Abstract
It has long been known that certain antibiotics, at subinhibitory concentrations, differentially inhibit the synthesis of α-hemolysin and other staphylococcal virulence factors. In this report, we show that subinhibitory clindamycin (SBCL) eliminates production of nearly all exoproteins by Staphylococcus aureus but has virtually no effect on cytoplasmic proteins. The effect was abolished by a gene conferring resistance to macrolides-lincosamides-streptogramin B, showing that differential inhibition of protein synthesis is responsible; remarkably, however, subinhibitory clindamycin blocked production of several of the individual exoprotein genes, including spa (encoding protein A), hla (encoding α-hemolysin), and spr (encoding serine protease), at the level of transcription, suggesting that the primary effect must be differential inhibition of the synthesis of one or more regulatory proteins. In contrast to earlier reports, however, we found that subinhibitory clindamycin stimulates synthesis of coagulase and fibronectin binding protein B, also at the level of transcription. agr and sar expression was minimally affected by subinhibitory clindamycin. These effects varied from strain to strain and do not seem to be responsible for the effects of subinhibitory clindamycin on the overall exoprotein pattern.
The pathogenicity of Staphylococcus aureus, like that of other gram-positive bacteria, depends largely on extracellular virulence factors, including both secreted and surface proteins. These factors may be regarded as accessory gene products, constituting a subset of proteins that are not required for growth and cell division under ordinary conditions but that enable the organism to adapt to special environmental conditions, including various types of stress as well as the presence of particular nutritional substrates. On one hand, the production of virulence factors and other accessory gene products is carefully regulated, so that the various genes are expressed in response to explicit environmental contingencies. This regulation is accomplished by a network of interlocking regulatory gene functions, often involving signal transduction pathways that monitor the local environment. On the other hand, the synthesis of these products is differentially suppressed by certain antibiotics at low concentrations that have little if any effect on overall growth. This suppression has been reported to include secreted proteins such as α- and δ-hemolysins, DNase, lipase, coagulase, and toxic shock syndrome toxin 1 (TSST-1) (6, 13). Such effects have also been seen with Streptococcus pyogenes and Bacteroides fragilis, as well as with staphylococci (9, 10, 37). Similar though less fully documented effects are seen in response to the limitation of essential amino acids (18). We note that suppression of exoprotein synthesis is seen only with antibiotics that block protein synthesis; those that affect the cell envelope, such as β-lactams and glycopeptides, have a stimulatory effect on the synthesis of most exoproteins (11, 13, 30).
It is interesting that this decrease of exoprotein expression causes a significant attenuation of virulence. For example, the decrease of protein A on the cell surface of S. aureus resulted in a greater number of free receptor sites for complement C3b and an increase in phagocytosis (7, 8, 22). Reduction of adherence to bone surfaces has also been observed (21), and pretreatment with subinhibitory clindamycin (SBCL) or lincomycin caused a significant reduction in the severity of skin lesions in a murine subcutaneous abscess model (5, 35). Though it may seem strange that SBCL would attenuate virulence, one should view the virulence factors as a subset of extracellular proteins; perhaps suppression of exoprotein production by low concentrations of antibiotics could be an adaptive response that evolved in relation to soil ecology long before the development of antibiotics for clinical use. Moreover, the environmental concentration of any antibiotic would have been much lower than that currently encountered by the bacteria in a typical clinical setting. In our view, the effects of low concentrations of antibiotics such as clindamycin are likely to reflect important principles governing the regulation of genes encoding extracellular proteins. Accordingly, we have begun to analyze the molecular basis of the response of S. aureus to SBCL. We find first that with a few exceptions, SBCL essentially eliminates the production of secreted staphylococcal proteins and stimulates the production of certain surface proteins but has very little effect on the production of cytoplasmic proteins. The effect is eliminated by a standard macrolide-lincosamide-streptogramin B (MLS) resistance gene, indicating that the basic biological activity of clindamycin, inhibition of protein synthesis, is responsible for the observed effects. Remarkably, however, the individual exoprotein genes are regulated at the transcriptional level. This suggests that the effects are mediated through one or more regulatory factors rather than by direct translational regulation of individual exoproteins.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. NCTC8325 is the propagating strain for phage 47 of the international phage typing set. It is a wild-type agr+ strain with a naturally occurring 11-nucleotide deletion in rsbU (14). RN12 is a derivative of NCTC8325 with a silent insertion of Tn551, conferring erythromycin resistance (26). RN9203 and RN9204 were obtained by phage transduction (80α or ø11) of plasmids pRN6832 (Phla::blaZ) and pRN6676 (Ptst::blaZ) to NCTC8325. In these plasmids, the α-hemolysin (hla) and TSST-1 (tst) promoters are transcriptionally fused to the promoterless β-lactamase (bla) gene in the vector plasmid pSA3800 (13). RN9202 is NCTC8325 carrying pRN6683 (agrP3::blaZ), where the accessory gene regulator (agr) P3 promoter, driving rnaiii, is fused to the β-lactamase gene (29). RN6911 is an agr-null strain, with a 3.3-kb deletion of the agr locus, replaced by a tetracycline resistance gene, tetM (29). RN9211 is RN6911 carrying pRN6788 (Pspa::blaZ), where the protein A (spa) promoter is fused to the β-lactamase gene (13). RN9398 (agr null) was obtained by transduction of the agr knockout from RN6911 into NCTC8325, using 80α. RN9205 is NCTC8325 containing staphylococcal pathogenicity island 1 (SaPl1) and expressing tst, constructed by phage transduction (80α) (19). COL and WCUH29 (24) are clinical S. aureus isolates. COL is a prototypical methicillin-resistant S. aureus (MRSA) strain whose genome is currently being sequenced and analyzed (www.tigr.org). The genes and proteins involved in this work are described in Table 2.
TABLE 1.
Bacterial strains and plasmids used in this work.
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| S. aureus strains | ||
| NCTC8325 | Wild-type agr+ strain | Propagating strain for phage 47a |
| WCUH29 | Clinical strain | 24 |
| COL | MRSA strain | www.tigr.org |
| RN12 | NCTC8325 with silent insertion of Tn551, conferring erythromycin resistance | 27 |
| RN6911 | Derivative of RN6390B with tetM replacing agr | 29 |
| RN0024 | NCTC8325 containing pRN3038 | 27 |
| RN9205 | NCTC8325 (SaPl1) | This work |
| RN9398 | NCTC8325 with tetM replacing agr | This work |
| RN9202 (agrP3::blaZ) | NCT8325(pRN6683) | This work |
| RN9203 (Phla::blaZ) | NCTC8325(pRN6832) | This work |
| RN9204 (Ptst::blaZ) | NCTC8325(pRN6676) | This work |
| RN9211 (Pspa::blaZ) | RN6911(pRN6788) | This work |
| Plasmids | ||
| pRN3038 | pl258 blal443 (constitutive for β-lactamase) | 27 |
| pRN6676 | pSA3800 containing the tst promoter transcriptionally fused to blaZ | 14 |
| pRN6683 | pSA3800 containing the agr P3 promoter transcriptionally fused to blaZ | 29 |
| pRN6788 | pSA3800 containing the spa promoter transcriptionally fused to blaZ | 14 |
| pRN6832 | pWN2019 containing the hla promoter transcriptionally fused to blaZ | 14 |
Obtained from Central Public Health Laboratory, Colindale, London, United Kingdom.
TABLE 2.
Genes and proteins used in this work
| Gene or protein | Description |
|---|---|
| agr | Multigene global regulator of exoprotein production, consisting of a two-component signal transduction module and an autoinducing peptide module, all driven by promoter P2, and a regulatory RNA, RNAIII, that controls transcription of the exoprotein genes and is driven by a divergent promoter, P3 |
| bla | β-Lactamase determinant whose promoter, Pbla, is inducible and is used to drive expression of cloned genes, and whose structural gene, blaZ, is used as a reporter gene in expression vectors |
| coa | Encodes staphylococcal coagulase, expressed during early exponential phase and probably not regulated by agr |
| DNase | The classical staphylococcal nuclease, studied in depth by Shortle and colleagues and by Anfinsen and colleagues; regulated minimally, if at all, by agr |
| fnbB | Encodes one of two staphylococcal fibronectin binding proteins; regulated similarly to spa |
| geh | Encodes glycerol ester hydrolase, generally referred to as lipase; regulated by agr to a very limited extent |
| hla | Encodes α-hemolysin, a pore-forming cytotoxin, lethal for mice in the low-μg/kg range; expressed postexponentially and regulated at both transcriptional and translational levels by agr |
| hld | Encodes δ-hemolysin, an amphipathic 26-amino-acid hemolytic peptide encoded by agr RNAIII |
| Lysostaphin | Staphylolytic enzyme used for the preparation of staphylococcal lysates |
| MLS | Macrolides-lincosamides-streptogramin B. Resistance to these three groups of antibiotics is determined by ribosome methylation, which prevents the drugs from binding to ribosomes. |
| RsbU | Homolog of the anti-anti-ςB factor of the same name in Bacillus subtilis |
| sar | Regulatory locus encoding a transcription factor, SarA, that acts on many staphylococcal genes including agr |
| SaPl1 | A 15-kb phage-related pathogenicity island that carries the gene (tst) for TSST-1 and other superantigen genes |
| spa | Encodes staphylococcal protein A, a ubiquitous surface protein that is down-regulated by agr during the exponential phase of growth |
| spr | Encodes a classical staphylococcal serine protease (V8); regulated similarly to hla |
Stock cultures were maintained in CYGP broth (25) at −70°C and cultivated overnight on GL agar (23), supplemented with antibiotics as required, for use in growth studies. In all flask cultures, CYGP broth was used without glucose, to eliminate the effects of catabolite repression on exoprotein synthesis. Thirty milliliters of CYGP or CYGP with subinhibitory clindamycin hydrochloride (0.02 or 0.04 μg/ml; Sigma) in 300-ml side arm flasks was inoculated with S. aureus to a density of ∼3 × 107 cells/ml and incubated with shaking (240 rpm) at 37°C. Growth was monitored turbidimetrically with a Klett-Summerson photoelectric colorimeter read at 540 nm. A Klett reading of 100 is equivalent to 3 × 108 cells/ml. Cultures were sampled at hourly intervals from 0 to 6 h (referred to as T = 0 to T = 6).
Protein analysis.
Supernatants obtained by centrifugation of postexponential cultures (T = 5 and T = 6) were analyzed for exoproteins. Bacteria obtained by centrifugation of late exponential cultures (T = 3 and T = 4) were used for the analysis of cellular proteins. Cell pellets were washed with Tris-EDTA buffer and lysed with lysostaphin. Samples were normalized to constant cell density and then prepared for analysis by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis as described by Laemmli (17), using SDS–12% polyacrylamide gels. Gels were stained with Coomassie brilliant blue and photographed. Coagulase was assayed by the standards slide agglutination method. β-Lactamase activity was determined as described by O'Callaghan et al. (29a), modified for use in a microtiter plate reader (12). Samples were normalized to constant cell density unless otherwise specified.
Whole-cell lysates and Northern blotting.
Bacteria were collected at the indicated time points (T = 0 to T = 6), and whole-cell lysates were prepared as described by Kornblum et al. (15). Cultures were centrifuged, fixed in acetone-ethanol (1:1), and washed in N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES)-sucrose buffer. Equalized cell samples were incubated on ice for 30 min with lysostaphin (150 μg/ml) in TES-sucrose buffer (20% [wt/vol] sucrose, 20 mM Tris [pH 7.6], 10 mM EDTA, 50 mM NaCl) and shaken for 1 h with proteinase K (50 μg/ml; Sigma) and 2% SDS at 4°C. For Northern blotting (40), the same amount of cell lysate (10 μl) was electrophoresed through a 0.66 M formaldehyde–1% agarose gel in MOPS (morpholinepropanesulfonic acid) buffer (20). Nucleic acids were transferred to a nitrocellulose membrane (Amersham) with a VacuGene apparatus (Pharmacia) in 20× SSC (3 M NaCl, 0.3 M sodium citrate [pH 7]) and fixed under UV light. The membrane was preincubated for 2 h at 52°C in 2× Denhardt's solution (0.02% bovine serum albumin, 0.02% Ficoll, 0.02% polyvinylpyrrolidone, 0.05 M EDTA [pH 8], 0.2% SDS, 5× SSC, with sonicated and heat-denatured salmon sperm DNA [100 μg/ml]) and 10% (wt/vol) dextran (Sigma) and then hybridized overnight with a 32P-labeled DNA probe in hybridization solution. The 32P-labeled DNA probes were prepared by PCR with gene-specific primers (Table 3). The blot was exposed to a storage phosphor screen (Molecular Dynamics) and quantitated with ImageQuant or NIH Image software. Quantitative values for NCTC8325 were normalized to corresponding values for 16S rRNA, obtained using a 16S-specific PCR product as the probe.
TABLE 3.
PCR primers used in this study
| Primer | Sequence | Locus | Coordinates |
|---|---|---|---|
| RNAIII | 5′ CTGAGTCCTAGGAAACTAACTC 3′ | SAAGRAB | 1260–1281 |
| 5′ ATGATCACAGAGATGTGA 3′ | 1571–1554 | ||
| RNAII | 5′ TGAATTATTTTGATAATAAAATTG 3′ | SAAGRAB | 1777–1800 |
| 5′ TTTAAGTCCTCCTTAATAAAGAAAATAG 3′ | 2341–2314 | ||
| sar | 5′ GAGTTGTTATCAATGGTCACTTATGCTG 3′ | SAU20782 | 49–76 |
| 5′ GTGATTCGTTTATTTACTCGACTC 3′ | 365–342 | ||
| hla | 5′ TTAGCCTGGCCTTCAGCC 3′ | SATOXA | 640–657 |
| 5′ TGCCATATACCGGGTTC 3′ | 985–969 | ||
| fnbB | 5′ CCGAAAACTGTGCAAGCACC 3′ | SAFNBB | 805–824 |
| 5′ CTCCAATTATTTCTCCTGTCGCC 3′ | 1273–1251 | ||
| tst | 5′ ATCGTAAGCCCTTTGTTG 3′ | U93688 | 2147–2164 |
| 5′ TGGATATAAGTTCCTTCGC 3′ | 2481–2463 | ||
| spa | 5′ GGCACTACTGCTGACAAAATTGCTGCAG 3′ | STASPAA | 1988–2015 |
| 5′ GTTCGCGACGACGTCCAGCTAATAACGCTGC 3′ | 2219–2189 | ||
| coa | 5′AGAAGGTCTTGAAGGTAGC 3′ | SACOA | 1364–1382 |
| 5′ GAATCTTGGTCTCGCTTC 3′ | 1616–1599 | ||
| spr | 5′ GACAACAGCGACACTTG 3′ | SASP | 401–417 |
| 5′ CTGAATTACCACCAGTTG 3′ | 1065–1048 | ||
| 16S | 5′ GGTGAGTAACACGTGGATAA 3′ | X68417 | 111–130 |
| 5′ ATGTCAAGATTTGGTAAGGTT 3′ | 1006–986 |
RESULTS
SBCL affects the synthesis of exoproteins but not cytoplasmic proteins.
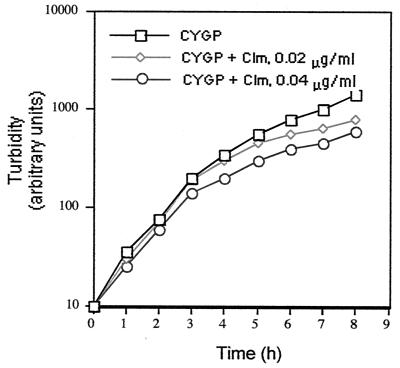
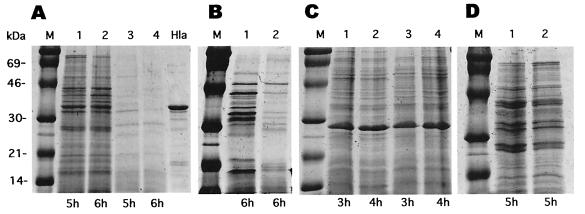
Although many antibiotics that inhibit protein synthesis have been observed to suppress the synthesis of virulence factors at subinhibitory concentrations, we have studied primarily the effects of clindamycin, because it seems to have the greatest effect (29; unpublished data) and because its effects were seen at concentrations that had little or no effect on growth. A general picture of the effects of SBCL is seen in Fig. 1 and 2. Clindamycin at a concentration of 0.02 μg/ml had a very slight effect (<5%) on the exponential doubling time of NCTC8325 and some of its derivatives (Fig. 1 and 3D), for others, there was no effect (not shown). Additionally, all of the strains tested multiplied and formed colonies of normal size on agar containing clindamycin at 0.02 or 0.04 μg/ml. This is typical of results obtained by other investigators (33). Figure 2A shows the exoprotein profiles of a postexponential culture of NCTC8325 after growth in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of clindamycin at a concentration of 0.02 μg/ml. As can be seen, there was a dramatic inhibition by SBCL of general exoprotein synthesis, in general agreement with earlier reports on the effects of SBCL on specific exoproteins, including α- and δ-hemolysins, lipase, DNase, and TSST-1 (4, 13, 30, 32, 34). Given that NCTC8325 has a deletion in rsbU (16), which encodes an anti-anti-ςB factor that could affect the clindamycin response (S. Herbert, B. Peter, and R. P. Novick, unpublished data; see below), we analyzed two rsbU+ clinical S. aureus isolates, WCUH29 and the MRSA strain COL, as well as NCTC8325. Similar, though less dramatic, results were obtained with both WCUH29 (Fig. 2B) and COL (data not shown). An example of an exoprotein whose synthesis is not blocked by SBCL is β-lactamase (Fig. 3D; see below). This is consistent with the possibility that the effects of SBCL are mediated through specific pleiotropic regulatory proteins (see below), since β-lactamase is regulated independently of other extracellular proteins.
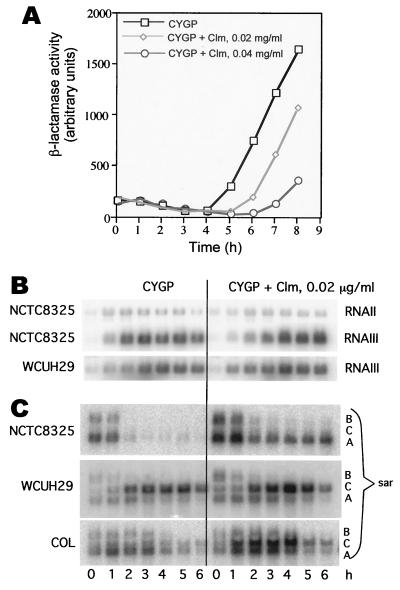
FIG. 1.
Effect of SBCL on growth of NCTC8325. Clm, clindamycin.
FIG. 2.
Effect of SBCL on exocellular and cellular protein expression. S. aureus strains NCTC8325 (A and C) and WCUH29 (B) were incubated with CYGP (A, lanes 1 and 2; B, lane 1; C, lanes 1 and 2) and CYGP with SBCL (0.02 μg/ml) (A, lanes 3 and 4; B, lane 2; C, lanes 3 and 4). M, molecular size markers; Hla, α-hemolysin (1 μg, 33 kDa). (A) Exoproteins in NCTC8325 culture supernatants; (B) exoproteins in WCUH29 culture supernatants; (C) cellular (cytoplasmic and membrane-bound) proteins of NCTC8325. (D) Effect of MLS resistance. RN12 was grown in CYGP with (lane 2) or without (lane 1) SBCL (0.02 μg/ml). Supernatants from 5-h cultures were analyzed as above.
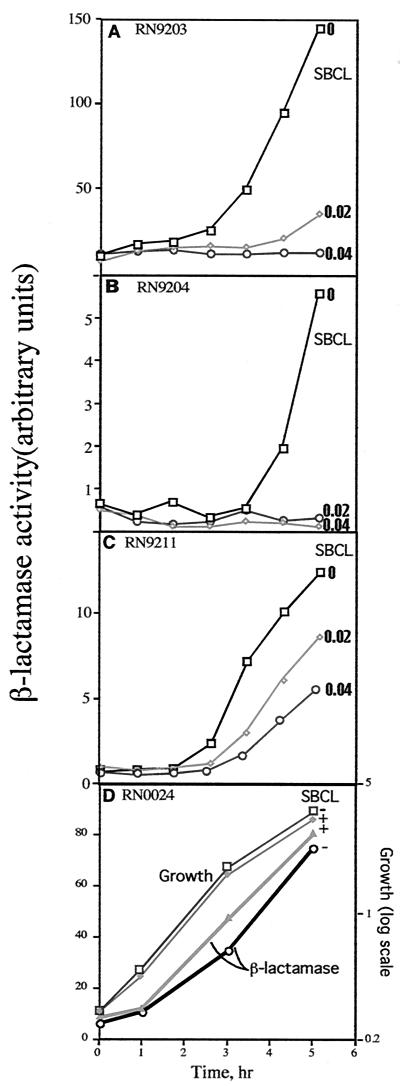
FIG. 3.
Effect of SBCL on exoprotein gene transcription (gene fusion analysis). Cultures of strains containing plasmid-carried exoprotein gene promoter-blaZ fusions were grown with or without SBCL and assayed for β-lactamase activity at hourly intervals. (A) Phla-blaZ (RN9203); (B) Ptst-blaZ (RN9204); (C) Pspa-blaZ (RN9211). (D) Strain RN0024, containing plasmid pRN3038, a derivative of pl258 that produces β-lactamase constitutively. Here, the absolute β-lactamase values were plotted (linear scale), not normalized to cell density, in comparison to growth of the culture (semilog scale).
In striking contrast to these results, we find that the profiles of cellular (cytoplasmic and membrane-bound) proteins with or without clindamycin are virtually indistinguishable (Fig. 2C, lanes 3 and 4 versus lanes 1 and 2), with the exception of two protein bands that were increased in the presence of SBCL (see below). This is consistent with the observed normal growth of the bacteria. Thus, it is clear that SBCL has a dramatic differential effect on the synthesis of secreted versus cellular proteins. For reasons described below, we predict that there is at least one cytoplasmic protein whose synthesis is inhibited by SBCL. Current experiments are directed toward the identification of such proteins.
SBCL acts by inhibiting protein synthesis.
Because the differential effect of clindamycin on extracellular proteins was so striking, it was important to determine whether the effect was a consequence of the standard activity of clindamycin or of inhibition of protein synthesis by binding to the 50S ribosomal subunit or was a manifestation of another, novel activity of the antibiotic. To test this, we used strain RN12, which has a silent insertion of Tn551, carrying a classical MLS resistance determinant, in strain NCTC8325, comparing the exoprotein profiles in the presence and absence of SBCL. As shown in Fig. 2D, the profiles are indistinguishable, indicating that Tn551 eliminates the effect of SBCL on exoprotein production, thus demonstrating that its effect on sensitive strains is, in fact, a consequence of protein synthesis inhibition. To evaluate the specificity of the Tn551 effect, we tested derivatives of NCTC8325 resistant to chloramphenicol and tetracycline, which also inhibit protein synthesis. We found that these strains are as sensitive to SBCL as NCTC8325 (data not shown), indicating that MLS resistance specifically abolishes the clindamycin effect.
SBCL inhibits exoprotein gene transcription.
In principle, clindamycin could affect the synthesis, secretion, or stability of the exoproteins. The most direct way to determine the mechanism seemed to be to test for inhibition of transcription, since if transcription were blocked, the other possibilities would automatically be eliminated. For this test, we used transcriptional fusions to β-lactamase, at clindamycin concentrations of 0.02 μg/ml and sometimes 0.04 μg/ml, and Northern blot hybridization analysis of the transcripts at a clindamycin concentration of 0.02 μg/ml. As spa is expressed very poorly in NCTC8325, owing to its down-regulation by agr (40), we also used agr-null derivatives, RN9211 and RN9398, for the analysis of spa. The Phla and Ptst β-lactamase fusions (14) in strain NCTC8325 were dramatically inhibited by SBCL at either concentration (Fig. 3A and B), whereas SBCL had no effect on the synthesis of β-lactamase encoded by a constitutive mutant of plasmid p1258 (Fig. 3D). The Pspa β-lactamase fusion in RN9211 was also inhibited, but only partially (Fig. 3C).
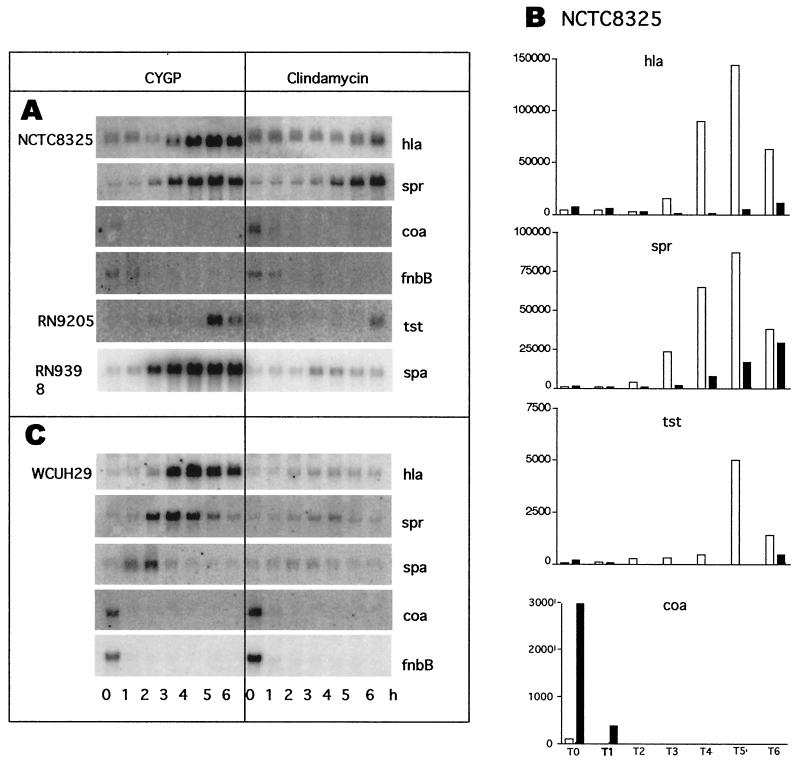
To confirm the promoter fusion results, we measured the gene-specific transcripts by Northern blot hybridization. To broaden the study, we included WCUH29 and three additional genes, coa, fnbB, and spr. Figure 4A shows Northern blot analysis of whole-cell RNAs prepared from NCTC8325 and probed for hla, spr, coa, and fnbB and from RN9205 and probed for tst. In Fig. 4B is a graphical representation of these results after normalization to 16S rRNA, which was detected on the same blots by hybridization with a 16S-specific probe (not shown). Also shown are Northern blotting patterns for RNA isolated from the isogenic agr-null strain, RN9398, and probed for spa (Fig. 4A) and for WCUH29 RNA probed for hla, spr, spa, coa, and fbnB (Fig. 4C). In agreement with the results of the promoter activity tests, SBCL (0.02 μg/ml) sharply decreased hla and tst mRNA transcripts in NCTC8325. However, the effects of clindamycin on the spa transcript (Fig. 4A) seem considerably stronger than its effects on the Pspa β-lactamase fusion (Fig. 3), whereas the inhibition of tst transcription was greater as measured by the Ptst β-lactamase fusion (Fig. 3B) than by Northern blotting (Fig. 4A). We note that the Northern blotting pattern with a spa probe was somewhat different for RN9398 than for other agr-null strains such as RN6911, in that the spa transcript level remained high throughout the postexponential phase for RN9398, whereas it decreased considerably for the others (not shown). There was no difference between the two strains in their response to SBCL. We note also that the clindamycin inhibition of hla and spr transcription was greater in WCUH29 than in NCTC8325 (Fig. 4A and B). This is especially striking for spr, whose expression was delayed rather than blocked by clindamycin in NCTC8325.
FIG. 4.
Effect of SBCL on exoprotein gene transcription as analyzed by Northern blot hybridization. Cultures were grown in CYGP with or without SBCL (0.02 μg/ml) and sampled hourly for analysis. (A) NCTC8325 and derivatives; (B) graphs of data from panel A, normalized to 16S RNA; (C) WCUH29.
Transcription of coa and fnbB seemed to be stimulated in NCTC8325 and WCUH29 by SBCL. The coa and fbnB transcription signals seemed stronger in WCUH29 than in NCTC8325, and we suggest that this and other differences observed between NCTC8325 derivatives and WCUH29 could be related to the rsbU deletion in the former (16). Because these results are inconsistent with earlier reports of inhibition of coagulase production and fibronectin binding by SBCL (4, 31), we determined coagulase activity in culture supernatants as well as transcription of coa. Since the coa mRNA signal was present only at the earliest time points, we measured coagulase activity in T1-h as well as in T5-h samples. In T1-h samples, activity was detected at a 1:32 dilution, whereas in the T5-h sample, activity was detected only in the undiluted supernatants. In neither case was there any detectable effect of SBCL. We suggest that the decrease in coagulase activity may be a consequence of increased protease activity late in growth. We have no explanation for the apparent disparity between coa transcription and coagulase activity. Note also that, as we show elsewhere (11), expression of both coa and fbnB is dependent on the alternative sigma factor, ςB, and it is known that coa has a ςB-dependent promoter (23). Moreover, considerable interstrain differences have been observed for these proteins. In any case, we suggest that the effects of SBCL seen in Fig. 2 involve transcription rather than protein degradation or secretion.
Since the primary mode of action of clindamycin is blockage of protein synthesis at the level of the ribosome, the blockage of exoprotein synthesis by SBCL would have been expected to be at this step. It is therefore especially significant that the low concentrations of clindamycin used in these experiments block transcription of the exoprotein genes rather than translation of their products. The possibility that the primary effect is translational and that the results shown in Fig. 4 represent mRNA destabilization seems unlikely on the basis of the stimulation of certain extracellular proteins and of the gene fusion results shown in Fig. 3.
Effects of SBCL on global regulators.
Although the observed effects of SBCL are clearly mediated by translational inhibition, which is the basis of the antibiotic activity of clindamycin, the results presented so far suggest that these effects are not at the level of direct interference with exoprotein translation. In other words, clindamycin must interfere with translation of one or more regulatory gene products that, in turn, affect transcription of the exoprotein genes. Accordingly, we have begun to address this question by determining the effects of SBCL on agr and sar, well-characterized global regulators of exoprotein synthesis in S. aureus. The agr locus specifies two transcripts, RNAII (2.3 kb) and RNAIII (514 nucleotides), of which the former determines activation of agr transcription (28) and the latter is the effector of agr regulation (29).
It is clear that agr cannot be the sole mediator of the clindamycin effect, since SBCL blocks transcription of genes such as hla that are up-regulated as well as genes, such as spa, that are reciprocally down-regulated by agr. As noted earlier (Fig. 3C and 4A), SBCL strongly inhibited spa transcription in the agr-null strain, confirming that its effects on spa are independent of agr. Nevertheless, it remained possible that SBCL could act through agr, at least on some of the exoprotein genes. Accordingly, we tested an agrP3-blaZ fusion in NCTC8325 for the effects of SBCL and also analyzed the agr transcription patterns directly by Northern blotting with agr-specific probes. As seen in Fig. 5A, the onset of β-lactamase synthesis is delayed by SBCL at either dose and is somewhat inhibited by the higher dose but not by the lower one. The Northern blotting patterns (0.02 μg of clindamycin/ml) (Fig. 5B) are fully consistent with the gene fusion results for NCTC8325. Note that there is a 1-h delay in both cases and that RNAIII synthesis is roughly parallel with and without clindamycin thereafter. This delay was not seen with WCUH29, and there was even a modest stimulation of RNAIII transcription at the early time points. One possible explanation for the apparent difference in strains is that NCTC8325 and its derivatives are mutated in rsbU, as noted. Since hla, spr, and tst transcription are inhibited at all time points in both strains, it is clear that the effects of SBCL on RNAIII cannot be responsible for the effects of the drug on transcription of these three genes.
FIG. 5.
Effect of SBCL on agr and sar expression. (A) Strain RN9202, containing a plasmid-carried agrP3-blaZ fusion, was grown in CYGP with or without SBCL at 0.2 or 0.4 μg/ml, and hourly samples were assayed for β-lactamase. (B) Clm, clindamycin. Strains NCTC8325 and WCUH29 were grown as above, with or without SBCL (0.02 μg/ml), and hourly samples were analyzed by Northern blotting for the two major agr transcripts, RNAII and RNAIII. (C) Strains NCTC8325, WCUH29, and COL were grown as above, and hourly samples were analyzed by Northern blotting for the three sar transcripts, using a probe specific for the 3′ end of the sar locus.
The effects of SBCL on transcription of sar in these same two strains and also in COL are shown in Fig. 5C. sar is transcribed from three tandem promoters, PsarA,, PsarB, and PsarC, producing three overlapping transcripts, all of which encode SarA, a transcriptional regulator (1). SBCL stimulated each of these promoters but did so differently in each of the three strains. The strongest effects were on PsarA in NCTC8325 and in WCUH29 and on PsarC in COL and in WCUH29. These effects are difficult to interpret since the roles of the three sar promoters in the production of SarA have not been clearly defined. Since sar strongly enhances the expression of agr RNAIII (3) and therefore contributes significantly to the agr response, it is clear that the general effects of SBCL on exoprotein synthesis cannot be mediated through the effects of sar on agr. Nevertheless, sar has effects on several exoprotein genes independently of agr (2, 41), and it is conceivable that SBCL could act partly through sar in some cases.
DISCUSSION
It has long been known that the synthesis of many staphylococcal exoproteins, including virulence factors, is inhibited by subinhibitory concentrations of antibiotics whose mode of action is to block protein synthesis. The clindamycin effect has been observed for extracellular proteins in a variety of organisms, including streptolysin S and M protein production by Streptococcus pyogenes (9, 37) and extracellular lipase production by Propionibacterium acnes and P. granulosum (39). Similar effects have been observed for tetracycline and aminoglycosides, which down-regulate protease production by Pseudomonas aeruginosa (36) and hemolysin production by Escherichia coli (38). In some cases, disparate results have been reported by different investigators, which may well represent interstrain differences. In any case, we suggest that the observed effects of subinhibitory antibiotics have profound implications for the overall regulatory strategies that are used by bacteria in their interactions with the environment, and we have begun to study the phenomenon with this idea in mind.
In this report, we have confirmed that the synthesis of many exoproteins is inhibited by SBCL. We have shown that the effects of SBCL on exoprotein synthesis are manifested as effects on transcription of the exoprotein genes. This is puzzling since the standard effect of clindamycin is blockage of protein translation at the level of the ribosome. Moreover, the effects on exoprotein gene transcription were abolished by a standard MLS resistance determinant. The suggested explanation of this seeming paradox is that clindamycin specifically interferes with translation of one or more proteins that regulate transcription of the exoprotein genes. For this to be true, translation of these regulatory proteins would have to be differentially sensitive to SBCL. The basis of such differential sensitivity would be an important subject for further study. An alternative possibility is that SBCL differentially blocks transcriptionally coupled translation of proteins that use (membrane-bound) ribosomes dedicated to exoprotein synthesis. This possibility is ruled out by gene fusion experiments using β-lactamase as a reporter, since β-lactamase is an extracellular protein whose synthesis is not blocked by SBCL.
We have shown here that agr cannot be responsible for the clindamycin effect, although sar could conceivably have a role with respect to certain exoproteins. We show elsewhere that SBCL up-regulates the ςB operon (Herbert et al., unpublished), which is clearly important for the observed effects of the drug on exoprotein synthesis.
Finally, it may be instructive to consider the biological context in which the effects of subinhibitory antibiotics could have a role. As has been noted, there are profound effects on virulence—as would be expected, since many virulence factors are extracellular proteins. However, it is suggested that the role of subinhibitory antibiotics must be viewed within the competitive environment where the production of antibiotics must have evolved, namely, the soil. Since antibiotic concentrations comparable to those used therapeutically are probably never encountered in the soil, one must assume that the evolution of antibiotics has been driven by forces based on very low environmental, i.e., subinhibitory, concentrations. Further, it is very difficult to imagine that inhibition of the production of exoproteins involved in virulence for metazoan hosts could have provided the evolutionary driving force. Rather, it would seem instructive to consider other types of exoproteins, namely, (i) enzymes that degrade macromolecules and thus enable the organism to obtain importable nutrients and (ii) bacteriocins and other antibacterials. It is possible that other types of secondary metabolism, such as the production of antibiotics, are also affected. Could it be that the development of antibiotics by soil organisms has been driven by the selective advantage gained from the inhibition by low antibiotic concentrations of the production by competitors of degradative enzymes, bacteriocins, or antibiotics?
ACKNOWLEDGMENTS
We thank Hope F. Ross for discussion and critical reading of the manuscript. We also thank D. McDevitt for S. aureus strain WCUH29.
This work was supported by National Institutes of Health grant RO1-AI30138 to R.P.N.
REFERENCES
- 1.Bayer M G, Heinrichs J H, Cheung A L. The molecular architecture of the sar locus in Staphylococcus aureus. J Bacteriol. 1996;178:4563–4570. doi: 10.1128/jb.178.15.4563-4570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blevins J S, Gillaspy A F, Rechtin T M, Hurlburt B K, Smeltzer M S. The staphylococcal accessory regulator (sar) represses transcription of the Staphylococcus aureus collagen adhesin gene (cna) in an agr-independent manner. Mol Microbiol. 1999;33:317–326. doi: 10.1046/j.1365-2958.1999.01475.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheung A L, Heinrichs J H, Bayer M G. Characterization of the sar locus and its interaction with agr in Staphylococcus aureus. J Bacteriol. 1996;178:418–423. doi: 10.1128/jb.178.2.418-423.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gemmel C G, Shibl A M A. The control of toxin and enzyme biosynthesis in staphylococci by antibiotics. In: Jeljaszewicz J, editor. Staphylococci and staphylococal diseases. Stuttgart, Germany: Gustav Fischer Verlag; 1976. pp. 657–664. [Google Scholar]
- 5.Gemmell C. Chemotherapy. 1978. Effect of subinhibitory concentrations of antibiotics on experimental pyogenic infections in mice; pp. 512–514. [Google Scholar]
- 6.Gemmell C G. Antibiotics and the expression of staphylococcal virulence. J Antimicrob Chemother. 1995;36:283–291. doi: 10.1093/jac/36.2.283. [DOI] [PubMed] [Google Scholar]
- 7.Gemmell C G. Clindamycin and its action on the susceptibility of pathogenic bacteria to phagocytosis. Scand J Infect Dis Suppl. 1984;43:17–23. [PubMed] [Google Scholar]
- 8.Gemmell C G, O'Dowd A. Regulation of protein A biosynthesis in Staphylococcus aureus by certain antibiotics: its effect on phagocytosis by leukocytes. J Antimicrob Chemother. 1983;12:587–597. doi: 10.1093/jac/12.6.587. [DOI] [PubMed] [Google Scholar]
- 9.Gemmell C G, Peterson P K, Schmeling D, Kim Y, Mathews J, Wannamaker L, Quie P G. Potentiation of opsonization and phagocytosis of Streptococcus pyogenes following growth in the presence of clindamycin. J Clin Investig. 1981;67:1249–1256. doi: 10.1172/JCI110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gemmell C G, Peterson P K, Schmeling D, Mathews J, Quie P G. Antibiotic-induced modification of Bacteroides fragilis and its susceptibility to phagocytosis by human polymorphonuclear leukocytes. Eur J Clin Microbiol. 1983;2:327–334. doi: 10.1007/BF02019462. [DOI] [PubMed] [Google Scholar]
- 11.Hallander H O, Laurell G, Lofstrom G. Stimulation of staphylococcal haemolysin production by low concentrations of penicillin. Acta Pathol Microbiol Scand. 1966;68:142–148. doi: 10.1111/apm.1966.68.1.142. [DOI] [PubMed] [Google Scholar]
- 12.Ji G, Beavis R, Novick R. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayasi A, Barnett J, Sanford J. Effect of antibiotics on the in vitro production of alpha hemolysin by Staphylococcus aureus. J Lab Clin Med. 1966;68:890. [Google Scholar]
- 14.Kornblum J, Kreiswirth B, Projan S J, Ross H, Novick R P. Agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 373–402. [Google Scholar]
- 15.Kornblum J S, Projan S J, Moghazeh S L, Novick R P. A rapid method to quantitate non-labeled RNA species in bacterial cells. Gene. 1988;63:75–85. doi: 10.1016/0378-1119(88)90547-1. [DOI] [PubMed] [Google Scholar]
- 16.Kullik I, Giachino P, Fuchs T. Deletion of the alternative sigma factor ςB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J Bacteriol. 1998;180:4814–4820. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Leboeuf-Trudeau T, deRepentigny J, Frenette R M, Sonea S. Tryptophan metabolism and toxin formation in S. aureus Wood 46 strain. Can J Microbiol. 1969;15:1–7. doi: 10.1139/m69-001. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay J A, Ruzin A, Ross H F, Kurepina N, Novick R P. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol. 1998;29:527–543. doi: 10.1046/j.1365-2958.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 20.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a Laboratory Manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 21.Mayberry-Carson K J, Mayberry W R, Tober-Meyer B K, Costerton J W, Lambe D W., Jr An electron microscopic study of the effect of clindamycin on adherence of Staphylococcus aureus to bone surfaces. Microbios. 1986;45:21–32. [PubMed] [Google Scholar]
- 22.Milatovic D, Braveny I, Verhoef J. Clindamycin enhances opsonization of Staphylococcus aureus. Antimicrob Agents Chemother. 1983;24:413–417. doi: 10.1128/aac.24.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazaki E, Chen J-M, Ko C, Bishai W. The Staphylococcus aureus rsbW (orf159) gene encodes an anti-sigma factor of SigB. J Bacteriol. 1999;181:2846–2851. doi: 10.1128/jb.181.9.2846-2851.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholas R O, Li T, McDevitt D, Marra A, Sucoloski S, Demarsh P L, Gentry D R. Isolation and characterization of a sigB deletion mutant of Staphylococcus aureus. Infect Immun. 1999;67:3667–3669. doi: 10.1128/iai.67.7.3667-3669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 26.Novick R P, Edelman I, Schwesinger M D, Gruss D, Swanson E C, Pattee P A. Genetic translocation in Staphylococcus aureus. Proc Natl Acad Sci USA. 1979;76:400–404. doi: 10.1073/pnas.76.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novick R P, Murphy E, Gryczan T J, Baron E, Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979;2:109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- 28.Novick R P, Projan S, Kornblum J, Ross H, Kreiswirth B, Moghazeh S. The agr P-2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol Gen Genet. 1995;248:446–458. doi: 10.1007/BF02191645. [DOI] [PubMed] [Google Scholar]
- 29.Novick R P, Ross H F, Projan S J, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.O'Callaghan C H, Morris A, Kirby S M, Shingler A H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972;1:283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohlsen K, Ziebuhr W, Koller K P, Hell W, Wichelhaus T A, Hacker J. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother. 1998;42:2817–2823. doi: 10.1128/aac.42.11.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proctor R A, Olbrantz P J, Mosher D F. Subinhibitory concentrations of antibiotics alter fibronectin binding to Staphylococcus aureus. Antimicrob Agents Chemother. 1983;24:823–826. doi: 10.1128/aac.24.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlievert P M, Kelly J A. Clindamycin-induced suppression of toxic-shock syndrome-associated exotoxin production. J Infect Dis. 1984;149:471. doi: 10.1093/infdis/149.3.471. [DOI] [PubMed] [Google Scholar]
- 33.Shibl A. Effect of antibiotics on enzyme and toxin production by S. aureus. Ph.D. dissertation. Glasgow, Scotland: Council for National Academic Awards; 1977. [Google Scholar]
- 34.Shibl A M. Effect of antibiotics on production of enzymes and toxins by microorganisms. Rev Infect Dis. 1983;5:865–875. doi: 10.1093/clinids/5.5.865. [DOI] [PubMed] [Google Scholar]
- 35.Shibl A M. Subcutaneous staphylococcal infections in mice: the influence of antibiotics on staphylococcal extracellular products. Chemotherapy. 1982;28:46–53. doi: 10.1159/000238059. [DOI] [PubMed] [Google Scholar]
- 36.Shibl A M, Al-Sowaygh I A. Antibiotic inhibition of protease production by Pseudomonas aeruginosa. J Med Microbiol. 1980;13:345–348. doi: 10.1099/00222615-13-2-345. [DOI] [PubMed] [Google Scholar]
- 37.Shibl A M, Al-Sowaygh I A. Differential inhibition of bacterial growth and hemolysin production by lincosamide antibiotics. J Bacteriol. 1979;137:1022–1023. doi: 10.1128/jb.137.2.1022-1023.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibl A M, Gemmell C G. Effect of four antibiotics on haemolysin production and adherence to human uroepithelial cells by Escherichia coli. J Med Microbiol. 1983;16:341–349. doi: 10.1099/00222615-16-3-341. [DOI] [PubMed] [Google Scholar]
- 39.Unkles S E, Gemmell C G. Effect of clindamycin, erythromycin, lincomycin, and tetracycline on growth and extracellular lipase production by propionibacteria in vitro. Antimicrob Agents Chemother. 1982;21:39–43. doi: 10.1128/aac.21.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandenesch F, Kornblum J, Novick R. A temporal signal, independent of agr, is required for hla but not for spa transcription in Staphylococcus aureus. J Bacteriol. 1991;173:4204–4209. doi: 10.1128/jb.173.20.6313-6320.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolz C, Pohlmann-Dietze P, Steinhuber A, Chien Y T, Manna A, van Wamel W, Cheung A. Agr-independent regulation of fibronectin-binding protein(s) by the regulatory locus sar in Staphylococcus aureus. Mol Microbiol. 2000;36:230–243. doi: 10.1046/j.1365-2958.2000.01853.x. [DOI] [PubMed] [Google Scholar]