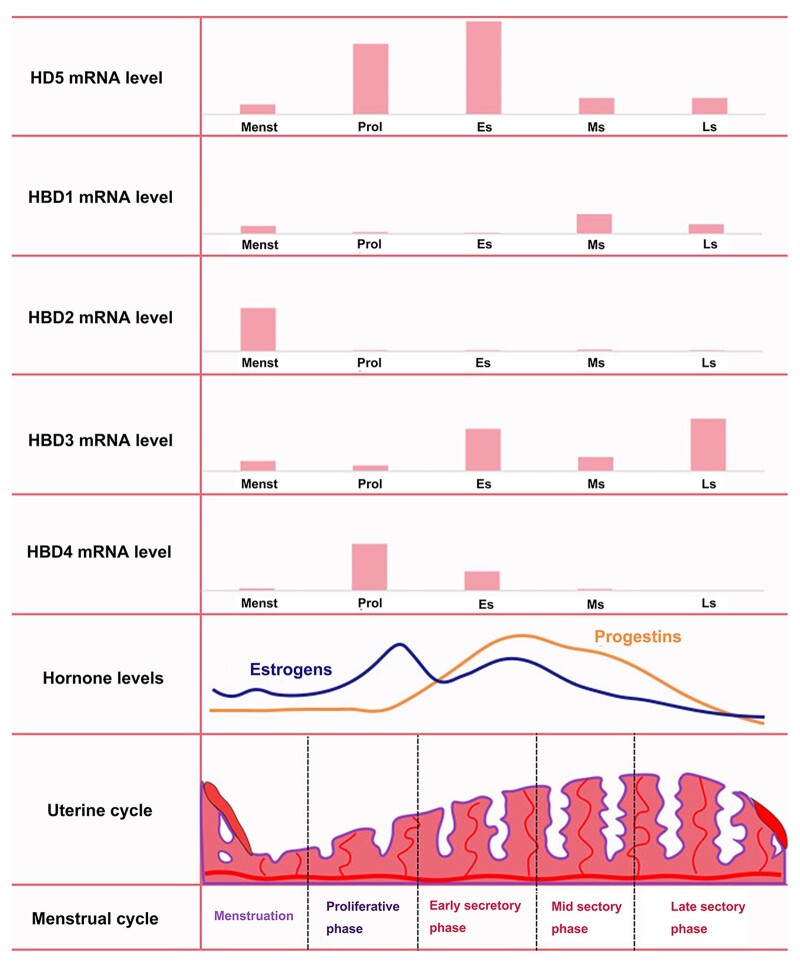
The innate and adaptive immune responses in the FRT are greatly affected by the hypothalamic–pituitary–ovarian axis. The immune responsive protection in the FRT is downregulated during the secretory phase, which is also called a ‘window of vulnerability’, to allow the passage of ‘non-self’ sperm for fertilization, implantation and pregnancy (Yarbrough et al., 2015). Hence, apart from the sectional distribution of defensins in the FRT, the levels of defensins vary during the menstrual cycle. In the cervicovaginal secretions, the protein levels of secretory HNP1–3 and HBD3 are maximal in the secretory phase (Shust et al., 2010). In the endometrium, maximal mRNAs encoding different defensins have been monitored (Fig. 3): HBD3 in the secretory phase (King et al., 2003b), HD5 in the early secretory phase (Quayle et al., 1998) and HBD1 in the late secretory and menstrual phase (Fleming et al., 2003). The secretory HBD4 was maximal in the proliferative phase (King et al., 2003b) and HBD2 was maximal in the menstrual phase (Fleming et al., 2003). Moreover, human immunodeficiency virus (HIV)-negative postmenopausal women had significantly lower levels of HBD2 compared with HIV-negative premenopausal women (Ghosh et al., 2019). Hormonal contraception can suppress natural antimicrobial gene transcription in the human endometrium (Fleming et al., 2003). The levels of defensins were decreased in the cervicovaginal lavage of women on hormonal contraception (Shust et al., 2010). With progesterone treatment to prevent preterm birth, the vaginal progesterone treatment can increase the expression of HBD1 (Nold et al., 2013). Thus, both endogenous and exogenous hormones affect the expression of defensins. The underlying mechanism of hormonal regulation of FRT defensins is mainly related to the varied expression of PRRs, like TLRs, during the menstrual cycle (Wira et al., 2015).
Figure 3.
mRNA expression of defensins in the endometrium changes during the menstrual cycle. Menst, menstrual phase; Prol, proliferative phase; Es, early secretory phase; Ms, mid secretory phase; Ls, late secretory phase.
Defensins expression and hormonal regulation in the male reproductive tract
The male reproductive system includes the testes, excretory genital ducts (epididymis, ductus deferens and ejaculatory duct), accessory glands (seminal vesicles, prostate glands and bulbourethral glands) and penis (urethra within). Seminiferous tubules in the testes produce spermatozoa which subsequently mature in the epididymis to acquire motility and capacitation for the later fertilization process. The genital ducts and accessory glands produce a mixture of secretions to form the seminal plasma. Upon ejaculation, spermatozoa are propelled through the vas deferens into the urethra in conjunction with seminal plasma to form the semen (a suspension of spermatozoa) for ejaculation through the penile urethra. Hence, the major function of the male reproductive system is to produce viable male germ cells to deposit in the FRT and finally complete fertilization (Mawhinney and Mariotti, 2013). The MRT is also constantly facing the challenge of external insults. However, due to the distinct mucosal environment, the distribution of defensins along the MRT is quite different from the situation in the FRT. Androgen receptor (AR) binding sites have been found in β-defensin promoters or intronic regions in mouse epididymis, indicating a direct hormonal regulation of the protein expression (Hu et al., 2014). Various β-defensins have been found to be preferentially expressed in the male reproductive system, especially in the testis and epididymis (Yamaguchi et al., 2002; Zaballos et al., 2004; Patil et al., 2005). In addition, many β-defensins in epididymis and testis are developmentally regulated, with elevated expression during puberty and the sexual maturation period (Sangeeta and Yenugu, 2019).
α-defensins
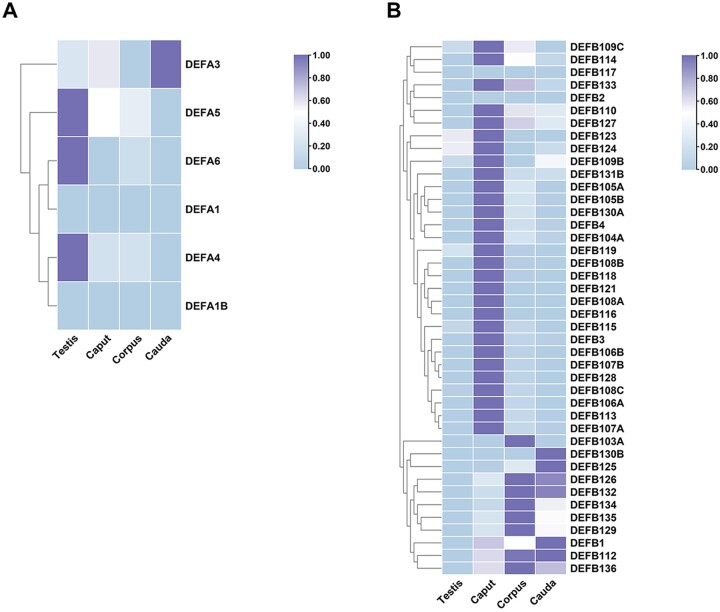
Published experimental data on the distribution and protein levels of α-defensins in the MRT are limited. The transcriptome data in the GEO database (GEO accession No.: GSE150852) (Robertson et al., 2020) showed very low levels of mRNAs encoding HNP1–3 in the testis and a relatively high level of mRNA encoding HNP3 in the epididymal caput and cauda (Fig. 4A). Com et al. (2003) reported low levels of mRNAs encoding HNP1–3, HD5 and HD6 in human testis and high levels of mRNAs encoding HNP1–3 mRNA but not HD5 and HD6 in normal spermatozoa isolated from semen (Table I). The presence of HNP1–3 in testis tissue may come from the large numbers of macrophages and lymphocytes in the interstitial tissue of the testis. Moreover, the leucocytes may contribute to the high level of HNP1–3 in semen isolated spermatozoa due to ‘round cell’ contamination (Barratt et al., 1990). However, we did not find published data on HNP1–3, HD5 and HD6 in the epididymis, ductus deferens, ejaculatory duct and urethra. Several studies have reported the acute inflammatory role and inhibition effect of neutrophil-origin α-defensins on prostate cancer (Sfanos et al., 2009; Kohli et al., 2015; Sasani et al., 2021).
Figure 4.
Transcriptome data of defensins in the male reproductive tract. (A) α-defensins. (B) β-defensins. The mean mRNA levels of 6 α-defensins and 41 β-defensins in the testis and the caput, corpus and cauda of the epididymis were summarized from transcriptome data GSE150852 in the GEO database. Number of samples: 3 testes, 3 capita, 3 corpora and 3 caudae.
β-defensins
Unlike the well-studied situation in the FRT, the research data on HBD1–4 in the MRT are very limited. HBD1 mRNA and protein were detected in human testis tissue, spermatozoa, semen and prostate gland (Table I) (Zhao et al., 1996; Com et al., 2003; Hong et al., 2017; Zupin et al., 2019a). The expression of HBD2 was not found in the transcriptome data or published research. Although mRNA encoding HBD3 was high in the transcriptome data, we did not find published results consistent with this either. The presence of HBD4 was confirmed at the mRNA level in human epididymis (Yamaguchi et al., 2002). Notably, the transcriptome data showed high mRNA levels for 39 out of 42 β-defensins listed in the data source (Fig. 4B) in the epididymal caput, corpus and cauda but not in the testis. Over the years, many novel β-defensins have been identified in the male reproductive system in different mammal species (reviewed in Hall et al., 2007; Tollner et al., 2012; Dorin and Barratt, 2014). In humans, DEFB129 mRNA was reported in the testis (Miyai et al., 2021); the proteins DEFB106A, DEFB114 and DEFB129 were detected in the epididymis (Dubé et al., 2008; Yu et al., 2013; Xin et al., 2014); the proteins DEFB118 and DEFB126 were found on sperm (Yenugu et al., 2004; Aram et al., 2020); DEFB132 (also called HEL-75) protein was detected in both epididymis and sperm (Lin et al., 2008); and DEFB124 protein was found in the prostate epithelia (Kim et al., 2014).
Hormonal regulation of defensins expression in MRT
The expression of defensins in the MRT varies with androgen levels and ages. Since the androgen/AR signaling pathway plays an important regulatory role in the structure and function of the epididymis, many epididymal secretory proteins involved in sperm maturation have been identified as androgen regulatory proteins, including some β-defensins (Patrão et al., 2009). For example, the expression of human DFEB118 is regulated by androgen level (Yenugu et al., 2004). Due to limited access to human epididymal samples or effective in vitro human epididymal cell models, we did not find published results on the androgen regulation mechanism on human defensins. Most mechanistic research has been done on model animals such as mice and rats. Rat epididymis-specific β-defensin 15 (Defb15) exhibits an androgen-dependent expression pattern (Zhao et al., 2011). In mice, some β-defensins, including Spag11a (Bin1b), Defb20, 22, 41 and 42, are regulated by androgens (Jalkanen et al., 2005; Oh et al., 2006; Hu et al., 2010; Pujianto et al., 2013). The androgen/AR differentially regulates β-defensins in the epididymal caput of mice (Hu et al., 2014). In addition to androgens and testicular factors can regulate some β-defensins (Hu et al., 2014; Pujianto et al., 2020). The destruction of the hypothalamic–pituitary testis axis changed defensin expression in the boar testis and epididymis (Weber et al., 2018). In addition, there are differences in the expression profiles of defensin proteins in the epididymis and testis of extreme age rats (Sangeeta and Yenugu, 2019), which may be due to the different physiological conditions of rats and the difference in serum testosterone content.
Host defence by defensins in the reproductive system
Microbial-induced reproductive tract diseases, including sexually transmitted infections (STIs), bring huge burdens to human reproductive health by escaping the protection conferred by the mucosal immune system. Defensins play important roles in protecting the reproductive tracts from microbial invasion (Table II).
Table II.
Defensins against reproductive tract infection.
| Microorganism1 | Female reproduction2 | Male reproduction3 | ||
|---|---|---|---|---|
| Defensin4 | Reference | Defensin4 | Reference | |
| HIV (Human Immunodeficiency Virus) | HNP1, HNP2, HNP3, HD5 and HD6 | (Klotman et al., 2008; Levinson et al., 2009; Pellett Madan et al., 2015) | α-defensin | (Hirbod et al., 2014; Prodger et al., 2014) |
| HBD1, HBD2 and HBD3 | (Ricci et al., 2009; Aguilar-Jiménez et al., 2011; Jiang et al., 2012; Hughes et al., 2016; Jais et al., 2016; Zupin et al., 2019b) | HBD2 and HBD3 | (Kreuter et al., 2009) | |
| θ-defensin (DEFT) | (Yang et al., 2005) | θ-defensin | Not reported | |
| HPV (Human Papilloma Virus) | HNP1 and HD5 | (Hubert et al., 2014; Zhao et al., 2015) | α-defensin | Not reported |
| HBD1, HBD2, HBD3 and HBD4 | (Erhart et al., 2011; Abe et al., 2013; Segat et al., 2014; Buckley et al., 2016; Szukiewicz et al., 2016) | HBD2 and HBD3 | (Kreuter et al., 2009) | |
| θ-defensin | Not reported | θ-defensin | Not reported | |
| HSV (Herpes Simplex Virus) | HNP1, HNP2 and HNP3 | (John et al., 2005) | α-defensin | Not reported |
| HBD1 and HBD2 | (Keller et al., 2019; Fichorova et al., 2020) | β-defensin | Not reported | |
| θ-defensin | Not reported | θ-defensin | Not reported | |
| Neisseria gonorrhoeae | HNP1, HNP2, HNP3, HD5 and HD6 | (Wiesenfeld et al., 2002; Klotman et al., 2008) | HNP1, HNP2, HNP3, and HD5 | (Porter et al., 2005) |
| β-defensin | Not reported | β-defensin | Not reported | |
| θ-defensin | Not reported | θ-defensin | Not reported | |
| Chlamydia trachomatis | HNP1, HNP2 and HNP3 | (Wiesenfeld et al., 2002; Wiechuła et al., 2007) | HNP1, HNP2, HNP3, and HD5 | (Porter et al., 2005) |
| HBD1, HBD2, HBD3 and HBD4 | (Wiechuła et al., 2010; Mukura et al., 2017; Noda-Nicolau et al., 2017; Fichorova et al., 2020) | β-defensin | Not reported | |
| θ-defensin | Not reported | θ-defensin | Not reported | |
| Gardnerella vaginalis | HNP1, HNP2, HNP3 and HD5 | (Sawada et al., 2006; Fan et al., 2008; Xu et al., 2008) | α-defensin | Not reported |
| HBD1, HBD2 and HBD3 | (Balu et al., 2002; Valore et al., 2006; Fan et al., 2008; Eade et al., 2012; Jiang et al., 2012; Zaga-Clavellina et al., 2012a; Mitchell et al., 2013; Castro et al., 2018; Fichorova et al., 2020; Noda-Nicolau et al., 2021) | β-defensin | Not reported | |
| θ-defensin | Not reported | θ-defensin | Not reported | |
| Escherichia coli | α-defensin | Not reported | Recombinant defensin-21 | (Biswas et al., 2015) |
| HBD1, HBD2 and HBD3 | (Garcia-Lopez et al., 2010; Ghartey et al., 2012; Suff et al., 2020) | DEFB118 and DEFB114 | (Yenugu et al., 2004; Hou et al., 2021) (Yu et al., 2013) | |
| cBD-1, cBD-2, and cBD-3 | (Sang et al., 2005) | |||
| rBD-1, rBD-2, rBD-22, and rBD-42 | (Palladino et al., 2003; Diao et al., 2011; Xin et al., 2015) | |||
| mBin1b | (Li et al., 2001; Fei et al., 2012) | |||
| Candida | α-defensin | Not reported | α-defensin | Not reported |
| HBD1, HBD2 and HBD3 | (Pivarcsi et al., 2005; Wiechuła et al., 2010; Zaga-Clavellina et al., 2012b; Fichorova et al., 2020; Kotani et al., 2020; Miró et al., 2021) | DEFB114 | (Yu et al., 2013) | |
| mBD-1 | (Miró et al., 2017) | |||
| θ-defensin | Not reported | θ-defensin | Not reported | |
| Ureaplasma | α-defensin | Not reported | α-defensin | Not reported |
| HBD3 | (Tantengco et al., 2021) | β-defensin | Not reported | |
| θ-defensin | Not reported | θ-defensin | Not reported | |
| Trichomonas vaginalis | HNP1, HNP2 and HNP3 | (Wiesenfeld et al., 2002) | α-defensin | Not reported |
| HBD2 | (Fichorova et al., 2015) | β-defensin | Not reported | |
| θ-defensin | Not reported | θ-defensin | Not reported | |
The different colors summarize different defensins. The yellow is for α-defensins, the blue is for β-defensins and the green is for θ-defensin.
Microorganism associated with male and female reproductive tract infections.
Research on defensins related to female reproduction, germ-related cell lines, germ-related tissues, germ-related organs and germ-related secretions were included.
Research on defensins-related male reproduction, germ-related cell lines, germ-related tissues, germ-related organs and germ-related secretions were included.
Human neutrophil defensins (HNP), human α-defensin 5 (HD5), human α-defensin 6 (HD6), human β-defensin (HBD), canine β-defensin (cBD), rat β-defensin (rBD), mouse β-defensin (mBD), mBin1b: a β-defensin gene identified in the mouse epididymis.
Bacteria
Gardnerella vaginalis
Gardnerella vaginalis infection can cause bacterial vaginosis (BV), leading to an imbalanced vaginal microbiota (Mendling, 2016). The loss of normal immunostimulatory flora in BV is associated with a local deficiency of multiple innate immune factors that may predispose individuals to STIs (Valore et al., 2006). Clinical studies have found defensin deficiency in vaginal lavage fluids of women with BV. Effective treatment normalizes defensin levels in BV, suggesting that abnormal defensin levels are a consequence of G. vaginalis infection (Balu et al., 2002; Valore et al., 2006; Fan et al., 2008; Libby et al., 2008; Xu et al., 2008; Mitchell et al., 2013; Noda-Nicolau et al., 2021). However, in vitro experiments showed that G. vaginalis infection could induce HBD1–3 secretion in human chorioamnion (Zaga-Clavellina et al., 2012a).
Inconsistent changes in defensin levels were found in lavage fluids from patients with G. vaginalis infection, for instance, decreased levels of HBD1–3 in the cervix of childbearing age women (Noda-Nicolau et al., 2021), increased HNP1–3 concentrations in vaginal fluid of pregnant women (Balu et al., 2002; Xu et al., 2008) and low HBD3 concentrations in vaginal fluid of African American pregnant women (Mitchell et al., 2013). In postmenopausal women, HBD1 levels were significantly decreased, and HBD2 and HBD3 levels were significantly increased (Brunner et al., 2021). The inconsistent changes in defensins may be due to the inability of the studies described above to standardize the collection of vaginal effusions, the differences in the physiologic status of the study subjects, and the differences in the immune defence systems between non-pregnant and pregnant women, as well as the effect of ethnic differences.
Escherichia coli, Chlamydia trachomatis and Neisseria gonorrhoeae
The Gram-negative bacteria, Escherichia coli, Chlamydia trachomatis and Neisseria gonorrhoeae, are the most common causes of urogenital infections (Mcconaghy and Panchal, 2016). When sexually transmitted, these bacteria can cause genitourinary tract infections, such as urethritis or cervicitis, in women (whereas salpingitis is a more serious complication) and urethritis combined with epididymitis or proctitis, in men. The pathogenicity of Gram-negative bacteria is usually related to a major component of their cell walls, specifically a lipopolysaccharide (also known as LPS or endotoxin) layer. In humans, LPS can stimulate an innate immune response (Silva et al., 2018), including inducing the expression of defensins in the reproductive tract. Defensins regulate the immune response of macrophages against Gram-negative bacterial infection by neutralizing LPS (Wilson et al., 2013).
Infections with C. trachomatis and N. gonorrhoeae cause abnormal expression of defensins in the female reproductive system. Noda-Nicolau et al. (2017) found that the levels of HBD1, HBD2 and HBD3 in the cervicovaginal fluid of C. trachomatis-positive women were significantly lower than those of negative women. Wiechuła et al. (2010) found that C. trachomatis infection activates HBD2 production but causes variable increases in HBD1 concentrations. Selective stimulation of the placental outer membrane by E. coli results in tissue-specific secretion of HBD1, HBD2 and HBD3 primarily in the choriodecidual, the first area of infection during ascending infection. Among the three defensins, HBD3 was a potential candidate for enhancing cervical innate immunity to prevent ascending infection-related preterm birth and its associated neonatal consequences (Garcia-Lopez et al., 2010). HD5 and HNP1–3 were significantly elevated in male urethral and female vaginal secretions with C. trachomatis and N. gonorrhoeae. The HD5-mediated antibacterial activity against C. trachomatis depends on its N-terminal processing modification (Porter et al., 2005). In vitro studies involving the treatment of cells with the pro-inflammatory factor LPS can upregulate the expression and production of HBD2 in primary endometrial epithelial cells (Wilson et al., 2013), vaginal epithelial cell lines (Pivarcsi et al., 2005) and human amniotic epithelial cells (Flores-Espinosa et al., 2014).
There are many studies related to LPS and male reproduction. LPS induces epididymitis and affects sperm motility. Experiments show that LPS can upregulate tissue concentrations of inflammatory mediators and cytokines/chemokines, thereby activating acute inflammation and reducing epididymal sperm count and transit time (Silva et al., 2018). LPS infection also affects the expression of epididymal defensin. In LPS-induced rat epididymitis, the gene expression of Defb2, Defb21 and Defb27 was decreased in the epididymal head (Biswas and Yenugu, 2011). Sperm-associated antigen 11e (Spag11e), an epididymal-specific defensin, is downregulated and simultaneously reduces sperm function parameters (Cao et al., 2010). Conversely, some studies found that LPS induced the expression of defensins, Spag11e and defensin-like Spag11 genes in male rat reproductive tissues (Biswas and Yenugu, 2011, 2013, 2014). The DEFB126 core peptide binds to LPS to exert anti-inflammatory and intracellular regulatory functions (Liu et al., 2013). Truncated α-defensin analogues have inhibitory and killing activities against multidrug-resistant E. coli (UPEC) biofilmsin an in vitro study (Moazzezy et al., 2020). The conserved cysteine and arginine residues of HD5 are indispensable for its antibacterial and LPS neutralizing effects (Chen et al., 2019). In addition, the core peptide of a novel antimicrobial peptide DEFB118 significantly inhibited the mRNA levels of LPS-induced inflammatory cytokines, including IL-α, IL-1β, IL-6 and TNF-α, in RAW264.7 cells, correspondingly decreasing the secretion of IL-6 and TNF-α (Lin et al., 2020; Hou et al., 2021). Defensins can inhibit LPS-induced inflammation and promote sperm motility. When male mice were infected by uropathogenic UPEC, both mBD-3 and mBD-14 exhibited dose-dependent bactericidal activity against UPEC (Becknell et al., 2013). Recombinant defensin 21 reduces the bacterial load in the rat epididymis and testis more effectively than gentamicin (Biswas et al., 2015). Recombinant human DEFB114 rescues human sperm motility through its LPS-neutralizing activity (Yu et al., 2013).
Virus
Human immunodeficiency virus
The main routes of HIV transmission are sexual transmission, blood transmission and mother-to-child transmission (Pope and Haase, 2003; Page-Shafer et al., 2006). HIV specifically invades and destroys helper T lymphocytes and, at the same time, damages a variety of immune cells in the human body. The invasion is eventually complicated by various serious opportunistic infections and malignant tumors, which are called acquired immunodeficiency syndrome (Appay and Sauce, 2008). Defensins influence HIV transmission, replication and disease progression through their lectin properties, immune regulation, genetic polymorphisms and copy number variation (CNV), etc.
The α-defensins fight HIV infection mainly through lectin properties, fusion inhibition and viral replication. The anti-HIV activity of HNP1–3 depends on its lectin properties. CD4+ T cells can be protected from HIV-1 infection by these α-defensins binding to the sugar moiety on CD4 and the viral envelope glycoprotein gp120 inhibiting viral entry (Wang et al., 2004; Furci et al., 2007; Demirkhanyan et al., 2012). Therefore, the antiviral activity of HNP1–3 may be disrupted by glycosylated serum proteins. However, in the presence of serum, HNP1 can act on cells to interfere with the PKC signaling in primary CD4+ T cells, thereby inhibiting HIV-1 replication (Chang et al., 2005). HNP4 is more potent than HNP1–3 in protecting human peripheral blood mononuclear cells from X4 and R5 HIV-1 strains, but its anti-HIV activity is independent of lectin properties (Wang et al., 2004). In vitro studies showed that HD5 inhibited HIV-1 entry into target cells at physiological concentrations in a serum-free environment by interfering with the interaction between gp120 and CD4, which is similar to the mechanism of the HNPs, probably due to a high degree of structural homology (Furci et al., 2012). Furthermore, HD5 was significantly more effective than HNP1 in downregulating the CXC chemokine receptor 4 (CXCR4) in activated primary T cells, which is the primary target of HIV-1 infection. Reduction of cell surface CXCR4 has been reported to significantly inhibit X4 HIV-1-mediated fusion efficiency and viral replication (Zhou et al., 2004b; Anderson and Akkina, 2005); thus, CXCR4 regulation represents another possible mechanism for HD5-mediated HIV-1 inhibition at virus entry level.
However, contradictory pieces of evidence also exist. HD5 and HD6 were reported to enhance HIV-1 infectivity by promoting virus attachment (Ellegård et al., 2011; Ding et al., 2013). HD5 and HD6 are elevated in the genital mucosa of individuals with STIs. And HD5 has been shown to contribute to the STI-mediated increase in HIV infectivity in vitro (Klotman et al., 2008; Valere et al., 2015). This may be related to the elevated concentrations of HD5 and HD6 upon STIs infection. It is possible that at high concentrations, HD5 and HD6 form cationic aggregates that enhance HIV-1 infectivity by neutralizing membrane charges or promoting viral aggregation (Rapista et al., 2011). Furthermore, the enhancement of HIV by HD5 and HD6 is influenced by peptide structure, as loss of intramolecular cysteine linkages is associated with loss of HIV enhancement (Klotman et al., 2008). The mutation of residues involved in the hydrophobicity and self-association of defensins significantly deprives the ability of HD5 and HD6 to promote HIV attachment and infection (Valere et al., 2017).
Polymorphisms and CNV in β-defensin genes are associated with susceptibility to HIV infection and disease progression. Three single-nucleotide polymorphisms in the 5′ untranslated region of the DEFB1 gene, namely -52(G/A), -44(C/G) and -20(G/A), can be used as markers of HIV-1 infection risk and vary by population (Milanese et al., 2006; Baroncelli et al., 2008; Segat et al., 2009; Zupin et al., 2019b). In Brazilian HIV-positive children, the median copy number of DEFB104 was lower than HIV-exposed, uninfected children and healthy controls, suggesting that DEFB104 CNV may have been involved in protection from vertical transmission of HIV (Milanese et al., 2009). A North American, predominantly white, cohort observed that higher DEFB4/103A CNV was associated with slower progression to acquired immune deficiency syndrome (AIDS) (Mehlotra et al., 2012). The β-defensin gene copy number results for 1002 Ethiopian and Tanzanian patients show that higher β-defensin CNV was associated with increased HIV load before highly active antiretroviral therapy (HAART) (P = 0.005) and poor immune reconstitution after HAART initiation (P = 0.003) (Hardwick et al., 2012).
β-defensins inhibit HIV infection through immunomodulatory effects. HBD3 blocks HIV infection through direct interaction with virions and by acting as an antagonist of CXCR4 on T cells. HBD3 competes with the natural ligand of CXCR4, stromal-derived factor 1 (SDF-1), for cell binding and blocks CXCR4 activation (Feng et al., 2006). The ability of HBD3 to inhibit the SDF-1α-CXCR4 interaction is related to the presence of HBD3 cysteine residues, the specific surface distribution of cationic residues, and the electrostatic properties and availability of both HBD3 termini (Feng et al., 2013). Additionally, in vitro, in primary human monocyte-derived macrophages, HBD2 and HBD3 inhibit HIV replication in a dose-dependent manner (Bharucha et al., 2021). HBD3 also induces chemokines in monocytes and macrophages, allowing cells to reach sites of inflammation (Petrov et al., 2013). HBD2 suppresses HIV at an early post-entry stage via G-protein coupled receptor-mediated signaling (Bharucha et al., 2021) and selectively protects primary CCR6+CD4+T cells infected with HIV-1 (Lafferty et al., 2017).
The synthetic human-derived θ-defensin polypeptides similar to rhesus theta-defensins (RTDs), ‘retrocyclin’, were identified as a potential novel small molecule capable of inhibiting HIV-1 entry and fusion (Münk et al., 2003; Penberthy et al., 2011). θ-defensins have lectin properties as well as immunomodulatory effects. Retrocyclin has a high affinity for the HIV-1 glycoproteins gp120 and gp41 and the host cell glycoprotein CD4; therefore, it can inhibit the fusion of virus and target T cells (Münk et al., 2003; Wang et al., 2003). RTD-1 can also downregulate CXCR4 and inhibit HIV replication (Seidel et al., 2010). In addition, retrocyclins can block infection by inhibiting HIV-1 fusion with host cells. After HIV-1 gp120 binds to its receptor and co-receptor, the outer domain of gp41 transmembrane protein undergoes a significant conformational change to form a membrane-penetrating six-helix bundle required for host cell membrane fusion. Retrocyclins can prevent HIV-1 fusion with host cells by binding to gp41 and blocking the six-helix bundle formation (Cole et al., 2006; Gallo et al., 2006).
θ-defensins are currently considered the greatest clinical potential as biocides of HIV fusion/entry inhibitors. A formulation of the anti-HIV vaginal microbicide RC1 analog RC-101 has been developed (Sassi et al., 2011a,b). A polyvinyl alcohol vaginal membrane containing RC-101 (100 μg/membrane) was shown to be safe in human and monkey tissue and effective against HIV-1 in vitro and ex vivo in human cervical tissue. RC-101 films are stable for 1 month at 25°C and maintain in vitro bioactivity for up to 6 months (Sassi et al., 2011b). Therefore, RC-101 has the potential to be a safe and effective topical microbicide.
Human papilloma virus
The main routes of human papilloma virus (HPV) transmission are sexual transmission, contact transmission and mother-to-child transmission. According to their oncogenic potential, HPV can be divided into low-risk and high-risk types (LR-HPV and HR-HPV) (Tommasino, 2014; Doorbar et al., 2015). Infection with different types of HPV can cause a variety of diseases, such as genital warts and cervical cancer. The α-defensins resist HPV infection mainly through immune regulation, preventing HPV from entering target cells and altering HPV transport in cells. HNP1 can directly promote the apoptosis of condyloma acuminatum cells and exert an antiviral effect in condyloma acuminatum tissue by restricting viral replication (Zhao et al., 2015). HNP2 can recruit DCs in tumor lesions caused by HPV infection (Hubert et al., 2007). HD5 is particularly effective against sexually transmitted HPV types (Buck et al., 2006) by stabilizing the viral capsid and completely blocking endosome uncoating via consolidating the interaction between the two capsid proteins (L1/L2) of HPV (Gulati et al., 2019). HD5 has at least two effects on HPV entry: firstly, blocking the cleavage of the minor capsid protein L2 on the cell surface (Wiens and Smith, 2015); and secondly, even though the virus is internalized and partially uncoated, the genome remains abnormally associated with the major L1 capsid protein and cannot enter the nucleus (Wiens and Smith, 2017). After HPV entry into cells, HD5 treatment significantly altered the trafficking of the viral genome and capsid proteins downstream of the early endosome, redirecting them from the trans-Golgi network to the lysosome. Hence capsid proteins are degraded more rapidly during infection in the presence of HD5 (Wiens and Smith, 2017). HPV infection can induce the expression of defensins, mainly including HBD2 and HBD3, in relevant lesions (Kreuter et al., 2009; Erhart et al., 2011; Mhatre et al., 2012; Buckley et al., 2016; Dasgupta et al., 2016; Szukiewicz et al., 2016). β-defensin gene polymorphisms and CNV are associated with HPV susceptibility (Abe et al., 2013; Segat et al., 2014). However, the relevant mechanisms and therapeutic potential of β-defensins against HPV infection still need to be studied and evaluated. θ-defensins inhibit high-risk HPV infection through charge-driven capsid clustering (Skeate et al., 2020).
Candidiasis
Candida yeast invades the vagina and cause mucosal inflammation called vulvovaginal candidiasis. The vaginal epithelial cells provide the first barrier against fungi, actively recognize the pathogenic phenotype of fungi, and initiate local responses by releasing antimicrobial peptides, chemokines and other cytokines (Netea and Kullberg, 2010; Yano et al., 2010). Local innate immunity plays a key role in the defence and pathogenesis of vaginal Candida infection.
Vaginas infected by C. albicans have high numbers of polysexual neutrophils (PMNs). PMN-derived proteins can cause macrophages to engulf bacteria. Human neutrophil peptides (HNP1–3) secreted by the PMN are mediators of macrophage activation (Soehnlein et al., 2008). In vitro studies have shown that HNP1 and HNP2 exhibit potent Candida killing activity, whereas HNP3 is completely inactive against C. albicans due to an asparagine residue at its N-terminus (Raj et al., 2000). HD6 prevents adhesion, invasion and biofilm formation of the fungal pathogen (Cegelski, 2017; Chairatana et al., 2017).
Candida infection induces the expression and production of β-defensins, which are also lethal to the fungi (Pivarcsi et al., 2005; Kotani et al., 2020; Miró et al., 2021). Selective stimulation of the amnion chorion by C. albicans results in tissue-specific secretion of HBD1 and HBD2 (Zaga-Clavellina et al., 2012b). HBD2 and HBD3 have potent killing activity against Candida at micromolar concentrations, with HBD3 being ten times more powerful than HBD2 (Joly et al., 2004; Vylkova et al., 2006). In vitro experiments have shown that HBD2 kills C. albicans through membrane permeability mediated by phosphatidylinositol 4,5-bisphosphate (Järvå et al., 2018). In addition, Vylkova et al. (2007) proved that HBD2 and HBD3 kill Candida in an energy-dependent and salt-sensitive manner without disrupting the membrane. Kotani et al. (2020) found that C. albicans induced HBD2 expression in a human vaginal epithelial cell line, VK2/E6E7, by activating Dectin-1 and (NF-κB) signaling. In animal experiments, increased fungal burden and mucosal invasion trigger local transcriptional levels of mBD1 in TLR2−/− mice (Miró et al., 2017).
Defensins in human reproduction
Defensins at the maternal–fetal interface
The maternal–fetal interface promotes tolerance to the semiexogeneic fetuses while also preventing vertical transmission of infection. As an indirect ‘dialogue’ contact, the maternal–fetal interface is constituted by different components at different stages of pregnancy: first, the endometrium during the ‘implantation window’ and the trophoblasts in the implantation stage, and later, the basal decidua and villous chorion, which is called placenta (Ander et al., 2019). Different defensin levels have been reported in the above interfaces. During the implantation window, HNP1–3 are expressed by endometrial stromal cells (Das et al., 2007). HBD1–4 are expressed in the endometrium with varied peaks throughout the menstrual cycle (as discussed above). However, none of them seem to participate in the implantation process other than the antimicrobial function (King et al., 2003b; Das et al., 2007). Existing studies have proven that HNP1–3 proteins are expressed in amniotic fluid (Table I) (Espinoza et al., 2003), placental trophoblasts (Svinarich et al., 1997) and vernix caseosa (Akinbi et al., 2004). HD5 mRNA is expressed in chorion (Svinarich et al., 1997). The HBD1–3 mRNAs and proteins are detected in the trophoblasts of the placenta and chorion, amnion epithelium, amniotic fluid and decidua in term and preterm pregnancy (Buhimschi et al., 2004; King et al., 2007a,b). Concentrations of HBD1 in amniotic fluid vary with gestational age and are higher in the second trimester (Varrey et al., 2018). The fetal foreskin only expresses HBD2 among the β-defensins at mRNA levels (Dorschner et al., 2003).
Under the circumstances of pregnancy disorders, abnormal levels of defensins levels were found to be accompanied with specific situations. Microbial invasion of the amniotic membrane, premature delivery and premature rupture of premature fetal membranes are related to the increased concentration of amniotic fluid immunoreactive HNP1–3 (Espinoza et al., 2003). Elevated vaginal fluid neutrophil defensins (HNP1–3) concentrations at 24–29 weeks gestation may predict preterm birth before 32 weeks (Balu et al., 2003). Human beta defensin-1 protein levels show no significant differences between asymptomatic women with a history of spontaneous preterm birth or cervical surgery, both of whom have a high risk of preterm birth (Manning et al., 2019). The concentration of HBD2 in the amniotic fluid in the second trimester was associated with premature rupture of membranes but not with preterm birth (Iavazzo et al., 2010). A recent study on the relationship between vaginal microbiota and spontaneous preterm birth (sPTB) in US women reported that higher vaginal levels of HBD2 lowered the risk of sPTB associated with cervicovaginal microbiota in African American women, but not in non-African American women, independently of the presence or absence of Lactobacillus species, which are the major species in vaginal microbiota. However, the mechanism behind this observation is still unknown (Elovitz et al., 2019). As a physiological component in the amniotic fluid, HBD3 increases during the process of termed labor and also increases in women with spontaneous preterm labor with intact membranes or preterm prelabor rupture of membranes with intra-amniotic inflammation or intra-amniotic infection. In addition, according to the proteomics of amniotic fluid, four biomarkers (defensin-1, defensin-2, calgranulin C and calgranulin A) that are highly predictive of intrauterine inflammation have been shown to detect inflammation accurately, through the Mr score. The Mr score shows the gradient of disease activity, from ‘absent’ to ‘mild’ to ‘severe’ inflammation (Buhimschi et al., 2006). This method provides an opportunity for the early identification of chorioamnionitis (Buhimschi et al., 2008), which can identify patients who may benefit from uterine intervention in the modern diagnosis-treatment framework. Other studies also show that defensins play a certain role in the antimicrobial infection and inflammation of the mother-fetal interface. Tissue-specific HBD1–3, secreted by the outer cell membrane of the human placenta, is stimulated by E. coli (Garcia-Lopez et al., 2010). Stimulation with G. vaginalis induces a tissue-specific secretion profile of HBD1–3 in the chorioamniotic membranes (Zaga-Clavellina et al., 2012a). Additionally, in patients undergoing assisted reproduction, the expression level of HBD1 in the follicular fluid of women with a good fertilization rate is significantly higher than that in women with poor fertilization rates (Zupin et al., 2019a).
The role of defensins in male fertility
During storage in the epididymis, the sperm undergoes a series of profound changes in morphological structure, biochemical metabolism and physiological functions, and finally obtains the capacity for movement and sperm–oocyte binding. In animal experiments, defensins in the epididymis affect the maturation, transport, capacitation and fertilization of sperm (Yudin et al., 2005a; Tollner et al., 2008a; Fernandez-Fuertes et al., 2016; Lyons et al., 2018). In some species, certain β-defensin clusters are preferentially expressed in the MRT and are involved in reproduction (Patil et al., 2005; Zhou et al., 2013). However, due to the difficulties in obtaining human epididymal epithelium or alternative in vitro cellular models, the majority of research on β-defensin function in male fertility has been done in animals.
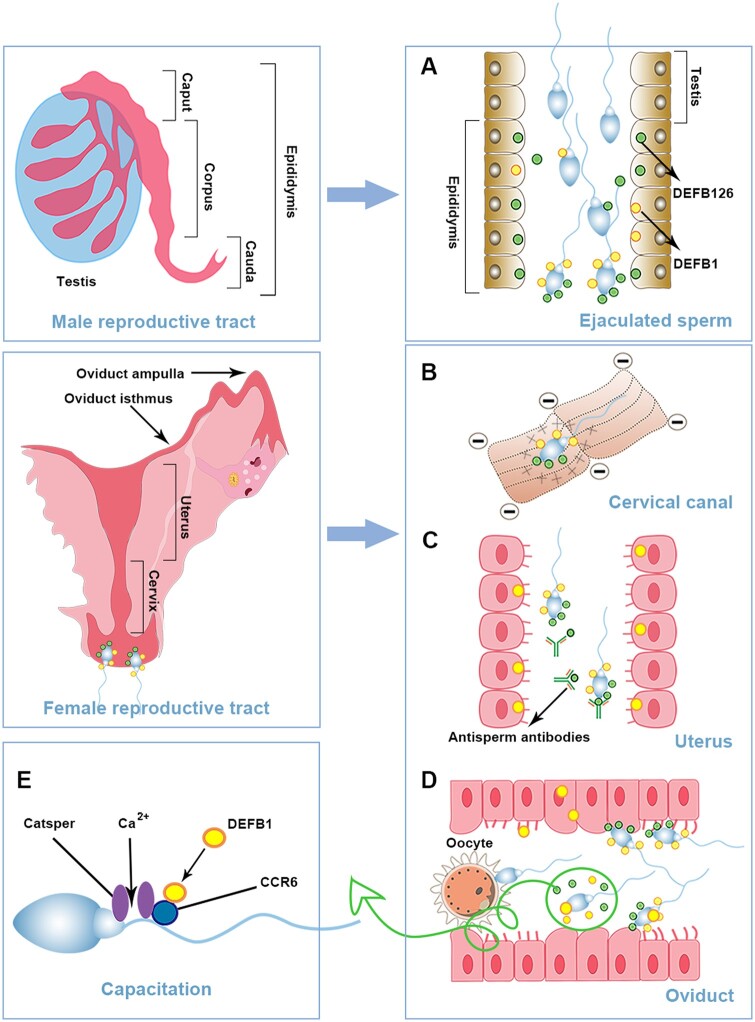
Beta-defensins expressed in the epididymis play a major role in male infertility due to their involvement in sperm maturation, transportation and capacitation (Zhou et al., 2004a; Dorin and Barratt, 2014; Zhang et al., 2018). When sperm passes through the epididymal duct, defensins are adsorbed to the entire sperm surface (Yudin et al., 2005b, 2008; Tollner et al., 2008b) (Fig. 5A). DEFB126 prevents the female immune system from recognizing sperm, hence, it is essential for the effective transport of sperm in the FRT in macaque (Yudin et al., 2005a); it also mediates attachment of sperm to oviductal epithelia in macaque and bull (Tollner et al., 2008a; Lyons et al., 2018) and promotes the delivery of active sperm to the fertilization site in macaque (Yudin et al., 2005b) (Fig. 5B–D). Mouse DEFB41 affects the combination of sperm and zona pellucida (Björkgren et al., 2016).
Figure 5.
The role of defensins in human reproduction. Defensins are widely distributed along the male and female reproductive tracts. Sperm are produced from the testis, mature in the epididymis and enter the female reproductive tract for fertilization. (A) During the maturation in the epididymis, β-defensins are loaded onto the sperm surface. (B) Defensins are cationic polypeptides, while cervical mucus proteins are negatively charged. Therefore, defensins can promote sperm to pass through the cervical mucus to the uterus. (C) DEFB126 can also form a protective shield on the surface of sperm to protect the sperm from immune recognition and binding by anti-sperm antibodies. (D) When the sperm reaches the isthmus of the fallopian tube, the DEFB126 on the surface of the sperm can mediate the combination of the sperm with the fallopian tube epithelium to form a sperm pool. When sperm capacitate, the surface DEFB126 falls off, the sperm is released from the isthmus of the fallopian tube, and the receptor of the sperm and oocyte on the surface are exposed to promote fertilization. (E) DEFB1 secreted from the uterine epithelium interacts with sperm chemokine receptor type 6 (CCR6) and causes Catsper-dependent Ca2+ influx to maintain movement.
Due to ethical reasons, research on defensin in human sperm maturation has not yet been reported. However, related clinical studies have found that β-defensins on human sperm correlate with sperm motility, bactericidal effects and fertilization rates. The loss of DEFB126 is related to male infertility. The proportion of DEFB126-positive sperm in normal semen samples was significantly higher than in patients with varicocele and infertile semen deficiency (Aram et al., 2020). A form of DEFB126 mutation, double nucleotide deletion (DEFB126-2del) or tetranucleotide deletion (DEFB126-4del), is associated with decreased sperm motility (Ozerlat, 2011; Tollner et al., 2011), and full-length DEFB126 can increase sperm motility in vitro (Aram et al., 2020). The allele frequency of the double nucleotide deletion (DEFB126-2del) variant sequence is very high in the European (0.47) and Chinese (0.45) population cohorts. Homozygous patients for this mutation are not sterile, but their fertilization rate is extremely low (Tollner et al., 2011). Moreover, homozygous patients with this mutation among infertility patients were significantly higher than those in the control group (P ≤ 0.05), and the existence of this deletion led to a significant reduction in the clinical pregnancy rate of intrauterine fertilization (P ≤ 0.05) (Boroujeni et al., 2019). In addition, the sperm penetration rate of DEFB126-2del patients through a hyaluronic acid gel was 84% lower than that of other genotypes, mainly due to the changes in the glycocalyx on the sperm surface (Tollner et al., 2011). Lectin chip technology analyzed the difference between mutant DEFB126 and wild-type sperm surface glycocalyx and finally found that six types of lectins (Jacalin/AIA, GHA, ACAL, MPL, MPL, VVL, ABA) and homozygous DEFB126 mutant spermatozoa have a lower binding affinity (Xin et al., 2016). In summary, DEFB126, especially its glycosylation, plays a vital role in the sperm function.
Besides its antimicrobial function, HBD1 was found to relate to sperm function. Diao et al. (2014) showed that compared with normal fertile sperm, the content of HBD1 in the sperm of infertile men with leukospermia or asthenospermia is much lower. Interference in HBD function leads to reduced motility and bactericidal activity of normal sperm. Recombinant HBD1 treatment could significantly restore HBD1 expression, bactericidal activity, sperm quality and the oocyte penetration ability in the sperm of patients with asthenospermia and leukospermia (Diao et al., 2014). Consistent with the above finding, Zupin et al. (2019a) found that the concentrations of HBD1 are lower in oligo-asthenozoospermic semen than in normozoospermic semen, and the incubation of exogenous exposure HBD1 significantly increased normal sperm motility after 1 h and 24 h. However, the results of Khayamabed et al. are different from those of Zupin et al. Their results showed that incubating sperm with HBD1 maintained the percentage of sperm motility within 2 h (P < 0.05). In contrast, after 3 h, the difference was reduced and insignificant (Khayamabed et al., 2020). The differences between these studies may be related to the source, stability of recombinant HBD1 and experimental conditions (room temperature vs 37°C), as well as the method of sperm preparation. The underlying mechanism of HBD1 affecting various sperm functions is that it is a ligand of chemokine receptor type 6 (CCR6), which mediates the ligand-induced, CatSper-dependent Ca2+ influx required for these functions (Diao et al., 2014; 2017). On the other hand, in the FRT, HBD1 is reported to be secreted by the cumulus–oocyte complex and the uterine epithelium in response to elevated progesterone levels around the ovulation period (Calogero et al., 2000; Horne et al., 2008; Yin et al., 2009). Therefore, after sperm enters FRT, HBD1 of female origin also promotes sperm motility and helps them to reach the fallopian tube.
Conclusion
At present, antibiotic resistance is an important issue in treating reproductive tract infections (Unemo and Jensen, 2017). Macrolide antibiotics and fluoroquinolones are used to treat common mycoplasma infections in the genital tract, and related mutations that are resistant to these two drugs cause treatment failure (Li et al., 2020; Machalek et al., 2020; Gossé et al., 2021). In some low-income and middle-income countries, the increase in drug-resistant strains of AIDS is mainly due to poor antiretroviral therapy, incomplete adherence to treatment and poor viral load testing (Hamers et al., 2018). Therefore, there is an urgent need to adopt global surveillance and measures to optimize the effects of reproductive tract infection treatment, including drug resistance-guided strategies and new treatment methods to ensure that a high proportion of reproductive tract infections are treated successfully. As a broad-spectrum antimicrobial peptides without drug resistance, defensins have great potential in developing a new natural antimicrobial therapy to treat reproductive tract infections (Wilmes and Sahl, 2014; Suarez-Carmona et al., 2015). However, problems remain in developing and translating defensins as anti-infective therapies and fertility-improving drugs. Defensins suffer from potential toxicity, poor stability, low bioavailability and difficult synthesis. Especially with HIV infection, defensins are potentially pathogenic in special biological environments, and their safety needs to be carefully evaluated. The development and innovation of defensin drugs can provide new ideas for the clinical treatment of reproductive tract infections and related infertility problems.
Contributor Information
Yu-Jia Zhai, Center for Translational Medicine, Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, P.R. China; Department of Obstetrics and Gynecology, West China Second Hospital, Sichuan University, Chengdu, Sichuan, China.
Ying Feng, Department of Histology, Embryology, and Neurobiology, West China School of Basic Medical Sciences & Forensic Medicine, Sichuan University.
Xue Ma, Department of Pediatric Urology, West China Hospital, Sichuan University, Chengdu, Sichuan, China.
Fang Ma, Center for Translational Medicine, Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, P.R. China; Department of Obstetrics and Gynecology, West China Second Hospital, Sichuan University, Chengdu, Sichuan, China.
Data availability
The data underlying this article are available in GEO database, and can be accessed with GeneChip array data GSE7307 and high-throughput sequencing data GSE150852.
Authors’ roles
Y.-J.Z. and Y.F. conceived the manuscript, participated in writing, prepared the tables and the figures and reviewed the content of the work. X.M. and F.M. conceived the manuscript, participated in the article revision and reviewed the content of the work. All authors reviewed the final text and agreed to take responsibility for all work.
Funding
This review was funded by the National Natural Science Foundation of China (31771662), the National Natural Science Foundation of China (31800677) and the National Natural Science Foundation of China (31470797).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Abe S, Miura K, Kinoshita A, Mishima H, Miura S, Yamasaki K, Hasegawa Y, Higashijima A, Jo O, Sasaki K. et al. Copy number variation of the antimicrobial-gene, defensin beta 4, is associated with susceptibility to cervical cancer. J Hum Genet 2013;58:250–253. [DOI] [PubMed] [Google Scholar]
- Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jörnvall H, Wigzell H, Gudmundsson GH.. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000;96:3086–3093. [PubMed] [Google Scholar]
- Aguilar-Jiménez W, Zapata W, Rugeles MT.. Differential expression of human beta defensins in placenta and detection of allelic variants in the DEFB1 gene from HIV-1 positive mothers. Biomedica 2011;31:44–54. [DOI] [PubMed] [Google Scholar]
- Akinbi HT, Narendran V, Pass AK, Markart P, Hoath SB.. Host defense proteins in vernix caseosa and amniotic fluid. Am J Obstet Gynecol 2004;191:2090–2096. [DOI] [PubMed] [Google Scholar]
- Ander SE, Diamond MS, Coyne CB.. Immune responses at the maternal-fetal interface. Sci Immunol 2019;4:eaat6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J, Akkina R.. HIV-1 resistance conferred by siRNA cosuppression of CXCR4 and CCR5 coreceptors by a bispecific lentiviral vector. AIDS Res Ther 2005;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Sauce D.. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol 2008;214:231–241. [DOI] [PubMed] [Google Scholar]
- Aram R, Chan PTK, Cyr DG.. Beta-defensin126 is correlated with sperm motility in fertile and infertile men. Biol Reprod 2020;102:92–101. [DOI] [PubMed] [Google Scholar]
- Balu RB, Savitz DA, Ananth CV, Hartmann KE, Miller WC, Thorp JM, Heine RP.. Bacterial vaginosis and vaginal fluid defensins during pregnancy. Am J Obstet Gynecol 2002;187:1267–1271. [DOI] [PubMed] [Google Scholar]
- Balu RB, Savitz DA, Ananth CV, Hartmann KE, Miller WC, Thorp JM, Heine RP.. Bacterial vaginosis, vaginal fluid neutrophil defensins, and preterm birth. Obstet Gynecol 2003;101:862–868. [DOI] [PubMed] [Google Scholar]
- Baroncelli S, Ricci E, Andreotti M, Guidotti G, Germano P, Marazzi MC, Vella S, Palombi L, De Rossi A, Giuliano M.. Single-nucleotide polymorphisms in human beta-defensin-1 gene in Mozambican HIV-1-infected women and correlation with virologic parameters. AIDS 2008;22:1515–1517. [DOI] [PubMed] [Google Scholar]
- Barratt CL, Bolton AE, Cooke ID.. Functional significance of white blood cells in the male and female reproductive tract. Hum Reprod 1990;5:639–648. [DOI] [PubMed] [Google Scholar]
- Becknell B, Spencer JD, Carpenter AR, Chen X, Singh A, Ploeger S, Kline J, Ellsworth P, Li B, Proksch E. et al. Expression and antimicrobial function of beta-defensin 1 in the lower urinary tract. PLoS One 2013;8:e77714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch KW, Raida M, Mägert HJ, Schulz-Knappe P, Forssmann WG.. hBD-1: a novel beta-defensin from human plasma. FEBS Lett 1995;368:331–335. [DOI] [PubMed] [Google Scholar]
- Bevins CL, Jones DE, Dutra A, Schaffzin J, Muenke M.. Human enteric defensin genes: chromosomal map position and a model for possible evolutionary relationships. Genomics 1996;31:95–106. [DOI] [PubMed] [Google Scholar]
- Bharucha JP, Sun L, Lu W, Gartner S, Garzino-Demo A.. Human beta-defensin 2 and 3 inhibit HIV-1 replication in macrophages. Front Cell Infect Microbiol 2021;11:535352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas B, Bhushan S, Rajesh A, Suraj SK, Lu Y, Meinhardt A, Yenugu S.. Uropathogenic Escherichia coli (UPEC) induced antimicrobial gene expression in the male reproductive tract of rat: evaluation of the potential of Defensin 21 to limit infection. Andrology 2015;3:368–375. [DOI] [PubMed] [Google Scholar]
- Biswas B, Yenugu S.. Antimicrobial responses in the male reproductive tract of lipopolysaccharide challenged rats. Am J Reprod Immunol 2011;65:557–568. [DOI] [PubMed] [Google Scholar]
- Biswas B, Yenugu S.. Lipopolysaccharide induces epididymal and testicular antimicrobial gene expression in vitro: insights into the epigenetic regulation of sperm-associated antigen 11e gene. Immunogenetics 2013;65:239–253. [DOI] [PubMed] [Google Scholar]
- Biswas B, Yenugu S.. Transcriptional regulation of the rat sperm-associated antigen 11e (Spag 11e) gene during endotoxin challenge. Mol Genet Genomics 2014;289:837–845. [DOI] [PubMed] [Google Scholar]
- Björkgren I, Alvarez L, Blank N, Balbach M, Turunen H, Laajala TD, Toivanen J, Krutskikh A, Wahlberg N, Huhtaniemi I. et al. Targeted inactivation of the mouse epididymal beta-defensin 41 alters sperm flagellar beat pattern and zona pellucida binding. Mol Cell Endocrinol 2016;427:143–154. [DOI] [PubMed] [Google Scholar]
- Boroujeni PB, Ebrahimian S, Abedini M, Chayjan MR, Hassani M, Gourabi H, Sadighi-Gilani MA, Sabbaghian M, Meybodi AM.. The role of DEFB126 variation in male infertility and medically assisted reproduction technique outcome. Reprod Biomed Online 2019;39:649–657. [DOI] [PubMed] [Google Scholar]
- Brunham RC, Paavonen J.. Reproductive system infections in women: lower genital tract syndromes. Pathog Dis 2020;78:ftaa022. [DOI] [PubMed] [Google Scholar]
- Brunner A, Medvecz M, Makra N, Sárdy M, Komka K, Gugolya M, Szabó D, Gajdács M, Ostorházi E.. Human beta defensin levels and vaginal microbiome composition in post-menopausal women diagnosed with lichen sclerosus. Sci Rep 2021;11:15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR, Schiller JT.. Human alpha-defensins block papillomavirus infection. Proc Natl Acad Sci USA 2006;103:1516–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley N, Huber A, Lo Y, Castle PE, Kemal K, Burk RD, Strickler HD, Einstein MH, Young M, Anastos K. et al. Association of high-risk human papillomavirus with genital tract mucosal immune factors in HIV-infected women. Am J Reprod Immunol 2016;75:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi CS, Weiner CP, Buhimschi IA.. Proteomics, part II: the emerging role of proteomics over genomics in spontaneous preterm labor/birth. Obstet Gynecol Surv 2006;61:543–553. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Jabr M, Buhimschi CS, Petkova AP, Weiner CP, Saed GM.. The novel antimicrobial peptide beta3-defensin is produced by the amnion: a possible role of the fetal membranes in innate immunity of the amniotic cavity. Am J Obstet Gynecol 2004;191:1678–1687. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Zambrano E, Pettker CM, Bahtiyar MO, Paidas M, Rosenberg VA, Thung S, Salafia CM, Buhimschi CS.. Using proteomic analysis of the human amniotic fluid to identify histologic chorioamnionitis. Obstet Gynecol 2008;111:403–412. [DOI] [PubMed] [Google Scholar]
- Burris HH, Riis VM, Schmidt I, Gerson KD, Brown A, Elovitz MA.. Maternal stress, low cervicovaginal β-defensin, and spontaneous preterm birth. Am J Obstet Gynecol MFM 2020;2:100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero AE, Burrello N, Barone N, Palermo I, Grasso U, D'agata R.. Effects of progesterone on sperm function: mechanisms of action. Hum Reprod 2000;15(Suppl 1):28–45. [DOI] [PubMed] [Google Scholar]
- Cao D, Li Y, Yang R, Wang Y, Zhou Y, Diao H, Zhao Y, Zhang Y, Lu J.. Lipopolysaccharide-induced epididymitis disrupts epididymal beta-defensin expression and inhibits sperm motility in rats. Biol Reprod 2010;83:1064–1070. [DOI] [PubMed] [Google Scholar]
- Castro J, Jefferson KK, Cerca N.. Innate immune components affect growth and virulence traits of bacterial-vaginosis-associated and non-bacterial-vaginosis-associated Gardnerella vaginalis strains similarly. Pathog Dis 2018;76:10. [DOI] [PubMed] [Google Scholar]
- Cegelski L. Disentangling nanonets: human α-defensin 6 targets Candida albicans virulence. Biochemistry 2017;56:1027–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chairatana P, Chiang IL, Nolan EM.. Human α-defensin 6 self-assembly prevents adhesion and suppresses virulence traits of Candida albicans. Biochemistry 2017;56:1033–1041. [DOI] [PubMed] [Google Scholar]
- Chang TL, Vargas J, Delportillo A, Klotman ME.. Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. J Clin Invest 2005;115:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Tang Y, Zheng H, Xu Y, Wang J, Wang C.. Roles of the conserved amino acid residues in reduced human defensin 5: cysteine and arginine are indispensable for its antibacterial action and LPS neutralization. ChemMedChem 2019;14:1457–1465. [DOI] [PubMed] [Google Scholar]
- Chertov O, Michiel DF, Xu L, Wang JM, Tani K, Murphy WJ, Longo DL, Taub DD, Oppenheim JJ.. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem 1996;271:2935–2940. [DOI] [PubMed] [Google Scholar]
- Cole AL, Yang OO, Warren AD, Waring AJ, Lehrer RI, Cole AM.. HIV-1 adapts to a retrocyclin with cationic amino acid substitutions that reduce fusion efficiency of gp41. J Immunol 2006;176:6900–6905. [DOI] [PubMed] [Google Scholar]
- Cole AM, Hong T, Boo LM, Nguyen T, Zhao C, Bristol G, Zack JA, Waring AJ, Yang OO, Lehrer RI.. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc Natl Acad Sci USA 2002;99:1813–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Com E, Bourgeon F, Evrard B, Ganz T, Colleu D, Jégou B, Pineau C.. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod 2003;68:95–104. [DOI] [PubMed] [Google Scholar]
- Conejo-Garcia JR, Benencia F, Courreges M-C, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L et al Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med 2004;10:950–958. [DOI] [PubMed] [Google Scholar]
- Das S, Bates MD, Vince GS, Lewis-Jones I, Gazvani R.. Follicular fluid expression of alpha-defensins and their role in ovulation. J Assist Reprod Genet 2008;25:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Vince GS, Lewis-Jones I, Bates MD, Gazvani R.. The expression of human alpha and beta defensin in the endometrium and their effect on implantation. J Assist Reprod Genet 2007;24:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta T, Nweze EI, Yue H, Wang L, Jin J, Ghosh SK, Kawsar HI, Zender C, Androphy EJ, Weinberg A. et al. Human papillomavirus oncogenic E6 protein regulates human β-defensin 3 (hBD3) expression via the tumor suppressor protein p53. Oncotarget 2016;7:27430–27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leeuw E, Li C, Zeng P, Li C, Diepeveen-De Buin M, Lu WY, Breukink E, Lu W.. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS Lett 2010;584:1543–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirkhanyan LH, Marin M, Padilla-Parra S, Zhan C, Miyauchi K, Jean-Baptiste M, Novitskiy G, Lu W, Melikyan GB.. Multifaceted mechanisms of HIV-1 entry inhibition by human α-defensin. J Biol Chem 2012;287:28821–28838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H, Yu H-G, Sun F, Zhang Y-L, Tanphaichitr N.. Rat recombinant β-defensin 22 is a heparin-binding protein with antimicrobial activity. Asian J Androl 2011;13:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao R, Fok KL, Chen H, Yu MK, Duan Y, Chung CM, Li Z, Wu H, Li Z, Zhang H. et al. Deficient human β-defensin 1 underlies male infertility associated with poor sperm motility and genital tract infection. Sci Transl Med 2014;6:249ra108. [DOI] [PubMed] [Google Scholar]
- Diao R, Wang T, Fok KL, Li X, Ruan Y, Yu MK, Cheng Y, Chen Y, Chen H, Mou L. et al. CCR6 is required for ligand-induced CatSper activation in human sperm. Oncotarget 2017;8:91445–91458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Tasker C, Valere K, Sihvonen T, Descalzi-Montoya DB, Lu W, Chang TL.. Anti-HIV activity of human defensin 5 in primary CD4+ T cells under serum-deprived conditions is a consequence of defensin-mediated cytotoxicity. PLoS One 2013;8:e76038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I.. Human papillomavirus molecular biology and disease association. Rev Med Virol 2015;25(Suppl 1):2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorin JR, Barratt CLR.. Importance of β-defensins in sperm function. Mol Hum Reprod 2014;20:821–826. [DOI] [PubMed] [Google Scholar]
- Dorschner RA, Lin KH, Murakami M, Gallo RL.. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr Res 2003;53:566–572. [DOI] [PubMed] [Google Scholar]
- Driss V, Legrand F, Hermann E, Loiseau S, Guerardel Y, Kremer L, Adam E, Woerly G, Dombrowicz D, Capron M.. TLR2-dependent eosinophil interactions with mycobacteria: role of alpha-defensins. Blood 2009;113:3235–3244. [DOI] [PubMed] [Google Scholar]
- Dubé E, Hermo L, Chan PTK, Cyr DG.. Alterations in gene expression in the caput epididymides of nonobstructive azoospermic men. Biol Reprod 2008;78:342–351. [DOI] [PubMed] [Google Scholar]
- Eade CR, Diaz C, Wood MP, Anastos K, Patterson BK, Gupta P, Cole AL, Cole AM.. Identification and characterization of bacterial vaginosis-associated pathogens using a comprehensive cervical-vaginal epithelial coculture assay. PLoS One 2012;7:e50106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegård R, Shankar EM, Larsson M.. Targeting HIV-1 innate immune responses therapeutically. Curr Opin HIV AIDS 2011;6:435–443. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Gajer P, Riis V, Brown AG, Humphrys MS, Holm JB, Ravel J.. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat Commun 2019;10:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhart W, Alkasi Ö, Brunke G, Wegener F, Maass N, Arnold N, Arlt A, Meinhold-Heerlein I.. Induction of human β-defensins and psoriasin in vulvovaginal human papillomavirus-associated lesions. J Infect Dis 2011;204:391–399. [DOI] [PubMed] [Google Scholar]
- Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S. et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med 2003;13:2–21. [DOI] [PubMed] [Google Scholar]
- Fan SR, Liu XP, Liao QP.. Human defensins and cytokines in vaginal lavage fluid of women with bacterial vaginosis. Int J Gynaecol Obstet 2008;103:50–54. [DOI] [PubMed] [Google Scholar]
- Fei Z, Hu S, Xiao L, Zhou J, Diao H, Yu H, Fang S, Wang Y, Wan Y, Wang W. et al. mBin1b transgenic mice show enhanced resistance to epididymal infection by bacteria challenge. Genes Immun 2012;13:445–451. [DOI] [PubMed] [Google Scholar]
- Feng Z, Dubyak GR, Jia X, Lubkowski JT, Weinberg A.. Human β-defensin-3 structure motifs that are important in CXCR4 antagonism. FEBS J 2013;280:3365–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Dubyak GR, Lederman MM, Weinberg A.. Cutting edge: human beta defensin 3—a novel antagonist of the HIV-1 coreceptor CXCR4. J Immunol 2006;177:782–786. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fuertes B, Narciandi F, O'farrelly C, Kelly AK, Fair S, Meade KG, Lonergan P.. Cauda epididymis-specific beta-defensin 126 promotes sperm motility but not fertilizing ability in cattle. Biol Reprod 2016;95:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova RN, Chen P-L, Morrison CS, Doncel GF, Mendonca K, Kwok C, Chipato T, Salata R, Mauck C.. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. mBio 2015;6:e00221–e00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova RN, Morrison CS, Chen P-L, Yamamoto HS, Govender Y, Junaid D, Ryan S, Kwok C, Chipato T, Salata RA. et al. Aberrant cervical innate immunity predicts onset of dysbiosis and sexually transmitted infections in women of reproductive age. PLoS One 2020;15:e0224359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova RN, Rheinwald JG, Anderson DJ.. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod 1997;57:847–855. [DOI] [PubMed] [Google Scholar]
- Fleming DC, King AE, Williams ARW, Critchley HOD, Kelly RW.. Hormonal contraception can suppress natural antimicrobial gene transcription in human endometrium. Fertil Steril 2003;79:856–863. [DOI] [PubMed] [Google Scholar]
- Flores-Espinosa P, Pineda-Torres M, Vega-Sánchez R, Estrada-Gutiérrez G, Espejel-Nuñez A, Flores-Pliego A, Maida-Claros R, Paredes-Vivas Y, Morales-Méndez I, Sosa-González I. et al. Progesterone elicits an inhibitory effect upon LPS-induced innate immune response in pre-labor human amniotic epithelium. Am J Reprod Immunol 2014;71:61–72. [DOI] [PubMed] [Google Scholar]
- Fruitwala S, El-Naccache DW, Chang TL.. Multifaceted immune functions of human defensins and underlying mechanisms. Semin Cell Dev Biol 2019;88:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furci L, Sironi F, Tolazzi M, Vassena L, Lusso P.. Alpha-defensins block the early steps of HIV-1 infection: interference with the binding of gp120 to CD4. Blood 2007;109:2928–2935. [DOI] [PubMed] [Google Scholar]
- Furci L, Tolazzi M, Sironi F, Vassena L, Lusso P.. Inhibition of HIV-1 infection by human α-defensin-5, a natural antimicrobial peptide expressed in the genital and intestinal mucosae. PLoS One 2012;7:e45208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay JE, Scott RW, Campanelli D, Griffith J, Wilde C, Marra MN, Seeger M, Nathan CF.. Antibiotic proteins of human polymorphonuclear leukocytes. Proc Natl Acad Sci USA 1989;86:5610–5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo SA, Wang W, Rawat SS, Jung G, Waring AJ, Cole AM, Lu H, Yan X, Daly NL, Craik DJ. et al. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J Biol Chem 2006;281:18787–18792. [DOI] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 2003;3:710–720. [DOI] [PubMed] [Google Scholar]
- Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI.. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest 1985;76:1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AE, Osapay G, Tran PA, Yuan J, Selsted ME.. Isolation, synthesis, and antimicrobial activities of naturally occurring theta-defensin isoforms from baboon leukocytes. Infect Immun 2008;76:5883–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lopez G, Flores-Espinosa P, Zaga-Clavellina V.. Tissue-specific human beta-defensins (HBD)1, HBD2, and HBD3 secretion from human extra-placental membranes stimulated with Escherichia coli. Reprod Biol Endocrinol 2010;8:146–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghartey JP, Carpenter C, Gialanella P, Rising C, Mcandrew TC, Mhatre M, Tugetman J, Einstein MH, Chazotte C, Herold BC.. Association of bactericidal activity of genital tract secretions with Escherichia coli colonization in pregnancy. Am J Obstet Gynecol 2012;207:297.e1–297.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Jais M, Delisle J, Younes N, Benyeogor I, Biswas R, Mohamed H, Daniels J, Wang C, Young M. et al. Dysregulation in genital tract soluble immune mediators in postmenopausal women is distinct by HIV status. AIDS Res Hum Retroviruses 2019;35:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Schaefer TM, Fahey JV, Wright JA, Wira CR.. Antiviral responses of human Fallopian tube epithelial cells to toll-like receptor 3 agonist poly(I:C). Fertil Steril 2008;89:1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossé M, Nordbø SA, Pukstad B.. Evaluation of treatment with two weeks of doxycycline on macrolide-resistant strains of Mycoplasma genitalium: a retrospective observational study. BMC Infect Dis 2021;21:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati NM, Miyagi M, Wiens ME, Smith JG, Stewart PL.. α-defensin HD5 stabilizes capsid/core interactions. Pathog Immun 2019;4:196–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie BL, Introini A, Roxby AC, Choi RY, Bosire R, Lohman-Payne B, Hirbod T, Farquhar C, Broliden K.. Depot medroxyprogesterone acetate use is associated with elevated innate immune effector molecules in cervicovaginal secretions of HIV-1-uninfected women. J Acquir Immune Defic Syndr 2015;69:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SH, Yenugu S, Radhakrishnan Y, Avellar MCW, Petrusz P, French FS.. Characterization and functions of beta defensins in the epididymis. Asian J Androl 2007;9:453–462. [DOI] [PubMed] [Google Scholar]
- Hamers RL, Rinke De Wit TF, Holmes CB.. HIV drug resistance in low-income and middle-income countries. Lancet HIV 2018;5:e588–e596. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, Schroder JM.. A peptide antibiotic from human skin. Nature 1997;387:861. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, Schroder JM.. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 2001;276:5707–5713. [DOI] [PubMed] [Google Scholar]
- Hardwick RJ, Amogne W, Mugusi S, Yimer G, Ngaimisi E, Habtewold A, Minzi O, Makonnen E, Janabi M, Machado LR. et al. β-defensin genomic copy number is associated with HIV load and immune reconstitution in sub-saharan Africans. J Infect Dis 2012;206:1012–1019. [DOI] [PubMed] [Google Scholar]
- Hayashi R, Tsuchiya K, Fukushima K, Horita N, Hibiya S, Kitagaki K, Negi M, Itoh E, Akashi T, Eishi Y. et al. Reduced human alpha-defensin 6 in noninflamed jejunal tissue of patients with Crohn's disease. Inflamm Bowel Dis 2016;22:1119–1128. [DOI] [PubMed] [Google Scholar]
- Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T.. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol 2002;187:137–144. [DOI] [PubMed] [Google Scholar]
- Hirbod T, Kong X, Kigozi G, Ndyanabo A, Serwadda D, Prodger JL, Tobian AA, Nalugoda F, Wawer MJ, Shahabi K. et al. HIV acquisition is associated with increased antimicrobial peptides and reduced HIV neutralizing IgA in the foreskin prepuce of uncircumcised men. PLoS Pathog 2014;10:e1004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly MK, Diaz K, Smith JG.. Defensins in viral infection and pathogenesis. Annu Rev Virol 2017;4:369–391. [DOI] [PubMed] [Google Scholar]
- Hong SA, Kim KH, Lee TJ, Park ES, Kim MK, Myung SC.. A role of human beta defensin-1 in predicting prostatic adenocarcinoma in cases of false-negative biopsy. APMIS 2017;125:1063–1069. [DOI] [PubMed] [Google Scholar]
- Horne AW, Stock SJ, King AE.. Innate immunity and disorders of the female reproductive tract. Reproduction 2008;135:739–749. [DOI] [PubMed] [Google Scholar]
- Hou J, Liu H-Y, Diao H, Yu H.. The truncated human beta-defensin 118 can modulate lipopolysaccharide mediated inflammatory response in RAW264.7 macrophages. Peptides 2021;136:170438. [DOI] [PubMed] [Google Scholar]
- Hu H, Di B, Tolbert WD, Gohain N, Yuan W, Gao P, Ma B, He Q, Pazgier M, Zhao L. et al. Systematic mutational analysis of human neutrophil α-defensin HNP4. Biochim Biophys Acta Biomembr 2019;1861:835–844. [DOI] [PubMed] [Google Scholar]
- Hu S, Yao G, Guan X, Ni Z, Ma W, Wilson EM, French FS, Liu Q, Zhang Y.. Research resource: genome-wide mapping of in vivo androgen receptor binding sites in mouse epididymis. Mol Endocrinol 2010;24:2392–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S-G, Zou M, Yao G-X, Ma W-B, Zhu Q-L, Li X-Q, Chen Z-J, Sun Y.. Androgenic regulation of beta-defensins in the mouse epididymis. Reprod Biol Endocrinol 2014;12:76–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert P, Herman L, Maillard C, Caberg J-H, Nikkels A, Pierard G, Foidart J-M, Noel A, Boniver J, Delvenne P.. Defensins induce the recruitment of dendritic cells in cervical human papillomavirus-associated (pre)neoplastic lesions formed in vitro and transplanted in vivo. FASEB J 2007;21:2765–2775. [DOI] [PubMed] [Google Scholar]
- Hubert P, Herman L, Roncarati P, Maillard C, Renoux V, Demoulin S, Erpicum C, Foidart J-M, Boniver J, Noël A. et al. Altered α-defensin 5 expression in cervical squamocolumnar junction: implication in the formation of a viral/tumour-permissive microenvironment. J Pathol 2014;234:464–477. [DOI] [PubMed] [Google Scholar]
- Hughes BL, Dutt R, Raker C, Barthelemy M, Rossoll RM, Ramratnam B, Wira CR, Cu-Uvin S.. The impact of pregnancy on anti-HIV activity of cervicovaginal secretions. Am J Obstet Gynecol 2016;215:748.e1–748.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavazzo C, Tassis K, Gourgiotis D, Boutsikou M, Baka S, Hassiakos D, Hadjithomas A, Botsis D, Malamitsi-Puchner A.. The role of human beta defensins 2 and 3 in the second trimester amniotic fluid in predicting preterm labor and premature rupture of membranes. Arch Gynecol Obstet 2010;281:793–799. [DOI] [PubMed] [Google Scholar]
- Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol 2010;10:699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jais M, Younes N, Chapman S, Cu-Uvin S, Ghosh M.. Reduced levels of genital tract immune biomarkers in postmenopausal women: implications for HIV acquisition. Am J Obstet Gynecol 2016;215:324.e1–324.e10. [DOI] [PubMed] [Google Scholar]
- Jalkanen J, Huhtaniemi I, Poutanen M.. Discovery and characterization of new epididymis-specific beta-defensins in mice. Biochim Biophys Acta 2005;1730:22–30. [DOI] [PubMed] [Google Scholar]
- Järvå M, Phan TK, Lay FT, Caria S, Kvansakul M, Hulett MD.. Human β-defensin 2 kills through phosphatidylinositol 4,5-bisphosphate-mediated membrane permeabilization. Sci Adv 2018;4:eaat0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Ghosh SK, Flyckt R, Kalinowska M, Starks D, Jurevic R, Weinberg A, Lederman MM, Rodriguez B.. Bacterial colonization and beta defensins in the female genital tract in HIV infection. Curr HIV Res 2012;10:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Weinberg A.. Human antimicrobial peptides and cancer. Semin Cell Dev Biol 2019;88:156–162. [DOI] [PubMed] [Google Scholar]
- John M, Keller MJ, Fam EH, Cheshenko N, Hogarty K, Kasowitz A, Wallenstein S, Carlucci MJ, Tuyama AC, Lu W. et al. Cervicovaginal secretions contribute to innate resistance to herpes simplex virus infection. J Infect Dis 2005;192:1731–1740. [DOI] [PubMed] [Google Scholar]
- Joly S, Maze C, Mccray PB, Guthmiller JM.. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol 2004;42:1024–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DE, Bevins CL.. Defensin-6 mRNA in human Paneth cells: implications for antimicrobia peptides in host defense of the human bowel. FEBS Lett 1993;315:187–192. [DOI] [PubMed] [Google Scholar]
- Jones DE, Bevins CL.. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem 1992;267:23216–23225. [PubMed] [Google Scholar]
- Keller MJ, Huber A, Espinoza L, Serrano MG, Parikh HI, Buck GA, Gold JA, Wu Y, Wang T, Herold BC.. Impact of herpes simplex virus type 2 and human immunodeficiency virus dual infection on female genital tract mucosal immunity and the vaginal microbiome. J Infect Dis 2019;220:852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayamabed R, Tavalaee M, Taherian S-S, Nasr-Esfahani MH.. Effect of recombinant β-defensin 1 protein on human sperm motility and viability. Andrologia 2020;52:e13455. [DOI] [PubMed] [Google Scholar]
- Kim C, Gajendran N, Mittrucker HW, Weiwad M, Song YH, Hurwitz R, Wilmanns M, Fischer G, Kaufmann SH.. Human alpha-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc Natl Acad Sci USA 2005;102:4830–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim K-H, Kim HJ, Lee J, Myung SC.. Expression of beta-defensin 131 promotes an innate immune response in human prostate epithelial cells. PLoS One 2015;10:e0144776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-H, Lee J, Han JH, Myung SC.. Beta-defensin 124 is required for efficient innate immune responses in prostate epithelial RWPE-1 cells. Korean J Urol 2014;55:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AE, Critchley HOD, Kelly RW.. Innate immune defences in the human endometrium. Reprod Biol Endocrinol 2003a;1:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AE, Fleming DC, Critchley HO, Kelly RW.. Differential expression of the natural antimicrobials, beta-defensins 3 and 4, in human endometrium. J Reprod Immunol 2003b;59:1–16. [DOI] [PubMed] [Google Scholar]
- King AE, Fleming DC, Critchley HOD, Kelly RW.. Regulation of natural antibiotic expression by inflammatory mediators and mimics of infection in human endometrial epithelial cells. Mol Hum Reprod 2002;8:341–349. [DOI] [PubMed] [Google Scholar]
- King AE, Kelly RW, Sallenave JM, Bocking AD, Challis JRG.. Innate immune defences in the human uterus during pregnancy. Placenta 2007a;28:1099–1106. [DOI] [PubMed] [Google Scholar]
- King AE, Paltoo A, Kelly RW, Sallenave JM, Bocking AD, Challis JRG.. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta 2007b;28:161–169. [DOI] [PubMed] [Google Scholar]
- Klotman ME, Chang TL.. Defensins in innate antiviral immunity. Nat Rev Immunol 2006;6:447–456. [DOI] [PubMed] [Google Scholar]
- Klotman ME, Rapista A, Teleshova N, Micsenyi A, Jarvis GA, Lu W, Porter E, Chang TL.. Neisseria gonorrhoeae-induced human defensins 5 and 6 increase HIV infectivity: role in enhanced transmission. J Immunol 2008;180:6176–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli M, Young CY, Tindall DJ, Nandy D, Mckenzie KM, Bevan GH, Donkena KV.. Whole blood defensin mRNA expression is a predictive biomarker of docetaxel response in castration-resistant prostate cancer. Onco Targets Ther 2015;8:1915–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani H, Koshizuka T, Matsubara K, Nishiyama K, Sugiyama T, Suzutani T.. Relationship between human β-defensin 2 and the vaginal environment. Jpn J Infect Dis 2020;73:214–220. [DOI] [PubMed] [Google Scholar]
- Kreuter A, Skrygan M, Gambichler T, Brockmeyer NH, Stücker M, Herzler C, Potthoff A, Altmeyer P, Pfister H, Wieland U.. Human papillomavirus-associated induction of human beta-defensins in anal intraepithelial neoplasia. Br J Dermatol 2009;160:1197–1205. [DOI] [PubMed] [Google Scholar]
- Lafferty MK, Sun L, Christensen-Quick A, Lu W, Garzino-Demo A.. Human beta defensin 2 selectively inhibits HIV-1 in highly permissive CCR6+CD4+ T cells. Viruses 2017;9:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Gallo RL.. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol 2009;30:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer RI. Primate defensins. Nat Rev Microbiol 2004;2:727–738. [DOI] [PubMed] [Google Scholar]
- Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME.. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest 1989;84:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer RI, Cole AM, Selsted ME.. θ-Defensins: cyclic peptides with endless potential. J Biol Chem 2012;287:27014–27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer RI, Ganz T.. Defensins of vertebrate animals. Curr Opin Immunol 2002;14:96–102. [DOI] [PubMed] [Google Scholar]
- Lehrer RI, Lu W.. α-Defensins in human innate immunity. Immunol Rev 2012;245:84–112. [DOI] [PubMed] [Google Scholar]
- Levinson P, Choi RY, Cole AL, Hirbod T, Rhedin S, Payne B, Guthrie BL, Bosire R, Cole AM, Farquhar C. et al. HIV-neutralizing activity of cationic polypeptides in cervicovaginal secretions of women in HIV-serodiscordant relationships. PLoS One 2012;7:e31996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson P, Kaul R, Kimani J, Ngugi E, Moses S, Macdonald KS, Broliden K, Hirbod T; Kibera HIV Study Group. Levels of innate immune factors in genital fluids: association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS 2009;23:309–317. [DOI] [PubMed] [Google Scholar]
- Li P, Chan HC, He B, So SC, Chung YW, Shang Q, Zhang YD, Zhang YL.. An antimicrobial peptide gene found in the male reproductive system of rats. Science 2001;291:1783–1785. [DOI] [PubMed] [Google Scholar]
- Li WN, Shi L, Long XY, Li Y, Zhu WB, Liu G.. Mycoplasma genitalium incidence, treatment failure, and resistance: a retrospective survey of men of infertile couples from a hospital in China. Andrology 2020;8:91–100. [DOI] [PubMed] [Google Scholar]
- Libby EK, Pascal KE, Mordechai E, Adelson ME, Trama JP.. Atopobium vaginae triggers an innate immune response in an in vitro model of bacterial vaginosis. Microbes Infect 2008;10:439–446. [DOI] [PubMed] [Google Scholar]
- Lin Q, Xie K, Chen D, Yu B, Mao X, Yu J, Luo J, Zheng P, Luo Y, Yan H. et al. Expression and functional characterization of a novel antimicrobial peptide: human beta-defensin 118. Biomed Res Int 2020;2020:1395304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YQ, Li JY, Wang HY, Liu J, Zhang CL, Wang WT, Liu J, Li N, Jin SH.. Cloning and identification of a novel sperm binding protein, HEL-75, with antibacterial activity and expressed in the human epididymis. Hum Reprod 2008;23:2086–2094. [DOI] [PubMed] [Google Scholar]
- Linzmeier R, Michaelson D, Liu L, Ganz T.. The structure of neutrophil defensin genes. FEBS Lett 1993;326:299–300. [DOI] [PubMed] [Google Scholar]
- Liu H, Yu H, Gu Y, Xin A, Zhang Y, Diao H, Lin D.. Human beta-defensin DEFB126 is capable of inhibiting LPS-mediated inflammation. Appl Microbiol Biotechnol 2013;97:3395–3408. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhao C, Heng HH, Ganz T.. The human beta-defensin-1 and alpha-defensins are encoded by adjacent genes: two peptide families with differing disulfide topology share a common ancestry. Genomics 1997;43:316–320. [DOI] [PubMed] [Google Scholar]
- Lucovnik M, Kornhauser-Cerar L, Premru-Srsen T, Gmeiner-Stopar T, Derganc M.. Neutrophil defensins but not interleukin-6 in vaginal fluid after preterm premature rupture of membranes predict fetal/neonatal inflammation and infant neurological impairment. Acta Obstet Gynecol Scand 2011;90:908–916. [DOI] [PubMed] [Google Scholar]
- Lyons A, Narciandi F, Donnellan E, Romero-Aguirregomezcorta J, Farrelly CO, Lonergan P, Meade KG, Fair S.. Recombinant β-defensin 126 promotes bull sperm binding to bovine oviductal epithelia. Reprod Fertil Dev 2018;30:1472–1481. [DOI] [PubMed] [Google Scholar]
- Machalek DA, Tao Y, Shilling H, Jensen JS, Unemo M, Murray G, Chow EPF, Low N, Garland SM, Vodstrcil LA. et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis 2020;20:1302–1314. [DOI] [PubMed] [Google Scholar]
- Manning R, James CP, Smith MC, Innes BA, Stamp E, Peebles D, Bajaj-Elliott M, Klein N, Bulmer JN, Robson SC. et al. Predictive value of cervical cytokine, antimicrobial and microflora levels for pre-term birth in high-risk women. Sci Rep 2019;9:11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars WM, Patmasiriwat P, Maity T, Huff V, Weil MM, Saunders GF.. Inheritance of unequal numbers of the genes encoding the human neutrophil defensins HP-1 and HP-3. J Biol Chem 1995;270:30371–30376. [DOI] [PubMed] [Google Scholar]
- Mawhinney M, Mariotti A.. Physiology, pathology and pharmacology of the male reproductive system. Periodontol 2000 2013;61:232–251. [DOI] [PubMed] [Google Scholar]
- Mcconaghy JR, Panchal B.. Epididymitis: an overview. Am Fam Physician 2016;94:723–726. [PubMed] [Google Scholar]
- Mehlotra RK, Dazard J-E, John B, Zimmerman PA, Weinberg A, Jurevic RJ.. Copy number variation within human β-defensin gene cluster influences progression to AIDS in the multicenter AIDS cohort study. J AIDS Clin Res 2012;3:1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendling W. Vaginal microbiota. Adv Exp Med Biol 2016;902:83–93. [DOI] [PubMed] [Google Scholar]
- Meng W, Du R, Wang Y, Chen Z, Ding Y.. Human β-defensin messenger RNA is overexpressed in the cervical epithelia of patients with nongonococcal cervicitis. J Low Genit Tract Dis 2013;17:440–445. [DOI] [PubMed] [Google Scholar]
- Mhatre M, Mcandrew T, Carpenter C, Burk RD, Einstein MH, Herold BC.. Cervical intraepithelial neoplasia is associated with genital tract mucosal inflammation. Sex Transm Dis 2012;39:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanese M, Segat L, Arraes LC, Garzino-Demo A, Crovella S.. Copy number variation of defensin genes and HIV infection in Brazilian children. J Acquir Immune Defic Syndr 2009;50:331–333. [DOI] [PubMed] [Google Scholar]
- Milanese M, Segat L, Pontillo A, Arraes LC, De Lima Filho JL, Crovella S.. DEFB1 gene polymorphisms and increased risk of HIV-1 infection in Brazilian children. AIDS 2006;20:1673–1675. [DOI] [PubMed] [Google Scholar]
- Miró MS, Caeiro JP, Rodriguez E, Vargas L, Vigezzi C, Icely PA, Castillo GDV, Azcurra AI, Abiega CD, Riera FO. et al. Candida albicans modulates murine and human beta defensin-1 during vaginitis. J Fungi (Basel) 2021;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miró MS, Rodríguez E, Vigezzi C, Icely PA, García LN, Peinetti N, Maldonado CA, Riera FO, Caeiro JP, Sotomayor CE.. Contribution of TLR2 pathway in the pathogenesis of vulvovaginal candidiasis. Pathog Dis 2017;75:10. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Gottsch ML, Liu C, Fredricks DN, Nelson DB.. Associations between vaginal bacteria and levels of vaginal defensins in pregnant women. Am J Obstet Gynecol 2013;208:132.e1–132.e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyai K, Yonekura Y, Ito K, Matsukuma S, Tsuda H.. Gene expression microarray analysis of adult testicular germ cell tumor: a comparison between pure-type seminomas and seminoma components in mixed tumors. Virchows Arch 2021;479:1177–1186. [DOI] [PubMed] [Google Scholar]
- Moazzezy N, Asadi Karam MR, Rafati S, Bouzari S, Oloomi M.. Inhibition and eradication activity of truncated α-defensin analogs against multidrug resistant uropathogenic Escherichia coli biofilm. PLoS One 2020;15:e0235892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin L, Whettlock EM, Male V.. Immune responses in the human female reproductive tract. Immunology 2020;160:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukura LR, Hickey DK, Rodriguez-Garcia M, Fahey JV, Wira CR.. Chlamydia trachomatis regulates innate immune barrier integrity and mediates cytokine and antimicrobial responses in human uterine ECC-1 epithelial cells. Am J Reprod Immunol 2017;78:e12764. [DOI] [PubMed] [Google Scholar]
- Münk C, Wei G, Yang OO, Waring AJ, Wang W, Hong T, Lehrer RI, Landau NR, Cole AM.. The theta-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res Hum Retroviruses 2003;19:875–881. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006;6:173–182. [DOI] [PubMed] [Google Scholar]
- Netea MG, Kullberg BJ.. Epithelial sensing of fungal invasion. Cell Host Microbe 2010;8:219–220. [DOI] [PubMed] [Google Scholar]
- Niyonsaba F, Ogawa H, Nagaoka I.. Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology 2004;111:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda-Nicolau NM, Bastos LB, Bolpetti AN, Pinto GVS, Marcolino LD, Marconi C, Ferreira CST, Polettini J, Vieira EP, Da Silva MG.. Cervicovaginal levels of human β-defensin 1, 2, 3, and 4 of reproductive-aged women with Chlamydia trachomatis infection. J Low Genit Tract Dis 2017;21:189–192. [DOI] [PubMed] [Google Scholar]
- Noda-Nicolau NM, Silva MDC, Bento GFC, Ferreira JSB, Novak J, Morales J, Tronco JA, Bolpetti AN, Pinto GVS, Polettini J. et al. Cervicovaginal levels of human beta defensins during bacterial vaginosis. PLoS One 2021;16:e0260753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold C, Maubert M, Anton L, Yellon S, Elovitz MA.. Prevention of preterm birth by progestational agents: what are the molecular mechanisms? Am J Obstet Gynecol 2013;208:223.e1–223.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus A, Hayden MS, Ghosh S.. Crosstalk in NF-κB signaling pathways. Nat Immunol 2011;12:695–708. [DOI] [PubMed] [Google Scholar]
- Oh J, Lee J, Woo J-M, Choi E, Park I, Han C, Baek N, Lee H, Kim DH, Cho C.. Systematic identification and integrative analysis of novel genes expressed specifically or predominantly in mouse epididymis. BMC Genomics 2006;7:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil DA. Regulation of expression of beta-defensins: endogenous enteric peptide antibiotics. Mol Immunol 2003;40:445–450. [DOI] [PubMed] [Google Scholar]
- Ordonez SR, Veldhuizen EJA, Van Eijk M, Haagsman HP.. Role of soluble innate effector molecules in pulmonary defense against fungal pathogens. Front Microbiol 2017;8:2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette AJ. Paneth cell α-defensins in enteric innate immunity. Cell Mol Life Sci 2011;68:2215–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerlat I. Male factor infertility: mutation of sperm defensin causes subfertility. Nat Rev Urol 2011;8:474–474. [DOI] [PubMed] [Google Scholar]
- Page-Shafer K, Sweet S, Kassaye S, Ssali C.. (C2) Saliva, breast milk, and mucosal fluids in HIV transmission. Adv Dent Res 2006;19:152–157. [DOI] [PubMed] [Google Scholar]
- Palladino MA, Mallonga TA, Mishra MS.. Messenger RNA (mRNA) expression for the antimicrobial peptides beta-defensin-1 and beta-defensin-2 in the male rat reproductive tract: beta-defensin-1 mRNA in initial segment and caput epididymidis is regulated by androgens and not bacterial lipopolysaccharides. Biol Reprod 2003;68:509–515. [DOI] [PubMed] [Google Scholar]
- Para R, Romero R, Miller D, Panaitescu B, Varrey A, Chaiworapongsa T, Hassan SS, Hsu C-D, Gomez-Lopez N.. Human β-defensin-3 participates in intra-amniotic host defense in women with labor at term, spontaneous preterm labor and intact membranes, and preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2020;33:4117–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil AA, Cai Y, Sang Y, Blecha F, Zhang G.. Cross-species analysis of the mammalian beta-defensin gene family: presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol Genomics 2005;23:5–17. [DOI] [PubMed] [Google Scholar]
- Patrão MTCC, Silva EJR, Avellar MCW.. Androgens and the male reproductive tract: an overview of classical roles and current perspectives. Arq Bras Endocrinol Metabol 2009;53:934–945. [DOI] [PubMed] [Google Scholar]
- Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J.. Human beta-defensins. Cell Mol Life Sci 2006;63:1294–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazgier M, Li X, Lu W, Lubkowski J.. Human defensins: synthesis and structural properties. Curr Pharm Des 2007;13:3096–3118. [DOI] [PubMed] [Google Scholar]
- Pellett Madan R, Masson L, Tugetman J, Werner L, Grobler A, Mlisana K, Lo Y, Che D, Arnold KB, Abdool Karim SS. et al. Innate antibacterial activity in female genital tract secretions is associated with increased risk of HIV acquisition. AIDS Res Hum Retroviruses 2015;31:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penberthy WT, Chari S, Cole AL, Cole AM.. Retrocyclins and their activity against HIV-1. Cell Mol Life Sci 2011;68:2231–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov V, Funderburg N, Weinberg A, Sieg S.. Human β defensin-3 induces chemokines from monocytes and macrophages: diminished activity in cells from HIV-infected persons. Immunology 2013;140:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivarcsi A, Nagy I, Koreck A, Kis K, Kenderessy-Szabo A, Szell M, Dobozy A, Kemeny L.. Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human beta-defensin-2 in vaginal epithelial cells. Microbes Infect 2005;7:1117–1127. [DOI] [PubMed] [Google Scholar]
- Polettini J, Takitane J, Peraçoli JC, Silva MG.. Expression of β defensins 1, 3 and 4 in chorioamniotic membranes of preterm pregnancies complicated by chorioamnionitis. Eur J Obstet Gynecol Reprod Biol 2011;157:150–155. [DOI] [PubMed] [Google Scholar]
- Pope M, Haase AT.. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med 2003;9:847–852. [DOI] [PubMed] [Google Scholar]
- Porter E, Yang H, Yavagal S, Preza GC, Murillo O, Lima H, Greene S, Mahoozi L, Klein-Patel M, Diamond G. et al. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect Immun 2005;73:4823–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premratanachai P, Joly S, Johnson GK, Mccray PB, Jia HP, Guthmiller JM.. Expression and regulation of novel human beta-defensins in gingival keratinocytes. Oral Microbiol Immunol 2004;19:111–117. [DOI] [PubMed] [Google Scholar]
- Prodger JL, Hirbod T, Kigozi G, Nalugoda F, Reynolds SJ, Galiwango R, Shahabi K, Serwadda D, Wawer MJ, Gray RH. et al. ; Rakai Genital Immunology Research Group. Immune correlates of HIV exposure without infection in foreskins of men from Rakai, Uganda. Mucosal Immunol 2014;7:634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujianto DA, Loanda E, Sari P, Midoen YH, Soeharso P.. Sperm-associated antigen 11A is expressed exclusively in the principal cells of the mouse caput epididymis in an androgen-dependent manner. Reprod Biol Endocrinol 2013;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujianto DA, Muliawati D, Rizki MD, Parisudha A, Hardiyanto L.. Mouse defensin beta 20 (Defb20) is expressed specifically in the caput region of the epididymis and regulated by androgen and testicular factors. Reprod Biol 2020;20:536–540. [DOI] [PubMed] [Google Scholar]
- Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, Mok SC.. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol 1998;152:1247–1258. [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan Y, Hamil KG, Yenugu S, Young SL, French FS, Hall SH.. Identification, characterization, and evolution of a primate beta-defensin gene cluster. Genes Immun 2005;6:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj PA, Antonyraj KJ, Karunakaran T.. Large-scale synthesis and functional elements for the antimicrobial activity of defensins. Biochem J 2000;347 Pt 3:633–641. [PMC free article] [PubMed] [Google Scholar]
- Rapista A, Ding J, Benito B, Lo Y-T, Neiditch MB, Lu W, Chang TL.. Human defensins 5 and 6 enhance HIV-1 infectivity through promoting HIV attachment. Retrovirology 2011;8:45–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci E, Malacrida S, Zanchetta M, Montagna M, Giaquinto C, De Rossi A.. Role of beta-defensin-1 polymorphisms in mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr 2009;51:13–19. [DOI] [PubMed] [Google Scholar]
- Robertson MJ, Kent K, Tharp N, Nozawa K, Dean L, Mathew M, Grimm SL, Yu Z, Légaré C, Fujihara Y. et al. Large-scale discovery of male reproductive tract-specific genes through analysis of RNA-seq datasets. BMC Biol 2020;18:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-García M, Oliva H, Climent N, García F, Gatell JM, Gallart T.. Human immature monocyte-derived dendritic cells produce and secrete alpha-defensins 1-3. J Leukoc Biol 2007;82:1143–1146. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Jiménez F-J, , KrauseA, , SchulzS, , ForssmannW-G, , Conejo-GarciaJ-R, , SchreebR, , Motzkus D.. Distribution of new human β-defensin genes clustered on chromosome 20 in functionally different segments of epididymis. Genomics 2003;81:175–183. [DOI] [PubMed] [Google Scholar]
- Sang Y, Ortega MT, Blecha F, Prakash O, Melgarejo T.. Molecular cloning and characterization of three beta-defensins from canine testes. Infect Immun 2005;73:2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangeeta K, Yenugu S.. Male reproductive tract antimicrobial expression in the extremes of ages of rats. Gene 2019;710:218–232. [DOI] [PubMed] [Google Scholar]
- Sasani N, Roghanian R, Emtiazi G, Aghaie A.. A novel approach on leukodepletion filters: investigation of synergistic anticancer effect of purified α-defensins and nisin. Adv Pharm Bull 2021;11:378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi AB, Bunge KE, Hood BL, Conrads TP, Cole AM, Gupta P, Rohan LC.. Preformulation and stability in biological fluids of the retrocyclin RC-101, a potential anti-HIV topical microbicide. AIDS Res Ther 2011a;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi AB, Cost MR, Cole AL, Cole AM, Patton DL, Gupta P, Rohan LC.. Formulation development of retrocyclin 1 analog RC-101 as an anti-HIV vaginal microbicide product. Antimicrob Agents Chemother 2011b;55:2282–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Otsuki K, Mitsukawa K, Yakuwa K, Nagatsuka M, Okai T.. Cervical inflammatory cytokines and other markers in the cervical mucus of pregnant women with lower genital tract infection. Int J Gynaecol Obstet 2006;92:117–121. [DOI] [PubMed] [Google Scholar]
- Schutte BC, Mccray PB.. [beta]-defensins in lung host defense. Annu Rev Physiol 2002;64:709–748. [DOI] [PubMed] [Google Scholar]
- Schutte BC, Mitros JP, Bartlett JA, Walters JD, Jia HP, Welsh MJ, Casavant TL, Mccray PB.. Discovery of five conserved beta-defensin gene clusters using a computational search strategy. Proc Natl Acad Sci USA 2002;99:2129–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segat L, Brandão LA, Guimarães RL, Crovella S.. Are defensin beta 1 gene polymorphisms associated with HIV infection and virus replication? AIDS 2009;23:647–650. [DOI] [PubMed] [Google Scholar]
- Segat L, Zupin L, Moura RR, Coelho AVC, Chagas BS, De Freitas AC, Crovella S.. DEFB1 polymorphisms are involved in susceptibility to human papillomavirus infection in Brazilian gynaecological patients. Mem Inst Oswaldo Cruz 2014;109:918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel A, Ye Y, De Armas LR, Soto M, Yarosh W, Marcsisin RA, Tran D, Selsted ME, Camerini D.. Cyclic and acyclic defensins inhibit human immunodeficiency virus type-1 replication by different mechanisms. PLoS One 2010;5:e9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted ME, Ouellette AJ.. Mammalian defensins in the antimicrobial immune response. Nat Immunol 2005;6:551–557. [DOI] [PubMed] [Google Scholar]
- Selsted ME, Tang YQ, Morris WL, Mcguire PA, Novotny MJ, Smith W, Henschen AH, Cullor JS.. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem 1993;268:6641–6648. [PubMed] [Google Scholar]
- Semple CAM, Rolfe M, Dorin JR.. Duplication and selection in the evolution of primate beta-defensin genes. Genome Biol 2003;4:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple F, Dorin JR.. beta-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun 2012;4:337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfanos KS, Wilson BA, De Marzo AM, Isaacs WB.. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci USA 2009;106:3443–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafee TMA, Lay FT, Phan TK, Anderson MA, Hulett MD.. Convergent evolution of defensin sequence, structure and function. Cell Mol Life Sci 2017;74:663–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Gupta S, Agarwal A, Henkel R, Finelli R, Parekh N, Saleh R, Arafa M, Ko E, Zini A. et al. Relevance of leukocytospermia and semen culture and its true place in diagnosing and treating male infertility. World J Mens Health 2022;40:191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shust GF, Cho S, Kim M, Madan RP, Guzman EM, Pollack M, Epstein J, Cohen HW, Keller MJ, Herold BC.. Female genital tract secretions inhibit herpes simplex virus infection: correlation with soluble mucosal immune mediators and impact of hormonal contraception. Am J Reprod Immunol 2010;63:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva EJR, Ribeiro CM, Mirim AFM, Silva AAS, Romano RM, Hallak J, Avellar MCW.. Lipopolysaccharide and lipotheicoic acid differentially modulate epididymal cytokine and chemokine profiles and sperm parameters in experimental acute epididymitis. Sci Rep 2018;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeate JG, Segerink WH, Garcia MD, Fernandez DJ, Prins R, Lühen KP, Voss FO, Da Silva DM, Kast WM.. Theta-defensins inhibit high-risk human papillomavirus infection through charge-driven capsid clustering. Front Immunol 2020;11:561843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehnlein O, Kai-Larsen Y, Frithiof R, Sorensen OE, Kenne E, Scharffetter-Kochanek K, Eriksson EE, Herwald H, Agerberth B, Lindbom L.. Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J Clin Invest 2008;118:3491–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J, Romero R.. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2007;20:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes RS, Kronenberg M, Heinzmann C, Daher KA, Klisak I, Ganz T, Mohandas T.. Assignment of defensin gene(s) to human chromosome 8p23. Genomics 1989;5:240–244. [DOI] [PubMed] [Google Scholar]
- Spencer JD, Hains DS, Porter E, Bevins CL, Dirosario J, Becknell B, Wang H, Schwaderer AL.. Human alpha defensin 5 expression in the human kidney and urinary tract. PLoS One 2012;7:e31712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Carmona M, Hubert P, Delvenne P, Herfs M.. Defensins: “Simple” antimicrobial peptides or broad-spectrum molecules? Cytokine Growth Factor Rev 2015;26:361–370. [DOI] [PubMed] [Google Scholar]
- Suff N, Karda R, Diaz JA, Ng J, Baruteau J, Perocheau D, Taylor PW, Alber D, Buckley SMK, Bajaj-Elliott M. et al. Cervical gene delivery of the antimicrobial peptide, human β-defensin (HBD)-3, in a mouse model of ascending infection-related preterm birth. Front Immunol 2020;11:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svinarich DM, Gomez R, Romero R.. Detection of human defensins in the placenta. Am J Reprod Immunol 1997;38:252–255. [DOI] [PubMed] [Google Scholar]
- Szukiewicz D, Alkhalayla H, Pyzlak M, Watroba M, Szewczyk G, Wejman J.. Human beta-defensin 1, 2 and 3 production by amniotic epithelial cells with respect to human papillomavirus (HPV) infection, HPV oncogenic potential and the mode of delivery. Microb Pathog 2016;97:154–165. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S.. Pattern recognition receptors and inflammation. Cell 2010;140:805–820. [DOI] [PubMed] [Google Scholar]
- Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT.. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev 2012;249:158–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YQ, Yuan J, Osapay G, Osapay K, Tran D, Miller CJ, Ouellette AJ, Selsted ME.. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 1999;286:498–502. [DOI] [PubMed] [Google Scholar]
- Tantengco OAG, Kechichian T, Vincent KL, Pyles RB, Medina PMB, Menon R.. Inflammatory response elicited by Ureaplasma parvum colonization in human cervical epithelial, stromal, and immune cells. Reproduction 2021;163:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Territo MC, Ganz T, Selsted ME, Lehrer R.. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest 1989;84:2017–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollner TL, Bevins CL, Cherr GN.. Multifunctional glycoprotein DEFB126–a curious story of defensin-clad spermatozoa. Nat Rev Urol 2012;9:365–375. [DOI] [PubMed] [Google Scholar]
- Tollner TL, Venners SA, Hollox EJ, Yudin AI, Liu X, Tang G, Xing H, Kays RJ, Lau T, Overstreet JW. et al. A common mutation in the defensin DEFB126 causes impaired sperm function and subfertility. Sci Transl Med 2011;3:92ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollner TL, Yudin AI, Tarantal AF, Treece CA, Overstreet JW, Cherr GN.. Beta-defensin 126 on the surface of macaque sperm mediates attachment of sperm to oviductal epithelia. Biol Reprod 2008a;78:400–412. [DOI] [PubMed] [Google Scholar]
- Tollner TL, Yudin AI, Treece CA, Overstreet JW, Cherr GN.. Macaque sperm coating protein DEFB126 facilitates sperm penetration of cervical mucus. Hum Reprod 2008b;23:2523–2534. [DOI] [PubMed] [Google Scholar]
- Tommasino M. The human papillomavirus family and its role in carcinogenesis. Semin Cancer Biol 2014;26:13–21. [DOI] [PubMed] [Google Scholar]
- Unemo M, Jensen JS.. Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium. Nat Rev Urol 2017;14:139–152. [DOI] [PubMed] [Google Scholar]
- Valere K, Lu W, Chang TL.. Key determinants of human α-defensin 5 and 6 for enhancement of HIV infectivity. Viruses 2017;9:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valere K, Rapista A, Eugenin E, Lu W, Chang TL.. Human alpha-defensin HNP1 increases HIV traversal of the epithelial barrier: a potential role in STI-mediated enhancement of HIV transmission. Viral Immunol 2015;28:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valore EV, Park CH, Quayle AJ, Wiles KR, Mccray PB, Ganz T.. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest 1998;101:1633–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valore EV, Wiley DJ, Ganz T.. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun 2006;74:5693–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrey A, Romero R, Panaitescu B, Miller D, Chaiworapongsa T, Patwardhan M, Faro J, Pacora P, Hassan SS, Hsu C-D. et al. Human β-defensin-1: a natural antimicrobial peptide present in amniotic fluid that is increased in spontaneous preterm labor with intra-amniotic infection. Am J Reprod Immunol 2018;80:e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbanac D, Zanetti M, Romeo D.. Chemotactic and protease-inhibiting activities of antibiotic peptide precursors. FEBS Lett 1993;317:255–258. [DOI] [PubMed] [Google Scholar]
- Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, Mckenzie ANJ, Mebius RE. et al. Innate lymphoid cells: 10 years on. Cell 2018;174:1054–1066. [DOI] [PubMed] [Google Scholar]
- Vylkova S, Li XS, Berner JC, Edgerton M.. Distinct antifungal mechanisms: beta-defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-free cationic peptides. Antimicrob Agents Chemother 2006;50:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vylkova S, Nayyar N, Li W, Edgerton M.. Human beta-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob Agents Chemother 2007;51:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Cole AM, Hong T, Waring AJ, Lehrer RI.. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J Immunol 2003;170:4708–4716. [DOI] [PubMed] [Google Scholar]
- Wang W, Owen SM, Rudolph DL, Cole AM, Hong T, Waring AJ, Lal RB, Lehrer RI.. Activity of alpha- and theta-defensins against primary isolates of HIV-1. J Immunol 2004;173:515–520. [DOI] [PubMed] [Google Scholar]
- Weber A, Alves J, Abujamra AL, Bustamante-Filho IC.. Structural modeling and mRNA expression of epididymal β-defensins in GnRH immunized boars: a model for secondary hypogonadism in man. Mol Reprod Dev 2018;85:921–933. [DOI] [PubMed] [Google Scholar]
- Wehkamp J, Harder J, Weichenthal M, Schwab M, Schaffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P. et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut 2004;53:1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehkamp J, Wang G, Kübler I, Nuding S, Gregorieff A, Schnabel A, Kays RJ, Fellermann K, Burk O, Schwab M. et al. The Paneth cell alpha-defensin deficiency of ileal Crohn’s disease is linked to Wnt/Tcf-4. J Immunol 2007;179:3109–3118. [DOI] [PubMed] [Google Scholar]
- Wiechuła B, Cholewa K, Ekiel A, Romanik M, Dolezych H, Martirosian G.. [HBD-1 and hBD-2 are expressed in cervico-vaginal lavage in female genital tract due to microbial infections]. Ginekol Pol 2010;81:268–271. [PubMed] [Google Scholar]
- Wiechuła BE, Friedek DA, Ekiel AM, Romanik MK, Martirosian G.. Human neutrophil peptides in vaginitis/cervicitis of different etiology. Pol J Microbiol 2007;56:185–189. [PubMed] [Google Scholar]
- Wiens ME, Smith JG.. Alpha-defensin HD5 inhibits furin cleavage of human papillomavirus 16 L2 to block infection. J Virol 2015;89:2866–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens ME, Smith JG.. α-Defensin HD5 inhibits human papillomavirus 16 infection via capsid stabilization and redirection to the lysosome. mBio 2017;8:e02304–e02316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld HC, Heine RP, Krohn MA, Hillier SL, Amortegui AA, Nicolazzo M, Sweet RL.. Association between elevated neutrophil defensin levels and endometritis. J Infect Dis 2002;186:792–797. [DOI] [PubMed] [Google Scholar]
- Wilde CG, Griffith JE, Marra MN, Snable JL, Scott RW.. Purification and characterization of human neutrophil peptide 4, a novel member of the defensin family. J Biol Chem 1989;264:11200–11203. [PubMed] [Google Scholar]
- Wilmes M, Sahl H-G.. Defensin-based anti-infective strategies. Int J Med Microbiol 2014;304:93–99. [DOI] [PubMed] [Google Scholar]
- Wilson SS, Wiens ME, Smith JG.. Antiviral mechanisms of human defensins. J Mol Biol 2013;425:4965–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, , Rodriguez-GarciaM, , Patel MV.. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol 2015;15:217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L.. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev 2005;206:306–335. [DOI] [PubMed] [Google Scholar]
- Xin A, Cheng L, Diao H, Wu Y, Zhou S, Shi C, Sun Y, Wang P, Duan S, Zheng J. et al. Lectin binding of human sperm associates with DEFB126 mutation and serves as a potential biomarker for subfertility. Sci Rep 2016;6:20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin A, Zhao Y, Yu H, Shi H, Diao H, Zhang Y.. Characterization of β-defensin 42 expressed in principal cells at the initial segment of the rat epididymis. Acta Biochim Biophys Sin (Shanghai) 2015;47:861–869. [DOI] [PubMed] [Google Scholar]
- Xin A, Zhao Y, Yu H, Shi H, Liu H, Diao H, Zhang Y.. Soluble fusion expression, characterization and localization of human β-defensin 6. Mol Med Rep 2014;9:149–155. [DOI] [PubMed] [Google Scholar]
- Xu D, Lu W.. Defensins: a double-edged sword in host immunity. Front Immunol 2020;11:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Holzman CB, Arvidson CG, Chung H, Goepfert AR.. Midpregnancy vaginal fluid defensins, bacterial vaginosis, and risk of preterm delivery. Obstet Gynecol 2008;112:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Nagase T, Makita R, Fukuhara S, Tomita T, Tominaga T, Kurihara H, Ouchi Y.. Identification of multiple novel epididymis-specific beta-defensin isoforms in humans and mice. J Immunol 2002;169:2516–2523. [DOI] [PubMed] [Google Scholar]
- Yang C, Boone L, Nguyen TX, Rudolph D, Limpakarnjanarat K, Mastro TD, Tappero J, Cole AM, Lal RB.. theta-Defensin pseudogenes in HIV-1-exposed, persistently seronegative female sex-workers from Thailand. Infect Genet Evol 2005;5:11–15. [DOI] [PubMed] [Google Scholar]
- Yang D, Biragyn A, Kwak LW, Oppenheim JJ.. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol 2002;23:291–296. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Chertov O, Oppenheim JJ.. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol 2000;68:9–14. [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schröder JM, Wang JM, Howard OM. et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999;286:525–528. [DOI] [PubMed] [Google Scholar]
- Yang D, Liu Z-H, Tewary P, Chen Q, De La Rosa G, Oppenheim JJ.. Defensin participation in innate and adaptive immunity. Curr Pharm Des 2007;13:3131–3139. [DOI] [PubMed] [Google Scholar]
- Yano J, Lilly E, Barousse M, Fidel PL.. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun 2010;78:5126–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbrough VL, Winkle S, Herbst-Kralovetz MM.. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update 2015;21:353–377. [DOI] [PubMed] [Google Scholar]
- Yenugu S, Hamil KG, Radhakrishnan Y, French FS, Hall SH.. The androgen-regulated epididymal sperm-binding protein, human beta-defensin 118 (DEFB118) (formerly ESC42), is an antimicrobial beta-defensin. Endocrinology 2004;145:3165–3173. [DOI] [PubMed] [Google Scholar]
- Yin L, Chung CM, Huo R, Liu H, Zhou C, Xu W, Zhu H, Zhang J, Shi Q, Wong HYC. et al. A sperm GPI-anchored protein elicits sperm-cumulus cross-talk leading to the acrosome reaction. Cell Mol Life Sci 2009;66:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Shen FS, Sturch S, Aquino A, Glazer RI, Felsted RL.. Regulation of HIV-1 gag protein subcellular targeting by protein kinase C. J Biol Chem 1995;270:4792–4796. [DOI] [PubMed] [Google Scholar]
- Yu H, Dong J, Gu Y, Liu H, Xin A, Shi H, Sun F, Zhang Y, Lin D, Diao H.. The novel human β-defensin 114 regulates lipopolysaccharide (LPS)-mediated inflammation and protects sperm from motility loss. J Biol Chem 2013;288:12270–12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin AI, Generao SE, Tollner TL, Treece CA, Overstreet JW, Cherr GN.. Beta-defensin 126 on the cell surface protects sperm from immunorecognition and binding of anti-sperm antibodies. Biol Reprod 2005a;73:1243–1252. [DOI] [PubMed] [Google Scholar]
- Yudin AI, Tollner TL, Treece CA, Kays R, Cherr GN, Overstreet JW, Bevins CL.. Beta-defensin 22 is a major component of the mouse sperm glycocalyx. Reproduction 2008;136:753–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin AI, Treece CA, Tollner TL, Overstreet JW, Cherr GN.. The carbohydrate structure of DEFB126, the major component of the cynomolgus Macaque sperm plasma membrane glycocalyx. J Membr Biol 2005b;207:119–129. [DOI] [PubMed] [Google Scholar]
- Zaballos A, Villares R, Albar JP, Martinez AC, Marquez G.. Identification on mouse chromosome 8 of new beta-defensin genes with regionally specific expression in the male reproductive organ. J Biol Chem 2004;279:12421–12426. [DOI] [PubMed] [Google Scholar]
- Zaga-Clavellina V, Martha RV-M, Flores-Espinosa P.. In vitro secretion profile of pro-inflammatory cytokines IL-1β, TNF-α, IL-6, and of human beta-defensins (HBD)-1, HBD-2, and HBD-3 from human chorioamniotic membranes after selective stimulation with Gardnerella vaginalis. Am J Reprod Immunol 2012a;67:34–43. [DOI] [PubMed] [Google Scholar]
- Zaga-Clavellina V, Ruiz M, Flores-Espinosa P, Vega-Sanchez R, Flores-Pliego A, Estrada-Gutierrez G, Sosa-Gonzalez I, Morales-Méndez I, Osorio-Caballero M.. Tissue-specific human beta-defensins (HBD)-1, HBD-2 and HBD-3 secretion profile from human amniochorionic membranes stimulated with Candida albicans in a two-compartment tissue culture system. Reprod Biol Endocrinol 2012b;10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002;415:389–395. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhou Y, Xie S, Yin Q, Tang C, Ni Z, Fei J, Zhang Y.. CRISPR/Cas9-mediated genome editing reveals the synergistic effects of β-defensin family members on sperm maturation in rat epididymis. FASEB J 2018;32:1354–1363. [DOI] [PubMed] [Google Scholar]
- Zhao C, Wang I, Lehrer RI.. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett 1996;396:319–322. [DOI] [PubMed] [Google Scholar]
- Zhao S, Zhou HY, Li H, Yi T, Zhao X.. The therapeutic impact of HNP-1 in condyloma acuminatum. Int J Dermatol 2015;54:1205–1210. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Diao H, Ni Z, Hu S, Yu H, Zhang Y.. The epididymis-specific antimicrobial peptide β-defensin 15 is required for sperm motility and male fertility in the rat (Rattus norvegicus). Cell Mol Life Sci 2011;68:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CX, Zhang Y-L, Xiao L, Zheng M, Leung KM, Chan MY, Lo PS, Tsang LL, Wong HY, Ho LS. et al. An epididymis-specific beta-defensin is important for the initiation of sperm maturation. Nat Cell Biol 2004a;6:458–464. [DOI] [PubMed] [Google Scholar]
- Zhou N, Fang J, Mukhtar M, Acheampong E, Pomerantz RJ.. Inhibition of HIV-1 fusion with small interfering RNAs targeting the chemokine coreceptor CXCR4. Gene Ther 2004b;11:1703–1712. [DOI] [PubMed] [Google Scholar]
- Zhou YS, Webb S, Lettice L, Tardif S, Kilanowski F, Tyrrell C, Macpherson H, Semple F, Tennant P, Baker T. et al. Partial deletion of chromosome 8 β-defensin cluster confers sperm dysfunction and infertility in male mice. PLoS Genet 2013;9:e1003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupin L, Polesello V, Martinelli M, Luppi S, Giolo E, Zito G, Romano F, Segat L, Crovella S, Ricci G.. Human β-defensin 1 in follicular fluid and semen: impact on fertility. J Assist Reprod Genet 2019a;36:787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupin L, Polesello V, Segat L, Kamada AJ, Kuhn L, Crovella S.. polymorphisms and HIV-1 mother-to-child transmission in Zambian population. J Matern Fetal Neonatal Med 2019b;32:2805–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in GEO database, and can be accessed with GeneChip array data GSE7307 and high-throughput sequencing data GSE150852.