Abstract
Background
Addition of temozolomide (TMZ) to radiotherapy (RT) improves overall survival (OS) in patients with glioblastoma (GBM), but previous studies suggest that patients with tumors harboring an unmethylated MGMT promoter derive minimal benefit. The aim of this open-label, phase III CheckMate 498 study was to evaluate the efficacy of nivolumab (NIVO) + RT compared with TMZ + RT in newly diagnosed GBM with unmethylated MGMT promoter.
Methods
Patients were randomized 1:1 to standard RT (60 Gy) + NIVO (240 mg every 2 weeks for eight cycles, then 480 mg every 4 weeks) or RT + TMZ (75 mg/m2 daily during RT and 150–200 mg/m2/day 5/28 days during maintenance). The primary endpoint was OS.
Results
A total of 560 patients were randomized, 280 to each arm. Median OS (mOS) was 13.4 months (95% CI, 12.6 to 14.3) with NIVO + RT and 14.9 months (95% CI, 13.3 to 16.1) with TMZ + RT (hazard ratio [HR], 1.31; 95% CI, 1.09 to 1.58; P = .0037). Median progression-free survival was 6.0 months (95% CI, 5.7 to 6.2) with NIVO + RT and 6.2 months (95% CI, 5.9 to 6.7) with TMZ + RT (HR, 1.38; 95% CI, 1.15 to 1.65). Response rates were 7.8% (9/116) with NIVO + RT and 7.2% (8/111) with TMZ + RT; grade 3/4 treatment-related adverse event (TRAE) rates were 21.9% and 25.1%, and any-grade serious TRAE rates were 17.3% and 7.6%, respectively.
Conclusions
The study did not meet the primary endpoint of improved OS; TMZ + RT demonstrated a longer mOS than NIVO + RT. No new safety signals were detected with NIVO in this study. The difference between the study treatment arms is consistent with the use of TMZ + RT as the standard of care for GBM.
ClinicalTrials.gov NCT02617589
Keywords: newly diagnosed glioblastoma, nivolumab, radiotherapy, temozolomide, unmethylated MGMT
Key Points.
NIVO did not improve survival in newly diagnosed GBM with unmethylated MGMT promoter.
No new safety signals were detected with NIVO + standard of care in this study.
Immunotherapy with NIVO is not a suitable replacement for chemotherapy with TMZ.
Importance of the Study.
Given the survival benefits of immunotherapy in cancer, it was hypothesized that it may also offer promise in difficult-to-treat cancers, such as glioblastoma (GBM). Worse outcomes are observed in patients with GBM with unmethylated versus methylated MGMT promoter. Temozolomide (TMZ), the standard chemotherapy, is associated with limited efficacy in unmethylated MGMT tumors. Here we report data from the largest phase III study in patients with GBMs and unmethylated MGMT promoter and the first prospective phase III study examining TMZ omission in this chemoresistant phenotype. Nivolumab + radiotherapy (NIVO + RT) showed a shorter survival benefit vs TMZ + RT, suggesting that NIVO is not a suitable replacement for TMZ. Results also suggest that in the absence of other treatment options, TMZ should continue to be the standard of care for all patients with GBM regardless of MGMT promoter status.
Glioblastoma (GBM), the most common primary malignant brain tumor, is associated with a dismal prognosis and poor quality of life.1–4 The mainstay of treatment for newly diagnosed disease is surgical resection followed by radiotherapy (RT) with concomitant and adjuvant temozolomide (TMZ).4–6 The benefit of this treatment was demonstrated in a phase III study, which showed improved overall survival (OS) from 12.1 months with RT alone to 14.6 months with TMZ chemoradiotherapy (hazard ratio [HR], 0.63; P < .001).5
Chemosensitivity to alkylating agents has been strongly linked to epigenetic silencing of the MGMT gene in various cancers.7,8 Methylation of the MGMT promoter results in decreased MGMT expression, which reduces DNA repair capacity and confers chemosensitivity.8–10 Analyses from the pivotal phase III study validating TMZ in GBM suggested that patients with tumors harboring a methylated MGMT promoter derived a survival benefit from TMZ + RT (median, 21.7 vs. 15.3 months), whereas patients with unmethylated MGMT promoter derived minimal and statistically insignificant benefit (median, 12.7 vs 11.8 months).8MGMT promoter methylation is also an independent prognostic factor in GBM; patients with a methylated MGMT promoter achieve significantly better outcomes.8,10,11 Given the lack of treatment alternatives, TMZ is offered to all patients with GBM, regardless of tumor MGMT promoter methylation status—with or without tumor-treating fields.4,6,12 Because of TMZ’s minimal benefit and known toxicities, reassessment of its role in GBM with unmethylated MGMT promoter remains of interest, and novel treatment alternatives are clearly needed for this patient population.13
Nivolumab (NIVO) is a fully human immunoglobulin G4 monoclonal antibody targeting the programmed cell death 1 protein (PD-1) immune checkpoint. NIVO has been shown to improve survival in multiple cancers, including melanoma, lung cancer, and renal cell carcinoma, and has demonstrated activity in brain metastasis from melanoma.14–16 Gliomas have been shown to express PD-1 ligand (PD-L1), and expression levels have been associated with tumor grade.17,18 Preclinical studies in GBM models suggest that efficacy of PD-1 inhibitors could be enhanced through combination with RT.19 RT may expose antigenic mutations, induce the expression of peptides that can activate T cells, and recruit antigen-presenting and immune effector cells to the tumor microenvironment (TME).20–22 Given the chemoresistance observed in tumors with unmethylated MGMT promoter, we conducted a phase III study to evaluate whether immunotherapy with NIVO could improve survival when combined with RT (NIVO + RT) compared with conventional chemoradiotherapy with TMZ + RT in this patient population.
Materials and Methods
Study Design and Participants
In this open-label, phase III study, patients were stratified by degree of tumor resection (complete vs. partial) at baseline and randomized 1:1 to receive NIVO + RT or TMZ + RT. In both arms, focal RT consisted of 60 Gy in 2-Gy fractions. In the NIVO + RT arm, RT was combined with NIVO 240 mg every 2 weeks for eight doses followed by NIVO 480 mg every 4 weeks until unacceptable toxicity or disease progression. In the TMZ + RT arm, RT was combined with the standard TMZ regimen, 75 mg/m2 once daily during RT (concomitant),23 followed by a 4-week treatment break and then adjuvant treatment with TMZ 150 to 200 mg/m2 once daily on days 1 to 5 of a 28-day cycle for ≤ 6 cycles (maintenance). The median dose and duration of RT was 60.0 Gy and 6.1 weeks in both arms, respectively. Per investigator’s discretion, patients receiving NIVO were permitted to continue treatment beyond suspected progression until confirmation of progression on follow-up MRI.
Eligible patients were aged ≥ 18 years and had newly diagnosed, histologically confirmed, supratentorial GBM with unmethylated MGMT promoter determined centrally by a methylation-specific polymerase chain reaction assay.24 Other key eligibility criteria included no prior treatment for GBM beyond surgery and Karnofsky Performance Scale (KPS) ≥ 70. At randomization, patients must have been receiving ≤ 20 mg prednisone or ≤ 3 mg dexamethasone (or equivalent). Patients were excluded if they had recurrent or secondary GBM; undergone biopsy only for GBM at surgery; tumors harboring IDH-1 or -2 mutation; unresolved CNS hemorrhage; metastatic extracranial or leptomeningeal disease; active, known, or suspected autoimmune disease; tumor-treating fields therapy (not a recommended treatment at time of study start); or used a biodegradable carmustine wafer.
Procedures
Tumor samples were assessed for MGMT promoter methylation status; testing was performed by Covance laboratory services. A sample was determined to be MGMT unmethylated when the ratio of the gene copy numbers of methylated MGMT to control (β-actin) × 1000 was < 2 and the gene copy numbers of MGMT and control were within the reportable range (β-actin ≥ 10 copies and MGMT ≥ 10 copies). Disease status was assessed using contrast-enhanced MRI at baseline and beginning 4 weeks (± 7 days) after RT completion. Then, disease status was evaluated every 8 weeks (± 7 days) until progression per Response Assessment in Neuro-Oncology (RANO) criteria.25 As detailed in the RANO criteria, classification of tumor progression during the first 12 weeks after completion of RT requires either that the new enhancement be located outside of the radiation field (beyond the high-dose region or the 80% isodense line) or unequivocal pathological confirmation of progressive disease. Confirmation was determined at a subsequent MRI performed within 8 weeks after the initial radiological assessment of progression. Theoretically, patients treated with immunotherapy may derive clinical benefit despite initial evidence of disease progression; therefore, patients in the NIVO + RT arm were allowed to continue NIVO in the setting of suspected progression at investigator discretion until progression was confirmed. Progression-free survival (PFS) was defined as time from randomization to documented progression or death from any cause. OS was defined as time from randomization to death from any cause. Tumor-sample sections for PD-L1 expression were retrospectively assessed centrally (LabCorp Clinical Trials, Research Triangle Park, NC, USA); PD-L1 positivity was defined as percentage of membranous staining of tumor cells with 1% and 5% cutoff values. Adverse events (AEs) were assessed continuously during the study per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Patient-reported outcomes (PROs) related to patients’ health-related quality of life (HRQoL) were assessed using the European Organisation for Research and Treatment of Cancer QLQ-C30 and EQ-5D-3L questionnaires, collected at baseline, week 11, and then every 8 weeks during treatment until disease progression. The time to deterioration in PRO score was assessed by time from randomization to first worsening of PRO score from baseline during treatment—meeting or exceeding the minimal change in responder definition threshold—without subsequent improvement based on the responder definition.26
Outcomes
The primary endpoint was OS. Secondary endpoints included investigator-assessed PFS based on RANO criteria and OS at 24 months using Kaplan-Meier methodology. Key exploratory endpoints included safety and tolerability, HRQoL, and efficacy based on tumor PD-L1 expression.
Treatment Beyond Suspected Progression
Patients in the NIVO + RT arm with evidence of progression in imaging findings were allowed to continue study therapy until disease progression was confirmed.
Statistical Analyses
OS, defined as the time between the date of randomization and the date of death due to any cause, was compared between treatment arms using a two-sided log-rank test stratified by extent of surgical resection (complete or partial). The final OS analysis was planned after follow-up of ≥ 23 months or when ≥ 390 deaths were reported, providing ≈ 90% power with an overall type I error of 0.05. At the time of the database lock, some patients had < 24 months of follow-up. However, given the number of events at the time of the database lock, it was considered that the number of patients with follow-up of < 24 months at the time of the current analysis would not have affected the data maturity or interpretability of the results. Kaplan-Meier methodology was used to estimate OS and PFS curves, medians with 95% CIs, and OS and PFS rates at fixed time points with 95% CIs. HRs and corresponding two-sided 95% CIs were estimated using a stratified Cox proportional hazards model. A stratified Cox proportional hazards regression model was used to estimate the HR between treatment groups. Baseline characteristics in all randomized patients and safety in all treated patients were assessed using descriptive statistics.
Study Oversight
The study was conducted in accordance with Good Clinical Practice guidelines per the International Conference on Harmonisation and with ethical principles of the European Union Directive and US Code of Federal Regulations. The study is registered at ClinicalTrials.gov (NCT02617589). The protocol was approved by an institutional review board or independent ethics committee at each site before study activation. All patients provided written informed consent in accordance with the Declaration of Helsinki.
Role of the Funding Source
The study was designed by the authors in collaboration with the funder (Bristol Myers Squibb). The authors and funder were responsible for data collection, and the funder was responsible for data analysis. The authors and funder were involved in data interpretation, development of the report, and the decision to submit. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Patients and Treatment
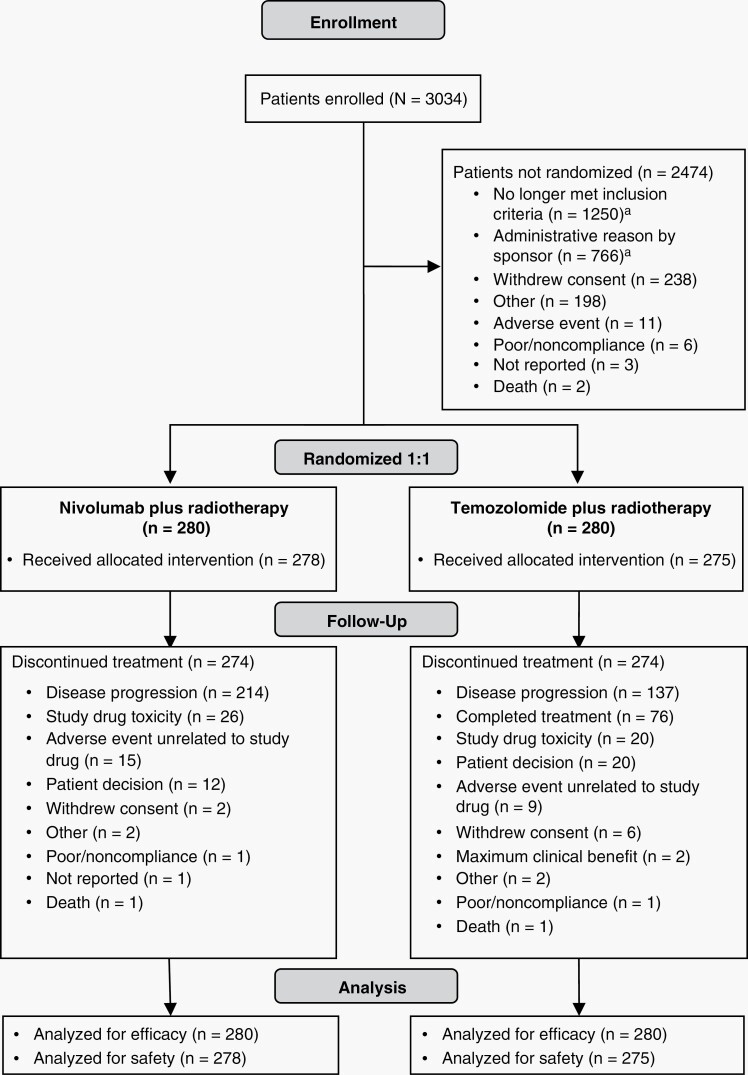
From March 1, 2016, through October 25, 2018, 560 patients with newly diagnosed GBM with unmethylated MGMT promoter were randomized to receive NIVO + RT (n = 280) or TMZ + RT (n = 280) (Figure 1). Patients were enrolled at 124 sites across 19 countries. Of 560 randomized patients, 278 of 280 (99.3%) in the NIVO + RT arm and 275 of 280 (98.2%) in the TMZ + RT arm eventually received treatment. No marked imbalances were observed in baseline characteristics or demographics between arms (Table 1 and Supplementary Table S1).
Fig. 1.
Trial profile. aThe majority of the nonrandomized population was excluded due to methylation status (cutoff, ratio of the gene copy numbers of methylated MGMT to control (β-actin) × 1000 < 2).
Table 1.
Patient Demographics and Baseline Characteristics
| Nivolumab plus radiotherapy (n = 280) | Temozolomide plus radiotherapy (n = 280) | |
|---|---|---|
| Age, years | ||
| Median | 59.5 | 56.0 |
| Range | 18–83 | 23–81 |
| Age, no. (%) | ||
| < 65 years | 190 (67.9) | 207 (73.9) |
| ≥ 65 to < 75 years | 76 (27.1) | 61 (21.8) |
| ≥ 75 years | 14 (5.0) | 12 (4.3) |
| Sex, no. (%) | ||
| Male | 190 (67.9) | 175 (62.5) |
| Female | 90 (32.1) | 105 (37.5) |
| Histopathologic diagnosis, no. (%) | ||
| Glioblastoma | 272 (97.1) | 270 (96.4) |
| Gliosarcoma | 8 (2.9) | 10 (3.6) |
| RPA class, no. (%)a | ||
| III | 20 (7.1) | 42 (15.0) |
| IV | 219 (78.2) | 202 (72.1) |
| V | 41 (14.6) | 36 (12.9) |
| Other | 0 | 0 |
| Extent of surgery, no. (%)b | ||
| Complete resection | 151 (53.9) | 144 (51.4) |
| Partial resection | 129 (46.1) | 136 (48.6) |
| KPS, no. (%) | ||
| 100 | 76 (27.1) | 91 (32.5) |
| 90 | 122 (43.6) | 118 (42.1) |
| 80 | 54 (19.3) | 47 (16.8) |
| 70 | 28 (10.0) | 20 (7.1) |
| Not reported | 0 | 4 (1.4) |
| Time from diagnosis to randomization, weeks | ||
| Median | 4.93 | 5.14 |
| Range | (4.1–5.6) | (4.3–5.9) |
| Patients with evaluable PD-L1 expression, no. (%) | ||
| PD-L1 expression level, no. (%)c | 275 (99.6) | 280 (100.0) |
| < 1% | 171 (62.2) | 155 (55.4) |
| ≥ 1% | 104 (37.8) | 125 (44.6) |
| Not quantifiable | 1 (0.4) | 0 |
| Corticosteroid use, no. (%)d | ||
| Yes | 78 (27.9) | 95 (33.9) |
| ≤ 3 mg/day | 62 (22.1) | 73 (26.1) |
| > 3 mg/day | 16 (5.7) | 22 (7.9) |
| No | 202 (72.1) | 185 (66.1) |
KPS, Karnofsky Performance Scale; PD-L1, programmed cell death ligand 1; RPA, recursive-partitioning analysis.
aThe RPA classes were as follows: class III, age < 50 years and KPS ≥ 90 (on a scale of 0–100, with higher scores indicating better function); class IV, < 50 years and KPS < 90 (or ≥ 50 years, KPS ≥ 70, complete or partial tumor resection, and ability to work); class V, ≥ 50 years, KPS ≥ 70, complete or partial tumor resection, and inability to work (or ≥ 50 years, KPS ≥ 70, and tumor-biopsy specimen only; or ≥ 50 years and KPS < 70).35
bThis characteristic was used as a stratification factor as recorded in the interactive voice response system at time of randomization. Information presented as collected in the case report form.
cPercentages were based on the number of patients with evaluable PD-L1 expression.
dBased on average corticosteroid use 5 days before start of dosing or randomization date for patients not treated (in dexamethasone equivalent). Patients enrolled at doses > 3 mg/day were tapered off; treatment did not commence until the dose was ≤ 3 mg/day.
Complete surgical resection had been performed in 151 patients (53.9%) in the NIVO + RT arm and 144 patients (51.4%) in the TMZ + RT arm. Baseline PD-L1 expression was ≥ 1% in 104 patients (37.8%) in the NIVO + RT arm and 125 patients (44.6%) in the TMZ + RT arm; PD-L1 expression was < 1% in 171 patients (62.2%) and 155 patients (55.4%), respectively (PD-L1 was not evaluable in one patient and tumor tissue samples were not collected for four patients in the NIVO + RT arm). Seventy-eight patients (27.9%; n = 280) in the NIVO + RT arm and 95 patients (33.9%; n = 280) in the TMZ + RT arm were receiving corticosteroids at baseline, with 5.7% and 7.9% of patients receiving > 3 mg/day of dexamethasone equivalents, respectively.
The median duration of study treatment was 22.1 weeks (range, 0.1–140.9) in the NIVO + RT arm and 6.1 weeks (range, 0.6–8.3; concomitant) and 15.4 weeks (range, 0.1–121.1; maintenance) in the TMZ + RT arm. A median of 10.0 doses of NIVO was received (range, 1–40); the median number of TMZ cycles for all patients who entered the maintenance phase was 4.0 (range, 1–31).
At data cutoff, four patients (1.4%) in the NIVO + RT arm and one patient (0.4%) in the TMZ + RT arm were still receiving treatment. Among treated patients, discontinuations occurred in 274 patients (98.6%) in the NIVO + RT arm and 274 patients (99.6%) in the TMZ + RT arm. The most common reasons for treatment discontinuation were disease progression (NIVO + RT, n = 214 [77.0%]; TMZ + RT, n = 137 [49.8%]) and study drug toxicity (NIVO + RT, n = 26 [9.4%]; TMZ + RT, n = 20 [7.3%]) (Figure 1). In the TMZ + RT arm, 76 patients (27.6%) completed treatment.
Efficacy
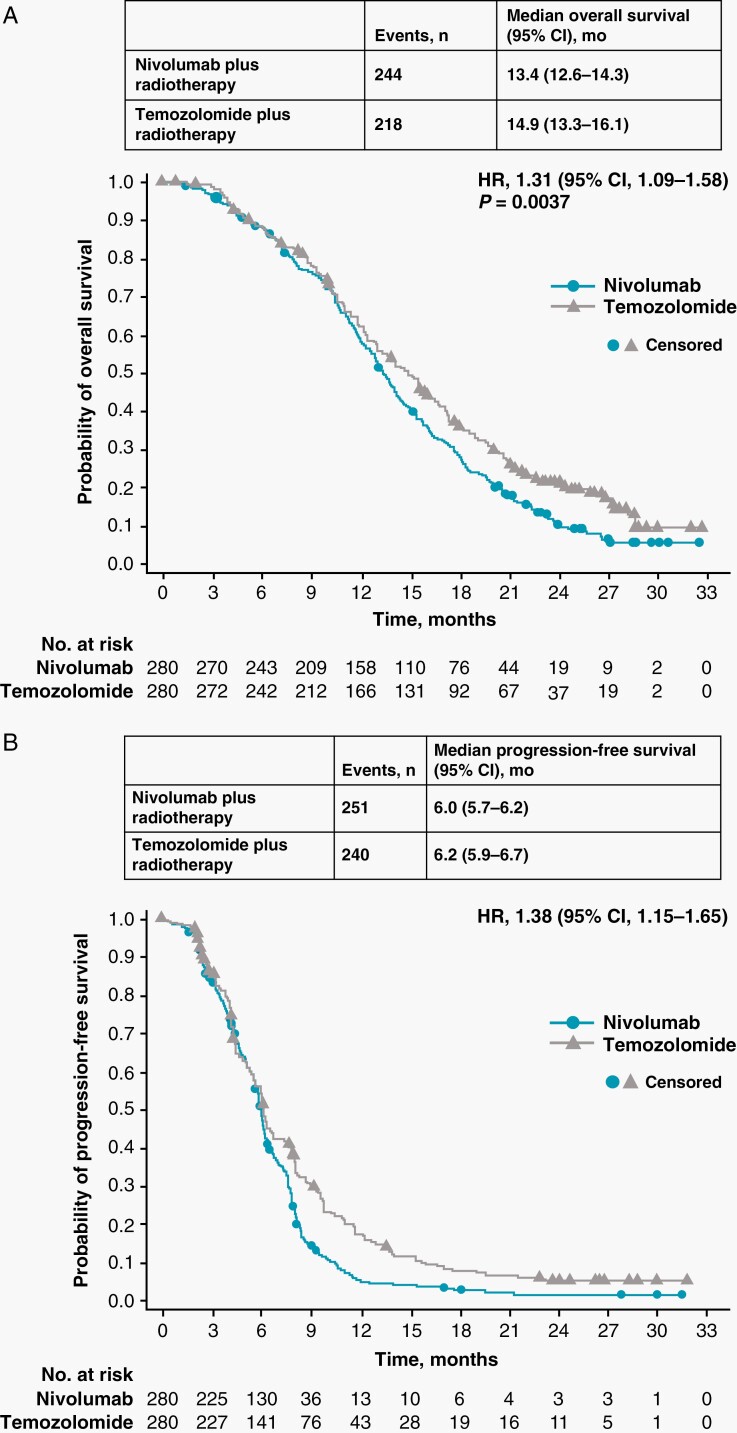
At data cutoff (March 21, 2019), the median follow-up time for OS was 13.0 months (range, 0.6–32.4) in the NIVO + RT arm and 14.2 months (range, 0–32.6) in the TMZ + RT arm. The final analysis was performed after 462 OS events had occurred. The median OS (mOS) was 13.4 months (95% CI, 12.6 to 14.3) in the NIVO + RT arm and 14.9 months (95% CI, 13.3 to 16.1) in the TMZ + RT arm (HR, 1.31; 95% CI, 1.09 to 1.58; P = .0037) (Table 2 and Figure 2A). The 24-month OS rates were 10.3% (95% CI, 6.8 to 4.6) in the NIVO + RT arm and 21.2% (95% CI, 16.4 to 26.5) in the TMZ + RT arm.
Table 2.
Overall Survival and Progression-Free Survival Rates Per Investigator Assessment
| Nivolumab plus radiotherapy (n = 280) | Temozolomide plus radiotherapy (n = 280) | |
|---|---|---|
| Overall survival, months | ||
| Median (95% CI) | 13.4 (12.6 to 14.3) | 14.9 (13.3 to 16.1) |
| Overall survival rate, (95% CI) % | ||
| 6 months | 88.5 (84.1 to 91.7) | 88.7 (84.4 to 91.9) |
| 12 months | 58.3 (52.2 to 63.9) | 62.3 (56.3 to 67.8) |
| 18 months | 28.5 (23.3 to 34.0) | 36.4 (30.7 to 42.2) |
| 24 months | 10.3 (6.8 to 14.6) | 21.2 (16.4 to 26.5) |
| Progression-free survival, months | ||
| Median (95% CI) | 6.0 (5.7 to 6.2) | 6.2 (5.9 to 6.7) |
| Progression-free survival rate, (95% CI) % | ||
| 6 months | 50.5 (44.3 to 56.3) | 54.6 (48.4 to 60.4) |
| 9 months | 14.8 (10.7 to 19.4) | 30.9 (25.3 to 36.6) |
| 12 months | 5.7 (3.2 to 9.1) | 17.7 (13.3 to 22.7) |
| 18 months | 3.0 (1.3 to 5.8) | 8.1 (5.1 to 11.9) |
Fig. 2.
OS and PFS in all patients. (A) Shows the number of events, median OS, and the Kaplan-Meier curve for OS in all patients treated with nivolumab plus radiotherapy or temozolomide plus radiotherapy. (B) Shows the number of events, median PFS, and the Kaplan-Meier curve for PFS per investigator assessment in patients treated with nivolumab plus radiotherapy or temozolomide plus radiotherapy. Symbols indicate censored observations. Hazard ratios and 95% CIs were estimated using a Cox proportional hazards model. OS, overall survival; PFS, progression-free survival.
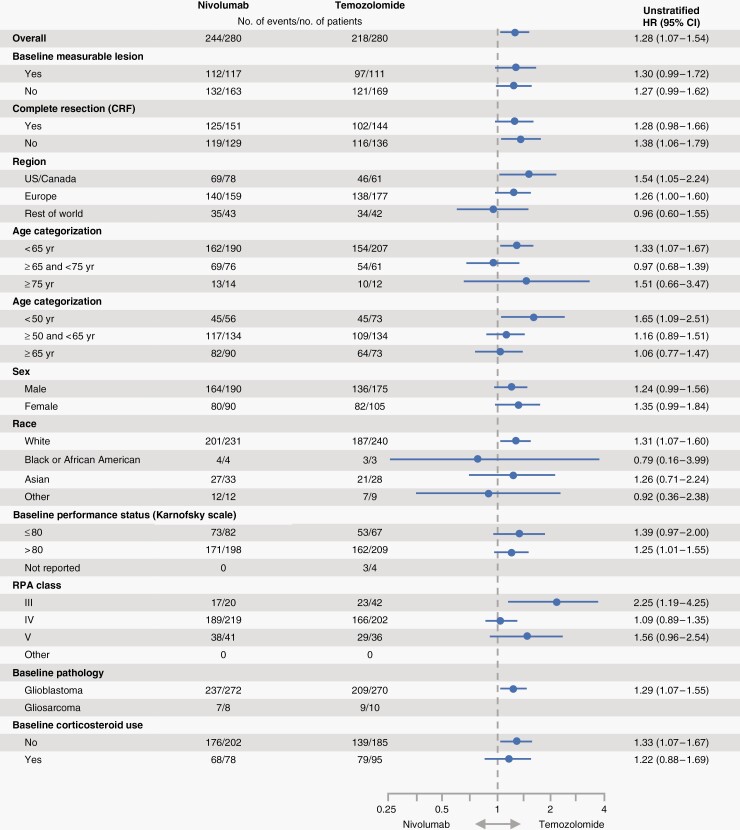
Among patients with baseline PD-L1 expression ≥ 1%, mOS was 12.6 months (n = 104; 95% CI, 11.3 to 14.2) in the NIVO + RT arm and 15.5 months (n = 125; 95% CI, 13.2 to 17.2) in the TMZ + RT arm (HR, 1.4; 95% CI, 1.1 to 1.9) (Supplemental Figure S1A). The mOS in patients with PD-L1 < 1% was 13.8 months (n = 171; 95% CI, 13.0 to 14.6) in the NIVO + RT arm and 14.7 months (n = 155; 95% CI, 12.6 to 16.0) in the TMZ + RT arm (HR, 1.2; 95% CI, 0.9 to 1.5) (Supplemental Figure S1B). OS data by baseline PD-L1 expression ≥ 5% are shown in Supplemental Figures S1C and S1D. The results were consistent across several subgroup analyses, including complete tumor resection (Figure 3).
Fig. 3.
Overall survival in prespecified patient subgroups defined by baseline clinical characteristics. This figure shows a forest plot of unstratified hazard ratios for death in the analysis of treatment effect in prespecified patient subgroups according to baseline characteristics.
Exploratory analyses (not protocol defined) showed balanced distributions of MGMT scores across both arms. Median PFS was 6.0 months (95% CI, 5.7 to 6.2) with NIVO + RT versus 6.2 months (95% CI, 5.9 to 6.7) with TMZ + RT (HR, 1.38; 95% CI, 1.15 to 1.65) (Table 2; Figure 2B). The 12-month PFS rate was 5.7% (95% CI, 3.2 to 9.1) with NIVO + RT and 17.7% (95% CI, 13.3 to 22.7) with TMZ + RT.
The investigator-assessed objective response rate per RANO criteria was 7.8% (9/116; 95% CI, 3.6 to 14.2) in the NIVO + RT arm and 7.2% (8/111; 95% CI, 3.2 to 13.7) in the TMZ + RT arm (Supplemental Table S2). Duration of response data are presented in Supplemental Table S2. Demographic and disease characteristics of responders are presented in Supplemental Table S3.
Subsequent cancer therapy (any therapy) was received by 63.6% and 53.6% of patients in the NIVO + RT and TMZ + RT groups, respectively, of which 52.9% and 46.1%, respectively, received subsequent systemic cancer therapy (Supplemental Table S4). In the NIVO + RT group, 41.1% of patients received subsequent treatment with alkylating agent (including 38.9% receiving TMZ therapy), and 27.5% received vascular endothelial growth factor antibody. In the TMZ + RT group, 30.7% of patients received alkylating agent, and 28.9% received vascular endothelial growth factor antibody. Three patients (1.1%) in the NIVO + RT arm and 7 (2.5%) in the TMZ + RT arm received subsequent immunotherapy (Supplemental Table S4).
Safety
Any-grade treatment-related AEs (TRAEs) were reported in 72.7% of patients treated with NIVO + RT and 75.6% of patients treated with TMZ + RT. The most frequent TRAE was fatigue (any grade, 19.1%) in the NIVO + RT arm and nausea (any grade, 29.1%) in the TMZ + RT arm (Table 3; Supplemental Table S5). Rates of grade 3/4 TRAEs were 22.0% with NIVO + RT and 25.1% with TMZ + RT. Three treatment-related deaths were reported in the NIVO + RT arm: vasogenic cerebral edema, sudden death, and respiratory failure (1 each), after receiving 10, 7, and 10 infusions of NIVO, respectively. The patient who died from sudden death had previously experienced hyperglycemia and grade 3 rash and had been treated with insulin and corticosteroids. No deaths attributed to treatment were reported in the TMZ + RT arm. Neurological TRAEs occurred in 16.5% (grade 3/4, 1.8%) of patients treated with NIVO + RT and 9.5% (grade 3/4, 0%) of patients treated with TMZ + RT. Any-grade serious TRAEs occurred in 17.3% (NIVO + RT) and 7.6% (TMZ + RT) of patients. Any-grade TRAEs leading to discontinuation occurred in 24 patients (8.6%) in the NIVO + RT arm and 16 patients (5.8%) in the TMZ + RT arm. Treatment-related, immune-mediated AEs reported by category are shown in Supplemental Table S6. Most patients were not receiving corticosteroids at baseline (NIVO + RT = 200/278 [71.9%]; TMZ + RT = 180/275 [65.5%]). The median dose of corticosteroid was 0 mg/day (dexamethasone equivalents) in the NIVO + RT arm throughout the study treatment, except at weeks 11–18 and 91–98 when median corticosteroid use was 0.21 and 0.44 mg/day, respectively. Similarly, in the TMZ + RT arm, median dose of corticosteroid was 0 mg/day except at weeks 1–6 and 11–18 when median corticosteroid use was 0.24 and 0.66 mg/day, respectively.
Table 3.
Treatment-Related Adverse Eventsa
| Nivolumab plus radiotherapy (n = 278) | Temozolomide plus radiotherapy (n = 275) | |||
|---|---|---|---|---|
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Any treatment-related adverse event, no. (%)b | 202 (72.7) | 61 (21.9) | 208 (75.6) | 69 (25.1) |
| Fatigue | 53 (19.1) | 1 (0.4) | 77 (28.0) | 3 (1.1) |
| Pyrexia | 15 (5.4) | 4 (1.4) | 2 (0.7) | 0 |
| Alopecia | 31 (11.2) | 0 | 48 (17.5) | 1 (0.4) |
| Rash | 28 (10.1) | 5 (1.8) | 6 (2.2) | 1 (0.4) |
| Pruritus | 20 (7.2) | 1 (0.4) | 11 (4.0) | 0 |
| Diarrhea | 22 (7.9) | 2 (0.7) | 8 (2.9) | 0 |
| Nausea | 18 (6.5) | 0 | 80 (29.1) | 2 (0.7) |
| Headache | 16 (5.8) | 0 | 11 (4.0) | 0 |
| Radiation skin injury | 21 (7.6) | 0 | 17 (6.2) | 0 |
| Hypothyroidism/autoimmune hypothyroidism | 16 (5.8) | 2 (0.8) | 0 | 0 |
| Decreased appetite | 15 (5.4) | 0 | 34 (12.4) | 0 |
| Lymphocyte count decreased/lymphopenia | 12 (4.3) | 4 (1.5) | 51 (18.6) | 28 (10.2) |
| Asthenia | 10 (3.6) | 0 | 17 (6.2) | 1 (0.4) |
| Vomiting | 8 (2.9) | 0 | 39 (14.2) | 0 |
| Constipation | 7 (2.5) | 0 | 40 (14.5) | 0 |
| Neutrophil count decreased/neutropenia | 4 (1.4) | 3 (1.1) | 28 (10.2) | 12 (4.4) |
| Platelet count decreased/thrombocytopenia | 3 (1.1) | 1 (0.4) | 66 (24.0) | 30 (10.9) |
| Treatment-related adverse event leading to discontinuation, no. (%) | 24 (8.6) | 20 (7.2) | 16 (5.8) | 12 (4.4) |
aData are based on a March 21, 2019, database lock. The safety analysis included all patients who received ≥ 1 dose of study drug. Some patients had > 1 adverse event. Includes events reported between first dose and 30 days after last dose of study therapy. Three treatment-related deaths were reported in the nivolumab arm due to vasogenic cerebral edema, sudden death, and respiratory failure (1 each); no treatment-related deaths were reported in the temozolomide arm.
bThese treatment-related adverse events were reported in ≥ 5% of the patients in either study arm. The full-length treatment-related adverse events table is included in Supplement Table S5.
PROs
In all randomized patients, median time to deterioration of HRQoL scores was 4.6 months with NIVO + RT and 3.1 months with TMZ + RT (HR, 0.76; 95% CI, 0.59 to 0.99; P = .039). A trend of delayed time to deterioration was observed in the NIVO + RT arm compared with the TMZ + RT arm for most domains of HRQoL and similarly for general health utilities (EQ-5D-3L index and visual analog scale; Supplemental Figure S2). However, these results for time to deterioration were affected by heavy censoring and should be interpreted with caution.
Discussion
CheckMate 498 is a randomized phase III study investigating the efficacy of NIVO + RT compared with conventional TMZ + RT chemoradiation in patients with newly diagnosed GBM with unmethylated MGMT promoter. Although patients in both arms fared better than historical controls, the primary endpoint was not met. TMZ + RT was associated with superior OS compared with NIVO + RT (mOS, 14.9 vs. 13.4 months), suggesting that NIVO is not a substitute for TMZ in this patient population.
Although NIVO has shown notable efficacy in several other cancer types, it did not demonstrate a survival benefit in patients with newly diagnosed GBM with unmethylated MGMT promoter compared with TMZ. Likewise, in a subgroup analysis of the CheckMate 143 phase III study, NIVO did not demonstrate a survival benefit versus bevacizumab in patients with recurrent GBM with unmethylated MGMT promoter.27,28 PD-L1 expression in this study (≈ 41% of all patients expressed PD-L1 ≥ 1%) was similar to that observed in other GBM studies.18 However, it did not predict survival benefit with NIVO, suggesting that other factors may hinder successful immune responses in this tumor type. Notably, recent results from the CheckMate 548 study (NCT02667587) in newly diagnosed GBM with methylated MGMT have also shown the addition of NIVO to TMZ + RT does not prolong PFS or OS compared with TMZ + RT alone.29 Taken together, these results clearly highlight a need for better understanding the mechanisms of immune evasion in GBM to improve the efficacy of immunotherapies. In addition to PD-L1 expression, multiple factors have been implicated in the maintenance of an immunosuppressive microenvironment in gliomas. These include both tumor- and brain-specific mechanisms (eg, low tumor mutational burden, recruitment of other immune checkpoints, decreased T-cell responsiveness, inhibitory cytokine production, interactions between CNS microenvironment and microglia, predominance of myeloid cells, and paucity of lymphocytes in TME), in addition to frequent corticosteroid use.30–33
A key limitation of our study was the lack of immune-predictive biomarkers and comprehensive genomic characterization due to limited availability of tumor samples; therefore, novel biomarkers remain to be further explored. In addition, this study did not consider potential effects of the timing of PD-1 blockade relative to RT administration, which has subsequently been demonstrated to be of possible consequence in some preclinical, non-GBM settings.34
The overall safety profile with NIVO + RT in this study was similar to that reported in CheckMate 143 for NIVO alone, with no new safety signals observed.28 However, in some AE categories, as expected, more AEs were reported in the NIVO + RT arm than in the TMZ + RT arm. One of the reasons for the omission of TMZ in the NIVO + RT arm was to manage lymphopenia and immunosuppression; indeed, lymphopenia was more frequent in the TMZ + RT arm than in the NIVO + RT arm. Interestingly, HRQoL deteriorated numerically more rapidly in the TMZ + RT arm than in the NIVO + RT arm, consistent with expected effects of chemotherapy. However, time to deterioration results were affected by heavy censoring and should be interpreted with caution.
Our study found a statistically significant survival benefit with TMZ + RT over NIVO + RT despite tumor MGMT unmethylated status. mOS was 14.9 months (95% CI, 13.3 to 16.1) with TMZ + RT and 13.4 months (95% CI, 12.6 to 14.3) with NIVO + RT. These results were similar to those of previous studies, including the study conducted by Gilbert et al.,35 which reported an mOS of 14.6 months (95% CI, 13.2 to 16.5) with TMZ + RT. The study conducted by Hegi et al.8 also produced similar results: an mOS of 12.7 months (95% CI, 11.6 to 14.4) with TMZ + RT and 11.8 months (95% CI, 9.7 to 14.1) with RT alone. Several potential differences in studies exist, including patient selection, study design, and patient management. However, one caveat is that NIVO was compared with TMZ, and any potential benefit of combining NIVO with TMZ was not evaluated to fully assess the effects of NIVO.
In summary, we report on the largest phase III study conducted to date in patients with GBM molecularly selected for unmethylated MGMT promoter and the first to prospectively examine the omission of TMZ in this population. Overall, our results indicate that immunotherapy with NIVO is not a suitable replacement for chemotherapy with TMZ despite the chemoresistance of this difficult-to-treat patient population. Further immunotherapy efforts in GBM include alternative immune checkpoint inhibitors, vaccines, oncolytic viruses, and cell therapies. Additionally, immune checkpoint inhibitors combined with each other or with vaccines may also be explored.
Supplementary Material
Acknowledgments
We thank the patients and their families who made this study possible; Corina Taitt for her contribution to study development and design; investigators and research staff at all study sites; ONO Pharmaceutical Company Ltd. (Osaka, Japan); and the staff of Dako, an Agilent Technologies, Inc., company, for collaborative development of the PD-L1 IHC 28-8 pharmDx assay. Editorial assistance was provided by Bridget Sackey-Aboagye, PhD, Judith Matray-Devoti, PhD, and Larra Yuelling, PhD, of SciMentum, Inc, a Nucleus Holding Ltd company, and was funded by Bristol Myers Squibb.
Contributor Information
Antonio Omuro, Department of Neurology, Yale School of Medicine, New Haven, Connecticut, USA; Department of Neurology, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Alba A Brandes, AUSL–IRCCS Institute of Neurological Sciences, Bologna, Italy.
Antoine F Carpentier, Université de Paris, Assistance Publique-Hôpitaux de Paris (AP-HP), Hôpital Saint-Louis, Service de Neurologie, Paris, France .
Ahmed Idbaih, Sorbonne Université, Institut du Cerveau – Paris Brain Institute – ICM, Inserm, CNRS, AP-HP, Hôpital Universitaire La Pitié Salpêtrière, DMU Neurosciences, Paris, France.
David A Reardon, Dana-Farber Cancer Center, Harvard Medical School, Boston, Massachusetts, USA.
Timothy Cloughesy, Department of Neurology, University of California, Los Angeles, California, USA.
Ashley Sumrall, Levine Cancer Institute, Charlotte, North Carolina, USA.
Joachim Baehring, Department of Neurology, Yale School of Medicine, New Haven, Connecticut, USA.
Martin van den Bent, Brain Tumor Center at Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Oliver Bähr, Dr. Senckenberg Institute of Neurooncology, Goethe University Hospital, Frankfurt, Germany.
Giuseppe Lombardi, Department of Oncology, Oncology 1, Veneto Institute of Oncology IOV-IRCCS, Padua, Italy.
Paul Mulholland, University College London Hospitals, London, UK.
Ghazaleh Tabatabai, Department of Neurology and Interdisciplinary Neuro-Oncology, Hertie Institute for Clinical Brain Research, Center for Neuro-Oncology, Comprehensive Cancer Center Tübingen-Stuttgart, University Hospital Tuebingen, Eberhard Karls University, Tuebingen, Germany.
Ulrik Lassen, Department of Oncology, Rigshospitalet, Copenhagen, Denmark.
Juan Manuel Sepulveda, Hospital Universitario 12 de Octubre, Madrid, Spain.
Mustafa Khasraw, The University of Sydney, Sydney, New South Wales, Australia.
Elodie Vauleon, Centre Eugene Marquis, Rennes, France.
Yoshihiro Muragaki, Tokyo Women’s Medical University Hospital, Tokyo, Japan.
Anna Maria Di Giacomo, Center for Immuno-Oncology, University Hospital of Siena, Siena, Italy.
Nicholas Butowski, Department of Neurological Surgery, University of California, San Francisco, California, USA.
Patrick Roth, Department of Neurology and Brain Tumor Center, University Hospital and University of Zurich, Zurich, Switzerland.
Xiaozhong Qian, Bristol Myers Squibb, Princeton, New Jersey, USA.
Alex Z Fu, Bristol Myers Squibb, Princeton, New Jersey, USA.
Yanfang Liu, Bristol Myers Squibb, Princeton, New Jersey, USA.
Von Potter, Bristol Myers Squibb, Princeton, New Jersey, USA.
Alexandros-Georgios Chalamandaris, Bristol Myers Squibb, Braine-L’Alleud, Belgium.
Kay Tatsuoka, Bristol Myers Squibb, Princeton, New Jersey, USA.
Michael Lim, The Johns Hopkins Hospital, Baltimore, Maryland, USA.
Michael Weller, Department of Neurology and Brain Tumor Center, University Hospital and University of Zurich, Zurich, Switzerland.
Funding
This work was supported by Bristol Myers Squibb, Princeton, NJ.
Conflict of interest statement. A.O. has served as a consultant on ad hoc advisory boards for Bristol Myers Squibb, AstraZeneca, Inovio, Merck, Stemline, Novocure, Alexion, and KIYATEC and has received grant funding from Arcus Biosciences. A.A.B. has nothing to disclose. A.F.C. received personal fees from Bristol Myers Squibb. A.I. received grants and other funding from Carthera, Transgene, Sanofi, Air Liquide, and Leo Pharma. D.A.R. received other funding from Acerta Pharma, Agenus, Celldex, EMD Serono, Incyte, InovFio, Midatech, Omniox, and Tragara and personal fees from AbbVie, Advantagene, Agenus, Amgen, Bayer, Bristol Myers Squibb, Celldex, DelMar, EMD Serono, Genentech/Roche, Inovio, Merck, Merck KGaA, Monteris, Novocure, Oncorus, Oxigene, Regeneron, Stemline, and Taiho Oncology, Inc. T.C. received personal fees from Roche, Bayer, Amgen, Chimerix, Novocure, Novartis, Jubilant, Immvira, Gan & Lee, Brainstorm, Tyme, SDP, Inovio, Sapience, DNATrix, QED, DelMar, Tocagen, Karyopharm, GW Pharma, KIYATEC, AbbVie, Boehringer Ingelheim, VBI, Deciphera, VBL, Agios, Merck, Bristol Myers Squibb, and Cortice; is a co-founder, with milestone royalties, stock and stock options, consultant, scientific advisory board to Katmai; has stock, milestones and royalties from Chimerix, and is a member of the board for the 501(c)(3) nonprofit Global Coalition for Adaptive Research and SAB for Break Through Cancer. A.S. has nothing to disclose. J.B. has served as a consultant for Bristol Myers Squibb. M.V.D.B. received personal fees from Bristol Myers Squibb, Carthera, Nerviano, AbbVie, Bayer, Boehringer Ingelheim, Agios, and Genenta. O.B. has received personal fees from medac Pharma and Novocure. G.L. received personal fees from Bayer Italy S.p.A., AbbVie, Orbus, and Brainfarm. P.M. has nothing to disclose. G.T. received personal fees from AbbVie, Bayer, Bristol Myers Squibb, medac Pharma, and Novocure and grants from Bristol Myers Squibb, medac Pharma, Roche Diagnostics, and Novocure. U.L. received grant funding from Bristol Myers Squibb. J.M.S received grant funding from Pfizer, other fees from Astellas, and personal fees from AbbVie and Celgene, a Bristol-Myers Squibb company. M.K. received grant funding from Bristol Myers Squibb and AbbVie and personal fees from Roche, Ipsen, Novartis, Pfizer, and AbbVie. E.V. has nothing to disclose. Y.M. received grant funding from MSD, Chugai Pharma, Otsuka, Tsumura, and Hitachi and personal fees from Novocure, AbbVie, Ono Pharmaceutical, Daiichi Sankyo, MSD, Chugai Pharma, Otsuka, Eisai, and Novartis. A.M.D.G. received other funding from Bristol Myers Squibb, Pierre Fabre, MSD, Inscyte, and GSK. N.B. has nothing to disclose. P.R. received other funding from Bristol Myers Squibb and personal fees from Bristol Myers Squibb, Debiopharm, medac Pharma, Merck, QED, Roche, MSD, Virometix, and Novocure and grant funding from MSD and Novocure. X.Q. was an employee of Bristol Myers Squibb and received stocks from Bristol Myers Squibb. A.Z.F. was an employee of Bristol Myers Squibb. Y.L. was an employee of Bristol Myers Squibb. V.P. was an employee of Bristol Myers Squibb. A.-G.C. is an employee of and has received stocks from Bristol Myers Squibb. K.T. was an employee of Bristol Myers Squibb. M.L. received grants from Arbor Pharmaceuticals, Accuray, DNAtrix, Biohaven, Bristol Myers Squibb, Kyowa Kyrin, and UroGen; received personal fees from VBI, Stryker, Bristol Myers Squibb, Tocagen, InCephalo Therapeutics, Pyramid Bio, Merck, Insightec, Biohaven, Sanianoia, Hemispherian, Black Diamond Therapeutics, and Novocure; and has a patent. M.W. received other funding from Bristol Myers Squibb; grants from MSD, Merck (EMD), Novocure, and Quercis; and personal fees from Bristol Myers Squibb, AbbVie, MSD, Merck (EMD), Orbus, and Y-mAbs.
Authorship statement. A.O., D.A.R., M.L., and M.W. participated in the initial study design; A.-G.C. participated in the statistical analysis of the data; A.O., A.A.B., A.F.C., A.I., D.A.R., T.C., A.S., J.B., M.V.D.B., O.B., G.L., P.M., G.T., U.L., J.M.S., M.K., E.V., Y.M., A.M.D.G., N.B., P.R., M.L., and M.W. participated in acquisition of the data; all authors provided critical review or revision of the manuscript; and all authors approved to submit the manuscript for publication.
Data Availability
The data sets presented in this article are not readily available because requestors must complete a data request on the BMS investigator portal. Requests to access the data sets should be directed to https://fasttrack-bms.force.com/Login. Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- 1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018; 20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11(9):504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 4. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 6. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4(4):296–307. [DOI] [PubMed] [Google Scholar]
- 8. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 9. Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350–1354. [DOI] [PubMed] [Google Scholar]
- 10. Weller M, Stupp R, Reifenberger G, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6(1):39–51. [DOI] [PubMed] [Google Scholar]
- 11. Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10(6):1871–1874. [DOI] [PubMed] [Google Scholar]
- 12. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weller M. Where does O6-methylguanine DNA methyltransferase promoter methylation assessment place temozolomide in the future standards of care for glioblastoma? Cancer. 2018;124(7):1316–1318. [DOI] [PubMed] [Google Scholar]
- 14. OpdivoTM (Nivolumab) US Prescribing Information. Princeton, NJ: Bristol-Myers Squibb; 2019. [Google Scholar]
- 15. Lauko A, Thapa B, Jia X, Ahluwalia MS. Efficacy of immune checkpoint inhibitors in patients with brain metastasis from NSCLC, RCC, and melanoma. J Clin Oncol. 2018;36(5):214–214. [Google Scholar]
- 16. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17(8):1064–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nduom EK, Wei J, Yaghi NK, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–7523. [DOI] [PubMed] [Google Scholar]
- 21. Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol. 2015;25(1):11–17. [DOI] [PubMed] [Google Scholar]
- 22. Lhuillier C, Rudqvist N-P, Elemento O, Formenti SC, Demaria S. Radiation therapy and anti-tumor immunity: exposing immunogenic mutations to the immune system. Genome Med. 2019;11(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. TEMODAR ® (Temozolomide) US Prescribing Information . Whitehouse Station, NJ: Merck; 2017. [Google Scholar]
- 24. Hegi ME, Genbrugge E, Gorlia T, et al. MGMT promoter methylation cutoff with safety margin for selecting glioblastoma patients into trials omitting temozolomide: a pooled analysis of four clinical trials. Clin Cancer Res. 2019;25(6):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 26. Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012;48(11):1713–1721. [DOI] [PubMed] [Google Scholar]
- 27. Weller M, Reardon D, Brandes A, et al. Nivolumab vs bevacizumab in patients with recurrent glioblastoma: exploratory analysis of MGMT methylation status and baseline corticosteroid use. Neuro Oncol. 2019; 21(suppl_6):vi12. [Google Scholar]
- 28. Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weller M, Lim M, Idbaih A, et al. A randomized phase 3 study of nivolumab or placebo combined with radiotherapy plus temozolomide in patients with newly diagnosed glioblastoma with methylated MGMT promoter: CheckMate 548. Neuro Oncol. 2021; 23(suppl_6):vi55–vi56. [Google Scholar]
- 30. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hodges TR, Ott M, Xiu J, et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro Oncol. 2017;19(8):1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mangani D, Weller M, Roth P. The network of immunosuppressive pathways in glioblastoma. Biochem Pharmacol. 2017;130:1–9. [DOI] [PubMed] [Google Scholar]
- 33. Wong ET, Lok E, Gautam S, Swanson KD. Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br J Cancer. 2015;113(2):232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei J, Montalvo-Ortiz W, Yu L, et al. Sequence of αPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci Immunol. 2021;6(58):eabg0117. [DOI] [PubMed] [Google Scholar]
- 35. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets presented in this article are not readily available because requestors must complete a data request on the BMS investigator portal. Requests to access the data sets should be directed to https://fasttrack-bms.force.com/Login. Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.