Abstract
We present an update of EpiFactors, a manually curated database providing information about epigenetic regulators, their complexes, targets, and products which is openly accessible at http://epifactors.autosome.org. An updated version of the EpiFactors contains information on 902 proteins, including 101 histones and protamines, and, as a main update, a newly curated collection of 124 lncRNAs involved in epigenetic regulation. The amount of publications concerning the role of lncRNA in epigenetics is rapidly growing. Yet, the resource that compiles, integrates, organizes, and presents curated information on lncRNAs in epigenetics is missing. EpiFactors fills this gap and provides data on epigenetic regulators in an accessible and user-friendly form. For 820 of the genes in EpiFactors, we include expression estimates across multiple cell types assessed by CAGE-Seq in the FANTOM5 project. In addition, the updated EpiFactors contains information on 73 protein complexes involved in epigenetic regulation. Our resource is practical for a wide range of users, including biologists, bioinformaticians and molecular/systems biologists.
Graphical Abstract
Graphical Abstract.
EpiFactors: a comprehensive database resource for epigenetic regulators.
INTRODUCTION
Epigenetics is a rapidly growing area of molecular biology covering changes in chromatin often leading to alteration in gene expression without any change in the DNA sequence. An epigenetic factor could be described as a molecule that initiates, modifies, and acts upon epigenetic modifications. Epigenetic regulatory pathways generally affect DNA accessibility or recruitment of other regulatory molecules by modification of DNA or histones (1).
The ultimate result of such regulation may be both fine-tuning and major changes in expression gene profiles (2–5). Various fundamental cell processes such as proliferation, differentiation, cell death, and response to conditional changes in metabolic demand are controlled by epigenetic mechanisms (6–8). Moreover, dysregulation of epigenetic factors is often associated with neurological, cardiovascular and metabolic diseases, various cancers, and other human disorders (6,9–12). Thus, the information about epigenetic regulators and their complexes is extremely relevant for understanding fundamental biological processes and human disorders.
EpiFactors database has been originally developed to provide a compilation of functional information about human and mouse epigenetic regulators, their complexes, and expression in multiple cell types to facilitate the work of researchers in the field of epigenetics (13). The information included in EpiFactors served different user needs and had multiple applications. EpiFactors has been used in such rapidly growing areas as organoid formation, pan-cancer driver mutation screening, and Sars-CoV-2 infection research (14–16). Recently, EpiFactors served as a reference epigenetic protein atlas to profile the genetic determinants of chromatin accessibility with single-cell CRISPR screening (17).
Guided by an original definition of epigenetic factors, we avoided an enormous expansion down the regulatory networks with no clear boundaries between epigenetic and non-epigenetic regulation. The majority of borderline cases were not included at that time since their role in epigenetic regulation was not understood. However, recently an increasing evidence supports new molecules and mechanisms of epigenetic regulation. Thus, it is necessary to further curate and update Epifactors in order to maintain its relevance.
Long noncoding RNA (lncRNA) are finally acknowledged as key players in epigenetics, making it critical to systematize information about their role in epigenetic regulation. LncRNAs represent transcripts longer than 200 nucleotides that do not encode functional proteins. This broad definition covers a large and highly heterogeneous group of RNAs with different biogenesis and functions (18,19). According to different estimates, there are 16 000–100 000 lncRNAs in human cells. The ability to bind various proteins, DNA and RNA molecules enables lncRNAs to affect replication, transcription, translation, DNA repair, and chromatin regulation (20). During the development of the first version of Epifactors database, they were considered borderline cases since at that time the role of RNAs was not clear for the majority of the cases. Despite the fact that this field is still largely unexplored and information about the functions of numerous lncRNAs remains unknown, recent studies have shown that a growing number of lncRNAs affect gene expression through epigenetic regulatory pathways (20–22).
There are several mechanisms for lncRNAs to affect gene expression in epigenetic manner. Synthesis of lncRNAs can affect transcription on the same or the opposite strand causing the displacement of Pol II from it or changing the choice of exons to be included in the transcript or acting as promoter switch (23). They can directly bind DNA generating hybrid DNA-RNA structures (both double and triple helices) or nascent RNA which could be recognized by transcription factors and histone modifiers and therefore influence chromatin accessibility and mediate gene silencing or activation (24–26). LncRNA can also act as a scaffold for protein complexes. It can serve as a factor driving chromatin looping between enhancer and promoter regions (27). By binding proteins, lncRNAs can either sequester them or modulate their activity, for example, regulating the ability of proteins to bind chromatin (28,29). Being bound to an epigenetic protein or a protein complex, lncRNA can also function as a guide and attract its partner to specific genomic locations (30). Taking into consideration a vast number of reported lncRNAs in the genome and a huge variability of their function, carefully curated and summarized data on lncRNAs may help researches to uncover their role in transcription regulation and in reconstruction of regulatory networks.
Since information about lncRNAs is extremely relevant for researchers in the field of regulatory genomics, various databases compile lncRNA data. NONCODE and LNCipedia databases (31,32) provide information about the majority of annotated lncRNAs regardless of their functions. They annotate lncRNA transcripts with general information about their sequence, structure, expression, conservation, and disease relevance with expression profile. In addition, LNCipedia accompanies entries with information about their secondary structure information, protein-coding potential and microRNA binding sites (32). LncRNAdb (33) was a database containing comprehensive annotations of eukaryotic lncRNAs including functional evidence. However, it is currently unavailable. Databases DIANA-LncBase (34) and LnCeCell (35) compile information on lncRNAs acting as miRNA sponges. DIANA-LncBase represents a source of experimentally supported miRNA targets on non-coding transcripts, while LnCeCell illustrates cell-specific lncRNA-associated ceRNA networks. dbEssLnc (36) is a database focused only on lncRNAs which are important in establishing minimal genomes of living cells or involved in cancerogenesis. It considers lncRNAs with non-epigenetic mechanisms of action as well as lncRNAs acting as epigenetic regulators. HiMoRNA (37) database is focused on lncRNAs presumably involved in regulation of histone modifications. It provides a comprehensive collection of lncRNA–genomic loci for which lncRNA expression is significantly correlated with the histone modification signal across multiple cell types and tissues but it does not incorporate information on lncRNA known functions in epigenetic regulations.
Thus, despite the availability of resources on both epigenetic regulation and lncRNAs, neither of them provides a compilation of functional information about the epigenetic functions of lncRNAs. Here we present an update of the EpiFactors database with a new section for long noncoding RNAs as a new epigenetic regulator class. The section contains 124 entries annotated with information on their function, mechanism of action, targets, and general data with relevant references and PubMed IDs supporting this information. We also updated the database sections for protein and protein complexes with 91 entries in addition to the previously added entries.
EPIFACTORS CONTENT UPDATE
The main feature in the EpiFactors update is the addition of lncRNAs as a new type of regulators. The respective novel section is populated with 124 new entries. Previously existing sections of the database were updated with current information about the existing entries and 91 new regulators: 81 proteins, six histones and four protein complexes.
Updating the definition of the epigenetic factors
The original version of EpiFactors introduced the definition of epigenetic factors allowing to decide which proteins should be included. This definition covered the core proteins involved in epigenetic regulation such as histones, histone variants, protamines, histone chaperones, histone modifiers, readers of histone modifications, chromatin remodelers, DNA and RNA modifiers, readers of DNA and RNA modifications, and protein cofactors forming complexes with epigenetic factors if they are important for the activity of the complexes. To include a new type of regulators in the database, this definition has been extended with lncRNAs that regulate gene expression at the transcriptional level or regulate the expression of other epigenetic factors at any level.
Data sources
To find new protein epigenetic regulators we manually screened the UniProt database with ‘human’ as species and keywords ‘methylation’, ‘chromatin’, ‘histone’, and ‘protamine’ as they were used previously for the same purpose. We selected only reviewed instances published in 2015 and later. To include RNA modifiers, we used ‘RNA modification’ and ‘RNA methylation’ keywords. To update protein complexes, we used the Complex Portal database with ‘Homo sapiens’ as species and the ‘chromatin’ keyword. The database search was performed in May 2022.
As a starting point to select lncRNAs involved in epigenetic regulation, recent reviews have been used (20–22) and complemented by lncRNAs collected from literature over the years in the lab. Further, results were manually checked to extract records that fit the definition of an epigenetic factor. Thus, only molecules with a proven role in epigenetic regulation were actually included in the database.
Change of annotation
To annotate entries of the new lncRNAs section the following fields were used: ‘HGNC approved symbol’, ‘HGNC ID’, ‘HGNC approved name’, ‘Entrez gene ID’, ‘Alternative names’, ‘HGNC gene family tag’, ‘HGNC gene family description’, ‘Function’, ‘PMID for information on the function’, ‘Target molecule’, ‘Target entity’, ‘PMID for information on target’, ‘Comment’.
Field ‘function’ contains possible ways of epigenetic regulation mediated by lncRNAs. As mechanisms of lncRNAs action are very diverse, we decided to characterize them with the following categories to define groups of lncRNAs:
Chromatin remodeler/Histone modifier/DNA modifier/TF/Splicing factor recruitment
Protein sequestration
Modulation of protein functions
Chromatin looping
Promoter switching
Transcription machinery interference
miRNA sponging
RNA binding
Protein binding
A category ‘Protein binding’ was used to characterize lncRNAs which are shown to bind their target proteins but the exact mechanism by which these lncRNA–protein complexes implement their function is unknown.
EPIFACTORS STRUCTURE AND CONTENT MODIFICATION
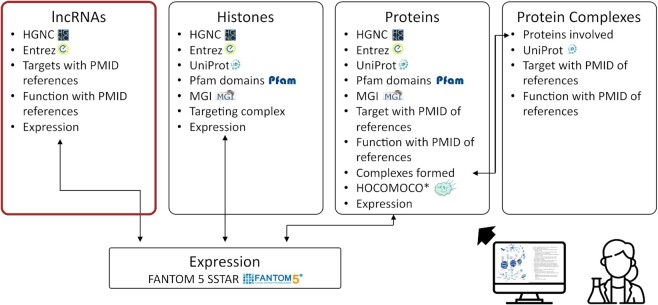
The Epifactors database was modified by adding a new section ‘lncRNAs’ for a new class of epigenetic regulators. The simplified/detailed scheme of updated database tables is shown in Figure 1 and in Supplementary Figure S1 respectively.
Figure 1.
Simplified structure of the Epifactors DB.
Content summary statistics
In total, six histone variants, 118 complexes, 124 lncRNAs, and 81 proteins were found during the search for updated information on epigenetic factors with UniProt, Complex Portal, and literature sources. After manual filtering, only 81 proteins, 124 lncRNAs, six histone variants, and four protein complexes were included in the database. The added 124 lncRNA were annotated based on their function (Supplementary Table S1) and the function of their targets (Supplementary Table S2).
Also, six proteins in the databases were updated with new information about complexes they are involved in, and UniProt references for the links supporting complex formation have been reported.
Proteins added to the database since 2015 are presented in table S3 in several functional groups. Chromatin remodeling proteins MIS18A, MIS18BP1, and OIP5, members of a complex Mis18 (also included in the database), regulate epigenetic states of centromeric chromatin. The complex interacts with DNMT3A and DNMT3B recruiting them to the centromeric region. As Mis18 provides access of CENPA-specific chaperone HJURP to centromeres, it defines centromeric localisation of CENPA and maintains normal chromosome segregation during mitosis (38).
RNA modificators METTL3, METTL14, WTAP, ZC3H13, CBLL1 and VIRMA are members of a methyltransferase complex WMM. It establishes degradation mark m6A in RNA molecules affecting their stability. Thus, WMM is implicated in regulation of cell differentiation of embryonic and hematopoietic stem cells by destabilization of transcripts (39). WMM also mediates random X-inactivation by methylation of Xist-RNA (40).
A large group of proteins involved in alternative splicing was added to the database as well. For example, ACINU, EIF4A3, KHDRBS1, MAGOH, PQBP1, RBM11, RBM8a, RNPS1, RBM5 and ZBTB7A are shown to regulate alternative splicing of apoptotic regulators (41–45). CELF family proteins and RBM24 control muscle differentiation (46,47). SRRM regulates alternative splicing events in genes with important neuronal functions such as the RE1-silencing transcription factor (48). DDX5 and DDX17 are coregulators of master transcriptional regulators of differentiation and control several layers of gene expression (47).
Besides WMM and Mis18 complexes mentioned above, complexes containing proteins reported in the first release of EpiFactors were also added. Particularly, there is MSL histone acetyltransferase complex is composed of MSL1, MSL2, MSL3, KAT8 proteins. The complex is responsible for genome-wide H4K16 acetylation and regulates transcription and DNA damage repair processes (49). Proteins DDB2, DDB1, RBX1, CUL4B and CUL4A form CUL4-DDB-ROC1 histone ubiquitination complex. It is involved in DNA repair. CUL4-DDB-ROC1 recognizes UV-induced cyclobutane pyrimidine dimers in chromatin, ubiquitinates histones and other chromatin-associated proteins located around the DNA lesion. Ubiquitination marks promote the removal of ubiquitinated histones from the nucleosome and initiate DNA repair (50). All new complexes are represented in table S4.
USE CASE
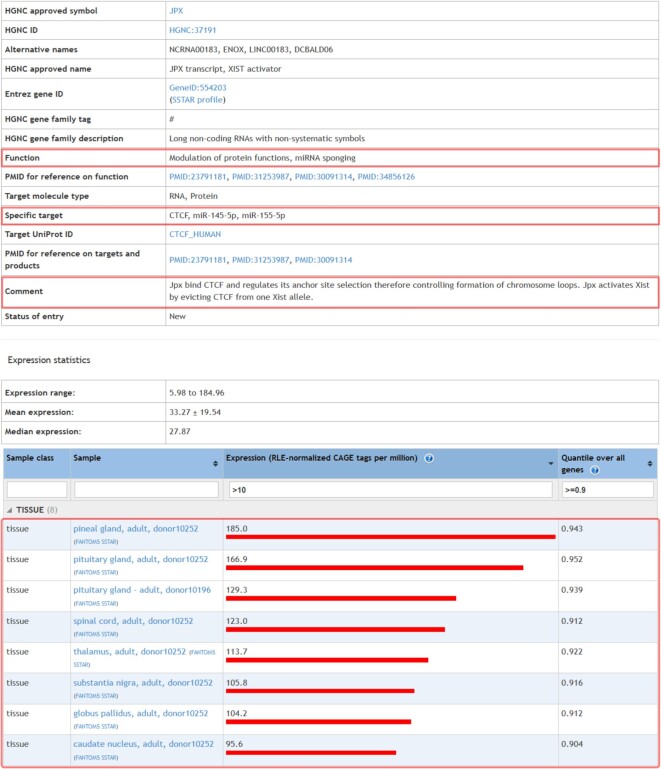
EpiFactors can be used in multiple ways. For example, lncRNA JPX has been known for a long time as an activator of XIST in a dosage compensation mechanism (51). Only recently the mechanism of JPX functioning has been discovered: JPX binds CTCF and extricates CTCF from one of the XIST alleles, in this way activating it (52,53). Additionally, JPX interacts with miRNAs miR-145-5p and miR-155-5p acting as a molecular sponge (54,55). All this information with appropriate links is summarized on a JPX page available by direct search by lncRNA name as well as by screening the lncRNAs page (see Figure 2). By filtering only cell types and tissues with the highest expression of JPX (expression, RLE > 10, quantile over all genes > 0.9) a user gets an idea that JPX is highly overexpressed in pineal and pituitary glands, thalamus, globus pallidus, substantial nigra and other parts of the subcortical structures of the brain. Thus, EpiFactors provides an essential summary information on the lncRNA JPX and facilitates further research.
Figure 2.
Summary for an lncRNA Jpx in the EpiFactors. Information mentioned in the text is highlighted.
IMPLEMENTATION, WEB INTERFACE AND VISUALIZATION
EpiFactors is available online via a user-friendly web interface implemented as a Ruby-on-Rails front-end with an SQLite back-end. We updated the front page of the database, so now the additional section can be accessed through ‘lncRNA’ link as well as through ‘Genes’, ‘Complexes’, ‘Histones and protamines’ and ‘Expression’, either directly or by using keyword search. Each data table contains a customizable set of columns presenting information on respective entities. A user can also browse individual histones, protamines, epigenetic modifiers, their complexes and lncRNAs.
DOWNLOADS
All tables from the current version of the EpiFactors can be downloaded in a csv (comma-separated values) format. The downloaded file contains all rows and columns that are currently visible, as well as corresponding external links to facilitate downstream analysis. The previous version of the EpiFactors can also be downloaded as a .csv file.
EPIFACTORS SUMMARY
EpiFactors is a web-accessible database that provides carefully curated information about human and mouse proteins and complexes involved in epigenetic regulation (see Figure 1). The current version is expanded by the addition of lncRNAs involved in epigenetic regulation. We believe that the database will be a valuable resource for researchers working in the rapidly growing field of epigenetics.
FUTURE DEVELOPMENTS
We keep collecting the information related to the content of the EpiFactors. Repeated literature searches are planned to allow for the identification and integration of new entries into the database on a regular basis. Cross-references to HiMoRNA or other databases related to lncRNAs will be added shortly. We will also consider the inclusion of data for other model organisms, to broaden the scope of the database to a larger audience. Any input from groups and individuals with specific areas of epigenetic expertise is welcome.
DATA AVAILABILITY
Code of the databases is openly available at https://github.com/autosome-ru/epifactors_webapp/tree/master.
Supplementary Material
Contributor Information
Daria Marakulina, Department of Biological and Medical Physics, Moscow Institute of Physics and Technology, 141701, Dolgoprudny, Moscow Region, Russia.
Ilya E Vorontsov, Vavilov Institute of General Genetics, Russian Academy of Sciences, 119991 Moscow, Russia.
Ivan V Kulakovskiy, Vavilov Institute of General Genetics, Russian Academy of Sciences, 119991 Moscow, Russia; Institute of Protein Research, Russian Academy of Sciences, Pushchino 142290, Russia.
Andreas Lennartsson, Department of Biosciences and Nutrition, NEO, Karolinska Institutet, 14157, Huddinge, Sweden.
Finn Drabløs, Department of Clinical and Molecular Medicine, NTNU - Norwegian University of Science and Technology, PO Box 8905, NO-7491 Trondheim, Norway.
Yulia A Medvedeva, Department of Biological and Medical Physics, Moscow Institute of Physics and Technology, 141701, Dolgoprudny, Moscow Region, Russia; Institute of Bioengineering, Research Center of Biotechnology, Russian Academy of Science, 117312 Moscow, Russia; The National Medical Research Center for Endocrinology, 117036 Moscow, Russia.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
This project was supported by the Ministry of Science and Higher Education of the Russian Federation (Grant Number: 075-15-2020-784); A.L. is funded by the Swedish Barncancerfonden, Cancerfonden, Research council and Karolinska Institutet. Funding for open access charge: Ministry of Science and Higher Education of the Russian Federation (Grant Number: 075-15-2020-784).
Conflict of interest statement. None declared.
REFERENCES
- 1. Cavalli G., Heard E.. Advances in epigenetics link genetics to the environment and disease. Nature. 2019; 571:489–499. [DOI] [PubMed] [Google Scholar]
- 2. Zhang X., Gao Y., Zhang X., Zhang X., Xiang Y., Fu Q., Wang B., Xu Z.. FGD5-AS1 is a Hub lncRNA ceRNA in hearts with tetralogy of fallot which regulates congenital heart disease genes transcriptionally and epigenetically. Front. Cell Dev. Biol. 2021; 9:630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhan A., Mandal S.S.. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim. Biophys. Acta. 2015; 1856:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cerase A., Pintacuda G., Tattermusch A., Avner P.. Xist localization and function: new insights from multiple levels. Genome Biol. 2015; 16:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vu vcićeviç D., Corradin O., Ntini E., Scacheri P.C., Ørom U.A.. Long ncRNA expression associates with tissue-specific enhancers. Cell Cycle. 2015; 14:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maass P.G., Luft F.C., Bähring S.. Long non-coding RNA in health and disease. J. Mol. Med. 2014; 92:337–346. [DOI] [PubMed] [Google Scholar]
- 7. Koo G.-B., Morgan M.J., Lee D.-G., Kim W.-J., Yoon J.-H., Koo J.S., Kim S.I., Kim S.J., Son M.K., Hong S.S.et al.. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 2015; 25:707–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lapierre L.R., Kumsta C., Sandri M., Ballabio A., Hansen M.. Transcriptional and epigenetic regulation of autophagy in aging. Autophagy. 2015; 11:867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Surace A.E.A., Hedrich C.M.. The role of epigenetics in autoimmune/inflammatory disease. Front. Immunol. 2019; 10:1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsushima S., Sadoshima J.. The role of sirtuins in cardiac disease. Am. J. Physiol.-Heart Circ. Physiol. 2015; 309:H1375–H1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ling C., Rönn T.. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 2019; 29:1028–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dawson M.A., Kouzarides T.. Cancer epigenetics: from mechanism to therapy. Cell. 2012; 150:12–27. [DOI] [PubMed] [Google Scholar]
- 13. The FANTOM Consortium Medvedeva Y.A., Lennartsson A., Ehsani R., Kulakovskiy I.V., Vorontsov I.E., Panahandeh P., Khimulya G., Kasukawa T., Drabløs F.. EpiFactors: A comprehensive database of human epigenetic factors and complexes. Database. 2015; 2015:bav067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aloia L., McKie M.A., Vernaz G., Cordero-Espinoza L., Aleksieva N., van den Ameele J., Antonica F., Font-Cunill B., Raven A., Aiese Cigliano R.et al.. Epigenetic remodelling licences adult cholangiocytes for organoid formation and liver regeneration. Nat. Cell Biol. 2019; 21:1321–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Youn A., Kim K.I., Rabadan R., Tycko B., Shen Y., Wang S.. A pan-cancer analysis of driver gene mutations, DNA methylation and gene expressions reveals that chromatin remodeling is a major mechanism inducing global changes in cancer epigenomes. BMC Med. Genomics. 2018; 11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salgado-Albarrán M., Navarro-Delgado E.I., Del Moral-Morales A., Alcaraz N., Baumbach J., González-Barrios R., Soto-Reyes E.. Comparative transcriptome analysis reveals key epigenetic targets in SARS-CoV-2 infection. NPJ Syst. Biol. Appl.L. 2021; 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liscovitch-Brauer N., Montalbano A., Deng J., Méndez-Mancilla A., Wessels H.-H., Moss N.G., Kung C.-Y., Sookdeo A., Guo X., Geller E.et al.. Profiling the genetic determinants of chromatin accessibility with scalable single-cell CRISPR screens. Nat. Biotechnol. 2021; 39:1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kung J.T.Y., Colognori D., Lee J.T.. Long noncoding RNAs: Past, present, and future. Genetics. 2013; 193:651–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu H., Yang L., Chen L.-L.. The diversity of long noncoding RNAs and their generation. ATrends Genet. 2017; 33:540–542. [DOI] [PubMed] [Google Scholar]
- 20. Statello L., Guo C.-J., Chen L.-L., Huarte M.. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021; 22:96–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neve B., Jonckheere N., Vincent A., Van Seuningen I.. Long non-coding RNAsc: the tentacles of chromatin remodeler complexes. Cell. Mol. Life Sci. CMLS. 2021; 78:1139–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pisignano G., Ladomery M.. Epigenetic regulation of alternative splicing: How LncRNAs Tailor the message. Non-Coding RNA. 2021; 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Canzio D., Nwakeze C.L., Horta A., Rajkumar S.M., Coffey E.L., Duffy E.E., Duffié R., Monahan K., O’Keeffe S., Simon M.D.et al.. Antisense lncRNA transcription mediates DNA demethylation to drive stochastic protocadherin promoter choice. Cell. 2019; 177:639–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pandey R.R., Mondal T., Mohammad F., Enroth S., Redrup L., Komorowski J., Nagano T., Mancini-Dinardo D., Kanduri C.. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell. 2008; 32:232–246. [DOI] [PubMed] [Google Scholar]
- 25. Blank-Giwojna A., Postepska-Igielska A., Grummt I.. LncRNA KHPS1 activates a poised enhancer by triplex-dependent recruitment of epigenomic regulators. Cell Rep. 2019; 26:904–2915. [DOI] [PubMed] [Google Scholar]
- 26. Antonov I., Medvedeva Y.. Direct interactions with nascent transcripts is potentially a common targeting mechanism of long non-coding RNAs. Genes. 2020; 11:E1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Villamizar O., Chambers C.B., Riberdy J.M., Persons D.A., Wilber A.. Long noncoding RNA Saf and splicing factor 45 increase soluble Fas and resistance to apoptosis. Oncotarget. 2016; 7:13810–13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puvvula P.K., Desetty R.D., Pineau P., Marchio A., Moon A., Dejean A., Bischof O.. Long noncoding RNA PANDA and scaffold-attachment-factor SAFA control senescence entry and exit. Nat. Commun. 2014; 5:5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marchese F.P., Grossi E., Marín-Béjar O., Bharti S.K., Raimondi I., González J., Martínez-Herrera D.J., Athie A., Amadoz A., Brosh R.M.et al.. A long noncoding RNA regulates sister chromatid cohesion. Mol. Cell. 2016; 63:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu F.-Y., Zhang S.-R., Wang L.-H., Wu W.-D., Zhao H.. LINC00511 promotes the progression of non-small cell lung cancer through downregulating LATS2 and KLF2 by binding to EZH2 and LSD1. Eur. Rev. Med. Pharmacol. Sci. 2019; 23:8377–8390. [DOI] [PubMed] [Google Scholar]
- 31. Fang S., Zhang L., Guo J., Niu Y., Wu Y., Li H., Zhao L., Li X., Teng X., Sun X.et al.. NONCODEV5: a comprehensive annotation database for long non-coding RNAs. Nucleic Acids Res. 2018; 46:D308–D314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Volders P.-J., Anckaert J., Verheggen K., Nuytens J., Martens L., Mestdagh P., Vandesompele J.. LNCipedia 5: towards a reference set of human long non-coding RNAs. Nucleic Acids Res. 2019; 47:D135–D139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quek X.C., Thomson D.W., Maag J.L.V., Bartonicek N., Signal B., Clark M.B., Gloss B.S., Dinger M.E.. lncRNAdb v2.0: Expanding the reference database for functional long noncoding RNAs. Nucleic Acids Res. 2015; 43:D168–D173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karagkouni D., Paraskevopoulou M.D., Tastsoglou S., Skoufos G., Karavangeli A., Pierros V., Zacharopoulou E., Hatzigeorgiou A.G.. DIANA-LncBase v3: indexing experimentally supported miRNA targets on non-coding transcripts. Nucleic Acids Res. 2020; 48:D101–D110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang P., Guo Q., Hao Y., Liu Q., Gao Y., Zhi H., Li X., Shang S., Guo S., Zhang Y.et al.. LnCeCell: a comprehensive database of predicted lncRNA-associated ceRNA networks at single-cell resolution. Nucleic Acids Res. 2021; 49:D125–D133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y.-Y., Zhang W.-Y., Xin X.-H., Du P.-F.. dbEssLnc: A manually curated database of human and mouse essential lncRNA genes. Comput. Struct. Biotechnol. J. 2022; 20:2657–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mazurov E., Sizykh A., Medvedeva Y.A.. HiMoRNA: A Comprehensive Database of Human lncRNAs Involved in Genome-Wide Epigenetic Regulation. Non-Coding RNA. 2022; 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim I.S., Lee M., Park K.C., Jeon Y., Park J.H., Hwang E.J., Jeon T.I., Ko S., Lee H., Baek S.H.et al.. Roles of Mis18 in epigenetic regulation of centromeric chromatin and CENP-A loading. Mol. Cell. 2012; 46:260–273. [DOI] [PubMed] [Google Scholar]
- 39. Yu T., Qi X., Zhang L., Ning W., Gao D., Xu T., Ma Y., Knott J.G., Sathanawongs A., Cao Z.et al.. Dynamic reprogramming and function of RNA N6-methyladenosine modification during porcine early embryonic development. Zygote. 2021; 29:417–426. [DOI] [PubMed] [Google Scholar]
- 40. Coker H., Wei G., Moindrot B., Mohammed S., Nesterova T., Brockdorff N.. The role of the Xist 5′ m6A region and RBM15 in X chromosome inactivation. Wellcome Open Res. 2020; 5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fushimi K., Ray P., Kar A., Wang L., Sutherland L.C., Wu J.Y.. Up-regulation of the proapoptotic caspase 2 splicing isoform by a candidate tumor suppressor, RBM5. Proc. Natl. Acad. Sci. U.S.A. 2008; 105:15708–15713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Michelle L., Cloutier A., Toutant J., Shkreta L., Thibault P., Durand M., Garneau D., Gendron D., Lapointe E., Couture S.et al.. Proteins associated with the exon junction complex also control the alternative splicing of apoptotic regulators. Mol. Cell. Biol. 2012; 32:954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pedrotti S., Busà R., Compagnucci C., Sette C.. The RNA recognition motif protein RBM11 is a novel tissue-specific splicing regulator. Nucleic Acids Res. 2012; 40:1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bielli P., Busà R., Di Stasi S.M., Munoz M.J., Botti F., Kornblihtt A.R., Sette C.. The transcription factor FBI-1 inhibits SAM68-mediated BCL-X alternative splicing and apoptosis. EMBO Rep. 2014; 15:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Q., Moore M.J., Adelmant G., Marto J.A., Silver P.A.. PQBP1, a factor linked to intellectual disability, affects alternative splicing associated with neurite outgrowth. Genes Dev. 2013; 27:615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ladd A.N., Charlet-B. N., Cooper T.A.. The CELF Family of RNA Binding Proteins Is Implicated in Cell-Specific and Developmentally Regulated Alternative Splicing. Mol. Cell. Biol. 2001; 21:1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang T., Lin Y., Liu J., Zhang Z.G., Fu W., Guo L.Y., Pan L., Kong X., Zhang M.K., Lu Y.H.et al.. Rbm24 regulates alternative splicing switch in embryonic stem cell cardiac lineage differentiation. Stem Cells. 2016; 34:1776–1789. [DOI] [PubMed] [Google Scholar]
- 48. Nakano Y., Kelly M.C., Rehman A.U., Boger E.T., Morell R.J., Kelley M.W., Friedman T.B., Bánfi B.. Defects in the alternative splicing-dependent regulation of REST cause deafness. Cell. 2018; 174:536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith E.R., Cayrou C., Huang R., Lane W.S., Côté J., Lucchesi J.C.. A human protein complex homologous to the drosophila MSL complex is responsible for the majority of Histone H4 Acetylation at Lysine 16. Mol. Cell. Biol. 2005; 25:9175–9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scrima A., Fischer E.S., Lingaraju G.M., Böhm K., Cavadini S., Thomä N.H.. Detecting UV-lesions in the genome: The modular CRL4 ubiquitin ligase does it best!. FEBS Lett. 2011; 585:2818–2825. [DOI] [PubMed] [Google Scholar]
- 51. Richard J.L.C., Ogawa Y.. Understanding the complex circuitry of lncRNAs at the X-inactivation center and Its implications in disease conditions. Curr. Top. Microbiol. Immunol. 2016; 394:1–27. [DOI] [PubMed] [Google Scholar]
- 52. Sun S., Del Rosario B.C., Szanto A., Ogawa Y., Jeon Y., Lee J.T.. Jpx RNA activates Xist by evicting CTCF. Cell. 2013; 153:1537–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oh H.J., Aguilar R., Kesner B., Lee H.-G., Kriz A.J., Chu H.-P., Lee J.T.. Jpx RNA regulates CTCF anchor site selection and formation of chromosome loops. Cell. 2021; 184:6157–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jin M., Ren J., Luo M., You Z., Fang Y., Han Y., Li G., Liu H.. Long non-coding RNA JPX correlates with poor prognosis and tumor progression in non-small-cell lung cancer by interacting with miR-145-5p and CCND2. Carcinogenesis. 2020; 41:634–645. [DOI] [PubMed] [Google Scholar]
- 55. Lin X.Q., Huang Z.M., Chen X., Wu F., Wu W.. XIST induced by JPX suppresses hepatocellular carcinoma by sponging miR-155-5p. Yonsei Med. J. 2018; 59:816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Code of the databases is openly available at https://github.com/autosome-ru/epifactors_webapp/tree/master.