Abstract
Background
Reducing radiation dose to the hippocampus with hippocampal avoidance prophylactic cranial irradiation (HA-PCI) is proposed to prevent cognitive decline. It has, however, not been investigated whether hippocampal atrophy is actually mitigated by this approach. Here, we determined whether HA-PCI reduces hippocampal atrophy. Additionally, we evaluated neurotoxicity of (HA-)PCI to other brain regions. Finally, we evaluated associations of hippocampal atrophy and brain neurotoxicity with memory decline.
Methods
High-quality research MRI scans were acquired in the multicenter, randomized phase 3 trial NCT01780675. Hippocampal atrophy was evaluated for 4 months (57 HA-PCI patients and 46 PCI patients) and 12 months (28 HA-PCI patients and 27 PCI patients) after (HA-)PCI. We additionally studied multimodal indices of brain injury. Memory was assessed with the Hopkins Verbal Learning Test–Revised (HVLT-R).
Results
HA-PCI reduced hippocampal atrophy at 4 months (1.8% for HA-PCI and 3.0% for PCI) and at 12 months (3.0% for HA-PCI and 5.8% for PCI). Both HA-PCI and PCI were associated with considerable reductions in gray matter and normal-appearing white matter, increases in white matter hyperintensities, and brain aging. There were no significant associations between hippocampal atrophy and memory.
Conclusions
HA-PCI reduces hippocampal atrophy at 4 and 12 months compared to regular PCI. Both types of radiotherapy are associated with considerable brain injury. We did not find evidence for excessive brain injury after HA-PCI relative to PCI. Hippocampal atrophy was not associated with memory decline in this population as measured with HVLT-R. The usefulness of HA-PCI is still subject to debate.
Keywords: brain aging, brain injury, hippocampal atrophy, hippocampal avoidance prophylactic cranial irradiation, memory
Key Points.
HA-PCI reduced hippocampal atrophy compared to conventional PCI.
Hippocampal atrophy was not directly associated with memory decline.
Both HA-PCI and PCI were associated with considerable brain injury and aging.
Importance of the Study.
Prophylactic cranial irradiation (PCI) is advocated in small cell lung cancer patients because the risk of brain metastases is very high. Neurocognitive decline is a dreaded side effect of PCI. Reducing radiation dose to the hippocampus with hippocampal avoidance PCI (HA-PCI) is proposed to prevent cognitive decline. It has, however, not been investigated whether hippocampal atrophy is actually mitigated by this approach. In addition, there are concerns that HA-PCI might be more detrimental to brain regions other than the hippocampus because of suboptimal dosimetry. We found that HA-PCI reduced hippocampal atrophy compared to PCI at 4 and 12 months after treatment, where both HA-PCI and PCI were associated with considerable brain injury and brain aging. There were no significant associations between hippocampal atrophy and memory decline. For PCI, selective reduction of radiation dose might only be of clinical benefit for cognitive functioning when entire networks are spared.
Prophylactic cranial irradiation (PCI) is used to prevent clinical symptoms of brain metastases. For small cell lung cancer (SCLC) patients, the risk of brain metastases is >50%. As PCI reduces the incidence of brain metastases and increases overall survival (OS), PCI is advocated in patients without distant metastases as well considered in those with spread disease.1,2
The reluctance to use PCI is due to concerns about neurocognitive decline as a result of radiation-induced injury to healthy brain tissue.3 Hippocampal avoidance prophylactic cranial radiation (HA-PCI) is an advanced type of PCI where the radiation dose to the hippocampus is reduced as much as technically possible (eg, from 25 Gy to <10 Gy) to diminish neurocognitive side effects, particularly with respect to learning and memory.4 In patients with brain metastases, hippocampal avoidance whole-brain radiotherapy (HA-WBRT) combined with memantine was shown to better preserve cognitive functioning than conventional whole-brain radiotherapy (WBRT) with memantine (NRG-CC001 trial).5 In patients not yet diagnosed with brain metastases, the neurocognitive benefit of HA-PCI vs PCI has been reported in one prospective phase 3 trial, the PREMER study (n = 150).6 In this trial, decline in delayed free recall on the free and cued selective reminding test (FCSRT) at 3 months was significantly lower in the HA-PCI arm (5.8%) than in the PCI arm (23.5%). Our Dutch phase 3 randomized controlled clinical trial (RCT) NCT01780675 failed to show benefit using the Hopkins Verbal Learning Test–Revised (HVLT-R).7 As a possible reason for this negative trial, it has been suggested that the HA-PCI technique used insufficiently protected the hippocampus from radiation injury,8 and that “hot spots” in brain regions outside the hippocampus might have counteracted the positive neurocognitive effects of hippocampal sparing.9
In the present study, we longitudinally evaluated whether HA-PCI reduces hippocampal atrophy and is associated with an increase in brain injury outside the hippocampus, 4 and 12 months after completion of (HA-)PCI compared to a pre-(HA-)PCI baseline measurement. We analyzed dedicated high-quality MRI scans that were aligned between all participating institutions.10 To measure hippocampal atrophy we performed hippocampal volume measurements. To measure brain injury, we measured the volumes of gray matter, normal-appearing white matter, and white matter hyperintensities. In addition, we estimated accelerated brain aging using a machine learning algorithm. To assess the clinical relevance of these measures, we investigated the association of these neuroimaging outcomes with the primary endpoint of the Dutch trial, HVLT-R total recall.11
Materials and Methods
Patients
We describe the secondary results of the multicenter phase 3 trial (NCT01780675).7 Eligible patients had histologically or cytologically proven SCLC, stages I-III (limited stage) or stage IV (extensive stage), without clinical or radiological evidence of brain metastases on a contrast-enhanced MRI scan. All patients had no progressive disease after chemoradiotherapy in stages I-III or after chemotherapy alone in stage IV. Patients younger than 18 years old and those with previous radiotherapy to the brain or receiving anticancer agents concurrently with PCI were excluded. Patients first received four courses of chemotherapy and subsequently PCI. The interval between the last chemotherapy course and the start of PCI was at least 3 weeks.
All patients gave written informed consent. The trial was conducted according to the Declaration of Helsinki and approved by the Medical Ethics Committee of the Netherlands Cancer Institute.
Patients were irradiated using image-guided radiotherapy to a total dose of 25 Gy in 10 fractions, five times a week. Image-guided intensity-modulated radiotherapy was performed using 6 or 10 megavolt photon beams. The constraints in the HA-PCI group were: a mean physical dose in the left and right hippocampi of ≤8.5 Gy (biological dose ≤6.1 Gy for α/β = 2 Gy), a D1% hippocampus ≤10 Gy, maximum dose (Dmax) planning target volume (PTV) of <28.75 Gy (115%), and V115% PTV ≤1%. The treatment plans complied with the trial constraints in the vast majority of cases.7
The study MRI scan protocol used defined high-quality brain MRI scan acquisitions before (HA-)PCI and 4 and 12 months after (HA-)PCI. All sequences of the MRI scanners of participating institutions were aligned and assessed for multicenter and longitudinal reproducibility before the start of the study, including physical and human phantom measurements.10 For the present study, a high-resolution, three-dimensional T1-weighted MRI with excellent contrast between gray and white matter (1.2-mm slice thickness) and a high-resolution fluid-attenuated inversion recovery (3D FLAIR) scan were used. The T1-weighted MRI was used for the determination of hippocampal volume, gray matter, and normal-appearing white matter volume and brain age estimations. The 3D FLAIR scan was used for the determination of white matter hyperintensities volume. All measures were extracted with fully automated procedures and visually checked for accuracy. Hippocampal volume, gray matter, and normal-appearing white matter volume were automatically extracted with the longitudinal pipeline of FreeSurfer 6.0.12 We extensively verified the reproducibility of hippocampal volume measurements in our methodological neuroimaging study.10 For instance, we found a coefficient of variation (CV) of the hippocampal volume of 0.94% for 5 consecutive MRI acquisitions in the same individual, indicating satisfactory longitudinal consistency. Briefly, the preprocessing steps involved interpolation of images, non-uniform intensity correction, intensity normalization, removal of non-brain tissue, and nonlinear registration of the hippocampal volume, gray matter, and normal-appearing white matter segmentations in the FreeSurfer package to subject space. White matter hyperintensities were extracted with the longitudinal pipeline of the lesion prediction algorithm (LPA).13,14 The BrainageR toolbox, version 1.0 was used for estimating brain age.15 For each time point, the brain-predicted age difference (BrainPad) was calculated by subtracting brain age from chronological age (positive numbers indicate accelerated brain aging).15 A neuropsychological test battery was administered that included the HVLT-R.11 This verbal learning and memory test provided the primary endpoint, total recall.
Statistics
A per-protocol, complete case analysis approach was used. For baseline measures, group differences were compared using independent-samples t-tests. For the primary outcome measure (hippocampal volume change), a P < .05 was considered statistically significant. For baseline characteristics and the secondary outcome measures, including correlations, we used a P < .01 to reduce the risk of type I errors as a result of multiple testing.
For each outcome measure, separate factorial general linear model (GLM) repeated-measures analyses were conducted for (1) the baseline and 4-month measurement and (2) the baseline, 4-month, and 12-month measurement. We modeled the main effect of the within-subject factor Time, consisting of two or three levels (baseline and 4 months, or baseline, 4 months, and 12 months), and the main effect of the between-subject factor Group, consisting of two levels (HA-PCI or PCI) and the Time × Group interaction. For hippocampal volume, the factor hemisphere (left/right) and its interaction with the other factors were additionally modeled, but has no significant interactions with Group or Time were found these effects are not reported. Sensitivity analyses were run excluding patients who were diagnosed with brain metastases at the 4- or 12-month follow-up.
To examine the effect of dose variations to the hippocampus in the HA-PCI group with volume change, correlations were calculated between mean dose to the left and right hippocampus, and D1% to the left and right hippocampus, with changes in left and right hippocampal volume in the HA-PCI group at 4 and 12 months compared to the pre-HA-PCI baseline measurement.
Baseline left and right hippocampal volume, normal-appearing white matter, and white matter hyperintensities were correlated with age to verify whether normal-appearing white matter and hippocampal volume were negatively correlated with age, and white matter hyperintensities were positively correlated with age. To assess the association of (changes in) these imaging outcomes with (changes in) HVLT-R total recall, we ran Pearson correlations. Because other related studies sometimes focus on HVLT-R delayed recall, we also calculated correlations with this outcome measure. Hippocampal and white matter volumes at baseline were adjusted for intracranial volume. For changes in hippocampal volume and white matter volumes, unadjusted volumes were used. Please note that to increase sensitivity, we used change in recall scores as a continuous outcome measure whereas in the main outcome paper, a dichotomized outcome measure was used (“decline” or “stable”).
Results
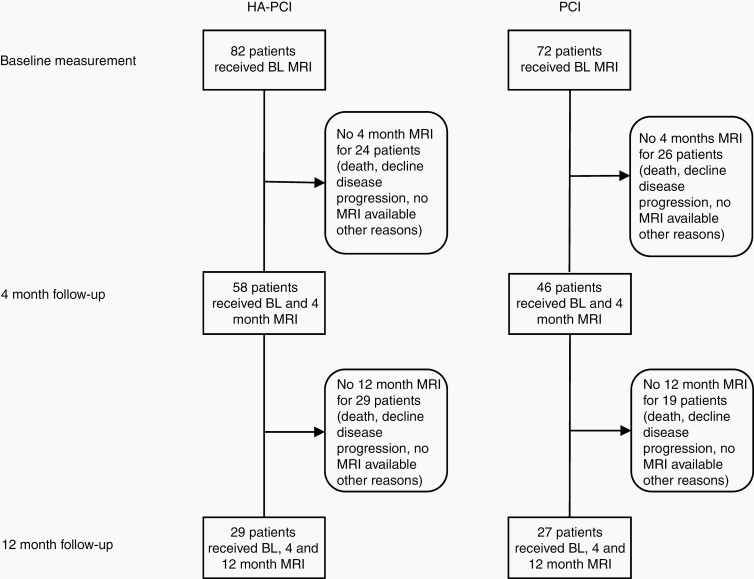
Figure 1 shows a flowchart depicting patient attrition. At 4 months, MRIs of 58 (HA-PCI) and 46 (PCI) patients were available for analysis. At 12 months, MRIs of 29 (HA-PCI) and 27 (PCI) patients were available for analysis. The planned mean dose to the left and right hippocampi was 8.0 Gy (range: 5.4-11.4 Gy). This was lower than the trial constraint ≤8.5 Gy.
Fig. 1.
Study flowchart.
FreeSurfer failed to run for one patient in the HA-PCI group. The 3D FLAIR scan was corrupt at 4 months for one of the patients in the HA-PCI group affecting the three time points. White matter hyperintensities estimation failed at 4 months in 4 patients (2 HA-PCI and 2 PCI patients), and at 12 months in 4 HA-PCI patients.
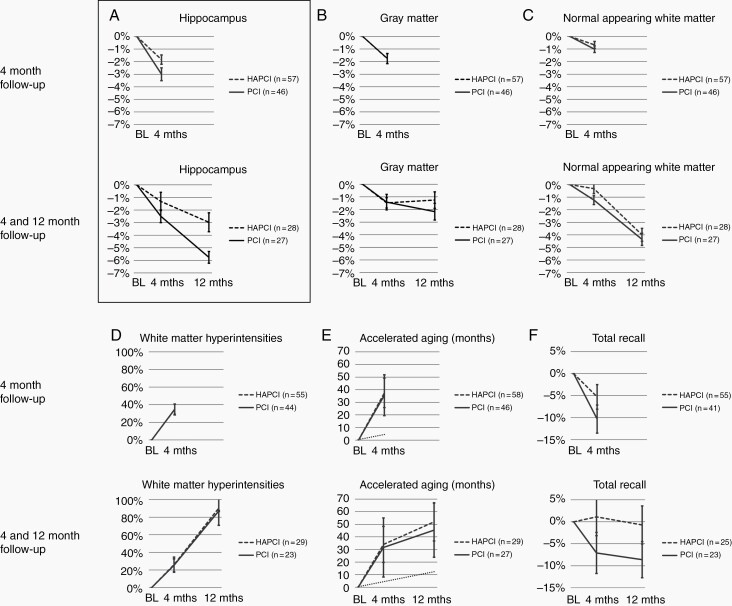
Baseline characteristics (before (HA-)PCI and after primary treatment of SCLC are shown in Table 1. The two groups did not significantly differ on any outcome measure. Table 2 shows the statistical results of the Time × Group analyses. Figure 2 visualizes these changes over time for the two groups normalized for the baseline assessment.
Table 1.
Baseline Characteristics
| HA-PCI | PCI | P | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | ||
| Age | 63.1 | 8.4 | 58 | 64.3 | 7.9 | 46 | .455 |
| Hippocampal volume (mL) | 3.8 | 0.5 | 57 | 3.8 | 0.4 | 46 | .762 |
| Gray matter (mL) | 566 | 57.8 | 57 | 585 | 47.3 | 46 | .072 |
| Normal appearing white matter (mL) | 442 | 63.0 | 57 | 450 | 55.1 | 46 | .536 |
| White matter hyperintensities (mL) | 12.7 | 12.5 | 55 | 17.4 | 20.7 | 44 | .167 |
| Accelerated aging (months) | 0.1 | 7.0 | 58 | 3.0 | 8.8 | 46 | .062 |
| Total recall (nr. words) | 22.9 | 4.8 | 55 | 24.6 | 6.2 | 41 | .132 |
Abbreviation: HA-PCI, hippocampal avoidance prophylactic cranial radiation.
Characteristics for patients for whom MRI data from the baseline and 4-month measurement were available.
Table 2.
Time × Group Analyses, Complete Sample
| Time Point | Factor | Hippocampus | Gray Matter | NAWM | WMHI | Accelerated Aging | Total Recall | |
|---|---|---|---|---|---|---|---|---|
| Baseline, 4-month follow-up | Time | F | 62.127 | 58.777 | 20.047 | 42.311 | 140.217 | 17.663 |
| P | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||
| Group | F | .002 | 3.374 | .315 | 1.980 | 3.287 | .778 | |
| P | .966 | .069 | .576 | .163 | .073 | .380 | ||
| Time × Group | F | 4.453 | .029 | .768 | .434 | .089 | 2.252 | |
| P | .037 | 0.866 | .383 | .512 | .766 | .137 | ||
| HA-PCI | n | 57 | 57 | 57 | 55 | 58 | 55 | |
| PCI | n | 46 | 46 | 46 | 44 | 46 | 41 | |
| Baseline, 4- and 12-month follow-up | Time | F | 77.611 | 16.501 | 71.091 | 18.399 | 47.22 | 1.521 |
| P | <.001 | <.001 | <.001 | <.001 | <.001 | .229 | ||
| Group | F | 1.072 | 2.806 | .004 | 1.905 | 2.749 | .098 | |
| P | .305 | .112 | .948 | .174 | .103 | .755 | ||
| Time × Group | F | 7.138 | 1.305 | 1.536 | 1.354 | .149 | .792 | |
| P | .002 | .280 | .225 | .268 | .862 | .459 | ||
| HA-PCI | n | 28 | 28 | 28 | 29 | 29 | 25 | |
| PCI | n | 27 | 27 | 27 | 23 | 27 | 23 |
Abbreviations: HA-PCI, hippocampal avoidance prophylactic cranial radiation; NAWM, normal-appearing white matter; WMHI, white matter hyperintensities.
Significant P values are displayed in bold.
Fig. 2.
Time × Group analyses for 4-month follow-up and 4- and 12-month follow-up. For clarity, the figure shows percentage change compared to pre-(HA-)PCI baseline (BL) measurement. Statistics as shown in Table 2 were performed on raw data. Panel A shows the primary outcome measure, hippocampal volume. For accelerated aging (panel E), chronological aging is shown as a dotted line as a reference. See text for details.
For the 4 months vs baseline analysis, the main effect of Time was highly significant for all outcome measures (P < .001), indicating volume reduction of the hippocampus, gray matter and normal-appearing white matter, volume increase of white matter hyperintensities, accelerated brain aging, and decrease in memory performance irrespective of the type of PCI. Brain-predicted age difference was 3.1 years (CI 2.4-3.8 years) for HA-PCI patients and 3.0 years (CI 2.2-3.7 years) for PCI patients. This indicates that brain aging was on average 8.4 faster than chronological aging for both groups. A significant Time × Group interaction for hippocampal volume indicated less volume decline for the HA-PCI (−67 mm3, CI −95 to −40 mm3) than the PCI group (−116 mm3, CI −156 to −77 mm3). This indicates a volume decline of 1.8% for the HA-PCI group and a volume decline of 3.0% for the PCI group. No other significant Time × Group interactions were found.
For the 4- and 12-month follow-up vs baseline, the main effect of Time was highly significant for all outcome measures (P < .001), except for total recall. This again indicated volume reduction of the hippocampus, gray matter and normal-appearing white matter, volume increase of white matter hyperintensities, and brain-predicted age difference of 4.2 years (CI 2.8-5.6 years) for HA-PCI patients and 3.8 years (CI 2.2-5.3 years) for PCI patients. This indicates that brain aging was on average 4.9 and 4.5 times faster than chronological aging for the HA-PCI and PCI groups, respectively. Similar to the 4 months vs baseline analysis, a significant Time × Group interaction for hippocampal volume indicated less volume decline for the HA-PCI group (−115 mm3, CI −154 to −78 mm3) than for the PCI group (−215 mm3, CI −273 to −158 mm3). This indicates a volume decline of 3.0% for the HA-PCI group and 5.8% for the PCI group. No other significant Time × Group interactions were found.
A sensitivity analysis, excluding patients who developed brain metastases, showed comparable statistical results (Table 3).
Table 3.
Time × Group Analyses, Excluding Patients Who Developed Brain Metastases
| Time Point | Factor | Hippocampus | Gray Matter | NAWM | WMHI | Accelerated Aging | Memory Recall | |
|---|---|---|---|---|---|---|---|---|
| Baseline, 4-month follow-up | Time | F | 59.388 | 63.217 | 19.764 | 38.739 | 121.964 | 16.067 |
| P | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||
| Group | F | .242 | 5.720 | 1.436 | 4.210 | 5.110 | .957 | |
| P | .624 | .019 | .234 | .043 | .026 | .331 | ||
| Time × Group | F | 4.989 | 0.248 | 0.977 | 0.466 | 0.001 | 2.810 | |
| P | .028 | .619 | .326 | .496 | .982 | .097 | ||
| HA-PCI | n | 54 | 54 | 54 | 52 | 55 | 52 | |
| PCI | n | 40 | 40 | 40 | 38 | 40 | 37 | |
| Baseline, 4- and 12-month follow-up | Time | F | 102.982 | 15.691 | 67.998 | 16.615 | 39.498 | 2.603 |
| P | <.001 | <.001 | <.001 | <.001 | <.001 | .087 | ||
| Group | F | 1.867 | 1.780 | 0.012 | 2.096 | 3.618 | .054 | |
| P | .178 | .189 | .915 | .154 | .063 | .817 | ||
| Time × Group | F | 15.593 | 2.162 | 1.926 | 1.059 | 0.261 | 1.190 | |
| P | <.001 | .127 | .157 | .355 | .772 | .315 | ||
| HA-PCI | n | 25 | 25 | 25 | 26 | 26 | 23 | |
| PCI | n | 24 | 24 | 24 | 22 | 24 | 20 |
Abbreviations: HA-PCI, hippocampal avoidance prophylactic cranial radiation; NAWM, normal-appearing white matter; WMHI, white matter hyperintensities.
Significant P values are displayed in bold.
Associations of Dosimetric Variables With Changes in Hippocampal Volume in the HA-PCI Group
No significant correlations were found between mean dose to the left and right hippocampus, and D1% to the left and right hippocampus, with changes in left and right hippocampal volume in the HA-PCI group at 4 and 12 months compared to the pre-HA-PCI baseline measurement (Supplementary Table 1).
Associations of MRI Outcomes Measures With Recall
No significant associations were found between MRI outcomes and memory outcomes. Left and right hippocampal volume at baseline showed the expected negative correlation with age, although this was only significant for the HA-PCI group. Hippocampal volume at baseline was not associated with total recall at baseline. It was also not associated with change in total recall from baseline to 4 months and from baseline to 12 months. Hippocampal volume change from baseline to 4 months and from baseline to 12 months was not associated with change in total recall from baseline to 4 months and from baseline to 12 months (Supplementary Tables 2 and 3). Hippocampal volume at baseline was also not significantly correlated with delayed recall (Supplementary Tables 4 and 5). White matter hyperintensities and normal-appearing white matter at baseline showed correlations in the expected direction with age. White matter hyperintensities and normal-appearing white matter at baseline, as well as changes between time points were, however, not significantly associated with (changes in) total recall (Supplementary Tables 6 and 7).
Discussion
To the best of our knowledge, this is the first study to show that HA-PCI reduces hippocampal atrophy compared to conventional PCI. Although atrophy was still apparent in the HA-PCI group, it was only about 50% of the rate of atrophy observed in patients exposed to conventional PCI. A previous study failed to show a difference in hippocampal volume between HA-PCI and PCI. This was, however, a cross-sectional comparison in a much smaller retrospective sample of MRIs acquired in a clinical context (n = 2 × 9).9
Our longitudinal neuroimaging measures converged to show that PCI (being conventional PCI or HA-PCI) has severe side effects as shown by various MRI indices for neurotoxicity.
In itself, it is not a novel finding that fractionated radiotherapy is associated with (MRI indices of) neurotoxicity,16–19 but our study is unique since we used state-of-the-art research MRIs acquired at fixed time points in a prospective randomized trial with a large number of patients. This allowed us to measure percentage volume change depending on brain tissue type and time since treatment. We showed that gray matter and normal-appearing white matter decreased with several percentages in volume, independent of hippocampal sparing. Machine learning-based brain age estimations put this in perspective by suggesting quite a dramatic accelerated brain aging that was on average 8.4 times higher than chronological aging (ie, the time that passed between the baseline and follow-up measurements) at 4 months. In addition, white matter hyperintensities almost doubled 12 months after baseline but did not differentially increase between hippocampal sparing and no sparing. This is in contrast to the aforementioned study that did not find hippocampal volume differences between HA-PCI and PCI.9 This study focused on periventricular white matter hyperintensities and used a manual rating method that might explain the discrepancy between our study. On the other hand, the Mayinger et al’s study hardly found any increase in white matter hyperintensities in the conventional PCI group which is in contrast to the literature.20 Therefore, the pattern of results of that study is somewhat puzzling.
Hippocampal sparing was clearly associated with a reduction in hippocampal atrophy at 4 and 12 months, as demonstrated by significant differences between the PCI and HA-PCI groups. It should be noted, however, that “sparing” is not absolute: even in the HA-PCI group the hippocampus received radiation (mean dose 8.0 Gy), and hippocampal volume also decreased significantly in the HA-PCI group albeit to a lesser extent than in the PCI group. Moreover, radiation dose within the HA-PCI group varied between patients. We did, however, not observe significant associations between dosimetric parameters and hippocampal volume change within the HA-PCI group. There are various explanations for the absence of significant associations: (i) volume decline was possibly mainly the result of normal aging and therefore not associated with radiotherapy dose to the hippocampus in the HA-PCI group. The 3% volume decline we found at 12 months was, however, larger than the 1.4% that has been reported for normal aging, making this explanation not very likely,21 (ii) the hippocampus is sensitive to neurotoxicity at relatively low doses of radiation, but the variability in the HA-PCI group was too small to demonstrate a dose-response relationship, (iii) the sample size was too small to detect relatively small differences in hippocampal volume change, (iv) the used methodology (type of MRI scan and/or segmentation method) was not sufficiently sensitive to detect relatively small differences in hippocampal volume change as a result of dose variations to the hippocampus.
The neuroimaging outcomes presented here provide important additional information to our RCT where we did not observe a benefit of HA-PCI vs PCI on HVLT-R total recall (the primary endpoint) or on HVLT-R delayed recall (one of the secondary endpoints) at any of the evaluated time points (4, 8, 12, and 24 months).7 A major concern raised was that we insufficiently succeeded to reduce the radiation dose to the hippocampus.22,23 Here we provide neuroanatomical evidence that HA-PCI actually did reduce radiation-induced hippocampal atrophy. In addition, we performed extensive quality assurance on the radiotherapy planning and execution7,24,25 and maintained wide margins for hippocampal delineation uncertainties.26 We therefore consider the explanation that we did not find a beneficial effect of HA-PCI on memory because of insufficient sparing of the hippocampus unlikely.
Several alternative explanations can be brought forward to explain disparate findings between our RCT and closely related RCTs. Considering the positive NRG-CC001 trial in patients with brain metastases who were randomized between HA-WBRT and conventional WBRT (both with memantine treatment),5 the combination of hippocampal sparing and memantine might have had synergistic effects on cognitive functioning.27 Another explanation for a specific advantage of HA-WBRT over WBRT in patients with brain metastases as compared to advantage of HA-PCI over PCI in patients without brain metastases, might be the higher level of brain injury outside the hippocampus due to the presence of brain metastases and higher RT dose in the former case. It might be argued that as a result of this higher level of brain injury, patients with brain metastases rely more on the hippocampus for cognitive functioning as a compensatory mechanism, which would explain the beneficial effect of hippocampal sparing.28 It should be noted that the primary endpoint for this trial was not memory-specific (ie, time to cognitive failure on any neuropsychological test). With regard to HVLT-R total recall and delayed recall, for the 2-, 4-, and 6-month time points evaluated, a significant advantage for HA-WBRT over WBRT was only observed for HVLT-R total recall at 6 months. In the RTOG 0614 trial where patients with brain metastases were randomized between WBRT with or without memantine and evaluated at 2, 4, and 6 months, HVLT-R delayed recall was the primary endpoint. No statistically significant beneficial effect of memantine over no memantine was observed at any time point although performance on the HVLT-R delayed recall, not the HVLT-R total recall, leaned toward significance for the memantine vs the no memantine group at 2 and 6 months.29
Combining the results of these studies, it seems uncertain which HVLT-R subtest is more suited to isolate hippocampal (dys)function. If anything, the beneficial effects of hippocampal sparing and/or memantine on HVLT-R performance do not appear to be particularly sound. Finally, the study most closely related to our study is the PREMER study, which compared HA-PCI with PCI (both arms without memantine) in SCLC patients not yet diagnosed with brain metastases. In contrast to our study, the NRG-CC003 trial, the RTOG 0614 trial, and many other brain radiotherapy studies with cognitive endpoints,30–34 the PREMER study used an alternative memory test, the FCSRT. No other cognitive domains were evaluated. This study showed a statistically significant advantage of HA-PCI compared to PCI on the primary endpoint, “delayed free recall” at 3 months. HA-PCI was also significantly better than PCI at other time points and for other FCSRT-based memory outcomes.6 The FCSRT, therefore, seems superior to HVLT-R in isolating hippocampal (dys)function in the context of brain radiotherapy, although it should be applied more often in this field to allow a fair comparison.
In our view, it is beyond doubt that all described (sub)tests measure (episodic) memory and therefore tap into hippocampal (dys)function, although some tests may succeed better than others. Comparing HVLT-R total recall and HVLT-R delayed recall, it should be noted that performance on both subtests is typically strongly correlated, which argues against the notion that they tap into qualitatively different aspects of memory. A potential advantage of the HVLT-R total recall is that it is closely linked to hippocampus-dependent memory consolidation. A potential advantage of the HVLT-R delayed recall is that it is less influenced by working memory, which does not rely on the hippocampus.35 The FCSRT has some potential advantages over the HVLT-R in general because it aims at limiting both confounding, non-hippocampal-driven effects of inattention and working memory on memory performance.6,36,37
The present study allowed us to directly compare (changes in) hippocampal volume and white matter volume with (changes in) memory functioning. Importantly, both the decline in brain volumes as well as the decline in memory were highly significant at 4 months (independent of the study arm). There were, however, no direct associations between brain volume declines and memory decline. First, it should be noted that in general, the literature on hippocampal volume (changes) and memory is inconclusive.38–45 Second, the decline in memory after (HA-)PCI may not primarily depend on radiation-induced atrophy of separate brain regions but may be better explained as the result of alterations in brain networks supporting cognitive functions instead of single “modules” like the hippocampus.46
Perhaps the most pertinent question that arises from this and related studies is Should we prescribe HA-PCI to SCLC patients? For HA-PCI to become the standard of care, further study is needed. While the results of the NRG-CC003 trial (NCT02635009) are eagerly awaited, the conclusion from contemporary theoretical frameworks on brain function seems to be, that selectively reducing radiotherapy dose might only be of clinical benefit for cognitive functioning when entire networks are spared, eg, the “default mode” network in the case of memory function.47 This network of interconnected brain regions has been uncovered with “resting-state fMRI,” a technique that allows the reconstruction of networks that are involved in cognitive function, and is based on fMRI scans that are acquired when an individual is not explicitly instructed to perform a cognitive test. The “default mode” network is considered to represent the backbone of cortical integration48 and next to the hippocampus it encompasses many cortical brain regions. Connectivity within the “default mode” network has been found to correlate with cognitive functioning in several cognitive domains, including memory. Of course, sparing of large brain volumes brings up safety issues, as receiving therapeutic doses of radiation will inevitably increase the risk of brain metastases. Moreover, as the “default mode” network might vary considerably at the individual level, radiotherapy dose distributions should preferably be tailored accordingly.49 Assuming that sparing the hippocampus from receiving high radiation dose truly results in preservation of (a subcomponent of) memory function (as indicated by the PREMER trial), the most important question for patients is to what extent this will benefit their quality of life. Indications that this is the case were reported for the NRG-CC001 trial5 but not the PREMER trial.6 For the present study, the manuscript of the quality of life results is in preparation. For the broader question whether we should still be describing PCI altogether, or should adapt a strategy of MRI surveillance, we refer to a recent review on this matter.50
Several limitations should be noted for the current study. We did not directly explore the (individually variable) locations of hot spots and their association with MRI measures at these specific locations. This would require registration of dose distributions to the MRI scans at the individual level which was outside the scope of this study. Also, more advanced MRI sequences may be more sensitive to relatively subtle regional overdosing.
In conclusion, we showed for the first time that HA-PCI reduces hippocampal atrophy compared to regular PCI, whereas both techniques are associated with considerable brain injury as shown by various MRI indices. The neurocognitive benefit of sparing the hippocampus in the context of PCI is still subject to debate.
Supplementary Material
Acknowledgments
We are indebted to the patients who have participated in our clinical trial, to the radiation oncologists from all participating institutions for supporting the study, and to Marianne Kuenen for her tireless efforts as a study coordinator and research assistant.
Contributor Information
Michiel B de Ruiter, Division of Psychosocial Research and Epidemiology, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Paul F C Groot, Department of Radiology and Nuclear Medicine, Amsterdam University Medical Center, location AMC, University of Amsterdam, The Netherlands.
Sabine Deprez, Department of Imaging and Pathology, KU Leuven, Leuven, Belgium; Leuven Cancer Institute, KU Leuven, Leuven, Belgium.
Pim Pullens, Department of Radiology, Ghent University, Ghent, Belgium.
Stefan Sunaert, Department of Imaging and Pathology, KU Leuven, Leuven, Belgium.
Dirk de Ruysscher, Radiation Oncology (MAASTRO), School for Oncology and Developmental Biology, Maastricht University Medical Center, Maastricht, The Netherlands.
Sanne B Schagen, Division of Psychosocial Research and Epidemiology, The Netherlands Cancer Institute, Amsterdam, The Netherlands; Brain and Cognition, Department of Psychology, University of Amsterdam, Amsterdam, The Netherlands.
José Belderbos, Radiation Oncology, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Funding
This work was supported by the Institute for the Promotion of Innovation by Science and Technology in Flanders (Grant No. IWT 130262), the Vlaamse Liga Tegen Kanker (VLK), Fonds NutsOhra (Grant No. 1003-105), and the Dutch Cancer Society (Grant No. 2013-6096).
Conflict of interest statement. D.R. reports receiving grants and other fees from AstraZeneca and Bristol Myers Squibb; other fees from BeiGene, Seattle Genetics, and Philips Health; and grants from Olink outside of the submitted work. All financial support and fees were institutional, not personal. The remaining authors declare no conflict of interest.
Authorship statement. D.R., S.B.S., and J.B. initiated and designed the study and were involved in data interpretation, manuscript drafting, reading, and approving the final version. M.B.R. was involved in study design, data collection, data analysis, data interpretation, manuscript drafting, reading, and approving the final version. P.F.C.G. was involved in data analysis, data interpretation, manuscript drafting, reading, and approving the final version. S.D., P.P., and S.S. were involved in study design, data collection, data interpretation, manuscript drafting, reading, and approving the final version.
References
- 1. Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341(7):476–484. [DOI] [PubMed] [Google Scholar]
- 2. Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357(7):664–672. [DOI] [PubMed] [Google Scholar]
- 3. Le Péchoux C, Sun A, Slotman BJ, et al. Prophylactic cranial irradiation for patients with lung cancer. Lancet Oncol. 2016;17(7):e277–e293. [DOI] [PubMed] [Google Scholar]
- 4. Tome WA, Gokhan S, Gulinello ME, et al. Hippocampal-dependent neurocognitive impairment following cranial irradiation observed in pre-clinical models: current knowledge and possible future directions. Br J Radiol. 2016;89(1057):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown PD, Gondi V, Pugh S, et al. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol. 2020;38(10):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodrıguez de Dios N, Couñago F, Murcia-Mejía M, et al. Randomized phase III trial of prophylactic cranial irradiation with or without hippocampal avoidance for small-cell lung cancer (PREMER): a GICOR-GOECP-SEOR study. J Clin Oncol. 2021;39(28):3118–3127. [DOI] [PubMed] [Google Scholar]
- 7. Belderbos JSA, De Ruysscher DKM, De Jaeger K, et al. Phase 3 randomized trial of prophylactic cranial irradiation with or without hippocampus avoidance in SCLC (NCT01780675). J Thorac Oncol. 2021;16(5):840–849. [DOI] [PubMed] [Google Scholar]
- 8. Breen WG, Anderson SK, Carrero XW, et al. Final report from Intergroup NCCTG 86-72-51 (Alliance): a phase III randomized clinical trial of high-dose versus low-dose radiation for adult low-grade glioma. Neuro Oncol. 2020;22(6):830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayinger M, Kraft J, Lohaus N, et al. Leukoencephalopathy after prophylactic whole-brain irradiation with or without hippocampal sparing: a longitudinal magnetic resonance imaging analysis. Eur J Cancer. 2020;124:194–203. [DOI] [PubMed] [Google Scholar]
- 10. Deprez S, de Ruiter MB, Bogaert S, et al. Multi-center reproducibility of structural, diffusion tensor, and resting state functional magnetic resonance imaging measures. Neuroradiology. 2018;60(6):617–634. [DOI] [PubMed] [Google Scholar]
- 11. Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test—revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12(1):43–55. [Google Scholar]
- 12. Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmidt P. Bayesian Inference for Structured Additive Regression Models for Large-Scale Problems with Applications to Medical Imaging. Chapter 6.1 [dissertation]. München: Faculty of Mathematics, Computer Science and Statistics, LMU München; 2016. https://edoc.ub.uni-muenchen.de/20373/ [Google Scholar]
- 14. Ribaldi F, Altomare D, Jovicich J, et al. Accuracy and reproducibility of automated white matter hyperintensities segmentation with lesion segmentation tool: a European multi-site 3T study. Magn Reson Imaging. 2021;76:108–115. [DOI] [PubMed] [Google Scholar]
- 15. Cole JH, Underwood J, Caan MWAA, et al. Increased brain-predicted aging in treated HIV disease. Neurology. 2017;88(14):1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagtegaal SHJ, David S, van Grinsven EE, et al. Morphological changes after cranial fractionated photon radiotherapy: localized loss of white matter and grey matter volume with increasing dose. Clin Transl Radiat Oncol. 2021;31:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagtegaal SHJ, David S, Snijders TJ, et al. Effect of radiation therapy on cerebral cortical thickness in glioma patients: treatment-induced thinning of the healthy cortex. Neurooncol Adv. 2020;2(1):vdaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagtegaal SHJ, David S, van der Boog ATJ, Leemans A, Verhoeff JJC. Changes in cortical thickness and volume after cranial radiation treatment: a systematic review. Radiother Oncol. 2019;135:33–42. [DOI] [PubMed] [Google Scholar]
- 19. Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol. 2016;13(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheline GE. Radiation therapy of brain tumors. Cancer. 1977;39(2S):873–881. [DOI] [PubMed] [Google Scholar]
- 21. Barnes J, Bartlett JW, van de Pol LA, et al. A meta-analysis of hippocampal atrophy rates in Alzheimer’s disease. Neurobiol Aging. 2009;30(11):1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Breen WG, Brown PD, Laack NN. Hippocampal avoidance prophylactic cranial irradiation for SCLC. J Thorac Oncol. 2021;16(6):e41–e42. [DOI] [PubMed] [Google Scholar]
- 23. Mladkova N, Lo S, Brown PD, Gondi V, Palmer JD. Hippocampal avoidance prophylactic cranial irradiation: interpreting the evidence. J Thorac Oncol. 2021;16(8):e60–e63. [DOI] [PubMed] [Google Scholar]
- 24. Belderbos JSA, De Ruysscher DKM, De Jaeger K, et al. Why did the randomized trial of prophylactic cranial irradiation with or without hippocampus avoidance in SCLC not reveal a difference? J Thorac Oncol. 2021;16(6):e42–e45. [DOI] [PubMed] [Google Scholar]
- 25. Belderbos JSA, De Ruysscher DKM, De Jaeger K, et al. Reaction on the interpretation of the hippocampus avoidance prophylactic cranial irradiation trial in SCLC (NCT01780675). J Thorac Oncol. 2021;16(8):e63–e65. [DOI] [PubMed] [Google Scholar]
- 26. Bartel F, van Herk M, Vrenken H, et al. Inter-observer variation of hippocampus delineation in hippocampal avoidance prophylactic cranial irradiation. Clin Transl Oncol. 2019;21(2):178–186. [DOI] [PubMed] [Google Scholar]
- 27. Duman JG, Dinh J, Zhou W, et al. Memantine prevents acute radiation-induced toxicities at hippocampal excitatory synapses. Neuro Oncol. 2018;20(5):655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Baene W, Rutten GJM, Sitskoorn MM. Cognitive functioning in glioma patients is related to functional connectivity measures of the non-tumoural hemisphere. Eur J Neurosci. 2019;50(12):3921–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 33. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun A, Bae K, Gore EM, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29(3):279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gazzaniga M, Ivry R, Mangun G.. Cognitive Neuroscience: The Biology of the Mind. 4th ed.2014; New York, NY: W.W. Norton & Company. [Google Scholar]
- 36. Brown PD, Parsons MW, Rusthoven CG, Gondi V. Hippocampal avoidance prophylactic cranial irradiation: a new standard of care? J Clin Oncol. 2021;39(28):3093–3096. [DOI] [PubMed] [Google Scholar]
- 37. Novellino F, Vasta R, Sarica A, et al. Relationship between hippocampal subfields and category cued recall in AD and PDD: a multimodal MRI study. Neuroscience. 2018;371:506–517. [DOI] [PubMed] [Google Scholar]
- 38. Bonner-Jackson A, Mahmoud S, Miller J, Banks SJ. Verbal and non-verbal memory and hippocampal volumes in a memory clinic population. Alzheimers Res Ther. 2015;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okoukoni C, McTyre ER, Ayala Peacock DN, et al. Hippocampal dose volume histogram predicts Hopkins Verbal Learning Test scores after brain irradiation. Adv Radiat Oncol. 2017;2(4):624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pohlack ST, Meyer P, Cacciaglia R, et al. Bigger is better! Hippocampal volume and declarative memory performance in healthy young men. Brain Struct Funct. 2014;219(1):255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16(5):292–305. [DOI] [PubMed] [Google Scholar]
- 42. Clark IA, Monk AM, Hotchin V, et al. Does hippocampal volume explain performance differences on hippocampal-dependent tasks? Neuroimage. 2020;221:117211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zahodne LB, Schupf N, Brickman AM. Control beliefs are associated with preserved memory function in the face of low hippocampal volume among diverse older adults. Brain Imaging Behav. 2018;12(4):1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Den Heijer T, Van Der Lijn F, Koudstaal PJ, et al. A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain. 2010;133(4):1163–1172. [DOI] [PubMed] [Google Scholar]
- 45. Abraham CD, Pugh SL, Bovi JA, et al. Association of pre-treatment hippocampal volume with neurocognitive function in patients treated with hippocampal avoidance whole brain radiotherapy for brain metastases: secondary analysis of NRG Oncology/RTOG 0933 [published online ahead of print December 11, 2021]. Adv Radiat Oncol. doi: 10.1016/j.adro.2021.100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bassett DS, Sporns O. Network neuroscience. Nat Neurosci. 2017;20(3):353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kocher M, Jockwitz C, Caspers S, et al. Role of the default mode resting-state network for cognitive functioning in malignant glioma patients following multimodal treatment. Neuroimage Clin. 2020;27:102287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network. Ann N Y Acad Sci. 2008;1124(1):1–38. [DOI] [PubMed] [Google Scholar]
- 49. Sours Rhodes C, Zhang H, Patel K, et al. The feasibility of integrating resting-state fMRI networks into radiotherapy treatment planning. J Med Imaging Radiat Sci. 2019;50(1):119–128. [DOI] [PubMed] [Google Scholar]
- 50. Taylor JM, Rusthoven CG, Moghanaki D. Prophylactic cranial irradiation or MRI surveillance for extensive stage small cell lung cancer. J Thorac Dis. 2020;12(10):6225–6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.