Abstract
Although Salmonella enterica serovar Typhimurium can undergo phase variation to alternately express two different types of flagellin subunit proteins, FljB or FliC, no biological function for this phenomenon has been described. In this investigation, we constructed phase-locked derivatives of S. enterica serovar Typhimurium that expressed only FljB (termed locked-ON) or FliC (termed locked-OFF). The role of phase variation in models of enteric and systemic pathogenesis was then evaluated. There were no differences between the wild-type parent strain and the two phase-locked derivatives in adherence and invasion of mouse epithelial cells in vitro, survival in mouse peritoneal macrophages, or in a bovine model of gastroenteritis. By contrast, the locked-OFF mutant was virulent in mice following oral or intravenous (i.v.) inoculation but the locked-ON mutant was attenuated. When these phase-locked mutants were compared in studies of i.v. kinetics in mice, similar numbers of the two strains were isolated from the blood and spleens of infected animals at 6 and 24 h. However, the locked-OFF mutant was recovered from the blood and spleens in significantly greater numbers than the locked-ON strain by day 2 of infection. By 5 days postinfection, a majority of the mice infected with the locked-OFF mutant had died compared with none of the mice infected with the locked-ON mutant. These results suggest that phase variation is not involved in the intestinal stage of infection but that once S. enterica serovar Typhimurium reaches the spleens of susceptible mice those organisms in the FliC phase can grow and/or survive better than those in the FljB phase. Additional experiments with wild-type S. enterica serovar Typhimurium, fully capable of switching flagellin type, supported this hypothesis. We conclude that organisms that have switched to the FliC+ phase have a selective advantage in the mouse model of typhoid fever but have no such advantage in invasion of epithelial cells or the induction of enteropathogenesis.
Salmonella spp. produce diseases that range from a mild enteritis to a severe systemic infection in a variety of animal hosts. Although Salmonella enterica serovar Typhimurium can undergo phase variation to alternately express two different major surface proteins, the flagellin subunit proteins FljB and FliC, no biological function has been ascribed to this capacity. Over 40 genes, arranged in 17 operons, are required for the structure, assembly, and function of flagella (34). We have demonstrated that flagellar regulation is linked to virulence of S. enterica serovar Typhimurium (6, 7, 45, 46, 57). A region adjacent to flagellar genes (flg), originally named mviS, was shown to be required for virulence in mice (6). Subsequently, that gene was identified as flgM (46). The flgM product is an anti-sigma factor that negatively regulates flagellar synthesis by inhibiting FliA, an alternate sigma factor required for the transcription of late-class flagellar genes (25). A mutation in flgM causes decreased survival of Salmonella in mouse peritoneal macrophages and attenuation of virulence in a mouse model of typhoid fever (46).
In S. enterica serovar Typhimurium, expression of flagella is also controlled by phase variation, a mechanism by which the organism alternately expresses two different types of flagellin subunit proteins, FljB and FliC. Flagellar phase variation was first described in Salmonella by Andrewes over 75 years ago (2). Since that time, many studies have focused on the molecular mechanism of switching of flagellin type, and a model has been generated (13, 14, 18, 22, 24, 26, 27, 29, 30, 39, 40, 47–49, 59, 60). Flagellar phase variation involves the inversion of approximately 1 kb of DNA containing the promoter of fljB (49, 59, 60). In one orientation, the promoter is situated directly upstream of the fljBfljA operon (39). In this position (referred to as ON), transcription of fljB and fljA can occur, and transcription of fliC, located elsewhere on the chromosome, is repressed by the fljA product (13, 14, 40, 49). In the opposite orientation (referred to as OFF), fljB and fljA are not transcribed and thus derepression of fliC occurs. The invertible DNA segment is flanked by 26-bp inverted repeats (hix sites) and contains the hin gene, a locus that encodes a recombinase required for switching (27, 47, 60).
The biological significance of flagellar phase variation in S. enterica serovar Typhimurium has not been determined. Although flagella are not necessary for the virulence of S. enterica serovar Typhimurium in the mouse model of typhoid fever (6, 33), inappropriate expression of flagella or a particular flagellin type may affect the pathogenic process. In addition, the role of flagella in other models of salmonellosis has not been determined. In this study, the importance of phase variation in the pathogenicity of S. enterica serovar Typhimurium was investigated by studying phase-locked derivatives incapable of switching flagellin types in tissue culture assays, mouse infection studies, and a bovine enteropathogenesis assay. Flagellar phase variation was also examined by determining the incidence of each flagellin type following oral inoculation of mice with a strain that was capable of switching between the two flagellin types.
MATERIALS AND METHODS
Bacterial strains.
Strains of S. enterica serovar Typhimurium used in this study are shown in Table 1. Escherichia coli DH5α was used in DNA cloning procedures except where noted.
TABLE 1.
S. enterica serovar Typhimurium strains used in this study
| Strain | Characteristics | Source and/or reference |
|---|---|---|
| SL3201 | Wild-type, mouse virulent; Fim− Mot+; phase variable (FljB+/FliC+) | B. A. D. Stocker, 23 |
| SL3201N | Resistant to 50 μg of nalidixic acid/ml | This study |
| SL3201 ON | hin::kan (phase-locked ON); FljB+ | This study |
| SL3201 OFF | hin::kan (phase-locked OFF); FliC+ | This study |
| SL3201 ON FljB− | hin::kan (phase-locked ON); fljB::aphA-3 | This study |
| SL3201 ON FliC− | hin::kan (phase-locked ON); fliC::aphA-3 | This study |
| SL3201 ON FljA− | hin::kan (phase-locked ON); fljA::Tn10 | This study |
| SL3201 ON FljA− FliC− | hin::kan (phase-locked ON); fljA::Tn10 fliC::aphA-3 | This study |
| SL3201 FliC− | fliC::aphA-3 | This study |
| SL3201 FljB− | fljB::aphA-3 | This study |
| KK2506 | fljA::Tn10 | K. Kutsukake |
| JR501 | galE, restriction-deficient modification-proficient hsdSA, hsdSB, and hsdLT | 55 |
Media and growth conditions.
Strains were grown at 37°C in Luria-Bertani (LB) broth (Difco Laboratories, Detroit, Mich.) with shaking (225 rpm) or on LB agar. Antimicrobial agents (Sigma Chemical Co., St. Louis, Mo.) were used when needed at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; nalidixic acid, 50 μg/ml; tetracycline, 10 μg/ml. Motility agar consisted of 10 g of tryptone, 5 g of NaCl, and 0.35% (wt/vol) agar per liter (pH 7.4) with or without antiserum against FliC (1/500) or FljB (1/250) (salmonella H antiserum i or single factor 2, respectively; Difco Laboratories). Sucrose media used for selecting resolution of cointegrates consisted of 10 g of tryptone, 5 g of yeast extract, and 6% (wt/vol) sucrose per liter (pH 7.0) with or without 1.5% (wt/vol) agar.
DNA techniques.
Standard DNA cloning procedures were conducted as described by Sambrook et al. (44). Restriction enzymes BamHI, SacI, and SalI were purchased from New England Biolabs (Beverly, Mass.). Chromosomal DNA used in PCR was prepared from bacterial cells with Gene Releaser (BioVentures, Inc., Murfreesboro, Tenn.). DNA fragments and plasmids were isolated using QIAEX gel extraction and Qiagen plasmid kits, respectively (Qiagen, Inc., Valencia, Calif.). DNA was separated by agarose gel (1%; IBI Eastman Kodak Company, Scientific Imaging Systems, Rochester, N.Y.) electrophoresis in a Tris-borate-EDTA buffer and stained with ethidium bromide (1 μg/ml of gel). DNA standards were purchased from BioVentures, Inc. (BioMarker EXT) and New England Biolabs (1-kb DNA ladder).
Construction of phase-locked mutants and phase-locked mutant derivatives.
Phase variation involves the inversion of a DNA segment upstream of the fljBfljA operon (Fig. 1A). The invertible segment contains the hin gene, which encodes a recombinase required for the inversion process. Phase-locked mutants incapable of switching flagellin types were constructed by disruption of hin so as to lock the invertible DNA segment in the ON position (FljB+) or in the OFF position (FliC+). The hin gene (approximately 550 bp) was amplified by PCR from wild-type strain SL3201 (GeneAmp PCR kit and Perkin-Elmer DNA thermal cycler; Perkin-Elmer Cetus, Norwalk, Conn.). The following primers were used: CKS23 (5′-CGAGCTGGATCCATGGCTACTATTGGGTATATTC-3′) and CKS24 (5′-CGAGCTGTCGACACTGCTTGCCGGAAAGTATCTG-3′). BamHI and SalI restriction sites were included at the 5′ ends of CKS23 and CKS24, respectively. The amplified product was cloned directly into vector pCR2.1, as described by the manufacturer (TA cloning kit; Invitrogen, Carlsbad, Calif.). The presence of a SacI site in pCR2.1 necessitated the movement of the hin fragment into pBR328 (51) via BamHI and SalI sites. To insertionally inactivate the hin gene, a kanamycin resistance gene cassette (KSAC; Amersham Pharmacia Biotech, Piscataway, N.J.) was inserted into the internal SacI site of the gene. Disruption of the hin gene at the SacI site was confirmed by sequencing with CKS23 and CKS24 primers (ABI PRISM dye terminator cycle sequencing kit; Perkin-Elmer, Foster City, Calif.). The hin::kan fragment was cloned into the BamHI and SalI sites of suicide vector pAM450 (35) to yield plasmid pHin::kan. The pAM450 vector contains a temperature-sensitive origin of replication, a sacB gene, and an ampicillin resistance gene. To avoid restriction of the plasmid by Salmonella, the suicide vector construct was transformed into S. enterica serovar Typhimurium strain JR501 before reisolation and electroporation into strain SL3201 (Gene Pulser and E. coli Pulser cuvette; 0.2-cm electrode, gap, 50; Bio-Rad Laboratories, Hercules, Calif.). Motility agar that contained antiflagellin antibodies (see below) was used to select FljB- (hin/fljB promoter in the ON position) or FliC (hin/fljB promoter in the OFF position)-expressing SL3201(pHin::kan) cells. Cointegrates were selected by growing the host containing the vector construct at 42°C in LB broth with ampicillin followed by plating onto LB agar with ampicillin. Cointegrates that lost the integrated vector but retained the hin::kan sequence were selected by growing them at 37°C in broth that contained sucrose and then plating them onto a sucrose agar medium that contained kanamycin. Resolution of cointegrates was checked by loss of ampicillin resistance. The chromosomal hin mutations in the ON and OFF positions were introduced into SL3201 by transduction with bacteriophage P22HT int as described previously (9). PCR with primers to regions flanking the hin gene (Fig. 1A; HIXL, 5′-CGATTTATTGGTTCTTGAAA-3′, and BMID, 5′-CCGATACATCCAGTGTAGTA-3′) was used to monitor the formation and resolution of cointegrates, the transduction of the mutated hin gene into SL3201, and the orientation of the invertible DNA segment (see “Determination of flagellar phase”).
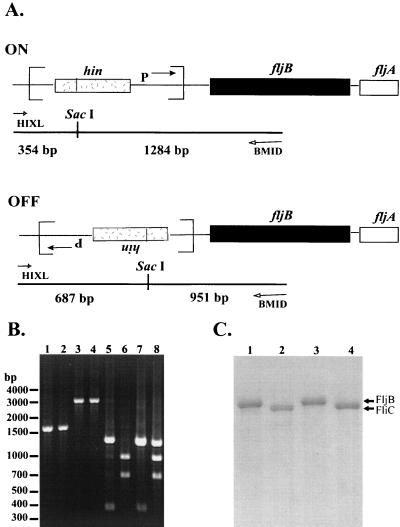
FIG. 1.
Analysis of the invertible hin region and the flagellin type expressed by the wild-type strain and phase-locked S. enterica serovar Typhimurium mutants. (A) Diagrams of the fljBfljA operon with the invertible DNA segment (enclosed by brackets) containing the hin gene and promoter (P→) in the ON and OFF orientations. The orientation of the invertible segment was determined by PCR with primers flanking the region (HIXL and BMID) followed by digestion of the amplified product with SacI. The predicted sizes of the fragments generated for the ON and OFF positions are indicated below each diagram. (B) PCR amplification of the invertible hin region untreated (lanes 1 to 4) or digested with SacI (lanes 5 to 8) prior to separation by agarose gel (1%) electrophoresis. Lane 1, SL3201 (FljB+); lane 2, SL3201 (FliC+); lane 3, SL3201 ON; lane 4, SL3201 OFF; lane 5, SL3201 (FljB+); lane 6, SL3201 (FliC+); lane 7, SL3201 ON; lane 8, SL3201 OFF. Strain SL3201 expressing FliB or FliC was selected from motility agar that contained antiflagellin antibodies. Molecular size (in base pairs) is shown on the left. (C) Flagellin proteins were separated by SDS–12% PAGE and stained with Coomassie stain. Lanes 1 to 4 are as indicated for panel B.
SL3201 locked-ON derivatives were made as follows. SL3201 ON with a mutation in fljB or fliC was constructed by transducing a nonpolar fljB or fliC mutation into SL3201 ON. The nonpolar flagellin mutants were made by inserting an aphA-3 cassette (36) modified to contain a flagellin-like ribosomal binding site (i.e., AGGA-N7) into the fljB or fliC gene of SL3201. Motile nonpolar flagellin mutants were selected from motility agar and used to produce P22 phage lysates. SL3201 ON was transduced with the phage lysates and butirosin (50 μg/ml; Sigma), and resistant organisms were selected to obtain SL3201 ON fljB::aph or SL3201 ON fliC::aph. The hin::kan (ON position) and fljB::aph mutations are closely linked and were transduced together into SL3201. A derivative of SL3201 locked in the ON position with a mutation in fljA was produced by transducing an fljA::Tn10 mutation from strain KK2506 into SL3201 ON. SL3201 ON fljA::Tn10 was further transduced with the fliC::aph nonpolar mutation to obtain SL3201 ON fljA::Tn10 fliC::aph.
Analysis of flagellins.
Surface flagellin was prepared by mechanical shearing of bacteria with a homogenizer (Omni International, Inc., Warrenton, Va.). Bacteria from 4 ml of a broth culture of S. enterica serovar Typhimurium were collected by centrifugation, and the bacterial pellet was resuspended in 300 μl of phosphate-buffered saline (pH 7.4). The suspension was homogenized for 15 s at the half-maximum setting, cooled on ice for 15 s, and homogenized again for 15 s. The mixture was then centrifuged at 10,000 × g for 10 min in a microcentrifuge to pellet bacteria. The flagellin in the supernatant was analyzed by sodium dodecyl sulfate-polyacrylamide (12%) gel electrophoresis (SDS-PAGE; acrylamide/bisacrylamide ratio, 37.5:1; Bio-Rad Laboratories) and stained with Coomassie stain (Coomassie blue G250; Sigma Chemical Co.) (38). Prestained SDS-PAGE standards were used (broad range; Bio-Rad Laboratories).
Motility assay.
Motility agar was inoculated by stabbing it with an inoculating needle dipped into 100 μl of a broth culture grown overnight. The plate was incubated at 37°C, and the distance of migration away from the point of inoculation was measured after various times.
Determination of flagellar phase.
The flagellar phase of bacterial cells in a colony was determined by a modification of an overlay method described by Stocker (52). Colonies of S. enterica serovar Typhimurium growing on LB agar media were overlaid with motility agar (10 ml per 100- by 15-mm petri dish; Becton Dickinson) that contained anti-FliC (1/500) or anti-FljB (1/250) antibodies. The motility agar was left to harden for 20 min at room temperature, and then the plate was incubated at 37°C for 1 to 2 h. Bacterial cells that were FliC+ swam away from the colony in the presence of anti-FljB antibodies but not in the presence of anti-FliC antibodies. Conversely, bacterial cells that were FljB+ swam away from the colony in the presence of anti-FliC antibodies but not in the presence of anti-FljB antibodies.
A second method of flagellar phase determination involved the analysis of the orientation of the invertible hin region by PCR. Primers outside the invertible segment (HIXL and BMID) were used to amplify fragments from wild-type strain SL3201 (see Fig. 1A for a schematic) and the phase-locked mutants. The amplified products were desalted and concentrated (QIAEX gel extraction kit; Qiagen, Inc.) and digested with SacI, and the resulting fragments were separated by agarose gel (1%) electrophoresis. As predicted, DNA fragments amplified from the wild-type strain were approximately 1.6 kb whether the invertible segment was in the ON or OFF position (Fig. 1B, lanes 1 and 2). Those products amplified from the phase-locked-ON and -OFF mutants were larger, approximately 3 kb (Fig. 1B, lanes 3 and 4), sizes that were consistent with the insertion of the kanamycin resistance cassette into hin. Digestion of the amplified products with SacI resulted in fragments indicative of the orientation of the invertible segment. For the wild-type strain, fragments of approximately 354 and 1,284 bp were generated when the hin region was in the ON position (Fig. 1A and B, lane 5), whereas fragments of approximately 687 and 951 bp were produced when the invertible segment was in the OFF position (Fig. 1A and B, lane 6). For the phase-locked mutants, digestion of the amplified fragments with SacI produced approximately 1.2-kb fragments, a size which corresponded to the size of the kanamycin resistance cassette, and additional fragments whose sizes corresponded to those for either the ON phase or the OFF phase (Fig. 1B, lanes 7 and 8, respectively). These results confirmed that the phase-locked-ON mutant had an insertion in the hin gene when the invertible segment was in the ON position; conversely, the phase-locked-OFF mutant was disrupted in the hin gene while the invertible segment was in the OFF orientation.
Capacity to switch flagellin type.
The capacity to switch flagellar phase was determined qualitatively by examining whether Salmonella could swim in motility agar in the presence of antiflagellin antibodies (1/500 dilution of anti-FliC or 1/250 dilution of anti-FljB). The agar was inoculated with a stab from a single colony and incubated for 18 h at 37°C. Antibodies to the flagellin type expressed prevent organisms from swimming in the agar unless organisms can switch to the other flagellin type. Therefore, swimming correlates with the capacity to undergo phase variation. The capacity to switch was measured quantitatively by analyzing the flagellar phase of cells following growth of FljB- or FliC-expressing cells. A strain that expressed a particular flagellin type was selected from motility agar that contained the heterologous antiserum and was grown on LB agar. Single colonies were inoculated into LB broth (in 12- by 75-mm glass tubes) and incubated at 37°C with shaking to an absorbance at 600 nm (A600) between 0.2 and 0.3 (mid-log phase of growth). The cultures were diluted in sterile saline (0.9%) and plated in duplicate onto LB agar, and the flagellar phase of cells on each plate was determined by the overlay method. The capacity to switch was calculated as the number of switched colonies relative to the total number of colonies tested.
Proteomic analysis.
Overnight bacterial cultures were diluted 1:100 into fresh, prewarmed LB medium and grown with shaking to an A600 of 0.5 before harvesting. Cells were harvested at 37°C by centrifugation at 5,000 rpm for 15 min using a JLA-10.500 rotor and Beckman J2-21 centrifuge, washed once in ice-cold saline (0.9%), and collected by centrifugation at 4°C. For each strain, at least three independent batches of cells were prepared and analyzed. Samples were prepared by resuspending cell pellets in 5 ml of lysis buffer (9 M urea, 2% [vol/vol] Triton X-100, 2% [vol/vol] 2-mercaptoethanol, 2% [vol/vol] Ampholine 3.5-10, 8 mM phenylmethylsulfonyl fluoride), and cells were disrupted by sonication using an MSE Soniprep 150 with four 20-s cycles and 40 s of cooling on ice. Samples of cell lysates were then flash frozen by immersion in liquid nitrogen and stored at −70°C until use. Two-dimensional gel electrophoresis was done essentially as described previously (19, 41, 42). Immobilized pH gradient strips (pH 4.0 to 7.0) and 12 to 14% gradient gels (Amersham Pharmacia) were used for the first and second dimensions, respectively. Gels were digitized as described previously (1, 41), and images were analyzed using Phoretix-2D software, version 4.0 (Phoretix, Newcastle-Upon-Tyne, United Kingdom). Only protein spots that consistently had a greater-than-threefold difference in abundance relative to the parent strain were considered to be differentially expressed. Images were exported to Adobe Photoshop, version 5.0, for annotation and printing.
Assay for mouse virulence.
Mouse experiments were carried out according to the principles outlined in the Guide for the Care and Use of Laboratory Animals (37). Values of 50% lethal dose (LD50) were determined with groups of 4 to 10 mice (C57BL/6J, 6- to 8-week-old females; Jackson Laboratories, Bar Harbor, Maine; BALB/c, 6- to 8-week-old females; Charles River Laboratory, Wilmington, Mass.) inoculated orally, intraperitoneally (i.p.), or intravenously (i.v.) with different doses of Salmonella. For orally inoculated mice, food was taken away approximately 16 h before inoculation and mice were given 25 μl of 2% NaHCO3 just prior to feeding them the inoculum. BALB/c mice were inoculated i.v. in the tail vein. Mice were monitored for death for 14 days (i.v.) and for up to 32 days (i.p. and oral), and the LD50 values were calculated by the method of Reed and Muench (43). Virulence was also assessed by oral inoculation of C57BL/6J mice with a single dose of approximately 5 × 108 CFU per mouse and by monitoring for death.
Study of i.v. kinetics.
The phase-locked mutants were compared in a kinetic study that involved i.v. inoculation of BALB/c mice. Two groups of 40 mice were inoculated with each mutant at a dose of approximately 7.6 × 104 CFU. At selected times postinoculation (6h and 1, 2, 3, 4, and 5 days), 5 mice from each group were sacrificed, with up to 15 mice sacrificed at the last time point. Mice were anesthetized with Metofane (Mallinckrodt Veterinary, Inc., Mundelein, Ill.), and 100 μl of blood was recovered from the jugular vein followed by euthanasia of the mice by cervical dislocation. The spleen was removed and disrupted in sterile saline (0.9%) with a blender (Stomacher Lab-Blender 80; Tekmar Company, Cincinnati, Ohio). The numbers of CFU recovered from the blood and spleen were determined by plate counts.
Assay for adherence and invasion of epithelial cells.
Adherence and invasion assays were conducted as described previously (10, 54, 58) and included the step of centrifugation of the bacteria onto the epithelial cell monolayer.
Bovine-ligated-ileal-loop assay for enteropathogenesis.
All experiments with calves were conducted according to the requirements of the Animal Scientific Procedures Act, United Kingdom (1986). The assay has been described in detail elsewhere (56). Briefly, calves were terminally anesthetized with pentobarbital, and intestinal loops, 6 cm in length and spaced 1 to 2 cm apart, were constructed in the ileum using braided surgical silk. To prepare the inoculum, bacterial strains were grown overnight, with shaking, at 25°C. The cultures were diluted approximately 1:3 in fresh LB medium and incubated at 37°C, with shaking, for 90 min. The A600 was adjusted by addition of LB broth to give a concentration of approximately 3 × 108 CFU/ml. A total of 5 ml of this suspension was injected into each loop. The same volume of sterile LB broth was used as a negative control. All bacterial strains and controls were tested in three loops per animal. Polymorphonuclear cells (PMNs) were isolated from 50 ml of blood removed from the calf, labeled with 111indium, and reinjected into the jugular vein. Twelve hours after inoculation the anesthetized animal was humanely killed, and all loops were exteriorized. Fluid secretion was measured as a ratio of the volume of accumulated fluid to loop length. PMN influx was measured as a ratio of 111In activity in test loops to that in control loops.
Assay for survival in macrophages.
Survival in mouse peritoneal macrophages was measured as described previously (32, 46). Briefly, bacteria were grown overnight in LB broth. Bacteria were diluted to give a multiplicity of infection of approximately 5, opsonized with normal mouse serum, and allowed to infect resident mouse peritoneal macrophages; five wells per time point (T0, T4, and T24, where the subscript indicates hours) were infected with each strain. After 50 min, the wells were washed three times. For the T0 time point, bacteria from three wells for each strain were enumerated and the numbers of macrophages were determined with the remaining two wells. Medium that contained gentamicin was added to the remaining wells. The same procedure described for the T0 time point was repeated at 4 and 24 h postinfection. Controls included uninfected macrophages.
RESULTS
Characterization of phase-locked mutants (SL3201 ON and SL3201 OFF).
Based on the molecular model of phase variation (Fig. 1A), when the orientation of the invertible segment is in the ON phase, FljB is expressed, while FliC is expressed in the OFF phase. The two flagellin types are distinguishable by PAGE analysis because FljB is slightly larger than FliC (Fig. 1C, lanes 1 and 2). As predicted, analysis of flagellins produced by the phase-locked mutants demonstrated that FljB and FliC were the types synthesized by the locked-ON and -OFF mutants, respectively (Fig. 1C, lanes 3 and 4).
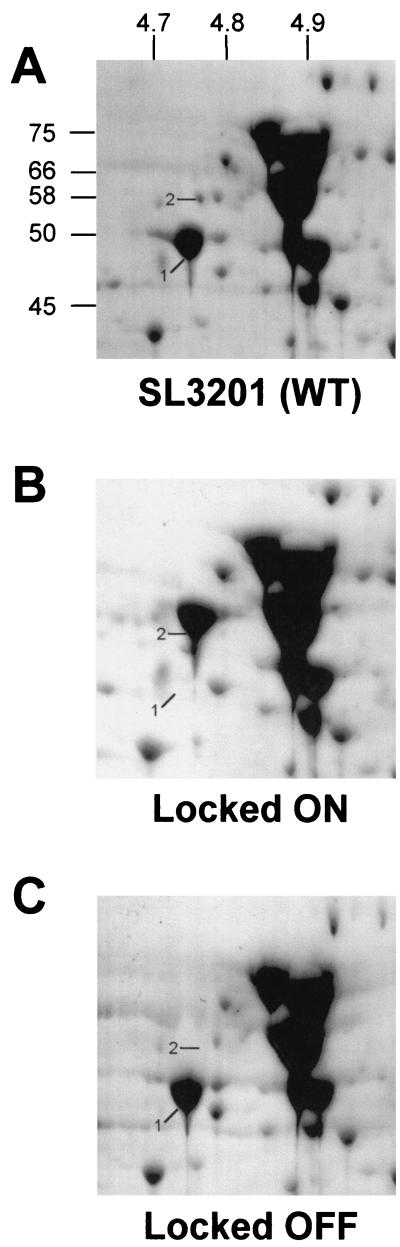
A proteomic analysis was carried out to confirm the absence of FliC and FljB in the locked-ON and -OFF mutants, respectively, and to examine the possibility that these strains might also have altered levels of other proteins. The protein expression profiles of whole-cell extracts from wild-type locked-ON and locked-OFF cells were analyzed using high-resolution two-dimensional gel electrophoresis and image analysis (Fig. 2). Previous studies localized flagellin proteins in S. enterica serovar Typhimurium SL1344 by peptide mass fingerprinting and N-terminal sequencing (1, 42). Proteins with relative molecular weights (Mrs) and isoelectric points (pIs) identical to those of the previously characterized flagellin proteins of SL1344 were identified in the parent strain SL3201 (Fig. 2A). In contrast, the locked-ON cells lacked the spot previously identified as FliC (Fig. 2B) while the locked-OFF ones lacked the FljB protein (Fig. 2C). Of the various preparations of lysates analyzed, some showed variations in other proteins; however, only differences in FliC and FljB were reproducibly found in all the samples analyzed. These results confirmed the flagellar genotypes of the mutants and additionally indicated that there were no gross alterations in the protein profiles of the locked-ON and -OFF mutants.
FIG. 2.
Two-dimensional gel analysis of wild-type (WT) strain SL3201 and its locked-ON and -OFF derivatives. The figure shows the acidic region of representative gels with relative molecular weights shown on the y axis and isoelectric points on the x axis. Spots 1 and 2, FliC and FljB, respectively.
The locked-ON and locked-OFF mutants were also examined for additional phenotypes. The motilities of the phase-locked mutants were similar to that of the wild-type strain that expresses either FliC or FljB: all strains tested (SL3201 ON, SL3201 OFF, SL3201 FljB+, and SL3201 FliC+) swam approximately 4.5 mm in motility agar after 6 h of incubation. No swimming was detected for the phase-locked mutants in the presence of antibodies to the flagellin type expressed even after 18 h of incubation. Only growth of the mutants at the inoculation site was observed. The mutants swam in the presence of antibodies to the heterologous flagellin type. Taken together, these results indicate that the phase-locked organisms in the original inoculum and those that grew following inoculation of motility agar did not undergo phase variation.
The capacity to switch to expression of the heterologous flagellin type was also measured quantitatively for the wild-type strain and the phase-locked mutants. For the wild-type strain, levels of switching from FljB to FliC and from FliC to FljB were 3.6 × 10−2 and 1.1 × 10−2 switched colonies per total colonies tested, respectively. For the phase-locked mutants, no switching was observed in over 2,300 colonies tested (<4.2 × 10−4 colonies switched per total colonies tested), an observation that confirmed that the phase-locked mutants failed to undergo phase variation. The growth rates of the phase-locked mutants in LB medium were also nearly identical to that of wild type (data not shown). Isolation and analysis of plasmid DNA from each of the strains confirmed the presence of the large virulence plasmid (data not shown).
Virulence of phase-locked mutants and derivatives.
To determine if the failure to undergo phase variation and/or expression of a particular flagellin type affected the virulence of S. enterica serovar Typhimurium, the LD50 values for the locked-ON and locked-OFF mutants were determined for C57BL/6J and BALB/c mice inoculated orally, i.p., and i.v. (Table 2). In both strains of mice, the phase-locked-ON mutant had LD50 values for oral inoculation of >107 CFU, unlike the wild-type strain and phase-locked-OFF mutant, which had LD50 values for oral inoculation between 3 × 104 and 1 × 106 CFU. LD50 values for i.v. inoculation revealed that the locked-ON mutant was also less virulent than the locked-OFF mutant and wild-type strain. In contrast, all Salmonella strains tested were virulent for both strains of mice when inoculated i.p. (LD50 value for i.p. inoculation, <10 CFU).
TABLE 2.
Oral, i.p., and i.v. LD50 values of phase-locked mutants in mice
| Strain | LD50 (CFU) for mouse strain:
|
||||
|---|---|---|---|---|---|
| C57BL/6J
|
BALB/c
|
||||
| Oral | i.p. | Oral | i.p. | i.v. | |
| SL3201 | 3 × 104 | <10 | 6 × 105 | <10 | ∼20 |
| SL3201 ON | >1 × 107 | <10 | >2 × 107 | <10 | 5 × 102 |
| SL3201 OFF | 3 × 104 | <10 | 1 × 106 | <10 | <10 |
The attenuated phenotype of the locked-ON mutant was investigated further using derivatives of the locked-ON mutant that contained additional mutations in fljB, fliC, fljA, or fljAfliC (Table 1). These derivatives were assessed for virulence by oral inoculation of C57BL/6J mice with a single dose of approximately 5 × 108 CFU per mouse (Table 3). As expected, SL3201 ON FljB− was nonmotile and produced no detectable flagellin. Unlike SL3201 ON, this derivative was virulent in mice inoculated orally, a result which suggests that expression of FljB interferes with pathogenicity of S. enterica serovar Typhimurium in this model. Derivative SL3201 ON FliC still produced FljB flagellin and was attenuated. When the derivative is locked in the ON phase, fljB is expressed and also fljA, located downstream from fljB in the same operon. The fljA product represses fliC transcription. SL3201 ON FljA− produced both flagellins in equal amounts and was attenuated in mice. An additional mutation in fliC (SL3201 ON FljA− FliC−) resulted in expression of only fljB and an attenuated phenotype. The growth rates of all the locked-ON derivatives in LB media were similar to that of the parental strain, SL3201 ON (data not shown). From these data, we conclude that expression of FljB alone or in combination with FliC attenuates SL3201.
TABLE 3.
Virulence of phase-locked-ON mutants following oral inoculation of C57BL/6J mice
| Strain | Flagellin(s)a | No. of dead mice/no. inoculatedb | Mean no. of days to death ± SD |
|---|---|---|---|
| SL3201 | FliC | 10/15 | 17 ± 6 |
| SL3201 ON | FljB | 4/15 | 17 ± 3 |
| SL3201 ON FljB− | NDc | 14/15 | 13 ± 3 |
| SL3201 ON FliC− | FljB | 3/15 | 12 ± 1 |
| SL3201 ON FljA− | FljB, FliC | 5/15 | 13 ± 3 |
| SL3201 ON FljA− FliC− | FljB | 4/15 | 12 ± 2 |
Flagellin type of organisms in the inoculum dose as assessed by PAGE.
Dose was approximately 5 × 108 CFU per mouse. Results are from three separate experiments.
ND, none detected.
Adherence to and invasion of mouse epithelial cells.
Following oral ingestion of Salmonella, the bacteria are proposed to reach the small intestine and adhere to and invade the intestinal epithelial cells (12). Many of the genes required for invasion of intestinal cells reside in a pathogenicity island (SPI-1) (31), and SPI-1 mutants are attenuated after oral but not i.p. inoculation (15, 16, 28). Because the phase-locked-ON, but not the phase-locked-OFF, mutant was also attenuated in the mouse after oral inoculation, we postulated that it might be deficient in its capacity to adhere to and invade murine gut intestinal cells. We compared the phase-locked mutants (SL3201 ON and SL3201 OFF) to wild-type S. enterica serovar Typhimurium (SL3201N) for adherence and invasion of the murine MODE-K intestinal epithelial cell line. Results were calculated as the mean percent adherence (number of bacteria after washes/number of bacteria in the initial inoculum ×100) or mean percent invasion (number of bacteria surviving gentamicin treatment/number of bacteria in initial inoculum × 100) from duplicate assays. No differences between SL3201N and the phase-locked mutants (SL3201 ON and SL3201 OFF) in adherence (19 to 31%) or invasion (8 to 12%) were observed. These in vitro results suggest that the phase-locked mutants are capable of binding to and penetrating the intestinal barrier in vivo.
Induction of enteropathogenic responses.
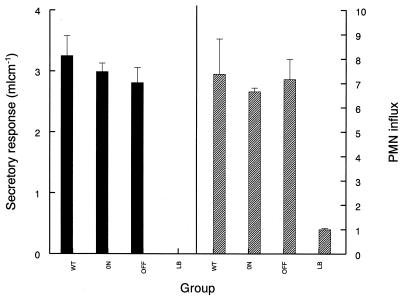
Salmonella infection can also cause enteritis in several animal hosts. Mice are not routinely used as a model for salmonella-induced enteritis, and so the contribution of flagellar phase variation to enteropathogenesis was assessed by using the well-established bovine-ileal-loop model (Fig. 3). There was no significant difference (P > 0.05) in the induction of fluid secretion or the influx of PMN leukocytes into the ileal mucosa and lumen between either phase-locked mutant (SL3201 ON or SL3201 OFF) and the wild-type strain.
FIG. 3.
Secretory response and PMN influx elicited by S. enterica serovar Typhimurium strains in bovine intestinal loops. The secretory response is shown as volume per length of loop. The PMN influx is a ratio of the PMN radioactivity within the infected loops to the PMN radioactivity within the control loops. Data are averages for three loops and the standard errors of the means. Each strain was tested at least three times; a representative experiment is shown. WT, wild type.
Study of i.v. kinetics.
Previous studies demonstrated that wild-type S. enterica serovar Typhimurium cells injected i.v. into mice are rapidly cleared from the circulation (>85% in 1 h) and are subsequently found in the liver and spleen (53, 57). Since the phase-locked-ON mutant was attenuated following i.v. inoculation, an i.v. kinetic study was done to compare levels of survival and growth of the mutants in the spleen and blood (Fig. 4). BALB/c mice were inoculated i.v. with the phase-locked-ON or the phase-locked-OFF strain at a dose of approximately 7.6 × 104 CFU. At 6 h postinoculation, the mean CFU per spleen were similar for the two strains: 4.5 × 103 for the phase-locked-ON strain and 7.8 × 103 CFU for the phase-locked-OFF strain (Fig. 4A). Both mutants continued to increase in numbers in the spleen, but at days 1 to 5 postinoculation, there were greater numbers of the locked-off strain than of the locked-ON strain (significant differences at days 2, 3, and 5; P = 0.05). By 5 days postinoculation, 9 of the remaining 15 mice given the phase-locked-OFF strain had died compared to none of the mice given the phase-locked-ON strain. In the blood, there were also greater numbers of the locked-OFF strain than of the locked-ON strain at days 1 to 5 postinoculation (Fig. 4B; significant differences at days 2, 4, and 5; P = 0.05). Concentrations of greater than 104 CFU/100 μl were isolated from the blood of the majority of the mice inoculated with the locked-OFF strain at days 4 and 5 of the study. Only 2 out of 20 mice given the locked-ON strain had high numbers of bacteria in the blood at those times. These findings indicate that, once S. enterica serovar Typhimurium reaches the spleens of susceptible mice, those organisms locked in the OFF phase (FliC+) grow and/or survive better than those locked in the ON phase (FljB+).
FIG. 4.
Kinetic study of phase-locked mutants recovered from the spleen and blood following i.v. inoculation of mice. BALB/c mice were inoculated i.v. with SL3201 ON (○) or SL3201 OFF (▵) mutants at a dose of approximately 7.6 × 104 CFU per mouse. At various times postinoculation (6 h and 1, 2, 3, 4, and 5 days) mice were sacrificed, and the CFU per spleen (A) and CFU per 100 μl of blood (B) were determined. Each symbol represents data collected from one mouse, and the geometric means are indicated by horizontal bars. ∗, values of 108 CFU/spleen and 107 CFU/100 μl of blood were assigned to mice that died.
Survival in mouse macrophages.
Fields et al. demonstrated the importance of survival in macrophages for the virulence of Salmonella in mice (11), and we have previously shown a correlation between degree of survival of S. enterica serovar Typhimurium in resident peritoneal macrophages and the rate of net growth of wild-type S. enterica serovar Typhimurium in spleens (32). Therefore, we compared the survival of the phase-locked mutants (SL3201 ON and SL3201 OFF) and that of wild-type Salmonella in resident peritoneal macrophages. Our conclusion from three separate macrophage assays (data not shown) is that there is no major difference between the survival of the phase-locked mutants and that of wild-type Salmonella in mouse peritoneal macrophages.
Flagellar phase of wild-type S. enterica serovar Typhimurium recovered from infected mice.
To determine if expression of a particular flagellin type by the wild-type strain of S. enterica serovar Typhimurium is important in vivo, bacteria recovered from the spleens and Peyer's patches of mice originally inoculated with Salmonella that expressed mainly FljB or FliC were checked for the flagellin type expressed. Strain SL3201N was used to permit detection of the Salmonella from infected mouse tissues by incorporation of nalidixic acid in the agar. Strain SL3201N was selected by growth of SL3201 on medium that contained 50 μg of nalidixic acid/ml followed by transduction of the nalidixic acid resistance mutation into SL3201. The motilities and the capacities to switch of SL3201N and the parent strain were similar: the switch rates of FljB to FliC and FliC to FljB were 3.4 × 10−2 and 1.6 × 10−2 colony switched/colony tested, respectively. The oral LD50 value for SL3201N, 105 CFU for C57BL/6J mice, was also similar to that for the parent strain.
Isolates of SL3201N that expressed FliC or FljB were selected from motility agar that contained antiserum and administered orally to C57BL/6J mice at doses of 6.7 × 107 and 4.1 × 107 CFU, respectively. Based on the overlay method of Stocker (52), Salmonella cells that expressed the flagellin type selected as described above comprised 99% of the bacterial composition of the inoculum (1% of the Salmonella cells expressed the other flagellin type). Between 12 and 21 days postinoculation, mice were sacrificed, and bacteria from the Peyer's patches and spleen were recovered on LB agar that contained nalidixic acid (Fig. 5). Four out of 14 mice inoculated with FliC-expressing Salmonella died during the course of the experiment, whereas all 14 mice inoculated with FljB-expressing bacteria survived the duration of the experiment (Fig. 5A). Approximately 87% or more of the Salmonella cells isolated from the Peyer's patches and spleens of all the C57BL/6J mice originally inoculated with FliC-expressing bacteria were in the FliC phase (Fig. 5A). By contrast, mice infected with the FljB-producing bacteria showed differences in distribution of FljB- versus FliC-expressing bacteria based on the two sites examined and the length of time postinfection. The majority (≥60% of the bacterial population) of the Salmonella cells recovered from the Peyer's patches from 13 out of 14 mice at 15 and 21 days postinoculation were in the FljB+ phase. One mouse that was moribund had mainly FliC-expressing bacteria isolated from the Peyer's patches and spleen (95 and 73% of the bacterial population, respectively; not shown on graph). From the spleen, three out of eight mice had mostly FliC-expressing bacteria (≥68% of the bacterial population) at 15 days postinoculation. By 21 days, five out of six mice were infected with mainly FliC-producing bacteria (≥62% of the bacterial population). Mainly FljB-producing organisms (56% of the bacterial population) were recovered from the remaining mouse. Similar patterns of FljB- and FliC-phase distributions were obtained following oral inoculation of BALB/c mice with FljB- or FliC-expressing Salmonella (Fig. 5B). These observations reveal an apparent selective advantage in the mouse model for switching to FliC by the wild-type strain of Salmonella.
FIG. 5.
Flagellin type expressed by S. enterica serovar Typhimurium from orally infected mice. Mice were inoculated with SL3201N expressing FliC or FljB at a dose of approximately 5 × 107 CFU per mouse. Groups of mice were sacrificed between 12 and 22 days postinfection, and the flagellin types expressed by Salmonella isolated from the spleen and Peyer's patches were determined. Bars, combined average percentages of colonies that expressed the original input flagellin type: inoculum (stippled), bacteria isolated from the Peyer's patches after infection (hatched), and bacteria isolated from the spleen after infection (solid). The ranges are shown above the bars. (A) Salmonella isolated from infected C57BL/6J mice through day 21 postinfection (n = 10 to 14 mice). (B) Salmonella isolated from infected BALB/c mice through day 16 postinfection (n = 22 mice).
DISCUSSION
The major finding of this study was that a mutation which locked the phase of S. enterica serovar Typhimurium flagellin expression in the ON (FljB+) position attenuated the strain in the murine model of typhoid fever but did not influence invasion of epithelial cells or the induction of enteropathogenic responses. This attenuation did not result solely from the failure of an isolate to undergo phase variation because an S. enterica serovar Typhimurium strain with a mutation that locked the phase in the OFF position was still virulent. Attenuation of the locked-ON mutant was observed when the bacteria were administered to mice by oral and i.v. routes but not i.p. The results of assays of i.v. kinetics are consistent with the hypothesis that a mutation that locks the flagellar phase into the ON position affects the growth and/or survival of S. enterica serovar Typhimurium in the spleen. This defect would account for attenuation by the oral and i.v. routes. Conversely, the i.p. route may place the bacteria in a host environment that allows the bacteria to grow rapidly, with a subsequent increase in dissemination of the organisms throughout the host. This could account for the virulence of the locked-ON mutant following i.p. inoculation and is consistent with the lack of a difference between the phase-locked mutants in survival in peritoneal macrophages.
The attenuated phenotype of the locked-ON mutant but not that of the locked-OFF mutant may be due to differences between FljB and FliC flagellin molecules. In support of the proposal that FljB is involved in the attenuation of a locked-ON mutant is the finding that the phase-locked-ON mutant with a fljB null mutation was virulent (Table 3). The predicted amino acid sequences of the two flagellins are very similar. The fljB and fliC genes encode 506- and 495-amino-acid sequences, respectively, and a comparison of the two protein sequences reveals 80% identical amino acids, with the N- and C-terminal ends highly conserved. However, the central region is more divergent, with only 51% identity in 220 amino acids. This region confers antigenic differences to the flagella, which form part of the basis for Salmonella serotyping. Other differences between the two flagellins have been reported. Flagella were responsible for the differential agglutination of cultures of Salmonella in the two phases as shown by acridine dyes (3). More recently, Salmonella flagellin was demonstrated to elicit a tumor necrosis factor alpha response from human promonocytic cells, and FliC elicited a greater cytokine response than FljB (8). Whether differences in host responses to each flagellin type influence host defense mechanisms remains to be tested.
Although we did not detect any major differences in proteins expressed by the phase-locked mutants except for flagellin, we cannot completely rule out the involvement of other proteins that might be expressed in amounts below the sensitivity of our assay. Indeed, we considered the possibility that the attenuated phenotype of the locked-ON mutant was due to decreased levels of FljA. The fljA gene is located in the same operon as fljB but is transcribed at a significantly reduced level compared to fljB (21). FljA normally represses fliC expression but may also be involved in repression of other genes required for virulence. However, a locked-ON mutant with additional mutations in fljA or fljA and fliC (Table 3) was still attenuated, observations which suggest that FljA is not involved in the attenuation of the locked-ON mutant.
Another explanation for the attenuated phenotype of the locked-ON mutant is that the locked-OFF strain, but not the locked-ON derivative, expresses a factor required for virulence. Three possible products are, or may be, synthesized in the OFF position. First, FliC flagellin is produced. Second, downstream of fliC is fliB, which encodes an enzyme that methylates lysine residues in flagellin molecules (5). Whether fliB is in the same operon as fliC has not been determined. Third, transcription from the fljB promoter, in an orientation opposite to that for the fljBfljA operon, can occur while in the OFF orientation (48), but no product has been identified. In all cases, however, the flagellin-specific sigma factor FliA is required for transcription at the fliC and fljB promoters. A fliA mutant is still virulent in the mouse, an observation which indicates that a factor required for virulence is not transcribed from the fljB promoter while in the OFF orientation (45, 46). In addition, the idea that FliC is specifically required for virulence in S. enterica serovar Typhimurium is not likely since flagellar mutants that lack FliC are still virulent (45).
The difference in virulence between the phase-locked mutants implies that the virulence of the wild-type strain is affected by phase variation and the expression of a particular flagellin type. Indeed, differences in virulence between a wild-type strain expressing FljB and one expressing FliC were observed. Although the phase-locked-ON (FljB+) mutant was attenuated, we hypothesize that the wild-type strain that initially expresses FljB remains potentially virulent because the organism has the capacity to switch to FliC and produce a systemic infection. In support of this proposal, we demonstrated an increase in the proportion of FliC-expressing Salmonella cells recovered from the spleens of mice originally inoculated with predominantly FljB-expressing bacteria. This increase was greater in mice sacrificed at 21 days than at 15 days postinoculation.
The change in population within mice orally infected with FljB+ wild-type S. enterica serovar Typhimurium to bacteria that express FliC could be due to several mechanisms. First, as reported by others, the frequency of switching in vitro is greater in the direction of FljB to FliC than from FliC to FljB (17, 52, 59). This bias in switching could create a population composed mainly of FliC-expressing bacteria after several generations of growth (52). Second, switching from the ON to the OFF phase may be induced by in vivo conditions. Third, bacteria that have switched to the OFF phase may grow and/or survive better in the host than bacteria in the ON phase. One argument against this possibility is that the phase-locked-ON mutant with an fljB null mutation was virulent. Fourth, the presence of FljB is virulence attenuating (for reasons that are not clear), and bacteria that make that protein are outcompeted by FliC-expressing bacteria. Several of these explanations may be operating to some extent in vivo, but the last explanation is most consistent with the i.v. kinetic results in which a greater number of phase-locked-OFF than phase-locked-ON bacteria were isolated from the spleens of infected mice and with the virulence of the phase-locked-ON mutant with an fljB null mutation.
In the studies with FljB+ bacteria capable of switching flagellin type, a majority of the salmonellae isolated from the spleen expressed FliC. In contrast, most of the bacteria isolated from the Peyer's patches of these mice expressed FljB, a result that suggests that the frequency of phase variation was not very high in that organ. This result is also consistent with our conclusion that phase variation is not involved in infection of the intestines.
The net growth rate in vivo is an important factor in the virulence of bacterial pathogens (50). There are several possible explanations for FliC-expressing Salmonella attaining higher numbers than FljB-expressing organisms in the spleen. Although the in vitro growth rates of the phase-locked mutants in a nutrient-rich medium such as LB broth were similar, differences within the spleen due to limitations of specific nutrients may have occurred. Although the in vitro survival capacities in resident peritoneal macrophages for the phase-locked mutants were similar, there may be differences in survival and/or growth in other intracellular sites such as splenic macrophages or PMNs. Indeed, Buchmeier and Heffron demonstrated that S. enterica serovar Typhimurium survived better in splenic macrophages than in peritoneal macrophages (4). Alternatively, the host responses to FljB may be greater than those to FliC, which could suppress the growth and/or survival of FljB-expressing Salmonella. This is consistent with the observation that nonflagellated S. enterica serovar Typhimurium (FlhD−) strains are even more virulent than the wild-type strain (unpublished observation). Last, the selective loss in SL3201 ON of the virulence plasmid of S. enterica serovar Typhimurium that is known to increase the growth rate of this pathogen within mice (20) could have accounted for its slower growth rate compared to that of SL3201 OFF. However, we found that both mutants contained the plasmid (data not shown).
In summary, an S. enterica serovar Typhimurium flagellar mutant that is locked into expression of FljB is attenuated in susceptible mice following oral and i.v. inoculations. In contrast, a mutant locked into expression of FliC is as virulent as the wild-type parent strain. The results of the study of i.v. kinetics suggest that the locked-ON mutant is affected in its growth and/or survival in the spleens of infected mice. Consistent with these results are those from the in vivo study of the wild-type strain, which suggested a selective advantage for those organisms that express FliC. Therefore, phase variation in S. enterica serovar Typhimurium is a mechanism that not only controls flagellin type but also influences the virulence of the pathogen in mice.
ACKNOWLEDGMENTS
The work performed in the United States was funded by Public Health Service grants from the National Institutes of Health AI 33525 (A. D. O'Brien) and AI 32951 (E. S. Metcalf) and by grant RO73FE (E. S. Metcalf) from the Uniformed Services University of the Health Sciences. The work performed at the Institute for Animal Health was jointly funded by the BBSRC, grant 201/S10274 (T.S.W.), the MAFF, grant OZ0308 (T.S.W.), and the EU, Fair 3 grant CT96-1743 (T.S.W.). Work in the O'Connor laboratory was supported by BBSRC and Wellcome Trust grants 51/JEI09403 and 049382, respectively.
REFERENCES
- 1.Adams P, Fowler R, Howell G, Kinsella N, Skipp P, Coote P, O'Connor C D. Defining protease specificity with proteomics: a protease with a dibasic amino acid recognition motif is regulated by a two-component signal transduction system in Salmonella. Electrophoresis. 1999;20:2241–2247. doi: 10.1002/(SICI)1522-2683(19990801)20:11<2241::AID-ELPS2241>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Andrewes F W. Studies in group-agglutination. I. The Salmonella group and its antigenic structure. J Pathol Bacteriol. 1922;25:505–521. [Google Scholar]
- 3.Bernstein A, Lederberg J. Agglutination of motile salmonellas by acridines. J Bacteriol. 1955;69:142–146. doi: 10.1128/jb.69.2.142-146.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchmeier N A, Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnens A P, Stanley J, Sack R, Hunziker P, Brodard I, Nicolet J. The flagellin N-methylase gene fliB and an adjacent serovar-specific IS200 element in Salmonella typhimurium. Microbiology. 1997;143:1539–1547. doi: 10.1099/00221287-143-5-1539. [DOI] [PubMed] [Google Scholar]
- 6.Carsiotis M, Stocker B A D, Weinstein D L, O'Brien A D. A Salmonella typhimurium virulence gene linked to flg. Infect Immun. 1989;57:3276–3280. doi: 10.1128/iai.57.11.3276-3280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carsiotis M, Weinstein D L, Karch H, Holder I A, O'Brien A D. Flagella of Salmonella typhimurium are a virulence factor in infected C57BL/6J mice. Infect Immun. 1984;46:814–818. doi: 10.1128/iai.46.3.814-818.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciacci-Woolwine F, Blomfield I C, Richardson S H, Mizel S B. Salmonella flagellin induces tumor necrosis factor alpha in a human promonocytic cell line. Infect Immun. 1998;66:1127–1134. doi: 10.1128/iai.66.3.1127-1134.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis R W, Botstein D, Roth J R, editors. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. pp. 13–16. [Google Scholar]
- 10.Elsinghorst E A, Baron L S, Kopecko D J. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finlay B B, Falkow S. Salmonella as an intracellular parasite. Mol Microbiol. 1989;3:1833–1841. doi: 10.1111/j.1365-2958.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 13.Fujita H, Yamaguchi S, Iino T. Studies on H-O variants in Salmonella in relation to phase variation. J Gen Microbiol. 1973;76:127–134. doi: 10.1099/00221287-76-1-127. [DOI] [PubMed] [Google Scholar]
- 14.Fujita H, Yamaguchi S, Taira T, Hirano T, Iino T. Isolation and genetic analysis of operator-constitutive mutants of the H1 operon in Salmonella typhimurium. J Gen Microbiol. 1987;133:3071–3080. doi: 10.1099/00221287-133-11-3071. [DOI] [PubMed] [Google Scholar]
- 15.Galán J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 16.Galán J E, Curtiss R., III Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillen K L, Hughes K T. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J Bacteriol. 1991;173:2301–2310. doi: 10.1128/jb.173.7.2301-2310.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glasgow A C, Hughes K T, Simon M I. Bacterial DNA inversion systems. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C.: American Society for Microbiology; 1989. pp. 637–659. [Google Scholar]
- 19.Görg A, Postel W, Günther S. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 1988;9:531–546. doi: 10.1002/elps.1150090913. [DOI] [PubMed] [Google Scholar]
- 20.Gulig P A, Doyle T J. The Salmonella typhimurium virulence plasmid increases the growth rate of salmonellae in mice. Infect Immun. 1993;61:504–511. doi: 10.1128/iai.61.2.504-511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanafusa T, Saito K, Tominaga A, Enomoto M. Nucleotide sequence and regulated expression of the Salmonella fljA gene encoding a repressor of the phase 1 flagellin gene. Mol Gen Genet. 1993;236:260–266. doi: 10.1007/BF00277121. [DOI] [PubMed] [Google Scholar]
- 22.Heichman K A, Johnson R C. The Hin invertasome: protein-mediated joining of distant recombination sites at the enhancer. Science. 1990;249:511–517. doi: 10.1126/science.2166334. [DOI] [PubMed] [Google Scholar]
- 23.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature (London) 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 24.Hughes K T, Gaines P C W, Karlinsey J E, Vinayak R, Simon M I. Sequence-specific interaction of the Salmonella Hin recombinase in both major and minor grooves of DNA. EMBO J. 1992;11:2695–2705. doi: 10.1002/j.1460-2075.1992.tb05335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes K T, Mathee K. The anti-sigma factors. Annu Rev Microbiol. 1998;52:231–286. doi: 10.1146/annurev.micro.52.1.231. [DOI] [PubMed] [Google Scholar]
- 26.Johnson R C, Bruist M F. Intermediates in Hin-mediated DNA inversion: a role for Fis and the recombinational enhancer in the strand exchange reaction. EMBO J. 1989;8:1581–1590. doi: 10.1002/j.1460-2075.1989.tb03542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson R C, Simon M I. Hin-mediated site-specific recombination requires two 26 bp recombination sites and a 60 bp recombinational enhancer. Cell. 1985;41:781–791. doi: 10.1016/s0092-8674(85)80059-3. [DOI] [PubMed] [Google Scholar]
- 28.Jones B D, Falkow S. Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect Immun. 1994;62:3745–3752. doi: 10.1128/iai.62.9.3745-3752.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lederberg J, Edwards P R. Serotypic recombination in Salmonella. J Immunol. 1953;71:232–240. [PubMed] [Google Scholar]
- 30.Lederberg J, Iino T. Phase variation in Salmonella. Genetics. 1956;41:743–757. doi: 10.1093/genetics/41.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee V T S O. Type III secretion machines and the pathogenesis of enteric infections caused by Yersinia and Salmonella spp. Immunol Rev. 1999;168:241–255. doi: 10.1111/j.1600-065x.1999.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 32.Lissner C R, Swanson R N, O'Brien A D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983;131:3006–3013. [PubMed] [Google Scholar]
- 33.Lockman H A, Curtiss R., III Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect Immun. 1990;58:137–143. doi: 10.1128/iai.58.1.137-143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 123–145. [Google Scholar]
- 35.McKee M L, Melton-Celsa A R, Moxley R A, Francis D H, O'Brien A D. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect Immun. 1995;63:3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ménard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institutes of Health. Guide for the care and use of laboratory animals. National Institutes of Health publication 85–23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 38.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie brilliant blue G-250 and R-250. Electrophoresis. 1988;9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 39.Osuna R, Lienau D, Hughes K T, Johnson R C. Sequence, regulation, and functions of fis in Salmonella typhimurium. J Bacteriol. 1995;177:2021–2032. doi: 10.1128/jb.177.8.2021-2032.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearce U B, Stocker B A D. Phase variation of flagellar antigens in Salmonella: abortive transduction studies. J Gen Microbiol. 1967;49:335–349. doi: 10.1099/00221287-49-2-335. [DOI] [PubMed] [Google Scholar]
- 41.Qi S Y, Li Y, Szyroki A, Giles I G, Moir A, O'Connor C D. Salmonella typhimurium responses to a bactericidal protein from human neutrophils. Mol Microbiol. 1995;17:523–531. doi: 10.1111/j.1365-2958.1995.mmi_17030523.x. [DOI] [PubMed] [Google Scholar]
- 42.Qi S Y, Moir A, O'Connor C D. Proteome of Salmonella typhimurium SL1344: identification of novel abundant cell envelope proteins and assignment to a two-dimensional reference map. J Bacteriol. 1996;178:5032–5038. doi: 10.1128/jb.178.16.5032-5038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniantis T, editors. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Schmitt C K, Darnell S C, O'Brien A D. The attenuated phenotype of a Salmonella typhimurium flgM mutant is related to expression of FliC flagellin. J Bacteriol. 1996;178:2911–2915. doi: 10.1128/jb.178.10.2911-2915.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt C K, Darnell S C, Tesh V L, Stocker B A D, O'Brien A D. Mutation of flgM attenuates virulence of Salmonella typhimurium, and mutation of fliA represses the attenuated phenotype. J Bacteriol. 1994;176:368–377. doi: 10.1128/jb.176.2.368-377.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silverman M, Simon M. Phase variation: genetic analysis of switching mutants. Cell. 1980;19:845–854. doi: 10.1016/0092-8674(80)90075-6. [DOI] [PubMed] [Google Scholar]
- 48.Silverman M, Zieg J, Hilmen M, Simon M. Phase variation in Salmonella: genetic analysis of a recombinational switch. Proc Natl Acad Sci USA. 1979;76:391–395. doi: 10.1073/pnas.76.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverman M, Zieg J, Simon M. Flagellar-phase variation: isolation of the rhI gene. J Bacteriol. 1979;137:517–523. doi: 10.1128/jb.137.1.517-523.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith H. What happens to bacterial pathogens in vivo? Trends Microbiol. 1998;6:239–243. doi: 10.1016/s0966-842x(98)01250-5. [DOI] [PubMed] [Google Scholar]
- 51.Soberon X, Covarrubias L, Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980;9:287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- 52.Stocker B A D. Measurements of rate of mutation of flagellar antigenic phase in Salmonella typhi-murium. J Hyg. 1949;47:398–413. doi: 10.1017/s002217240001473x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swanson R N, O'Brien A D. Genetic control of the innate resistance of mice to Salmonella typhimurium: Ity gene is expressed in vivo by 24 hours after infection. J Immunol. 1983;131:3014–3020. [PubMed] [Google Scholar]
- 54.Tartera C, Metcalf E S. Osmolarity and growth phase overlap in regulation of Salmonella typhi adherence to and invasion of human intestinal cells. Infect Immun. 1993;61:3084–3089. doi: 10.1128/iai.61.7.3084-3089.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsai S P, Hartin R J, Ryu J-I. Transformation in restriction-deficient Salmonella typhimurium LT2. J Gen Microbiol. 1989;135:2561–2567. doi: 10.1099/00221287-135-9-2561. [DOI] [PubMed] [Google Scholar]
- 56.Wallis T S, Paulin S M, Plested J S, Watson P R, Jones P W. The Salmonella dublin virulence plasmid mediates systemic but not enteric phases of salmonellosis in cattle. Infect Immun. 1995;63:2755–2761. doi: 10.1128/iai.63.7.2755-2761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinstein D L, Carsiotis M, Lissner C R, O'Brien A D. Flagella help Salmonella typhimurium survive within murine macrophages. Infect Immun. 1984;46:819–825. doi: 10.1128/iai.46.3.819-825.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinstein D L, O'Neill B L, Metcalf E S. Salmonella typhi stimulation of human intestinal epithelial cells induces secretion of epithelial cell-derived interleukin-6. Infect Immun. 1997;65:395–404. doi: 10.1128/iai.65.2.395-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zieg J, Silverman M, Hilmen M, Simon M. Recombinational switch for gene expression. Science. 1977;196:170–172. doi: 10.1126/science.322276. [DOI] [PubMed] [Google Scholar]
- 60.Zieg J, Simon M. Analysis of the nucleotide sequence of an invertible controlling element. Proc Natl Acad Sci USA. 1980;77:4196–4200. doi: 10.1073/pnas.77.7.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]