Abstract
Ensembl (https://www.ensembl.org) has produced high-quality genomic resources for vertebrates and model organisms for more than twenty years. During that time, our resources, services and tools have continually evolved in line with both the publicly available genome data and the downstream research and applications that utilise the Ensembl platform. In recent years we have witnessed a dramatic shift in the genomic landscape. There has been a large increase in the number of high-quality reference genomes through global biodiversity initiatives. In parallel, there have been major advances towards pangenome representations of higher species, where many alternative genome assemblies representing different breeds, cultivars, strains and haplotypes are now available. In order to support these efforts and accelerate downstream research, it is our goal at Ensembl to create high-quality annotations, tools and services for species across the tree of life. Here, we report our resources for popular reference genomes, the dramatic growth of our annotations (including haplotypes from the first human pangenome graphs), updates to the Ensembl Variant Effect Predictor (VEP), interactive protein structure predictions from AlphaFold DB, and the beta release of our new website.
INTRODUCTION
For over 20 years, Ensembl has been at the forefront of creating high-quality genome annotations, including genes, variants, regulatory regions and comparative genomics resources. We enable free access to the data in a variety of ways, from visualisations in the genome browser, programmatic access through the Application Programming Interfaces (APIs), querying via our tools and services, and downloading data through our FTP site.
Over the last two decades, the field of genomics has progressed from sequencing and assembling a handful of draft-quality eukaryotic genomes to the thousands available today. There has been a dramatic improvement in the protocols and technology used to sequence and assemble genomes (1), and in tandem the costs and expertise required to build a high-quality genome assembly have been greatly reduced. This has led to an unprecedented number of available genomes, better capturing genomic variation both within and across species (1–6).
Nowhere is this more evident than the human genome itself, with the recent release of the CHM13 genome assembly from the Telomere to Telomere Consortium (T2T) (6), marking the first essentially complete human genome. This has quickly been followed by 94 high-quality haplotype assemblies from 47 individuals, represented in the first large scale human pangenome graph, released as part of the Human Pangenome Reference Consortium (HPRC) (7). These efforts are broadening the representation of human haplotypes and mark an important step toward a more inclusive and diverse era of human genomics.
Species with large research communities continue to both regularly improve the quality of reference genomes, while also increasing the number of alternative assemblies per species. This is particularly evident in the livestock community where there are many examples of high-quality reference genomes and breed-specific genome assemblies (2,4,8–10), both of which are key to advancing breeding programs, which in turn enhance global food security (11–14).
While there is a trend of deeper representation of genomic variation within species, there is simultaneously an explosion of the breadth of eukaryotic genomes through the many global biodiversity efforts currently underway, which includes Darwin Tree of Life (DToL), the Vertebrate Genomes Project (VGP) and the Earth BioGenome Project (1,3,5). These initiatives have already led to hundreds of high-quality reference genomes across the eukaryotic tree, with almost 400 public reference genomes available from DToL alone. This will undoubtedly lead to a much clearer understanding of eukaryotic life and how genomes and their underlying functional elements evolve, in addition to helping with efforts to conserve global biodiversity.
Ensembl provides high-quality, consistent genomic analyses. These range from the annotation of repeats, to genes and gene trees, whole genome alignments, variants and regulatory features. Over the past year we have more than doubled our available genome annotations, with new data coming out as frequently as every two weeks through our Rapid Release website. We have adopted a strategy to ensure support for a rich variety of data types for key species, while continuing to expand our annotations across the tree of life.
In this manuscript we will highlight some of the resources added to the Ensembl platform over the past year and detail future directions.
Deep resources for key species
Increasing the quality and diversity of resources available for popular species, with rich data and large communities (such as human, livestock and model species), is a key part of our mission. The past year has witnessed some major advances in genomics, including the first essentially complete human genome, the first draft human pangenome graph and large-scale availability of protein structure predictions. Below we describe our ongoing efforts to ensure that our annotation and resources evolve in tandem with these advances. These updates are available from www.ensembl.org (as of Ensembl release 108), with the exception of the human pangenome data, which are hosted on rapid.ensembl.org.
Human reference resources
The Ensembl/GENCODE human reference annotation (15) is used globally by major projects and consortia including the NHGRI-EBI GWAS Catalog (16), UK BioBank (17), ICGC/TCGA (18), gnomAD (19) and the Human Cell Atlas (20), which demands that the annotations reflect the latest scientific understanding and data.
Our team of manual annotation experts have worked on refining existing gene structures, and the addition of alternate transcripts and new loci. A total of 313 new and 1,918 updated lncRNA annotations have been added through the TAGENE process (15). In addition, there are 48 new and 1286 updated annotations for protein-coding loci. We have removed or deprecated low-quality genes or those that exist due to errors in the GRCh38 assembly; for example, we have reclassified 29 genes on chromosome 21 under the ‘Artifact’ biotype, as the recent released CHM13 assembly demonstrated the corresponding genes reside on an artificial duplication on the reference genome.
We have continued to work on the Matched Annotation from NCBI and EMBL-EBI (MANE) project (21) to increase the consistency of annotation between the Ensembl/GENCODE and NCBI’s RefSeq (22) gene sets. A total of 19 062 (99.1%) of human protein-coding genes now have an agreed representative transcript. This ensures consistency on the agreed default transcript for each protein-coding gene, while also resolving differences between the reported transcript sequence and structure in each resource.
The Ensembl human variation resources have been updated to include a further 13 million short variants (714 million total), 1 million structural variants (6 million total), and 1 million phenotype associations (16 million total). We have added population frequency data from gnomAD version 3.1.2, which spans 76 156 genomes of diverse ancestry and created tracks for key Human Genome Structural Variation studies.
The Ensembl Regulatory Build (23) now consists of 622 461 regulatory features across 118 epigenomes. We have increased the strictness of our motif feature annotation algorithm and now only display motif features that overlap a ChIP-seq peak in at least one cell type. This increases the confidence that the reported motifs have biological significance. We removed the standalone promoter-flanking feature type, as these are not strictly adjacent to promoters, and instead relabelled these regions as enhancers, which better reflects how they are identified. Additionally, we have made minor updates to the display of regulatory features, ensuring that features of the same type are displayed on the same row. Taken together these changes both improve the confidence in our regulatory region annotation and ensure more consistent categorisation and display.
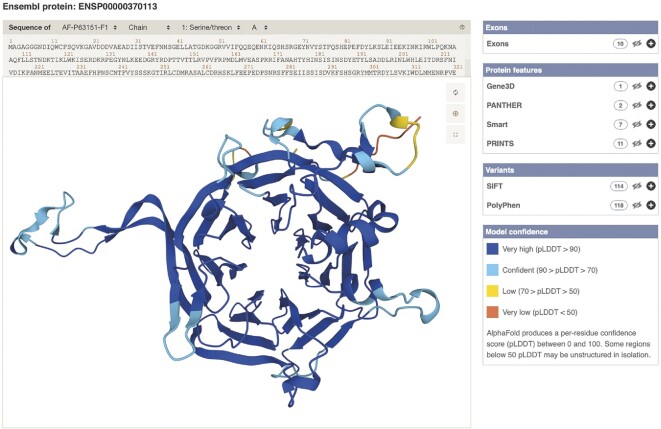
With the advent of AlphaFold2 (24), protein structure predictions are available for almost every human protein-coding gene. Structures from AlphaFold DB (25) for 19 013 human protein-coding genes are now viewable through our new AlphaFold browser widget, accessible via the ‘AlphaFold predicted model’ link in the left-hand menu of any transcript page. For transcripts where a model is available, it is possible to highlight individual residues, variants, exon boundaries, and protein features on the predicted structure. This includes the ability to focus on one feature at a time, for example highlighting a single exon. By default, the structure is colour coded based on a pLDDT score, to show the confidence of the predictions (Figure 1).
Figure 1.
AlphaFold structure prediction for the product of PPP2R2A. AlphaFold structures can be viewed through our new browser widget, via the ‘AlphaFold predicted model’ link in the left hand menu of any transcript page. Note that AlphaFold structures are currently limited to the primary coding transcript of each protein-coding gene, usually the one listed at the top of the transcript table.
Towards an annotated human pangenome
The HPRC has generated the first draft human pangenome graphs, which contain the maternal and paternal haplotypes for 47 individuals, along with the CHM13 T2T assembly and the GRCh38 reference assembly.
As part of these efforts, we have generated gene annotation for each of the 95 haplotypes, including the CHM13v2.0 assembly, through a process of mapping the Ensembl/GENCODE reference gene set (version 38) from GRCh38 to each haplotype, including the annotation of new loci arising from copy number variation (7). Due to the high-quality nature of the underlying sequences, high mapping percentages across the haplotypes are observed, with the largest variability coming from pseudogenes. We have additionally lifted over variation data from gnomAD Genomes (version 3.1.2) and ClinVar (version 2022-07-09) for an initial 89 haplotypes, including CHM13 T2T. To facilitate the annotation of variants called against the new assemblies, these data are available through our FTP site in VCF alongside Ensembl VEP caches containing the remapped Ensembl/GENCODE gene set. The underlying whole genome alignment graphs, generated by UCSC using Cactus (26), are also available through our FTP site.
We have released initial annotation sets for each of these genomes through our Rapid Release site and built a dedicated HPRC project page (https://projects.ensembl.org/hprc). We will regularly update this site with new haplotypes as they become available and will update the gene annotation for all assemblies to reflect the latest Ensembl/GENCODE data and utilise enhanced mapping methods.
Expanded support for other popular species
In collaboration with UniProt (27), our mouse Ensembl/GENCODE gene set has been refined, through the Genome Integration with FuncTion and Sequence (GIFTS, https://www.ebi.ac.uk/gifts/) project. GIFTS has helped improve consistency between protein products present in UniProt and the representation of the corresponding transcript structures on the genome. When the two are in conflict, annotators and curators have determined the cause and then implemented a change to either the genome annotation or the protein record (or both). A total of 104 protein-coding genes have been updated. The mouse lncRNA set has been significantly improved through TAGENE, with 1388 new and 1340 updated annotations.
We have improved and expanded our chicken and pig resources to incorporate tissue-specific developmental data generated as part of the GENE-SWitCH project (https://www.gene-switch.eu/), that is part of the EuroFAANG initiative (https://eurofaang.eu/). For chicken, we have updated the reference genome from the wild Jungle Fowl to a Broiler breed and also added the White Leghorn breed, both of which are commercially important. All three genomes have been annotated utilising the GENE-SWitCH data along with other publicly available transcriptomic data. For the Broiler reference, we display allele frequency data from the European Variation Archive (28) and have also generated ATAC-seq tracks for Broiler and Jungle Fowl. For pig, we have updated the Duroc reference breed annotation using developmental transcriptomic data and display new ATAC-seq tracks.
For fish we have new, high-quality genome assemblies and annotations for Atlantic salmon, common carp and European seabass, produced as part of the EuroFAANG AQUA-FAANG project (https://www.aqua-faang.eu/). We have updated our rainbow trout reference assembly and annotation and added AlphaFold predictions for Zebrafish proteins.
Annotating the eukaryotic tree of life
Below we describe our work supporting global biodiversity efforts. The data can be accessed via our Rapid Release website.
Efficient, high-quality genome annotation
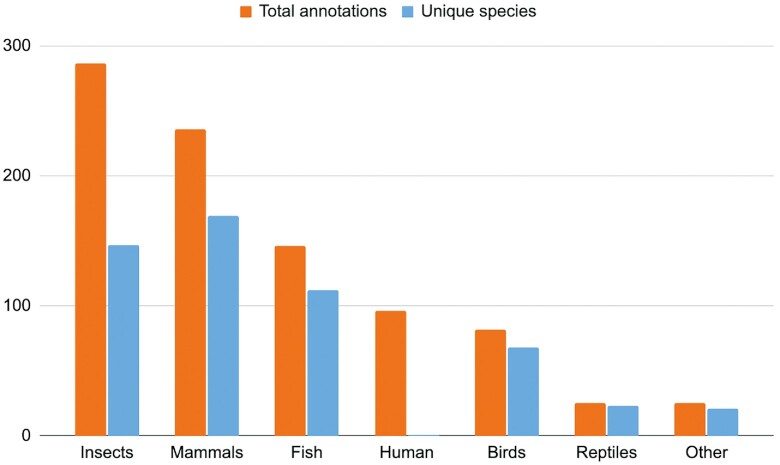
Over the past twelve months we have released our largest ever number of annotated assemblies (see Figure 2), with over 570 new annotations. The majority of these are in support of biodiversity, driven primarily by genomes generated as part of DToL. We have not only annotated the primary haplotypes of these species, but also the alternative haplotypes wherever a high-quality alternative haplotype is present. These data are released approximately every two weeks via our Rapid Release website.
Figure 2.
Total annotations and species offered through Ensembl. This represents total annotations across all species where we have generated annotations through the Ensembl Annotation System (as of October 2022). Human has been given a separate category to reflect the large number of annotations generated as part of the HPRC.
Traditionally, we have focused on annotating vertebrate genomes (still accounting for around 50 percent of our current total annotations), but we are rapidly expanding our analysis across several non-vertebrate groups. We have significantly improved our available resources for insects, releasing a total of 286 assemblies across 147 species. Lepidoptera (butterflies and moths) account for the bulk of these genomes and we have also extensively annotated the genus Bombus, with 26 species of bumble bee now available. Other annotations include species from Hymenoptera, Diptera, Coleoptera, a mollusc and a cnidarian.
To help assess the completeness of these annotations, which vary based on the amount of publicly available transcriptomic data and the suitability of the aligned proteins, we have started to generate BUSCO (29) scores for our annotations as standard. These have been made available through our Darwin Tree of Life project page (https://projects.ensembl.org/darwin-tree-of-life/), and in future will also be available directly on Rapid Release.
Rapid, draft annotation for species lacking transcriptomic data
The Ensembl annotation system uses transcriptomic data to build high-quality gene sets, but for many species there are no available transcriptomic data. This is particularly true of critically endangered species. Despite this, there is a need to support the communities studying these species with draft genome annotations. We have used the BRAKER2 (30) annotation tool to create draft annotations for species that do not yet have transcriptomic data. Protein data from both UniProt and OrthoDB (31) is used to help provide hints to guide BRAKER2. As of October 2022 there are 162 draft gene sets for 88 species of Lepidoptera using this approach.
Homologies and alignments at scale
The Ensembl homology pipeline identifies homologues using Diamond (32) to search against a set of 9 key eukaryotic species (namely human, chicken, zebrafish, D. melanogaster, C. elegans, A. thaliana, rice, P. falciparum and S. cerevisiae) and 29 other reference genomes selected based on the clade to which the target species belongs (33).
Every species available via the Rapid Release browser now has a set of homologues calculated for every protein-coding gene, with information on the type of homology (reciprocal best hit or best hit), the percent identity and coverage of the hit against the query and the gene name associated with the hit (if present). At time of writing this equates to over 108 million homologies.
The number of species included in our whole genome alignments have been expanded, with a new whole genome alignment of 38 fish species, generated as part of the AQUA-FAANG project. The alignment was built using Cactus and can be downloaded from the Rapid Release FTP site (https://ftp.ensembl.org/pub/rapid-release/data_files/multi/hal_files/).
Building an extensive collection of repeat libraries
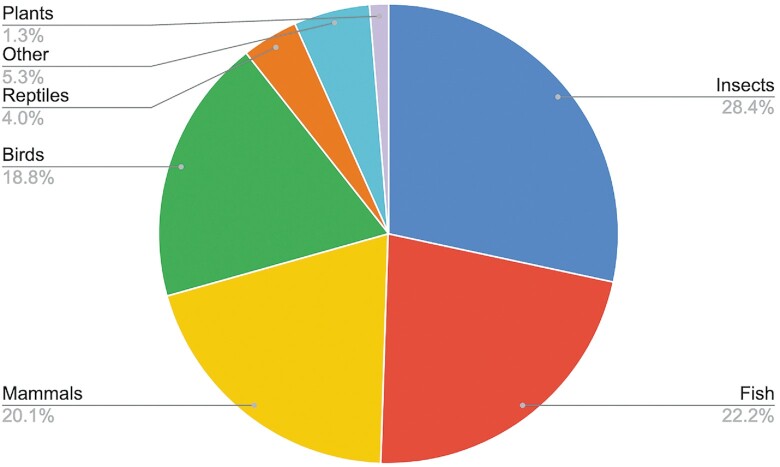
Our collection of freely available repeat libraries (https://ftp.ebi.ac.uk/pub/databases/ensembl/repeats/unfiltered_repeatmodeler/species/) has increased the number of species represented by 54%.
We distribute libraries for 2792 genome assemblies, across 1647 different species (as of October 2022), adding libraries for a further 579 species over the past year. To improve the quality of the libraries we updated to using the recently released version 2 of RepeatModeler (34). The libraries now cover a large number of non-vertebrate species, primarily insects, but also plants, protists and molluscs (Figure 3).
Figure 3.
Distribution of eukaryotic repeat libraries. RepeatModeler-derived libraries are now available on our FTP site for over 1100 species. These libraries are released as soon as the RepeatModeler analysis has completed. The ‘Other’ category includes molluscs, protists, arthropods and vertebrates not falling into the clades defined above.
While the majority of libraries generated over the past twelve months represent newly sequenced species, we have continued to update libraries for existing species in the collection as newer, higher-quality genome assemblies become available, as in general newer assemblies have significantly better representation of repeats compared to older short-read assemblies (35).
Empowering genome interpretation and analysis
In this section we describe updates to our new website, tools, services and training programs.
A beta release of our new website
The beta version of our new website has been released (https://beta.ensembl.org). This initial release represents an overview of some of the major design decisions behind the new browser. These include an app-based approach to functionality, a highly responsive genome browser, simplified displays and the ability to browse multiple species of interest simultaneously. Six key species have been included: human, D. melanogaster, C. elegans, wheat, P. falciparum and E. coli. For human, we have included both GRCh37 and GRCh38 assemblies, as a precursor to broader support for alternative assemblies in future iterations.
Initial functionality revolves around three apps: the species selector, the genome browser, and the entity viewer (Figure 4). The species selector allows the selection of one or several species (including alternate assemblies for a species), providing an overview of key metrics such as assembly contiguity and the number of features annotated. Selected species are then automatically carried into the genome browser and entity viewer. The new genome browser enables an intuitive overview of a genome, allowing rapid panning and zooming, from the level of viewing an entire chromosome right down to the underlying nucleotide sequence. The entity viewer displays a detailed view of a specific gene, from the exon structure of its transcripts to the function of the gene and resulting protein. The beta site also includes the ability to run BLAST (36) searches for multiple sequences against any of the seven currently available genomes, with results being presented in a straightforward tabular view.
Figure 4.
Homepage for the new Ensembl website. The homepage acts as a gateway for interacting with our data via the species selector, genome browser and entity viewer apps. The new website will eventually bring all species across the current set of Ensembl websites under a single infrastructure.
Updates to the ensembl VEP
The Ensembl VEP (37) is a powerful toolset for variant annotation and prioritisation. We have continued to extend the available analysis options and have this year integrated predictions from EVE (38), which employs an evolutionary model of variant effect, and CAPICE (39), a machine-learning based method for prioritising pathogenic variants. Ensembl VEP also now reports when a variant which causes a premature stop codon results in a transcript likely to escape nonsense-mediated decay; such variants are thought to be relevant in Mendelian disease (40).
We have extended the protein annotations available in Ensembl VEP beyond the previous reporting when a variant falls in a protein domain and listing SwissProt matches and protein family information. The web interface now displays a variant's location on AlphaFold-predicted protein structures in an interactive visualisation. Variants lying in a protein location shown to affect protein interaction, as described in the IntAct database (41), are reported with links to the IntAct website and any journals describing the variant's effect for further information. To aid identification of potential disease-associated variants, Gene Ontology terms are also now reported, describing the function, the cellular location and the associated biological processes of the product of any gene a variant lies within.
Tracking transcripts through tark
Our transcript archive service, Tark (http://tark.ensembl.org/), allows extremely fine-grained tracking of changes to the annotation of the transcript sequences and structures from both Ensembl and RefSeq. Recent updates include the ability to track changes to the gene biotype (e.g. if a protein-coding gene was reclassified as a pseudogene). We have also added support for comparing the sequence and structure of GRCh37 annotations to MANE Select and MANE Plus Clinical transcripts annotated on GRCh38.
Outreach and training
During the course of the last year we delivered training, both in-person and virtual to 1805 participants from across the globe. In February 2022, we returned to in-person training, holding 3 in-person workshops, attended by over 200 participants. In addition, we ran a total of 46 virtual workshops, 20 of which were targeted at low and middle income countries including Colombia, India and Nigeria.
The training we deliver primarily covers our genome browser, but we also run more specialised training courses covering how to use our REST API, our variation resources and the Ensembl VEP. All training materials are made available via our training pages (https://training.ensembl.org/) where you can also find information about attending or hosting a workshop. You can also contact us via our helpdesk (helpdesk@ensembl.org).
SUMMARY AND FUTURE DIRECTIONS
Over the past 12 months, we have released new genome annotations for key species, including the haplotypes from the draft human pangenome, updated our variant sets and regulatory regions, and imported protein structure predictions from AlphaFold DB. The number of genomes we have annotated has more than doubled to 829, expanding further across the eukaryotic tree of life. Our main website, www.ensembl.org, continues to focus on reference genome updates for vertebrate and model species, with high levels of data integration, including gene trees, multiple alignments and BioMart availability for the majority of species, and variants and regulatory data for select species. Our Rapid Release site has become the primary location for new species, particularly those arising from biodiversity initiatives, and also for accessing alternative haplotypes, breeds and strains. It continues to grow in terms of the resources available, with all species now having an associated set of homologies. The launch of our beta site points towards the future of our platform, and will eventually house all our species and annotations in one location, under an entirely new interface and infrastructure. It will retain the best elements of our resources from the previous two decades, while dealing with the shortcomings of our current infrastructure, particularly in terms of updating the look and feel, and dramatically improving the experience of browsing a genome.
Over the coming year, we expect a major increase in the number of pangenome initiatives for key species, particularly for model organisms and species related to agriculture. The number of haplotypes represented in the human pangenome will also continue to expand through the efforts of the HPRC and other initiatives. In addition to the biodiversity initiatives that are already well underway such as the VGP and DToL, we will see several new initiatives gather pace, including the Canadian BioGenome Project, African BioGenome Project and the European Reference Genome Atlas.
To ensure the value of these genomes are maximised, we will continue to release annotations, tools and services to allow researchers to ask increasingly complex questions. A particular focus will be on expanding our comparative resources, through the provision of gene trees and orthologue calls for all species via the integration of OrthoFinder (42) into our gene tree pipeline. We also plan to increase the number of Cactus alignments we provide to cover both more eukaryotic clades and include more species within each alignment. Similarly, as more variant and regulatory data become available, we will continue to broaden the types of data we produce across species. AlphaFold DB now includes predictions for over 200 million proteins from UniProt, as a result, we will expand our provision of AlphaFold models across all species where predictions are available.
We will continue to work towards our goal of providing freely accessible, high-quality data and tools across the tree of life.
DATA AVAILABILITY
All Ensembl integrated data are available without restriction from the main website (https://www.ensembl.org), the Rapid Release site (https://rapid.ensembl.org), in bulk from the FTP site (https://ftp.ensembl.org) and programmatically via the REST API (https://rest.ensembl.org). Ensembl code is available from GitHub (https://github.com/Ensembl) under an open source Apache 2.0 licence. News about our releases and services can be found on our blog (https://www.ensembl.info), our announce mailing list (https://lists.ensembl.org/mailman/listinfo/announce), Twitter (@ensembl; https://twitter.com/ensembl) and Facebook (https://facebook.com/Ensembl.org). Ensembl and Ensembl VEP are registered trademarks of EMBL.
ACKNOWLEDGEMENTS
We wish to thank all of our user community and data providers for making their data available for reuse within Ensembl; and the following members of EMBL-EBI’s technical services cluster for their continued support: Simone Badoer, Jonathan Barker, Sarah Butcher, Andy Cafferkey, Andrea Cristofori, Tim Dyce, Rafael Grimán Canto, Salvatore Di Nardo, Santiago Insua, Rodrigo Lopez, Zander Mears, Manuela Menchi, Sundeep Nanawa and Steven Newhouse. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Fergal J Martin, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
M Ridwan Amode, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Alisha Aneja, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Olanrewaju Austine-Orimoloye, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Andrey G Azov, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
If Barnes, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Arne Becker, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Ruth Bennett, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Andrew Berry, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Jyothish Bhai, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Simarpreet Kaur Bhurji, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Alexandra Bignell, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Sanjay Boddu, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Paulo R Branco Lins, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Lucy Brooks, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Shashank Budhanuru Ramaraju, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Mehrnaz Charkhchi, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Alexander Cockburn, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Luca Da Rin Fiorretto, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Claire Davidson, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Kamalkumar Dodiya, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Sarah Donaldson, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Bilal El Houdaigui, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Tamara El Naboulsi, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Reham Fatima, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Carlos Garcia Giron, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Thiago Genez, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Gurpreet S Ghattaoraya, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Jose Gonzalez Martinez, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Cristi Guijarro, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Matthew Hardy, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Zoe Hollis, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Thibaut Hourlier, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Toby Hunt, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Mike Kay, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Vinay Kaykala, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Tuan Le, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Diana Lemos, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Diego Marques-Coelho, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
José Carlos Marugán, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Gabriela Alejandra Merino, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Louisse Paola Mirabueno, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Aleena Mushtaq, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Syed Nakib Hossain, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Denye N Ogeh, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Manoj Pandian Sakthivel, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Anne Parker, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Malcolm Perry, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Ivana Piližota, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Irina Prosovetskaia, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
José G Pérez-Silva, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Ahamed Imran Abdul Salam, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Nuno Saraiva-Agostinho, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Helen Schuilenburg, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Dan Sheppard, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Swati Sinha, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Botond Sipos, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
William Stark, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Emily Steed, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Ranjit Sukumaran, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Dulika Sumathipala, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Marie-Marthe Suner, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Likhitha Surapaneni, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Kyösti Sutinen, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Michal Szpak, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Francesca Floriana Tricomi, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
David Urbina-Gómez, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Andres Veidenberg, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Thomas A Walsh, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Brandon Walts, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Elizabeth Wass, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Natalie Willhoft, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Jamie Allen, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Jorge Alvarez-Jarreta, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Marc Chakiachvili, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Bethany Flint, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Stefano Giorgetti, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Leanne Haggerty, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Garth R Ilsley, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Jane E Loveland, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Benjamin Moore, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Jonathan M Mudge, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
John Tate, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
David Thybert, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Stephen J Trevanion, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Andrea Winterbottom, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Adam Frankish, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Sarah E Hunt, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Magali Ruffier, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Fiona Cunningham, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Sarah Dyer, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Robert D Finn, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Kevin L Howe, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Peter W Harrison, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Andrew D Yates, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
Paul Flicek, European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, CB10 1SD, Cambridge, UK.
FUNDING
Ensembl receives majority funding from Wellcome Trust [WT222155/Z/20/Z] with additional funding for specific project components; research reported in this publication was supported by National Human Genome Research Institute of the National Institutes of Health [2U41HG007234, U41HG010972, R01 HG010485]; Ensembl receives further funding from the Biotechnology and Biological Sciences Research Council [BB/W019108/1, BB/S020152/1, BB/T01461X/1]; Open Targets; Wellcome Trust [WT200990/A/16/Z, WT108749/Z/15/A, WT212925/Z/18/Z, WT218328/B/19/Z]; British Council [414710385]; ELIXIR: the research infrastructure for life-science data, and the European Molecular Biology Laboratory; European Union's Horizon 2020 research and innovation programme [733161 (MultipleMS), 825575 (EJP RD), 817923 (AQUA-FAANG), 817998 (GENE-SWitCH) and 815668 (BovReg)]. Funding for open access charge: Wellcome [WT222155/Z/20/Z].
Conflict of interest statement. Paul Flicek is a member of the Scientific Advisory Boards of Fabric Genomics, Inc. and Eagle Genomics, Ltd.
REFERENCES
- 1. Rhie A., McCarthy S.A., Fedrigo O., Damas J., Formenti G., Koren S., Uliano-Silva M., Chow W., Fungtammasan A., Kim J.et al.. Towards complete and error-free genome assemblies of all vertebrate species. Nature. 2021; 592:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang Y., Xie M., Chen W., Talbot R., Maddox J.F., Faraut T., Wu C., Muzny D.M., Li Y., Zhang W.et al.. The sheep genome illuminates biology of the rumen and lipid metabolism. Science. 2014; 344:1168–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Darwin Tree of Life Project Consortium Sequence locally, think globally: the darwin tree of life project. Proc. Natl. Acad. Sci. U.S.A. 2022; 119:e2115642118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalbfleisch T.S., Rice E.S., DePriest M.S. Jr, Walenz B.P., Hestand M.S., Vermeesch J.R., O Connell B.L., Fiddes I.T., Vershinina A.O., Saremi N.F.et al.. Improved reference genome for the domestic horse increases assembly contiguity and composition. Commun Biol. 2018; 1:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lewin H.A., Richards S., Lieberman Aiden E., Allende M.L., Archibald J.M., Bálint M., Barker K.B., Baumgartner B., Belov K., Bertorelle G.et al.. The earth biogenome project 2020: starting the clock. Proc. Natl. Acad. Sci. U.S.A. 2022; 119:e2115635118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nurk S., Koren S., Rhie A., Rautiainen M., Bzikadze A.V., Mikheenko A., Vollger M.R., Altemose N., Uralsky L., Gershman A.et al.. The complete sequence of a human genome. Science. 2022; 376:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liao W.-W., Asri M., Ebler J., Doerr D., Haukness M., Hickey G., Lu S., Lucas J.K., Monlong J., Abel H.J.et al.. A draft human pangenome reference. 2022; bioRxiv doi:09 July 2022, preprint: not peer reviewed 10.1101/2022.07.09.499321. [DOI] [PMC free article] [PubMed]
- 8. Low W.Y., Tearle R., Liu R., Koren S., Rhie A., Bickhart D.M., Rosen B.D., Kronenberg Z.N., Kingan S.B., Tseng E.et al.. Haplotype-resolved genomes provide insights into structural variation and gene content in angus and brahman cattle. Nat. Commun. 2020; 11:2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pettersson M.E., Rochus C.M., Han F., Chen J., Hill J., Wallerman O., Fan G., Hong X., Xu Q., Zhang H.et al.. A chromosome-level assembly of the atlantic herring genome-detection of a supergene and other signals of selection. Genome Res. 2019; 29:1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warr A., Affara N., Aken B., Beiki H., Bickhart D.M., Billis K., Chow W., Eory L., Finlayson H.A., Flicek P.et al.. An improved pig reference genome sequence to enable pig genetics and genomics research. Gigascience. 2020; 9:giaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayes B.J., Bowman P.J., Chamberlain A.J., Goddard M.E.. Invited review: genomic selection in dairy cattle: progress and challenges. J. Dairy Sci. 2009; 92:433–443. [DOI] [PubMed] [Google Scholar]
- 12. Christensen O.F., Madsen P., Nielsen B., Ostersen T., Su G.. Single-step methods for genomic evaluation in pigs. Animal. 2012; 6:1565–1571. [DOI] [PubMed] [Google Scholar]
- 13. Clark E.L., Archibald A.L., Daetwyler H.D., Groenen M.A.M., Harrison P.W., Houston R.D., Kühn C., Lien S., Macqueen D.J., Reecy J.M.et al.. From FAANG to fork: application of highly annotated genomes to improve farmed animal production. Genome Biol. 2020; 21:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cleveland M.A., Hickey J.M.. Practical implementation of cost-effective genomic selection in commercial pig breeding using imputation. J. Anim. Sci. 2013; 91:3583–3592. [DOI] [PubMed] [Google Scholar]
- 15. Frankish A., Diekhans M., Jungreis I., Lagarde J., Loveland J.E., Mudge J.M., Sisu C., Wright J.C., Armstrong J., Barnes I.et al.. gencode 2021. Nucleic Acids Res. 2021; 49:D916–D923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buniello A., MacArthur J.A.L., Cerezo M., Harris L.W., Hayhurst J., Malangone C., McMahon A., Morales J., Mountjoy E., Sollis E.et al.. The NHGRI-EBI GWAS catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019; 47:D1005–D1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bycroft C., Freeman C., Petkova D., Band G., Elliott L.T., Sharp K., Motyer A., Vukcevic D., Delaneau O., O’Connell J.et al.. The UK biobank resource with deep phenotyping and genomic data. Nature. 2018; 562:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium Pan-cancer analysis of whole genomes. Nature. 2020; 578:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P.et al.. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020; 581:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rozenblatt-Rosen O., Stubbington M.J.T., Regev A., Teichmann S.A.. The human cell atlas: from vision to reality. Nature. 2017; 550:451–453. [DOI] [PubMed] [Google Scholar]
- 21. Morales J., Pujar S., Loveland J.E., Astashyn A., Bennett R., Berry A., Cox E., Davidson C., Ermolaeva O., Farrell C.M.et al.. A joint NCBI and EMBL-EBI transcript set for clinical genomics and research. Nature. 2022; 604:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Leary N.A., Wright M.W., Brister J.R., Ciufo S., Haddad D., McVeigh R., Rajput B., Robbertse B., Smith-White B., Ako-Adjei D.et al.. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016; 44:D733–D745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zerbino D.R., Wilder S.P., Johnson N., Juettemann T., Flicek P.R.. The ensembl regulatory build. Genome Biol. 2015; 16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A.et al.. Highly accurate protein structure prediction with alphafold. Nature. 2021; 596:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., Yuan D., Stroe O., Wood G., Laydon A.et al.. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022; 50:D439–D444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Armstrong J., Hickey G., Diekhans M., Fiddes I.T., Novak A.M., Deran A., Fang Q., Xie D., Feng S., Stiller J.et al.. Progressive cactus is a multiple-genome aligner for the thousand-genome era. Nature. 2020; 587:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Consortium UniProt UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021; 49:D480–D489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cezard T., Cunningham F., Hunt S.E., Koylass B., Kumar N., Saunders G., Shen A., Silva A.F., Tsukanov K., Venkataraman S.et al.. The european variation archive: a FAIR resource of genomic variation for all species. Nucleic Acids Res. 2022; 50:D1216–D1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manni M., Berkeley M.R., Seppey M., Zdobnov E.M.. BUSCO: assessing genomic data quality and beyond. Curr. Protoc. 2021; 1:e323. [DOI] [PubMed] [Google Scholar]
- 30. Brůna T., Hoff K.J., Lomsadze A., Stanke M., Borodovsky M.. BRAKER2: automatic eukaryotic genome annotation with genemark-EP+ and AUGUSTUS supported by a protein database. NAR Genom. Bioinform. 2021; 3:lqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zdobnov E.M., Kuznetsov D., Tegenfeldt F., Manni M., Berkeley M., Kriventseva E.V.. OrthoDB in 2020: evolutionary and functional annotations of orthologs. Nucleic Acids Res. 2021; 49:D389–D393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buchfink B., Xie C., Huson D.H.. Fast and sensitive protein alignment using DIAMOND. Nat. Methods. 2015; 12:59–60. [DOI] [PubMed] [Google Scholar]
- 33. Cunningham F., Allen J.E., Allen J., Alvarez-Jarreta J., Amode M.R., Armean I.M., Austine-Orimoloye O., Azov A.G., Barnes I., Bennett R.et al.. Ensembl 2022. Nucleic Acids Res. 2022; 50:D988–D995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flynn J.M., Hubley R., Goubert C., Rosen J., Clark A.G., Feschotte C., Smit A.F.. RepeatModeler2 for automated genomic discovery of transposable element families. Proc. Natl. Acad. Sci. U.S.A. 2020; 117:9451–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mascher M., Wicker T., Jenkins J., Plott C., Lux T., Koh C.S., Ens J., Gundlach H., Boston L.B., Tulpová Z.et al.. Long-read sequence assembly: a technical evaluation in barley. Plant Cell. 2021; 33:1888–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J.. Basic local alignment search tool. J. Mol. Biol. 1990; 215:403–410. [DOI] [PubMed] [Google Scholar]
- 37. McLaren W., Gil L., Hunt S.E., Riat H.S., Ritchie G.R.S., Thormann A., Flicek P., Cunningham F.. The ensembl variant effect predictor. Genome Biol. 2016; 17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frazer J., Notin P., Dias M., Gomez A., Min J.K., Brock K., Gal Y., Marks D.S.. Disease variant prediction with deep generative models of evolutionary data. Nature. 2021; 599:91–95. [DOI] [PubMed] [Google Scholar]
- 39. Li S., van der Velde K.J., de Ridder D., van Dijk A.D.J., Soudis D., Zwerwer L.R., Deelen P., Hendriksen D., Charbon B., van Gijn M.E.et al.. CAPICE: a computational method for consequence-agnostic pathogenicity interpretation of clinical exome variations. Genome Med. 2020; 12:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coban-Akdemir Z., White J.J., Song X., Jhangiani S.N., Fatih J.M., Gambin T., Bayram Y., Chinn I.K., Karaca E., Punetha J.et al.. Identifying genes whose mutant transcripts cause dominant disease traits by potential Gain-of-Function alleles. Am. J. Hum. Genet. 2018; 103:171–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Del Toro N., Shrivastava A., Ragueneau E., Meldal B., Combe C., Barrera E., Perfetto L., How K., Ratan P., Shirodkar G.et al.. The intact database: efficient access to fine-grained molecular interaction data. Nucleic Acids Res. 2022; 50:D648–D653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Emms D.M., Kelly S.. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 2019; 20:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All Ensembl integrated data are available without restriction from the main website (https://www.ensembl.org), the Rapid Release site (https://rapid.ensembl.org), in bulk from the FTP site (https://ftp.ensembl.org) and programmatically via the REST API (https://rest.ensembl.org). Ensembl code is available from GitHub (https://github.com/Ensembl) under an open source Apache 2.0 licence. News about our releases and services can be found on our blog (https://www.ensembl.info), our announce mailing list (https://lists.ensembl.org/mailman/listinfo/announce), Twitter (@ensembl; https://twitter.com/ensembl) and Facebook (https://facebook.com/Ensembl.org). Ensembl and Ensembl VEP are registered trademarks of EMBL.