Introduction
Inflammatory bowel disease (IBD) is becoming increasing prevalent in the Western world and developing countries, and places a significant burden on the healthcare system. IBD accounts for approximately 2.9 disability-adjusted life-years and affects approximately 1% of the population.1,2 Diagnosing IBD is very challenging due to the overlap of symptoms with irritable bowel syndrome (IBS), a common disorder that affects the large intestine. Both diseases are characterized by recurrent abdominal pain, and IBS-like symptoms are frequently reported in patients before the diagnosis of IBD. Consequently, careful clinical examination, including endoscopic and radiologic features, is needed for their differentiation, resulting in substantial costs. Multiple lines of evidence have established that mucins, essential components of the mucosal defense system, are key drivers governing the pathogenesis of intestinal diseases.3 Consequently, changes in mucin quantity and quality are associated with disease outcomes.3 Mucins have long been recognized as potential biomarkers for the diagnosis, prognosis, or management of IBD or colorectal cancer.4,5 However, until now, they have not been used in routine clinical practice, as their characterization required invasive procedures.
A new paradigm has emerged establishing that excreted feces are covered by 2 layers of colon mucus.6 Therefore, we hypothesized that any changes to mucins could be detected in the feces of patients with IBD or IBS. Thus, we conducted, for the first time, the analysis of human fecal mucin glycosylation and compared the profile of mucin O-glycans between healthy individuals, patients with Crohn’s disease (CD), and patients with unrelated IBD.
Methods
All stool samples used in this study were obtained from patients monitored at Lille University Hospital. No additional sampling was necessary. As stool samples were taken from a registered biological collection, and patient consent was not required according to French law. Agreement for the establishment of a biological collection of IBD samples was obtained from the French Ministry of Education and Research under reference DC2008-642. Institutional review board approval was given by the Comité de Protection des Personnes Nord-Ouest IV, the ethical committee of our institution.
Stool Sample Collection
Stools were collected from 60 patients admitted for abdominal pain or IBD management. Among them, 48 had experienced CD (18 with active CD and 30 with quiescent CD) and 12 had unrelated IBD. Five healthy control (HC) subjects were also included.
After the determination of calprotectin, the stool samples were stored at -80°C until analysis. CD activity was assessed by the Harvey-Bradshaw index and Pediatric Crohn’s Disease Activity Index (PCDAI). The demographics and clinical features of the CD patients are summarized in Table 1.
Table 1.
Clinical and demographic features of the study population
| Active CD | |||||
|---|---|---|---|---|---|
| Patient No. | Calprotectin (µg/g) | Disease Location (Montreal Classification)a | Age at Stool Collection (y) | Age at Diagnosis (y) | Treatment |
| 1 | 903 | L3 | 63 | 31 | Humira 80 mg/wk |
| 2 | 73 | L3 | 14 | 13 | Pentasa 2 g/d |
| 3 | >1800 | L2 | 61 | 24 | No treatment |
| 4 | 240 | L1 | 58 | 14 | Inflectra 10 mg/kg every 6 wk + Salazopyrin |
| 5 | 101 | L3 | 53 | 20 | Simponi 50 mg/month |
| 6 | >1800 | L1 | 20 | 17 | No treatment |
| 7 | >1800 | L1 | 18 | 16 | No treatment |
| 8 | 753 | L1 + L4 | 26 | 23 | Stelara 90 mg/month |
| 9 | 283 | L3 | 28 | 28 | Idacio 40 mg every 2 wk + Solupred 40 mg/d |
| 10 | >1800 | L1 | 14 | 13 | Entocort 9 mg/d |
| 11 | 852 | L1 | 9 | 9 | Methotrexate 7.5 mg/wk + Hydrocortisone 12.5 mg/m²/d |
| 12 | 1371 | L2 | 9 | 8 | Infliximab 10 mg/kg every 4 wk + Imurel 75 mg/d |
| 13 | 1482 | L2 | 15 | 12 | Humira 40 mg every 2 wk |
| 14 | >1800 | L3 | 14 | 10 | Stelara 45 mg every 4 wk + Methotrexate 10 mg/wk |
| 15 | 124 | L1 | 30 | 20 | Entyvio 300 mg every 4 wk + Solupred 30 mg/d |
| 16 | 1641 | L2 | 18 | 10 | Humira 40 mg every 2 wk |
| 17 | >1800 | L3 + L4 | 16 | 16 | Humira 40 mg every 2 wk + Solupred 10 mg/d |
| 18 | 40 | L1 + L4 | 10 | 10 | Humira 40 mg every 2 wk + Imurel 75 mg/d + Hydrocortisone 10 mg/d |
| Inactive CD | |||||
| 19 | 31 | L3 | 34 | 20 | No treatment |
| 20 | >1800 | L1 | 29 | 18 | Inflectra 10 mg/kg every 6 wk |
| 21 | 214 | L1 + L4 | 20 | 18 | Humira 80 mg every 2 wk |
| 22 | <30 | L3 | 31 | 21 | Inflectra 10 mg/kg every 8 wk |
| 23 | <30 | L1 | 54 | 36 | No treatment |
| 24 | <30 | L1 | 40 | 37 | Imurel 200 mg/d |
| 25 | <30 | L1 | 34 | 24 | Idacio 40 mg every 2 wk |
| 26 | <30 | L1 | 27 | 22 | Inflectra 5 mg/kg every 8 wk |
| 27 | 352 | L3 | 35 | 15 | Stelara 90 mg every 8 wk |
| 28 | 309 | L3 | 64 | 24 | Entyvio 300 mg every 4 wk |
| 29 | >1800 | L4 | 60 | 45 | Inflectra 10 mg/kg every 8 wk + Imurel 175 mg/d |
| 30 | 35 | L1 | 24 | 18 | Inflectra 10 mg/kg every 6 wk |
| 31 | <30 | L2 | 43 | 32 | Imurel 125 mg/d + Pentasa 2 g/d |
| 32 | 479 | L3 | 18 | 10 | Entyvio 5 mg/kg every 6 wk + Pentasa 4 g/d |
| 33 | >1800 | L1 | 14 | 14 | Humira 40 mg every 2 wk |
| 34 | 66 | L3 + L4 | 57 | 32 | Imurel 200 mg/d + Remsima 120 mg every 2 wk |
| 35 | 44 | L3 | 29 | 22 | Entyvio 300 mg every 4 wk |
| 36 | 1221 | L1 | 12 | 12 | Pentasa 3 g/d |
| 37 | 86 | L1 | 26 | 19 | No treatment |
| 38 | <30 | L3 | 68 | 46 | Pentasa 2 g/d + Inexium 40 mg/d |
| 39 | 430 | L1 | 25 | 16 | Infliximab 10 mg/kg every 6 wk |
| 40 | 444 | L3 | 36 | 20 | Stelara 90 mg every 8 wk + Infliximab 5 mg/kg every 8 wk |
| 41 | <30 | L1 | 44 | 28 | Entyvio 300 mg every 4 wk |
| 42 | 48 | L3 | 40 | 23 | Entyvio 300 mg every 8 wk |
| 43 | 128 | L3 + L4 | 17 | 13 | Inflectra 10 mg/kg every 4 wk + Imurel 150 mg/d |
| 44 | 939 | L1 | 36 | 33 | Cimzia 400 mg/month + Questran 1 bag/d |
| 45 | 368 | L1 | 36 | 20 | Entyvio 300 mg every 8 wk |
| 46 | 93 | L3 + L4 | 17 | 10 | Humira 80 mg every 2 wk + Pentasa 2 g/d |
| 47 | 1002 | L3 | 13 | 9 | Humira 80 mg/wk |
| 48 | 690 | L3 | 13 | 12 | Infliximab 10 mg/kg every 4 wk + Imurel 100 mg/d |
| Unrelated IBD | |||||
| Patient No. | Calprotectin (µg/g) | Symptoms/Diseases | Age at Stool Collection (y) | Age at Diagnosis (y) | Treatment |
| 49 | <30 | Chronic abdominal pains | 11 | 7 | Forlax 2 bags/d |
| 50 | <30 | Abdominal pains | 8 | 2 | No treatment |
| 51 | 37 | Irritable bowel syndrome | 32 | 31 | Gluten-free diet |
| 52 | 346 | Abdominal pains | 61 | 58 | Low Fodmap diet |
| 53 | <30 | Functional colopathy | 39 | Many years | Low Fodmap diet |
| 54 | 92 | Abdominal pains + mucus and bloody stools + constipation | 16 | 11 | Forlax 1 bag/d |
| 55 | 324 | Cystic fibrosis + gastroesophageal reflux + constipation | 11 | 0 | Forlax when needed |
| 56 | 350 | Cystic fibrosis | 2 | 0 | No treatment |
| 57 | 311 | Mucus and bloody stools + gastroesophageal reflux | 0 | 0 | No treatment |
| 58 | 35 | Chronic abdominal pains + transit disorders | 24 | 24 | Debridat |
| 59 | 70 | Functional colopathy | 34 | 29 | Pantoprazole 20 mg/d + Bedelix |
| 60 | <30 | Dyspepsia + chronic epigastralgia | 48 | 48 | No treatment |
aL1: ileal; L2: colonic; L3: ileocolonic; L4: upper gastrointestinal tract.
Abbreviations: CD, Crohn’s disease; IBD, inflammatory bowel disease.
Mucin Purification
Stool samples were suspended in sodium chloride solution (0.2 mol/L) containing 0.02 mol/L sodium azide at 4°C. After homogenization, the samples were immediately centrifuged at 10 000 g for 30 minutes. The supernatant was dialyzed and freeze dried, and mucins were solubilized in extraction buffer (containing 4 M guanidine chloride, 5 mM EDTA, 10 mM benzamidine, 5 mM N-ethylmaleimide, 0.1 mg/mL trypsin inhibitor, and 1 mM phenylmethanesulfonyl fluoride) and then purified by isopycnic density-gradient centrifugation (Beckman Coulter LE80K ultracentrifuge; 70.1 Ti rotor, 417 600 g at 15°C for 72 hours). Mucin-containing fractions were pooled, dialyzed, and lyophilized before use.
Glycan Release and Permethylation
Mucins were then submitted to β-elimination under reductive conditions (0.1 M potassium hydroxide, 1 M potassium borohydride for 24 hours at 45°C). After coevaporations with methanol, oligosaccharides were purified on a cation exchange resin column (Dowex 50 × 2, 200-400 mesh, H+ form). The oligosaccharide-alditol fractions were then permethylated, in their anhydrous form, in a solution containing 200 μL dimethyl sulfoxide, 300 μL iodomethane, and 1 g sodium hydroxide, for 2 hours, before adding 1 mL acetic acid (5% [v/v]) to stop the reaction. After derivatization, the reaction products were dissolved in 200 µL methanol and further purified on a C18 Sep-Pak column (Oasis HLB; Waters).
Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry
Permethylated oligosaccharides were analyzed by matrix-assisted laser desorption ionization–time of flight mass spectrometry in positive ion reflective mode as [M + Na]+. Samples were dissolved in a methanol/water solvent (50:50) and coated on a matrix-assisted laser desorption ionization target with a 2,5-dihydroxybenzoic acid matrix at a volume/volume dilution. The relative percent of each oligosaccharide was calculated based on the integration of peaks on mass spectrometry spectra.
Statistics
The results are expressed as mean ± SD. Statistical analyses were performed using R (version 4.2.1; R Foundation for Statistical Computing) and RStudio (R: 4.2.1) software. Differences in the level of expression of mucin O-glycosylation were analyzed using the Student’s t test. A P value <.05 was considered statistically significant.
Results
Mucins were purified from human stool samples and their glycosylation repertoire was analyzed by matrix-assisted laser desorption ionization–time of flight mass spectrometry. The profile of fecal O-glycans from HC subjects was compared with that of human intestinal mucins purified previously from tissues of healthy individuals,7 in order to evaluate if fecal O-glycans could reflect the glycans found in intestine. Around 100 different oligosaccharides have been described in tissues, mainly based on core 3 structures, with the most common being sialylated trisaccharides at m/z 936. No significant differences were observed between fecal and tissue samples from healthy individuals.
The ability of this novel stool-based analysis of mucin O-glycans to discriminate between CD and unrelated IBD patients was then determined. The objective was to identify O-glycans that were differently expressed in CD and unrelated IBD patients (comprising IBS patients and HC subjects). Four glycans were of particular interest: the ion at m/z 936 and ions at m/z 534, 779, and 983, whose level of expression increased significantly in CD. The ion at m/z 534 corresponds to the Thomsen–Friedenreich antigen whose increased expression has been widely reported in the inflamed mucosa of patients with IBD.8 Whereas the ions at m/z 779 and 983 are mainly based on core 3 structures in HC subjects and are only weakly expressed, they corresponded to core 1 O-glycans and were drastically increased in CD, suggesting a potential dysregulation of expression of core 1 and core 3 glycosyltransferases in IBD.
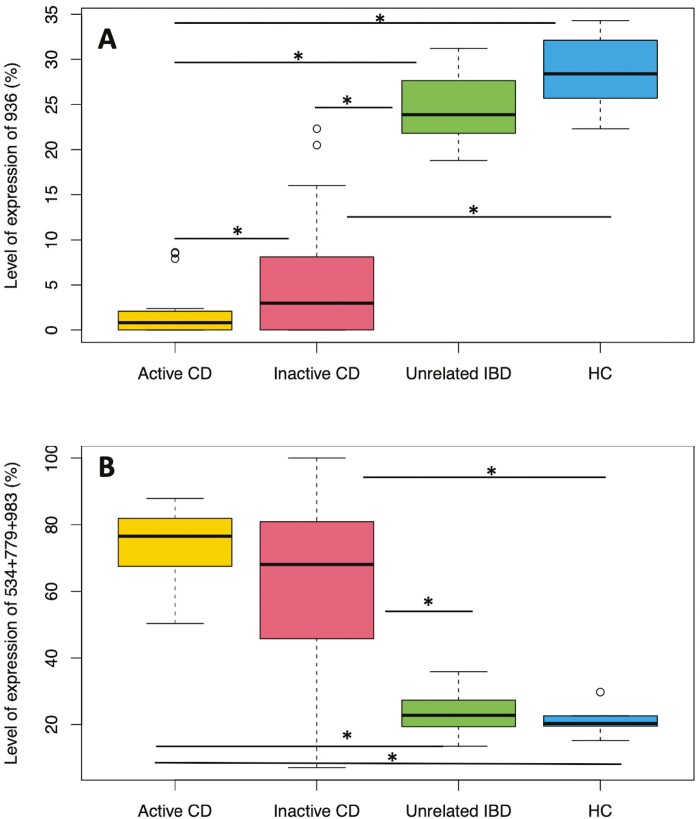
Compared with unrelated IBD individuals, patients with active and inactive CD all showed a significant decrease in sialylated glycans at m/z 936: from 23.7 ± 4.3% in unrelated IBD patients and 28.6 ± 4.8% in HC subjects to 2 ± 3% in active CD patients and 5.4 ± 6.5% in inactive CD patients (Figure 1A). In inactive CD, 2 groups of patients were observed: those for whom the level of m/z 936 was low or null and always <5% (0.9 ± 1.5%) and those for whom the level of m/z 936 was between 5% and 22% (12 ± 5.3%). This suggests that terminal sialylation of glycans could be affected in some CD patients, probably due to mutations or deficiency of ST6GALNAC1, the dominant sialyltransferase expressed in goblet cells. This concurs with the recent paper of Yao et al.9 Thus, this new approach of mucin analysis could be a reliable tool to distinguish CD patients from unrelated IBD patients, and to monitor CD patients for whom treatments to improve the mucus barrier and sialylation should be considered.
Figure 1.
The association of fecal mucin O-glycans with active Crohn’s disease (CD), inactive CD, unrelated inflammatory bowel disease (IBD), and healthy control (HC) subjects. The 25th, 50th, and 75th percentiles and whiskers at the first quartile – 1.5 × the interquartile range (IQR) and quartile 3 + 1.5 × IQR based on (A) the level of expression of m/z 936, corresponding to the structure GlcNAcβ1-3(NeuAcα2-6)GalNAc and (B) sum of expression of m/z 534, 779, and 983. The structures of oligosaccharides at m/z 534, 779, and 983 were Galβ1-3GalNAc, GlcNAcβ1-3Galβ1-3GalNAc, and Galβ1-3/4GlcNAcβ1-3Galβ1-3GalNAc, respectively. P values were calculated for the different associations. For the ion at m/z 936, P values were .042 (active CD/inactive CD), 4.37 × 10-17 (active CD/unrelated IBD), 7.63 × 10-13 (active CD/HC), 6.38 × 10-12 (inactive CD/unrelated IBD), 1.05 × 10-8 (inactive CD/HC), and .094 (unrelated IBD/HC). For the ions at m/z 534 + 779 + 983, P values were respectively of .1056 (active CD/inactive CD), 7.56 × 10-14 (active CD/unrelated IBD), 4.52 × 10-9 (active CD/HC), 4.59 × 10-6 (inactive CD/unrelated IBD), .0011 (inactive CD/HC), and .5831 (unrelated IBD/HC). Statistically significant associations are marked with an asterisk.
Patients with active and inactive CD all had a high level of truncated core 1 O-glycans. In HC subjects or unrelated IBD patients, their expression was relatively low, at an average of 21.4 ± 5.4% and 24.5 ± 6.5%, respectively, whereas the average was 72.4 ± 11.5% in active CD patients and 62.1 ± 24.9% in inactive CD patients (Figure 1B).
Discussion
Currently, the accurate diagnosis of IBD is a challenge, and endoscopy remains the gold standard to discriminate between IBD and IBS. However, its invasive nature increases the risk of adverse events for the patient and has a significant impact on health costs. In the present study, we demonstrated that the glycosylation profile of fecal mucins showed specific features in active and quiescent CD, not found in IBS patients or HC subjects, suggesting that evaluation of mucin glycosylation in feces could become a new noninvasive tool for screening of intestinal diseases, without recourse to endoscopy.
Based on our previous findings in colorectal cancer and the fact that the tumor O-glycans identified were mainly sialylated core 1 or Thomsen-nouveau antigens, we assume that this new tool could also be used for the diagnosis, prognosis, or management of colorectal cancer.10
Our data also highlight the need for a new classification of patients with CD, taking into account alterations in mucin glycosylation, potentially leading to the development of new treatments to improve the mucus barrier.
The limitations to our study include the small number of patients enrolled; thus, the results need to be confirmed in a larger prospective study. It would also be interesting to follow mucin glycosylation in CD patients and the impact of biological therapy on mucin barrier restoration longitudinally.
Acknowledgments
The authors sincerely thank Jean-Michel Dewitte and Zélie Mockelyn for their contribution to this work.
Contributor Information
Catherine Robbe Masselot, Unité de Glycobiologie Structurale et Fonctionnelle (CNRS UMR 8576), Université de Lille, Lille, France.
Camille Cordier, Laboratoire de Parasitologie-Mycologie, INSERM U1285, Unité de Glycobiologie Structurale et Fonctionnelle (CNRS UMR 8576), Centre Hospitalier Universitaire de Lille, Université de Lille, Lille, France.
Benjamin Marsac, Unité de Glycobiologie Structurale et Fonctionnelle (CNRS UMR 8576), Université de Lille, Lille, France.
Maria Nachury, INSERM U1286, Institute for Translational Research in Inflammation, Centre Hospitalier Universitaire de Lille, Université de Lille, Lille, France.
Renaud Léonard, Unité de Glycobiologie Structurale et Fonctionnelle (CNRS UMR 8576), Université de Lille, Lille, France.
Boualem Sendid, Laboratoire de Parasitologie-Mycologie, INSERM U1285, Unité de Glycobiologie Structurale et Fonctionnelle (CNRS UMR 8576), Centre Hospitalier Universitaire de Lille, Université de Lille, Lille, France.
Author Contributions:
C.R.M.: study concept and design, funding, collection and assembly of data, data analysis and integration, manuscript writing, editing, and final approval of the manuscript. C.C.: clinical study design and patient recruitment, editing, and final approval of the manuscript. B.M.: collection and assembly of data and final approval of the manuscript. M.N.: clinical patient recruitment, editing, and final approval of the manuscript. R.L.: study concept and design, collection and assembly of data, and final approval of the manuscript. B.S.: study concept and design, funding, clinical study design and patient recruitment, drafting, editing, and final approval of the manuscript.
Funding
This study was equally supported by INSERM and CNRS (France).
Conflict of Interest
All authors declare that they have no conflicts of interest.
Data Availability
All data will be available to other researchers upon request.
References
- 1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality form 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 19901-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197-2223. [DOI] [PubMed] [Google Scholar]
- 3. Sheng YH, Hasnain SZ, Florin THJ, McGuckin MA.. Mucins in inflammatory bowel diseases and colorectal cancer. J Gastroenterol Hepatol. 2012;27:28-38. [DOI] [PubMed] [Google Scholar]
- 4. Robbe-Masselot C, Herrmann A, Maes E, Carlstedt I, Michalski J-C, Capon C.. Expression of a core 3 disialyl-Le(x) hexasaccharide in human colorectal cancers: a potential marker of malignant transformation in colon. J Proteome Res. 2009;8:702-711. [DOI] [PubMed] [Google Scholar]
- 5. Theodoratou E, Campbell H, Ventham NT, et al. The role of glycosylation in IBD. Nat Rev Gastroenterol Hepatol. 2014;11:588-600. [DOI] [PubMed] [Google Scholar]
- 6. Bergstrom K, Shan X, Casero D, et al. Proximal colon–derived O-glycosylated mucus encapsulates and modulates the microbiota. Science 2020;370:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robbe C, Capon C, Coddeville B, Michalski J-C.. Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem J. 2004;384:307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhodes JM, Black RR, Savage A.. Altered lectin binding by colonic epithelial glycoconjugates in ulcerative colitis and Crohn’s disease. Dig Dis Sci. 1988;33:1359-1363. [DOI] [PubMed] [Google Scholar]
- 9. Yao Y, Kim G, Shafer S, et al. Mucus sialylation determines intestinal host–commensal homeostasis. Cell 2022;185:1172-1188.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mihalache A, Delplanque J-F, Ringot-Destrez B, et al. Structural characterization of mucin O-glycosylation may provide important information to help prevent colorectal tumor recurrence. Front Oncol. 2015;5:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be available to other researchers upon request.