Abstract
Three subtypes of distinct pathological proteins accumulate throughout multiple brain regions and shape the heterogeneous clinical presentation of frontotemporal lobar degeneration (FTLD). Besides the main pathological subtypes, co-occurring pathologies are common in FTLD brain donors. The objective of this study was to investigate how the location and burden of (co-)pathology correlate to early psychiatric and behavioural symptoms of FTLD.
Eighty-seven brain donors from The Netherlands Brain Bank cohort (2008–2017) diagnosed with FTLD were included: 46 FTLD-TAR DNA-binding protein 43 (FTLD-TDP), 34 FTLD-tau, and seven FTLD-fused-in-sarcoma (FTLD-FUS). Post-mortem brain tissue was dissected into 20 standard regions and stained for phosphorylated TDP-43, phosphorylated tau, FUS, amyloid-β, and α-synuclein. The burden of each pathological protein in each brain region was assessed with a semi-quantitative score. Clinical records were reviewed for early psychiatric and behavioural symptoms. Whole-brain clinico-pathological partial correlations were calculated (local false discovery rate threshold = 0.01). Elaborating on the results, we validated one finding using a quantitative assessment of TDP-43 pathology in the granular layer of the hippocampus in FTLD-TDP brain donors with (n = 15) and without (n = 15) hallucinations.
In subcortical regions, the presence of psychiatric symptoms showed positive correlations with increased hippocampal pathology burden: hallucinations with TDP-43 in the granular layer (R = 0.33), mania with TDP-43 in CA1 (R = 0.35), depression with TDP-43 in CA3 and with parahippocampal tau (R = 0.30 and R = 0.23), and delusions with CA3 tau (R = 0.26) and subicular amyloid-β (R = 0.25). Behavioural disinhibition showed positive correlations with tau burden in the thalamus (R = 0.29) and with both TDP-43 and amyloid-β burden in the subthalamus (R = 0.23 and R = 0.24). In the brainstem, the presence of α-synuclein co-pathology in the substantia nigra correlated with disinhibition (R = 0.24), tau pathology in the substantia nigra correlated with depression (R = 0.25) and in the locus coeruleus with both depression and perseverative/compulsive behaviour (R = 0.26 and R = 0.32). The quantitative assessment of TDP-43 in the granular layer validated the higher burden of TDP-43 pathology in brain donors with hallucinations compared to those without hallucinations (P = 0.007).
Our results show that psychiatric symptoms of FTLD are linked to subcortical pathology burden in the hippocampus, and hallucinations are linked to a higher burden of TDP-43 in the granular layer. Co-occurring non-FTLD pathologies in subcortical regions could contribute to configuring the clinical phenotype of FTLD.
Keywords: frontotemporal dementia, psychiatric symptoms, hallucinations, copathology, subcortical
A majority of patients with frontotemporal dementia display co-pathologies alongside the main pathology. Scarioni et al. show that neuropsychiatric symptoms in FTD are associated with subcortical pathology in the hippocampus, with hallucinations associated with a higher burden of TDP-43 pathology in the granular layer.
Introduction
Frontotemporal dementia is an early-onset neurodegenerative disease that is challenging to diagnose due to the heterogeneous clinical presentation and has a high social impact.1–3 Frontotemporal dementia encompasses several clinical presentations, including neuropsychiatric (i.e. behavioural and psychiatric), language and motor syndromes, which largely overlap after early disease stages.3–5 Behavioural symptoms are the most common and shared features between different clinical presentations of frontotemporal dementia, and psychiatric symptoms have recently been recognized as a central feature of frontotemporal dementia3,6,7: up to 50% of patients with the behavioural variant of frontotemporal dementia are initially misdiagnosed with a primary psychiatric disorder (PPD).6,8 Among psychiatric symptoms of frontotemporal dementia, hallucinations have been reported in up to 17% of sporadic patients, although the lack of a formal inclusion criterion in the consensus clinical criteria may lead to an underestimate.6,7,9,10
Not only the clinical presentation of frontotemporal dementia is complex, but also the underlying pathology, named frontotemporal lobar degeneration (FTLD), which includes three main distinct subtypes of pathologically misfolded proteins: TAR DNA-binding protein-43 (TDP-43), tau and fused in sarcoma (FUS).11 Adding further to the complexity, FTLD pathological subtypes are scarcely predictable on the basis of frontotemporal dementia clinical syndromes.7 On the other hand, we recently showed that single symptoms, rather than clinical syndromes, can be an important indicator of pathology, and that neuropsychiatric features such as hallucinations and perseverative/compulsive behaviour point to specific underlying FTLD pathological subtypes.7
Although FTLD brain donors share the common hallmark of frontal and/or temporal atrophy, multiple cortical and subcortical brain areas are affected by variable degrees of distinct subtypes of accumulating pathologies. Moreover, a common finding beside FTLD main pathological subtype is the presence of concomitant FTLD and non-FTLD pathologies, whose clinical significance has not yet been elucidated.7,12,13 Correlations between symptoms of frontotemporal dementia and regional brain abnormalities are traditionally assessed through imaging studies, which provide insights into atrophy patterns, but not into underlying pathology.14–20 A comprehensive evaluation of regional vulnerability across the clinical spectrum of frontotemporal dementia should not only incorporate the affected brain region, but also the burden and type of misfolded protein. Moreover, in order to understand the potential impact of each misfolded protein on the clinical presentation, co-occurring pathologies should be taken into account, without any presumptive distinction between main pathological subtype and co-pathology.
The primary aim of this post-mortem study is to investigate regional clinicopathological correlations between early neuropsychiatric symptoms of frontotemporal dementia and the burden of specific pathologies across several brain regions. Another objective of this work is to determine the role of non-FTLD co-pathologies in shaping the clinical presentation of frontotemporal dementia.
Materials and methods
Whole-brain correlations
Subjects
The Netherlands Brain Bank (NBB) is a non-profit-making facility that registers donors with neurological or psychiatric disorders referred from different clinical settings in the Netherlands, including academic and community hospitals, and upon death provides their anonymized brain tissue, clinical information and neuropathological diagnosis to researchers following an approved request for this material.21 In this retrospective post-mortem study, we selected from the NBB cohort brain donors autopsied between 2008 and 2017 who received both a clinical diagnosis of frontotemporal dementia and a pathological diagnosis of FTLD.7 The exclusion criteria were the presence of concomitant brain tumours or large infarcts.7 We evaluated 99 brain donors, of which 12 were further excluded due to the lack of whole-hemisphere tissue availability. This yielded a cohort of 87 donors of which the right hemisphere was available for analysis. All donors had given informed consent for autopsy and the use of their tissue and medical records for research purposes.22,23 Ethical approval for the NBB procedures and forms was given by the Medical Ethics Committee of the VU University Medical Center (Amsterdam, The Netherlands). Table 1 summarizes the demographics, clinical and pathological features of this cohort.
Table 1.
Demographics of included brain donors
| All donors | FTLD-TDP | FTLD-tau | FTLD-FUS | P-value | |
|---|---|---|---|---|---|
| n (%) | 87 (100%) | 46/87 (53%) | 34/87 (39%) | 7/87 (8%) | — |
| Gender (M:F) | 46:41 | 21:25 | 19:15 | 6:1 | 0.13a |
| Age at onset (years; mean ± SD) | 57.6 ± 10.7 | 59.4 ± 9.4 | 56.9 ± 11.5 | 48.6 ± 11.4 | 0.04b,* (FTLD-TDP versus FTLD-FUS 0.03*) |
| Age at death (years; mean ± SD) | 66.0 ± 9.7 | 67.4 ± 8.0 | 66.1 ± 10.6 | 56.1 ± 11.4 | 0.02b,* (FTLD-TDP versus FTLD-FUS 0.01*; FTLD-tau versus FTLD-FUS 0.03*) |
| Disease duration (years; mean ± SD) | 8.4 ± 5.0 | 8.0 ± 4.7 | 9.2 ± 5.2 | 7.6 ± 6.5 | 0.54 |
| Clinical diagnosis (%) | 61/87 (70%) bvFTD; 21/87 (24%) PPA; 1/87 (1%) PSP; 4/87 (5%) ALS-FTD | 28/46 (61%) bvFTD; 14/46 (30%) PPA; 4/46 (9%) ALS-FTD | 27/34 (79%) bvFTD; 6/34 (18%) PPA; 1/34 (3%) PSP | 5/7 (72%) bvFTD; 1/7 (14%) PPA; 1/7 (14%) CBS | — |
| Genetic status (%) | 15/87 (17%) C9orf72; 11/87 (13%) MAPT; 6/87 (7%) GRN; 1/87 (1%) TARDBP | 15/46 (33%) C9orf72; 6/46 (13%) GRN; 1/46 (2%) TARDBP | 11/34 (32%) MAPT | — | |
| Brain weight (g; mean ± SD) | 1061.6 ± 166.7 | 1053.4 ± 160.7 | 1052.1 ± 180.1 | 1161.7 ± 117.1 | 0.26b |
| Pathological subgroups (% main pathology) | — | 14/46 (31%) TDP-A; 13/46 (28%) TDP-B; 11/46 (24%) TDP-C; 6/46 (13%) TDP-E; 2/46 (4%) TDP-U | 20/34 (59%) PiD; 3 (9%) CBD; 4 (12%) PSP; 7 (20%) tauopathy-U | 5/7 (72%) aFTLD-U; 2/7 (28%) NIFID | — |
| Thal stage for amyloid-β (median ± IQR) | 1 (0–2) | 1 (0–3) | 0 (0–1) | 0 (0–0.5) | 0.01c,* (FTLD-TDP versus FTLD-tau 0.04*) |
| Braak stage for tau (median ± IQR) | 2 (0–2) | 2 (0.5–2) | NA | 1 (0–2.25) | NA |
| Braak stage for Lewy body pathology (median ± IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.07c |
Values are expressed as %, mean ± standard deviation (SD), or median ± interquartile range (IQR). ADL = activities of daily living; ALS = amyotrophic lateral sclerosis; bvFTD = behavioural variant frontotemporal dementia; CBS = corticobasal syndrome; U = unknown (did not meet criteria for any specific subtype); aFTLD-U = atypical FTLD with ubiquitin inclusions; NIFID = neuronal intermediate filament inclusion disease; PPA = primary progressive aphasia.
Pearson’s chi-squared test.
One-way ANOVA (Tukey's post hoc test).
Kruskal–Wallis one-way ANOVA and pairwise comparisons with Bonferroni correction.
P < 0.05.
Scoring of symptoms
A neurologist experienced in the evaluation of patients with frontotemporal dementia (M.S.) reviewed the clinical records of all donors, which included detailed evaluations from neurologists, psychiatrists and neuropsychologists, and standardized neuropsychological tests administered to support the diagnostic process.
Psychiatric and behavioural symptoms concerning the first 3 years from the beginning of symptoms were scored.4,5 Symptoms that were explicitly mentioned as absent or were never mentioned throughout all written evaluations were scored as absent. The psychiatric features ‘depression’, ‘mania’, ‘hallucinations’ and ‘delusions’ were scored as present or absent.24 The clinical criteria for the behavioural variant of frontotemporal dementia were operationalized and used as a score, where the subcriteria explicitly mentioned in the clinical records were summed for the following symptoms: disinhibition, apathy, perseverative/compulsive behaviour, and hyperorality.4 The behavioural feature ‘lack of empathy’ was not included in this evaluation because it was rarely explicitly mentioned in the clinical records. See Supplementary material for detailed definitions and scoring of symptoms.
Pathological procedures
NBB procedures were performed in accordance with the Code of Conduct for Brain Banking and Declaration of Helsinki.22,23 Autopsies were performed by the NBB at the designated premises of the VU Medical Center in Amsterdam, The Netherlands. Twenty-one brain regions from the right hemisphere were dissected for diagnostic purposes following standard procedures.25 These regions included: prefrontal cortex (middle frontal gyrus), temporal lobe (superior temporal gyrus), motor cortex (precentral gyrus), parietal lobe (inferior parietal lobule), occipital lobe (superior occipital gyrus), cingulum (anterior cingulate gyrus), insula (short insular gyrus), hippocampus (middle third of an anteroposterior axis)—including granular layer (GL) of the hippocampal dentate gyrus (DG), hippocampal cornu ammonis (CA) subfields CA1 to CA4, subiculum, and parahippocampal gyrus, corpus striatum, amygdala, thalamus, subthalamus, substantia nigra (SN), locus coeruleus (LC), and medulla oblongata (MO) (at the level of inferior olivary nucleus). We stained all brain regions for phosphorylated-TDP-43 (pTDP43; Cosmo Bio), phosphorylated-tau (pTau) (AT8; Pierce Biotechnology), and amyloid-β (IC16 antibody; kind gift of Prof. Dr Korth, Heinrich Heine University, Düsseldorf, Germany). After ascertaining from the pathology records that no donor had a Braak α-synuclein score higher than 4, we assessed α-synuclein pathology in the brainstem, amygdala, and hippocampus (LB509 Thermo Fisher Scientific).26 FTLD-FUS donors were stained with FUS antibody (HPA008784; Sigma Aldrich) in addition to pTDP-43, pTau, amyloid-β and α-synuclein.
Semi-quantitative scoring of pathology
A visual semi-quantitative score from 0 (none) to 3 (severe) was used to separately assess the burden of pTDP-43, pTau, FUS, and amyloid-β in each brain region. α-synuclein pathology was scored as present (1) or absent (0), as the burden of α-synuclein showed too little variability to be further categorized in a semi-quantitative score. Scoring methods were adapted from the literature and applied to the area with the highest burden of pathology for each brain region.27–29 See Supplementary Fig. 1 for scoring examples and definitions. Dipeptide pathology specific to C9orf72 repeat-expansion carriers was not quantified, as TDP-43 pathology is most closely linked to neurodegeneration in this condition.30
Statistical analysis
Whole-brain clinicopathological partial correlations
Whole brain clinicopathological partial correlations were calculated with R 3.6.1 (R Core Team, 2019). All neuropsychiatric symptoms and all semi-quantitative pathological scores were included in the statistical analysis, together with measures of disease duration and age at death. We calculated partial correlations between all included variables on the basis of their heterogeneous (polychoric and polyserial) marginal correlations. This approach allowed us to take into account the mixed metrics of the data (i.e. quantitative, ordinal and nominal variables) and to weed out spurious associations.31 Briefly, a heterogeneous correlation matrix based on pairwise-complete observations was calculated. Variables with no variability were omitted. The conditioning of this matrix was then assessed based on the condition number plot.32 Next, a regularized version of the heterogeneous inverse correlation matrix was calculated, whose entries are proportional to the full-order conditioned correlations, i.e. partial correlations, with a penalty-value informed by the previous step.33 Whenever we talk about correlations in the remainder, we imply the partial or conditioned correlations. Finally, the support of the matrix was determined by way of a local false discovery rate (FDR) procedure.34 The local FDR threshold was set at 0.01. The local FDR, in this setting, represents the empirical posterior probability that a partial correlation is non-zero given its observed value. We retain those partial correlations whose posterior probability of being present equals or exceeds 0.99.
Additional Spearman correlations were performed to assess the relationship between neuropsychiatric symptoms and the regional cumulative pathology burden, calculated as the sum of all pathology scores in each brain region.
Subgroup analyses
Within the FTLD-TDP group, we also evaluated the association between hippocampal sclerosis and neuropsychiatric symptoms by means of Pearson's chi-squared tests. The association between hippocampal sclerosis and regional TDP-43 pathology burden was assessed with Mann–Whitney U-tests. Moreover, Mann–Whitney U-tests were performed to compare TDP-43 deposition throughout the brain between C9orf72 and other FTLD-TDP donors.
Validation of results by quantification of TDP-43 pathology in the hippocampal granular layer
Subjects
From the NBB cohort, we selected brain donors who received a pathological diagnosis of FTLD-TDP, without concomitant brain tumors or large infarcts, with available brain tissue from the right middle hippocampus, and with detailed complete clinical records. An experienced neurologist (M.S.) searched their clinical records for the presence of hallucinations in any sensory modality in the first 3 years from disease onset. We included 15 FTLD-TDP brain donors with hallucinations (FTLD-Hal+) and 15 age- and sex- matched FTLD-TDP brain donors without hallucinations (FTLD-Hal−), whose main features are described in Table 2.
Table 2.
Overview of FTLD-TDP brain donors with and without hallucinations
| ID | Age at death | Gender | Disease duration | Clinical diagnosis | Hallucinations | Genetics | TDP-43 subtype | Brain weight |
|---|---|---|---|---|---|---|---|---|
| FTLD-TDP Hal+ | ||||||||
| ȃ1 | 67 | F | 4 | bvFTD | Auditory | Negative | E | 1149 |
| ȃ2 | 64 | F | 12 | bvFTD | Auditorya1 | Negative | C | 975 |
| ȃ3 | 60 | M | 6 | bvFTD | Auditorya2 | C9orf72 | B | 1065 |
| ȃ4 | 64 | M | 3 | PPAnos | Auditory | C9orf72 | B | 1100 |
| ȃ5 | 63 | M | 11 | bvFTD | Visual | Negative | E | 1239 |
| ȃ6 | 61 | F | 6 | bvFTD | Nos | Negative | A | 755 |
| ȃ7 | 69 | M | 11 | svPPA | Auditory | Negative | C | 974 |
| ȃ8 | 64 | M | 2 | PPAnos | Nos | Negative | E | 1071 |
| ȃ9 | 66 | F | 4 | PPAnos | Auditory and visual | C9orf72 | B | 1099 |
| ȃ10 | 61 | F | 2 | nfPPA | Auditory | Negative | E | 1311 |
| ȃ11 | 67 | F | 16 | Schizoaffective disorder | Nos | Negative | C | 878 |
| ȃ12 | 63 | M | 10 | svPPA | Auditorya3 | Negative | C | 1190 |
| ȃ13 | 66 | M | 10 | bvFTD | Visual | Negative | B | 1055 |
| ȃ14 | 81 | M | 8 | bvFTD | Auditory and visual | Negative | A | 1120 |
| ȃ15 | 70 | F | 4 | bvFTD | Nos | Negative | A | 972 |
| Descriptive statisticsb,c | 65.7 ± 5.1b | 7/15 (47%) F, 8/15 (53%) Mc | 7.3 ± 4.2b | 8/15 (53%) bvFTD, 6/15 (40%) PPA, 1/15 (7%) psychiac | 7/15 (47%) auditory, 2/15 (13%) visual, 2/15 (13%) visual and auditory, 4/15 (27%) nosc | 12/15 (80%) negative, 3/15 (20%) C9orf72 carriersc | 1063.5 ± 140.2b | |
| FTLD-TDP Hal− | ||||||||
| ȃ1 | 59 | M | 14 | bvFTD | None | C9orf72 | B | 1170 |
| ȃ2 | 71 | F | 6 | PPAnos | None | Negative | A | 840 |
| ȃ3 | 65 | M | 5 | bvFTD | None | C9orf72 | A | 1247 |
| ȃ4 | 66 | M | 12 | bvFTD | None | Negative | C | 1085 |
| ȃ5 | 71 | F | 8 | bvFTD | None | Negative | E | 874 |
| ȃ6 | 67 | F | 10 | bvFTD | None | Negative | B | 1115 |
| ȃ7 | 65 | F | 9 | bvFTD | None | Negative | A | 826 |
| ȃ8 | 64 | M | 2 | nfPPA | None | Negative | B | 1285 |
| ȃ9 | 77 | F | 10 | PPAnos | None | Negative | B | 1008 |
| ȃ10 | 45 | F | 10 | bvFTD | None | Negative | nos | 800 |
| ȃ11 | 62 | M | 16 | svPPA | None | Negative | C | 755 |
| ȃ12 | 73 | M | 11 | PPAnos | None | Negative | A | 1107 |
| ȃ13 | 60 | M | 4 | bvFTD | None | Negative | B | 1007 |
| ȃ14 | 87 | F | 7 | bvFTD | None | Negative | A | 980 |
| ȃ15 | 64 | F | 2 | PPAnos | None | Negative | B | 1097 |
| Descriptive statisticsb,c | 66.4 ± 9.3b | 8/15 (53%) F, 7/15 (47%) Mc | 8.4 ± 4.1b | 9/15 (60%) bvFTD, 6/15 (40%) PPAc | 13/15 (86.6%) negative, 2/15 (13.3%) C9orf72 carriersc | 1013.1 ± 165.2b | ||
| P-value (FTLD-TDP Hal+ versus FTLD-TDP Hal−)d | ||||||||
| 0.06e | 0.72f | 0.55e | 0.59f | — | 1.00f | 0.36f | 0.81e | |
Nos = not otherwise specified; psychia = psychiatric; nfPPA = non-fluent variant PPA; svPPA = semantic variant PPA.
Also reported with somatoform complaints: (1. Diffuse pains and itching. Feeling shaky, dizzy, and with heart palpitations; 2. Somatoform and vegetative symptoms, irritable bowel syndrome; 3. Hemianaesthesia alternans, anxiety syndrome with somatizations, prickly, tingling sensations in the right leg which could not be neurologically explained).
Mean ± standard deviation.
Frequency.
Between-groups comparisons.
One-way ANOVA.
Pearson's chi-squared test.
Quantitative assessment of pathology
In order to confirm the main result obtained with the semi-quantitative assessment of pathology and to validate the semi-quantitative method itself, we further assessed the burden of TDP-43 pathology in FTLD-Hal+ and FTLD-Hal− in the hippocampal GL with a quantitative method. High-resolution pictures of the GL of the hippocampal DG were acquired for each pTDP-43 stained hippocampal section (n = 30) and processed with the ImageJ software (2019, version 1.52r).35 Regions of interest (ROIs) were drawn around the GL of the DG in the middle portion of a medio-lateral axis. Then, each picture was processed with the ‘color deconvolution’ plugin and converted into a black/white image. Finally, the percentage of black pixels was calculated for each picture. Next, to assess substantial GL neuronal loss, we measured GL thickness between FTLD-Hal+ and FTLD-Hal− donors at six locations in two images using the line tool in ImageJ and averaged the results.
Statistical analysis
The percentage of pixels showing TDP-43 inclusions in the GL of the hippocampal DG was compared between FTLD-Hal+ and FTLD-Hal− using independent-samples t-test. An ANOVA was used to assess the differences in GL thickness between FTLD-Hal+ and FTLD-Hal− donors. Demographic, clinical and pathological data were compared between the two groups of interest using Pearson's chi-squared test—for categorical variables—or one-way ANOVA—for continuous variables. Statistical tests were two-tailed and a P-value of 0.05 was considered significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS version 26 for Windows, Chicago, IL).
Data availability
Data and R script are available upon request from the corresponding author.
Results
Whole-brain correlations
Demographics, clinical and pathological overview
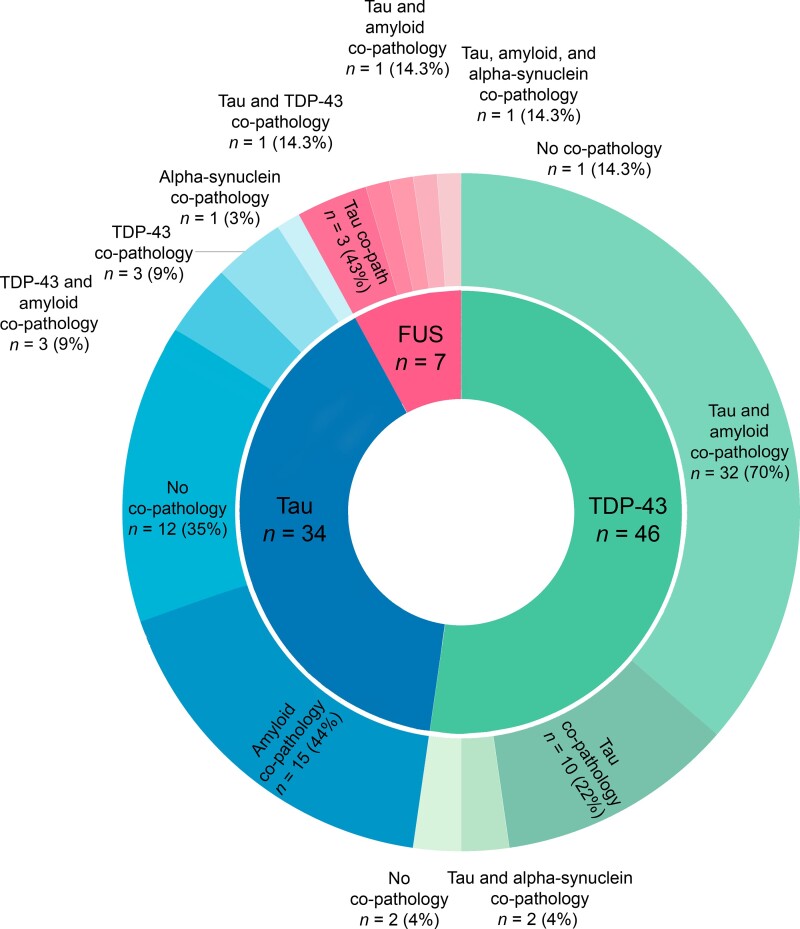
Our cohort consisted of 87 FTLD brain donors, whose main pathological subtypes were FTLD-TDP (n = 46; 53%), FTLD-tau (n = 34; 39%), and FTLD-FUS (n = 7; 8%). Demographics are described in Table 1. FTLD-FUS brain donors were younger than other groups of brain donors at disease onset and death, in line with previous literature (Table 1).36C9orf72 repeat expansions were most common in our cohort (15/87 = 16%) compared to GRN (6/87 = 7%); genetic status was obtained from a genetic cohort.37 The prevalence of genetic mutations in our cohort is in line with previous work.37,38 The frequency of early symptoms of frontotemporal dementia has been compared between FTLD pathological subtypes in a previous study.7Table 3 summarizes the frequency of early neuropsychiatric symptoms and the distribution of behavioural symptoms scores in FTLD brain donors. In this cohort, co-occurring pathologies next to the main pathological diagnosis were assessed in detail and we also scored minimal pathology burden in a single brain region. Overall, co-occurring pathologies were present in 73 (83%) brain donors. Figure 1 shows the distribution of co-occurring pathologies among the main FTLD pathological subtypes. Table 4 describes the distribution of regional pathology burden scores. The burden of non-FTLD co-occurring pathologies—amyloid-β and α-synuclein—was low in all examined brain regions (Table 3). We observed a higher regional burden of amyloid co-pathology and a higher Thal stage for amyloid in FTLD-TDP brain donors than in FTLD-tau donors, which were diagnosed with Pick's disease (PiD) in the majority of cases (20/34; 59%) (Tables 1 and 4). Our observation could be explained in light of previous literature, which shows less widespread amyloid co-pathology in PiD donors compared to other FTLD-tau donors, such as those diagnosed with corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP).12
Table 3.
Frequency of neuropsychiatric symptoms (%) and behavioural symptoms scores (median ± IQR)
| Symptom | All donors | FTLD-TDP | FTLD-tau | FTLD-FUS |
|---|---|---|---|---|
| Depression (%) | 18/87 (20.7%) | 6/46 (13%)a | 7/34 (20.6%)a | 5/7 (71.4%)b,c |
| Mania (%) | 4/87 (4.6%) | 3/46 (6.5%) | 0/34 (0%) | 1/7 (14.3%) |
| Hallucinations (%) | 11/87 (12.6%) | 11/46 (23.9%)b | 0/34 (0%)c | 0/7 (0%) |
| Delusions (%) | 9/87 (10.3%) | 4/46 (8.7%) | 4/34 (11.8%) | 1/7 (14.3%) |
| Disinhibition (%) | 65/87 (74.7%) | 33/46 (71.7%) | 26/34 (76.5%) | 6/7 (85.7%) |
| Disinhibition score (0–3) | 1 (0–2) | 1 (0–2) | 1 (0.75–2) | 1 (1–3) |
| Apathy (%) | 63/87 (72.4%) | 31/46 (67.4%) | 26/34 (76.5%) | 6/7 (85.7%) |
| Apathy score (0–2) | 1 (0–2) | 1 (0–2) | 1 (0.75–2) | 1 (1–2) |
| Pers/comp (%) | 57/87 (65.5%) | 27/46 (58.7%) | 25/34 (73.5%) | 5/7 (71.4%) |
| Pers/comp score (0–3) | 1 (0–2) | 1 (0–2) | 1 (0–1) | 2 (0–2) |
| Hyperorality (%) | 57/87 (65.5%) | 26/46 (56.5%) | 26/34 (76.5%) | 5/7 (71.4%) |
| Hyperorality score (0–3) | 1 (0–1) | 1 (0–1) | 1 (0.75–1) | 1 (0–2) |
Values are expressed as % or median ± IQR. The frequency of neuropsychiatric symptoms was compared between groups with Pearson’s chi-square and P-values have been adjusted for multiple comparisons with Bonferroni correction; behavioural symptoms scores were compared between groups with Kruskal–Wallis one-way ANOVA and pairwise comparisons with Bonferroni correction.
Significant (P < 0.05) difference with FTLD-FUS.
Significant (P < 0.05) difference with FTLD-tau.
Significant (P < 0.05) difference with FTLD-TDP.
Figure 1.
Overview of concomitant pathologies across FTLD pathological subtypes. The inner circle represents the main pathological diagnosis, the outer circle represents the proportion of different concomitant pathologies, where minimal pathology burden in a single brain region was also scored.
Table 4.
(Co-)pathology burden per brain region
| (Co-)pathology score | All donors | FTLD-TDP | FTLD-tau | FTLD-FUS |
|---|---|---|---|---|
| Prefrontal Aβ (0–3) | 0 (0–2.75) | 2 (0–3)a | 0 (0–1.5)b | 0 (0–0) |
| Prefrontal TDP (0–3) | 1 (0–2) | 2 (1–2.25)a,c | 0 (0–0)b | 0 (0–0)b |
| Temporal Aβ (0–3) | 0 (0–1) | 1 (0–2)a | 0 (0–0)b | 0 (0–0) |
| Temporal tau (0–3) | 1 (0–3) | 0 (0–1)a | 3 (2–3)b,c | 1 (0–1)a |
| Temporal TDP (0–3) | 0 (0–2) | 2 (1–2.5)a,c | 0 (0–0)b | 0 (0–0)b |
| Precentral tau (0–3) | 0 (0–2) | 0 (0–0)a | 2 (1–2)b,c | 0 (0–0)a |
| Precentral TDP (0–3) | 0 (0–2) | 2 (1–2)a,c | 0 (0–0)b | 0 (0–0)b |
| Parietal tau (0–3) | 0 (0–2) | 0 (0–0)a | 2 (2–3)b,c | 0 (0–1)a |
| Parietal TDP (0–3) | 0 (0–2) | 2 (1–3)a,c | 0 (0–0)b | 0 (0–0)b |
| Occipital TDP (0–3) | 0 (0–1) | 0.5 (0–1)a,c | 0 (0–0)b | 0 (0–0)b |
| Cingulum Aβ (0–3) | 0 (0–2) | 2 (0–3)a | 0 (0–0)b | 0 (0–0) |
| Cingulum TDP (0–3) | 0 (0–2.75) | 2.5 (2–3)a,c | 0 (0–0)b | 0 (0–0)b |
| Dentate gyrus Aβ (0–3) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Dentate gyrus TDP (0–3) | 0.5 (0–1) | 1 (1–2)a,c | 0 (0–0)b | 0 (0–0)b |
| CA4 Aβ (0–3) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| CA4 TDP (0–3) | 0 (0–1) | 0 (0–1)a,c | 0 (0–0)b | 0 (0–0)b |
| CA3 tau (0–3) | 1 (0–1.75) | 0 (0–1)a | 2 (1–2)b,c | 0 (0–1)a |
| CA3 TDP (0–3) | 0 (0–0) | 0 (0–1)a | 0 (0–0)b | 0 (0–0) |
| CA2 TDP (0–3) | 0 (0–1) | 1 (0–1)a,c | 0 (0–0)b | 0 (0–0)b |
| CA1 Aβ (0–3) | 0 (0–1) | 0 (0–2) | 0 (0–0) | 0 (0–0) |
| CA1 TDP (0–3) | 0 (0–1) | 1 (0.75–2)a,c | 0 (0–0)b | 0 (0–0)b |
| Subiculum Aβ (0–3) | 0 (0–1) | 0 (0–2)a | 0 (0–0)b | 0 (0–0) |
| Subiculum tau (0–3) | 1 (0–3) | 1 (0–1)a | 3 (2–3)b,c | 0 (0–0)a |
| Parahippocampal tau (0–3) | 2 (1–3) | 1 (0–2)a | 3 (2–3)b,c | 0 (0–0)a |
| Amygdala TDP (0–3) | 1 (0 -2) | 2 (1–3)a,c | 0 (0–0)b | 0 (0–0)b |
| Amygdala αsyn (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Thalamus tau (0–3) | 1 (0–1) | 0 (0–0)a | 1.5 (1–2)b,c | 0 (0–1)a |
| Thalamus TDP (0–3) | 0 (0–1) | 1 (0–1)a,c | 0 (0–0)b | 0 (0–0)b |
| Subthalamus Aβ (0–3) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Subthalamus TDP (0–3) | 0 (0–0) | 0 (0–1)a,c | 0 (0–0)b | 0 (0–0)b |
| Insula Aβ (0–3) | 0 (0–2) | 1 (0–2)a | 0 (0–0)b | 0 (0–0) |
| Sub nigra tau (0–3) | 1 (0–2) | 0 (0–1)a | 2 (2–2.5)b,c | 0 (0–0)a |
| Sub nigra TDP (0–3) | 0 (0–1) | 1 (0–1.5)a,c | 0 (0–0)b | 0 (0–0)b |
| Sub nigra FUS (0–3) | 0 (0–0) | 0 (0–0)c | 0 (0–0)c | 2 (1.25–2.75)a,b |
| Sub nigra αsyn (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0.25) |
| L coeruleus tau (0–3) | 1 (0–2) | 0 (0–1)a | 2 (2–3)b,c | 1 (1–1)a |
| M oblongata TDP (0–3) | 0 (0–1) | 1 (0–1)a,c | 0 (0–0)b | 0 (0–0)b |
Values are expressed as median ± IQR. Between-groups comparisons were assessed with Kruskal-Wallis one-way ANOVA and pairwise comparisons with Bonferroni correction.
Significant (P < 0.05) difference with FTLD-tau.
Significant (P < 0.05) difference with FTLD-TDP.
Significant (P < 0.05) difference with FTLD-FUS.
Whole-brain clinicopathological correlations
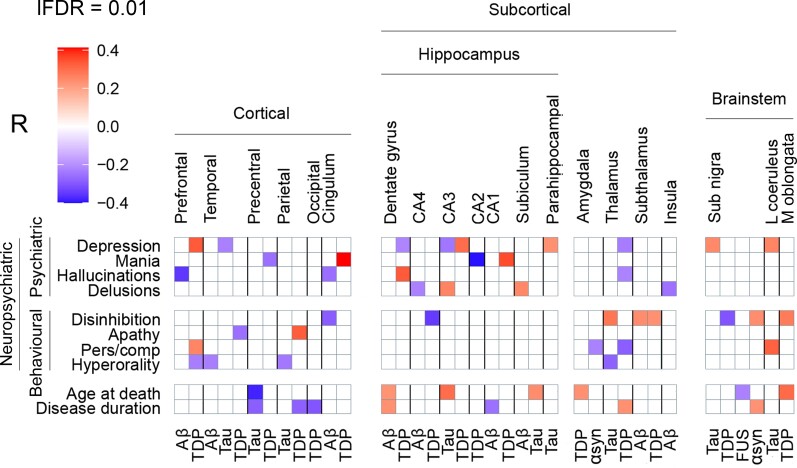
This study investigated the relationship between early neuropsychiatric (i.e. psychiatric and behavioural) symptoms of frontotemporal dementia and the regional brain burden of various pathologies, irrespective of the main pathological subtype diagnosed in each donor. In order to take into account all the reciprocal interactions, partial correlations were performed between all the variables of interest, which included neuropsychiatric symptoms, age at death, disease duration, and 83 scores of regional pathology burden. Supplementary Fig. 2 shows the unthresholded partial correlation matrix. Supplementary Fig. 3 shows the same partial correlations with a local FDR threshold of 0.01. Figure 2 gives an overview of all significant (P < 0.01) partial correlations between clinical and pathological variables.
Figure 2.
Whole-brain clinicopathological partial correlations between neuropsychiatric symptoms and regional pathology burden. Neuropsychiatric symptoms are shown on the left, brain regions are shown at the top, and pathological proteins are shown on the bottom. Partial correlation coefficients are represented with a colour gradient (top left). Red boxes represent positive correlations, purple boxes represent negative correlations. All correlations are significant at the 0.01 level. lFDR = local FDR threshold; sub nigra = substantia nigra; l coeruleus = locus coeruleus; m oblongata = medulla oblongata; pers/comp = perseverative/compulsive.
To investigate the extent to which our findings are specific to a distinct protein or could result from the cumulative burden of different proteins in the same brain region, we performed Spearman correlations between neuropsychiatric symptoms and the sum of all pathology scores in each brain region. No significant positive correlations were found between neuropsychiatric symptoms and regional cumulative regional pathology scores, except between delusions and CA3 cumulative pathology (R = 0.22) which, however, did not survive Bonferroni correction for multiple comparisons (P > 0.05) (Supplementary Table 1). As neuropsychiatric features are commonly reported in C9orf72 carriers,39 we performed an additional Mann–Whitney U-test between C9orf72 and other FTLD-TDP donors, which showed no difference in TDP-43 deposition throughout the brain, suggesting that C9orf72 donors are not specifically affected in one particular brain region compared to other FTLD-TDP donors (Supplementary Table 2).
Cortical regions
Significant positive correlations (P < 0.01) were found between cortical TDP-43 pathology and neuropsychiatric symptoms: prefrontal TDP-43 correlated with depression and perseverative/compulsive behaviour (R = 0.33 and R = 0.26, P < 0.01), parietal TDP-43 with apathy (R = 0.33, P < 0.01), and TDP-43 in the cingulate lobe with mania (R = 0.41, P < 0.01). The burden of TDP-43 was inversely correlated with the presence of mania (R = −0.26, P < 0.01) in the precentral gyrus and with the presence of apathy in the temporal lobe (R = −0.26, P < 0.01). Negative correlations were also observed between the presence of hallucinations and cortical amyloid-β burden in the prefrontal (R = −0.35, P < 0.01) and cingulate lobes (R = −0.26, P < 0.01). The behavioural symptom hyperorality showed solely negative correlations in cortical and subcortical brain regions (Fig. 2).
Subcortical regions
When looking at subcortical regions, psychiatric symptoms showed significant correlations with pathology burden in the hippocampus: hallucinations directly correlated with TDP-43 burden in the granular layer (R = 0.33, P < 0.01) and mania with TDP-43 burden in CA1 (R = 0.35, P < 0.01), while an inverse correlation was observed between mania and TDP-43 burden in the adjacent CA2 region (R = −0.4, P < 0.01). Depression directly correlated with parahippocampal tau and with TDP-43 in CA3 (R = 0.23 and R = 0.30, P < 0.01), while an inverse correlation was observed in CA3 between depression and tau burden (R = −0.24, P < 0.01). Delusions showed positive correlations with CA3 tau burden (R = 0.26, P < 0.01) and with the subicular burden of amyloid-β co-pathology (R = 0.25, P < 0.01) (Fig. 2).
No significant associations were found between the presence of hippocampal sclerosis and the presence of major depression, mania, hallucinations or delusions (Supplementary Table 3). Moreover, no significant associations were found between hippocampal sclerosis and hippocampal TDP-43 burden in all subfields (Supplementary Table 4).
Behavioural disinhibition showed positive correlations with both TDP-43 and amyloid-β co-pathology burden in the subthalamus (R = 0.23 and R = 0.24, P < 0.01) and with tau burden in the thalamus (R = 0.29, P < 0.01). TDP-43 pathology burden in the thalamus showed negative correlations with depression, hallucinations and perseverative-compulsive behaviour (R = −0.23, R = −0.22, R = −0.29, P < 0.01) (Fig. 2).
Brainstem
In the brainstem, behavioural disinhibition correlated with a higher burden of α-synuclein co-pathology and with a lower burden of TDP-43 in the SN (R = 0.24, R = −0.3, P < 0.01), while the burden of TDP-43 in the MO showed a positive correlation with behavioural disinhibition (R = 0.27, P < 0.01). Tau pathology in the SN directly correlated with depression (R = 0.25, P < 0.01), and tau pathology in the LC directly correlated with both depression and perseverative/compulsive behaviour (R = 0.26 and R = 0.32, P < 0.01) (Fig. 2).
Validation of results by quantification of TDP-43 pathology in the hippocampal granular layer
We showed in a previous work how the presence of early hallucinations in FTLD brain donors points to FTLD-TDP main pathological subtype.7 We showed in the present work a significant partial correlation between the presence of early hallucinations and the semi-quantitative assessment of TDP-43 burden in the hippocampal GL. In order to further elaborate on this result and to validate our semi-quantitative method, we performed a quantitative assessment of TDP-43 pathology in the granular layer of the hippocampus and compared it between FTLD-TDP brain donors with (n = 15) and without (n = 15) hallucinations.
Demographics, clinical and pathological overview
The quantitative analysis of TDP-43 pathology burden in the hippocampal GL was performed on an larger number of FTLD-TDP donors with hallucinations (FTLD-Hal+, n = 15) than those included in the main cohort, where 11/87 FTLD donors had hallucinations. FTLD-Hal+ brain donors were compared with an equal number of age- and sex-matched FTLD-TDP brain donors without hallucinations (FTLD-Hal−, n = 15), the majority of which (10/15) were selected from a different cohort of brain donors. Table 2 describes the demographics and the clinical and pathological features of all brain donors included in the quantitative analysis of TDP-43 burden in the GL of the DG (n = 30). No significant differences were found with respect to age at onset, disease duration, and brain weight when comparing FTLD-Hal+ and FTLD-Hal−. No significant differences were found in the distribution of gender, clinical diagnosis, genetic status, and TDP-43 subtypes between the two groups of interest.
Comparison of TDP-43 burden in the granular layer: FTLD-Hal+ versus FTLD-Hal−
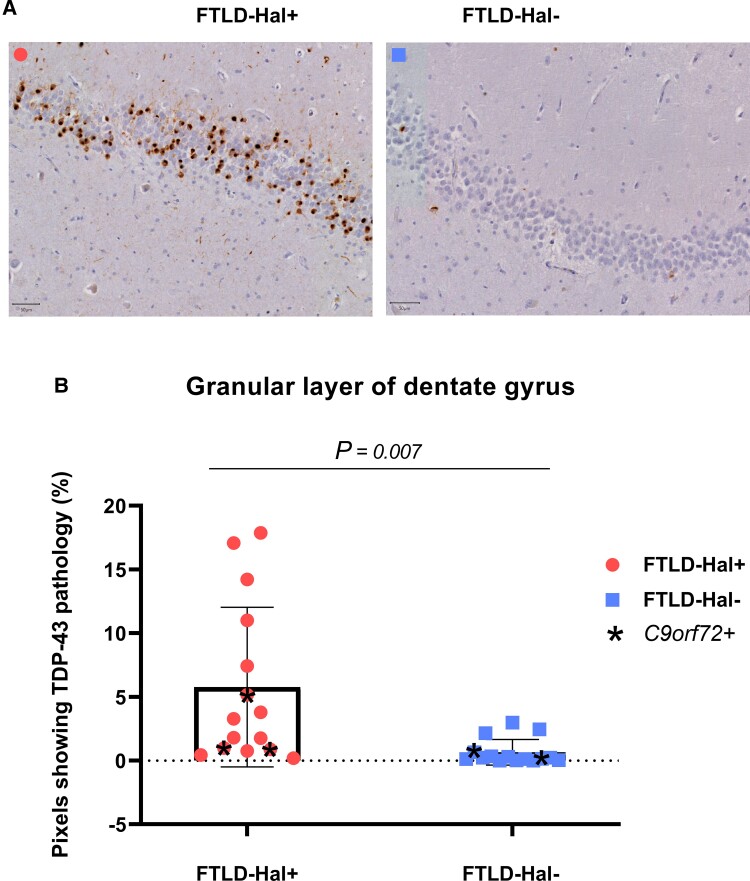
Figure 3A shows pTDP-43 staining in the GL of two hippocampal sections, one from a FTLD-Hal+ brain donor and one from a FTLD-Hal− brain donor. Figure 3B represents the percentage of pixels showing pTDP-43 pathology in the GL of the DG in the two groups of brain donors FTLD-Hal+ (n = 15) and FTLD-Hal− (n = 15).
Figure 3.
Quantification of TDP-43 burden in the granular layer of the hippocampal dentate gyrus. (A) TDP-43 stained sections of the middle hippocampus from two FTLD donors, one from the FTLD-Hal+ group (left) and one from the FTLD-Hal− group (right). Scale bar = 50 µm. (B) Differential distribution of TDP-43 pathology burden—measured with ImageJ software as the percentage of pixels showing TDP-43 pathology—between FTLD-Hal+ and FTLD Hal− brain donors.
Our results confirmed our previous analysis where the burden of TDP-43 pathology in the GL was higher in FTLD-Hal+ brain donors (5.78% ± 6.26%) compared to FTLD-Hal− brain donors (0.65% ± 1%), and this difference was significant [t(14.7) = 3.1, P = 0.007]. No correlation was found in FTLD-TDP brain donors (n = 30) between the burden of TDP-43 pathology in the GL and disease duration (R = 0.09, P = 0.61). In addition, we found no significant differences in GL thickness between FTLD-Hal+ and FTLD-Hal− [F(1) = 0.60, P = 0.45].
Discussion
In this study in 87 consecutive FTLD brain donors, we quantified main and co-pathologies throughout the brain and correlated regional pathology burden with early neuropsychiatric symptoms of frontotemporal dementia, including psychiatric and behavioural symptoms. We found novel subcortical associations between the semi-quantitative assessment of pathology burden in the hippocampus and the presence of psychiatric symptoms, and we validated the positive association between hallucinations and TDP-43 burden in the GL of the hippocampal DG with a quantitative analysis of histological staining. Moreover, we found novel associations between subcortical pathology burden and behavioural disinhibition in the thalamus and subthalamus. In the brainstem, neuropsychiatric symptoms were linked to tau burden in the monoaminergic nuclei SN and LC, while in cortical brain regions neuropsychiatric symptoms correlated with the burden of TDP-43. We showed for the first time that non-FTLD co-pathologies—amyloid-β and α-synuclein—in subcortical brain regions are not simple bystanders, but could play a role in the configuration of the clinical phenotype of frontotemporal dementia.
Subcortical pathology burden is linked to neuropsychiatric symptoms
Hippocampal pathology burden is linked to psychiatric symptoms
We found positive correlations between the semi-quantitative assessment of pathology burden in the hippocampus and multiple neuropsychiatric symptoms: depression was linked to TDP-43 in CA3 and to parahippocampal tau, mania to TDP-43 in CA1, hallucinations to TDP-43 in the GL of the DG, and delusions to tau in CA3 and to amyloid-β in the subiculum. The presence of hippocampal sclerosis does not mediate the positive correlations between hippocampal TDP-43 burden and psychiatric symptoms. To the best of our knowledge, this is the first neuropathology study showing a link between neuropsychiatric symptoms of frontotemporal dementia and multiple pathologies in the hippocampus. A recent neuroimaging study limited to genetic forms of frontotemporal dementia has found positive associations between left hippocampal atrophy and neuropsychiatric symptoms such as depression, anxiety and delusions.14 Interestingly, results from imaging and post-mortem studies on PPD also show connections between psychiatric symptoms and hippocampal atrophy/pathological changes, which could point to a shared vulnerability between frontotemporal dementia and PPD.40–44
The negative correlation between depression and tau in CA3 suggests that the regional association with depression in CA3 is specific for TDP-43 protein, while the negative correlation between mania and TDP-43 in CA2 suggests that the association between mania and TDP-43 is specific for CA1 region. Further studies are needed to determine whether the type of accumulating protein or the affected brain region is a bigger contributor to psychiatric symptoms of frontotemporal dementia. We showed in a previous work how the presence of hallucinations is specific for the pathological subtype FTLD-TDP.7 Until now, an association between psychotic symptoms and TDP-43 pathology in the GL of the hippocampal DG had only been reported in small groups of brain donors with frontotemporal dementia.45,46
Hallucinations are linked to TDP-43 burden in the hippocampal granular layer
Because in our previous study we had found an association between the presence of early hallucinations and FTLD-TDP main pathological subtype, we here further investigated in depth the positive correlation between hallucinations and TDP-43 pathology burden in the GL of the DG with a quantitative immunohistochemical analysis.7 We confirmed that a positive correlation exists between the presence of early-onset hallucinations and the burden of TDP-43 pathology in the GL of the hippocampus. FTLD-Hal+ brain donors did not differ from FTLD-Hal− brain donors with respect to age at death, sex, clinical diagnosis and pathological TDP-43 subtype, which suggests that none of these factors are related to the presence of hallucinations or to a higher burden of TDP-43 pathology in the GL. In addition, no difference in GL thickness was measured between the groups, suggesting that GL neuronal loss is not substantially more severe in donors with hallucinations compared to those without, and that TDP-43 pathology drives this finding.
Hallucinations from different sensory modalities were associated to a higher TDP-43 pathology burden in the GL of the DG. Although the majority of brain donors in our cohort presented with auditory hallucinations, two of them were reported with visual hallucinations only and two with mixed auditory and visual hallucinations. Interestingly, three Hal+ brain donors also reported somatoform complaints and positive sensory symptoms, which could suggest somatic delusions or cenaesthopathic tactile hallucinations, although the retrospective nature of this study precludes further speculations on the nature of these symptoms.47 Overall, the hallucinations reported in our cohort of FTLD brain donors were heterogeneous, which suggests that the hippocampus affected by TDP-43 pathology could produce aberrant internal representations of reality from multiple sensory modalities.48–50 However, it cannot be excluded that our results are driven by the majority of brain donors in our cohort reported with auditory hallucinations. Further studies are needed to confirm whether hippocampal TDP-43 pathology is linked to hallucinations from multiple sensory modalities.
The hippocampus has traditionally been associated to the consolidation of new memories.51 Growing evidence exists that the hippocampus is involved in many other functions, including imagination and conscious perception.51–54 In line with this hypothesis, recent MRI studies have found that impaired spatial representation of reality and impaired future thinking in the behavioural variant of frontotemporal dementia are linked to hippocampal atrophy.55,56 Moreover, hippocampal abnormalities have been extensively linked to psychosis in functional neuroimaging studies on PPD.57–59 A unifying hypothesis for the role of the hippocampus is the processing of new sensory information on the basis of prior experiences to produce internal representations of reality: memories, internal images, conscious experiences and, in the presence of structural abnormalities, hallucinations.48–50 Our novel finding that hippocampal TDP-43 burden is linked to hallucinations in FTLD brain donors supports this hypothesis.
Subthalamic pathology burden is linked to behavioural disinhibition
Our study links disinhibition, a core symptom of frontotemporal dementia, to increased tau burden in the thalamus and to both TDP-43 and amyloid-β burden in the subthalamus. Interestingly, the subthalamus is involved in cost-benefit evaluation and is connected to the thalamus and to the frontal and temporal lobes, which are known to regulate behavioural inhibition.60–66 The fact that disinhibition showed a similar degree of positive correlation with distinct pathological proteins in the subthalamus could point to a bigger role of the aberrant functioning of the subthalamus regardless of the type of pathological protein. Our results show that the core criteria symptoms of the behavioural variant of frontotemporal dementia are not only linked to cortical pathology, but also to subcortical regions such as the subthalamus.
Depression is linked to tau burden in the monoaminergic nuclei of the brainstem
We showed for the first time an association between depressive symptoms and tau pathology burden in the SN and LC in FTLD donors. Perseverative/compulsive behaviour was also linked to higher tau burden in the LC. The SN and LC modulate the monoaminergic input to the brain and can contribute to vulnerability to depression and obsessive-compulsive symptoms.67,68 Changes in the levels of the monoaminergic neurotransmitters dopamine and noradrenaline have been previously identified in frontotemporal dementia.69,70 Interestingly, post-mortem and imaging studies in patients with Lewy body pathology have showed a link between depression and α-synuclein burden in the SN and LC.67,71,72 Our results indicate that SN and LC are involved in the clinical symptoms of depression in frontotemporal dementia.
Both non-FTLD and FTLD pathologies shape the clinical presentation of frontotemporal dementia
Subcortical non-FTLD co-pathologies are linked to symptoms of frontotemporal dementia
The regional burden of non-FTLD co-pathologies, namely amyloid-β and α-synuclein, was assessed in this cohort of FTLD brain donors. Although the burden of non-FTLD co-pathologies was low throughout all examined brain regions, it showed significant correlations with neuropsychiatric symptoms in subcortical brain regions. Our analysis revealed a positive association between the presence of delusions and amyloid-β burden in the subiculum. Increased subthalamic amyloid-β burden was associated with the core clinical criterion of disinhibition, which was also associated with the presence of α-synuclein pathology in the SN. Similar neuroanatomical associations of neuropsychiatric symptoms have been previously found in patients with Alzheimer's disease and Parkinson's disease, where amyloid-β and α-synuclein are the main pathologies.73–77 Here, we show for the first time that concomitant non-FTLD pathology is not only a common finding in FTLD, but could also contribute in shaping the early clinical presentation of both psychiatric symptoms and core behavioural features.
Cortical TDP-43 pathology burden is linked to neuropsychiatric symptoms
The novelty of our results mainly relies on the findings that neuropsychiatric symptoms of frontotemporal dementia are linked to pathology burden in subcortical regions and to the presence of non-FTLD co-pathologies.78 Beside these new insights, our data also showed significant positive correlations between neuropsychiatric symptoms and FTLD pathology burden in cortical brain regions, which are in line with previous literature on frontotemporal dementia and PPD and contribute to validate our methods.60,61,79–84 Interestingly, all positive cortical associations between neuropsychiatric symptoms and pathology concerned TDP-43. Moreover, even when looking at the whole brain, the highest correlations (R ≥ 0.3) found in this study concerned TDP-43 burden: depression and TDP-43 in the prefrontal region and in CA3, apathy and TDP-43 in the parietal lobe, mania and TDP-43 in the cingulum and in CA1, and hallucinations and TDP-43 in the GL of the DG; the only strong correlation with tau pathology burden was found in the LC with perseverative/compulsive behaviour. No significant positive correlations were found between neuropsychiatric symptoms and the burden of FUS pathology, which could be due to the low numbers of FUS patients included in our cohort. Replication of our results in a different cohort is needed to clarify whether the predominance of strong correlations with TDP-43 burden reflects the predominance of TDP-43 brain donors in our population or points to a greater contribution of TDP-43 in neuropsychiatric symptoms of frontotemporal dementia.
Limitations and strengths
Our approach allowed to identify brain regions which are involved in specific symptoms and to detect subtle microscopic changes beyond atrophy patterns, despite the heterogeneous pathological substrate of frontotemporal dementia. To account for the progression of disease over time, the burden of pathology was assessed and compared to early onset symptoms, and the variables ‘disease duration’ and ‘age at onset’ were computed in the partial correlation analysis. Although on one hand it is possible that the partial correlation analysis with a local FDR threshold at 0.01 retained too little significant observations (Supplementary Figs 2 and 3), on the other hand it is very unlikely that our results represent false positive findings.
Apart from the positive correlations discussed above, we also found negative correlations, such as those between the presence of hyperorality and Alzheimer’s disease-related pathology in multiple brain regions. This can be explained by our previous findings, where the presence of hyperorality was highly specific for FTLD compared to Alzheimer's disease.7 These negative correlations are therefore also a result of our study design, where we included FTLD groups that present with distinct clinical symptoms. Some of the results of our study highlighted the presence of seemingly opposite clinico-pathological relationships, such as seen with depression in CA3 (positive correlation with TDP-43 and negative correlation with tau) or disinhibition in the substantia nigra (positive correlation with alpha synuclein and negative correlation with tau). These opposite correlations could suggest that not only the clinical manifestations of FTD are related to specific brain regions but, at least for some neuropsychiatric symptoms, the clinical correlates of pathology burden could be specific to a single protein. The absence of significant correlations between regional cumulative pathology burden and neuropsychiatric symptoms could add some additional evidence in support to this hypothesis which, however, should be addressed in future studies with a different design. The present study required the assumption that one class of pathological protein shares a similar impact on a brain region as the other pathological proteins, while this relationship could be more complicated than assumed in this work.
A limitation of the present study is that we only assessed the brain regions included in the standard dissection protocol, and therefore we were not able to study all regions. For example the hypothalamus, which has been linked to hyperorality in neuroimaging and post-mortem frontotemporal dementia cohorts, is not part of the dissection protocol.71,72 Furthermore, for this exploratory analysis, we did not always separately assess functionally distinct subregions within each brain region, as we did in the hippocampus. Future investigations are needed to assess correlations between neuropsychiatric symptoms and pathology burden in highly specialized sub-regions, such as the thalamic nuclei. Moreover, according to standard procedure, only the right half of each brain was assessed, which does not take into account the hemispheric asymmetry of pathology and neurodegeneration, a common feature in FTLD.4,5 As we analysed end-stage donors and did not include any highly lateralized functions, we expect the effects of asymmetry to be minimal in our study. Another possible limitation of this study is related to the characterization of (co-)pathology, as we did not make any distinction between age-related, Alzheimer's disease-related and FTLD-related tau neuropathological changes. This allowed us to interpret our findings in light of the overall role of tau protein, without any presumptive disease-specific distinction. In support of our findings, the clinico-pathological correlations that we have found in FTLD brain donors for α-synuclein and amyloid-β pathology are in line with the literature on Parkinson's disease and Alzheimer's disease, respectively. For example, we have found that the presence of disinhibition is related to the presence of α-synuclein co-pathology in the substantia nigra of FTLD brain donors. In previous studies on Parkinson's disease, were α-synuclein is the main pathology, impulse control disorders have been linked to nigral degeneration.85–89 Moreover, we observed a negative correlation between frontal amyloid pathology burden and the presence of hallucinations in our cohort. In previous studies on Alzheimer's disease, brain donors with hallucinations showed α-synuclein and/or TDP-43 co-pathology on autopsy, suggesting that amyloid is not directly correlated with hallucinations.7,90–92 Finally, we found significant correlations for multiple brain regions with the same symptom, and for multiple symptoms with the same brain regions. This points to the fact that brain circuits, rather than single brain areas, are involved in the genesis of symptoms of frontotemporal dementia. For example, the hippocampus and the prefrontal cortex, which were linked in this study with multiple neuropsychiatric symptoms, are deeply interconnected.73–75
To the best of our knowledge, this is the first study where early neuropsychiatric symptoms of frontotemporal dementia are correlated with the regional burden of multiple pathologies in a large cohort of brain donors. The extensive and detailed assessment of clinical and pathological data is a major strength of this work. Here, we found that early neuropsychiatric symptoms of frontotemporal dementia are linked to pathology burden in subcortical regions, and that the presence of hallucinations points to a higher burden of TDP-43 pathology in the granular layer of the hippocampal DG. Moreover, we showed for the first time that non-FTLD co-pathologies are not simple bystanders, but contribute to shape early neuropsychiatric symptoms of frontotemporal dementia.
Supplementary Material
Contributor Information
Marta Scarioni, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam University Medical Centers, Amsterdam, The Netherlands.
Priya Gami-Patel, Department of Pathology, Amsterdam University Medical Centers, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Carel F W Peeters, Division of Mathematical and Statistical Methods—Biometris, Wageningen University and Research, Wageningen, The Netherlands; Department of Epidemiology and Biostatistics, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, The Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Florianne de Koning, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam University Medical Centers, Amsterdam, The Netherlands; Department of Pathology, Amsterdam University Medical Centers, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Harro Seelaar, Department of Neurology, Erasmus University Medical Center Rotterdam, Rotterdam, The Netherlands.
Merel O Mol, Department of Neurology, Erasmus University Medical Center Rotterdam, Rotterdam, The Netherlands.
John C van Swieten, Department of Neurology, Erasmus University Medical Center Rotterdam, Rotterdam, The Netherlands.
Netherlands Brain Bank, Netherlands Institute for Neuroscience, 1105 BA Amsterdam, The Netherlands.
Annemieke J M Rozemuller, Department of Pathology, Amsterdam University Medical Centers, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Jeroen J M Hoozemans, Department of Pathology, Amsterdam University Medical Centers, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Yolande A L Pijnenburg, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam University Medical Centers, Amsterdam, The Netherlands.
Anke A Dijkstra, Department of Pathology, Amsterdam University Medical Centers, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Funding
The European Academy of Neurology supported this work with a research fellowship granted to M.S. This study was supported by a grant from ZonMW (Memorabel - 733050507).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Galvin JE, Howard DH, Denny SS, Dickinson S, Tatton N. The social and economic burden of frontotemporal degeneration. Neurology. 2017;89:2049–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liljegren M, Landqvist Waldo M, Frizell Santillo A, et al. Association of neuropathologically confirmed frontotemporal dementia and alzheimer disease with criminal and socially inappropriate behavior in a swedish cohort. JAMA Netw Open. 2019;2:e190261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murley AG, Coyle-Gilchrist I, Rouse MA, et al. Redefining the multidimensional clinical phenotypes of frontotemporal lobar degeneration syndromes. Brain. 2020;143:1555–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ducharme S, Dols A, Laforce R, et al. Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain. 2020;143:1632–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scarioni M, Gami-Patel P, Timar Y, et al. Frontotemporal dementia: correlations between psychiatric symptoms and pathology. Ann Neurol. 2020;87:950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ducharme S, Pearl-Dowler L, Gossink F, et al. The frontotemporal dementia versus primary psychiatric disorder (FTD versus PPD) checklist: a bedside clinical tool to identify behavioral variant FTD in patients with late-onset behavioral changes. J Alzheimers Dis. 2019;67:113–124. [DOI] [PubMed] [Google Scholar]
- 9. Gossink FT, Vijverberg EG, Krudop W, et al. Psychosis in behavioral variant frontotemporal dementia. Neuropsychiatr Dis Treat. 2017;13:1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Devenney EM, Landin-Romero R, Irish M, et al. The neural correlates and clinical characteristics of psychosis in the frontotemporal dementia continuum and the C9orf72 expansion. Neuroimage Clin. 2017;13:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mackenzie IR, Neumann M. Molecular neuropathology of frontotemporal dementia: insights into disease mechanisms from postmortem studies. J Neurochem. 2016;138:54–70. [DOI] [PubMed] [Google Scholar]
- 12. Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141:2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tan RH, Yang Y, Halliday GM. Multiple neuronal pathologies are common in young patients with pathologically proven frontotemporal lobar degeneration. Neuropathol Appl Neurobiol. 2018;44:522–532. [DOI] [PubMed] [Google Scholar]
- 14. Sellami L, Bocchetta M, Masellis M, et al. Distinct neuroanatomical correlates of neuropsychiatric symptoms in the three main forms of genetic frontotemporal dementia in the GENFI cohort. J Alzheimers Dis. 2018;65:147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agosta F, Galantucci S, Magnani G, et al. MRI signatures of the frontotemporal lobar degeneration continuum. Hum Brain Mapp. 2015;36:2602–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Short RA, Broderick DF, Patton A, Arvanitakis Z, Graff-Radford NR. Different patterns of magnetic resonance imaging atrophy for frontotemporal lobar degeneration syndromes. Arch Neurol. 2005;62:1106–1110. [DOI] [PubMed] [Google Scholar]
- 17. Staffaroni AM, Ljubenkov PA, Kornak J, et al. Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain. 2019;142:443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Day GS, Farb NA, Tang-Wai DF, et al. Salience network resting-state activity: prediction of frontotemporal dementia progression. JAMA Neurol. 2013;70:1249–1253. [DOI] [PubMed] [Google Scholar]
- 19. Ranasinghe KG, Rankin KP, Pressman PS, et al. Distinct subtypes of behavioral variant frontotemporal dementia based on patterns of network degeneration. JAMA Neurol. 2016;73:1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cerami C, Dodich A, Greco L, et al. The role of single-subject brain metabolic patterns in the early differential diagnosis of primary progressive aphasias and in prediction of progression to dementia. J Alzheimers Dis. 2016;55:183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Netherlands Brain Bank. https://www.brainbank.nl/. Accessed August 2021.
- 22. Klioueva NM, Rademaker MC, Dexter DT, et al. BrainNet Europe’s code of conduct for brain banking. J Neural Transm. 2015;122:937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 24. American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association Publishing; 2013. [Google Scholar]
- 25. Alafuzoff I. Chapter 10—Minimal neuropathologic diagnosis for brain banking in the normal middle-aged and aged brain and in neurodegenerative disorders. In: Huitinga I, Webster MJ, eds. Handbook of clinical neurology. Elsevier; 2018:131–141. [DOI] [PubMed] [Google Scholar]
- 26. Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 27. Attems J, Jellinger KA, Lintner F. Alzheimer’s disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol. 2005;110:222–231. [DOI] [PubMed] [Google Scholar]
- 28. Alafuzoff I, Arzberger T, Al-Sarraj S, et al. Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol. 2008;18:484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fatima M, Tan R, Halliday GM, Kril JJ. Spread of pathology in amyotrophic lateral sclerosis: assessment of phosphorylated TDP-43 along axonal pathways. Acta Neuropathol Commun. 2015;3:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mackenzie IR, Frick P, Grässer FA, et al. Quantitative analysis and clinico-pathological correlations of different dipeptide repeat protein pathologies in C9ORF72 mutation carriers. Acta Neuropathol. 2015;130:845–861. [DOI] [PubMed] [Google Scholar]
- 31. Roverato A, Castelo R. The networked partial correlation and its application to the analysis of genetic interactions. J R Stat Soc Series C. 2017;66:647–665. [Google Scholar]
- 32. Peeters CFW, van de Wiel MA, van Wieringen WN. The spectral condition number plot for regularization parameter evaluation. Comput Stat. 2020;35:629–646. [Google Scholar]
- 33. van Wieringen WN, Peeters CFW. Ridge estimation of inverse covariance matrices from high-dimensional data. Comput Stat Data Anal. 2016;103:284–303. [Google Scholar]
- 34. Efron B. Large-scale inference: empirical bayes methods for estimation, testing, and prediction. Institute of Mathematical Statistics Monographs. Cambridge University Press; 2010. [Google Scholar]
- 35. Jensen EC. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken). 2013;296:378–381. [DOI] [PubMed] [Google Scholar]
- 36. Urwin H, Josephs KA, Rohrer JD, et al. FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration. Acta Neuropathol. 2010;120:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mol MO, van Rooij JGJ, Wong TH, et al. Underlying genetic variation in familial frontotemporal dementia: sequencing of 198 patients. Neurobiol Aging. 2021;97:148.e9–148.e16. [DOI] [PubMed] [Google Scholar]
- 38. Moore KM, Nicholas J, Grossman M, et al. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: an international retrospective cohort study. Lancet Neurol. 2020;19:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Devenney E, Hornberger M, Irish M, et al. Frontotemporal dementia associated with the C9ORF72 mutation: a unique clinical profile. JAMA Neurol. 2014;71:331–339. [DOI] [PubMed] [Google Scholar]
- 40. Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. [DOI] [PubMed] [Google Scholar]
- 41. Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF, Stanley Neuropathology Consortium . Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9(6):609–620, 544. [DOI] [PubMed] [Google Scholar]
- 42. Silbersweig DA, Stern E, Frith C, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. [DOI] [PubMed] [Google Scholar]
- 43. McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 44. Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. [DOI] [PubMed] [Google Scholar]
- 45. Velakoulis D. Abnormal hippocampal distribution of TDP-43 in patients with-late onset psychosis. Aust N Z J Psychiatry. 2009;43:739–745. [DOI] [PubMed] [Google Scholar]
- 46. Velakoulis D, Walterfang M, Mocellin R, Pantelis C, McLean C. Frontotemporal dementia presenting as schizophrenia-like psychosis in young people: clinicopathological series and review of cases. Br J Psychiatry. 2009;194:298–305. [DOI] [PubMed] [Google Scholar]
- 47. Berrios GE. Tactile hallucinations: conceptual and historical aspects. J Neurol Neurosurg Psychiatry. 1982;45:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gallagher DA, Parkkinen L, O’Sullivan SS, et al. Testing an aetiological model of visual hallucinations in Parkinson’s disease. Brain. 2011;134:3299–3309. [DOI] [PubMed] [Google Scholar]
- 49. Diederich NJ, Goetz CG, Stebbins GT. Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: focused review and a new integrative model. Mov Disord. 2005;20:130–140. [DOI] [PubMed] [Google Scholar]
- 50. Barnes J. Reality monitoring and visual hallucinations in Parkinson’s disease. Neuropsychologia. 2003;41:565–574. [DOI] [PubMed] [Google Scholar]
- 51. Zeidman P, Maguire EA. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 2016;17:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Behrendt RP. Conscious experience and episodic memory: hippocampus at the crossroads. Front Psychol. 2013;4:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Behrendt RP. Hippocampus and consciousness. Rev Neurosci. 2013;24:239–266. [DOI] [PubMed] [Google Scholar]
- 54. Lebreton M, Bertoux M, Boutet C, et al. A critical role for the hippocampus in the valuation of imagined outcomes. PLoS Biol. 2013;11:e1001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wilson N-A, Ramanan S, Roquet D, et al. Scene construction impairments in frontotemporal dementia: evidence for a primary hippocampal contribution. Neuropsychologia. 2020;137:107327. [DOI] [PubMed] [Google Scholar]
- 56. Irish M, Hodges JR, Piguet O. Episodic future thinking is impaired in the behavioural variant of frontotemporal dementia. Cortex. 2013;49:2377–2388. [DOI] [PubMed] [Google Scholar]
- 57. Amad A, Cachia A, Gorwood P, et al. The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations. Mol Psychiatry. 2014;19:184–191. [DOI] [PubMed] [Google Scholar]
- 58. Hare SM, Law AS, Ford JM, et al. Disrupted network cross talk, hippocampal dysfunction and hallucinations in schizophrenia. Schizophr Res. 2018;199:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu L, Cui LB, Xi YB, et al. Association between connectivity of hippocampal sub-regions and auditory verbal hallucinations in schizophrenia. Front Neurosci. 2019;13:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zamboni G, Huey ED, Krueger F. Apathy and disinhibition in frontotemporal dementia: Insights into their neural correlates. Neurology. 2008;71:736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sheelakumari R, Bineesh C, Varghese T, Kesavadas C, Verghese J, Mathuranath PS. Neuroanatomical correlates of apathy and disinhibition in behavioural variant frontotemporal dementia. Brain Imaging Behav. 2020;14:2004–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zenon A, Duclos Y, Carron R, et al. The human subthalamic nucleus encodes the subjective value of reward and the cost of effort during decision-making. Brain. 2016;139:1830–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Massimo L, Powers C, Moore P, et al. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2009;27:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peters F, Perani D, Herholz K, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;21:373–379. [DOI] [PubMed] [Google Scholar]
- 66. Emmi A, Antonini A, Macchi V, Porzionato A, De Caro R. Anatomy and connectivity of the subthalamic nucleus in humans and non-human primates. Front Neuroanat. 2020;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wilson RS, Nag S, Boyle PA, et al. Brainstem aminergic nuclei and late-life depressive symptoms. JAMA Psychiatry. 2013;70:1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lustberg D, Iannitelli AF, Tillage RP, Pruitt M, Liles LC, Weinshenker D. Central norepinephrine transmission is required for stress-induced repetitive behavior in two rodent models of obsessive-compulsive disorder. Psychopharmacology (Berl). 2020;237:1973–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Murley AG, Rowe JB. Neurotransmitter deficits from frontotemporal lobar degeneration. Brain. 2018;141:1263–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Janssens J, Vermeiren Y, van Faassen M, van der Ley C, Kema IP, De Deyn PP. Monoaminergic and kynurenergic characterization of frontotemporal dementia and amyotrophic lateral sclerosis in cerebrospinal fluid and serum. Neurochem Res. 2020;45:1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Patterson L, Rushton SP, Attems J, Thomas AJ, Morris CM. Degeneration of dopaminergic circuitry influences depressive symptoms in Lewy body disorders. Brain Pathol. 2019;29:544–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–1322. [DOI] [PubMed] [Google Scholar]
- 73. Weintraub D, Koester J, Potenza MN, Siderowf AD. Impulse control disorders in Parkinson disease. Arch Neurol. 2010;67:589–595. [DOI] [PubMed] [Google Scholar]
- 74. Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. [DOI] [PubMed] [Google Scholar]
- 75. Trzepacz PT, Yu P, Bhamidipati PK, et al. Frontolimbic atrophy is associated with agitation and aggression in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2013;9:S95–S104.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Serra L, Perri R, Cercignani M, et al. Are the behavioral symptoms of Alzheimer’s disease directly associated with neurodegeneration? J Alzheimers Dis. 2010;21:627–639. [DOI] [PubMed] [Google Scholar]
- 77. Zubenko GS, Moossy J, Martinez AJ, et al. Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol. 1991;48:619–624. [DOI] [PubMed] [Google Scholar]
- 78. Bocchetta M, Malpetti M, Todd EG, Rowe JB, Rohrer JD. Looking beneath the surface: the importance of subcortical structures in frontotemporal dementia. Brain Commun. 2021;3:fcab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yi DS, Bertoux M, Mioshi E, Hodges JR, Hornberger M. Fronto-striatal atrophy correlates of neuropsychiatric dysfunction in frontotemporal dementia (FTD) and Alzheimer’s disease (AD). Dement Neuropsychol. 2013;7:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shin YW, Yoo SY, Lee JK, et al. Cortical thinning in obsessive compulsive disorder. Hum Brain Mapp. 2007;28:1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moretti R, Caberlotto R, Signori R. Apathy in corticobasal degeneration: possible parietal involvement. Funct Neurol. 2017;32:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Eslinger PJ, Moore P, Antani S, Anderson C, Grossman M. Apathy in frontotemporal dementia: behavioral and neuroimaging correlates. Behav Neurol. 2012;25:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Morbelli S, Ferrara M, Fiz F, et al. Mapping brain morphological and functional conversion patterns in predementia late-onset bvFTD. Eur J Nucl Med Mol Imaging. 2016;43:1337–1347. [DOI] [PubMed] [Google Scholar]
- 85. Antonelli F, Ray N, Strafella AP. Impulsivity and Parkinson’s disease: more than just disinhibition. J Neurol Sci. 2011;310:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Antonelli F, Strafella AP. Behavioral disorders in Parkinson’s disease: the role of dopamine. Parkinsonism Relat Disord. 2014;20:S10–S12. [DOI] [PubMed] [Google Scholar]
- 87. Buckholtz JW, Treadway MT, Cowan RL, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Steeves TD, Miyasaki J, Zurowski M, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132:1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ray NJ, Miyasaki JM, Zurowski M, et al. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson’s patients with medication-induced pathological gambling: a [11C] FLB-457 and PET study. Neurobiol Dis. 2012;48:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tsuang D, Larson EB, Bolen E, et al. Visual hallucinations in dementia: a prospective community-based study with autopsy. Am J Geriatr Psychiatry. 2009;17:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Naasan G, Shdo SM, Rodriguez EM, et al. Psychosis in neurodegenerative disease: differential patterns of hallucination and delusion symptoms. Brain. 2021;144:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jacobson SA, Morshed T, Dugger BN, et al. Plaques and tangles as well as Lewy-type alpha synucleinopathy are associated with formed visual hallucinations. Parkinsonism Relat Disord. 2014;20:1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and R script are available upon request from the corresponding author.