Abstract
Background
Tofacitinib is an oral, small molecule JAK inhibitor for the treatment of ulcerative colitis (UC). These post hoc analyses assessed associations between C-reactive protein (CRP), partial Mayo score (PMS), and efficacy outcomes during tofacitinib induction in UC.
Methods
Patients received tofacitinib 10 mg twice daily (BID) in an 8-week, phase 2 induction study and 2 identical, 8-week, phase 3 induction studies (OCTAVE Induction 1&2); induction nonresponders (IndNR) received an additional 8 weeks of tofacitinib 10 mg BID in an open-label, long-term extension study. Associations between CRP and PMS, and efficacy outcomes (clinical response, clinical remission, endoscopic improvement, and endoscopic remission) were analyzed using univariate and multivariable logistic regression and receiver operating characteristic curves.
Results
Changes from baseline in the logarithm of CRP ([log]CRP) and PMS at week 4 were associated with clinical response at week 8 (univariate: per unit, odds ratio [OR], 0.55 [95% confidence interval (CI), 0.48-0.62]; and 0.42 [0.37-0.47], respectively). Among IndNR, change from baseline in PMS at week 8 was associated with clinical response at week 16 (univariate: per unit, OR, 0.59; 95% CI, 0.46-0.75). C-reactive protein at week 4 (area under the curve [AUC] > 0.6) and PMS at weeks 2 and 4 (AUC, > 0.7) generally exhibited predictive value for week 8 efficacy outcomes.
Conclusions
Patients who achieved clinical response at week 8 had larger decreases in CRP and PMS at week 4 than patients who did not. IndNR who achieved clinical response at week 16 with extended tofacitinib induction had a larger decrease in PMS at week 8 vs those who did not. ClinicalTrials.gov:NCT00787202;NCT01465763;NCT01458951;NCT01470612.
Keywords: C-reactive protein, inflammatory bowel disease, partial Mayo score
Introduction
Ulcerative colitis (UC) is a chronic disease characterized by relapsing and remitting inflammation of the colonic mucosa.1 The Mayo score (incorporating the endoscopic subscore) is used to evaluate UC disease activity,1 but there is a need to identify early markers in patients with UC to assess this, as well as disease relapse, response to therapy, and the distinction between inflammatory and noninflammatory origin of symptoms.2
Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of UC. The efficacy and safety of tofacitinib have been evaluated in patients with moderately to severely active UC in an 8-week, phase 2 induction study,3 three phase 3 studies (2 identical, 8-week induction studies and a 52-week maintenance study),4 and an open-label, long-term extension (OLE) study.5 These studies provide the opportunity for post hoc analyses to be performed to select patients for whom altering tofacitinib dose or duration of induction would be beneficial, based on demographics and clinical characteristics such as prior exposure to other UC therapies, eg, tumor necrosis factor inhibitors (TNFi). The induction studies in particular provide an opportunity to potentially identify early changes in markers of disease activity, which may help clinicians predict successful induction therapy with tofacitinib or may help them to decide which patients might benefit from extended induction therapy.
It has been demonstrated that C-reactive protein (CRP), a biomarker of inflammation, correlates both with the extent of UC at diagnosis6 and with clinical and endoscopic activity in patients with UC.7–9 C-reactive protein has also been considered a predictive biomarker for response to biologics in these patients.10 Furthermore, guidelines recommend normalization of CRP as a vital treatment aim for patients with UC,11 illustrating the important role of this biomarker in UC. Fecal calprotectin (FCP), a marker of intestinal inflammation, can also be a helpful predictor of response to therapy or relapse in patients with UC.12 Partial Mayo score (PMS), which includes stool frequency, rectal bleeding, and Physician Global Assessment of disease activity subscores, can be used to classify patient- and physician-perceived clinical response in UC.13 As the PMS does not include the endoscopy component of the Mayo score, it may be considered a noninvasive and constructive means of identifying which patients will probably respond to UC therapy. C-reactive protein and PMS data were routinely collected for all patients throughout the tofacitinib UC clinical program.
A potential predictor of patients who may be less likely to respond to UC therapy is prior use of biologic therapy; as such, it is of value to characterize early predictors of response to control for TNFi exposure. Patients who have previously not responded to—or have lost response to—TNFi are generally considered more refractory to UC therapy than those who have not.14,15 These patients may represent a subgroup of patients less likely or requiring more time to respond to UC therapy.
To better identify patients for whom tofacitinib induction therapy might be successful, or patients who might benefit from extended induction therapy, we report on the association of early biomarkers of disease, CRP and PMS, with clinical and endoscopic outcomes in patients with moderately to severely active UC receiving tofacitinib induction therapy.
Materials and Methods
Study Design and Patients
The phase 2 and phase 3 induction studies were double-blind, placebo-controlled, parallel-group, multicenter studies of patients with moderately to severely active UC, which was defined as a Mayo score of 6 to 12 and endoscopic subscore of 2 or 3 (phase 2) or a Mayo score of 6 to 12, with a rectal bleeding subscore of 1 to 3 and an endoscopic subscore of 2 or 3 (phase 3). Inclusion and exclusion criteria were not the same between the studies. Full details of the trial designs, inclusion and exclusion criteria, and patient populations have been described previously.3,4
In the phase 2 induction study (NCT00787202), patients were randomized to receive tofacitinib 0.5, 3, 10, or 15 mg twice daily (BID), or placebo, for 8 weeks.3 The phase 3 OCTAVE Induction 1 and 2 studies (NCT01465763; NCT01458951) were 2 identical, 8-week studies in which patients were randomized to receive tofacitinib 10 mg BID or placebo.4 Patients without clinical response at the end of OCTAVE Induction 1 and 2 (induction nonresponders) were eligible to enroll in the OLE study (OCTAVE Open; NCT01470612), where they continued to receive tofacitinib 10 mg BID for an extra 8 weeks of induction therapy5 (Supplementary Figure 1). Only patients who received tofacitinib 10 mg BID induction therapy were included in these analyses, as this is the recommended induction dose for patients with moderately to severely active UC.16
Assessments
Early markers of disease activity and efficacy outcomes in the phase 2 and phase 3 OCTAVE Induction studies, and OLE studies, are summarized in Supplementary Table 1. Blood samples were collected and analyzed for CRP.
Partial Mayo score was assessed on a scale of 0 to 9, with each subscore ranging from 0 to 3. All patients underwent endoscopy at week 8 of the phase 2 and phase 3 OCTAVE Induction studies; tofacitinib induction nonresponders also underwent endoscopy at week 16 of tofacitinib induction (ie, month 2 of the OLE study).
Clinical and endoscopic outcomes were evaluated at baseline, week 8, and week 16 of tofacitinib induction.
Delayed responders were defined as tofacitinib induction nonresponders (at week 8) who demonstrated a clinical response at week 16 of tofacitinib induction. Complete nonresponders were defined as patients who did not demonstrate a clinical response at week 16 of tofacitinib induction and were subsequently discontinued from the study.
Statistical Analyses
The full analysis set for each study is defined in Supplementary Table 2. C-reactive protein and PMS were analyzed based on efficacy outcomes. For OCTAVE Induction 1 and 2, and the OLE study, median (interquartile range) change from baseline was reported for CRP, and arithmetic mean (standard deviation) change from baseline or week 8 was reported for PMS. For the phase 2 study, geometric mean and arithmetic mean were reported for absolute CRP and PMS, respectively. The proportions of patients with CRP <3 mg/L and PMS <2 at week 4 (OCTAVE Induction 1 and 2) were also summarized descriptively. Univariate logistic regression analyses were performed to evaluate whether absolute and change from baseline in the logarithm of CRP ([log]CRP) and PMS were associated with clinical and endoscopic efficacy outcomes; these were reported as odds ratios with 95% confidence intervals (CIs).
In the induction studies, PMS at baseline, week 2, and week 4, and CRP at baseline and week 4, were analyzed based on whether patients achieved the defined clinical and endoscopic outcomes at week 8. To assess early markers of disease activity associated with delayed response among initial tofacitinib induction nonresponders, PMS and CRP at week 8 and PMS at week 12 (ie, month 1 of the OLE study) of tofacitinib induction were analyzed based on whether patients achieved efficacy outcomes after receiving an extra 8 weeks tofacitinib of 10 mg BID in the OLE study (week 16 of tofacitinib induction; interim data as of May 27, 2019; database not locked).
These analyses were post hoc for exploratory purposes only, and no multiplicity adjustment was performed. For the univariate analyses, nominal P values ≤ .05 were considered significant. In order to assess the effect of various factors on the likelihood of achieving the defined efficacy outcomes, multivariable logistic regression modeling was performed for the pooled OCTAVE Induction 1 and 2 studies, using a stepwise selection procedure with a significance level of 0.05 to enter and remain in the model. Covariates used in the models were [log]CRP at week 4, PMS at week 4, age (continuous), body mass index, disease duration, extent of disease, gender, geographic region, oral corticosteroid use at baseline, prior immunosuppressant use, prior TNFi failure, race, and smoking status.
Analysis of receiver operating characteristic (ROC) curves, including area under the curve (AUC) analyses, were used to determine the predictive value of observed CRP at week 4, and PMS at weeks 2 and 4, in achieving efficacy outcomes at week 8 among patients who received tofacitinib 10 mg BID induction therapy. ROC curves were also used to establish the predictive value of observed CRP and PMS at week 8, and PMS at week 12 of tofacitinib induction, in achieving efficacy outcomes at week 16 among tofacitinib induction nonresponders receiving extended induction therapy. The predictive value of prior TNFi failure in achieving efficacy outcomes at week 8 or week 16 of tofacitinib induction was also determined using ROC curves.
Ethical Considerations
All studies were conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines and were approved by the Institutional Review Boards and/or independent ethics committees at each of the investigational centers participating in the studies, or by a central Institutional Review Board. All patients provided written informed consent. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Patient Population
Baseline characteristics have been described previously for the phase 2 and phase 3 OCTAVE Induction studies,3,4 and were similar across studies. In the phase 2 study, 33 patients received tofacitinib 10 mg BID; at baseline, mean PMS was 5.6 (standard deviation, 1.5), mean CRP was 11.3 mg/L (range 0.2-72.4), and 68.8% of patients had CRP >3 mg/L at baseline. In the OCTAVE Induction 1 and 2 studies, 905 patients received tofacitinib 10 mg BID; at baseline, mean PMS was 6.4 (standard deviation, 1.2), mean CRP was 11.2 mg/L (range 0.1-208.4), and 63.5% of patients had CRP >3 mg/L (pooled).
Baseline characteristics of tofacitinib induction nonresponders are shown in Supplementary Table 3.
Association Between Early Markers of Disease Activity and Clinical and Endoscopic Outcomes
CRP
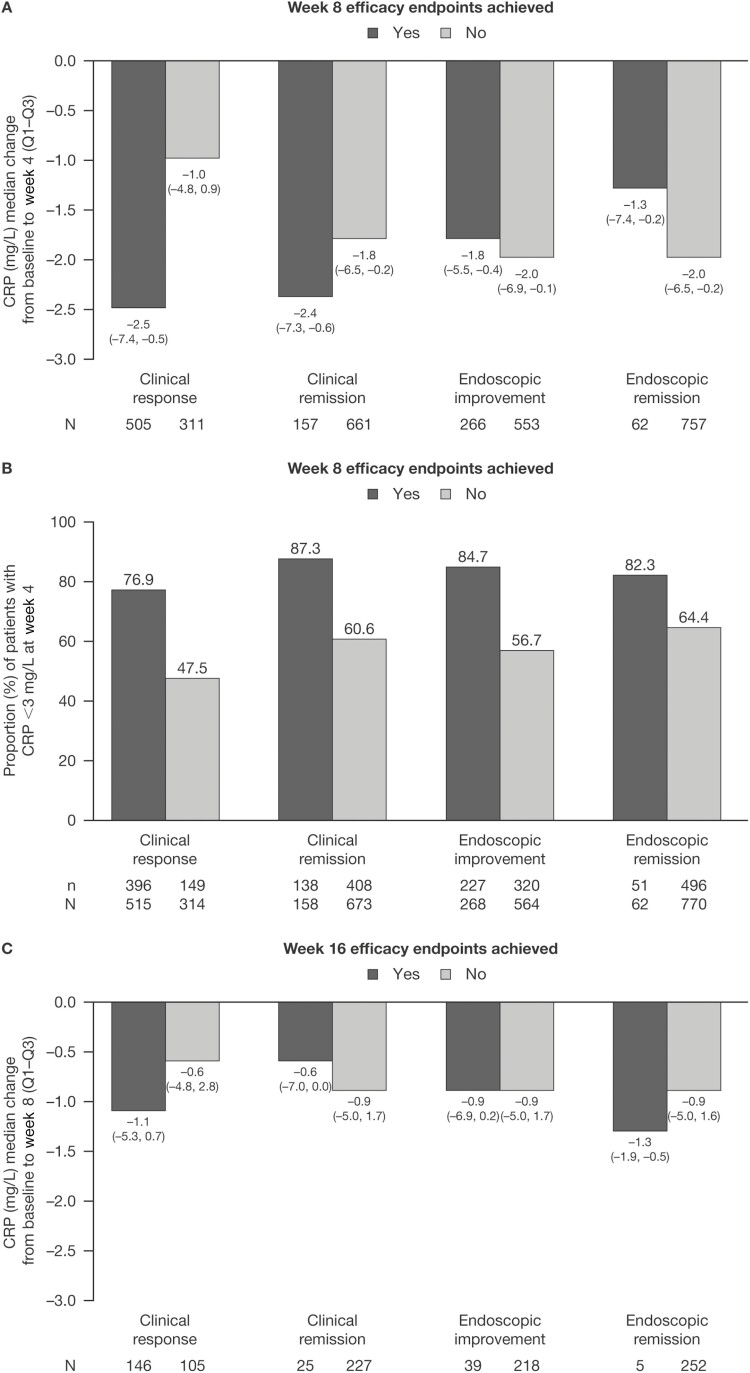
In OCTAVE Induction 1 and 2, patients receiving tofacitinib 10 mg BID had numerically greater median reductions from baseline in CRP at week 4 for patients who achieved clinical response (−2.5 mg/L) and clinical remission (−2.4 mg/L) at week 8, compared with those who did not (−1.0 mg/L and −1.8 mg/L, respectively; Figure 1A). A similar trend (based on geometric means) was observed for clinical response in the phase 2 study (Supplementary Figure 2). In OCTAVE Induction 1 and 2, median change from baseline in CRP at week 4 was −1.8 mg/L and −1.3 mg/L among patients who achieved endoscopic improvement and endoscopic remission at week 8, respectively, compared with those who did not (−2.0 mg/L and −2.0 mg/L, respectively; Figure 1A). A numerically higher proportion of patients treated with tofacitinib 10 mg BID who achieved efficacy outcomes had CRP <3 mg/L at week 4, compared with patients who did not (Figure 1B).
Figure 1.
CRP in patients receiving tofacitinib 10 mg BID in OCTAVE Induction 1 and 2, stratified by whether patients achieved clinical and endoscopic outcomes at specified timepoints (full analysis set, observed case analysis). A, Change from baseline in CRP at induction week 4, by week 8 efficacy endpoints. B, Proportion of patients with CRP <3 mg/L at week 4, by week 8 efficacy endpoints. C, Change from baseline in CRP at induction week 8, by week 16 efficacy endpoints. Abbreviations: BID, twice daily; CRP, C-reactive protein; N, number of patients with nonmissing data in each category; n, number of patients in the analysis population; Q, quartile.
Univariate logistic regression analysis demonstrated that change from baseline in CRP at week 4 was significantly associated with achieving efficacy outcomes at week 8 in OCTAVE Induction 1 and 2 (Table 1). Results for the association between CRP and induction efficacy from the phase 2 study are shown in Supplementary Table 4.
Table 1.
Univariate logistic regression analysisc results for association of CRP and PMS with clinical and endoscopic outcomes at weeks 8 and 16 of tofacitinib induction in patients receiving tofacitinib 10 mg BID (full analysis set, observed case analysis).
| Odds Ratio(95% CI) | CRP and PMS Change From Baseline, By Week 8 Efficacy Endpoints | CRP and PMS Change From Baseline, By Week 16 Efficacy Endpoints | |||
|---|---|---|---|---|---|
| CRP (mg/L) | PMS | CRP (mg/L) | PMS | ||
| Week 4 | Week 2 | Week 4 | Induction Week 8 | Induction Week 8 | |
| N = 819 | N = 832 | N = 838 | N = 257 | N = 259 | |
| Clinical responsed | 0.55a (0.48, 0.62) | 0.53a (0.47, 0.59) | 0.42a (0.37, 0.47) | 0.89 (0.75, 1.06) | 0.59a (0.46, 0.75) |
| Clinical remissione | 0.65a (0.57, 0.74) | 0.61a (0.56, 0.68) | 0.58a (0.52, 0.65) | 0.92 (0.69, 1.24) | 0.88 (0.61, 1.27) |
| Endoscopic improvement | 0.69a (0.61, 0.77) | 0.72a (0.67, 0.78) | 0.69a (0.63, 0.75) | 0.90 (0.70, 1.15) | 0.80 (0.59, 1.09) |
| Endoscopic remission | 0.81b (0.67, 0.98) | 0.71a (0.63, 0.82) | 0.70a (0.61, 0.80) | 0.85 (0.44, 1.62) | 0.92 (0.42, 2.01) |
Log-transformed data were used for CRP analysis. Odds ratios <1 signify that a greater decrease from baseline in CRP or PMS (per unit) is associated with higher odds of response.
Abbreviations: CI, confidence interval; CRP, C-reactive protein; N, number of evaluable patients; PMS, partial Mayo score.
P < .0001.
P < .05.
CRP and PMS were analyzed in OCTAVE Induction 1 and 2 and OCTAVE Open.
CRP week 4, N = 816; PMS week 2, N = 831; PMS week 4, N = 837; CRP week 8, N = 251; PMS week 8, N = 254.
CRP week 4, N = 818; PMS week 2, N = 831; PMS week 4, N = 837; CRP week 8, N = 252; PMS week 8, N = 254.
In tofacitinib induction nonresponders, numerically greater median reductions from baseline were observed in CRP at week 8 in patients who went on to achieve clinical response (–1.1 mg/L) and endoscopic remission (–1.3 mg/L) at week 16, compared with those who did not (–0.6 mg/L and –0.9 mg/L, respectively; Figure 1C). Median change from baseline in CRP at week 8 was −0.6 mg/L and −0.9 mg/L among patients who went on to achieve clinical remission and endoscopic improvement at week 16, respectively, compared with those who did not (−0.9 mg/L and −0.9 mg/L, respectively; Figure 1C).
Among tofacitinib induction nonresponders, there was insufficient evidence from the univariate logistic regression analysis to conclude that there was an association between change from baseline in CRP at week 8 and achieving efficacy outcomes at week 16 of tofacitinib induction (Table 1).
PMS
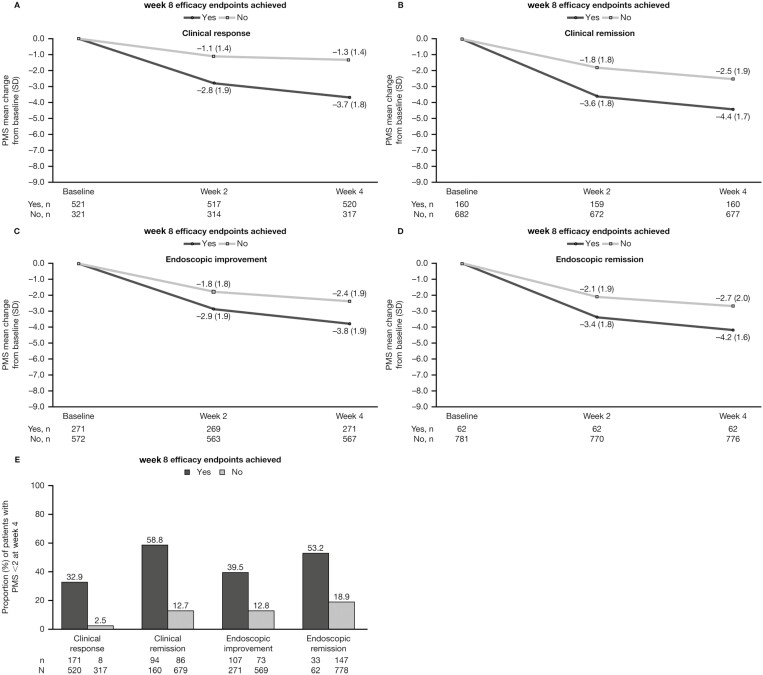
In OCTAVE Induction 1 and 2, numerically greater mean reductions from baseline were observed in PMS at week 2 in patients who achieved clinical response (−2.8), clinical remission (−3.6), endoscopic improvement (−2.9), and endoscopic remission (−3.4) at week 8, compared with those who did not (−1.1, −1.8, −1.8, and −2.1, respectively; Figures 2A-D). This pattern was also observed for PMS at week 4. Analysis of PMS in the phase 2 study yielded similar trends (Supplementary Figure 3). A numerically higher proportion of patients who achieved efficacy outcomes had PMS <2 at week 4, compared with patients who did not (Figure 2E).
Figure 2.
PMS in patients receiving tofacitinib 10 mg BID in OCTAVE Induction 1 and 2, stratified by whether patients achieved clinical and endoscopic outcomes at OCTAVE Induction week 8 (full analysis set, observed case analysis). A-D, Change from baseline in PMS at weeks 2 and 4. E, Proportion of patients with PMS <2 at week 4. Abbreviations: BID, twice daily; N, number of patients with nonmissing data in each category; n, number of patients in the analysis population; PMS, partial Mayo score; SD, standard deviation.
Univariate logistic regression analysis demonstrated that change from baseline in PMS at weeks 2 and 4 was significantly associated with achieving efficacy outcomes at week 8 in OCTAVE Induction 1 and 2 (Table 1). Results for the association between PMS and induction efficacy from the phase 2 study are shown in Supplementary Table 4.
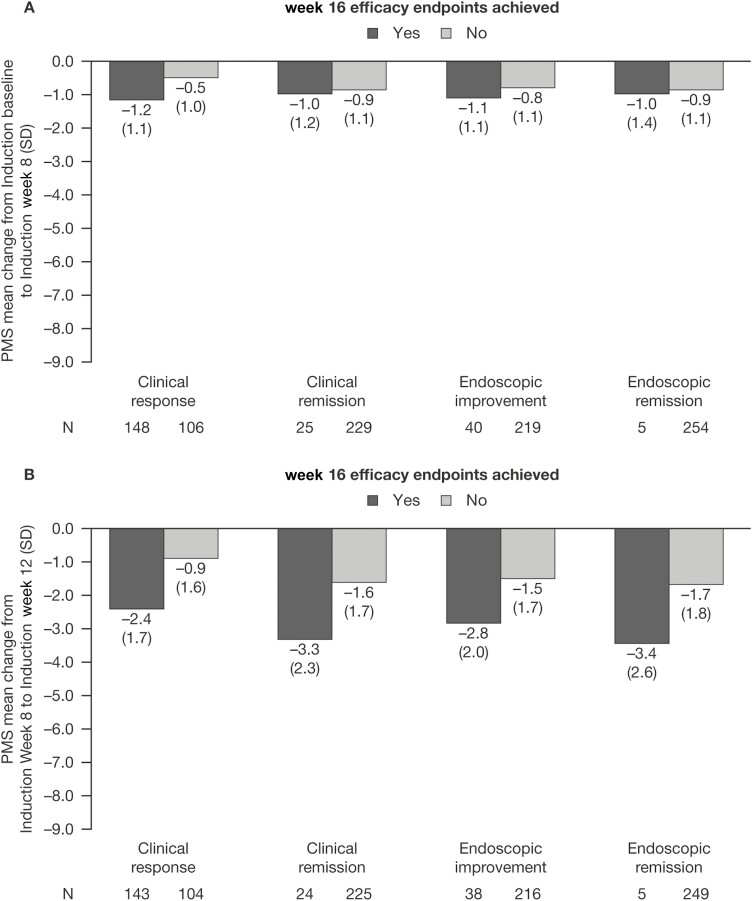
In tofacitinib induction nonresponders who went on to achieve clinical response at week 16 of tofacitinib induction, a numerically greater decrease from baseline in PMS was observed at week 8, compared with those who did not (−1.2 vs −0.5; Figure 3A). Change in PMS from baseline to week 8 was similar in tofacitinib induction nonresponders with and without clinical remission, endoscopic improvement, or endoscopic remission at week 16 (Figure 3A).
Figure 3.
PMS in tofacitinib induction nonresponders receiving tofacitinib 10 mg BID, stratified by whether patients achieved clinical and endoscopic outcomes at OCTAVE Induction week 16 (full analysis set, observed case analysis). A, Change in PMS from OCTAVE Induction baseline to week 8. B, Change in PMS from OCTAVE Induction week 8 to week 12. Abbreviations: BID, twice daily; N, number of patients in the analysis population; PMS, partial Mayo score; SD, standard deviation.
Numerically greater reductions in PMS from week 8 to week 12 of tofacitinib induction were observed in patients who went on to achieve clinical response (−2.4), clinical remission (−3.3), endoscopic improvement (−2.8), and endoscopic remission (−3.4) at week 16, compared with those who did not (−0.9, −1.6, −1.5, and −1.7, respectively; Figure 3B).
Among tofacitinib induction nonresponders, change from baseline in PMS at week 8 of OCTAVE Induction 1 and 2 was significantly associated with achieving clinical response at week 16 of tofacitinib induction in the univariate logistic regression analysis (Table 1). There was insufficient evidence to conclude that there was an association between change from baseline in PMS at week 8 and achieving clinical remission, endoscopic improvement, or endoscopic remission at week 16 of tofacitinib induction (Table 1).
Multivariable Modeling: Association Between Early Markers of Disease Activity and Clinical and Endoscopic Outcomes
In multivariable modeling, CRP and PMS at week 4 of OCTAVE Induction 1 and 2 were significantly associated with achieving clinical response, clinical remission, and endoscopic improvement at week 8, even when accounting for other covariates (Table 2). Partial Mayo score but not CRP, was significantly associated with achieving endoscopic remission. Not having prior TNFi failure was significantly associated with achieving efficacy outcomes. Age, baseline steroid use, and race were also each significantly associated with one of the efficacy outcomes (Table 2).
Table 2.
Multivariable logistic regression analyses for factors associated with clinical and endoscopic outcomes at week 8 in OCTAVE induction 1 and 2 in patients receiving tofacitinib 10 mg BID (full analysis set, observed case analysis).
| Odds Ratio (95% CI) | CRP (mg/L) Week 4 | PMS Week 4 | Prior TNFi Failure(No vs Yes) |
|---|---|---|---|
| Clinical responsea | 0.86 (0.77, 0.97) P = 0.0147 | 0.51 (0.46, 0.57) P < .0001 | 1.56 (1.09, 2.24) P = 0.0155 |
| Clinical remission | 0.78 (0.67, 0.91) P = 0.0020 | 0.46 (0.40, 0.54) P < .0001 | 2.07 (1.35, 3.16) P = 0.0008 |
| Endoscopic improvementb | 0.66 (0.58, 0.75) P < .0001 | 0.66 (0.60, 0.73) P < .0001 | 1.82 (1.29, 2.57) P = 0.0007 |
| Endoscopic remission | NS | 0.62 (0.53, 0.73) P < .0001 | 2.63 (1.44, 4.79) P = 0.0016 |
Log-transformed data were used for CRP analysis. Odds ratios <1 signify that a lower CRP or PMS is associated with greater odds of a response. Other covariates used in the models were: age (continuous), body mass index, disease duration, extent of disease, gender, geographic region, oral corticosteroid use at baseline, prior immunosuppressant use, prior TNFi failure, race, and smoking status. The final models included all selected covariates after the stepwise selection procedure at the 0.05 level of significance to enter and remain in the model; additional selected variables for each model were.
Abbreviations: CI, confidence interval; CRP, C-reactive protein; NS, not selected; PMS, partial Mayo score; TNFi, tumor necrosis factor inhibitor.
Age (years, OR, 1.01; 95% CI, 1.00-1.03), baseline steroid use (no vs yes, OR, 0.62; 95% CI, 0.43-0.89).
Race (Black vs White, OR, 0.91; 95% CI, 0.08-10.65; Asian vs White, OR, 0.40; 95% CI, 0.24-0.68; Other vs White, OR, 1.21; 95% CI, 0.48-3.02).
Performance Assessment of Early Predictors of Disease Activity
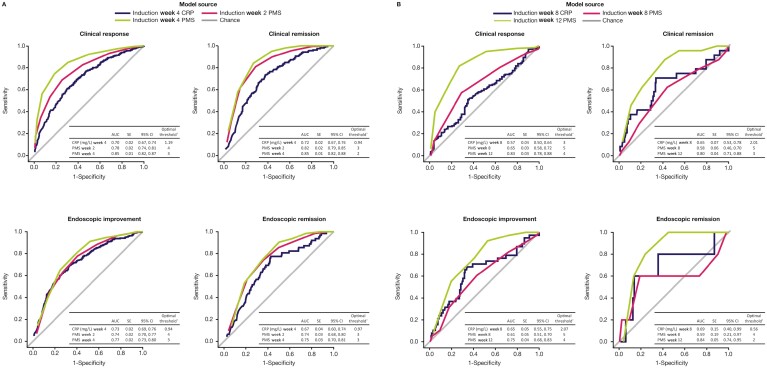
The predictive values of noninvasive early markers of disease activity for achieving clinical and endoscopic outcomes were investigated using ROC curve analysis (Figure 4).
Figure 4.
ROC curves for observed CRP and PMS as predictors of clinical and endoscopic outcomes in patients receiving tofacitinib 10 mg BID: A, at induction week 8; B, at induction week 16 (full analysis set, observed case analysis). AUC values between 0.9 and 1.0 = outstanding predictive value; between 0.8 and 0.9 = excellent predictive value; between 0.7 and 0.8 = acceptable predictive value; >0.5–0.7 = poor predictive value; ≤0.5 = no discrimination.17aThe optimal threshold is based on the maximum value of the Youden Index (sensitivity + specificity -1), calculated for all possible threshold values. Abbreviations: AUC, area under the curve; CI, confidence interval; CRP, C-reactive protein; PMS, partial Mayo score; ROC, receiver operating characteristic; SE, standard error.
In OCTAVE Induction 1 and 2, observed CRP at week 4 had an AUC of 0.70 for clinical response, 0.72 for clinical remission, 0.73 for endoscopic improvement, and 0.67 for endoscopic remission at week 8. Observed PMS at week 2 had an AUC of 0.78 for clinical response, 0.82 for clinical remission, 0.74 for endoscopic improvement, and 0.74 for endoscopic remission at week 8. Observed PMS at week 4 had an AUC of 0.85 for clinical response, 0.85 for clinical remission, 0.77 for endoscopic improvement, and 0.75 for endoscopic remission at week 8 (Figure 4A).
The sensitivities, specificities, and positive and negative predictive values for CRP and PMS in predicting clinical and endoscopic outcomes can be seen in Table 3. In OCTAVE Induction 1 and 2, the sensitivity and specificity of CRP at week 4 for clinical response at week 8 were 60.6% and 70.4%, respectively. The positive predictive value of CRP at week 4 for clinical response at week 8 was 77.0%, and the negative predictive value was 52.1%. The sensitivity of PMS at week 4 for clinical response at week 8 was 74.2%, and the specificity was 81.1%, with a positive predictive value of 86.6% and a negative predictive value of 65.7%.
Table 3.
ROC analysis for ability of CRP and PMS to predict clinical and endoscopic outcomes at weeks 8 and 16 of tofacitinib induction in patients receiving tofacitinib 10 mg BID (full analysis set, observed case analysis).
| Parameter (Observed) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Clinical response at week 8 | ||||
| CRP (mg/L) week 4 | 60.6 | 70.4 | 77.0 | 52.1 |
| PMS week 2 | 69.1 | 73.9 | 81.3 | 59.2 |
| PMS week 4 | 74.2 | 81.1 | 86.6 | 65.7 |
| Clinical remission at week 8 | ||||
| CRP (mg/L) week 4 | 73.4 | 62.4 | 31.4 | 90.9 |
| PMS week 2 | 81.1 | 70.8 | 39.6 | 94.1 |
| PMS week 4 | 84.4 | 72.8 | 42.2 | 95.2 |
| Endoscopic improvement at week 8 | ||||
| CRP (mg/L) week 4 | 69.0 | 67.2 | 50.0 | 82.0 |
| PMS week 2 | 78.1 | 59.1 | 47.6 | 85.0 |
| PMS week 4 | 81.2 | 59.9 | 49.1 | 87.0 |
| Endoscopic remission at week 8 | ||||
| CRP (mg/L) week 4 | 77.4 | 57.3 | 12.7 | 96.9 |
| PMS week 2 | 74.2 | 63.6 | 14.1 | 96.8 |
| PMS week 4 | 90.3 | 49.6 | 12.5 | 98.5 |
| Clinical response at week 16 | ||||
| CRP (mg/L) week 8 | 52.0 | 63.8 | 67.0 | 48.6 |
| PMS week 8 | 56.1 | 71.7 | 73.5 | 53.9 |
| PMS week 12 | 81.8 | 72.1 | 80.1 | 74.3 |
| Clinical remission at week 16 | ||||
| CRP (mg/L) week 8 | 72.0 | 66.4 | 19.0 | 95.6 |
| PMS week 8 | 60.0 | 57.1 | 13.2 | 93.0 |
| PMS week 12 | 87.5 | 57.8 | 18.1 | 97.7 |
| Endoscopic improvement at week 16 | ||||
| CRP (mg/L) week 8 | 70.0 | 66.2 | 27.5 | 92.4 |
| PMS week 8 | 60.0 | 58.4 | 20.7 | 89.0 |
| PMS week 12 | 92.1 | 46.8 | 23.3 | 97.1 |
| Endoscopic remission at week 16 | ||||
| CRP (mg/L) week 8 | 60.0 | 86.6 | 8.1 | 99.1 |
| PMS week 8 | 60.0 | 81.3 | 5.9 | 99.1 |
| PMS week 12 | 80.0 | 76.3 | 6.4 | 99.5 |
Abbreviations: CRP, C-reactive protein; NPV, negative predictive value; PMS, partial Mayo score; PPV, positive predictive value; ROC, receiver operating characteristic.
Among tofacitinib induction nonresponders who continued to receive tofacitinib 10 mg BID in the OLE study, observed PMS at week 12 of tofacitinib induction had an AUC of 0.83 for clinical response, 0.80 for clinical remission, 0.75 for endoscopic improvement, and 0.84 for endoscopic remission at week 16 (Figure 4B). The sensitivity of PMS at week 12 of tofacitinib induction for clinical response at week 16 was 81.8%, and the specificity was 72.1%, with a positive predictive value of 80.1% and a negative predictive value of 74.3% (Table 3).
Observed CRP at week 8 had an AUC of 0.57 for clinical response, 0.65 for clinical remission, 0.65 for endoscopic improvement, and 0.69 for endoscopic remission at week 16 (Figure 4B). Prior TNFi failure had an AUC >0.5–0.7 for all efficacy outcomes at both week 8 and week 16 (Supplementary Table 5 and Supplementary Figure 4).
Discussion
Although endoscopy remains key to assessing the extent of UC and the efficacy of UC therapies, it is an invasive procedure.1 Analysis of early markers of disease activity is considered to be a helpful tool in the overall management of inflammatory bowel disease, as it can provide a noninvasive indication of disease activity and may provide early insight into the efficacy of UC therapies in the absence of endoscopy.18
This report presents post hoc analyses assessing the associations between CRP and PMS, and achievement of efficacy outcomes in patients with moderately to severely active UC receiving tofacitinib induction therapy. Early decreases in PMS and CRP were associated with achieving efficacy outcomes during 8 weeks of tofacitinib 10 mg BID induction therapy. Among tofacitinib induction nonresponders, a decrease in PMS at week 8 was correlated with going on to achieve clinical response to tofacitinib induction therapy at week 16.
In the OCTAVE Induction 1 and 2 studies, univariate logistic regression analyses demonstrated that decreases as early as week 4 in CRP and week 2 in PMS were associated with achieving clinical response, clinical remission, endoscopic improvement, and endoscopic remission. The association between CRP and PMS at week 4 and efficacy outcomes at week 8 persisted even when accounting for additional covariates. Furthermore, ROC analysis suggested that CRP at week 4 and PMS at weeks 2 and 4 had generally acceptable to excellent predictive value for achieving efficacy outcomes at week 8. Partial Mayo score at weeks 2 and 4 had excellent predictive value (AUC, 0.82 and 0.85, respectively) for achieving clinical remission at week 8, and PMS at week 4 had excellent predictive value (AUC, 0.85) for achieving clinical response at week 8. It should be noted that CRP at week 4 had poor predictive value (AUC, 0.67) only for achieving endoscopic remission at week 8. C-reactive protein has previously been suggested to be a more useful biomarker in Crohn’s disease than in UC,18 which may explain the low, but still generally acceptable, predictive value of early changes in this biomarker. In evaluating the associations of CRP and PMS with other efficacy outcomes that use the endoscopic subscore, these results expand on previous findings that early improvement in stool frequency and rectal bleeding in response to tofacitinib induction therapy were associated with achieving clinical response at week 8.19
Among tofacitinib induction nonresponders, a decrease in PMS at week 8 was associated with achieving clinical response to tofacitinib induction therapy at week 16, whereas a numerically greater reduction in PMS from week 8 to week 12 of tofacitinib induction was observed in patients who went on to achieve efficacy outcomes at week 16 (ie, with extended induction), compared with those who did not go on to achieve these outcomes. Partial Mayo score may be helpful in determining which patients might benefit from extended tofacitinib induction therapy.
A numerically higher proportion of patients treated with tofacitinib 10 mg BID who achieved efficacy outcomes after 8 weeks of induction had CRP <3 mg/L and PMS <2 at week 4, compared with patients who did not. However, of the patients who achieved clinical response after 8 weeks of induction, 23.1% and 67.1% did not have CRP <3 mg/L or PMS <2 at week 4, respectively.
This suggests that while CRP and/or PMS below these thresholds may be associated with achieving response, higher values during the induction period should not be considered an indicator of early discontinuation or treatment inefficacy.
A numerically higher proportion of tofacitinib complete nonresponders had CRP >3 mg/L at baseline in OCTAVE Induction 1 and 2 vs delayed responders. This is consistent with results from a recently published study in 134 patients with UC outside of the clinical trial setting, which showed that a higher CRP level at the start of the study was independently associated with primary nonresponse to tofacitinib.20
Fecal calprotectin is a useful biomarker of intestinal inflammation and response to therapy;12 however, a limitation of this study is that it was only measured in the phase 2 study and not in phase 3 OCTAVE Induction studies. In a previous analysis of the phase 2 study, FCP at OCTAVE induction end (week 8) was shown to be associated with efficacy outcomes in patients with UC, with an FCP cutoff of 150 mg/kg achieving the highest sensitivity and specificity for clinical remission and endoscopic remission of the various FCP concentrations analyzed (50-300 mg/kg).21 A post hoc analysis of the phase 2 study showed some evidence that reductions in FCP as early as week 2 may be observed in tofacitinib-treated patients with improved efficacy outcomes at week 8; however, drawing absolute conclusions is not possible because of low patient numbers.
An additional limitation is that these are post hoc analyses; therefore, the studies were not planned to determine whether CRP or PMS were associated with achieving clinical or endoscopic outcomes. The small sample sizes of patients with clinical remission, endoscopic improvement, and endoscopic remission at week 16 of tofacitinib induction in the OLE study is also a limitation, and may explain why the association of CRP and PMS with clinical and endoscopic outcomes was greater at week 8 than at week 16.
Biomarkers as predictors of response to biologic UC therapies have also been evaluated. In a prospective study evaluating the efficacy of infliximab induction therapy in patients with UC, FCP reduction at week 2 was predictive of endoscopic remission at week 10.22 Furthermore, a review looking at predictors of response to TNFi treatment in UC found that low levels of FCP and CRP were indicators of better response to TNFi treatment.23 Despite the somewhat subjective nature of PMS when comparing total Mayo score (incorporating endoscopic subscore) and noninvasive PMS using data from a placebo-controlled clinical trial, PMS was comparable with total Mayo score in identifying patient- and physician-perceived clinical response.13
Conclusion
In summary, these analyses suggest that noninvasive disease activity markers, including CRP and PMS, were associated with achieving clinical and endoscopic outcomes in patients receiving tofacitinib 10 mg BID induction therapy in the UC clinical program. Furthermore, for patients who did not achieve clinical response after the first 8 weeks of tofacitinib induction therapy, PMS was associated with achieving clinical response after an additional 8 weeks of tofacitinib induction therapy. Our findings, together with other evidence, suggest that these early markers of disease activity are associated with clinical and endoscopic outcomes during tofacitinib induction, and may be useful as a tool to assist clinicians in the monitoring and treatment of patients with UC.
Supplementary Material
Acknowledgments
The authors would like to thank the patients, investigators, and study teams involved in the tofacitinib UC clinical program. Rajiv Mundayat at Pfizer Inc. assisted with statistical analysis. Leonardo Salese and Daniel Quirk at Pfizer Inc contributed to the study design and data interpretation, and reviewed the article. Medical writing support, under the guidance of the authors, was provided by Sarah Mancini, PhD, CMC Connect, McCann Health Medical Communications and was funded by Pfizer Inc. (New York, NY, USA) in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–4. Jerome Paulissen is an employee of Syneos Health, which was a paid contractor to Pfizer in connection with the development of this manuscript and related statistical analysis.
Contributor Information
Marla C Dubinsky, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Fernando Magro, University of Porto and Centro Hospitalar São João, Porto, Portugal.
Flavio Steinwurz, Unit of Inflammatory Bowel Disease, Hospital Israelita Albert Einstein, São Paulo, Brazil.
David P Hudesman, New York University, New York, NY, USA.
Jami A Kinnucan, Mayo Clinic Division of Gastroenterology and Hepatology, Jacksonville, FL, USA.
Ryan C Ungaro, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Markus F Neurath, Department of Medicine, University of Erlangen-Nürnberg, University Hospital, Erlangen, Germany.
Nicole Kulisek, Pfizer Inc, Collegeville, PA, USA.
Jerome Paulissen, Pfizer Inc, New York, NY, USA.
Chinyu Su, Pfizer Inc, Collegeville, PA, USA.
Dario Ponce de Leon, Pfizer Inc, Lima, Peru.
Miguel Regueiro, Department of Gastroenterology, Hepatology and Nutrition, Cleveland Clinic, Cleveland, OH, USA.
Author Contributions
N.K., C.S., and D.P.L. planned the studies. M.C.D., F.M., F.S., D.P.H., J.A.K., R.C.U., M.F.N., and M.R. conducted the studies. M.C.D., F.M., F.S., D.P.H., J.A.K., R.C.U., M.F.N., N.K., J.P., C.S., D.P.L., and M.R. collected or interpreted data. All authors drafted and edited the article, and approved the final version of the article including the authorship list.
Funding
These studies were sponsored by Pfizer. Medical writing support was funded by Pfizer Inc.
Conflicts of Interest
M.C.D. has received consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Gilead, Janssen, Pfizer Inc, Takeda, and UCB. F.M. has received research support from GEDII and National Science Foundation; and has received personal fees from AbbVie, Amgen, Biogen, Celgene, Celltrion, Dr. Falk Pharma, Ferring Pharmaceuticals, Hospira, Janssen, Laboratórios Vitória, MSD, Pfizer Inc, Sandoz, Takeda, UCB, and Vifor. F.S. has served as an advisory board member for Pfizer Inc.; and has received consulting and speaker fees from AbbVie, Eurofarma, Ferring Pharmaceuticals, Janssen, Sandoz, Takeda, and UCB. D.P.H. has received research support from Pfizer Inc. and has received personal fees from AbbVie, Bristol-Myers Squibb, Janssen, Pfizer Inc, Samsung, and Takeda. J.A.K. has received advisory board fees from Pfizer Inc. R.C.U. is supported by an NIH K23 Career Development Award (K23KD111995-01A1); has served as an advisory board member or consultant for AbbVie, Bristol-Myers Squibb, Janssen, Pfizer Inc, and Takeda; and has received research support from AbbVie, Boehringer Ingelheim, Eli Lilly, and Pfizer Inc. M.F.N. has received consulting fees from Bionorica SE, Boehringer Ingelheim Pharma GmbH & Co. KG, e.Bavarian Health GmbH, F. Hoffmann-La Roche Ltd, Genentech, Hexal AG, InDex Pharmaceuticals AB, Janssen-Cilag GmbH, MSD Sharp & Dohme GmbH, PENTAX Europe GmbH, PPM Services S.A., Takeda Pharma Vertrieb GmbH & Co. KG, and Tillotts Pharma AG; and has received speaker fees from AbbVie Deutschland GmbH & Co. KG, Falk Foundation, Janssen-Cilag GmbH, and PENTAX Europe GmbH. N.K., C.S., and D.P.L. are employees and stockholders of Pfizer Inc. J.P. is an employee of Syneos Health, which was a paid contractor to Pfizer in connection with the development of this article and related statistical analysis. M.R. has served as an advisory board member for, or has received consulting fees from, AbbVie, Amgen, Celgene, Janssen, Miraca Labs, Pfizer Inc, Seres, Takeda, and UCB; has received research support from AbbVie, Janssen, and Takeda; and has received unrestricted educational grants from AbbVie, Janssen, Pfizer Inc., Salix, Shire, Takeda, and UCB.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019(3);114:384–413. [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367(7):616–624. [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–1736. [DOI] [PubMed] [Google Scholar]
- 5. Lichtenstein GR, Loftus EV Jr, Wei SC, et al. DOP61 Tofacitinib, an oral, small-molecule Janus kinase inhibitor, in the treatment of ulcerative colitis: analysis of an open-label, long-term extension study with up to 5.9 years of treatment (abstract). J Crohns Colitis. 2020;14(Suppl 1):S100–1 (DOP61). [Google Scholar]
- 6. Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57(11):1518–1523. [DOI] [PubMed] [Google Scholar]
- 7. Rosenberg L, Lawlor GO, Zenlea T, et al. Predictors of endoscopic inflammation in patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis. 2013;19(4):779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karoui S, Laz S, Serghini M, et al. Correlation of C-reactive protein with clinical and endoscopic activity in patients with ulcerative colitis. Dig Dis Sci. 2011;56(6):1801–1805. [DOI] [PubMed] [Google Scholar]
- 9. Schoepfer AM, Beglinger C, Straumann A, et al. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15(12):1851–1858. [DOI] [PubMed] [Google Scholar]
- 10. Shin SY, Park SJ, Kim Y, et al. Clinical outcomes and predictors of response for adalimumab in patients with moderately to severely active ulcerative colitis: a KASID prospective multicenter cohort study. Intest Res. 2021:Epub ahead of print. https://www.irjournal.org/journal/view.php?doi=10.5217/ir.2021.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5): 1570–1583. [DOI] [PubMed] [Google Scholar]
- 12. Walsham NE, Sherwood RA.. Fecal calprotectin in inflammatory bowel disease. Clin Exp Gastroenterol. 2016;9:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14(12):1660–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feagan BG, Rubin DT, Danese S, et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2017;15(2):229–239.e5. [DOI] [PubMed] [Google Scholar]
- 15. Sands BE, Peyrin-Biroulet L, Marano C, et al. Efficacy in biologic-failure and nonbiologic-failure populations in a phase 3 study of ustekinumab in moderate-severe ulcerative colitis: Unifi (abstract). Gastroenterology. 2019;156(Suppl 1):S181–2 (833a). [Google Scholar]
- 16. Pfizer Inc. Xeljanz® (tofacitinib): highlights of prescribing information. Accessed May 14, 2021.http://labeling.pfizer.com/ShowLabeling.aspx?id=959
- 17. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–1316. [DOI] [PubMed] [Google Scholar]
- 18. Vermeire S, Van Assche G, Rutgeerts P.. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55(3):426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanauer S, Panaccione R, Danese S, et al. Tofacitinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2019;17(1):139–147. [DOI] [PubMed] [Google Scholar]
- 20. Honap S, Chee D, Chapman TP, et al. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multi-centre UK experience. J Crohns Colitis. 2020;14(10):1385–1393. [DOI] [PubMed] [Google Scholar]
- 21. Sandborn WJ, Panés J, Zhang H, et al. Correlation between concentrations of fecal calprotectin and outcomes of patients with ulcerative colitis in a phase 2 trial. Gastroenterology. 2016;150(1): 96–102. [DOI] [PubMed] [Google Scholar]
- 22. De Vos M, Dewit O, D’Haens G, et al. Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naïve patients with ulcerative colitis. J Crohns Colitis. 2012;6(5):557–562. [DOI] [PubMed] [Google Scholar]
- 23. Zampeli E, Gizis M, Siakavellas SI, et al. Predictors of response to anti-tumor necrosis factor therapy in ulcerative colitis. World J Gastrointest Pathophysiol. 2014;5(3):293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.