Abstract
Background
Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of ulcerative colitis (UC). We report herpes zoster (HZ) incidence and risk factors in the tofacitinib UC clinical program (up to 7.8 years).
Methods
Proportions and incidence rates (IRs; unique patients with events/100 patient-years) of HZ were evaluated in 4 cohorts: Induction (phase 2 and 3 induction study data), Maintenance (phase 3 maintenance study data), Overall (data from all phase 2, 3, and open-label, long-term extension studies), and Overall plus interim 6-month phase 3b and 4 data. Herpes zoster risk factors were assessed by Cox regression analysis.
Results
In the Induction and Maintenance Cohorts, IRs for HZ (nonserious and serious) were numerically higher with tofacitinib 10 mg twice daily (BID) vs placebo and tofacitinib 10 vs 5 mg BID, respectively. With all tofacitinib doses (5 or 10 mg BID), IRs (95% confidence intervals) for HZ in the Overall and Overall plus phase 3b/4 Cohorts (total exposure, 2814.4 and 2999.7 patient-years, respectively) were 3.38 (2.73-4.15) and 3.30 (2.67-4.04), respectively. In the Overall plus phase 3b/4 Cohort, >90% of HZ were nonserious; >90% were mild/moderate; >90% resolved without discontinuing tofacitinib; 0.6% of patients had multiple HZ events. Herpes zoster IRs were stable when analyzed by 6-month intervals up to >30 months. Herpes zoster risk factors included older age, lower weight, geographic region, and prior tumor necrosis factor inhibitor (TNFi) failure.
Conclusions
Most HZ events were mild/moderate. Herpes zoster IRs remained stable over 7.8 years of exposure. Older age, lower weight, geographic region, and prior TNFi failure were associated with increased HZ risk.
ClinicalTrials.gov
NCT00787202;NCT01465763;NCT01458951;NCT01458574;NCT01470612;NCT03281304
Keywords: herpes zoster, tofacitinib, ulcerative colitis
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease of the colonic mucosa, causing rectal bleeding, diarrhea, fecal urgency, and other potentially debilitating symptoms.1 Patients with UC and other chronic inflammatory auto-immune diseases have a higher risk of various infections, including herpes zoster (HZ; ie, shingles) caused by the reactivation of latent varicella zoster virus (VZV).2,3 In addition, HZ risk may be further increased in patients receiving therapies used to manage UC, such as corticosteroids, thiopurines, and tumor necrosis factor inhibitors (TNFi).4–6
Tofacitinib is an oral, small molecule Janus kinase (JAK) inhibitor for the treatment of UC. The efficacy and safety of tofacitinib in patients with UC have been previously demonstrated in a phase 2 induction study,7 2 phase 3 induction studies (OCTAVE Induction 1 and 2), and a phase 3 maintenance study (OCTAVE Sustain);8 tofacitinib has also been further investigated in an open-label, long-term extension (OLE) study (OCTAVE Open)9 and in the 6-month interim analysis of a phase 3b/4 study (RIVETING).10
Previous analyses of data pooled from studies in the tofacitinib UC clinical program, which includes data up to December 2016 (≤4.4 years of exposure) and May 2019 (≤6.8 years of exposure), showed an elevated risk of HZ in patients with UC receiving tofacitinib, compared with placebo.11,12 Herpes zoster incidence was higher in those with baseline corticosteroid usage;11 but only prior TNFi failure and older age were identified as independent risk factors for HZ in multivariable analysis.11,12 Herpes zoster risk was also shown to be increased with tofacitinib, as well as other JAK inhibitors, compared with TNFi across several inflammatory disease states, including UC, in prior clinical trial and real-world analyses.13–17
Since the previous analyses of HZ risk,11,12 data for a substantial number of additional patient-years (PY) of tofacitinib exposure (up to 7.8 years) have been accrued from the completed OCTAVE Open study and the 6-month interim analysis of the RIVETING study. In this post hoc analysis, we assessed HZ incidence associated with tofacitinib in the UC clinical program, and we evaluated HZ risk longitudinally with prolonged tofacitinib exposure.
Materials and Methods
Patient Populations and Study Designs
Data were derived from patients with UC in the global tofacitinib UC clinical program, which included the phase 2 dose-ranging induction study (ClinicalTrials.gov; NCT00787202), OCTAVE Induction 1 and 2 (ClinicalTrials.gov; NCT01465763 and NCT01458951), OCTAVE Sustain (ClinicalTrials.gov; NCT01458574), OCTAVE Open (ClinicalTrials.gov; NCT01470612), and the RIVETING study (ClinicalTrials.gov; NCT03281304). Full details of all studies have been reported previously.7-10
The phase 2 induction study was an 8-week, randomized, placebo-controlled, double-blind, parallel-group, multicenter study that enrolled patients with moderately to severely active UC (defined as a total Mayo score ≥6) 18 years of age or older. Patients could receive oral mesalamine or prednisone (≤30 mg/day) during the study. Patients were randomized to receive placebo or tofacitinib 0.5, 3, 10, or 15 mg twice daily (BID).7 This analysis included only those patients randomized to receive placebo or tofacitinib 10 mg BID in the phase 2 induction study.
OCTAVE Induction 1 and 2 were two identical, 8-week, phase 3 induction studies of patients with moderately to severely active UC 18 years of age or older, who had previously failed, or were intolerant to, treatment with corticosteroids, immunosuppressants, and/or TNFi. Patients could receive oral aminosalicylates or corticosteroids (prednisone or prednisone equivalent, ≤25 mg/day) during the study, but concomitant therapy with azathioprine, 6-mercaptopurine, methotrexate, or TNFi was prohibited. Patients were initially randomized to receive placebo, tofacitinib 10 mg BID, or tofacitinib 15 mg BID; however, randomization to the 15 mg BID dose was stopped due to feedback from regulatory authorities for rheumatoid arthritis (RA). Therefore, data in this analysis included only those patients randomized to receive placebo or tofacitinib 10 mg BID.8
OCTAVE Sustain was a 52-week, phase 3 maintenance study that enrolled patients in OCTAVE Induction 1 or 2 who had a clinical response (defined as a decrease from induction study baseline total Mayo score of ≥3 points and ≥30%, plus a decrease in rectal bleeding subscore of ≥1 point or an absolute rectal bleeding subscore 0 or 1). Per protocol, with the exception of corticosteroids, patients could remain on stable doses of concomitant medications. Tapering of corticosteroids was mandatory upon entry into OCTAVE Sustain; but any patient experiencing worsening signs or symptoms of UC which, in the opinion of the investigator, were attributable to the reduction in corticosteroid dose, could have their corticosteroid dosage stepped up once during the study and then resume corticosteroid taper to achieve steroid-free status. Patients were rerandomized to placebo, tofacitinib 5 mg BID or tofacitinib 10 mg BID.8
OCTAVE Open was a phase 3 multicenter OLE study that enrolled nonresponders from OCTAVE Induction 1 or 2, as well as patients who had either completed OCTAVE Sustain or had early withdrawal due to treatment failure (defined as an increase in total Mayo score of ≥3 points from baseline of OCTAVE Sustain, along with an increase in rectal bleeding subscore of ≥1 and endoscopic subscore of ≥1, after a minimum of 8 weeks of treatment). Patients in remission at week 52 of OCTAVE Sustain received tofacitinib 5 mg BID in OCTAVE Open, whereas all others received tofacitinib 10 mg BID; patients were allowed to switch doses during the study if they met protocol-defined criteria. Tapering of corticosteroids was required in OCTAVE Open (doses ≤10 mg/day were permitted for those patients who could not tolerate tapering their corticosteroid dose).9
RIVETING was a phase 3b/4 randomized, double-blind, parallel-group, multicenter study that enrolled patients who received tofacitinib 10 mg BID for ≥2 consecutive years in OCTAVE Open, who were in stable remission for ≥6 months prior to baseline, and who had not received corticosteroids for UC for ≥4 weeks prior to baseline; corticosteroids were not permitted in RIVETING. Patients were randomized to receive either tofacitinib 5 or 10 mg BID.10
Pre-treatment HZ vaccination was not mandated in any of the individual study protocols.
Analysis Cohorts
Herpes zoster events were evaluated in 4 patient cohorts. The Induction Cohort included all patients from the 8-week phase 2/3 induction studies (phase 2 induction; OCTAVE Induction 1 and 2) who received ≥1 dose of placebo or tofacitinib 10 mg BID. The Maintenance Cohort included all patients from the 52-week phase 3 maintenance study (OCTAVE Sustain) who received ≥1 dose of placebo, tofacitinib 5 mg BID, or tofacitinib 10 mg BID. The Overall Cohort included all patients from the phase 2/3 induction studies, the phase 3 maintenance study, and the OLE study (OCTAVE Open) who received ≥1 dose of tofacitinib. Finally, the Overall plus phase 3b/4 Cohort included all patients in the Overall Cohort and patients from the RIVETING study who received ≥1 dose of tofacitinib 5 or 10 mg BID (data as of February 20, 2020). As doses may be switched across studies and during OCTAVE Open and RIVETING, tofacitinib doses in the Overall and Overall plus phase 3b/4 Cohorts were categorized based on the average daily dose of tofacitinib received: patients receiving an average daily dose of tofacitinib <15 mg were categorized as receiving a predominant dose (PD) of tofacitinib 5 mg BID, whereas those receiving an average daily dose of tofacitinib ≥15 mg were categorized as receiving PD of tofacitinib 10 mg BID.
Identification and Adjudication of HZ Events
We used Medical Dictionary for Regulatory Activities (v23.0) to identify HZ events using terms consistent with potential HZ. Severity of HZ events was reported by investigators and was defined as mild, moderate, or severe. An independent, blinded, external adjudication committee assessed all HZ events involving >1 dermatome, or those reported as serious adverse events, defined as any event that requires hospitalization, is life-threatening, or results in death, persistent/significant disability, or birth defect. We classified HZ events as multidermatomal if they involved nonadjacent or >2 adjacent dermatomes (not classified as disseminated). Herpes zoster events that involved >6 dermatomes or nonskin involvement (eg, pneumonia, encephalitis) were classified as disseminated. Any HZ events that met the criteria for serious adverse events were graded as serious infection events, and these patients were discontinued from the study.
Statistical Analyses
For patients in the Induction, Maintenance, Overall, and Overall plus phase 3b/4 Cohorts, we summarized baseline demographics and disease characteristics. We calculated the proportions of patients with HZ events and calculated IRs with 95% confidence intervals (CIs) for the entire analysis period in each cohort and by 6-month time intervals in the Overall and Overall plus phase 3b/4 Cohorts, for all HZ (nonserious and serious) events, serious HZ, multidermatomal HZ, and disseminated HZ. Patients were censored at the time of an HZ event; only those events occurring within 28 days after the last study treatment dose were included in the calculation of proportions and IRs for HZ. The denominator for the calculation of IRs was the exposure (in days) accrued from the dates of the first to last doses of tofacitinib (on or before the data cutoff date, or the last dose plus 28 days for patients who discontinued prematurely), or to the date of the first event, whichever occurred earlier. Events occurring within 28 days of study discontinuation were included in the numerator. The Exact Poisson method was used to obtain 95% CIs.18
Exploratory subgroup analyses were carried out to evaluate the effect of baseline corticosteroid use on HZ incidence. In addition, use of varicella vaccine was approved in the US in 1995 for those aged 12 months and older.19 Herpes zoster incidence was evaluated in patients born before 1994 compared with those born after 1994.
To assess the effect of various demographic and disease-related factors on the occurrence of HZ, time-to-event analyses were performed in the Overall and Overall plus phase 3b/4 Cohorts. Univariate Cox proportional-hazards regression analysis was performed for each candidate covariate, and those covariates with a P value <.10 in the univariate regression model were used as a candidate for Cox proportional-hazards multivariable regression analyses, adjusting for multiple variables simultaneously. Exposure time was defined as described in the calculation for IR denominators previously mentioned. Candidate covariates included age (categorical and continuous), sex, weight, body mass index (BMI; continuous and categorical), race (White, Black, Asian, and other), geographic region (defined as North America, Europe and other; continuous and categorical), smoking status (current smoker, ex-smoker, and never smoked), baseline total Mayo score (continuous and categorical), time since diagnosis, baseline high-density lipoprotein level (categorical), baseline absolute lymphocyte count, baseline absolute neutrophil count, prior history of diabetes, prior history of baseline corticosteroid use and corticosteroid dose, prior immunosuppressant use, prior TNFi exposure, prior TNFi failure, and PD (ie, PD tofacitinib 10 vs 5 mg BID).
If multiple continuous covariates were highly correlated, only one was retained in the model to avoid issues with colinearity. Within the Cox proportional-hazards regression analysis, a stepwise selection process was implemented, with an entry criterion P value of .15 and stay criterion P value of .05. No adjustments were made for multiple comparisons.
Ethical Considerations
Informed consent was provided by patients in all studies, and these studies were conducted in accordance with the Declaration of Helsinki, International Council for Harmonisation Guidelines for Good Clinical Practice, and local regulations. Participating institutions provided Institutional Review Board approval prior to participation.
Results
Patients
In total, this analysis included 1157 patients receiving tofacitinib in the tofacitinib UC clinical program. Total exposure in the Overall and Overall plus phase 3b/4 Cohorts was 2814.4 PY and 2999.7 PY, respectively. Across cohorts and treatment groups, the majority of patients were male, younger than 65 years, White, and had never smoked. In the Overall and Overall plus phase 3b/4 Cohorts, mean time since diagnosis was 8.2 years (standard deviation [SD], 7.0) (Table 1); mean time since diagnosis was longer in patients who had previously failed TNFi (8.9 years, SD 7.1) compared with those without prior TNFi failure (7.4 years, SD 6.7). Mean total Mayo score was 8.6 (Table 1); mean total Mayo score was higher in patients who had previously failed TNFi (8.9 years, SD 1.8), compared with those without prior TNFi failure (8.3 years, SD 2.1). In total, 74.6% of patients had previously received immunosuppressant treatment; 54.4% had prior TNFi exposure; and 51.9% had experienced prior TNFi failure. In addition, 45.2% of patients were receiving corticosteroids at baseline (Table 1).
Table 1.
Patient Demographics and Baseline Clinical Characteristics.
| Induction Cohort a | Maintenance Cohort a | Overall and Overall plus phase 3b/4 Cohorts b | ||||
|---|---|---|---|---|---|---|
| Placebo (N = 282) | Tofacitinib10 mg BID (N = 938) | Placebo (N = 198) | Tofacitinib5 mg BID (N = 198) | Tofacitinib10 mg BID (N = 196) | Tofacitinib All (N = 1157) | |
| Total exposure (PY) | 44.8 | 156.2 | 100.4 | 146.2 | 154.3 | 2999.7c |
| Treatment duration (days), mean (SD) [range] | 57.9 (13.7) [7–80] | 60.8 (11.0) [1–96] | 185.1 (127.9) [14–382] | 269.7 (125.1) [22–420] | 287.4 (123.1) [1–399] | 946.9 (865.6) [1–2850]c |
| Age (years), mean (SD) | 41.4 (14.4) | 41.3 (13.8) | 43.4(14.0) | 41.9 (13.7) | 43.0 (14.4) | 41.3 (13.9) |
| Age ≥ 65 years, n (%) | 21 (7.4) | 62 (6.6) | 18 (9.1) | 13 (6.6) | 17 (8.7) | 77 (6.7) |
| Sex (male) | 155 (55.0) | 557 (59.4) | 116 (58.6) | 103 (52.0) | 110 (56.1) | 679 (58.7) |
| Weight (kg), mean (SD) | 73.2 (16.3)d | 73.7 (16.7) | 76.2(16.7) | 73.4 (17.8) | 74.6 (15.2) | 73.5 (16.7) |
| BMI (kg/m2), n (%) | ||||||
| <25 | 158 (56.2)d | 549 (58.5) | 100 (50.5) | 111 (56.1) | 103 (52.6) | 681 (58.9) |
| ≥25 to <30 | 84 (29.9)d | 260 (27.7) | 65 (32.8) | 61 (30.8) | 61 (31.1) | 316 (27.3) |
| >30 | 39 (13.9)d | 129 (13.8) | 33 (16.7) | 26 (13.1) | 32 (16.3) | 159 (13.8) |
| Race, n (%) | ||||||
| White | 229 (81.2) | 756 (80.6) | 155 (78.3) | 164 (82.8) | 153 (78.1) | 927 (80.1) |
| Black | 4 (1.4) | 6 (0.6) | 3 (1.5) | 2 (1.0) | 0 (0.0) | 10 (0.9) |
| Asian | 28 (9.9) | 114 (12.2) | 26 (13.1) | 23 (11.6) | 25 (12.8) | 144 (12.4) |
| Other | 11 (3.9) | 36 (3.8) | 9 (4.5) | 5 (2.5) | 9 (4.6) | 42 (3.6) |
| Geographic region, n (%) | ||||||
| Asia | 26 (9.2) | 95 (10.1) | 20 (10.1) | 22 (11.1) | 21 (10.7) | 123 (10.6) |
| Eastern Europee | 90 (31.9) | 283 (30.2) | 57 (28.8) | 66 (33.3) | 63 (32.1) | 342 (29.6) |
| Western Europef | 79 (28.0) | 281 (30.0) | 55 (27.8) | 47 (23.7) | 57 (29.1) | 344 (29.7) |
| North America | 53 (18.8) | 187 (19.9) | 45 (22.7) | 39 (19.7) | 44 (22.4) | 241 (20.8) |
| Rest of the World | 34 (12.1) | 92 (9.8) | 21 (10.6) | 24 (12.1) | 11 (5.6) | 107 (9.2) |
| Smoking status, n (%) | ||||||
| Never smoked | 195 (69.1) | 593 (63.2) | 113 (57.1) | 142 (71.7) | 127 (64.8) | 740 (64.0) |
| Current smoker | 11 (3.9) | 48 (5.1) | 12 (6.1) | 7 (3.5) | 6 (3.1) | 59 (5.1) |
| Ex-smoker | 76 (27.0) | 296 (31.6) | 73 (36.9) | 49 (24.7) | 63 (32.1) | 357 (30.9) |
| Time since diagnosis (years), mean (SD) | 8.2 (6.8) | 8.2 (7.0) | 8.8 (7.5) | 8.3 (7.2) | 8.7 (7.0) | 8.2 (7.0) |
| Total Mayo score at baseline, mean (SD) | 8.9 (1.5) | 9.0 (1.5) | 3.3 (1.8) | 3.3 (1.8) | 3.4 (1.8) | 8.6 (2.0)g |
| Prior immunosuppressant use, n (%) | 160 (68.4)h | 683 (75.5)i | 134 (67.7) | 149 (75.3) | 144 (73.5) | 838 (74.6)j,l |
| Prior TNFi exposure, n (%) | 130 (55.6)h | 488 (53.9)i | 92 (46.5) | 90 (45.5) | 100 (51.0) | 612 (54.4)j,l |
| Prior TNFi failure, n (%) | 124 (53.0)h | 465 (51.4)i | 89 (44.9) | 83 (41.9) | 92 (46.9) | 583 (51.9)j,l |
| Corticosteroid use at baseline, n (%)k | 127 (45.0) | 430 (45.8) | 100 (50.5) | 101 (51.0) | 86 (43.9) | 523 (45.2)l |
| Corticosteroid dose at baseline (mg/day), mean (SD)m | 16.9 (6.2)n | 16.0 (6.4)o | 15.9 (6.2)p | 14.9 (6.2)q | 14.5 (5.9)r | 16.0 (6.3)s |
Abbreviations: BID, twice daily; BMI, body mass index; N, total number of patients in a treatment group; n, number of patients with each characteristic; PY, patient-years; SD, standard deviation; TNFi, tumor necrosis factor inhibitor.
Includes data previously reported by Winthrop KL, et al 2018.11
The patient population was the same for the Overall and Overall plus phase 3b/4 Cohorts because patients could enter RIVETING if they had received tofacitinib 10 mg BID for ≥2 consecutive years in OCTAVE Open, were in stable remission for ≥6 months, and had not received corticosteroids for UC for ≥4 weeks prior to baseline; the Overall Cohort includes final data from OCTAVE Open as of August 24, 2020, and the Overall plus phase 3b/4 Cohort includes final data from OCTAVE Open as of August 24, 2020, and data from RIVETING, as of February 20, 2020.
Total exposure in the Overall Cohort was 2814.4 PY, and mean (SD [range]) treatment duration was 888.4 (811.2 [1-2850]) days.
N = 281.
Croatia, Czechia, Estonia, Hungary, Latvia, Poland, Romania, Russia, Serbia, Slovakia, and Ukraine.
Austria, Belgium, Denmark, France, Germany, Israel, Italy, Netherlands, Spain, and United Kingdom.
N = 1155.
N = 234.
N = 905.
N = 1124.
Corticosteroid tapering was mandatory during OCTAVE Sustain and OCTAVE Open, and corticosteroids were not permitted during RIVETING.
Data are from Day 1, start of active tofacitinib treatment in the tofacitinib UC clinical program.
Based on prednisone equivalent total daily dose (excludes medications such as budesonide and beclomethasone).
N = 114.
N = 394.
N = 95.
N = 94.
N = 78.
N = 477.
Vaccination history was assessed in 1124 patients enrolled in OCTAVE Induction 1 and 2, OCTAVE Sustain, and OCTAVE Open. In total, 12 of 1124 (1.1%; 1 of 186 [0.5%] and 11 of 938 [1.2%] in PD tofacitinib 5 and 10 mg BID groups, respectively) had received an HZ vaccine; and 934 of 1124 (83.1%) had not received an HZ vaccine. Herpes zoster vaccination history was unavailable for 178 of 1124 (15.8%) patients. In addition, 67 of 1124 (6.0%) had received vaccination for primary varicella, and 761 of 1124 (67.7%) had not received vaccination for primary varicella; varicella vaccination status was unknown for 296 of 1124 (26.3%) patients. Of note, the currently approved Shingrix vaccine20 was not available when vaccination history was assessed at baseline of OCTAVE Induction 1 and 2.
Herpes Zoster Events
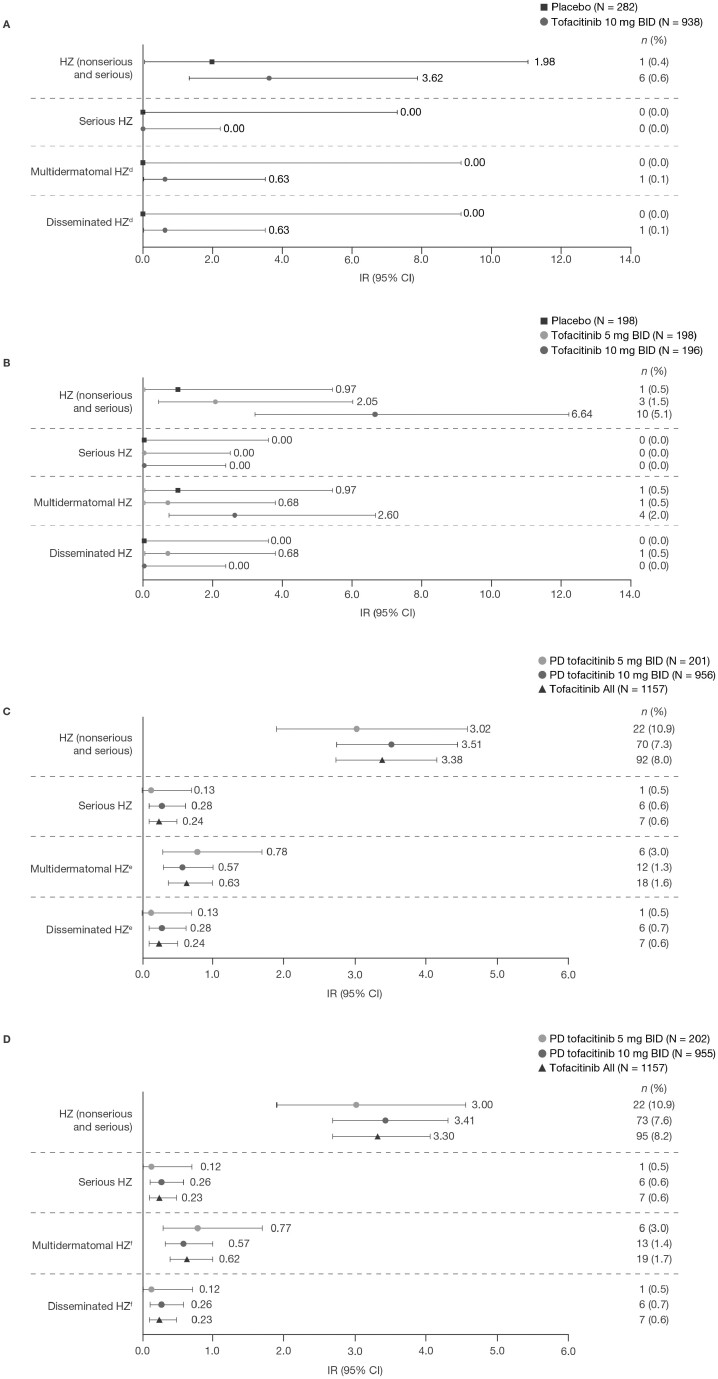
In the Induction Cohort, IR for all HZ (nonserious and serious) was numerically higher with tofacitinib (incidence rate [IR], 3.62; 95% CI, 1.33-7.88) vs placebo (IR, 1.98; 95% CI, 0.05-11.05; Figure 1A). In the Maintenance Cohort, IRs for HZ (nonserious and serious) with both tofacitinib doses (tofacitinib 5 BID; IR, 2.05; 95% CI, 0.42-6.00; tofacitinib 10 mg BID; IR, 6.64; 95% CI, 3.19-12.22) were numerically higher than placebo (IR, 0.97; 95% CI, 0.02–5.42]). Incidence rate for HZ was numerically higher with tofacitinib 10 vs 5 mg BID (Figure 1B). There were no serious HZ events in either the Induction or Maintenance Cohorts (Figure 1A-B).
Figure 1.
Proportions and IRs for HZ events in (A) the Induction Cohorta; (B) the Maintenance Cohorta; (C) the Overall Cohort (data as of August 2020)b; and (D) the Overall plus phase 3b/4 Cohort.c
aIncludes data previously reported by Winthrop KL, et al 2018.11bIncludes final data from OCTAVE Open, as of August 24, 2020 (≤7.8 years of exposure). cIncludes final data from OCTAVE Open, as of August 24, 2020, and data from RIVETING, as of February 20, 2020 (≤7.8 years of exposure). dN = 234 and N = 905 for placebo and tofacitinib 10 mg BID, respectively. eN = 923 and N = 1124 for tofacitinib 10 mg BID and Tofacitinib All, respectively. fN = 202, N = 922, and N = 1124 for PD tofacitinib 5 mg BID, PD tofacitinib 10 mg BID, and Tofacitinib All, respectively. Abbreviations: BID, twice daily; CI, confidence interval; HZ, herpes zoster; IR, incidence rate (unique patients with events per 100 PY); N, total number of patients in the analysis; n, number of patients with an event; PD, predominant dose; PY, patient-years.
In the Overall Cohort, there were 92 of 1157 (8.0%) patients with HZ (nonserious and serious) events, of which 22 of 201 (10.9%) and 70 of 956 (7.3%) patients were receiving PD tofacitinib 5 and 10 mg BID, respectively. In the Overall plus phase 3b/4 Cohort, there were 95 of 1157 (8.2%) patients with HZ (nonserious and serious) events, of which 22 of 202 (10.9%) and 73 of 955 (7.6%) were receiving PD tofacitinib 5 and 10 mg BID, respectively. In the Overall and Overall plus phase 3b/4 Cohorts, >90% of HZ events were nonserious; >90% resolved without discontinuing tofacitinib; and >90% were mild/moderate in severity. Median time to an HZ event was 516.0 days and 529.0 days in the Overall and Overall plus phase 3b/4 Cohorts, respectively. In the Overall and Overall plus phase 3b/4 Cohorts, HZ was limited to 1-2 adjacent dermatomes in 67 of 1157 (5.8%) and 69 of 1157 (6.0%) patients, respectively. In both the Overall and Overall plus phase 3b/4 Cohorts, 7 of 1157 (0.6%) patients had serious HZ. In the 7 of 1157 (0.6%) patients with disseminated HZ in the Overall and Overall plus phase 3b/4 Cohorts, 4 cases were limited to cutaneous involvement; 3 patients were previously reported to have events with other organ involvement: keratitis (n = 1), ophthalmic HZ (n = 1), and HZ meningitis (encephalitis; n = 1) in analyses of data with ≤4.4 and ≤6.8 years of tofacitinib exposure;11,12 therefore, there were no new noncutaneous cases of disseminated HZ up to 7.8 years. A total of 8 of 1157 (0.7%) patients were discontinued from the UC clinical program due to HZ (including 6 patients with serious HZ who were required to discontinue per protocol). Of the 8 patients who discontinued, 2 patients were receiving PD tofacitinib 5 mg BID, and 6 were receiving PD tofacitinib 10 mg BID. In total, 4 of 1157 (0.3%) patients had post-herpetic neuralgia, and 7 of 1157 (0.6%) patients had multiple or repeated HZ events. Incidence rates for HZ (nonserious and serious) with Tofacitinib All were 3.38 (95% CI, 2.73-4.15) and 3.30 (95% CI, 2.67-4.04) in the Overall and Overall plus phase 3b/4 Cohorts, respectively.
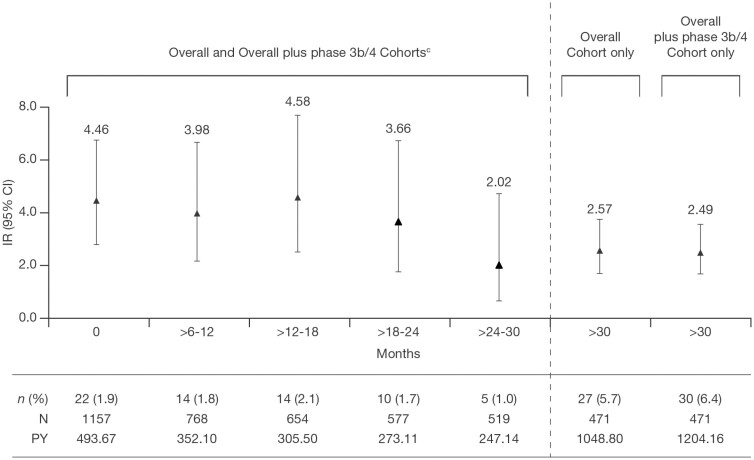
Consistent with previously reported analyses of data with shorter tofacitinib exposure,12 IRs of serious HZ, multidermatomal HZ, and disseminated HZ were <1.0 with Tofacitinib All in the Overall and Overall plus phase 3b/4 Cohorts, and IRs of HZ were generally comparable between PD tofacitinib doses (Figure 1A-D). In addition, IRs for HZ (nonserious and serious) were stable over time when analyzed by 6-month intervals, up to >30 months (Figure 2).
Figure 2.
Proportions and IRs for all HZ (nonserious and serious) events over time in the Overall Cohorta and Overall plus phase 3b/4 Cohort.b Incidence rates (95% CI), numbers of patients, and PY of exposure were the same in both Overall and Overall plus phase 3b/4 Cohorts at time points ≤30 months.
aIncludes final data from OCTAVE Open, as of August 24, 2020. bIncludes final data from OCTAVE Open, as of August 24, 2020, and data from RIVETING, as of February 20, 2020. cThe patient population was the same for the Overall and Overall plus phase 3b/4 Cohorts because patients could enter RIVETING if they had received tofacitinib 10 mg BID for ≥2 consecutive years in OCTAVE Open, were in stable remission for ≥6 months, and had not received corticosteroids for UC for ≥4 weeks prior to baseline. Abbreviations: CI, confidence interval; HZ, herpes zoster; IR, incidence rate (unique patients with events per 100 PY); N, total number of patients in the analysis; n, number of patients with an event; PY, patient-years.
Subgroup Analyses
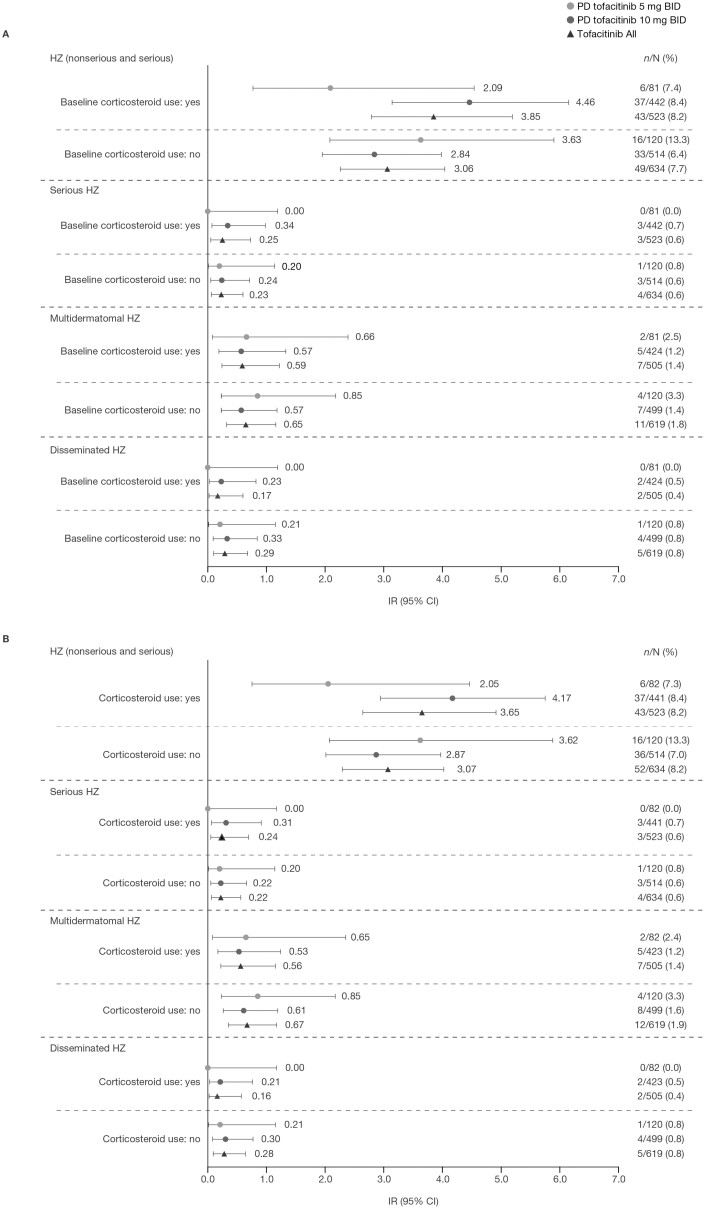
In total, 43 patients with HZ events were receiving corticosteroids at baseline (43 of 92 [46.7%] and 43 of 95 [45.3%] in the Overall and Overall plus phase 3b/4 Cohorts, respectively). Among the 43 patients with HZ events who were receiving corticosteroids at baseline, there were 3 cases of serious HZ and 2 cases of disseminated HZ.
In the Overall Cohort, IRs for HZ (nonserious and serious) were numerically higher in the PD group receiving tofacitinib 10 mg BID and in the Tofacitinib All group with vs without baseline corticosteroid use. Incidence rates for serious HZ, multidermatomal HZ, and disseminated HZ were generally similar with both doses of tofacitinib, irrespective of baseline corticosteroid use (Figure 3A). Similar results were observed in the Overall plus phase 3b/4 Cohort (Figure 3B). In both the Overall and Overall plus phase 3b/4 Cohorts, IRs for HZ were generally numerically higher in patients who were receiving baseline corticosteroid doses of <15 vs ≥15 mg/day (Supplementary Figure 1).
Figure 3.
Herpes zoster events by baseline corticosteroid use in (A) the Overall Cohorta and (B) the Overall plus phase 3b/4 Cohort.b
Events were counted up to 28 days beyond the last dose of study drug. aIncludes final data from OCTAVE Open, as of August 24, 2020. bIncludes final data from OCTAVE Open, as of August 24, 2020 and data from RIVETING, as of February 20, 2020. Abbreviations: BID, twice daily; CI, confidence interval; HZ, herpes zoster; IR, incidence rate (patients with events per 100 PY); N, total number of patients in the analysis; n, number of patients with an event; PD, predominant dose; PY, patient-years.
Incidence of HZ was evaluated in patients born before 1995 compared with those born after 1995, in relation to the US approval of varicella vaccine. In the Overall plus phase 3b/4 Cohort, 1 of 95 (1.1%) patient with HZ events was born after 1995. This patient was from Belgium and had multidermatomal HZ.
Evaluation of HZ Risk Factors
In univariate analyses, risk factors statistically significant for HZ in the Overall and Overall plus phase 3b/4 Cohorts were age, weight (continuous), geographic region (with those from North America at higher risk vs European region [both cohorts] and those from other regions at higher risk vs European region [Overall Cohort only]), Asian race, prior TNFi exposure, prior TNFi failure, and smoking status (ex-smoker vs never smoked), while patients of White race had a significantly lower risk of HZ. Body mass index (continuous) was a significant risk factor for HZ in the Overall plus phase 3b/4 Cohort, but not in the Overall Cohort (see Supplementary Table 1).
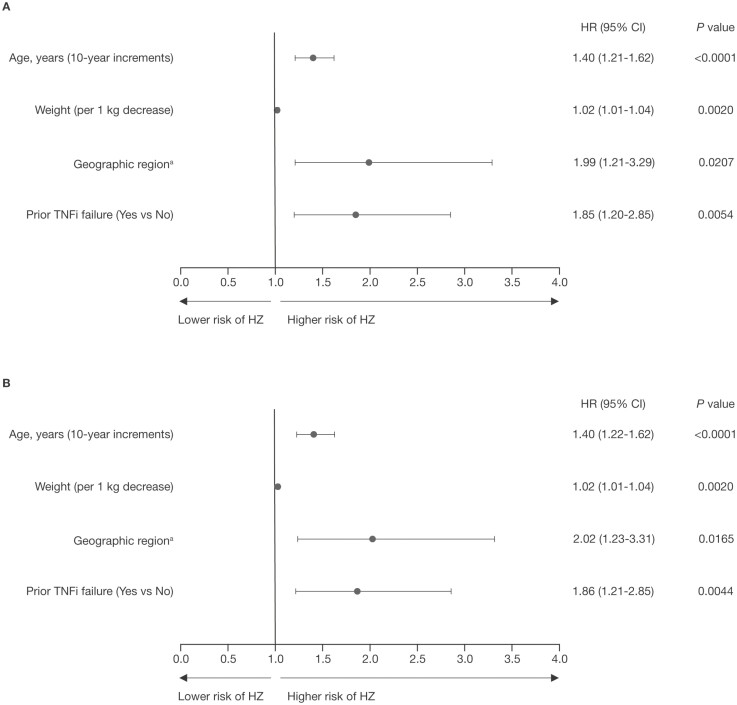
In multivariable regression analyses with stepwise selection, increasing age (per 10-year increments), lower weight (per 1 kg decrease), geographic region (which included North America, Europe, and other; those from North America were at higher risk vs those from the European region in subgroup analyses) and prior TNFi failure were significant risk factors for HZ in both cohorts (Figure 4A-B).
Figure 4.
Risk factors for HZ by multivariable regression analysis (stepwise selection) in (A) the Overall Cohort and (B) the Overall plus phase 3b/4 Cohort.
In total, 92 of 1157 patients in the Overall Cohort with an HZ (nonserious and serious) event and 95 of 1157 patients in the Overall plus phase 3b/4 Cohort with an HZ (nonserious and serious) event were included in Cox multivariable models. aGeographic region (which included North America, Europe, and other) was a significant risk factor; data shown are for North American vs European region. Abbreviations: CI, confidence interval; HR, hazard ratio; HZ, herpes zoster; TNFi, tumor necrosis factor inhibitor.
Discussion
This post hoc analysis of HZ incidence evaluated data from the entire tofacitinib UC clinical program, including final data from the phase 3, multicenter, OCTAVE Open OLE study and from the 6-month interim analysis of the RIVETING study. As previously reported,11,12 IRs for HZ in the Induction Cohort were numerically higher with tofacitinib 10 mg BID vs placebo and numerically higher with tofacitinib 10 vs 5 mg BID in the Maintenance Cohort. Incidence rates for HZ in the Overall and Overall plus phase 3b/4 Cohorts were generally similar between tofacitinib PDs and remained stable over time (up to 7.8 years of exposure). In addition, the majority of patients had nonserious HZ, limited to 1-2 dermatomes; and post-herpetic neuralgia was infrequent and was reported in 0.3% of patients in the UC clinical program. The majority of HZ events resolved without discontinuing tofacitinib therapy.
Previous analysis of data from the tofacitinib UC clinical program (using a data cutoff with up to 4.4 PY of exposure) reported higher IRs for HZ among patients with UC who were 65 years of age and older, Asian vs non-Asian race, with prior TNFi failure, PD tofacitinib 10 vs 5 mg BID, and corticosteroid use at baseline.11 In this current analysis of data including final data from OCTAVE Open and data from an interim analysis of the RIVETING study, increasing age, lower weight, geographic region, and prior TNFi failure were identified as significant independent risk factors in patients with UC receiving tofacitinib, and this is consistent with a previous analysis of data from the tofacitinib UC clinical program with up 6.8 PY of treatment.12 Increasing age is a known risk factor for HZ in both the general population21,22 and in patients with inflammatory bowel disease (IBD),23 and has also been previously identified as a risk factor in patients with RA or psoriasis receiving tofacitinib.24,25 The finding that geographic region (North America vs Europe) was a risk factor for HZ is consistent with a previous analysis of infection rates in patients with UC in the OCTAVE clinical program,12 and an integrated safety analysis in patients with RA,26 both of which reported that HZ risk was lower in Europe than in North America.12,26 In addition, a recent meta-analysis of HZ incidence worldwide reported that IRs for HZ were numerically lower in Europe (IR, 6.77; 95% CI, 6.14-7.48) compared with North America (IR, 7.33; 95% CI, 6.26-8.58).27 The increased risk of HZ in North America vs Europe may reflect differences in vaccination recommendations or vaccine uptake, and/or HZ incidence across these regions. Alternatively, this finding may reflect some other, as yet unidentified, confounding factor, and warrants further investigation.
Lower weight has also not previously been associated with HZ risk in patients with other conditions; however, IBD is a known HZ risk factor,28 and lower weight may be an indicator of more severe UC.29 Therefore, the increased risk of HZ in patients with UC who have lower body weight that was identified in this analysis may reflect an association between body weight and disease severity. The increased risk of HZ in patients with prior TNFi failure is consistent with the findings of previous analyses of data from the Overall Cohort.11,12 However, the underlying cause of this increased HZ risk is unclear. Inflammatory bowel disease is a known HZ risk factor,28 and it has been postulated previously that increased HZ risk in patients with prior TNFi failure could be due to longer disease duration.30 In this analysis, mean time since diagnosis and mean total Mayo score were higher in patients with vs without prior TNFi failure. Therefore, prior TNFi failure may be acting as a surrogate marker of disease duration and/or disease activity.
In patients with RA, age, baseline corticosteroid use, smoking status (ex-smoker vs never smoked), geographic region (Asia vs US/Canada), and tofacitinib dose (10 vs 5 mg BID) were identified as significant risk factors for HZ; and IRs for HZ were reported to be higher in patients from the Asia region, which was mainly due to elevated IRs for HZ in Japan and the Republic of Korea.26 In this analysis, tofacitinib dose was not a significant risk factor for HZ in patients with UC. In addition, although smoking status (ex-smoker vs never smoked) was identified as a risk factor for HZ in univariate analyses, this covariate was not a significant risk factor for HZ in the subsequent multivariable analysis. Incidence rates for HZ (nonserious and serious) in this analysis were numerically higher in patients receiving PD tofacitinib 10 mg BID with corticosteroids at baseline, compared with PD tofacitinib 10 mg BID without baseline corticosteroids. However, tapering of corticosteroids was a requirement for patients entering OCTAVE Sustain and OCTAVE Open, and corticosteroids were not permitted during RIVETING. Consequently, it would be expected that use of corticosteroids would reduce over time in both the Overall and Overall plus phase 3b/4 Cohorts; and baseline corticosteroid use was not a significant risk factor for HZ in either univariate or multivariable analyses.
Opportunistic HZ infection has also been reported in clinical trials of other JAK inhibitors, including baricitinib31 and upadacitinib,32 suggesting that the increased risk of HZ observed in patients receiving tofacitinib is likely to be a class-specific effect. Tofacitinib (and other JAK inhibitors) disrupt JAK/signal transducers and activators of transcription (STAT) signaling pathways, which play a critical role in several important processes, including regulation of innate antiviral immune responses and cell-mediated responses.33–36 Interestingly, VZV interacts with interferon (IFN)-induced intracellular JAK/STAT signaling, resulting in the disruption of innate antiviral immune responses, which can lead to viral reactivation.37,38 Therefore, disruption of type 1/2 IFN-induced JAK/STAT signaling by JAK inhibitors may mimic VZV-induced inhibition of IFN-regulated antiviral activity, thus potentially influencing the risk of VZV reactivation.35,36 Increased HZ risk has also been reported with other treatments for UC, including azathioprine, 6-mercaptopurine, corticosteroids, and TNFi.5,6
Postmarketing surveillance evaluations among patients with UC receiving tofacitinib suggest that, after nasopharyngitis (134 cases), HZ was the second most frequent infection in patients with UC, with 127 reported events of HZ (nonserious and serious). The majority (119 of 127; >90%) of these events were nonserious, which is consistent with the findings of the current analysis of clinical trial data.39
Use of HZ vaccines may protect against the probability of VZV reactivation in patients with chronic inflammatory diseases, such as UC. In the US, use of varicella vaccine was approved in 1995.40 In this analysis, there was 1 patient with HZ born after 1995; this patient was from Belgium and therefore not eligible for vaccination. Therefore, it was not possible to assess the effects of varicella vaccination on HZ risk from the data presented here.
Alternatively, use of vaccines specifically targeted at preventing HZ remain an option, and both recombinant and live-attenuated HZ vaccines have been used in individuals 50 years and older in the US.41 With respect to tofacitinib use, a previous assessment of live zoster vaccine (LZV) use in patients with RA prior to initiation of tofacitinib found that patients receiving LZV 2 to 3 weeks prior to initiation of tofacitinib or placebo, with background conventional synthetic disease-modifying antirheumatic drugs, had VZV-specific vaccine immune responses that were similar or numerically lower than those observed in healthy individuals in pivotal trials of that vaccine, although disseminated vaccine-strain primary varicella infection was reported in 1 patient with no previous VZV exposure.42 However, of 100 patients with RA who entered a tofacitinib OLE study 14 weeks post-vaccination, 5 patients experienced HZ infection, 3 of whom had undetectable VZV cell-mediated immunity at baseline and week 6.43 Use of live vaccines concurrently with tofacitinib is not recommended however,44 and LZV was recently discontinued in the US.45
Another option for HZ vaccination is a nonlive, adjuvant recombinant subunit zoster vaccine (RZV) that has been shown to induce 97% protection against HZ in healthy individuals 50 years and older, which was maintained with no apparent decline in protection for >3 years.46 Use of RZV was recently approved in the US for use in adults 18 years of age and older at an increased risk of HZ due to disease-related or therapy-related immunodeficiency or immunosuppression.20 This has the potential to impact the future risk of HZ associated with JAK inhibitors. The clinical efficacy and safety of RZV in patients with UC receiving tofacitinib are currently being evaluated in a separate study.47
Our analysis was limited in several ways that could impact on the interpretation of these results. This was a post hoc analysis of data pooled from studies that were not designed to evaluate HZ risk. The majority of patients in the Overall and Overall plus phase 3b/4 Cohorts received PD tofacitinib 10 mg BID, which limited the ability to examine the extent of the effect of tofacitinib dose on HZ risk. In addition, patients were permitted to switch tofacitinib dose during the study, therefore, dose dependency of HZ IRs could not be evaluated. Also, the PD method was used in order to account for all tofacitinib exposure across the tofacitinib UC clinical program. However, this approach may not reflect the long-term risk of HZ with continuous use of an actual tofacitinib dose of 5 or 10 mg BID. Another limitation is that it was not possible to assess the impact of HZ vaccination in the patient population in this analysis due to incomplete capture of prior HZ vaccination status and overall low vaccination rates. A limitation of the risk analyses was that HZ vaccination status and prior HZ infection were not available to be included in the univariate and multivariable models. Also, certain risk factors of interest could not be evaluated robustly; for example, assessment of concomitant corticosteroid use could not be carried out as patients were not on any background corticosteroids for the majority of their time on tofacitinib as a consequence of mandatory steroid tapering in OCTAVE Sustain and OCTAVE Open, and prohibition of corticosteroid use during RIVETING. In addition, only nominal P values were provided, with no adjustment for multiplicity. Finally, although RIVETING was a separate study, patients entering RIVETING had previously been enrolled in OCTAVE Open.
Conclusion
In conclusion, this analysis of HZ incidence included data from patients with UC across the tofacitinib UC clinical program, with up to 7.8 years of tofacitinib exposure. Herpes zoster is a documented risk with tofacitinib treatment and HZ IRs in this analysis were consistent with the known safety profile of tofacitinib. The majority of cases were cutaneous, nonserious, and affected 1 to 2 dermatomes. Herpes zoster incidence was stable over time. The risk of HZ was increased in older patients, those with lower weight, those from North America vs European region, and those with prior TNFi failure. Use of vaccination strategies prior to initiation of tofacitinib could mitigate against increased risk of HZ. Additional analysis of postmarketing surveillance data for tofacitinib will be required to further assess the long-term risk of HZ in the setting of UC.
Supplementary Material
Acknowledgments
The authors would like to thank the patients, investigators, and study teams who were involved in the tofacitinib UC clinical program. This study was sponsored by Pfizer. Medical writing support, under the guidance of the authors, was provided by Anthony G. McCluskey, PhD, CMC Connect, McCann Health Medical Communications, and was funded by Pfizer Inc, New York, New York, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–4).
Contributor Information
Kevin L Winthrop, Oregon Health & Science University, Portland, Oregon, USA.
Séverine Vermeire, Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium.
Millie D Long, University of North Carolina, Center for Gastrointestinal Biology and Disease, Chapel Hill, North Carolina, USA.
Julian Panés, Department of Gastroenterology, Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Spain.
Siew C Ng, Institute of Digestive Disease, Department of Medicine and Therapeutics, LKS Institute of Health Science, Chinese University of Hong Kong, Hong Kong.
Nicole Kulisek, Pfizer Inc, Collegeville, Pennsylvania, USA.
Rajiv Mundayat, **Pfizer Inc, New York, New York, USA.
Nervin Lawendy, Pfizer Inc, Collegeville, Pennsylvania, USA.
Ivana Vranic, Pfizer Ltd, Tadworth, Surrey, UK.
Irene Modesto, **Pfizer Inc, New York, New York, USA.
Chinyu Su, Pfizer Inc, Collegeville, Pennsylvania, USA.
Gil Y Melmed, Division of Gastroenterology, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, California, USA.
Author Contributions
N.K., R.M., N.L., I.M., and C.S.: study design. K.L.W., G.Y.M., S.V., M.D.L., J.P., S.C.N., N.K., N.L., I.M., and C.S.: analysis and interpretation of data. R.M.: statistical analysis. K.L.W., G.Y.M., S.V., M.D.L., J.P., S.C.N., N.K., R.M., N.L., I.M., and C.S.: writing, reviewing, and editing the manuscript.
Supported By
This work was supported by Pfizer. This study was sponsored by Pfizer. Medical writing support was funded by Pfizer Inc.
Conflicts of Interest
K.L.W. has received grant support or consulting fees from AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead Sciences, Pfizer Inc, Roche, and UCB. S.V. has received grant support from AbbVie, MSD, Pfizer Inc, and Takeda; speaker fees from AbbVie, Dr. Falk Pharma, Ferring Pharmaceuticals, Hospira, MSD, Takeda, and Tillotts; and consulting fees from AbbVie, Arena Pharmaceuticals, Celgene, Eli Lilly, Ferring Pharmaceuticals, Galapagos, Genentech/Roche, Gilead Sciences, Hospira, Janssen, MSD, Mundipharma, Pfizer Inc, Progenity, Second Genome, Shire, Takeda, and Theravance Biopharma. M.D.L. has received consulting fees from AbbVie, Janssen, Prometheus, Salix, Takeda, Target PharmaSolutions, and UCB; and has received grant support from Pfizer Inc and Takeda. J.P. has received personal fees from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Celltrion, Ferring Pharmaceuticals, Galapagos, Genentech/Roche, GSK, Immunic, Janssen, Origo, Pandion, Pfizer Inc, Progenity, Takeda, Theravance Biopharma, and Wassermann. S.C.N. has received grant support from AbbVie, Ferring Pharmaceuticals, and Olympus; speaker fees from AbbVie, Ferring Pharmaceuticals, Janssen, Menarini, and Takeda; and holds a directorship with Microbiota I Center. G.Y.M. has received consulting fees from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb/Celgene, Ferring Pharmaceuticals, Genentech, Janssen, Medtronic, Pfizer Inc, Samsung Bioepis, Shield, Takeda, and Techlab; and has received grant support from Pfizer Inc. NK, RM, NL, IV, IM, and CS are employees and stockholders of Pfizer Inc.
Data Availability
Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1. Ordás I, Eckmann L, Talamini M, et al. . Ulcerative colitis. Lancet. 2012;380:1606–1619. [DOI] [PubMed] [Google Scholar]
- 2. Yun H, Yang S, Chen L, et al. . Risk of herpes zoster in auto-immune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol. 2016;68:2328–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ning L, Liu R, Li S, et al. . Increased risk of herpes zoster infection in patients with inflammatory bowel disease: a meta-analysis of cohort studies. Eur J Clin Microbiol Infect Dis. 2020;39:219–227. [DOI] [PubMed] [Google Scholar]
- 4. Marra F, Lo E, Kalashnikov V, et al. . Risk of herpes zoster in individuals on biologics, disease-modifying antirheumatic drugs, and/or corticosteroids for autoimmune diseases: a systematic review and meta-analysis. Open Forum Infect Dis. 2016;3:ofw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta G, Lautenbach E, Lewis JD.. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:1483–1490. [DOI] [PubMed] [Google Scholar]
- 6. Long MD, Martin C, Sandler RS, et al. . Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sandborn WJ, Ghosh S, Panes J, et al. . Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–624. [DOI] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Su C, Sands BE, et al. . Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 9. Colombel J-F, Osterman MT, Thorpe AJ, et al. . maintenance of remission with tofacitinib therapy in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2022;20:116–125.e5. [DOI] [PubMed] [Google Scholar]
- 10. Vermeire S, Su C, Lawendy N, et al. . Outcomes of tofacitinib dose reduction in patients with ulcerative colitis in stable remission from the randomised RIVETING trial. J Crohns Colitis. 2021;15:1130–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Winthrop KL, Melmed GY, Vermeire S, et al. . Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis. 2018;24:2258–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Winthrop KL, Loftus EV, Baumgart DC, et al. . Tofacitinib for the treatment of ulcerative colitis: analysis of infection rates from the ulcerative colitis clinical programme. J Crohns Colitis. 2021;15:914–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olivera PA, Lasa JS, Bonovas S, et al. . Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2020;158:1554–1573.e12. [DOI] [PubMed] [Google Scholar]
- 14. Curtis JR, Xie F, Yun H, et al. . Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1843–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13:320. [DOI] [PubMed] [Google Scholar]
- 16. Conaghan PG, Mysler E, Tanaka Y, et al. . Upadacitinib in rheumatoid arthritis: a benefit-risk assessment across a phase III program. Drug Saf. 2021;44:515–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winthrop K, Buch MH, Curtis J, et al. . POS0092 Herpes zoster in the filgotinib rheumatoid arthritis program (abstract). Ann Rheum Dis. 2021;80(Suppl 1):255.1255–256 (POS0092). [Google Scholar]
- 18. Liu GF, Wang J, Liu K, et al. . Confidence intervals for an exposure adjusted incidence rate difference with applications to clinical trials. Stat Med. 2006;25:1275–1286. [DOI] [PubMed] [Google Scholar]
- 19. Seward JF, Watson BM, Peterson CL, et al. . Varicella disease after introduction of varicella vaccine in the United States, 1995-2000. JAMA. 2002;287:606–611. [DOI] [PubMed] [Google Scholar]
- 20. US Food and Drug Administration. SHINGRIX: highlights of prescribing information. Accessed February 9, 2022. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Shingrix/pdf/SHINGRIX.PDF
- 21. Rahier JF, Magro F, Abreu C, et al. . Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014;8:443–468. [DOI] [PubMed] [Google Scholar]
- 22. Schmader K. Herpes zoster in the elderly: issues related to geriatrics. Clin Infect Dis. 1999;28:736–739. [DOI] [PubMed] [Google Scholar]
- 23. Khan N, Patel D, Trivedi C, et al. . Overall and comparative risk of herpes zoster with pharmacotherapy for inflammatory bowel diseases: a nationwide cohort study. Clin Gastroenterol Hepatol. 2018;16:1919–1927.e3. [DOI] [PubMed] [Google Scholar]
- 24. Winthrop KL, Yamanaka H, Valdez H, et al. . Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2675–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Winthrop KL, Lebwohl M, Cohen AD, et al. . Herpes zoster in psoriasis patients treated with tofacitinib. J Am Acad Dermatol. 2017;77:302–309. [DOI] [PubMed] [Google Scholar]
- 26. Cohen SB, Tanaka Y, Mariette X, et al. . Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020;6:e001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Curran D, Callegaro A, Fahrbach K, et al. . Meta-regression of herpes zoster incidence worldwide. Infect Dis Ther. 2022;11:389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Forbes HJ, Bhaskaran K, Thomas SL, et al. . Quantification of risk factors for herpes zoster: population based case-control study. BMJ. 2014;348:g2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dong J, Chen Y, Tang Y, et al. . Body mass index is associated with inflammatory bowel disease: a systematic review and meta-analysis. PLoS One. 2015;10:e0144872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandborn WJ, Peyrin-Biroulet L, Sharara AI, et al. . Efficacy and safety of tofacitinib in ulcerative colitis based on prior tumor necrosis factor inhibitor failure status. Clin Gastroenterol Hepatol. 2022;20:591–601.e8. [DOI] [PubMed] [Google Scholar]
- 31. Winthrop KL, Harigai M, Genovese MC, et al. . Infections in baricitinib clinical trials for patients with active rheumatoid arthritis. Ann Rheum Dis. 2020;79:1290–1297. [DOI] [PubMed] [Google Scholar]
- 32. Cohen SB, van Vollenhoven RF, Winthrop KL, et al. . Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis. 2021;80:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rawlings JS, Rosler KM, Harrison DA.. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283. [DOI] [PubMed] [Google Scholar]
- 34. Gotthardt D, Trifinopoulos J, Sexl V, et al. . JAK/STAT cytokine signaling at the crossroad of NK cell development and maturation. Front Immunol. 2019;10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parampalli Yajnanarayana S, Stübig T, Cornez I, et al. . JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. Br J Haematol. 2015;169:824–833. [DOI] [PubMed] [Google Scholar]
- 36. Schönberg K, Rudolph J, Vonnahme M, et al. . JAK inhibition impairs NK cell function in myeloproliferative neoplasms. Cancer Res. 2015;75:2187–2199. [DOI] [PubMed] [Google Scholar]
- 37. Verweij MC, Wellish M, Whitmer T, et al. . Varicella viruses inhibit interferon-stimulated JAK-STAT signaling through multiple mechanisms. PLoS Pathog. 2015;11:e1004901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zerboni L, Sen N, Oliver SL, et al. . Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rubin DT, Modesto I, Vermeire S, et al. . Worldwide post-marketing safety surveillance experience with tofacitinib in ulcerative colitis. Aliment Pharmacol Ther. 2021;55:302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Centers for Disease Control and Prevention. Varicella vaccine information for healthcare professionals. Accessed May 14, 2021. https://www.cdc.gov/vaccines/vpd/varicella/hcp/index.html
- 41. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Grading of Recommendations, Assessment, Development, and Evaluation (GRADE): recombinant zoster vaccine (RZV) and herpes zoster live-attenuated vaccine (ZVL). Accessed May 14, 2021. https://www.cdc.gov/vaccines/acip/recs/grade/herpes-zoster.html
- 42. Winthrop KL, Wouters AG, Choy EH, et al. . The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase II trial. Arthritis Rheumatol. 2017;69:1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Winthrop KL, Wouters A, Choy EH, et al. . Long-term effectiveness of live herpes zoster vaccine in patients with rheumatoid arthritis subsequently treated with tofacitinib. Ann Rheum Dis. 2020;79:669–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. US Food and Drug Administration. XELJANZ® (tofacitinib): highlights of prescribing information. Accessed October 26, 2021. http://labeling.pfizer.com/ShowLabeling.aspx?id=959
- 45. Centers for Disease Control and Prevention. Zostavax (zoster vaccine live) recommendations. Accessed August 3, 2021. https://www.cdc.gov/vaccines/vpd/shingles/hcp/zostavax/recommendations.html
- 46. Cunningham AL. The herpes zoster subunit vaccine. Expert Opin Biol Ther. 2016;16:265–271. [DOI] [PubMed] [Google Scholar]
- 47. ClinicalTrials.gov. Shingrix vaccine in patients with moderate to severe ulcerative colitis on tofacitinib. Accessed July 30, 2021. https://clinicaltrials.gov/ct2/show/NCT03591770
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.