Abstract
The pls gene, coding for a large surface protein of methicillin-resistant Staphylococcus aureus, was cloned from a strain which adheres poorly to several mammalian proteins. The structure of pls revealed three distinct repeat regions, one of which was a serine-aspartate repeat characteristic of the Clf-Sdr family of surface proteins in staphylococci. The lengths of the repeat regions varied in different clinical strains and could be used as epidemiological markers. pls was found to be closely associated with the mecA gene by pulsed-field gel electrophoresis analysis of SmaI-digested DNA. A pls mutant constructed by allele replacement adhered well to immobilized fibronectin and immunoglobulin G, in contrast to the parental strain, suggesting that Pls could have a role in preventing adhesion at some stages during an infection.
Bacterial adhesion to host cells or extracellular matrices in damaged tissues is a prerequisite for colonization of the host by infecting bacteria. Implanted biomaterial also becomes coated with host proteins, enabling a pathogen to adhere and initiate a device-related infection. Staphylococcus aureus expresses a number of surface proteins that promote binding to the host extracellular matrix or plasma proteins. Several have been characterized at the molecular level, namely, protein A (SpA), binding the Fc part of immunoglobulins and von Willebrand factor (12, 16, 43, 46); the fibronectin-binding proteins FnBPA and FnBPB (11, 14, 24, 42); the fibrinogen-binding proteins ClfA, ClfB, and Efb (previously Fib) (7, 32, 33); the collagen-binding protein Cna (44); the elastin-binding protein EbpS (37); and the bone sialoprotein-binding protein Bbp (45). S. aureus can bind a number of other host proteins, such as vitronectin, laminin, mucin, and thrombospondin, but the molecular bases of these interactions are still poorly understood.
Some methicillin-resistant S. aureus (MRSA) strains show poor in vitro adherence to surfaces coated with several host plasma or extracellular matrix proteins. They were originally identified as strains giving a negative result in rapid S. aureus identification assays using particles coated with immunoglobulin G (IgG) and/or fibrinogen (27). These strains express a novel surface protein called Pls. The protein was originally purified from lysostaphin digests of a clinical MRSA strain, 1061, by affinity chromatography on immobilized wheat germ agglutinin (WGA) (17). Pls is sensitive to proteolysis by plasmin and trypsin. Similarly, the activation of receptor-bound plasminogen to plasmin on the S. aureus surface (28) leads to cleavage of the apparently 230-kDa Pls protein into 175- and 68-kDa segments—hence the name Pls for plasmin sensitive. Also, without prior proteolytic treatments, part of Pls exists as 175- and 68-kDa fragments in lysostaphin digests of Pls-expressing strains (17).
Methicillin resistance is caused by the mecA gene, coding for a low-affinity penicillin-binding protein, PBP2a. mecA is part of a large mec DNA region which lacks a homolog in methicillin-sensitive strains. The mec element from two strains has been sequenced. It is clear that the element has undergone several types of microevolutionary changes, including acquisition of integrated plasmids and transposons as well as mutations in the mec regulatory region that allow higher levels of expression of PBP2a (1).
This study was undertaken to determine if the poor adherence of MRSA strains is caused by Pls and to determine if Pls is encoded by a gene associated with the mec element. The pls gene was cloned and sequenced. The protein had several repeats, including serine-aspartate (SD) dipeptide repeats characteristic of the SD repeat-containing (Sdr) protein family. The pls gene was inactivated by allele replacement, which allowed the nonbinding phenotype to be attributed to the Pls protein.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown in Luria broth or agar, and S. aureus strains were grown in tryptic soy broth or agar. A temperature of 37°C was used, except for strains containing temperature-sensitive plasmid pTS2 or pPLS3 and cosmid 17 (see below), where temperatures of either 28 or 30°C and of 24°C, respectively, were used. When appropriate, antibiotics were added at concentrations of 75 to 100 μg/ml (ampicillin), 3 μg/ml (tetracycline), and 5 μg/ml (chloramphenicol). For adhesion assays, bacteria were grown to stationary phase and washed with phosphate-buffered saline (PBS). Cells were quantitated in a Petroff-Hausser counting chamber (Hausser Scientific Partnership, Horsham, Pa.).
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Properties | Reference or source |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| LE392 | Host for cosmid library | 40 |
| XL1Blue MRF′ | Cloning host | Stratagene |
| Staphylococcus aureus | ||
| 1061 | MRSA clinical isolate expressing Pls | 17, 27 |
| A900159 | MRSA clinical isolate expressing Pls | J. Étienne |
| RN4220 | Restriction-deficient mutant of 8325-4 capable of stably maintaining shuttle plasmids | 26 |
| 1061 pls::Tcr | Mutant of 1061 defective in expression of Pls | This work |
| BB255 | Derivative of 8325; Mecs | 6 |
| BB270 | mec(EK142) transduced into BB255 | 5 |
| Newman | High-level ClfA producer | 10 |
| Plasmids (host) | ||
| pHC79 (E. coli) | Cosmid vector; Apr Tcr; 6.4 kb | 19; Boehringer |
| Cosmid 17 (E. coli) | pls and surrounding DNA from 1061 cloned in pHC79 | This work |
| pBluescript II KS(+) (E. coli) | Cloning vector; Apr; 2.96 kb | Stratagene |
| pKS1 (E. coli) | 5.8-kb HindIII fragment from cosmid 17 subcloned in pBluescript II KS(+) | This work |
| pG0164 (E. coli) | 1.1-kb internal fragment of mecA cloned in pUC18; Apr | 2; L. Johnston |
| pPLS2 (E. coli) | pls::Tcr in pBluescript II KS(+) | This work |
| pKS1.13 (E. coli) | 3.5-kb HindIII-MluI fragment from the 5′ end of the pKS1 insert cloned in pBluescript II KS(+) | This work |
| pCW59 (S. aureus) | 2.35-kb TcrHindIII fragment; Cmr Tcr; 5.3 kb | 51 |
| pTS2 (S. aureus) | Cmr; temperature-sensitive replication system | 14 |
| pCU1 (shuttle) | Apr Cmr; 4.9 kb | 3 |
| pPLS3 (shuttle) | Apr Tcr Cmr; pTS2 cloned in pPLS2; 12.8 kb | This work |
| pPLS4 (shuttle) | pls with its promoter cloned in pCU1; 10.5 kb | This work |
| LE392 | Host for cosmid library | 40 |
| XL1Blue MRF′ | Cloning host | Stratagene |
| Staphylococcus aureus | ||
| 1061 | MRSA clinical isolate expressing Pls | 17, 27 |
| A900159 | MRSA clinical isolate expressing Pls | J. Étienne |
| RN4220 | Restriction-deficient mutant of 8325-4 capable of stably maintianing shuttle plasmids | 26 |
| 1061 pls::Tcr | Mutant of 1061 defective in expression of Pls | This work |
| BB255 | Derivative of 8325; Mecs | 6 |
| BB270 | mec(EK142) transduced into BB255 | 5 |
| Newman | High-level ClfA producer | 10 |
| Plasmids (host) | ||
| pHC79 (E. coli) | Cosmid vector; Apr Tcr; 6.4 kb | 19; Boehringer |
| Cosmid 17 (E. coli) | pls and surrounding DNA from 1061 cloned in pHC79 | This work |
| pBluescript II KS(+) (E. coli) | Cloning vector; Apr; 2.96 kb | Stratagene |
| pKS1 (E. coli) | 5.8-kb HindIII fragment from cosmid 17 subcloned in pBluescript II KS(+) | This work |
| pG0164 (E. coli) | 1.1-kb internal fragment of mecA cloned in pUC18; Apr | 2; L. Johnston |
| pPLS2 (E. coli) | pls::Tcr in pBluescript II KS(+) | This work |
| pKS1.13 (E. coli) | 3.5-kb HindIII-MluI fragment from the 5′ end of the pKS1 insert cloned in pBluescript II KS(+) | This work |
| pCW59 (S. aureus) | 2.35-kb TcrHindIII fragment; Cmr Tcr; 5.3 kb | 51 |
| pTS2 (S. aureus) | Cmr; temperature-sensitive replication system | 14 |
| pCU1 (shuttle) | Apr Cmr; 4.9 kb | 3 |
| pPLS3 (shuttle) | Apr Tcr Cmr; pTS2 cloned in pPLS2; 12.8 kb | This work |
| pPLS4 (shuttle) | pls with its promoter cloned in pCU1; 10.5 kb | This work |
DNA techniques.
A Wizard Plus Minipreps DNA purification system (Promega) was used for plasmid extraction, with the addition of lysostaphin (Ambicin L; Ambi Inc., Tarrytown, N.Y.) for S. aureus. Total DNA was extracted from S. aureus cells by first treating with lysostaphin and lysozyme (Boehringer Mannheim Biochemicals) in the presence of sucrose, then lysing the protoplasts with sodium dodecyl sulfate (SDS), treating with proteinase K, and extracting with chloroform-isoamyl alcohol (24:1) in the presence of sodium perchlorate. Standard methods (40) were used for DNA manipulations. Pfu DNA polymerase (Stratagene) was used in PCR amplifications for cloning purposes, and Dynazyme II DNA polymerase (Finnzymes, Espoo, Finland) was used in amplifications for other purposes. DNA was introduced into E. coli by calcium chloride transformation, S. aureus strain RN4220 by electroporation (13), and other S. aureus strains by φ85 phage transduction (13).
Cloning and sequencing of pls.
A cosmid library was constructed from MRSA strain 1061 total DNA. Fragments of approximately 40 kb, made by partial digestion with Sau3AI, were purified in a 10 to 40% sucrose gradient and ligated with BamHI-linearized pHC79. The ligated DNA was packaged in lambda phage heads in vitro using a DNA packaging kit (Boehringer) and introduced into E. coli LE392. The library was screened by dot blot hybridization with degenerate oligonucleotides based on tryptic peptides of Pls (FNPDLK, TTTPTTI, and EPETGE) (17). Hybond N+ nylon membrane and enhanced chemiluminescence (ECL) 3′ oligolabeling and detection systems (Amersham) were used. A cosmid clone containing pls (cosmid 17) was purified. The oligonucleotides were used to identify pls in a 5,759-bp HindIII fragment, which was subcloned in pBluescript II KS(+), forming pKS1. Sequencing of the subcloned DNA required the use of further subcloning, chromosome walking technique with oligonucleotides and generation of exonuclease deletion series. The 5′ end and promoter region of pls missing from pKS1 were obtained from a subclone of cosmid 17 made by cutting the cosmid with EcoRI and religating it.
The FASTA program (38) was used for searching homologies in EMBL and SWISS-PROT databases, LALIGN (20) was used for calculating local alignments, and ALIGN (38) was used for aligning two sequences. SignalP (34) was used for signal sequence prediction.
Mutation of pls by allelic replacement.
An E. coli-S. aureus shuttle plasmid was constructed in E. coli using a method described by Greene et al. (14). Two pls fragments were PCR amplified with pKS1.13 as the template. A 1,023-bp 5′-end fragment containing nucleotides 596 to 1618 (nucleotide numbers are based on sequence data in GenBank) was amplified using a forward primer with an added EcoRI site and a reverse primer with an added HindIII site. A 2,307-bp fragment containing nucleotides 1705 to 4011 was amplified using a forward primer with an added HindIII site and a reverse primer with an added KpnI site. The two fragments were cloned into pBluescript II KS(+) cut with EcoRI and KpnI. A 2.35-kb fragment from pCW59 encoding tetracycline resistance was cloned in the novel HindIII site between the pls fragments to form pPLS2. EcoRI-linearized S. aureus plasmid pTS2 was inserted into the EcoRI site in pPLS2 to construct a temperature-sensitive shuttle plasmid, pPLS3. pPLS3 was first introduced into S. aureus strain RN4220 and then transduced into strain 1061. Allele replacement was performed by first growing 1061(pPLS3) to stationary phase at a nonrestrictive temperature (28°C) in the presence of tetracycline, then diluting it 1:100 in broth without antibiotics, and then growing it to stationary phase at 42°C five times. Dilutions were plated on agar containing tetracycline at 42°C, and colonies were tested for chloramphenicol sensitivity. The pls::Tcr mutation of one Cms isolate was confirmed by Southern hybridization.
Complementation of the pls mutation.
The pls gene, with its putative promoter and transcription terminator (nucleotides 1 to 5602), was PCR amplified from cosmid 17 using primers with an added 5′ KpnI site and then cloned into the pCU1 KpnI site to make pPLS4. pPLS4 was introduced into S. aureus RN4220 and transduced into 1061 pls::Tcr.
Adhesion studies.
Bacterial adherence to protein-coated glass slides was tested essentially as described earlier (50). Slides were coated with 50 μl of rabbit IgG or its Fc fragments (10 μg/ml in PBS), human plasma fibronectin (Sigma; 14.6 μg/ml), human fibrinogen (10 μg/ml), or bovine serum albumin (BSA) (Sigma; 25 μg/ml) and saturated by incubation with PBS containing BSA (20 mg/ml) for 2 h at room temperature. IgG was purified from antiserum raised against E. coli Dr fimbriae (47). Fc fragments were generated by papain digestion of IgG and purification by affinity chromatography on Sepharose-protein A (Pharmacia, Uppsala, Sweden). Bacterial suspensions of 1 × 108 to 5 × 109 cells/ml in PBS were incubated on saturated slides for 2 h at 22°C. Methylene blue-stained adherent bacteria were examined using a Jenaval (Carl Zeiss Jena) microscope equipped with a charge-coupled device camera, and the images were digitized using the National Institutes of Health Image 1.55 program as detailed previously (49). The number of bacteria in 24 randomly chosen microscopic fields of 1.6 × 104 μm2 was determined.
Lysostaphin digestion, electrophoresis, and blotting.
Lysostaphin digests were prepared essentially as described earlier (27). Proteins were separated by SDS–8% polyacrylamide gel electrophoresis (PAGE) (29). For Western blot analysis, proteins were transferred electrophoretically to nitrocellulose membranes, which subsequently were blocked with 5% (wt/vol) defatted milk. Sheep antiserum to affinity-purified Pls (17) and peroxidase-conjugated MIG5, a recombinant Streptococcus dysgalactiae IgG-binding protein produced in E. coli (from Hans-Peter Müller, University of Greifswald, Greifswald, Germany), were used for immunodetection of Pls. To detect ClfA and SpA, rabbit antibodies to ClfA region A or mouse monoclonal antibodies to protein A (Sigma), together with peroxidase-conjugated MIG5, were used. 3,3′-Diaminobenzidine (Sigma) was used as a chromogenic substrate. Fibronectin-binding proteins were detected from lysostaphin digests of exponential-phase cells using ligand blotting with human plasma fibronectin (Sigma; 5 μg/ml), rabbit anti-Fn antiserum, and peroxidase-linked donkey anti-rabbit IgG (Amersham Pharmacia). The ECL technique was used for band visualization.
Identification of the proteolytic cleavage site in Pls using N-terminal sequencing.
Pls was isolated from a lysostaphin digest of strain 1061 using WGA affinity chromatography (17). The bacteria were grown to stationary phase in order to produce more spontaneously cleaved 175-kDa form of Pls. Fractions containing Pls were run in SDS–8% PAGE (29) and blotted onto polyvinylidene difluoride membranes (Bio-Rad Laboratories). The proteins were visualized using Ponceau S, and the 175-kDa protein band was excised from the membranes. The N terminus of the protein was sequenced with a modified Applied Biosystems 477A apparatus using gas-phase Edman degradation (4).
Repeat region size analysis.
pls (nucleotides 596 to 5338 in strain 1061 pls) was PCR amplified using forward primer 5′-CCGCTGAGAATAACACAACT-3′ and reverse primer 5′-GACGTCTGCCAACTAAGAAT-3′. The R1, R2, R3, and A regions of pls (nucleotides 709 to 1327, 2400 to 4346, 4327 to 5338, and 1329 to 2416, respectively, in strain 1061 pls) were amplified using primers 5′-CCTGAACAAGTTGATGTAAC-3′ and 5′-TGCTCTTAGCATTTGTGTCT-3′ for R1 (forward and reverse, respectively), 5′-AATTACAACGCCTCAAGCTG-3′ and 5′-GCACCATGGATGATTACTTC-3′ for R2, 5′-GAAGTAATCATCCATGGTGC-3′ and 5′-GACGTCTGCCAACTAAGAAT-3′ for R3, and 5′-TTCAAATGCTCAACCATCAG-3′ and 5′-CTTGAGGCGTTGTAATTGTT-3′ for region A. Genomic DNA was used as a template. PCR products were separated by electrophoresis in a 0.7 or 1.5% agarose gel.
PFGE.
Agarose plugs for pulsed-field gel electrophoresis (PFGE) were prepared as described earlier (39). One-third of a plug was digested with SmaI (Boehringer) for 18 h at room temperature. PFGE was carried out with 1% SeaKem agarose (FMC BioProducts) in a Bio-Rad CHEF-DR III system. Running time was 24 h with a ramped switch time from 10 to 60 s at an angle of 120o. The voltage was 6 V/cm, and the temperature was 14°C. Lambda ladder PFG marker (New England Biolabs) was used as a marker.
Southern hybridizations.
Hybond N+ nylon membrane and ECL direct nucleic acid labeling and detection systems (Amersham) were used in Southern hybridizations. Plasmid pG0164 (Table 1) and PCR-amplified region A of strain 1061 pls were used as probes for blotted, PFGE-separated DNA. Plasmid pG0164 has an internal fragment of mecA cloned in pUC18. The plain vector was shown earlier not to hybridize with S. aureus DNA (2). pls region A was used as a probe to avoid hybridization of the R3 region with the homologous area in several other S. aureus genes. A part of pls (nucleotides 596 to 4011) PCR amplified from strain 1061 DNA, the 2.35-kb HindIII fragment of pCW59, pBluescript II KS(+), and pTS2 were used as probes for verifying the structure of the pls locus in 1061 pls::Tcr. The PCR-amplified pls gene without the R3 region was used as a probe to search for the pls gene in various S. aureus strains.
Nucleotide sequence accession number.
The nucleotide sequence of the pls 4,914-bp coding region as well as 453 nucleotides of upstream sequence and 235 nucleotides of downstream sequence is available in the GenBank database under accession number AF115379.
RESULTS
Cloning and sequencing of pls.
We previously purified the 230-kDa surface protein Pls from MRSA clinical isolate 1061 (17). In order to characterize this protein at the molecular level, we now cloned the pls gene from MRSA strain 1061 and determined its nucleotide sequence.
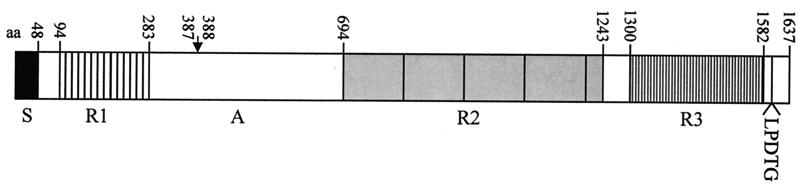
The pls gene start codon AUG is preceded by a putative ribosome-binding site AGGGG, and the coding sequence is followed by palindromic sequences possibly forming a hairpin structure of a transcription terminator. The deduced amino acid sequence of the Pls protein predicts a polypeptide of 1,637 amino acids (Fig. 1). The calculated molecular mass of the primary translation product is 175 kDa. The Pls protein has features typical of surface proteins of gram-positive bacteria, namely, a signal sequence of 48 amino acids at the amino-terminal end and an LPDTG sequence, known to mediate the covalent linkage of cell wall proteins to peptidoglycan in gram-positive bacteria (41). In addition, Pls has several positively charged amino acids at the extreme C terminus preceded by hydrophobic residues. Three repeat regions can be found in the amino acid sequence. Repeat region R1 consists of 14 repeats of a 12- to 14-amino-acid stretch with the consensus sequence AEETXKAXTEEAPK. The eighth and ninth repeats from the N terminus (R1-8 and R1-9) have exactly the same amino acid sequences, but variability increases toward the beginning and the end of R1. Repeat region R2 is formed by four repeats of 128 or 129 amino acids and a fifth, partial repeat of 36 amino acids. R2-2 and R2-3 are identical. R3 is a serine-aspartate dipeptide repeat region of 282 amino acids encoded by 47 DNA repeats of 18 bp.
FIG. 1.
Schematic model of the Pls structure in S. aureus strain 1061. aa, amino acids; S, signal sequence; R, repeat region; A, nonrepeat region; LPDTG, cell wall attachment sequence. The arrow shows the site for proteolytic cleavage.
A sequence with 91.6% identity to pls was found within mec DNA of S. aureus strain NCTC 10442 (accession number AB033763) by a FASTA search of the DDBJ/EMBL/GenBank databases. A DNA region very homologous to pls is also present in MRSA strain COL (preliminary sequence data obtained from The Institute for Genomic Research through the website at http://www.tigr.org). Pls has 39% overall amino acid sequence identity with a Staphylococcus epidermidis protein, Aap (GenBank accession number AJ249487). The Aap sequence has a structure similar to that of Pls, with three repeat regions. The most homologous region is R2, consisting of 128 amino acid repeats in both proteins. Individual repeats in R2 of Pls and Aap are 63 to 73% identical.
Repeat region size analysis.
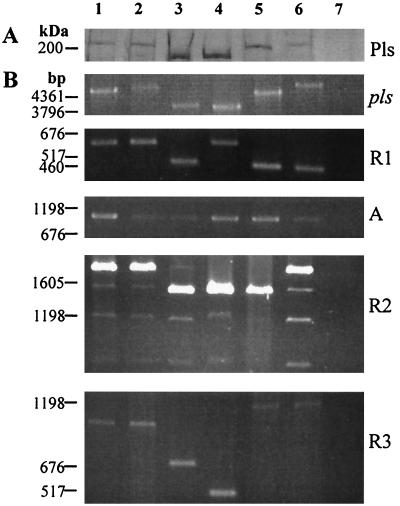
The apparent size of Pls varied in different MRSA strains (Fig. 2). To determine if this variation depends on the size variation of the repeat regions, the whole gene, the three repeat regions, and region A were amplified by PCR. The size of the gene varied in accordance with the apparent size of the protein. Region A was constant in size in all strains, whereas the sizes of the repeat regions were variable.
FIG. 2.
Repeat region size analysis. (A) Coomassie blue-stained gel from SDS-PAGE of lysostaphin digests of MRSA strains showing Pls (only the uppermost part of the gel is shown). (B) PCR amplification of pls and its R1, R2, R3, and A regions. Lanes 1 to 3 are analyses of Finnish Pls-expressing MRSA strains 1061, 1179, and 658. Lanes 4 to 6 are analyses of similar French MRSA isolates A900159, A920096, and A900557. The French isolates were kindly provided by Jerome Étienne, Centre National de Référence des Staphylocoques, Lyon, France. Lane 7 shows a Finnish MRSA isolate, 3821, not containing the pls gene. All the strains were clinical isolates. Molecular size markers are shown on the left.
Repeat R1 occurred in two different sizes among the studied isolates. The sizes of the PCR fragments from R1 were approximately 650 and 480 bp, suggesting that the strains with the shorter repeat lack 4 of the 14 amino acid repeats of strain 1061. PCR of repeat R2 produced several fragments from each strain; the sizes of the largest fragments, 1,920 and 1,570 bp, corresponded to four and three copies of R2, respectively. The primers had been designed to anneal to unique sequences flanking the repeat regions but had enough homology with sequences in R2 to produce nonspecific products. Repeat R3 was the most variable; the sizes of the PCR products ranged from 300 to 1,070 bp. The variable lengths of the repeats accounted for the size variation of the intact protein.
To determine whether the repeat regions are unstable in MRSA isolates, strains 1061 and A900159 were cultivated in broth for several generations. A culture grown to stationary phase was diluted 1:100 in fresh broth and grown again to stationary phase. This process was repeated 19 times altogether, and total DNA was extracted from the first and the last cultures. Repeat region size analysis was performed. The sizes of the PCR fragments from the first and the last cultures were identical (results not shown).
Association of pls with the mec region.
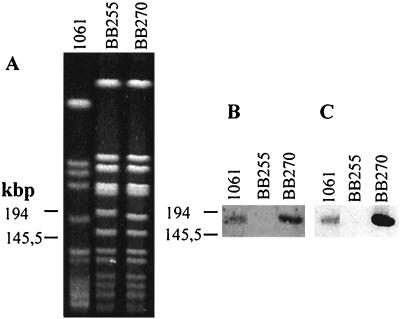
Southern hybridization of genomic DNAs from several S. aureus strains indicated that the pls gene occurs only in MRSA strains (results not shown). To investigate if mecA (specifying methicillin resistance) and pls are linked, SmaI-digested DNAs from strains 1061, BB255, and BB270 were analyzed by Southern hybridization. Strain BB270 had been made from BB255 by transduction of a mec element. The probes for mecA and pls both hybridized with the same SmaI fragments of 1061 DNA and BB270 DNA, but neither of them reacted with BB255 DNA (Fig. 3).
FIG. 3.
Association of pls with the mec region. (A) SmaI-digested DNAs from strains 1061, BB255, and BB270 separated by PFGE. (B and C) Southern hybridizations of the PFGE blot using the mecA (B) and pls (C) probes. Molecular size markers are shown on the left.
Identification of the proteolytic cleavage site in Pls using N-terminal sequencing.
In lysostaphin digests of MRSA cells, part of Pls usually exists as smaller fragments (175 and 68 kDa in strain 1061). Pls is expressed in all growth phases, but the amount of cleaved protein compared to whole Pls increases in stationary phase (data not shown). Sequence analysis of the 175-kDa form of strain 1061 Pls showed a clear N-terminal sequence of AAAQDT, indicating cleavage between amino acids 387R and 388A of the 230-kDa protein (Fig. 1).
S. aureus mutant defective in pls and complementation of the mutation.
To study the function of Pls, we constructed a mutant by allelic replacement of pls in the chromosome of MRSA strain 1061 (Fig. 4A). An E. coli-S. aureus shuttle plasmid carrying a pls::Tcr mutation was constructed and transferred to 1061. A double crossover allowed pls::Tcr to be introduced into the chromosomal locus. This step was confirmed by Southern hybridization analysis (Fig. 4B). Chromosomal DNA from the mutant digested with StuI and PstI had a band that hybridized with the pls probe and was 2.35 kb larger than that of the wild type. The same fragment also bound the pCW59 probe, whereas the pBluescript and pTS2 probes did not react (results not shown). The mutation was complemented by introducing wild-type pls in the pCU1 shuttle plasmid. Western blot analyses of lysostaphin digests showed that Pls was not expressed in the pls::Tcr mutant, whereas in the complemented strain, it was even more strongly expressed than in parental strain 1061 (Fig. 4C). Pls could also be purified from the lysostaphin digest of the complemented strain by utilizing affinity to immobilized WGA lectin (17), suggesting that it contained a carbohydrate composition similar to that of wild-type Pls.
FIG. 4.
Allelic replacement mutation of pls and complementation analysis. (A) Structure of the pls::Tcr mutation formed by allelic replacement in the chromosome of strain 1061. The numbers refer to the nucleotide numbers in GenBank sequence data. The gray box shows the domain structure of the Pls protein corresponding to the gene. (B) Southern hybridization analysis of pls in strains 1061 (lanes 1 and 3) and 1061 pls::Tcr (lanes 2 and 4). Chromosomal DNA cut with StuI and PstI was probed with pls (lanes 1 and 2) and the HindIII fragment of plasmid pCW59 (lanes 3 and 4). Molecular size markers are shown on the left. (C) Lysostaphin digests of strains 1061 (lane 1), 1061 pls::Tcr (lane 2), and 1061 pls::Tcr(pPLS4) (lane 3) analyzed by Western blotting with anti-Pls antibodies. Molecular size markers are shown on the left.
Adhesion studies.
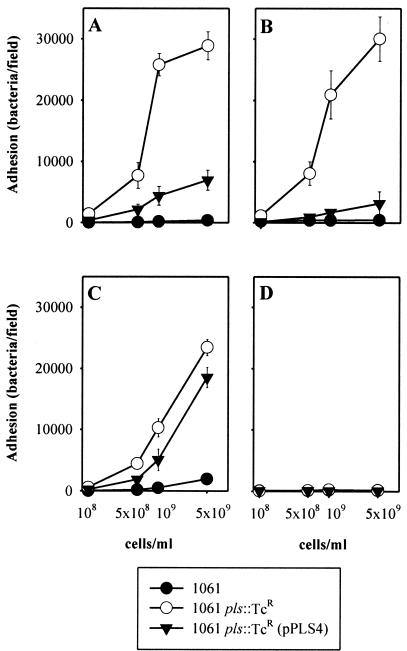
Pls was originally associated with MRSA strains which could not agglutinate particles coated with IgG and/or fibrinogen or adhere to glass surfaces coated with IgG, fibrinogen, or fibronectin (17). To investigate whether the poor adhesion was due to Pls expression, the adhesive capacities of strain 1061, the pls mutant, and the complemented strain were compared. Means and standard deviations from one representative experiment are shown in Fig. 5. The level of adherence of strain 1061 to surfaces coated with unrelated IgG or the corresponding Fc fragments was significantly lower than that of strain 1061 pls::Tcr (Fig. 5A and B). The same was true for adherence to surfaces coated with fibronectin (Fig. 5C). The level of adherence of both strains to fibrinogen was very low (results not shown). Complementation of 1061 pls::Tcr with pPLS4 decreased the level of adherence. The latter effect, however, varied between experiments, sometimes being rather small.
FIG. 5.
Adherence of bacterial cells to glass slides coated with rabbit IgG (A), Fc fragments of rabbit IgG (B), human fibronectin (C), and BSA (D). The vertical bars show standard deviations.
Presence of fibronectin-binding proteins, ClfA, and SpA in the staphylococcal cell wall.
Western blotting of lysostaphin digests was used to study the expression of surface proteins by S. aureus strains 1061 and Newman. Antibodies to ClfA revealed polypeptides of 170 and 140 kDa only in strain Newman and not in strain 1061. Antibodies to SpA reacted with a 43-kDa band in strain 1061 and a 49-kDa band in strain Newman. They also reacted with one smaller and a few larger peptides, probably corresponding to multimers of SpA, in both strains. Ligand blotting with fibronectin revealed in strain 1061 proteins of >200 and 160 kDa and (likely) their 60- and 53-kDa degradation products. In strain Newman, >200-, 140-, 60-, and 53-kDa proteins were recognized (results not shown).
DISCUSSION
Our earlier studies revealed that some MRSA strains give negative test results in slide agglutination assays designed to detect fibrinogen-binding proteins and SpA on the staphylococcal surface. These strains express Pls, a 230-kDa surface protein not present in strains giving the correct agglutination result (27). These strains also adhere poorly to surfaces coated with fibronectin, fibrinogen, IgG, and BSA (17; K. Savolainen, J. Vuopio-Varkila, and P. Kuusela, unpublished results). Here, the hypothesis that the Pls protein prevents surface adhesins from interacting with their ligands was investigated by measuring the adherence of wild-type, Pls−, and Pls-complemented MRSA cells to immobilized IgG, fibronectin, and fibrinogen. The results strongly favor the idea that the Pls protein causes the nonadherent phenotype of some MRSA strains. The adherence mediated by SpA was increased greatly in the Pls− mutant. The possibility of a specific antigen-antibody interaction between IgG and bacterial cells was excluded by the use of purified Fc fragments instead of complete IgG molecules. The adherence of the Pls− mutant strain to immobilized fibronectin was also greatly increased compared to the adherence of the wild-type strain, whereas a similar difference could not be observed for the adherence of the mutant strain to immobilized fibrinogen, the other ligand used in the original agglutination tests. This result can be explained by the failure of strain 1061 to express ClfA detectably in Western blotting.
There was great variation in the functional level of complementation of the pls::Tcr mutation with the pls gene. The possibility that the disruption of pls affected the expression of downstream genes is unlikely because the pls gene is followed by a possible transcription terminator and a new start codon preceded by a putative ribosome-binding site, AGGAGGA. The molecular mechanism by which Pls prevents the interaction between SpA and immobilized IgG as well as between fibronectin-binding proteins and fibronectin may be steric hindrance. Although the expression of Pls in the complemented strain was higher than that in the wild type strain, as judged by the amount of Pls in the lysostaphin digest, nothing is known about the surface distribution of Pls in the wild-type or complemented strain. Variations in the spatial distribution of the Pls molecule may greatly affect the adhesive properties of a strain.
The Pls protein contains three different types of repeat sequences. Extensive repeat regions are common in surface proteins of gram-positive bacteria, and some of them have a functional role in adhesion (24, 42, 43, 46). We have not found any adhesin function for Pls; on the contrary, Pls seems to work as an antiadhesin. However, it is possible that Pls has an as-yet-unknown adhesive function. Pls has high homology with the S. epidermidis accumulation-associated protein, Aap, which is essential for biofilm formation on glass or polystyrene surfaces (21).
All of the repeat regions of Pls were variable in length in various strains. The repeats seemed to be quite stable, since consecutive cultivation of two strains did not induce changes in the repeat lengths. Also, PCR fragments of two of the strains studied (Fig. 2, lanes 1 and 2) were exactly the same size. The two strains were clinical isolates from southern Finland but were isolated from different hospitals at different times (27). It might be possible to use the size variation as a marker in epidemiological studies.
The Pls protein can be regarded a member of the Sdr (SD repeat-containing) protein group. Repeat region R3 of Pls is composed mostly of the dipeptide SD, which is also found in the R regions of S. aureus and S. epidermidis surface proteins of the Clf-Sdr family (25, 30, 32, 33, 35, 45). The size of the ClfA R region varies in different strains (31), and this region is thought to be required for spanning the peptidoglycan layer and to act as an arm projecting the fibrinogen-binding region outside the cell wall in a flexible manner (15). The only common feature in the nonrepeat A region of the Sdr proteins is a consensus motif, TYTFTDYVD (25, 30), which does not exist in the A region of Pls.
The pls gene has been found only in MRSA strains and was located in the same 190-kb SmaI fragment as mecA in the chromosome of strain 1061. Furthermore, pls is part of the DNA region, originating from strain EK142, that was transduced into strain BB255 together with the mecA gene in the construction of strain BB270. A similar gene occurs in the mec element of strain NCTC 10442 (GenBank accession number AB033763) as well as in the genome of MRSA strain COL at a distance of 15.5 kb from the mecA gene. Yet another sequenced mec region, which does not contain a pls homolog, is found in a mecA-carrying, methicillin-susceptible “pre-MRSA” strain, N315 (GenBank accession number D86934) (22). Our results suggest that pls is closely associated with mecA and is part of the mec element in at least one lineage of MRSA.
The mec element is integrated at a specific site between spa and purA in the S. aureus chromosome and does not have a homolog in methicillin-sensitive strains. There is evidence for the horizontal transfer of mec DNA between different staphylococcal species, and a gene very similar to mecA has been found in all isolates of Staphylococcus sciuri, a species found on rodents and some primitive mammals (9). The mec element has undergone considerable changes by acquiring different insertions of plasmids and transposons in loci flanking mecA (reviewed in references 1, 8, and 18). Interestingly, Vaudaux et al. (48) showed that the introduction of a mec element into the 8325-genotyped strain BB255 to make BB270 inhibited the functions of fibrinogen and fibronectin adhesins without changing their levels of production. The finding is in good agreement with the present results, which suggest that this inhibition as well as the inhibition of adhesion in strain 1061 is caused by Pls, encoded by the pls gene, which is closely associated with or in this mec element.
Strain NCTC 10442 is the first reported MRSA strain, isolated in 1961 in England (18, 23), and EK142 is a clinical isolate from 1967, whereas the newest isolates used in this study were from the 1990s. In Canada, a molecular analysis of MRSA strains has shown that an epidemic strain, CMRSA-1, accounts for nearly half of the isolates. A predominant subtype (representing 72%) of CMRSA-1 expresses Pls. This subtype has low fibronectin-binding and protease activities and a limited profile of secreted proteins (36).
DNA in the mec element containing transposons and insertion sequences is supposed to be somewhat unstable (8). The existence of pls in isolates from different origins and from a long time range suggests that Pls is beneficial for the isolates. Pls is cleaved as a result of the activation of receptor-bound plasminogen on the MRSA surface in vitro. The cleavage of Pls might reveal its as-yet-unknown, possibly adhesive properties. Consequently, Pls could at one point of an infection prevent adhesion, allowing cells to spread, and at another point help staphylococci to adhere to tissues and structures of the host.
ACKNOWLEDGMENTS
This study was supported by the Academy of Finland (grants 29346, 42103, 42107, and 159257), the University of Helsinki, The Wellcome Trust (grant 52320 to T.J.F.), the Sigrid Juselius Foundation, the Maud Kuistila Memorial Foundation, and an FEMS research fellowship.
We thank Jaana Vesterinen, Department of Medical Chemistry, Institute of Biomedicine, University of Helsinki, for N-terminal sequencing and Saara Salmenlinna, National Public Health Institute, for help with PFGE. We also thank Jerome Étienne and Linda Johnston for bacterial strains and Hans-Peter Müller for MIG5.
REFERENCES
- 1.Archer G L, Niemeyer D M. Origin and evolution of DNA associated with resistance to methicillin in staphylococci. Trends Microbiol. 1994;2:343–346. doi: 10.1016/0966-842x(94)90608-4. [DOI] [PubMed] [Google Scholar]
- 2.Archer G L, Niemeyer D M, Thanassi J A, Pucci M J. Dissemination among staphylococci of DNA sequences associated with methicillin resistance. Antimicrob Agents Chemother. 1994;38:447–454. doi: 10.1128/aac.38.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustin J, Rosenstein R, Wieland B, Schneider U, Schnell N, Engelke G, Entian K-D, Götz F. Genetic analysis of epidermin biosynthetic genes and epidermin-negative mutants of Staphylococcus epidermidis. Eur J Biochem. 1992;204:1149–1154. doi: 10.1111/j.1432-1033.1992.tb16740.x. [DOI] [PubMed] [Google Scholar]
- 4.Baumann M. Comparative gas phase and pulsed liquid phase sequencing on a modified Applied Biosystems 477A sequencer. Anal Biochem. 1990;190:198–208. doi: 10.1016/0003-2697(90)90181-8. [DOI] [PubMed] [Google Scholar]
- 5.Beck W D, Berger-Bächi B, Kayser F H. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J Bacteriol. 1986;165:373–378. doi: 10.1128/jb.165.2.373-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger-Bächi B. Insertional inactivation of staphylococcal methicillin resistance by Tn551. J Bacteriol. 1983;154:479–487. doi: 10.1128/jb.154.1.479-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodén M K, Flock J-I. Cloning and characterization of a gene for a 19 kDa fibrinogen-binding protein from Staphylococcus aureus. Mol Microbiol. 1994;12:599–606. doi: 10.1111/j.1365-2958.1994.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 8.Chambers H F. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 1997;10:781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couto I, de Lancastre H, Severina E, Kloos W, Webster J A, Hubner R J, Sanches I S, Tomasz A. Ubiquitous presence of a mecA homologue in natural isolates of Staphylococcus sciuri. Microb Drug Resist. 1996;2:377–391. doi: 10.1089/mdr.1996.2.377. [DOI] [PubMed] [Google Scholar]
- 10.Duthie E S, Lorenz L L. Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 11.Flock J-I, Fröman G, Jönsson K, Guss B, Signäs C, Nilsson B, Raucci G, Höök M, Wadström T, Lindberg M. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 1987;6:2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsgren A, Sjöquist J. “Protein A” from S. aureus. J Immunol. 1966;97:822–827. [PubMed] [Google Scholar]
- 13.Foster T J. Molecular genetic analysis of staphylococcal virulence. Methods Microbiol. 1998;27:433–454. [Google Scholar]
- 14.Greene C, McDevitt D, Francois P, Vaudaux P E, Lew D P, Foster T J. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- 15.Hartford O, Francois P, Vaudaux P, Foster T J. The dipeptide repeat region of the fibrinogen-binding protein (clumping factor) is required for functional expression of the fibrinogen-binding domain on the Staphylococcus aureus cell surface. Mol Microbiol. 1997;25:1065–1076. doi: 10.1046/j.1365-2958.1997.5291896.x. [DOI] [PubMed] [Google Scholar]
- 16.Hartleib J, Köhler N, Dickinson R B, Chhatwal G S, Sixma J J, Hartford O M, Foster T J, Peters G, Kehrel B E, Herrmann M. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood. 2000;96:2149–2156. [PubMed] [Google Scholar]
- 17.Hildén P, Savolainen K, Tyynelä J, Vuento M, Kuusela P. Purification and characterisation of a plasmin-sensitive surface protein of Staphylococcus aureus. Eur J Biochem. 1996;236:904–910. doi: 10.1111/j.1432-1033.1996.00904.x. [DOI] [PubMed] [Google Scholar]
- 18.Hiramatsu K. Molecular evolution of MRSA. Microbiol Immunol. 1995;39:531–543. doi: 10.1111/j.1348-0421.1995.tb02239.x. [DOI] [PubMed] [Google Scholar]
- 19.Hohn B, Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980;11:291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- 20.Huang X, Miller W. A time-efficient, linear-space local similarity algorithm. Adv Appl Math. 1991;12:337–357. [Google Scholar]
- 21.Hussain M, Herrmann M, von Eiff C, Perdreau-Remington F, Peters G. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun. 1997;65:519–524. doi: 10.1128/iai.65.2.519-524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito T, Katayama Y, Hiramatsu K. Cloning and nucleotide sequence determination of the entire mec DNA of pre-methicillin-resistant Staphylococcus aureus N315. Antimicrob Agents Chemother. 1999;43:1449–1458. doi: 10.1128/aac.43.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jevons M P. “Celbenin”-resistant staphylococci. Br Med J. 1961;1:124–125. [Google Scholar]
- 24.Jönsson K, Signäs C, Müller H-P, Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991;202:1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- 25.Josefsson E, McCrea K W, Eidhin D N, O'Connell D, Cox J, Höök M, Foster T J. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Microbiology. 1998;144:3387–3395. doi: 10.1099/00221287-144-12-3387. [DOI] [PubMed] [Google Scholar]
- 26.Kreiswirth B N, Löfdahl S, Betley M J, O'Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 27.Kuusela P, Hildén P, Savolainen K, Vuento M, Lyytikäinen O, Vuopio-Varkila J. Rapid detection of methicillin-resistant Staphylococcus aureus strains not identified by slide agglutination tests. J Clin Microbiol. 1994;32:143–147. doi: 10.1128/jcm.32.1.143-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuusela P, Saksela O. Binding and activation of plasminogen at the surface of Staphylococcus aureus. Increase in affinity after conversion to the Lys form of the ligand. Eur J Biochem. 1990;193:759–765. doi: 10.1111/j.1432-1033.1990.tb19397.x. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.McCrea K W, Hartford O, Davis S, Eidhin D N, Lina G, Speziale P, Foster T J, Höök M. The serine-aspartate repeat (Sdr) protein family in Staphylococcus epidermidis. Microbiology. 2000;146:1535–1546. doi: 10.1099/00221287-146-7-1535. [DOI] [PubMed] [Google Scholar]
- 31.McDevitt D, Foster T J. Variation in the size of the repeat region of the fibrinogen receptor (clumping factor) of Staphylococcus aureus strains. Microbiology. 1995;141:937–943. doi: 10.1099/13500872-141-4-937. [DOI] [PubMed] [Google Scholar]
- 32.McDevitt D, Francois P, Vaudaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 33.Ní Eidhin D, Perkins S, Francois P, Vaudaux P, Höök M, Foster T J. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol Microbiol. 1998;30:245–257. doi: 10.1046/j.1365-2958.1998.01050.x. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson M, Frykberg L, Flock J-I, Pei L, Lindberg M, Guss B. A fibrinogen-binding protein of Staphylococcus epidermidis. Infect Immun. 1998;66:2666–2673. doi: 10.1128/iai.66.6.2666-2673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papakyriacou H, Vaz D, Simor A, Louie M, McGavin M J. Molecular analysis of the accessory gene regulator (agr) locus and balance of virulence factor expression in epidemic methicillin-resistant Staphylococcus aureus. J Infect Dis. 2000;181:990–1000. doi: 10.1086/315342. [DOI] [PubMed] [Google Scholar]
- 37.Park P W, Roberts D D, Grosso L E, Parks W C, Rosenbloom J, Abrams W R, Mecham R P. Binding of elastin to Staphylococcus aureus. J Biol Chem. 1991;266:23399–23406. [PubMed] [Google Scholar]
- 38.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmenlinna S, Lyytikäinen O, Kotilainen P, Scotford R, Siren E, Vuopio-Varkila J. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in Finland. Eur J Clin Microbiol Infect Dis. 2000;19:101–107. doi: 10.1007/s100960050438. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Schneewind O, Model P, Fischetti V A. Sorting of protein A to the staphylococcal cell wall. Cell. 1992;70:267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 42.Signäs C, Raucci G, Jönsson K, Lindgren P-E, Anantharamaiah G M, Höök M, Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci USA. 1989;86:699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sjödahl J. Repetitive sequences in protein A from Staphylococcus aureus. Arrangement of five regions within the protein, four being highly homologous and Fc-binding. Eur J Biochem. 1977;73:343–351. doi: 10.1111/j.1432-1033.1977.tb11324.x. [DOI] [PubMed] [Google Scholar]
- 44.Switalski L M, Speziale P, Höök M. Isolation and characterization of a putative collagen receptor from Staphylococcus aureus strain Cowan 1. J Biol Chem. 1989;264:21080–21086. [PubMed] [Google Scholar]
- 45.Tung H, Guss B, Hellman U, Persson L, Rubin K, Rydén C. A bone sialoprotein-binding protein from Staphylococcus aureus: a member of the staphylococcal Sdr family. Biochem J. 2000;345:611–619. [PMC free article] [PubMed] [Google Scholar]
- 46.Uhlén M, Guss B, Nilsson B, Gatenbeck S, Philipson L, Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem. 1984;259:1695–1702. [PubMed] [Google Scholar]
- 47.Väisänen-Rhen V. Fimbria-like hemagglutinin of Escherichia coli O75 strains. Infect Immun. 1984;46:401–407. doi: 10.1128/iai.46.2.401-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaudaux P E, Monzillo V, Francois P, Lew D P, Foster T J, Berger-Bächi B. Introduction of the mec element (methicillin resistance) into Staphylococcus aureus alters in vitro functional activities of fibrinogen and fibronectin adhesins. Antimicrob Agents Chemother. 1998;42:564–570. doi: 10.1128/aac.42.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virkola R, Lähteenmäki K, Eberhard T, Kuusela P, van Alphen L, Ullberg M, Korhonen T K. Interaction of Haemophilus influenzae with the mammalian extracellular matrix. J Infect Dis. 1996;173:1137–1147. doi: 10.1093/infdis/173.5.1137. [DOI] [PubMed] [Google Scholar]
- 50.Westerlund B, Kuusela P, Vartio T, van Die I, Korhonen T K. A novel lectin-independent interaction of P fimbriae of Escherichia coli with immobilized fibronectin. FEBS Lett. 1989;243:199–204. doi: 10.1016/0014-5793(89)80129-2. [DOI] [PubMed] [Google Scholar]
- 51.Wilson C R, Skinner S E, Shaw W V. Analysis of two chloramphenicol resistance plasmids from Staphylococcus aureus: insertional inactivation of Cm resistance, mapping of restriction sites, and construction of cloning vehicles. Plasmid. 1981;5:245–258. doi: 10.1016/0147-619x(81)90002-0. [DOI] [PubMed] [Google Scholar]