Abstract
Background
The incidence and biology of IDH1/2 mutations in pediatric gliomas are unclear. Notably, current treatment approaches by pediatric and adult providers vary significantly. We describe the frequency and clinical outcomes of IDH1/2-mutant gliomas in pediatrics.
Methods
We performed a multi-institutional analysis of the frequency of pediatric IDH1/2-mutant gliomas, identified by next-generation sequencing (NGS). In parallel, we retrospectively reviewed pediatric IDH1/2-mutant gliomas, analyzing clinico-genomic features, treatment approaches, and outcomes.
Results
Incidence: Among 851 patients with pediatric glioma who underwent NGS, we identified 78 with IDH1/2 mutations. Among patients 0–9 and 10–21 years old, 2/378 (0.5%) and 76/473 (16.1%) had IDH1/2-mutant tumors, respectively. Frequency of IDH mutations was similar between low-grade glioma (52/570, 9.1%) and high-grade glioma (25/277, 9.0%). Four tumors were graded as intermediate histologically, with one IDH1 mutation. Outcome: Seventy-six patients with IDH1/2-mutant glioma had outcome data available. Eighty-four percent of patients with low-grade glioma (LGG) were managed observantly without additional therapy. For low-grade astrocytoma, 5-year progression-free survival (PFS) was 42.9% (95%CI:20.3–63.8) and, despite excellent short-term overall survival (OS), numerous disease-related deaths after year 10 were reported. Patients with high-grade astrocytoma had a 5-year PFS/OS of 36.8% (95%CI:8.8–66.4) and 84% (95%CI:50.1–95.6), respectively. Patients with oligodendroglioma had excellent OS.
Conclusions
A subset of pediatric gliomas is driven by IDH1/2 mutations, with a higher rate among adolescents. The majority of patients underwent upfront observant management without adjuvant therapy. Findings suggest that the natural history of pediatric IDH1/2-mutant glioma may be similar to that of adults, though additional studies are needed.
Keywords: frequency, glioma, IDH1/2 mutation, outcomes, pediatrics
Key Points.
IDH1/2 mutations are present in a subset of pediatric glioma, validating prior data.
Most pediatric patients with IDH-mutant LGG are observed without adjuvant treatment at study institutions.
Long-term outcomes of pediatric and adult IDH-mutant glioma are similar.
Importance of the Study.
IDH1/2 mutations have traditionally been considered rare in pediatric glioma, with a paucity of clinical data in this population. In contrast, such mutations have commonly been described in adult gliomas and are typically associated with gliomagenesis and progression to higher grade. Importantly, the treatment approaches adopted by pediatric and adult oncologists tend to differ significantly, with unclear impact on outcomes. In this study, we systematically evaluate the frequency, treatment approaches, and outcomes of pediatric patients with IDH1/2-mutant glioma. We show that a significant proportion of pediatric gliomas harbor IDH1/2 mutations and most patients were managed observantly without adjuvant therapy, unlike their adult counterparts. However, long-term outcomes in pediatrics were similar to those reported in adult studies, supporting a role for adaptation and incorporation of adult treatment approaches in pediatric cases.
Gliomas are the most common primary central nervous system (CNS) tumor in children.1 Despite histological similarities to adult tumors, pediatric gliomas are often clinically and molecularly distinct. Children diagnosed with conventional pediatric low-grade glioma (LGG) have an excellent prognosis.2 Contrastingly, the majority of adult LGGs ultimately undergo malignant transformation to HGGs with poorer prognosis.2,3 A key driver in this difference in clinical behavior is the fact that pilocytic astrocytoma is the most common glioma in the pediatric population, and comprises 17.6% of all pediatric brain tumors.1 Overall, pediatric LGGs, whether circumscribed or diffuse, are typically driven by activating alterations in the MAPK pathway (such as the KIAA1549::BRAF fusion observed in pilocytic astrocytoma). In contrast, adult LGGs are classically characterized by IDH1/2 mutations with frequent cooccurring alterations.4–6 This has led the 5th edition of the WHO Classification of CNS tumors to distinguish between “pediatric type” and “adult type” tumors, with IDH-mutant gliomas considered one of the adult types.7
While IDH1/2 mutations were traditionally considered rare in pediatric glioma, recent studies report a wide possible range of frequency from 1% to 20%, with higher rates in adolescence.8–12 Furthermore, the prognostic significance of IDH1/2 mutation in the pediatric population is not fully understood. This has critical clinical implications, as the treatment approach for LGGs differs significantly between adults and pediatrics. Radiation therapy (RT) is often delayed or avoided in children and adolescents due to its associated long-term toxicities.13,14
For older adults, multimodal therapy is considered standard-of-care, with initial maximal safe resection followed by irradiation and chemotherapy. This practice is based on prospective trials showing improved outcomes with these approaches, especially among patients with residual tumor after upfront resection.15–17 Observant management is typically limited to younger patients with complete resections and favorable genomics. The pediatric oncology community has not generally adopted these practices given the paucity of data surrounding pediatric IDH-mutant glioma.
It is unclear if IDH1/2 mutations occurring in children and adolescent with gliomas confer similar prognostic significance to warrant such an aggressive upfront approach. In this multi-institutional, retrospective investigation, we sought to determine the frequency of IDH1/2 mutation among pediatric gliomas, and to describe the clinicopathologic features, genomic landscape, and efficacy of current treatment approaches. This study aims to improve our understanding of this disease and aid in establishing best practices for this age group.
Methods
This study includes two parallel analyses with distinct objectives and methodologies. One part involved five collaborating institutions to evaluate the overall frequency of IDH1/2-mutant glioma in pediatrics (sequencing cohort). The second part involved nine collaborating sites and aimed to evaluate the clinical features, treatment, genomics, and outcomes of pediatric patients with IDH-mutant glioma (clinical cohort). The institutional review board for each site approved this retrospective review (DF/HCC protocol 19-548).
Frequency of IDH1/2-mutant Glioma in the Pediatric Population (Sequencing Cohort)
The frequency of IDH1/2-mutant glioma in the pediatric population was determined by evaluating IDH1/2-mutational frequency through next-generation sequencing (NGS) of gliomas among patients under 21 years. This multi-institutional collaboration included four children’s hospitals or cancer centers [Children’s Hospital of Philadelphia (CHOP), Children’s Hospital Los Angeles (CHLA), Dana-Farber Cancer Institute/Boston Children’s Hospital (DFCI/BCH), Memorial Sloan Kettering Cancer Center (MSKCC)] and an industry partner (Tempus Labs, Inc.). These sites were chosen for this part of the study specifically because they utilized established NGS platforms and have routinely sequenced all gliomas (including pilocytic astrocytoma) during the noted study timeframes. All NGS platforms included unique cancer gene panels that included hotspot coverage of both IDH1 and IDH2 genes. A list of all genes tested on each individual platform and timeframe of testing is available in Figure 1A, Supplementary Table 1, respectively.
Fig. 1.
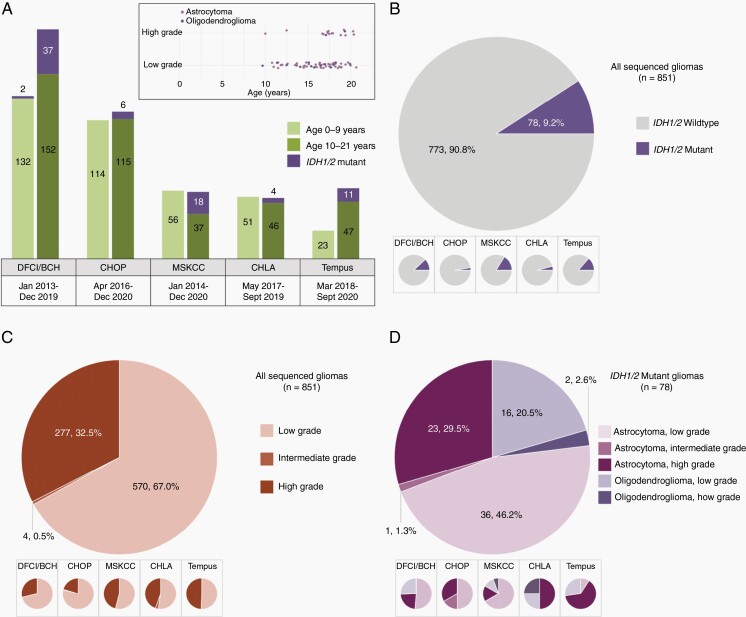
Age, histologic, and molecular features of the sequencing cohort. (A) Relative proportions of pediatric glioma (including IDH-mutant glioma) divided by site and age, with varied timeframe of testing as shown. (A, inset) Age distribution of IDH-mutant tumors, by histologic type. Note: Intermediate grade tumors were omitted (B–D) Site-specific and overall categorization of tumors included in the cohort, with site-specific breakdowns shown in inset panels: (B) Proportion of IDH mutant tumors identified among gliomas in sequencing cohort, (C) Breakdown of all gliomas included in sequencing cohort by histologic grade, (D) Histologic types and grades observed in IDH1/2-mutant gliomas.
Collaborators identified the number of IDH1/2-mutant gliomas in their respective cohorts over a specified timeframe and contributed data for their respective cohorts as it related to histological grade, subtype, and coexisting mutations/alterations. Timeframes included in the study varied by site (Figure 1A).
The frequency of IDH1/2-mutant glioma is reported as a fraction of all sequenced pediatric gliomas (encompassing all histologic grades, including circumscribed gliomas) as the denominator. Our methodology to determine the institution-specific and overall frequency of IDH1/2-mutant glioma is shown in Supplementary Figure 1.
Clinical Features and Outcome Data for Pediatric IDH1/2-mutant Glioma (Clinical Cohort)
To evaluate the clinical features and outcome data for pediatric IDH1/2 mutant glioma, we employed a multi-institutional retrospective approach with collaborators across nine institutions (CHOP, CHLA, OHSU Doernbecher Children’s Hospital, Orlando Health Arnold Palmer Hospital for Children, Lurie Children’s Hospital, Children’s Healthcare of Atlanta, St. Louis Children’s Hospital-Washington University, MSKCC, DFCI/BCH).
Patient selection
Using respective institutional databases, all patients with glioma diagnosed before the age of 21 years between 1990 and 2020 were identified. Patient selection timeframe was extended to 1990 to capture patients who may have been diagnosed with an IDH1/2 mutation at the time of recurrence. Patients treated at their respective institutions during that time were eligible for the clinical cohort if they had IDH1 or IDH2 mutation detected by immunohistochemistry and/or sequencing and had clinical follow-up data.
Data collection and analysis
Patient demographics, clinicopathological diagnosis, molecular features, clinical course, and outcomes were extracted from the medical record. All clinical data were de-identified at individual collaborating sites prior to secure, electronic data transfer between sites via Research Electronic Data Capture (REDCap).18
Progression-free survival (PFS) was calculated from date of diagnosis to date of disease progression. Overall survival (OS) was calculated from the date of diagnosis to date of death. Patients alive and/or without disease progression were censored at last follow-up. Both OS and PFS were estimated according to the Kaplan–Meier method, and differences in survival were assessed using the log-rank test.
Molecular features
Targeted NGS reports from respective institutions were analyzed for single nucleotide variants, indels, copy number variants (CNVs), and structural variants. Gene-level CNVs were not consistently reported among participating institutions, but this data was included when available. Reports from microarray-based comparative genomic hybridization were used to further evaluate CNVs in cases when available. All Tier 1, 2, and 3 alterations were collated and visualized using OncoPrint in the “ComplexHeatmap” package in R (v3.6). Only genes assayed in 80% of the samples were used for all subsequent analyses.
Results
Frequency of IDH1/2-mutant Glioma in the Pediatric Population (Sequencing Cohort)
We identified 851 patients with gliomas between the ages of 0–21 years whose tumor samples underwent NGS. Seventy-eight patient tumor samples harbored IDH1/2 mutations, accounting for 9.2% (78/851) of the entire cohort. Among these IDH1/2-mutant gliomas, 52 were histologically LGGs (WHO grade 1 & 2), comprising 9.1% (52/570) of all pLGG patients (n = 570). Twenty-five IDH1/2-mutant tumors were histologically HGGs (WHO 3 & 4), accounting for 9.0% (25/277) of all pHGG patients (n = 277). Pathologists designated four gliomas as intermediate grade (features indeterminate between low- and high-grade) that included one IDH1 mutation. Children aged 0–9 years accounted for 44.4% (378/851) of sequenced pediatric gliomas, of which only two possessed IDH1/2 mutations (2/378; 0.5%). Adolescents aged 10–21 years accounted for 55.6% (473/851) of the pediatric gliomas sequenced, with 76 IDH1/2-mutant tumors (76/473; 16.1%). Detailed analysis of data obtained from each institution is listed in Figure 1, Supplementary Table 2 and 3.
Patient Characteristics (Clinical Cohort)
In the clinical cohort, a total of 76 pediatric patients with IDH1/2 mutant gliomas were identified among the nine participating sites. Median age at diagnosis was 16.8 years (range:9.7–20.7 years). Sixty-one patients were diagnosed with astrocytoma, while 15 patients were diagnosed with oligodendroglioma (with 1p/19q codeletion).
Most tumors were supratentorial (n = 72), with only four tumors occurring in the posterior fossa (three brainstems, one cerebellar). None were reported in the suprasellar region, basal ganglia, thalamus, or spine. The majority of tumors were histologically low-grade [WHO grade 2 (58/76; 76.3%)]. One tumor was assigned an intermediate grade, and the remaining patients had high-grade tumors [WHO grade 3–4 (17/76; 22.4%)]. For patients with IDH1-mutant tumors, the canonical R132H was the most common alteration (61/68; 89.7%), followed by R132C (5/68; 7.4%), and R132S (2/68; 2.9%). For IDH2-mutant tumors, R172K and R172M were most common. All four posterior fossa tumors were HGGs, with two harboring the noncanonical R132C mutation.
All patients underwent an upfront surgical procedure for diagnostic and/or therapeutic indications. Thirty-one patients (31/76; 40.8%) underwent gross-total resections (GTR), 33 patients (33/76; 43.4%) had subtotal resections (STR), and 12 patients (12/76; 15.8%) had biopsies (Bx) only. Following surgery, 50/76 patients (65.8%) underwent expectant observation without additional adjuvant therapy. Of these patients, all but one had low-grade histology. Twenty-two patients (22/76; 28.9%) received upfront RT, with a median focal radiation dose of 5940 cGy (range:5400–6000). Most of the patients who received upfront RT had high-grade histology (16/22; 72.7%). Upfront chemotherapy was administered to a subset of patients (23/76; 30.2%). Of these, 19 patients received upfront RT followed by chemotherapy, 15 of whom had HGGs, three with LGGs, and one with intermediate grade histology. Patient characteristics, sorted by histologic type, are summarized in Table 1.
Table 1.
Patient Characteristics for Clinical Cohort
| Age at Diagnosis (years; N = 76) | ||
| Median | 16.8 | |
| Range | 9.7 – 20.7 | |
| Tumor Type | Astrocytoma (N = 61) | Oligodendroglioma (N = 15) |
| Age at Diagnosis | 16.8 [range: 10.0–20.7] | 16.7 [range:9.7–19.9] |
| Tumor Location | N (%) | N (%) |
| Supratentorial | 57 | 15 |
| Posterior Fossa (cerebellum/brain stem) | 4 | 0 |
| WHO Grade | ||
| 2 | 45 (73.8) | 13 (86.7) |
| 3 | 12 (19.7) | 2 (13.3) |
| 4 | 3 (4.9) | |
| Intermediate (2-3) | 1 (1.6) | |
| IDH1/2 Mutation | ||
| IDH1 | ||
| R132H | 49 (87.5) | 12 (100.0) |
| R132C | 5 (8.9) | 0 (0.0) |
| R132S | 2 (3.6) | 0 (0.0) |
| IDH2 | ||
| R172K | 0 (0.0) | 3 (100.0) |
| R172M | 3 (60.0) | 0 (0.0) |
| R172G | 1 (20.0) | 0 (0.0) |
| Other | 1 (20.0) | 0 (0.0) |
| Sequencing Results Available | 52 (85.2) | 10 (66.7) |
| MGMT Status | ||
| Methylated | 8 | 1 |
| Partially methylated | 1 | 1 |
| Unmethylated | 9 | 1 |
| Unknown | 43 | 12 |
| Extent of Resection | ||
| Biopsy | 10 (16.4) | 2 (13.3) |
| STR | 27 (44.3) | 6 (40.0) |
| GTR | 24 (39.3) | 7 (46.7) |
| Upfront Radiation | ||
| Yes | 19 (31.7) [14 HG, 4 LG, 1 intermediate] | 3 (18.7) [2 HGG, 1 LG] |
| Upfront Radiation Dose (Median cGy) | 5940 [Range: 5400–6000] | 5940 [Range: 5940–6000] |
| Upfront Chemotherapy | ||
| Yes | 19 (31.7) | 4 (25.0) |
| Temozolomide-based | 18 | 1 |
| Carboplatin/ Vincristine | 1 | 1 |
| PCV | 0 | 2 |
| Cycles of Chemotherapy (Median) | 12 [range: 0.5–19] | 13 [range: 6–18] |
| Upfront Observant Management | 40 (65.6) | 10 (66.7) |
| Low-grade (WHO grade 2) | 39 | 10 |
| Intermediate (WHO grade 2-3) | 0 | N/A |
| High-grade (WHO grade 3 & 4) | 1 | 0 |
Clinical Outcomes (Clinical Cohort)
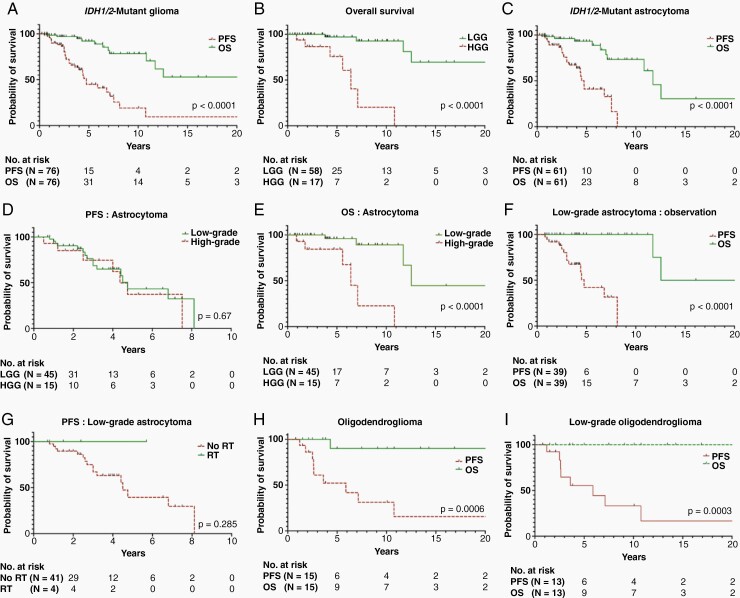
For the entire clinical cohort of pediatric IDH1/2-mutant gliomas (n = 76), which includes both astrocytoma and oligodendroglioma, with a median follow-up period of 4.0 years [range:0.2–32.4 years (IQR:2-7.5 years)], the 5- and 10-year PFS rates were 44.5% (95%CI:28.6–59.2) and 18.7% (95%CI:5.8–37.8), respectively; The 5- and 10-year OS rates were 92% (95%CI:79.3–96.6) and 78.2% (95%CI:59.7–88.5), respectively (Figure 2A). While histological grade was not associated with altered PFS [median survival: 4.8 (LGG) vs 4.4 years (HGG); P = .71 (Supplementary Figure 2A)], LGGs were associated with significantly better OS, with 5- and 10-year OS of 96.4% (95%CI:81.1–99.3) and 92.1% (95%CI:72.6–97.8), compared to 75.1% (95%CI:39.8–91.4) and 19.7% (95%CI:0.7–57.1) for HGGs, respectively [P < .0001 (Figure 2B)].
Fig. 2.
Progression-free and overall survival of (A) the entire cohort, (C) IDH1/2-mutant astrocytoma, (F) Low-grade astrocytoma observed upfront, (H) IDH1/2-mutant oligodendroglioma, and (I) Low-grade oligodendroglioma. Overall survival according to grade (low versus high) of (B) entire cohort and (E) astrocytoma. Progression-free survival of (D) astrocytoma according to grade (low/high) and (G) low-grade astrocytoma according to radiation therapy status.
IDH1/2-mutant Astrocytoma
For pediatric IDH1/2-mutant astrocytoma (n = 61), with a median follow-up period of 3.8 years (range:0.2–25.3), the 5- and 10-year PFS were 40.5% (95%CI:22.2–58.4) and 0%, respectively. The 5-, 10-, and 15-year OS were 92.9% (95%CI:79.4–97.5), 73.1% (95%CI:49.5–86.8), and 30.3% (95%CI:5.2–61.6), respectively (Figure 2C). Median PFS was 4.7 years, while the median OS for this cohort was 11.7 years. Histological grade did not significantly affect the PFS, with median PFS of 4.8 years for low-grade and 4.4 years for high-grade [P = .67, (Figure 2D)]. In contrast, high histological grade was associated with significantly shorter OS, with 5- and 10-year OS for high-grade tumors of 84% (95%CI:50.1–95.6) and 22.2% (95%CI:0.9–61.3), respectively, as compared to 95.4% (95%CI:74.5–99) and 88.6% (95%CI:61.2–97) for low-grade tumors (Figure 2E). This translates to a median OS for low-grade and high-grade IDH1/2-mutant astrocytoma of 12.5 and 6.4 years, respectively (P < .0001).
Outcomes for patients managed observantly (low-grade astrocytoma)
Most patients with low-grade diffuse astrocytoma underwent expectant observation without additional adjuvant therapy after initial surgery (39/45; 86.7%), including 20 patients (20/39; 51.3%) who underwent STR/Bx only. Among these 39 patients, the 5- and 10-year PFS was 41.7% (95%CI:17.5–64.7) and 0%, respectively, with a median PFS of 4.76 years. While OS for this observation-only cohort was 100% at 10 years with a median OS of 18.9 years, numerous deaths due to disease progression after year 10 were noted (Figure 2F). Interestingly within this cohort, extent of resection did not significantly impact survival outcomes [P = .51 (PFS) and P > .99 (OS)].
Impact of extent of upfront resection (low-grade astrocytoma)
Among all patients with low-grade astrocytoma (n = 45), upfront extent of resection was not associated with statistically significant improvement in survival outcomes. The 5-year PFS for patients with GTR was 45.7% (95%CI:8.5–77.6) with a median PFS of 4.4 years, while the 5-year PFS for patients with STR/Bx was 41% (95%CI:14.6–65.9) with a median PFS of 4.7 years (P = .4). In contrast, patients with GTR showed a trend towards better OS, with a 10-year OS of 100% compared to a 10-year OS of 81.3% (95%CI:41.6–95.2) for patients with STR/Bx (P = .23).
Impact of radiation (low-grade astrocytoma)
Only four patients with low-grade astrocytoma received upfront RT. At a median follow-up period of 1.94 years (range:0.7–5.7), none experienced disease progression/recurrence at time of last follow-up. Among the 41 patients who did not receive upfront RT, with a median follow-up period of 4 years (range:0.8–25.3), the 5-year PFS was 38.8% (95%CI:16.3–61.2) with a median PFS of 4.5 years [P = .28 (Figure 2G)].
Five patients with recurrent/progressive low-grade astrocytoma without progression to high-grade received radiation therapy at first progression [median time-to-progression: 2.5 years (range:0.8–6.8)]. Median follow-up period after RT was 8 years (range:3.5–9.7) with 5-year post-RT PFS and OS of 100%.
Impact of surgery on outcome for high-grade astrocytoma
In terms of upfront treatment for patients with high-grade astrocytoma (n = 15), better extent of resection showed a trend towards better PFS with a median of 7.5 years among patients receiving GTR compared to 4 years among patients with STR/Bx [P = .089 (Supplementary Fig. 2B)]. This translates to 5-year PFS of 66.4% (95%CI:5.2–94.3) among patients with GTR, while patients with STR/Bx had 5-year PFS of 19.9% (95%CI:0.7–57.6). Extent of resection did not significantly impact OS, with median OS of 8.6 for GTR vs 7.1 years for STR/Bx (P = .235).
IDH1/2-mutant Oligodendroglioma
Patients with IDH1/2 mutant oligodendroglioma (n = 15) had a median follow-up period of 7.5 years (range:1.5-32.4). Within this group, the 5- and 10-year PFS was 52% (95%CI:22.2–75.1) and 31% (95%CI:7.8–58.2), while the 5-, 10- and 15-year OS were all 89.8% (95%CI:47–98.2) (Figure 2H).
Outcome for low-grade oligodendroglioma
With a median follow-up of 9.1 years (range:1.5–32.4), these 13 patients had a 5- and 10-year PFS of 55% (95%CI:23.1–78.2) and 33.2% (95%CI:8.1–60.9), respectively, with a median PFS of 5.9 years (Figure 2I). All but one patient remained alive at the time of last follow-up (20-year OS of 100%; Figure 2I). Six patients had GTR upfront, while seven patients received STR or Bx at initial surgery. Extent of resection was not associated with significantly improved outcomes: GTR was associated with a 5- and 10-year PFS of 59.4% (95%CI:12.3–87.9) and 59.4% (95%CI:12.3–87.9) with a median PFS of 10.7 years, while STR/Bx led to a 5- and 10-year PFS of 51.1% (95%CI:11.4–81) and 16.8% (95%CI:0.7–52.4), respectively, with a median PFS of 5.9 years (P = .43).
Outcome for anaplastic oligodendroglioma
For the two patients with anaplastic oligodendroglioma, one was diagnosed at age 17.1 and the other at 19.3 years. Both patients received focal RT upfront followed by adjuvant chemotherapy. The patient who underwent upfront STR followed by RT and adjuvant temozolomide progressed after 1.8 years and ultimately died from disease 4.3 years after initial diagnosis. The other patient underwent upfront GTR followed by RT and adjuvant PCV for 6 cycles. This patient remains alive without evidence of disease 2.5 years after initial diagnosis.
Impact of Progression to Higher Histologic Grade
Among patients with low-grade histology (n = 58), 25 patients recurred/progressed. At first or subsequent disease progression, 6/16 (37.5%) astrocytomas and 3/9 oligodendrogliomas (33.3%) showed histologic high-grade features at recurrence. Of these 9 patients with progression to high-grade histology, only one with oligodendroglioma had received upfront RT at diagnosis (recurring 30.5 years later). The other eight patients had been treated with an upfront radiation-sparing approach. Additionally, only one of these patients had GTR upfront; the others had either STR (n = 6) or Bx (n = 2).
Median time-to-progression to high-grade for astrocytoma was 5.3 years (range:1.1–11.5), while the time-to-progression to high-grade for oligodendroglioma was 14.3 years (range:10.9–30.5). Upon progression to high-grade, all patients received RT. With a median follow-up of 3.1 years (range:1.9–4.3) after RT for the six patients with astrocytoma, 1-year PFS and OS were both 100%, while 3-year PFS and OS were 44.1% (95%CI:6.3–78.2) and 62.2% (95%CI:13.7–88.9), respectively. Of the three patients with oligodendroglioma, two remain alive at 5.2 and 2.3 years after progression to high-grade and RT. The one patient who received upfront radiation therapy progressed quickly following transformation and died of disease 1.9 years later despite re-irradiation.
Genomic Landscape (Clinical Cohort)
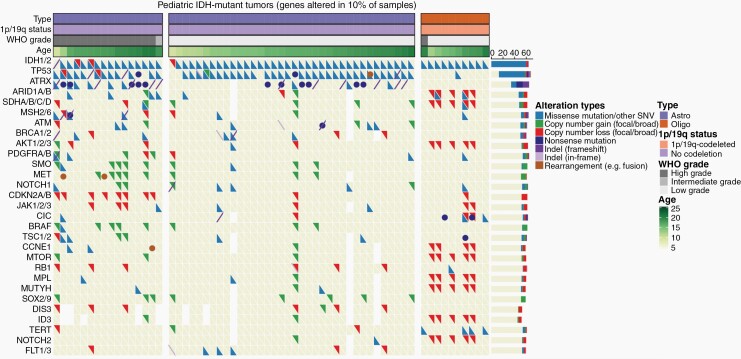
Comprehensive molecular reports were available for 62 of 76 patients in this cohort. Molecular analysis revealed numerous alterations cooccurring with IDH1/2 mutations (Figure 3, Supplementary Figure 3). In patients with astrocytoma, TP53 mutations were most frequent, identified in 92.3% (48/52) of cases, followed by alterations in ATRX (52.9%, 27/51), ATM (21.6%, 11/51), BRCA1/2 (20.0%, 10/50), MET (19.6%, 10/51), SMO (19.2%, 10/52), BRAF (17.3%, 9/52), CDKN2A/B (17.3%, 9/52), SOX2/9 (16.3%, 8/49), DIS3 (16.3%, 7/43), MSH2/6 (16.0%, 8/50), and ARID1A/B (16%, 8/50) (Supplementary Table 4). While oligodendrogliomas harbored copy number losses associated with chromosomal 1p/19q-codeletion in numerous genes (e.g., MUTYH, MPL, NOTCH2), additional mutations were observed most frequently in the TERT promoter region (40.0%, 4/10) and CIC (40.0%, 4/10). Notably, most BRAF alterations were copy number gains (n = 8/9, 88.9%) associated with gains in MET and/or SMO. These BRAF gains were part of broad 7q gains in all but one case. In addition, most of the CDKN2A/B alterations were single-copy deletions (n = 6/9, 66.7%).
Fig. 3.
Comutation plot displaying clinical features and molecular characteristics of 62 patients with pediatric IDH1/2-mutant glioma. Shown here are genes altered in at least 10% of the total sample, with the relative incidence (%) of coalterations.
To evaluate the impact of genetic alterations on outcomes, we first performed univariate time-to-event analyses on genes altered in more than 10% of the cohort. Notably, OS was significantly decreased in tumor samples harboring CDKN2A/B deletions (P < .001) or alterations in SMO (P < .0001), BRCA1/2 (P < .01), MSH2/6 (P < .01), ATRX (P < .05), or DIS3 (P < .05). There was also a trend towards reduced patient OS in samples having MET amplifications (P = .07) and ATM alterations (P = .08) (Table 2). Consistent with these results, both CDKN2A/B deletions and alterations in SMO, MET, BRAF, or ATRX alterations were more common in HGGs. ATRX alterations were also more likely to be found in older patients (Supplementary Table 5). In our analysis, only ATM (P < .05) and SOX2/9 alterations (P < .01) were associated with reduced PFS (Table 2). While these outcome-associated genes were relatively abundant in astrocytomas, no oligodendroglioma harbored SMO, BRCA1/2, ATRX, DIS3, ATM, or SOX2/9 alterations, and only one tumor had a CDKN2A/B deletion or MSH2/6 alteration (Figure 3).
Table 2.
Univariate Time-to-event Analyses of Specific Genetic Alterations
| Gene | Med. OS (Altered) | Med. OS (Unaltered) | P-value (OS) | Med. PFS (Altered) | Med. PFS (Unaltered) | P-value (PFS) |
|---|---|---|---|---|---|---|
| SMO | 6.69 | 32.36 | 3.06E-05 | 2.6 | 4.5 | 0.101 |
| CDKN2A/B | 6.97 | NA | 0.000348 | 4 | 6.8 | 0.234 |
| MET | 7.1 | 32.4 | 0.00247 | 4.75 | 4.5 | 0.427 |
| BRAF | 9.07 | 32.36 | 0.0034 | 4.75 | 4.41 | 0.462 |
| BRCA1/2 | 10.8 | 32.4 | 0.00522 | 4.75 | 4.41 | 0.541 |
| MSH2/6 | 6.97 | NA | 0.00694 | 2.6 | 4.5 | 0.43 |
| ATRX | 11.7 | 32.4 | 0.0174 | 4.5 | 4.41 | 0.201 |
| DIS3 | 6.97 | 32.36 | 0.0402 | 4.76 | 4.5 | 0.538 |
| MET (amplifications) | 9.35 | 32.36 | 0.0687 | 4.76 | 4.41 | 0.921 |
| ATM | 6.97 | 32.36 | 0.0815 | 2.6 | 4.76 | 0.0172 |
| PDGFRA/B | 6.97 | NA | 0.112 | 2.6 | 4.76 | 0.34 |
| MUTYH | 32.4 | 11.7 | 0.173 | 10.75 | 4.41 | 0.0648 |
| NOTCH2 | 32.4 | 11.7 | 0.18 | 10.75 | 4.41 | 0.12 |
| TSC1/2 | 6.97 | 12.54 | 0.197 | 2.6 | 4.5 | 0.855 |
| TP53 | 11.7 | 32.4 | 0.216 | 4.5 | 4.75 | 0.362 |
| NOTCH1 | 6.97 | 12.54 | 0.332 | 2.16 | 4.75 | 0.338 |
| RB1 | 12.5 | 32.4 | 0.359 | 2.6 | 4.5 | 0.141 |
| TERT | 32.4 | NA | 0.372 | 2.6 | 4.75 | 0.302 |
| MPL | 32.4 | 11.7 | 0.481 | 4.76 | 4.41 | 0.269 |
| FLT 1/3 | NA | 32.4 | 0.505 | 2.5 | 4.5 | 0.36 |
| SOX2/9 | NA | 12.5 | 0.546 | 2.71 | 4.76 | 0.00506 |
| ID3 | 32.4 | 11.7 | 0.548 | 4.76 | 4.41 | 0.183 |
| AKT1/2/3 | 12.5 | 11.7 | 0.577 | 4.75 | 4.41 | 0.586 |
| CCNE1 | 32.4 | NA | 0.781 | 4.76 | 4.5 | 0.175 |
| ARID1A/B | 32.4 | 11.7 | 0.794 | 4.76 | 4.41 | 0.264 |
| CIC | 32.4 | NA | 0.893 | 3.6 | 4.75 | 0.62 |
| SDHA/B/C/D | 32.4 | 11.7 | 0.932 | 2.63 | 4.5 | 0.855 |
| MTOR | 32.4 | 11.7 | 0.935 | 4.75 | 4.41 | 0.336 |
| JAK1/2/3 | 12.5 | NA | 0.966 | 3.52 | 4.75 | 0.867 |
To explore whether these outcome-associated genes were predictors of survival specifically rather than molecular markers for high-grade tumors, we next implemented Cox proportional hazards models on OS and PFS while adjusting for WHO grade (Supplementary Table 6). Notably, only alterations in SMO (P < .001) and BRAF (P < .01) remained significant predictors of OS in this multivariate analysis, with a trend towards reduced OS for CDKN2A/B loss (P = .09). However, both ATM (P < .05) and SOX2/9 (P < .01) alterations remained associated with reduced PFS even when adjusting for grade.
Discussion
Advanced genomic testing has deepened our understanding of cancer biology and allowed for molecular risk stratification of CNS tumors.19–22IDH1/2 mutations, for example, were identified in adult diffuse glioma in the 2000s and associated with superior outcomes compared to IDH-wildtype gliomas.4,5,23 While previously thought to be rare, IDH1/2 mutations have in recent years been shown to occur at a substantial rate in the pediatric population.8–12
To systematically evaluate the frequency of IDH1/2 mutation among pediatric gliomas, we amassed a large patient cohort through a multi-institutional collaboration. All collaborators utilized distinct sequencing platforms and in recent years routinely sequence every newly diagnosed CNS tumor. Our cohort of 851 sequenced pediatric gliomas demonstrated IDH1/2 mutations in nine percent of cases overall, with most cases identified among patients older than ten years. Among adolescents 10–21 years of age, our cohort reveals an incidence rate of approximately 16%, while children under ten years old had an incidence rate of less than 1%, which are within the range of previous reports.10,12,23–25 Our study is also concordant with observations from Children’s Oncology Group that IDH1 mutations are common in adolescents, and importantly, our data supports the findings of that study that IDH-mutant HGGs have a significantly more favorable prognosis when compared to IDH wild-type HGGs.12 Conversely, the overall incidence rate observed among pediatric LGGs in our study appears to be higher than several previous publications.11,26,27 These findings highlight the importance of routine testing for IDH1/2 mutations and coexisting mutations in pediatric gliomas, especially among older children with diffuse gliomas.
To describe the clinical features, current treatment approaches, and outcomes within the pediatric population, we utilized a parallel multi-institutional collaboration, where we identified 76 patients across nine institutions. To our knowledge, this represents the largest pediatric IDH1/2-mutant glioma cohort with comprehensive clinical data published to date. We showed that most pediatric patients diagnosed with IDH1/2-mutant glioma have low-grade histology and a preponderance of astrocytic (nonoligodendroglial) tumors, consistent with the previously established age distribution and incidence for these tumor types. As expected, histological grade was associated with poorer OS; most patients with high-grade astrocytoma died of their disease within 10 years of diagnosis. Most patients with low-grade histology were managed observantly regardless of extent of resection. In contrast, adult patients are typically only observed without adjuvant therapy if they were younger, clinically well, and underwent upfront gross total tumor resection.28–30
In our mixed cohort of 39 patients with low-grade astrocytoma who were observed upfront, the median PFS was 4.76 years with a 5- and 10-year PFS of 41.7% and 0%, respectively. These results mirror the outcomes reported for adult patients treated with upfront RT on the RTOG 9802 protocol.15,31,32 Additionally, the OS for this upfront observation cohort was 100% at ten years. Although these survival data appear more favorable than those reported for adults, numerous disease-related deaths after year 10 were noted. Only four patients with IDH-mutant LGG received upfront radiation, making any conclusions regarding its impact challenging. Five patients with recurrent/progressive IDH-mutant low-grade astrocytoma (without progression to higher grade), who were initially observed, received RT at first recurrence and experienced excellent long-term outcome (5-year PFS and OS at 100%, median follow-up 8 years). This raises the question of whether a patient could strictly be observed until recurrence and then receive irradiation as a radiation-delaying approach without compromising outcome.
For patients with low-grade oligodendroglioma, the median PFS at 5.9 years is comparable to the cohort treated with upfront RT on the RTOG 9802 protocol.15,31 However, ten out of our thirteen patients with low-grade oligodendroglioma underwent observant management. With a significant median follow-up period, despite disease recurrence for most patients in this cohort, all but one patient remained alive at the time of last follow-up. These results are similar to those for adult low-grade oligodendroglioma, and again raise the question regarding the optimal timing for RT.15,33
Among all patients with low-grade glioma, 25 patients experienced disease recurrence/progression with nine documented progression to high-grade histology. Time-to-progression to high-grade histology for astrocytic tumors was shorter compared to oligodendrogliomas, as expected. Despite radiation therapy at recurrence, these patients exhibited more aggressive tumor behavior with shorter PFS and OS. Our data suggest that while upfront observation after maximal safe surgical resection may be a reasonable radiation-delaying treatment approach for younger patients with IDH1/2-mutant LGGs, these patients, especially those with diffuse astrocytomas, should be monitored closely for disease progression and radiation therapy considered at first relapse to ideally delay progression to higher grade.
Extent of resection significantly affected neither disease progression nor OS of either low-grade histologic type, although there was a trend towards longer survival among patients with low-grade diffuse astrocytoma with better extent of resection. This is in contrast to the adult experience which has generally shown a positive correlation between extent of resection and overall outcome.34–37 This discrepancy likely reflects the relatively small sample size. Additionally, given the limited use of chemotherapy, we were unable to evaluate its effectiveness within this pediatric cohort. Notably, the proportion of pediatric MGMT-methylated gliomas is unclear, as routine MGMT testing has not been universally adopted.
Genomic characteristics of our pediatric IDH1/2-mutant glioma cohort mirrored those of adult IDH1/2-mutant gliomas, consistent with the current categorization of these tumors as “adult type” diffuse gliomas in the WHO classification.7 Of note, a limitation of our retrospective study is that TERT promoter mutations may be missed by the NGS panels used for clinical sequencing. Despite the relatively modest patient sample size, several genetic alterations were associated with shorter OS, including CDKN2A/B, validating previously reported findings.38,39 Among IDH1/2 mutant astrocytomas, MSH2/6 alterations were associated with poorer OS. While germline testing results were not evaluated in this study, it is possible that these tumors represent mismatch-repair deficiency-associated IDH-mutant gliomas, which have been recently reported to have more aggressive biologic behavior than other IDH-mutant gliomas.40 Validating our findings within a larger pediatric cohort will provide critical insight into risk stratification and treatment options. Additionally, it will be important to evaluate the safety and efficacy of emerging targeted therapies such as IDH1/2 inhibitors in pediatrics, as currently underway in the phase 2 subprotocol with ivosidenib in the NCI-COG Pediatric MATCH trial (APEC1621K).41,42
Conclusion
We systematically evaluated the frequency of IDH-mutant glioma within the pediatric population and found IDH1/2 mutations in over 16% of gliomas among patients 10–21 years old. These findings validate previously published data and support the routine testing for IDH1/2 mutations in all pediatric diffuse gliomas, especially among adolescents. Our data summarizes the pediatric experience with these tumors across several sites and highlights current therapeutic approaches in these United States pediatric institutions. Our clinical experience suggests that there may be a subset of patients with low-grade histology that can be managed observantly, especially if there is an excellent degree of surgical resection. However, long-term outcomes data shows that the clinical behavior of pediatric IDH1/2-mutant gliomas is similar to that of adults. This finding affirms the observation that pediatric IDH1/2-mutant low-grade glioma are biologically and clinically distinct from MAPK-activated pediatric low-grade gliomas. Importantly, like their adult counterparts, IDH1/2-mutant pediatric gliomas demonstrate eventual progression to higher histologic grade and corresponding poor clinical prognosis. This suggests a potential role for incorporation of adult treatment approaches including more aggressive therapy, especially at disease recurrence.
Supplementary Material
Acknowledgements
Authors gratefully acknowledge the members of the MSKCC Molecular Diagnostics Service in the Department of Pathology. The authors also thank Adam Walker, CHLA Department of Pathology, for assistance with figure preparation.
Affiliations at time of substantial contribution.
Contributor Information
Kee Kiat Yeo, Department of Pediatric Oncology, Dana-Farber/Boston Children’s Cancer and Blood Disorder Center, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Sanda Alexandrescu, Department of Pathology, Boston Children’s Hospital, Boston, MA, USA.
Jennifer A Cotter, Department of Pathology and Laboratory Medicine, Children’s Hospital Los Angeles, Los Angeles, CA, USA.
Jayne Vogelzang, Department of Pediatric Oncology, Dana-Farber/Boston Children’s Cancer and Blood Disorder Center, Boston, MA, USA.
Varun Bhave, Harvard Medical School, Boston, MA, USA.
Marilyn M Li, Division of Genomic Diagnostics, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Jianling Ji, Department of Pathology and Laboratory Medicine, Children’s Hospital Los Angeles, Los Angeles, CA, USA.
Jamal K Benhamida, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Marc K Rosenblum, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Tejus A Bale, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Nancy Bouvier, Department of Pediatrics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Kristiyana Kaneva, Department of Pediatrics, Ann & Robert H. Lurie Children’s Hospital of Chicago, USA; Tempus Labs, Inc., Chicago, IL, USA.
Tom Rosenberg, Department of Pediatric Oncology, Dana-Farber/Boston Children’s Cancer and Blood Disorder Center, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Mary Jane Lim-Fat, Department of Medical Oncology, Dana-Farber/Brigham and Women’s Hospital Cancer Center, Boston, MA, USA.
Hia Ghosh, Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA, USA.
Migdalia Martinez, Department of Pediatrics, Arnold Palmer Hospital for Children, Orlando, FL, USA.
Dolly Aguilera, Department of Pediatrics, Children’s Healthcare of Atlanta, Emory University School of Medicine, Atlanta, GA, USA.
Amy Smith, Department of Pediatrics, Arnold Palmer Hospital for Children, Orlando, FL, USA.
Stewart Goldman, Department of Child Health, Phoenix Children’s Hospital, University of Arizona College of Medicine, Phoenix, AZ, USA.
Eli L Diamond, Department of Neurology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Igor Gavrilovic, Department of Neurology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Tobey J MacDonald, Department of Pediatrics, Children’s Healthcare of Atlanta, Emory University School of Medicine, Atlanta, GA, USA.
Matthew D Wood, Department of Pathology and Laboratory Medicine, Oregon Health & Science University, Portland, OR, USA.
Kellie J Nazemi, Department of Pediatrics, Doernbecher Children’s Hospital, Portland, OR, USA.
AiLien Truong, Department of Pediatrics, Doernbecher Children’s Hospital, Portland, OR, USA.
Andrew Cluster, Department of Pediatrics, St. Louis Children’s Hospital, St. Louis, MO, USA.
Keith L Ligon, Department of Pathology, Dana-Farber/Brigham and Women’s Hospital Cancer Center, Boston, MA, USA.
Kristina Cole, Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Wenya Linda Bi, Harvard Medical School, Boston, MA, USA.
Ashley S Margol, Department of Pediatrics, Children’s Hospital Los Angeles, Los Angeles, CA, USA.
Matthias A Karajannis, Department of Pediatrics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Karen D Wright, Department of Pediatric Oncology, Dana-Farber/Boston Children’s Cancer and Blood Disorder Center, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Funding
This work was funded in part by the Marie-Josée and Henry R. Kravis Center for Molecular Oncology and the National Cancer Institute Cancer Center Core Grant (P30 CA008748); This work was also funded in part by the Pediatric Brain Tumor Foundation (PBTF), the PLGA Fund at the PBTF and the David Andrysiak CRA award, NCI P50CA165962, R01CA215489, and R01CA188228 to K.L.L.
Conflict of interest statement. ELD discloses paid advisory board membership with Day One Biopharmaceuticals and Springworks Therapeutics, both outside the submitted work.
Authorship statement. Experimental Design: KKY, KDW, SA, JAC, JV, KLL. Implementation: KKY, SA, JAC, MML, KK, JV, MM, AS, DA, TJM, SG, MDW, KJN, AT, AC, KLL, KC, AM, MAK. Analysis: KKY, VB, WLB, MLF, JV, SA, JAC, HG. Interpretation of the data: All Authors. Writing of manuscript: All authors.
References
- 1. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019; 21(Suppl 5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bandopadhayay P, Bergthold G, London WB, et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014; 61(7):1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schomas DA, Laack NN, Rao RD, et al. Intracranial low-grade gliomas in adults: 30-year experience with long-term follow-up at Mayo Clinic. Neuro Oncol. 2009; 11(4):437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu XY, Gerges N, Korshunov A, et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012; 124(5):615–625. [DOI] [PubMed] [Google Scholar]
- 5. Brat DJ, Verhaak RG, Aldape KD, et al. ; Cancer Genome Atlas Research Network. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015; 372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ryall S, Zapotocky M, Fukuoka K, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell. 2020; 37(4):569–583.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. WHO Classification of Tumours Editorial Board. World Health Organization Classification of Tumours of the Central Nervous System. 5th ed. Lyon: International Agency for Research on Cancer; 2021. [Google Scholar]
- 8. Lassaletta A, Zapotocky M, Bouffet E, Hawkins C, Tabori U. An integrative molecular and genomic analysis of pediatric hemispheric low-grade gliomas: an update. Childs Nerv Syst. 2016; 32(10):1789–1797. [DOI] [PubMed] [Google Scholar]
- 9. Ryall S, Tabori U, Hawkins C. A comprehensive review of paediatric low-grade diffuse glioma: pathology, molecular genetics and treatment. Brain Tumor Pathol. 2017; 34(2):51–61. [DOI] [PubMed] [Google Scholar]
- 10. Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009; 118(4):469–474. [DOI] [PubMed] [Google Scholar]
- 11. Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013; 45(6):602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pollack IF, Hamilton RL, Sobol RW, et al. IDH1 mutations are common in malignant gliomas arising in adolescents: a report from the Children’s Oncology Group. Childs Nerv Syst. 2011; 27(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Padovani L, Andre N, Constine LS, Muracciole X. Neurocognitive function after radiotherapy for paediatric brain tumours. Nat Rev Neurol. 2012; 8(10):578–588. [DOI] [PubMed] [Google Scholar]
- 14. Bhatia S, Sklar C.. Second cancers in survivors of childhood cancer. Nat Rev Cancer. 2002; 2(2):124–132. [DOI] [PubMed] [Google Scholar]
- 15. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016; 374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van den Bent MJ, Baumert B, Erridge SC, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet 2017; 390(10103):1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van den Bent MJ, Tesileanu CMS, Wick W, et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2021; 22(6):813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018; 555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sweet-Cordero EA, Biegel JA.. The genomic landscape of pediatric cancers: implications for diagnosis and treatment. Science. 2019; 363(6432):1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gajjar A, Bowers DC, Karajannis MA, et al. Pediatric brain tumors: innovative genomic information is transforming the diagnostic and clinical landscape. J Clin Oncol. 2015; 33(27):2986–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009; 360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balss J, Meyer J, Mueller W, et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008; 116(6):597–602. [DOI] [PubMed] [Google Scholar]
- 25. De Carli E, Wang X, Puget S. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009; 360(21):2248; author reply 2249. [DOI] [PubMed] [Google Scholar]
- 26. Bandopadhayay P, Ramkissoon LA, Jain P, et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet. 2016; 48(3):273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones DTW, Kieran MW, Bouffet E, et al. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 2018; 20(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dhawan S, Patil CG, Chen C, Venteicher AS. Early versus delayed postoperative radiotherapy for treatment of low-grade gliomas. Cochrane Database Syst Rev. 2020;1(1):CD009229. doi: 10.1002/14651858.CD009229.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ryken TC, Parney I, Buatti J, Kalkanis SN, Olson JJ.. The role of radiotherapy in the management of patients with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2015; 125(3):551–583. [DOI] [PubMed] [Google Scholar]
- 30. van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005; 366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 31. Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012; 30(25):3065–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bell EH, Zhang P, Shaw EG, et al. Comprehensive genomic analysis in NRG oncology/RTOG 9802: a phase III trial of radiation versus radiation plus procarbazine, lomustine (CCNU), and vincristine in high-risk low-grade glioma. J Clin Oncol. 2020; 38(29):3407–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000; 54(7):1442–1448. [DOI] [PubMed] [Google Scholar]
- 34. Aghi MK, Nahed BV, Sloan AE, et al. The role of surgery in the management of patients with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2015; 125(3):503–530. [DOI] [PubMed] [Google Scholar]
- 35. Choi J, Kim SH, Ahn SS, et al. Extent of resection and molecular pathologic subtype are potent prognostic factors of adult WHO grade II glioma. Sci Rep. 2020; 10(1):2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017; 18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 37. Wijnenga MMJ, French PJ, Dubbink HJ, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol. 2018; 20(1):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu VM, O’Connor KP, Shah AH, et al. The prognostic significance of CDKN2A homozygous deletion in IDH-mutant lower-grade glioma and glioblastoma: a systematic review of the contemporary literature. J Neurooncol. 2020; 148(2):221–229. [DOI] [PubMed] [Google Scholar]
- 39. Li KK, Shi ZF, Malta TM, et al. Identification of subsets of IDH-mutant glioblastomas with distinct epigenetic and copy number alterations and stratified clinical risks. Neurooncol Adv. 2019; 1(1):vdz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suwala AK, Stichel D, Schrimpf D, et al. Primary mismatch repair deficient IDH-mutant astrocytoma (PMMRDIA) is a distinct type with a poor prognosis. Acta Neuropathol. 2021; 141(1):85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mellinghoff IK, Ellingson BM, Touat M, et al. Ivosidenib in isocitrate dehydrogenase 1-mutated advanced glioma. J Clin Oncol. 2020;38(29):3398–3406. doi: 10.1200/JCO.19.03327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Study of Vorasidenib (AG-881) in Participants With Residual or Recurrent Grade 2 Glioma With an IDH1 or IDH2 Mutation (INDIGO): https://ClinicalTrials.gov/show/NCT04164901.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.