Abstract
Background
In patients with ulcerative colitis (UC), risks of infection and malignancies increase with age. Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of UC. This analysis assessed age as a risk factor for adverse events of special interest (AESI) in the tofacitinib UC clinical program.
Methods
Data were from phase 2 and 3 induction studies, a phase 3 maintenance study, and an open-label, long-term extension study. Efficacy and/or safety outcomes were analyzed in the Induction, Maintenance, and Overall Cohorts (patients who received ≥ 1 dose of tofacitinib), stratified by age. The effects of baseline demographic and disease-related factors on AESI incidence were assessed by Cox proportional-hazards regression analysis.
Results
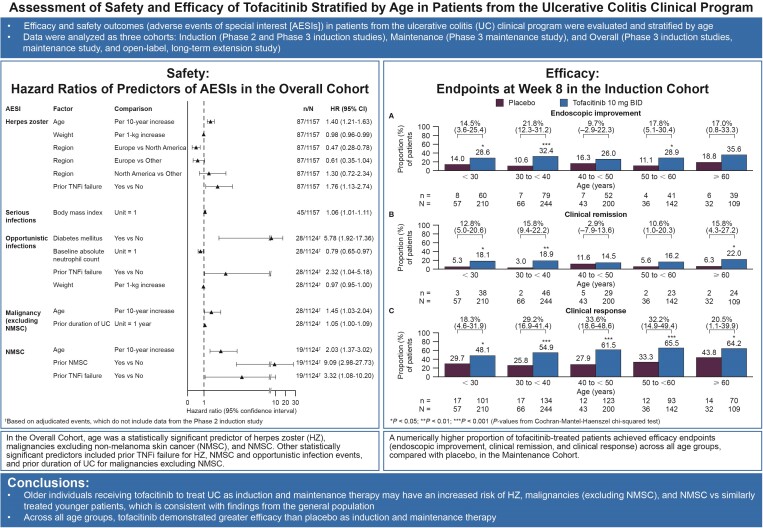
In the Overall Cohort (1157 patients with ≤ 6.8 years’ tofacitinib treatment), age was a statistically significant predictor of herpes zoster (HZ), malignancies excluding nonmelanoma skin cancer (NMSC), and NMSC. Other statistically significant predictors included prior tumor necrosis factor inhibitor failure for HZ, NMSC, and opportunistic infection events, and prior duration of UC for malignancies excluding NMSC. In the Induction and Maintenance Cohorts, a higher proportion of tofacitinib-treated than placebo-treated patients (numerical difference) achieved the efficacy endpoints (endoscopic improvement, clinical remission, clinical response) across all age groups.
Conclusions
Older individuals receiving tofacitinib as induction and maintenance therapy to treat UC may have an increased risk of HZ, malignancies (excluding NMSC), and NMSC versus similarly treated younger patients, consistent with findings from the general population. Across all age groups, tofacitinib demonstrated greater efficacy than placebo as an induction and maintenance therapy.
ClinicalTrials.gov Registration Numbers
NCT00787202; NCT01465763; NCT01458951; NCT01458574; NCT01470612.
Keywords: age, clinical response, safety, tofacitinib, ulcerative colitis
Graphical Abstract
Graphical Abstract.
Ulcerative colitis (UC) is a long-term disease that causes inflammation of the colonic tissue that affects individuals at any age, although onset is typically observed between the ages of 15 and 30.1,2 Previous studies in patients with inflammatory bowel disease (IBD), and in the general population, have suggested that an increased risk of serious infections, opportunistic infections, herpes zoster, and malignancies (including nonmelanoma skin cancer [NMSC]) may be linked with older age.3–11 Some commonly used treatments for IBD have also been correlated with a heightened risk of infections. For example, corticosteroid use has been correlated with a higher infection risk in patients with elderly-onset IBD,12,13 although rates seen in older patients were similar to those in younger patients, regardless of corticosteroid use.12 Corticosteroids have also been shown to inflate the risk of malignancy in older patients with IBD.6 Among patients with IBD treated with tumor necrosis factor inhibitors (TNFi), those aged > 65 years were found to have an increased rate of severe infections compared with younger patients or patients of a similar age who did not receive TNFi.14 The serious infection risk in patients with IBD has also been shown to be greater for patients treated with TNFi monotherapy, compared to thiopurine monotherapy, and the risk of serious infections or opportunistic infections was raised in those treated with TNFi and thiopurine combination therapy, compared to TNFi monotherapy; in both cases, the incidence of infections was greater in patients aged ≥ 65 years than in younger patients.15 Corticosteroid and TNFi use has also been shown to increase the risk of herpes zoster in IBD patients with UC.16
Tofacitinib is an oral small molecule Janus kinase inhibitor for the treatment of UC. The efficacy and safety of tofacitinib at 10 mg twice daily (BID) in patients with moderately to severely active UC has been demonstrated as induction therapy in 1 phase 2 and 2 phase 3 trials of 8 weeks’ duration.17,18 Tofacitinib at both 5 and 10 mg BID were investigated further in a phase 3 maintenance trial of 52 weeks’ duration18 and an additional open-label, long-term extension (OLE) study.19
A previous analysis of data from the tofacitinib UC clinical program showed higher rates of adverse events (AEs), including AEs of special interest (AESI; opportunistic infections, herpes zoster, malignancy, and major adverse cardiovascular events [MACE]), in patients aged > 65 years with UC, compared with younger patients, regardless of whether they received treatment with tofacitinib or placebo.20
The current analysis of data from patients enrolled in the tofacitinib UC clinical program further examines the effects of age on safety outcomes in patients with UC, up to a maximum of 6.8 years of treatment, as well as efficacy from phase 3 induction and maintenance pivotal studies.
Materials and Methods
Study Design and Patients
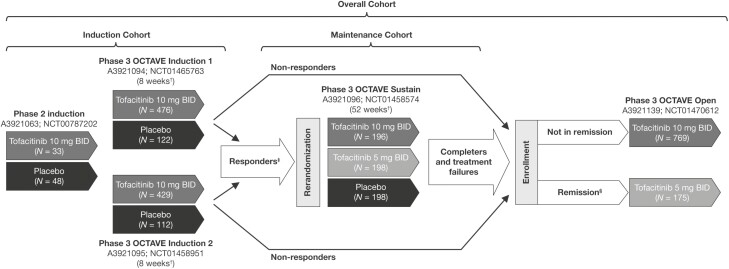
Data from an 8-week, phase 2 induction study (NCT00787202)17; 3 phase 3, randomized, placebo-controlled studies (Oral Clinical Trials for tofAcitinib in ulceratiVE colitis [OCTAVE] Induction 1 [NCT01465763]; OCTAVE Induction 2 [NCT01458951]; and OCTAVE Sustain, [NCT01458574])18; and an OLE study (OCTAVE Open [NCT01470612])21 in patients with moderately to severely active UC were included.
The study designs and patient inclusion or exclusion criteria for all studies in the tofacitinib UC clinical program have been described previously.17,18,21 Briefly, patients aged ≥ 18 years with moderately to severely active UC (defined as a total Mayo score of 6–12, with a rectal bleeding subscore of 1–3 and an endoscopic subscore of 2 or 3) for ≥ 3 months (phase 2) or ≥ 4 months (phase 3; OCTAVE Induction 1 and 2), were eligible for inclusion.17,18 Patients who achieved clinical response in the 8-week OCTAVE Induction 1 and 2 induction studies were eligible for inclusion in the 52-week OCTAVE Sustain maintenance study. Patients who were nonresponders in OCTAVE Induction 1 and 2, and those who completed or were treatment failures in OCTAVE Sustain, were eligible for inclusion in the OLE study (OCTAVE Open; data as of May 2019; database not locked; Figure 1).21
Figure 1.
Overview of the tofacitinib UC clinical program, showing phase 2, phase 3, and OLE studies, and the transfer of patients from induction studies to maintenance and/or OLE studies. †Final complete efficacy assessment at week 8/52. Treatment continued up to week 9/53. ‡Clinical response in OCTAVE Induction 1 and 2 was defined as a decrease from the induction study baseline total Mayo score ≥ 3 points and ≥ 30%, plus a decrease in rectal bleeding subscore ≥ 1 point or an absolute rectal bleeding subscore of 0 or 1. §Remission was defined as a total Mayo score ≤ 2 with no individual subscore > 1, and a rectal bleeding subscore of 0. Adapted from Winthrop et al.16 (in accordance with the Creative Commons Attribution-NonCommercial License [CC BY-NC] license). Abbreviations: BID, twice daily; N, total number of patients in each treatment group; OLE, open-label, long-term extension; UC, ulcerative colitis.
Full details of permitted and prohibited concomitant medications, and corticosteroid tapering, in the phase 2 and phase 3 studies are provided in the Supplementary Appendix. In brief, patients were allowed concomitant treatment with oral 5-aminosalicylates or sulfasalazine (provided the dose did not change in the induction and maintenance studies; dose modifications were permitted during OCTAVE Open). Oral corticosteroids were permitted in the phase 2 induction study (up to 30 mg/day prednisolone or equivalent; decrease of the corticosteroid dose due to tapering was permitted).
Patients were permitted concomitant treatment with oral corticosteroids in OCTAVE Induction 1 or 2 (up to 25 mg/day prednisone or equivalent; stable dose for ≥ 2 weeks prior to baseline); corticosteroid tapering was compulsory from baseline of OCTAVE Sustain and OCTAVE Open.
Patients in the Induction, Maintenance, and Overall Cohorts were included in these analyses of the effects of age on efficacy and safety outcomes. The Induction Cohort included patients who received placebo or tofacitinib 10 mg twice daily (BID) for 8 weeks in the phase 2 induction study or the OCTAVE Induction 1 and 2 studies. The Maintenance Cohort included patients who received placebo, tofacitinib 5 mg BID, or tofacitinib 10 mg BID in OCTAVE Sustain. The Overall Cohort included all patients who received ≥ 1 dose of tofacitinib 5 or 10 mg BID in the above-mentioned studies, or OCTAVE Open.
AEs and Adjudication
AESI in the Overall Cohort included serious infections (infections that met serious AE [SAE] reporting criteria), opportunistic infections, all herpes zoster (nonserious and serious), malignancies (excluding NMSC), NMSC, MACE, gastrointestinal (GI) perforations, deep vein thrombosis (DVT), pulmonary embolism (PE), and deaths.
Independent, external, blinded adjudication committees reviewed all potential opportunistic infections (including viral, bacterial, fungal, and parasitic infections), malignancies, cardiovascular events (including MACE), and GI perforation events in the phase 3 studies, in order to identify AESI using prespecified, consistent criteria. Adjudication of AESI in the tofacitinib UC clinical program has been described previously.22 AESI were not adjudicated in the phase 2 induction study.
Herpes zoster (including both nonserious and serious AEs) opportunistic infections included multidermatomal (nonadjacent or > 2 adjacent dermatomes that were not considered disseminated) or disseminated (any diffuse rash [> 6 dermatomes], encephalitis, pneumonia, or other nonskin organ involvement). Biopsies of all potentially malignant tumors were submitted for blinded central read of histopathology. In addition, external, independent adjudication was performed for all potential malignancies. Cardiovascular safety endpoints were based on the standard guidelines.23
Efficacy Evaluations
Efficacy evaluations were based on total Mayo score, as determined at baseline and week 8 in the Induction Cohort and at baseline (week 8 for the pooled Induction Cohorts) and week 52 in the Maintenance Cohort. Results from the phase 2 induction study and the phase 3 OLE study were not included in the efficacy analysis. Efficacy endpoints included endoscopic improvement, clinical remission, and clinical response. Total Mayo scores were calculated from central reads of the endoscopic subscore.
Statistical Analyses
AEs, SAEs, and discontinuations due to AEs were summarized for the Induction, Maintenance, and Overall Cohorts, respectively, by 2 sets of age grouping: (1) 5 age categories: 18 to < 30 years, 30 to < 40 years, 40 to < 50 years, 50 to < 60 years, and ≥ 60 years; and (2) 2 age categories: < 65 years and ≥ 65 years. These age subgroups were chosen, based on the age distribution of the patient population and clinical relevance of the age breakdowns, to investigate the association of age with risk of infection and malignancies in tofacitinib-treated patients. Additionally, the AESI were summarized by age group in the Maintenance and Overall Cohorts. Proportions and incidence rates (IRs; unique patients with events per 100 patient-years of exposure) were calculated for AESI, and confidence intervals (CIs) were obtained using the Exact Poisson method.24 The denominator for calculation of IRs was the exposure (in days) accrued from when the patient first received tofacitinib until they received their final tofacitinib dose (on or before the data cutoff date, or the last dose plus 28 days for patients who discontinued prematurely), or to the date of the first event, whichever occurred earlier. Events occurring within 28 days of study discontinuation were included in the numerator. In the Overall Cohort, all adjudicated events of malignancy (excluding NMSC), NMSC, MACE, and death were included, regardless of whether they occurred within or outside the 28-day period after the last dose.
In order to determine the effects of various demographic and disease-related factors on the occurrence of specific AESI (serious infections, opportunistic infections, all herpes zoster [nonserious and serious], malignancy, and NMSC) in the Overall Cohort, time-to-event analyses were performed using Cox proportional-hazards regression modeling, adjusting for multiple variables simultaneously, with exposure time as defined in the denominator for the IRs. The univariate Cox proportional-hazards regression analysis was performed for each candidate covariate listed below, and only the covariate with a P value < .10 in the univariate regression model was used as a candidate for the Cox proportional-hazards multiple regression model. Candidate covariates included age (continuous), sex, race, geographic region (European Union, North America, or other), weight, body mass index, history of diabetes mellitus at baseline, duration of UC, prior TNFi treatment, prior TNFi failure (defined as an “inadequate response” to TNFi medication started before day 1 of OCTAVE Induction), prior immunosuppressant treatment, predominant dose of tofacitinib (tofacitinib 5 or 10 mg BID based on an average total daily dose < 15 or ≥ 15 mg, respectively), UC severity, prior corticosteroid use, oral corticosteroid use at baseline, baseline corticosteroid dose group, baseline high-density lipoprotein (< 40 mg/dL), prior myocardial infarction, smoking status at baseline, prior NMSC, baseline absolute lymphocyte count, and baseline absolute neutrophil count. If multiple continuous covariates were highly correlated, only 1 was retained in the model in order to avoid problems with collinearity. Within the Cox proportional-hazards regression analysis, a stepwise selection process was implemented, with an entry-criterion P value of .15 and a stay-criterion P value of .05.
Ethical Considerations
This was a post hoc analysis of previously reported trials.17,18,21 All studies were conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines. Study protocols were approved by the Institutional Review Boards and/or independent ethics committees at each of the investigational centers participating in the studies, or a central Institutional Review Board. All the patients provided written informed consent.
Results
Patients
The Induction Cohort included 1220 patients (placebo, n = 282; tofacitinib 10 mg BID, n = 938) and the Maintenance Cohort included 592 patients (placebo, n = 198; tofacitinib 5 mg BID, n = 198; tofacitinib 10 mg BID, n = 196). The Overall Cohort comprised 1157 patients who received ≥ 1 dose of tofacitinib 5 or 10 mg BID during the phase 2, phase 3, and OLE studies, with 2581 patient-years of tofacitinib exposure and up to a maximum of 6.8 years of treatment. Of these patients, 82.9% received a predominant dose of tofacitinib 10 mg BID (average total daily dose ≥ 15 mg). Most patients in the Induction, Maintenance, and Overall Cohorts were aged < 65 years (93.2%, 91.9%, and 93.3%, respectively), with a smaller proportion aged ≥ 65 years (6.8%, 8.1%, and 6.7%, respectively; Table 1).
Table 1.
Demographics, baseline, and clinical characteristics of the Induction, Maintenance, and Overall Cohorts.
| Characteristic | Induction Cohorta | Maintenance Cohortb | Overall Cohortc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 65 years | ≥ 65 years | < 65 years | ≥ 65 years | < 65 years | ≥ 65 years | |||||||
| Placebo (N = 261) | Tofacitinib 10 mg BID (N = 876) | Placebo (N = 21) | Tofacitinib 10 mg BID (N = 62) | Placebo (N = 180) | Tofacitinib 5 mg BID (N = 185) | Tofacitinib 10 mg BID (N = 179) | Placebo (N = 18) | Tofacitinib 5 mg BID (N = 13) | Tofacitinib 10 mg BID (N = 17) | Tofacitinib All (N = 1080) | Tofacitinib All (N = 77) | |
| Age, years, mean (SD) | 39.1 (12.3) | 39.3 (11.9) | 69.9 (4.4) | 69.3 (4.0) | 40.9 (12.0) | 39.9 (11.8) | 40.3 (11.9) | 68.6 (3.4) | 70.2 (4.5) | 71.1 (4.7) | 39.3 (12.1) | 69.4 (4.0) |
| Males, n (%) | 142 (54.4) | 514 (58.7) | 13 (61.9) | 43 (69.4) | 104 (57.8) | 95 (51.4) | 98 (54.7) | 12 (66.7) | 8 (61.5) | 12 (70.6) | 626 (58.0) | 53 (68.8) |
| Race, n (%) | ||||||||||||
| White | 211 (80.8) | 709 (80.9) | 18 (85.7) | 47 (75.8) | 140 (77.8) | 154 (83.2) | 141 (78.8) | 15 (83.3) | 10 (76.9) | 12 (70.6) | 867 (80.3) | 60 (77.9) |
| Asian | 27 (10.3) | 105 (12.0) | 1 (4.8) | 9 (14.5) | 23 (12.8) | 21 (11.4) | 24 (13.4) | 3 (16.7) | 2 (15.4) | 1 (5.9) | 134 (12.4) | 10 (13.0) |
| Geographic region, n (%) | ||||||||||||
| Asia | 25 (9.6) | 88 (10.0) | 1 (4.8) | 7 (11.3) | 18 (10.0) | 20 (10.8) | 21 (11.7) | 2 (11.1) | 2 (15.4) | 0 (0.0) | 115 (10.6) | 8 (10.4) |
| Eastern Europe | 87 (33.3) | 273 (31.2) | 3 (14.3) | 10 (16.1) | 55 (30.6) | 65 (35.1) | 62 (34.6) | 2 (11.1) | 1 (7.7) | 1 (5.9) | 330 (30.6) | 12 (15.6) |
| Western Europe | 76 (29.1) | 267 (30.5) | 3 (14.3) | 14 (22.6) | 49 (27.2) | 45 (24.3) | 52 (29.1) | 6 (33.3) | 2 (15.4) | 5 (29.4) | 328 (30.4) | 16 (20.8) |
| North America | 44 (16.9) | 166 (18.9) | 9 (42.9) | 21 (33.9) | 40 (22.2) | 34 (18.4) | 35 (19.6) | 5 (27.8) | 5 (38.5) | 9 (52.9) | 212 (19.6) | 29 (37.7) |
| Rest of world | 29 (11.1) | 82 (9.4) | 5 (23.8) | 10 (16.1) | 18 (10.0) | 21 (11.4) | 9 (5.0) | 3 (16.7) | 3 (23.1) | 2 (11.8) | 95 (8.8) | 12 (15.6) |
| Disease duration, years, mean (SD) | 7.8 (6.3) | 8.1 (6.8) | 12.8 (10.6) | 9.8 (9.3) | 8.3 (6.9) | 8.3 (7.3) | 8.3 (6.6) | 13.3 (11.9) | 7.9 (6.3) | 12.7 (10.0) | 8.1 (6.7) | 10.7 (9.5) |
| Total Mayo score, mean (SD)d | 8.8 (1.5) | 9.0 (1.4) | 9.3 (1.6) | 8.7 (1.5) | 3.3 (1.8) | 3.3 (1.8) | 3.4 (1.8) | 3.1 (1.8) | 3.5 (1.9) | 3.5 (1.7) | 8.6 (2.0) | 8.3 (2.0) |
| Baseline CRP, mg/L, median (range)d | 4.9 (0.1–82.5) | 4.5 (0.1–208.4) | 6.7 (0.2–205.1) | 5.2 (0.4–58.1) | 1.0 (0.1–45.0) | 0.7 (0.1–33.7) | 0.8 (0.1–74.3) | 1.7 (0.1–16.3) | 0.3 (0.1–18.9) | 1.0 (0.1–10.0) | 4.4 (0.1–208.4) | 5.1 (0.4–58.1) |
| Prior TNFi treatment, n (%)e | 122 (56.0) | 452 (53.6) | 8 (50.0) | 36 (59.0) | 80 (44.4) | 85 (45.9) | 91 (50.8) | 12 (66.7) | 5 (38.5) | 9 (52.9) | 568 (54.2) | 44 (57.9) |
| Prior immunosuppressant treatment, n (%)e | 147 (67.4) | 640 (75.8) | 13 (81.3) | 43 (70.5) | 120 (66.7) | 142 (76.8) | 133 (74.3) | 14 (77.8) | 7 (53.8) | 11 (64.7) | 783 (74.7) | 55 (72.4) |
| Immunosuppressant treatment within 8 weeks prior to baseline, n (%)e | 53 (24.3) | 248 (29.4) | 3 (18.8) | 11 (18.0) | 41 (22.8) | 43 (23.2) | 52 (29.1) | 3 (16.7) | 2 (15.4) | 4 (23.5) | 299 (28.5) | 14 (18.4) |
| Oral corticosteroid use at baseline, n (%)d,f | 117 (44.8) | 399 (45.5) | 10 (47.6) | 31 (50.0) | 89 (49.4) | 94 (50.8) | 77 (43.0) | 11 (61.1) | 7 (53.8) | 9 (52.9) | 486 (45.0) | 37 (48.1) |
| Oral corticosteroid daily dose at baseline, prednisone equivalent, mg/day, mean (SD) d,f | 17.2 (6.3) | 16.2 (6.3) | 12.8 (4.4) | 13.6 (6.9) | 16.5 (6.1) | 14.9 (6.1) | 14.5 (5.8) | 11.6 (5.6) | 15.0 (7.6) | 14.4 (7.2) | 16.2 (6.3) | 12.9 (6.6) |
| Extent of disease, n (%)e | ||||||||||||
| Proctosigmoiditis | 35 (16.1) | 118 (14.0) | 0 (0.0) | 14 (23.3) | 17 (9.4) | 27 (14.7) | 31 (17.3) | 4 (22.2) | 1 (8.3) | 2 (12.5) | 148 (14.1) | 15 (20.3) |
| Left-sided colitis | 68 (31.2) | 284 (33.7) | 8 (53.3) | 23 (38.3) | 62 (34.4) | 59 (32.1) | 54 (30.2) | 6 (33.3) | 7 (58.3) | 6 (37.5) | 350 (33.4) | 30 (40.5) |
| Extensive/pancolitis | 115 (52.8) | 440 (52.2) | 7 (46.7) | 23 (38.3) | 100 (55.6) | 98 (53.3) | 94 (52.5) | 8 (44.4) | 4 (33.3) | 8 (50.0) | 548 (52.3) | 29 (39.2) |
| Proctitis | 0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0 (0.0) |
| Smoking status, n (%)e | ||||||||||||
| Current smoker | 10 (3.8) | 47 (5.4) | 1 (4.8) | 1 (1.6) | 11 (6.1) | 7 (3.8) | 6 (3.4) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 57 (5.3) | 2 (2.6) |
| Exsmoker | 65 (24.9) | 257 (29.3) | 11 (52.4) | 39 (62.9) | 58 (32.2) | 42 (22.7) | 52 (29.1) | 15 (83.3) | 7 (53.8) | 11 (64.7) | 310 (28.7) | 47 (61.0) |
| Never smoked | 186 (71.3) | 572 (65.3) | 9 (42.9) | 21 (33.9) | 111 (61.7) | 136 (73.5) | 121 (67.6) | 2 (11.1) | 6 (46.2) | 6 (35.3) | 713 (66.0) | 27 (35.1) |
Induction Cohort: placebo, N = 282; and tofacitinib 10 mg BID, N = 938.
Maintenance Cohort: placebo, N = 198; tofacitinib 5 mg BID, N = 198; tofacitinib 10 mg BID, N = 196; and Tofacitinib All, N = 364.
Overall Cohort: tofacitinib 5 mg BID, N = 198; tofacitinib 10 mg BID, N = 959; and Tofacitinib All, N = 1157.
Induction Cohort: parameters based on baseline of the induction trials (phase 2 induction, OCTAVE Induction 1, and OCTAVE Induction 2); Maintenance Cohort: parameters based on baseline of the maintenance trial; Overall Cohort: parameters based on day 1, start of active tofacitinib treatment in the UC program.
Induction, Maintenance, and Overall Cohorts: parameters based on baseline of the induction trials.
Based on prednisone-equivalent total daily doses, and excludes medications like budesonide and beclometasone.
Abbreviations: BID, twice daily; CRP, C-reactive protein; N, total number of patients in the treatment group; n, number of patients in the specified category; SD, standard deviation; TNFi, tumor necrosis factor inhibitor; UC, ulcerative colitis.
Patient demographics and characteristics were generally similar for patients in the Induction, Maintenance, and Overall Cohorts when stratified into groups of those aged < 65 and ≥ 65 years, although patients aged ≥ 65 years typically had a longer disease duration (Table 1). The proportions of patients with prior TNFi and immunosuppressant treatment ranged from 38.5% to 66.7% and 53.8% to 81.3%, respectively. Patient demographics, and baseline and clinical characteristics, for all cohorts, stratified by age, are described in Tables S1 to S3, respectively. In the Overall Cohort, older patients had been diagnosed with UC for a longer period of time; mean disease duration was 4.7 (standard deviation [SD], 3.5), 7.4 (SD, 5.0), 9.3 (SD, 7.2), 11.6 (SD, 8.1), and 10.6 (SD, 9.8) years in the groups aged 18 to < 30, 30 to < 40, 40 to < 50, 50 to < 60, and ≥ 60 years, respectively (Table S3).
Treatment-Emergent AEs
In the Induction Cohort, all-causality treatment-emergent AEs occurred in 54.9% and 55.0% of tofacitinib- and placebo-treated patients, respectively. In the Maintenance Cohort, the proportions were 75.9% and 75.3%, respectively. In the Overall Cohort, AEs occurred in 84.7% of tofacitinib-treated patients. The proportions of AEs, SAEs, severe AEs, and discontinuations/dose reductions due to AEs in the 3 cohorts, stratified by age, are shown in Table 2, Table 3 and Table 4. Across cohorts, the percentages of patients with SAEs, or who discontinued treatment with tofacitinib or placebo due to AEs, were higher in the older versus younger age group.
Table 2.
Treatment-emergent AEs reported in the Induction Cohort from the tofacitinib UC clinical program.
| 18 to < 30 years | 30 to < 40 years | 40 to < 50 years | 50 to < 60 years | ≥ 60 years | < 65 years | ≥ 65 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (N = 67) | Tofacitinib 10 mg BID (N = 216) | Placebo (N = 80) | Tofacitinib 10 mg BID (N = 251) | Placebo (N = 51) | Tofacitinib 10 mg BID (N = 210) | Placebo (N = 44) | Tofacitinib 10 mg BID (N = 149) | Placebo (N = 40) | Tofacitinib 10 mg BID (N = 112) | Placebo (N = 261) | Tofacitinib 10 mg, BID (N = 876) | Placebo (N = 21) | Tofacitinib 10 mg BID (N = 62) | |
| AEs, n (%) | 38 (56.7) | 118 (54.6) | 43 (53.8) | 136 (54.2) | 27 (52.9) | 113 (53.8) | 22 (50.0) | 85 (57.0) | 25 (62.5) | 63 (56.3) | 139 (53.3) | 477 (54.5) | 16 (76.2) | 38 (61.3) |
| SAEs, n (%) | 4 (6.0) | 6 (2.8) | 8 (10.0) | 12 (4.8) | 1 (2.0) | 9 (4.3) | 2 (4.5) | 3 (2.0) | 3 (7.5) | 6 (5.4) | 15 (5.7) | 32 (3.7) | 3 (14.3) | 4 (6.5) |
| Severe AEs, n (%) | 3 (4.5) | 8 (3.7) | 5 (6.3) | 12 (4.8) | 2 (3.9) | 10 (4.8) | 1 (2.3) | 4 (2.7) | 4 (10.0) | 4 (3.6) | 11 (4.2) | 36 (4.1) | 4 (19.0) | 2 (3.2) |
| Discontinuation due to AEs, n (%) | 3 (4.5) | 7 (3.2) | 8 (10.0) | 12 (4.8) | 0 (0.0) | 8 (3.8) | 1 (2.3) | 4 (2.7) | 2 (5.0) | 5 (4.5) | 12 (4.6) | 33 (3.8) | 2 (9.5) | 3 (4.8) |
| Dose reduction or temporary discontinuation due to AEs, n (%) | 2 (3.0) | 1 (0.5) | 0 (0.0) | 3 (1.2) | 0 (0.0) | 7 (3.3) | 3 (6.8) | 1 (0.7) | 0 (0.0) | 4 (3.6) | 5 (1.9) | 13 (1.5) | 0 (0.0) | 3 (4.8) |
SAEs were based on the Investigator’s assessment. Patients were counted only once per treatment for each category. Severity counts were based on the maximum severity or grade of events. Abbreviations: AE, adverse event; BID, twice daily; N, total number of patients in the treatment group; n, number of patients with the specified event; SAE, serious adverse event; UC, ulcerative colitis.
Table 3.
Treatment-emergent AEs reported in the Maintenance Cohort from the tofacitinib UC clinical program.
| 18 to < 30 years | 30 to < 40 years | 40 to < 50 years | 50 to <60 years | ≥60 years | <65 years | ≥65 years | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (N = 39) | Tofacitinib 5 mg BID (N = 44) | Tofacitinib 10 mg BID (N = 36) | Placebo (N = 48) | Tofacitinib 5 mg BID (N = 50) | Tofacitinib 10 mg BID (N = 56) | Placebo (N = 40) | Tofacitinib 5 mg BID (N = 46) | Tofacitinib 10 mg BID (N = 42) | Placebo (N = 42) | Tofacitinib 5 mg BID (N = 36) | Tofacitinib 10 mg BID (N = 31) | Placebo (N = 29) | Tofacitinib 5 mg BID (N = 22) | Tofacitinib 10 mg BID (N = 31) | Placebo (N = 180) | Tofacitinib 5 mg BID (N = 185) | Tofacitinib 10 mg BID (N = 179) | Placebo (N = 18) | Tofacitinib 5 mg BID (N = 13) | Tofacitinib 10 mg BID (N = 17) | |
| AEs, n (%) | 26 (66.7) | 30 (68.2) | 28 (77.8) | 35 (72.9) | 38 (76.0) | 43 (76.8) | 28 (70.0) | 33 (71.7) | 33 (78.6) | 36 (85.7) | 27 (75.0) | 27 (87.1) | 24 (82.8) | 15 (68.2) | 25 (80.6) | 134 (74.4) | 134 (72.4) | 141 (78.8) | 15 (83.3) | 9 (69.2) | 15 (88.2) |
| SAEs, n (%) | 4 (10.3) | 1 (2.3) | 2 (5.6) | 3 (6.3) | 0 (0.0) | 3 (5.4) | 3 (7.5) | 5 (10.9) | 1 (2.4) | 1 (2.4) | 2 (5.6) | 2 (6.5) | 2 (6.9) | 2 (9.1) | 3 (9.7) | 11 (6.1) | 8 (4.3) | 8 (4.5) | 2 (11.1) | 2 (15.4) | 3 (17.6) |
| Severe AEs, n (%) | 5 (12.8) | 6 (13.6) | 3 (8.3) | 3 (6.3) | 3 (6.0) | 5 (8.9) | 3 (7.5) | 5 (10.9) | 2 (4.8) | 4 (9.5) | 0 (0.0) | 2 (6.5) | 4 (13.8) | 0 (0.0) | 3 (9.7) | 17 (9.4) | 14 (7.6) | 12 (6.7) | 2 (11.1) | 0 (0.0) | 3 (17.6) |
| Discontinuation due to AEs, n (%) | 8 (20.5) | 4 (9.1) | 2 (5.6) | 10 (20.8) | 4 (8.0) | 7 (12.5) | 8 (20.0) | 5 (10.9) | 4 (9.5) | 4 (9.5) | 3 (8.3) | 3 (9.7) | 7 (24.1) | 2 (9.1) | 3 (9.7) | 33 (18.3) | 16 (8.6) | 16 (8.9) | 4 (22.2) | 2 (15.4) | 3 (17.6) |
| Dose reduction or temporary discontinuation due to AEs, n (%) | 0 (0.0) | 2 (4.5) | 2 (5.6) | 3 (6.3) | 0 (0.0) | 6 (10.7) | 0 (0.0) | 1 (2.2) | 2 (4.8) | 1 (2.4) | 0 (0.0) | 1 (3.2) | 0 (0.0) | 3 (13.6) | 4 (12.9) | 4 (2.2) | 4 (2.2) | 12 (6.7) | 0 (0.0) | 2 (15.4) | 3 (17.6) |
SAEs were based on the Investigator’s assessment. Patients were counted only once per treatment for each category. Severity counts were based on the maximum severity or grade of events. Abbreviations: AE, adverse event; BID, twice daily; N, total number of patients in the treatment group; n, number of patients with the specified event; SAE, serious adverse event; UC, ulcerative colitis.
Table 4.
Treatment-emergent AEs reported in the Overall Cohort from the tofacitinib UC clinical program.
| 18 to < 30 years | 30 to < 40 years | 40 to < 50 years | 50 to < 60 years | ≥ 60 years | < 65 years | ≥ 65 years | |
|---|---|---|---|---|---|---|---|
| Tofacitinib Alla (N = 270) | Tofacitinib Alla (N = 311) | Tofacitinib Alla (N = 251) | Tofacitinib Alla (N = 181) | Tofacitinib Alla (N = 144) | Tofacitinib Alla (N = 1080) | Tofacitinib Alla (N = 77) | |
| AEs, n (%) | 220 (81.5) | 253 (81.4) | 219 (87.3) | 159 (87.8) | 129 (89.6) | 912 (84.4) | 68 (88.3) |
| SAEs, n (%) | 42 (15.6) | 55 (17.7) | 49 (19.5) | 37 (20.4) | 40 (27.8) | 202 (18.7) | 21 (27.3) |
| Severe AEs, n (%) | 38 (14.1) | 48 (15.4) | 43 (17.1) | 26 (14.4) | 23 (16.0) | 164 (15.2) | 14 (18.2) |
| Discontinuation due to AEs, n (%) | 14 (5.2) | 23 (7.4) | 26 (10.4) | 26 (16.4) | 30 (23.3) | 99 (10.9) | 20 (29.4) |
| Dose reduction or temporary discontinuation due to AEs, n (%) |
16 (5.9) | 24 (7.7) | 29 (11.6) | 14 (7.7) | 23 (16.0) | 91 (8.4) | 15 (19.5) |
SAEs were based on the Investigator’s assessment. Patients were counted only once per treatment for each category. Severity counts were based on the maximum severity or grade of events. Overall Cohort data are as of May 27, 2019; database not locked.
Includes patients who received tofacitinib 5 or 10 mg BID in the phase 2 and phase 3 induction and maintenance studies and the ongoing OLE study; 82.9% of patients in the Overall Cohort received a predominant dose of tofacitinib 10 mg BID. Abbreviations: AE, adverse event; BID, twice daily; N, total number of patients in the treatment group; n, number of patients with the specified event; OLE, open-label, long-term extension; SAE, serious adverse event; UC, ulcerative colitis.
AEs of Special Interest
In the Maintenance Cohort, AESI (including serious infections, opportunistic infections, herpes zoster, malignancies [excluding NMSC], NMSC, MACE, GI perforations, DVT, and PE) were generally infrequent in the placebo and tofacitinib treatment groups, regardless of age (Figure S1). Herpes zoster (nonserious and serious) events occurred more frequently in the group receiving tofacitinib 10 mg BID, compared with the groups receiving tofacitinib 5 mg BID or placebo, across all age groups, up to 60 years of age. The IRs for all herpes zoster (nonserious and serious) events were numerically higher for patients aged 40 to < 50 and 50 to < 60 years in the group receiving tofacitinib 10 mg BID (40 to < 50 years: IR, 16.37 [95% CI, 5.32–38.21]; 50 to < 60 years: IR, 11.82 [95% CI, 2.44–34.53]), compared with those receiving placebo (40 to < 50 years: IR, 0.00 [95% CI, 0.00–20.12]; 50 to < 60 years: IR, 4.35 [95% CI, 0.11–24.26]) or tofacitinib 5 mg BID (40 to < 50 years: IR, 6.03 [95% CI, 0.73–21.77]; 50 to < 60 years: IR, 3.56 [95% CI, 0.09–19.84]; Figure S1). No cases of herpes zoster occurred in patients aged ≥ 60 years.
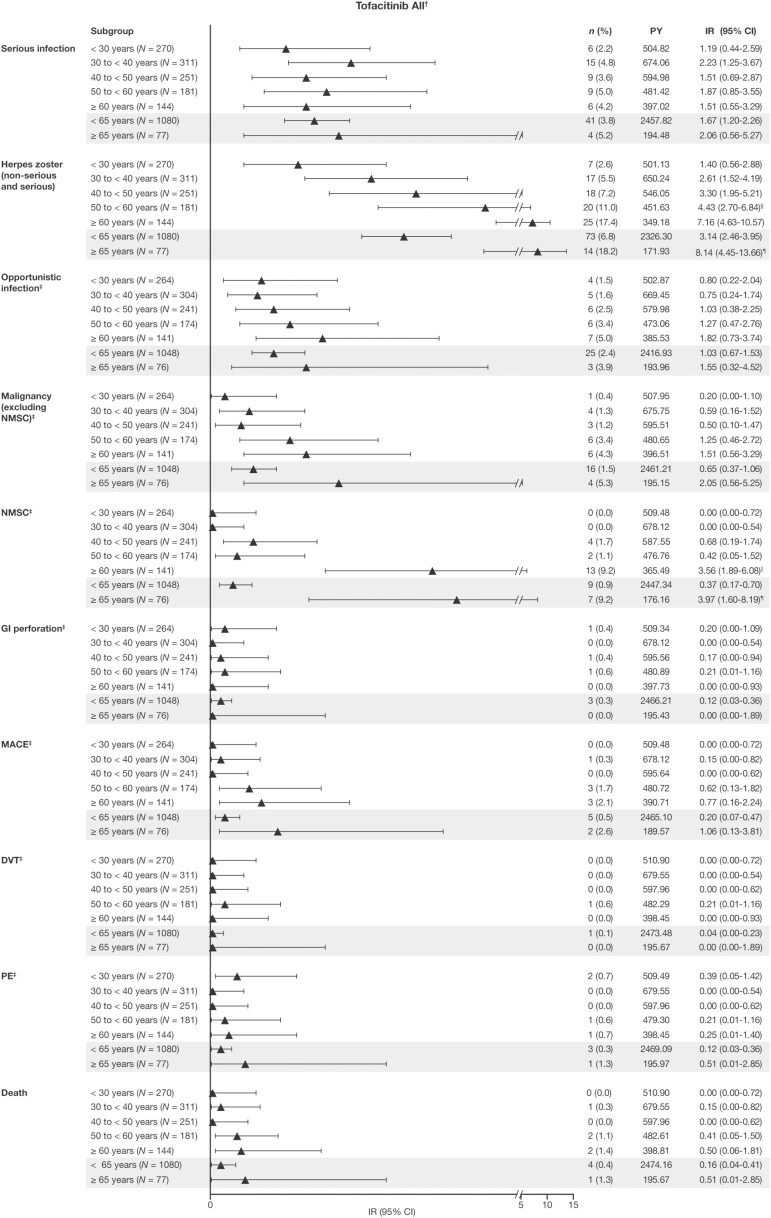
In the Overall Cohort, IRs for serious infections, GI perforations, DVT, PE, and death were generally comparable between age groups (Figure 2). The IRs for all herpes zoster (nonserious and serious) events increased with age across subgroups by 10-year intervals; the IR for herpes zoster was also greater in patients aged ≥ 65 years (IR, 8.14 [95% CI, 4.45–13.66]) compared with patients aged <65 years (IR, 3.14 [95% CI, 2.46–3.95]). Of the patients with a herpes zoster AE, 8 discontinued, of whom 6 were aged < 65 years and 2 were aged ≥ 65 years. Of the 4 cases of disseminated herpes zoster that occurred, none of them occurred in patients aged > 65 years. The IRs for opportunistic infections, malignancy (excluding NMSC), NMSC, and MACE also generally increased with increasing age (Figure 2). Of note, for patients aged ≥ 65 years, the IRs for malignancy (excluding NMSC; IR, 2.05 [95% CI, 0.56–5.25]), NMSC (IR, 3.97 [95% CI, 1.60–8.19]), and MACE (IR, 1.06 [95% CI, 0.13–3.81]) were greater than those for patients aged <65 years (IR, 0.65 [95% CI, 0.37–1.06]; IR, 0.37 [95% CI, 0.17–0.70]; and IR, 0.20 [95% CI, 0.07–0.47], respectively; Figure 2).
Figure 2.
Safety and AESI reported by age group in the Overall Cohort from the tofacitinib UC clinical program. Data are as of May 27, 2019; database not locked. All malignancy (excluding NMSC), NMSC, MACE, and death events were counted (including those that were outside the 28-day risk period); all other AESI were counted to 28 days beyond the last dose, with the exception of ongoing patients in the OLE study. The IR was defined as the number of unique patients with events per 100 patient-years of exposure. Exact Poisson (adjusted for patient-years) 95% CIs are provided. †Includes patients who received tofacitinib 5 or 10 mg BID in the phase 2 and phase 3 induction and maintenance studies, and the ongoing OLE study, unless stated otherwise. ‡Adjudicated events; the Overall Cohort does not include data from the phase 2 induction study. §The IR was significantly higher than for the group aged < 30 years, based on nonoverlapping 95% CIs. ¶The IR was significantly higher than for the group aged < 65 years, based on nonoverlapping 95% CIs. ††The IR was significantly higher than for the groups ages 18 to < 30, 30 to < 40, 40 to < 50, and 50 to < 60 years, based on nonoverlapping 95% CIs. Abbreviations: AESI, adverse event of special interest; BID, twice daily; CI, confidence interval; DVT, deep vein thrombosis; GI, gastrointestinal; IR, incidence rate; MACE, major adverse cardiovascular events; N, total number of patients; n, number of patients with the specified event; NMSC, nonmelanoma skin cancer; OLE, open-label, long-term extension; PE, pulmonary embolism; PY, patient-years; UC, ulcerative colitis.
Results of Cox Proportional-Hazards Regression Modeling
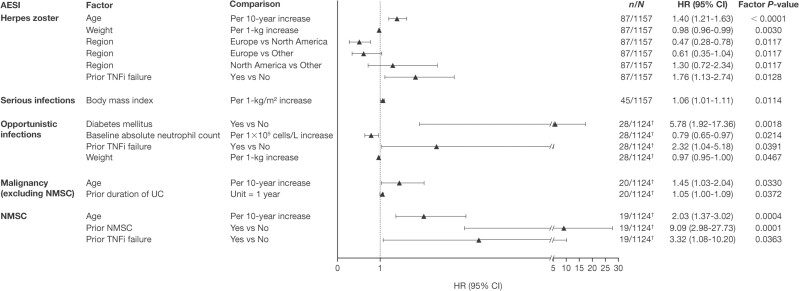
In the Cox proportional-hazards multiple regression model, a range of factors were significant predictors of AESI (Figure 3). Age, weight, and prior TNFi failure significantly predicted herpes zoster (HR per 10 years, 1.40 [95% CI, 1.21–1.63]; HR per 1-kg increase, 0.98 [95% CI, 0.96–0.99]; and HR 1.76 [95% CI 1.13–2.74], respectively). Region was also a predictor of herpes zoster, with 1 of the 3 comparisons reaching statistical significance based on the HR of 0.47 (95% CI, 0.28–0.78) for Europe versus North America.
Figure 3.
Predictors of AESI (Cox proportional-hazards regression models) in the Overall Cohort. †Based on adjudicated events, which do not include data from the phase 2 induction study. Abbreviations: AESI, adverse event of special interest; CI, confidence interval; HR, hazard ratio; N, number of patients in analysis; n, number of patients with event; NMSC, nonmelanoma skin cancer; TNFi, tumor necrosis factor inhibitor; UC, ulcerative colitis.
The only predictor of serious infection was body mass index (HR per 1 kg/m2 increase, 1.06; 95% CI, 1.01–1.11).
Diabetes mellitus, baseline absolute neutrophil count, prior TNFi failure, and weight were identified as significant predictors of opportunistic infections, with an HR of 5.78 (95% CI, 1.92–17.36), HR per 1 x 109 cells/L increase of 0.79 (95% CI, 0.65–0.97), HR of 2.32 (95% CI, 1.04–5.18), and HR per 1 kg increase of 0.97 (95% CI, 0.95–1.00), respectively.
The model also identified age and prior duration of UC as significant predictors of malignancy (excluding NMSC), with an HR per 10 years of 1.45 (95% CI, 1.03–2.04) and an HR per 1 year of 1.05 (95% CI, 1.00–1.09), respectively.
Significant predictors of NMSC were age (HR per 10-year increase, 2.03 [95% CI, 1.37–3.02]), prior NMSC (HR per 10-year increase, 9.09 [95% CI, 2.98–27.73]), and prior TNFi failure (HR, 3.32 [95% CI, 1.08, 10.20]).
In summary, the Cox proportional-hazards regression modeling revealed age significantly predicted herpes zoster, malignancy (excluding NMSC), and NMSC, but not serious infections or opportunistic infections.
Efficacy
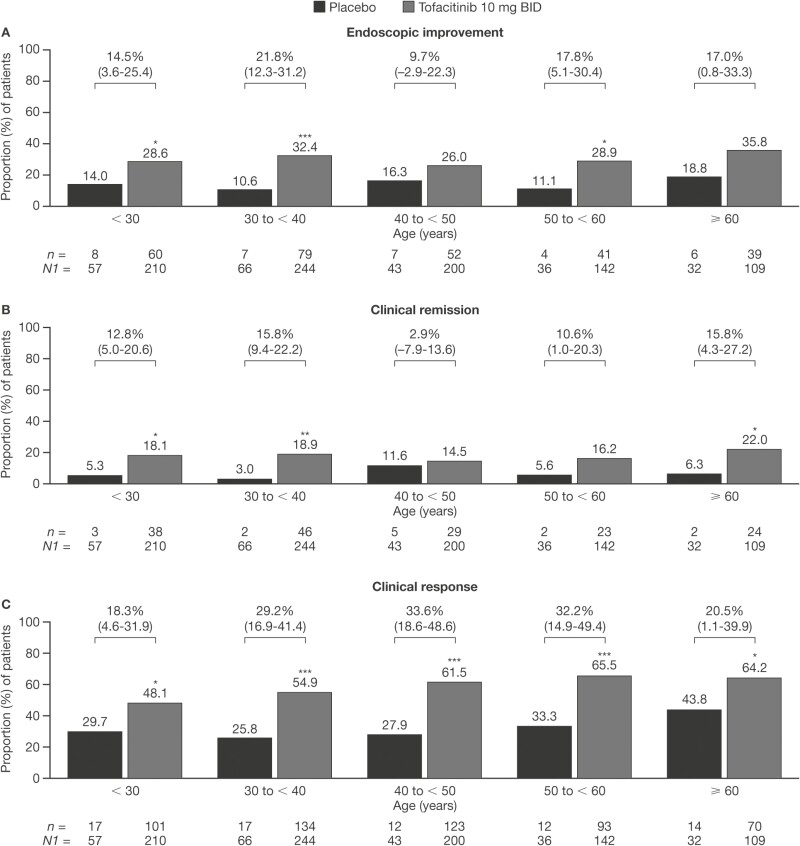
In the Induction Cohort, across age groups, the proportion of patients achieving endoscopic improvement, clinical remission, or clinical response was greater in the group receiving tofacitinib 10 mg BID than in the group receiving placebo (Figure 4). For clinical response, a statistically significant difference between the groups receiving tofacitinib 10 mg BID and placebo was seen across all 5 age categories, with 48.1% versus 29.8%, 54.9% versus 25.8%, 61.5% versus 27.9%, 65.5% versus 33.3%, and 64.2% versus 43.8% achieving clinical response in the groups aged < 30, 30 to < 40, 40 to < 50, 50 to < 60, and ≥ 60 years, respectively. For endoscopic improvement and clinical remission, differences between the groups receiving placebo and tofacitinib 10 mg BID were not always significant. Endoscopic improvement differences were significant in the groups aged < 30 (26.6% versus 14.0%), 30 to < 40 (32.4% versus 10.6%), and 50 to < 60 (28.9% versus 11.1%) years. Clinical remission differences were significant in the groups aged < 30 (18.1% versus 5.3%), 30 to < 40 (18.9% versus 3.0%), and ≥ 60 (22.0% versus 6.3%) years (Figure 4).
Figure 4.
Proportions of patients receiving placebo or tofacitinib 10 mg BID who achieved (A) endoscopic improvement, (B) clinical remission, or (C) clinical response at week 8 in the Induction Cohort (FAS, NRI). *P < .05; **P < .01; ***P < .001 (P values are from Cochran-Mantel-Haenszel chi-squared test). Values above bars show the difference from placebo (95% CI). Endoscopic improvement was defined as a Mayo endoscopic subscore of 0 or 1. Clinical remission was defined as a total Mayo score of ≤ 2, with no individual subscore exceeding 1 point. Clinical response was defined as a decrease from induction study baseline total Mayo score of ≥ 3 points and ≥ 30%, plus a decrease in rectal bleeding subscore ≥ 1 point or an absolute rectal bleeding subscore of 0 or 1. Abbreviations: BID, twice daily; CI, confidence interval; FAS, full analysis set; n, number of patients with the specified response within the given category; N1, number of patients in the specified category with nonmissing data; NRI, nonresponder imputation.
When efficacy endpoints in the Induction Cohort were assessed based on the groups aged < 65 and ≥ 65 years, significant differences between those receiving placebo and tofacitinib 10 mg BID were only observed in patients aged < 65 years, although it should be noted that the group aged ≥ 65 years included low patient numbers (Figure S2).
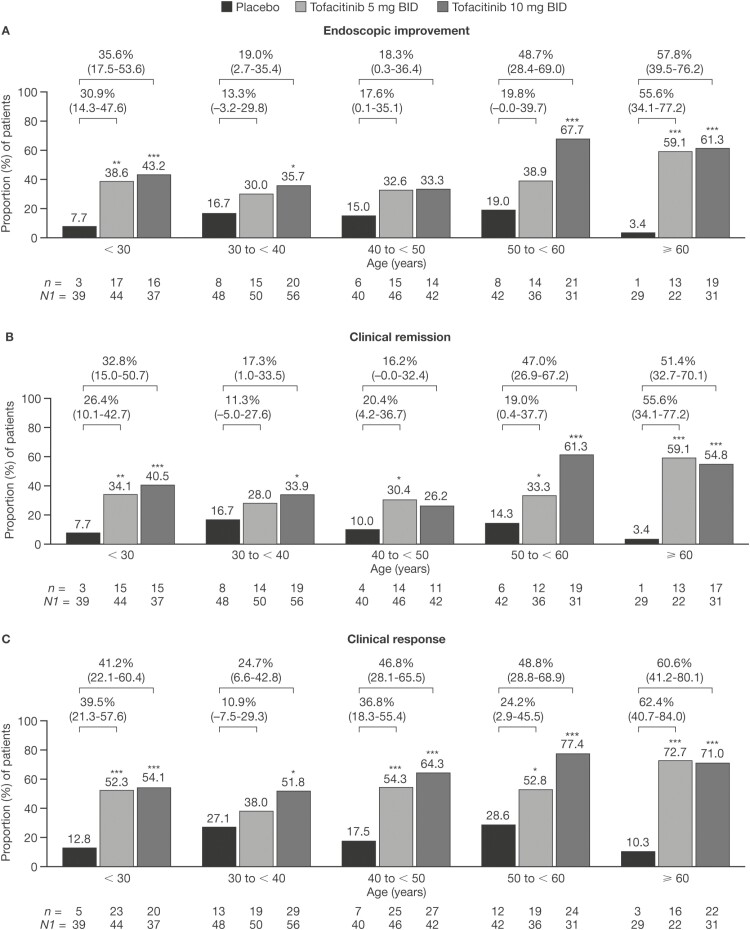
Across age groups, the percentages of patients in the Maintenance Cohort who achieved endoscopic improvement, clinical remission, or clinical response was significantly or numerically greater in patients treated with either tofacitinib 5 or 10 mg BID than in patients treated with placebo (Figure 5). The group aged ≥ 60 years had significant differences across the 3 endpoints. Endoscopic response was achieved in 59.1% and 61.3% of patients treated with tofacitinib 5 and 10 mg BID, respectively, compared to 3.4% of those receiving placebo. Clinical remission was achieved in 59.1% and 54.8% of patients receiving tofacitinib 5 and 10 mg BID, respectively, compared to 3.4% in those receiving placebo. Clinical response was achieved in 72.7% and 71.0% of patients receiving tofacitinib 5 and 10 mg BID, respectively, compared to 10.3% in those receiving placebo. The proportions of patients achieving clinical response in the Maintenance Cohort was generally significantly greater in the groups receiving tofacitinib 5 and 10 mg BID than in the group receiving placebo, across all age groups (Figure 5). For endoscopic improvement and clinical remission, the greatest differences between tofacitinib- and placebo-treated patients were observed in the youngest (18 to < 30 years) and oldest (≥ 60 years) age groups (Figure 5).
Figure 5.
Proportions of patients receiving placebo, tofacitinib 5 mg BID, or tofacitinib 10 mg BID who achieved (A) endoscopic improvement, (B) clinical remission, or (C) clinical response at week 52 in the Maintenance Cohort (FAS, NRI). *P < .05; **P < .01; ***P < .001 (P values are from Cochran-Mantel-Haenszel chi-squared test). Values above bars show the difference from placebo (95% CI). Endoscopic improvement was defined as a Mayo endoscopic subscore of 0 or 1. Clinical remission was defined as a total Mayo score of ≤ 2, with no individual subscore exceeding 1 point. Clinical response was defined as a decrease from induction study baseline total Mayo score of ≥ 3 points and ≥ 30%, plus a decrease in rectal bleeding subscore of ≥ 1 point or an absolute rectal bleeding subscore of 0 or 1. Abbreviations: BID, twice daily; CI, confidence interval; FAS, full analysis set; n, number of patients with the specified response within the given category; N1, number of patients in the specified category with non-missing data; NRI, nonresponder imputation.
The proportion of patients in the Maintenance Cohort achieving each efficacy endpoint was significantly greater in the groups receiving tofacitinib 5 and 10 mg BID than in the group receiving the placebo, for patients in both age groups (Figure S3).
Discussion
Tofacitinib was generally well tolerated across all age groups in this analysis of data pooled from the tofacitinib UC clinical program. On the whole, IRs of serious infections were similar between patients aged ≥ 65 and < 65 years in the Overall Cohort. The IRs of SAEs, opportunistic infections, herpes zoster (nonserious and serious), malignancy (excluding NMSC), NMSC, and MACE appeared to increase with increasing age in the Overall Cohort. These findings are consistent with previous studies,3,16,25,26 and suggest that older age may be linked to a heightened risk of some AESI in patients with moderately to severely active UC. Infection AESI, including serious infections, opportunistic infections, and all herpes zoster (nonserious and serious), were infrequent in the Maintenance Cohort. Other AESI, including malignancies (excluding NMSC), NMSC, and MACE, were also infrequent in the Maintenance Cohort with both placebo and tofacitinib treatment. The results should be interpreted with caution because older patients in the population in general, and not just those with IBD, have been shown to have generally higher rates of herpes zoster, malignancy (excluding NMSC), serious infections, opportunistic infections, and heart disease.3–11 Specifically, a study showed that the IR of herpes zoster increases with increasing age in a United States population captured in 2010, with rates of 7.1 and 10 cases per 1000/year in those aged 60–69 years and 70–79 years, respectively.9 Similarly, 1 study in Canada highlighted the incidence of invasive cancer to be highest in those aged 65–69 years, followed by those aged 60–64 years and 70–74 years.8 Of note, the long-term safety data presented here are for the Overall Cohort and do not include any comparators of patients who have not received tofacitinib. Moreover, it should be noted that corticosteroid use is a risk factor for serious infections in tofacitinib-treated patients with IBD.13 From the Overall Cohort, 45.0% and 48.1% of patients aged < 65 and ≥ 65 years had corticosteroid use at baseline, respectively. Baseline of the Overall Cohort was determined as baseline of the induction trials (phase 2 induction, OCTAVE Induction 1 and 2).
Cox proportional-hazards regression analysis with multiple variables showed that age statistically significantly predicted herpes zoster (nonserious and serious), malignancy (excluding NMSC), and NMSC events. These analyses were conducted on patients in the Overall Cohort (all patients who received ≥ 1 dose of tofacitinib in the phase 2 induction study or 1 or more of the studies in the phase 3 OCTAVE clinical program). In contrast to analyses in patients with UC treated with TNFi, the frequency of serious infections was not elevated in patients aged ≥ 65 versus < 65 years in the current cohorts of patients with UC,14 although this study was limited by a small number of older patients.
The Cox proportional-hazards regression analysis demonstrated that, in addition to age, there are a number of comorbidities and demographic characteristics that can contribute to increased risks of certain AESI. For example, along with age, the duration of UC was a significant predictor of malignancy (excluding NMSC). This could potentially be attributed to the effect of immunosuppressive therapies over a long duration, which may be seen in patients with a longer duration of UC.27 Other statistically significant predictors included prior TNFi failure for herpes zoster, NMSC, and opportunistic infection events. These findings highlight the importance of capturing information on these factors in clinical trials, as it can help identify which patient populations may be more at risk of particular AESI. It should be noted that although corticosteroid use is a risk factor for some AESI, baseline and prior corticosteroid use were not significant predictors of AESI in the Overall Cohort. This may be related to the mandatory corticosteroid tapering in the OCTAVE Sustain and OCTAVE Open studies. This analysis showed that geographic region was a statistically significant predictor of herpes zoster (nonserious and serious). This is in line with findings from previous analyses of the OCTAVE clinical program in patients with UC,28 and an integrated safety analysis in patients with rheumatoid arthritis.29
The efficacy of tofacitinib, relative to placebo, has previously been reported for the phase 2 induction study17 and the phase 3 induction and maintenance studies.18 Subgroup analyses of the Induction and Maintenance Cohorts show that tofacitinib was generally effective in older patients and that, overall, tofacitinib was numerically more effective than placebo for most efficacy endpoints, regardless of age.
A general limitation of interpreting these data is the post hoc nature of these analyses. They are also limited by low patient numbers in the group aged ≥ 65 years, suggesting that larger studies based on analyses from data outside the clinical trial setting should be carried out to further our knowledge of tofacitinib’s safety profile in older patients with UC. Furthermore, the patients in the tofacitinib UC clinical program may not be representative of the general UC population. The follow-up period for the Maintenance Cohort was relatively short, which limits analyses of certain AEs, particularly analyses of infrequent AESI, such as MACE and malignancies. However, the Overall Cohort encompasses data from up to 6.8 years of treatment with tofacitinib, which will allow longer-term study of age as a risk factor in these patients. It is important to note that the OLE program was not placebo controlled. In addition, the absence of data on the use of corticosteroids at the time of AESI is a limitation for interpreting the risk of infections. In the efficacy comparisons, nominal P values are provided, with no adjustment for multiplicity. As there were limited data on the placebo treatment, with very few AESI reported, there was not adequate statistical power to analyze the relationship between treatment and age. Lastly, as the majority of patients were treated with tofacitinib 10 mg BID, the extent of the effect of dose on AESI risks cannot be concluded.
Conclusions
These analyses of data from the tofacitinib UC clinical program show that tofacitinib was generally well tolerated across all age groups. Increasing age was a statistically significant predictor of herpes zoster, malignancy (excluding NMSC), and NMSC. Thus, the risks of these events increased with age in patients with moderately to severely active UC receiving tofacitinib; these findings are in keeping with product labelling, which states that caution should be used when treating elderly patients. Moreover, it is recommended that all patients be brought up to date with all immunizations, including prophylactic zoster vaccination, in agreement with current vaccination guidelines, prior to initiating tofacitinib.30,31 Increased risk with age is also observed in patients with UC receiving other therapies and in the general population, and further investigation is warranted.8,9,11,14,25 Across all age groups, tofacitinib was shown to be more efficacious than placebo as both induction and maintenance therapy. Future long-term studies may help better understand the relationship between malignancies and exposure to tofacitinib.
Supplementary Material
Acknowledgments
The authors thank the patients, investigators, and study teams involved in the tofacitinib UC clinical program. Medical writing support, under the guidance of the authors, was provided by Molly MacFadyen, MSc, and Pauline Craig, PhD, CMC Connect, McCann Health Medical Communications and was funded by Pfizer Inc, New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines [Ann Intern Med 2022; 175: 1298-1304].
Contributor Information
Gary R Lichtenstein, Division of Gastroenterology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
Brian Bressler, Division of Gastroenterology, Department of Medicine, University of British Columbia, Vancouver, BC, Canada.
Carlos Francisconi, Department of Internal Medicine, Federal University of Rio Grande do Sol, Gastroenterology Division, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil.
Severine Vermeire, Department of Gastroenterology & Hepatology, University Hospitals Leuven, Leuven, Belgium.
Nervin Lawendy, Pfizer Inc, Collegeville, PA, USA.
Leonardo Salese, Pfizer Inc, Collegeville, PA, USA.
Gosford Sawyerr, Pfizer Inc, New York, NY, USA.
Hongjiong Shi, Pfizer Inc, New York, NY, USA.
Chinyu Su, Pfizer Inc, Collegeville, PA, USA.
Donna T Judd, Pfizer Inc, Collegeville, PA, USA.
Thomas Jones, Pfizer Inc, Collegeville, PA, USA.
Edward V Loftus, Jr, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA.
Author Contributions
N.L. and G.R.L.: study concept and design. B.B., G.R.L., S.V., N.L., and E.V.L.: data collection, analysis, or interpretation. All authors drafted the work and/or revised it critically for important intellectual content; approved the final published version of the manuscript; and are accountable for all aspects of this work.
Funding
This study was sponsored by Pfizer. Medical writing support was funded by Pfizer Inc.
Conflicts of Interest
G.R.L. has received research support and/or funding from Celgene, Janssen, Pfizer Inc, Salix/Valeant, Santarus/Receptos, Shire, Takeda, and UCB; consultancy fees from Abbott/AbbVie, Actavis, Alaven, Celgene, Cellceutix, Eli Lilly, Ferring Pharmaceuticals, Gilead Sciences, Hospira, Janssen, Luitpold/American Regent, Pfizer Inc, Prometheus, Romark, Salix/Valeant, Santarus/Receptos, Shire, Takeda, and UCB; and honoraria from Ironwood, Luitpold/American Regent, Merck, Romark, and UCB. B.B. has been an advisor and/or speaker for AbbVie, Allergan, Amgen, Celgene, Ferring Pharmaceuticals, Genentech, Janssen, Merck, Microbiome Insights, Pendopharm, Pfizer Inc, Protagonist, Shire, and Takeda; has received research support from AbbVie, Alvine, Amgen, Atlantic Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Genentech, GSK, Janssen, Merck, Qu Biologic, RedHill Biopharma, and Takeda; and holds stock options in Qu Biologic. C.F. has been an advisory board member for, and has received Clinical Investigator support from, Pfizer Inc; and has received Clinical Investigator support from AbbVie, Celgene, Janssen, and Takeda. S.V. has received research support from AbbVie, Janssen, MSD, Pfizer Inc, and Takeda; and speaker fees or consultancy fees from AbbVie, Arena Pharmaceuticals, Avaxia, Boehringer Ingelheim, Celgene, Dr Falk Pharma, Ferring Pharmaceuticals, Galapagos, Genentech-Roche, Gilead Sciences, Hospira, Janssen, MSD, Mundipharma, Pfizer Inc, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Takeda, Theravance, and Tillotts Pharma AG. N.L., L.S., H.S., C.S., D.T.J., and T.J. are employees and shareholders of Pfizer Inc. G.S. is an employee of Syneos Health, which was a paid contractor to Pfizer in connection with the development of this manuscript and in providing statistical support. E.V.L. has received consultancy fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, CALIBR, Celgene, Eli Lilly, Gilead Sciences, Genentech, Gossamer Bio, Iterative Scopes, Janssen, Ono Pharmaceutical, Pfizer Inc, Sun Pharma, Takeda, and UCB; and research support from AbbVie, Bristol-Myers Squibb, Celgene, Genentech, Gilead Sciences, Gossamer Bio, Janssen, Pfizer Inc, Receptos, Robarts Clinical Trials, Takeda, Theravance, and UCB.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual, deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 2. Shivashankar R, Tremaine WJ, Harmsen WS, et al. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. 2017;15:857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Naganuma M, Kunisaki R, Yoshimura N, et al. A prospective analysis of the incidence of and risk factors for opportunistic infections in patients with inflammatory bowel disease. J Gastroenterol. 2013;48:595–600. [DOI] [PubMed] [Google Scholar]
- 4. Toruner M, LoftusEV, Jr., Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. [DOI] [PubMed] [Google Scholar]
- 5. Khan N, Vallarino C, Lissoos T, et al. Risk of infection and types of infection among elderly patients with inflammatory bowel disease: a retrospective database analysis. Inflamm Bowel Dis. 2020;26:462–468. [DOI] [PubMed] [Google Scholar]
- 6. Khan N, Vallarino C, Lissoos T, et al. Risk of malignancy in a nationwide cohort of elderly inflammatory bowel disease patients. Drugs Aging. 2017;34:859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Côté-Daigneault J, Bessissow T, Nicolae MV, et al. Herpes zoster incidence in inflammatory bowel disease patients: a population-based study. Inflamm Bowel Dis. 2019;25:914–918. [DOI] [PubMed] [Google Scholar]
- 8. White MC, Holman DM, Boehm JE, et al. Age and cancer risk: a potentially modifiable relationship. Am J Prev Med. 2014;46:S7–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yawn BP, Gilden D.. The global epidemiology of herpes zoster. Neurology. 2013;81:928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Institute of Medicine (US) Division of Health Promotion and Disease Prevention. 5 Risk Factors for Infection in the Elderly. In: Berg RLC, Cassells JS, eds. The Second Fifty Years: Promoting Health and Preventing Disability. National Academies Press (US); 1992: 65–75. [PubMed] [Google Scholar]
- 11. Zager JS, Ross MI.. Section 7: Integument. Chapter 88: Nonmelanoma skin cancer. In: Pollock RE, Curley SA, Ross MI, Perrier ND, eds. Advanced Therapy in Surgical Oncology. BC Decker Inc; 2008:825–836. [Google Scholar]
- 12. Govani SM, Wiitala WL, Stidham RW, et al. Age disparities in the use of steroid-sparing therapy for inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brassard P, Bitton A, Suissa A, et al. Oral corticosteroids and the risk of serious infections in patients with elderly-onset inflammatory bowel diseases. Am J Gastroenterol. 2014;109:1795–1802; quiz 1803. [DOI] [PubMed] [Google Scholar]
- 14. Cottone M, Kohn A, Daperno M, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:30–35. [DOI] [PubMed] [Google Scholar]
- 15. Kirchgesner J, Lemaitre M, Carrat F, et al. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155:337–346.e10. [DOI] [PubMed] [Google Scholar]
- 16. Winthrop KL, Melmed GY, Vermeire S, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis. 2018;24:2258–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–624. [DOI] [PubMed] [Google Scholar]
- 18. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 19. Sandborn WJ, Lawendy N, Danese S, et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE Open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther. 2022;55(4):464–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lichtenstein GR, Bressler B, Chan G, et al. Assessment of age as a risk factor for adverse events in patients from the tofacitinib ulcerative colitis clinical program (abstract). Am J Gastroenterol. 2019;114:S9(P034). [Google Scholar]
- 21. Lichtenstein GR, Loftus EV Jr, Wei SC, et al. Tofacitinib, an oral, small-molecule Janus kinase inhibitor, in the treatment of ulcerative colitis: analysis of an open-label, long-term extension study with up to 5.9 years of treatment (abstract). J Crohns Colitis. 2020;14 (Suppl 1):S100–S101. [Google Scholar]
- 22. Sandborn WJ, Panes J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019;17:1541–1550. [DOI] [PubMed] [Google Scholar]
- 23. Hicks KA, Mahaffey KW, Mehran R, et al. Cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018;137:961–972. [DOI] [PubMed] [Google Scholar]
- 24. Liu GF, Wang J, Liu K, et al. Confidence intervals for an exposure adjusted incidence rate difference with applications to clinical trials. Stat Med. 2006;25:1275–1286. [DOI] [PubMed] [Google Scholar]
- 25. Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390–399.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gupta G, Lautenbach E, Lewis JD.. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:1483–1490. [DOI] [PubMed] [Google Scholar]
- 27. Nimmons D, Limdi JK.. Elderly patients and inflammatory bowel disease. World J Gastrointest Pharmacol Ther. 2016;7:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winthrop KL, Loftus EV, Baumgart DC, et al. Tofacitinib for the treatment of ulcerative colitis: analysis of infection rates from the ulcerative colitis clinical programme. J Crohns Colitis. 2021;15:914–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020;6:e001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration. https://labeling.pfizer.com/showlabeling.aspx?id=959 Xeljanz® (tofacitinib): highlights of prescribing information. Accessed December 16, 2021.
- 31.European Medicines Agency. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf Xeljanz (tofacitinib): summary of product characteristics. Accessed December 7, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual, deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.