Abstract
Background:
Children with asthma are at risk for low lung function extending into adulthood but understanding of clinical predictors is incomplete.
Objective:
We aimed to determine phenotypic factors associated with FEV1 throughout childhood in the Severe Asthma Research Program (SARP) 3 pediatric cohort.
Methods:
Lung function was measured at baseline and annually. Multivariate linear mixed effects models were constructed to assess the effect of baseline and time-varying predictors of pre-bronchodilator FEV1 at each assessment for up to 6 years. All models were adjusted for age, predicted FEV1 by GLI reference equations, race, sex, and height. Secondary outcomes included post-bronchodilator FEV1 and pre-bronchodilator FEV1/FVC.
Results:
A total of 862 spirometry assessments were performed for 188 participants. Factors associated with FEV1 include baseline FeNO (B= −49mL/log2PPB, 95%CI −92,6), response to a characterizing dose of triamcinolone acetonide (B= −8.4mL/1%changeFEV1 post-triamcinolone, 95%CI-12.3,−4.5), and maximal bronchodilator reversibility (B= −27mL/1%change post bronchodilator FEV1, 95%CI −37,−16). Annually assessed time-varying factors of age, obesity, and exacerbation frequency predicted FEV1 over time. Notably, there was a significant age and sex interaction. Among girls there was no exacerbation effect. For boys, however, moderate (1–2) exacerbation frequency in the prior 12 months was associated with −20mL (95% CI −39, −2) FEV1 at each successive year. High exacerbation frequency (≥3) 12–24 months prior to assessment was associated with −34mL (95% CI −61, −7) FEV1 at each successive year.
Conclusion:
In children with severe and non-severe asthma, several clinically-relevant factors predict FEV1 over time. Boys with recurrent exacerbations are at high risk of lower FEV1 through childhood.
Keywords: Severe asthma, corticosteroid sensitivity, lung function, spirometry, asthma exacerbations
Capsule Summary
Children with asthma are at risk for poor lung function into adulthood. This study identifies longitudinal predictors of lower FEV1 among well-characterized children in the severe asthma research program (SARP) 3.
Introduction
The childhood origins of lifelong lung diseases has been widely acknowledged and the most fundamental and consistent finding is that reduced lung function during childhood is predictive of low lung function in adulthood1,2. Several general population-based studies have highlighted the risk of COPD in early- to mid-adulthood associated with low lung function trajectories that extend back to the earliest obtainable lung function assessments3,4. However, the majority of children with low lung function in early infancy have subsequently normal forced expiratory volume in 1 second (FEV1) in later childhood4,5, suggesting there are factors beyond genetic predisposition that influence pediatric lung function.
Identifying risk factors for low lung function in childhood is paramount to identifying high risk populations that may require closer clinical follow-up and to test targeted interventions to alter the course of lifelong lung function. Childhood asthma is consistently identified as a strong and significant negative influence on FEV1 through adolescence and adulthood1,6,7. McGeachie and colleagues8 demonstrated that 17% of children enrolled in the CAMP cohort of school-age children with mild to moderate persistent asthma attained stage 1 COPD by early adulthood. Children with severe asthma may be at an even greater risk6, however few patients with severe asthma are included in longitudinal asthma cohorts. The lack of data in contemporary cohorts with current definitions of severe asthma and repeated measures of time-varying traits limit our understanding of risk factors for poor lung function over time.
The Severe Asthma Research Program (SARP3) enrolled a cohort of carefully characterized, longitudinally re-assessed children and adults with severe asthma based on American Thoracic Society(ATS)/European Respiratory Society (ERS) criteria9 and a comparator population with non-severe asthma. Recently published findings of the SARP3 adult participants identified that triamcinolone acetonide-induced difference in post-bronchodilator FEV1% predicted (herein referred to as tdFEV1; derived by baseline subtraction) characterization was predictive of lung function decline10. We hypothesized that specific participant and asthma characteristics may be associated with lower lung function over time among children and adolescents. Based on the prior literature we evaluated the relationship of several key predictors of FEV1 across childhood, and specifically evaluated severe asthma at each timepoint and tdFEV1, unique features of this cohort, that have not been previously assessed in longitudinal lung function studies in children. To address the known shift in asthma epidemiology that occurs through adolescence11,12, we evaluated age and sex effect modification of the predictors’ relationship with lung function.
Methods
Cohort and clinical measurements
The SARP3 observational study enrolled participants with severe and non-severe asthma from November, 2012 through January, 2015. Informed consent, assent for children, was obtained prior to all research procedures and the study was approved by the investigational review boards at all participating centers. Severe asthma definition13 and baseline assessments for the SARP3 cohort have been previously described (See supplementary Table 1)14,15. SARP3 intentionally enrolled patients with severe asthma, based on the ATS/ERS guideline definition9, and non-severe asthma to better understand phenotypic and natural history outcomes by disease severity. To characterize baseline steroid responsiveness, subjects received a standard intramuscular dose of triamcinolone acetonide 1mg/kg up to a maximum 40mg15, and repeat assessments 18 ± 3 days later. Triamcinolone-induced differences from baseline were derived by simple subtraction (tdFEV1)15. Longitudinal assessments were collected at annual study visits which captured time-varying predictors. Pediatric participants who completed at least one research visit were included in this analysis. Visits were delayed at least 2 weeks from the last day of antibiotics for respiratory infection and 4 weeks from a burst of systemic steroid for any reason in order to capture data reflective of the participant usual level of function.
Lung Function Assessment
Spirometry and maximum bronchodilator reversibility, assessed following 4, 6, or 8 puffs of albuterol 90mcg, were performed annually14,16. Post-bronchodilator tdFEV1 was measured following the standard 4 puffs of albuterol17.
FEV1 percent-predicted indices are limited due to asymmetric growth in height and lung function across maturation stages between individuals, particularly across puberty18,19. FEV1/FVC has been used to internally adjust FEV1 for lung size (VC) 3,19, however, low VC may, itself, be a result of poor lung growth or airways closure due to poor airflow and it prohibits extrapolation to FEV1 relevant to adult lung function and risk of COPD8,20. Therefore, we selected the absolute value FEV1 as the primary outcome and adjusted for several time-varying factors related to lung growth. We adjusted for Global Lung Function Initiative (GLI) reference value16, the most commonly used reference equation in clinical practice, in order to determine the effect of each factor beyond the expected achieved level of FEV1.
Statistical Analysis
Descriptive statistics were used to assess participant characteristics. We evaluated the following determinants of longitudinal lung function outcomes: baseline continuous values of fractional exhaled nitric oxide (FeNO, log2-transformed), peripheral blood eosinophils, pre- and post-bronchodilator (BD) tdFEV1, total immunoglobulin E (IgE, log2-transformed), and maximal BD reversibility (for FEV1 and FVC); baseline dichotomous measures of environmental tobacco smoke (ETS) exposure and history of pneumonia; time-varying values assessed at each visit: severe asthma (vs. non-severe), exacerbations (categorical: 0, 1–2, 3+) in the prior 12 months and 1-year lag exacerbations (reported at previous annual assessment), inhaled corticosteroid (ICS) dose (categorical: none, low, medium, high dose), biologic medication use (yes/no), and BMI (categorical: normal, overweight, obese). FEV1 was modeled with linear mixed models clustered at the participant level. Age, predicted FEV1 by GLI reference equation16, race, sex, and height were adjustors in all models. Predictors of FEV1 were tested with a main effect and the interaction of the predictor across the age range. Individual predictors were tested separately and the main and age interaction effects were included in the multivariate model if the interaction effect was statistically significant (alpha=0.05); only the main effect was selected if it was significant and the interaction was not. In addition, we tested potential sex effects with 2- and 3-way interactions (with age and predictors) and included these terms in the multivariate model using the same criteria. Age in years was recoded to be age minus six so that coefficients for age and age interactions could be interpreted in terms of years since age six years (the youngest age at baseline). Because one-year lag exacerbations was missing for the baseline assessment (and n=14 follow-up assessments), data were imputed based on a multinomial regression model where lag exacerbations were predicted from current exacerbations. To account for data not missing completely at random, sensitivity analysis for the primary outcome utilizing inverse probability weighting was performed. This approach effectively increases the weight of the observations that are more likely to be missing21. Sensitivity analyses were performed to assess the longitudinal effect of asthma control (ACT score, continuous variable) and longitudinally collected FeNO (log2 FeNO, continuous variable) on pre-bronchodilator FEV1. All tests were two-tailed with alpha set at 0.05 and conducted using STATA 16.1. Additional detail on study procedures and data analysis is available in the Online Repository.
Results
Descriptive outcomes
The baseline characteristics of the cohort are presented in Table 1. SARP3 enrolled 111 children with severe asthma and 77 children with non-severe asthma. The average age was 11.5 years (SD=2.8), 62.2% male, and 53% Black race. The mean pre-bronchodilator FEV1 was 89.7 (SD=16.6) and post-bronchodilator FEV1 was 104.3 (SD=16.0) percent predicted. At enrollment, 31% reported no exacerbations, 42.2% reported moderate (1–2 per year) exacerbation frequency, and 26.7% reported high (≥ 3 per year) exacerbation frequency in the prior 12 months.
Table 1.
Baseline characteristics of pediatric SARP3 participants overall and by severe asthma status
| Characteristic | Total Cohort N = 188 | Severe asthma (N = 111) | Non-severe asthma (N = 77) |
|---|---|---|---|
|
| |||
| Age at Baseline, years | 11.5 ± 2.8 | 11.4 ± 2.8 | 11.7 ± 2.9 |
| Duration of asthma, years | 8.4 ± 3.5 | 8.4 ± 3.4 | 8.4 ± 3.8 |
| Male, N (%) | 117 (62.2%) | 67 (60.4%) | 50 (64.9%) |
| Female | 71 (37.8%) | 44 (39.6%) | 27 (35.1%) |
| White Race | 62 (33.0%) | 33 (29.7%) | 29 (37.7%) |
| Black Race | 100 (53.2%) | 60 (54.0%) | 40 (52.0%) |
| Other Race | 8 (4.3%) | 6 (5.4%) | 2 (2.6%) |
| Hispanic | 18 (9.6%) | 12 (10.8%) | 6 (7.8%) |
| BMI percentile | |||
| Normal (<85th percentile) | 83 (44.2%) | 43 (38.7%) | 40 (52.0%) |
| Overweight (85th-94th percentile) | 40 (21.3%) | 26 (23.4%) | 14 (18.2%) |
| Obese (≥95th percentile) | 65 (34.6%) | 42 (37.8%) | 23 (29.9%) |
| Severe Asthma at Baseline | 111 (59.0%) | -- | -- |
| ICS Dose - None, N (%) | 10 (5.3%) | 0 (0%) | 10 (13.0%) |
| ICS Dose - Low, N (%) | 24 (12.8%) | 0 (0%) | 24 (31.2%) |
| ICS Dose - Medium, N (%) | 21 (11.2%) | 0 (0%) | 21 (27.3%) |
| ICS Dose - High, N (%) | 133 (70.7%) | 111 (100%)*** | 22 (28.6%) |
| Number of Controller Therapies, median (IQR) | 2 (2, 3) | 3 (2, 3)*** | 2 (1, 2) |
| Baseline Exacerbations in prior 12 months | |||
| 0 | 58 (31.0%) | 18 (16.4%) | 40 (52.0%) |
| 1–2 (moderate) | 79 (42.2%) | 47 (42.7%) | 32 (41.6%) |
| 3+ (High) | 50 (26.7%) | 45 (40.9%)*** | 5 (6.5%) |
| Baseline ACT Score | 18.2 ± 4.3 | 17.0 ± 4.4*** | 19.9 ± 3.6 |
| FeNO, median (IQR) | 24 (12, 46.5) | 23 (12, 46) | 28 (12, 49) |
| Pre-BD FEVi% predicted | 89.7 ± 16.6 | 87.4 ± 17.7* | 93.0 ± 14.4 |
| Post-BD FEVi% predicted | 104.3 ± 16.0 | 103.7 ± 17.5 | 105.2 ± 13.6 |
| Pre-BD tdFEV1 | 1.9 ± 13.2% | 2.5 ± 13.7% | 1.0 ± 12.4% |
| Post-BD tdFEV1 | 0.1 ± 11.9% | −0.1 ± 13.2% | 0.4 ± 10.0% |
| Maximum bronchodilator reversibility | 14.6 ± 10.0% | 16.3 ± 11.0%** | 12.2 ± 7.8% |
Data are presented as mean ± sd or number (percentage of total) unless otherwise indicated.
p<0.05 vs. non-severe
p<0.01 vs. non-severe
p<0.001 vs. non-severe. Global Lung Index (GLI) reference values used for spirometry(15). BD, bronchodilator; tdFEV1, Triamcinolone Acetate (TA) - induced difference from baseline FEV1 (post-TA FEV1 % predicted – pre-TA FEV1% predicted)
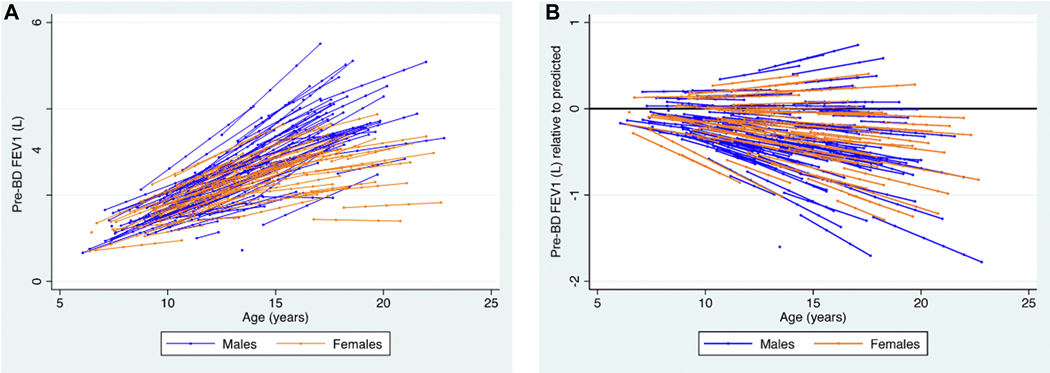
A total of 862 baseline and follow-up observations among 188 participants were available for analysis. The median number of observations per subject was 5 (IQR 3, 6; see Supplemental Table 2 in the Online Repository). The median age at last follow-up was 17.2 years. A plot of fitted FEV1 trajectories by participant is shown in Figure 1a. Figure 1b demonstrates the trajectories adjusted for the predicted value based on GLI reference equation. In total, 76% (655/862) of all observations were below predicted values, and 61% (114/188) of participants had all FEV1 values below predicted.
Figure 1. Participant pre-bronchodilator FEV1 trajectories, by sex.
Fitted model of FEV1 by age and random intercept for subject and random slope for age. Overlaid modeled participant FEV1 trajectories. Each point indicates the age of spirometry assessment X value) by the fitted predictions (i.e., fixed plus random portions) of FEV1 (Y value), with lines connecting these predictions for each participant for individually measured FEV1 observations. Panel A demonstrates the absolute change in FEV1 by age. Panel B depicts the absolute change in FEV1 adjusted for GLI16 predicted FEV1, thereby demonstrating the trajectory relative to the expected by GLI reference equation. The reference line at FEV1=0 indicates the expected FEV1 values as the child grows older. Trajectories did not vary by sex after adjustment for predicted FEV1 (p=0.49).
Determinants of Pre-bronchodilator FEV1
Univariate models identified baseline values of FeNO, post-BD tdFEV1, maximal bronchodilator reversibility, and predictors assessed at each FEV1 observation, termed time-varying, of severe asthma designation, exacerbation rate and obesity as significant factors related to FEV1 (Supplemental Table 3 in the Online Repository). There was a significant three-way interaction effect of exacerbation rate, age, and sex.
Multivariate regression model results are shown in Table 2 for FEV1, Table 3 for maximal post-bronchodilator FEV1 and Supplemental Table 4 for FEV1/FVC. For the primary outcome analysis, baseline FeNO level, post-BD tdFEV1, maximal bronchodilator reversibility of FEV1 and time-varying values of age, obesity, and the interactions of both exacerbations in the prior 12 months, and the 1-year lag exacerbations with age were significantly associated with FEV1 (Table 2, Supplementary figure 1). Severe asthma designation was not associated with FEV1 in adjusted models.
Table 2.
Multivariate model of predictors of longitudinal pre-bronchodilator FEV1 (mL)
| Main effect | Age interaction effect | |||
|---|---|---|---|---|
|
| ||||
| Baseline Predictors | ||||
| FeNO (log2) | −49 [−92,−6]* | - | ||
| Post-BD tdFEV1 | −8.4 [−12.3,−4.5] ‡ | - | ||
| Max BD reversibility FEV1 (Max Post% - Pre%) | −27 [−37,−16] ‡ | 2.5 [1.1,3.8] ‡ | ||
| Max BD reversibility FVC (Max Post% - Pre%) | −0.4 [−11.0,10.2] | - | ||
| Female | 72 [−164,310] | - | ||
| Race/ethnicity (Black=reference) | - | |||
| White | −68 [−192,56] | - | ||
| Hispanic | −66 [−242,110] | - | ||
| Other | 78 [−154,310] | - | ||
| Time-varying Predictors | ||||
| Age | −48 [−87,−9]* | NA | ||
| Severe asthma | 50 [−66,165] | −14 [−27,0.2] | ||
| Exacerbations | Main effect Boys | Age interaction Boys | Main effect Girls | Age interaction Girls |
| 1–2 | 99 [−55,252] | −20 [−39,−2]* | 27 [−148,202] | −7 [−28,14] |
| 3+ | 140 [−77,358] | −30 [−60,0.3] | −7 [−279,265] | 16 [−20,53] |
| Exacerbations 1-year lag | Main effect Boys | Age interaction Boys | Main effect Girls | Age interaction Girls |
| 1–2 | 58 [−85,200] | −9 [−27,9] | 35 [−135,204] | −6 [−27,15] |
| 3+ | 148 [−49,345] | −34 [−61,−7]* | 45 [−180,271] | −1 [−29,26] |
| BMI percentile | ||||
| Overweight | 79 [−75,233] | −10 [−29,9] | ||
| Obese | 185 [27,344]* | −24 [−45,−4]* | ||
| Height | 3.5 [−3.8,10.8] | - | ||
| Predicted FEV1 | 0.94 [0.76,1.12] ‡ | - | ||
P<0.05
P<0.001
Multivariate linear mixed model clustered at the participant level. A random intercept and random slope for age were allowed to correlate. Unit of change for continuous predictors: FeNO, log2; Post-BD tdFEV1, one percent absolute change in percent predicted; Max BD reversibility FEV1, one percent absolute change in percent predicted; Max BD reversibility FVC, one percent absolute change in percent predicted; age, 1 year. Age in years was recoded to be age minus six so that coefficients for age and age interactions could be interpreted in terms of years since age six years (the youngest age at baseline). The interpretation of the main effect can be 1) the effect of the variable at age 6 years if an age interaction term is present, or 2) the effect of the variable at every age if an interaction term is not present.
Table 3.
Multivariate model of predictors of longitudinal post-bronchodilator FEV1 (mL)
| Main effect | Age Interaction effect | |||
|---|---|---|---|---|
|
| ||||
| Baseline Predictors | ||||
| Post-BD tdFEVl Max BD | −7.8 [−10.8,−4.7]‡ | - | ||
| reversibility FEV1 (Max Post% - Pre%) | 3.5 [−3.1,10.1] | 0.3 [−0.8,1.4] | ||
| Female | −34 [−211,143] | - | ||
| Race/ethnicity (Black=reference) | - | |||
| White | 14 [−81,109] | - | ||
| Hispanic | −26 [−160,108] | - | ||
| Other | 68 [−119,256] | - | ||
| Time-varying Predictors | ||||
| Age | −33 [−64,−2]* | NA | ||
| Severe asthma | 51 [−34,135] | −9 [−20,1] | ||
| Exacerbations | Main effect Boys | Age interaction Boys | Main effect Girls | Age interaction Girls |
| 1–2 | 61 [−53,175] | −11 [−25,4] | 72 [−60,205] | −9 [−25,7] |
| 3+ | 100 [−56,255] | −22 [−44,−0.2]* | 38 [−169,245] | 6 [−21,34] |
| Exacerbations 1- year lag | Main effect Boys | Age interaction Boys | Main effect Girls | Age interaction Girls |
| 1–2 | 28 [−78,135] | −6 [−19,7] | 33 [−95,162] | −7 [−22,9] |
| 3+ | 121 [−24,265] | −30 [−51,−10]† | −16 [−187,154] | 6 [−15,27] |
| BMI percentile | ||||
| Overweight | 71 [−43,185] | −7 [−21,7] | ||
| Obese | 140 [25,255]* | −19 [−35,−4]* | ||
| Height Predicted FEV1 | 5.2 [−0.2,10.6] 1.01 [0.87,1.15]† |
- | ||
P<0.05
P<0.01
P<0.001
Multivariate linear mixed model clustered at the participant level. A random intercept and random slope for age were allowed to correlate. Unit of change for continuous predictors: Post-BD tdFEV1, one percent absolute change in percent predicted; Max BD reversibility FEV1, one percent absolute change in percent predicted; age, 1 year. Age in years was recoded to be age minus six so that coefficients for age and age interactions could be interpreted in terms of years since age six years (the youngest age at baseline). The interpretation of the main effect can be 1) the effect of the variable at age 6 years if an age interaction term is present, or 2) the effect of the variable at every age if an interaction term is not present.
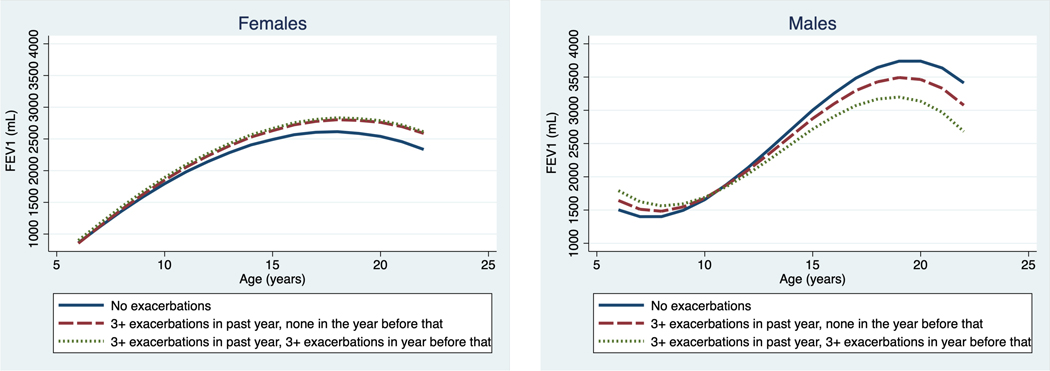
Most notably, we found an interaction of sex with exacerbation rate (Figure 2). There was no effect of exacerbation rate among females. However, males had a 20mL (B = −20mL, 95% CI −39, −2) and 30mL (B = −30mL, 95% CI −60, 0.3) decrease in FEV1 per year if they reported moderate or high exacerbation frequency, respectively, in the prior 12 months. Additionally, we found that boys with high exacerbation frequency in the prior 12 month assessment period had a 34mL decrease in FEV1 per year (B = −34mL, 95% CI −61, −7).
Figure 2. Age dependent change in FEV1 by exacerbation rate in males and females.
There was no significant main effect or interaction with age on female relationship of exacerbation rate with FEV1 (panel A). Male subjects (panel B) demonstrated a significantly lower FEV1 with increasing age associated with recurrent exacerbations in prior year and an additive effect if there were ≥3 exacerbations in the year before that. Solid line, no exacerbations in the past year; dashed line ≥3 exacerbations in the past year, none in the year prior; dotted line, ≥3 exacerbations in the past year, ≥3 exacerbations in the year prior.
Inverse probability weights for the sensitivity analysis were based on the relationship between missingness and baseline BMI, FEV1, FEV1 predicted, exacerbation rate, history of pneumonia, ETS, total IgE, and race. Major findings from the primary analysis were supported. Baseline FeNO, post-BD tdFEV1, maximal bronchodilator reversibility, and the interaction of exacerbations with age among boys only, remained significantly associated with FEV1 (Supplemental Table 5 in the Online Repository).
Post-BD tdFEV1 was inversely associated with FEV1 such that each percent predicted increase in post-BD tdFEV1was associated with an 8.4mL lower FEV1 (B = −8.4mL, 95% CI −12.3, −4.5). This translates to a clinically meaningful 100ml (95% CI −146.4. −53.6) lower FEV1 through childhood for each standard deviation in post-BD tdFEV1 (11.9%). The effect of baseline maximal bronchodilator FEV1 reversibility was an inverse association with predicted FEV1 (B = −27ml/% change in post-BD FEV1, 95% CI −37, −16), but there was a positive interaction with age such that as age increased there was a 2.5mL (95% CI 1.1, 3.8) increase in FEV1 per 1 percent predicted change in baseline maximal bronchodilator FEV1 reversibility. Obesity was also associated with a higher FEV1, but the interaction term demonstrated a decrease in FEV1 with each year increase of age.
Sensitivity analyses individually adding ACT and FeNO measured at each follow-up visit were significantly associated with pre-bronchodilator FEV1 but did not substantially change the effect of the primary model predictors (Supplementary Tables 6 and 7, respectively). The effect of ACT on FEV1 did not significantly vary by age (p=0.59) but was a significant time-varying determinant of FEV1 (beta=42.4, 95% confidence interval=[15.5,69.2], p=0.002. The effect of a time-varying FeNO (−41 [−65,−17], p=0.001) was similar to the effect for the baseline FeNO in our final model (−49 [−92,−6], p=0.02).
Determinants of maximal Post-bronchodilator FEV1
We also evaluated predictive factors for maximal post-bronchodilator FEV1 across the study in order to identify factors that may reflect airway remodeling (Table 3). The maximal post-bronchodilator FEV1 measure minimizes individual variations in FEV1 at each testing session that may be related to diurnal variation or other temporal factors affecting airway tone and represents the greatest airway caliber achievable by airway smooth muscle relaxation. Consistent with the pre-bronchodilator FEV1 outcome model, exacerbation frequency conferred a significantly lower maximal post-bronchodilator FEV1, but only for males with high exacerbation frequency (interaction of ≥ 3 exacerbations in prior 12 months with age at spirometry testing among males B = −22mL, 95% CI −44, −0.2; one-year lag ≥ 3 exacerbations*age at spirometry testing among males B = −30mL, 95% CI −51, −10). Post-BD tdFEV1 was also inversely associated with maximal post-bronchodilator FEV1 such that for each percent predicted increase in post-BD tdFEV1 was associated with a 7.8mL lower maximal post-bronchodilator FEV1 (B = −7.8mL, 95% CI −10.8, −4.7). The effect of obesity was a higher maximal post-bronchodilator FEV1 with an age interaction such that at increased age of testing, obesity was inversely associated with post-bronchodilator FEV1. Age, itself, was also independently associated with a 33mL decrease in maximal post-bronchodilator FEV1(B = −33mL, 95% CI −64, −2).
Determinants of Pre-bronchodilator FEV1/FVC
Age was associated with a small but significant decrease in absolute value of FEV1/FVC (B = −0.009, 95%CI −0.014, −0.004), after adjusting for race, sex, obesity status, height, and predicted FEV1/FVC by GLI reference equation (Supplemental Table 4). Higher pre-BD FEV1/FVC was significantly associated with lower post-BD tdFEV1 (B = −0.001, 95% CI −0.002, −0.0002) and baseline maximal bronchodilator reversibility of FEV1 (B = −0. 008 95%CI −0.010, −0.005). However, the interaction of age at testing with maximal bronchodilator reversibility of FEV1 indicated a small positive association with FEV1/FVC (B= 0.0006, 95% CI 0.0002, 0.0009) indicating that the negative association of bronchodilator reversibility and FEV1/FVC is more pronounced at younger ages.
Discussion
Asthma is one of the most important predictors of low lung function trajectories through childhood and extending into adulthood1. Identifying potentially modifiable risk factors of poor lung function in childhood is paramount to providing optimal care and improving lifelong respiratory health. The SARP3 prospective observational cohort presents a unique opportunity to understand the factors influencing lung function longitudinally across childhood in an extensively phenotyped cohort of children with severe and non-severe asthma. The annual assessments of lung function and clinical phenotyping through childhood are a unique feature of this cohort that supports the novel approach of assessing changing lung function in the context of changing phenotypic measures over time during a period of rapid physical maturation. In this cohort, three quarters of spirometry assessments were less than predicted and the majority of children had all FEV1 values below predicted levels. We identified that change in FEV1 following a single dose of triamcinolone acetonide (a baseline characterization procedure), FeNO, bronchodilator reversibility and age at testing predicted lower FEV1. There was an age interaction with obesity and maximum bronchodilator reversibility among all participants, and a negative impact of exacerbation rate present in boys, but not girls. Asthma severity did not remain statistically significant in our multivariate models, as its effect was attenuated by the inclusion of other variables associated with severity, namely, exacerbation rate. Notably, removing exacerbations from the set of independent variables restored the effect of asthma severity to the size observed in its univariate model predicting FEV1. Therefore, asthma severity should not be dismissed as a useful indicator of lung function decline.. There was no relationship of inhaled corticosteroid dose with lung function outcomes in unadjusted models.
While each of these findings are important, a few merit specific focus. The association of frequent asthma exacerbations and low lung function trajectories has been described8,22, but the male sex-specific association of recent and distant asthma exacerbations with lower lung function over time is a novel finding. Gold and colleagues23 found a greater FEV1 growth deficit in boys with asthma and wheeze compared with girls in the Six Cities Study recruited in the late 1970s. Within asthma cohorts, male sex has been identified as a risk factor for lower lung function trajectories8,23. Differences in hormonal chemistry has been associated with asthma outcomes, including lung function, between boys and girls as they progress through adolescence24, and recent data suggest that airway androgen receptor expression and circulating androgens may be important factors differentiating lung function between men and women25,26. How these differences affect lung function trajectories or the interaction of severe exacerbations and lung function over time remains unknown.
We evaluated the influence of exacerbations in the year prior to the current assessment year, including both the exacerbation rate 12 months prior to lung function assessment and for the year prior to that in our models, to understand whether there is a cumulative effect on lung function. Importantly, we found significant negative effects on lung function for both recent (past 12 months) and 1-year lag exacerbation rate in males. For example, if we look at the typical study participant, a 13 year old black male (158cm tall), a 10% increase in FEV1 following triamcinolone acetonide assessment and 2 exacerbations in the past year and 3 the year prior will have 138ml (approximately 5% predicted16) lower lung function than a 13y.o. black male with no change in FEV1 following triamcinolone and no exacerbations in the prior 2 years. The fact that distant exacerbations maintained a significant association with both pre- and post-bronchodilator FEV1 decrements in males indicates that frequent exacerbations during childhood has long lasting effects on lung function. The negative effect is cumulative which suggests secondary prevention of exacerbations may improve lung function outcomes through childhood. This finding should be studied in prospective intervention trials with long term lung function follow-up.
Among the adult participants in SARP3, insensitivity to a systemic dose of triamcinolone acetonide was a risk factor for severe decline in lung function10. Among pediatric participants, we found the opposite to be true; those with greater change in post-bronchodilator FEV1 assessed a little more than 2 weeks after administration had a significantly lower pre-bronchodilator FEV1 on subsequent annual evaluations. Taken together, the negative effects of greater post-bronchodilator tdFEV1 and FeNO at baseline and annual assessment of maximal bronchodilator reversibility on lung function identifies a high risk group of children with insufficiently treated type 2 inflammation that are at greater risk of impaired lung function over time. It bears mentioning that each of these effects were independent of the effect of exacerbations, indicating that even in the absence of frequent exacerbations in childhood, these markers identify individuals at high risk of low lung function. Sensitivity analysis found that better asthma control, measured by serial ACT at the time of spirometry, and lower levels of FeNO over time, were associated with higher FEV1, suggesting that targeting better symptomatic and reduced airway inflammation over time are likely to be beneficial to long-term lung function. We previously reported on the normal distribution of both pre- and post-bronchodilator change in FEV1 following intramuscular Triamcinolone in this cohort15. In the current analysis, we found only the post-bronchodilator tdFEV1 associated with longitudinal lung function. The post-bronchodilator tdFEV1 minimizes the influence of airway smooth muscle tone and highlights a steroid-responsive change in airway caliber. Addressing ongoing airway inflammation may improve longitudinal lung function during this critical period of growth.
Prebronchodilator spirometry is the most common clinical and population research test followed longitudinally. A maximal post-bronchodilator FEV1 offers a measure of the greatest attainable FEV1 and may offer a different phenotype, one of fixed airflow obstruction, than pre-bronchodilator lung function testing14. Our predictive models for post-bronchodilator lung function demonstrated consistent inverse associations with post-bronchodilator tdFEV1, Maximal bronchodilator reversibility, age, and recent and distant exacerbation rate in boys, but not girls. There was a positive association with obesity that became less pronounced with each successive year of age. In sum, these findings confirm that exacerbations are a concerning predictor of low lung function and that FEV1 improvement following systemic steroid or bronchodilator administration may identify a group of pediatric asthmatics with lower maximal lung function attainment over time.
The ratio of FEV1/FVC is the most sensitive marker for airflow obstruction in children with asthma27 and an essential part of the definition of COPD28. We found that advancing age was a determinant of lower FEV1/FVC, consistent with the natural history of lung function over time. Similar to the other lung function parameters, triamcinolone response at baseline was inversely related to FEV1/FVC over time, supporting airway inflammation as a key driver of airflow obstruction.
Notably, we did not find a significant association between asthma severity, defined as severe or non-severe asthma, in any multivariate lung function outcome model. Ross et al.13 previously found that the severe asthma categorical assignment varies substantially with age in this cohort. Therefore, we utilized the time-varying assignment of asthma severity in our analysis and still did not find a significant association when other, more objectively measured, traits were included in the model. While previous literature has suggested that patients with severe childhood asthma are at highest risk for poor lung function outcomes in adulthood29, here we are able to identify discrete, measurable, variables that are not constrained by the uncertainty of a composite definition of severity, which itself is a construct that can be redefined. The lack of association with categorical asthma severity also implies that patients with non-severe asthma may also be at risk for low lung function through childhood and into adulthood, highlighting the need to be vigilant in identifying lung function impairment in all children with asthma, consistent with national asthma guidelines30. Similar to prior studies31,32, we did not find a significant association between inhaled corticosteroid dose and lung function outcomes, though there was no control population for comparison to adequately evaluate a medication effect.
Assessing lung function trajectories throughout childhood is challenging. Lung growth does not mirror somatic growth, particularly over brief intervals through the adolescent period where maturation and somatic growth are highly variable across individuals18,19. There is no standard measure to assess lung function trajectories in childhood, but it is widely accepted that the FEV1 percent predicted value is inadequate. Some authors3 have selected the FEV1/FVC ratio in order to contextualize FEV1 by lung size, estimated by the vital capacity, but this, too, has limitations; low vital capacity may itself be a sign of poor lung growth or a reflection of poor airflow or airway closure which would lead to an overestimation of lung function. We chose the absolute value of FEV1 and included several covariates and utilized mixed effects models to capture the breadth of variability of the outcome at each timepoint. The choice of FEV1 also retains the clinical relevance to adult lung function trajectories and risk of COPD which is primarily mapped to this outcome8,20. We included GLI predicted value as a separate covariate to adjust for expected outcome based on the most commonly used clinical reference equations, which makes the interpretation of each variable as the effect beyond what would be expected. Within our analysis we determined factor-by-age effects which serves as a surrogate for developmental changes throughout childhood. We lacked complete data on pubertal development for subjects which may have offered more information regarding lung function changes by physical maturation during adolescence. One of the strengths of this study is the extensive clinical research assessments carried out in conjunction with the lung function measures annually across several years of follow-up. Nevertheless, in the context of lung growth and development the length of observation is relatively short and we are unable to clearly assess the predictors of maximally achieved adult lung function. The nature of the study design with repeated measures over several years and potential incomplete observations for some participants risks under-representation of observations from some individuals. Our sensitivity analysis utilizing inverse-probability weighting to address non-random loss to follow-up supported the main findings of the primary analysis. Both the clustered linear mixed model methods in the primary models which minimize sampling bias and the supportive sensitivity analysis affirm confidence in the results. Further, while environmental tobacco smoke was included in the analysis at baseline, it is possibly that changes is smoke exposure status over time, or influences from other environmental exposures may have influenced the findings of this study.
In summary, children with asthma are at risk for poor lung function trajectories into adulthood. We identified post-bronchodilator tdFEV1, FeNO, maximum bronchodilator reversibility, and obesity predict lower FEV1 in a highly phenotyped cohort of children and adolescents with severe and non-severe asthma and also describe a novel sex-specific risk of exacerbations on lower lung function in boys. These findings may offer a framework of clinically-available measures that can identify children with asthma who are at risk of lung function impairment over time and a potential for future intervention studies aiming to improve lung function in at-risk children.
Supplementary Material
Clinical Implications.
This article identifies clinically-available tools to predict lower lung function longitudinally among children with asthma.
Funding:
This work was supported by NHLBI to the Severe Asthma Research Program 3 (SARP3) Principal Investigators, Clinical Centers, and Data Coordinating Center as follows: U10 HL109164 (E.R.B., D.A.M. and W.C.M.), U10 HL109257 (M.C.), U10 HL109250 (S.C.E.), U10 HL109146 (J.V.F.), U10 HL109250 (B.G.), U10 HL109172 (E.I. and B.D.L.), U10 HL109168 (N.N.J.), U10 HL109250 (W.G.T.), U10 HL109152 (S.E.W.), and U10 HL109086-04 (D.T.M.). In addition, this program is supported through the following National Institutes of Health National Center for Advancing Translational Sciences awards: UL1 TR001420 (Wake Forest University), UL1 TR000427 (University of Wisconsin), UL1 TR001102 (Harvard University), and UL1 TR000454 (Emory University). The following companies provided financial support for study activities at the Coordinating and Clinical Centers beyond the third year of patient follow-up: AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Sanofi-Genzyme-Regeneron, and TEVA. These companies had no role in study design or data analysis, and the only restriction on the funds was that they be used to support the SARP initiative.
Author disclosures:
JMG receives grant support from NIEHS, Vertex pharmaceuticals, personal fees from Syneos health outside of the submitted work.
CRP reports no financial disclosures outside the submitted work.
RLS reports no financial disclosures outside the submitted work.
LCD reports grants from the NHLBI and American Lung Association / Asthma Clinical Research Centers, during the conduct of the study; and other considerations from AstraZeneca, Boehringer Ingelheim, Genentech, GSK, Sanofi-Genzyme-Regeneron, and Teva, outside the submitted work.
BRP reports no financial disclosures outside the submitted work.
NPL reports grants from Vertex, grants from Gilead, outside the submitted work.
BG is funded by NHLBI, but has no other conflicts relevant to this publication.
KR reports grants from Astra Zeneca, grants from Glaxo Smith Kline, grants from Boehringer Ingelheim, grants from Novartis, grants from Idorsia, personal fees from Sanofi, outside the submitted work.
AF reports grants from National Institutes of Health, during the conduct of the study.
LBB reports grants from NIH/NIAID and NHLBI, personal fees from GlaxoSmithKline Genentech/Novartis, DBV Technologies, Teva, Boehringer Ingelheim, AstraZeneca, WebMD/Medscape, Sanofi/Regeneron, Vectura, Circassia, Kinaset, Vertex; and royalties from Elsevier outside the submitted work.
MDD reports no financial disclosures outside the submitted work.
WGT reports no financial disclosures outside the submitted work.
SEW reports grants from Multiple companies provided broad support to SARP, during the conduct of the study; grants and personal fees from AstraZeneca, grants and personal fees from Novartis, personal fees from GSK, grants and personal fees from Knopp, personal fees from Sanofi, grants from Regeneron, non-financial support from Aer Therapeutics, outside the submitted work.
SR reports consulting fees from Sanofi.
EI reports grants from the NIH, Patient-Centered Outcomes Research Institute (PCORI), and Gossamer Bio; grants and nonfinancial support from Circassia; grants and personal fees from AstraZeneca, Avillion, and Novartis; personal fees and nonfinancial support from Genentech, GlaxoSmithKline (GSK), and Teva; personal fees from AB Science, Allergy and Asthma Network, Amgen, Arrowhead Pharmaceuticals, Biometry, Equillium, Merck, Pneuma Respiratory, the National Heart, Lung, and Blood Institute (NHLBI), PPS Health, Regeneron, Sanofi Genzyme, Sienna Biopharmaceutical, Teva, and Cowen; nonfinancial support from Boehringer Ingelheim; and other considerations from Vorso, outside the submitted work.
DTM reports no financial disclosures outside the submitted work.
WP reports grant and clinical trial support from NIH, Genentech, Novartis, Astra Zeneca, Regeneron, GSK, Merck, Sanofi; Consulting fees from Genentech, Novartis, Astra Zeneca, Regeneron, GSK, Merck, Sanofi, Teva.
Each author (JMG, CRP, RLS, LCD, BRP, NPL, BG, KR, AF, LBB, MDD, WGT, SEW, SR, EI, DTM and WP) report the following companies provided financial support for study activities at the Coordinating and Clinical Centers beyond the third year of patient follow-up: AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Sanofi–Genzyme–Regeneron, and TEVA. These companies had no role in study design or data analysis, and the only restriction on the funds was that they be used to support the SARP initiative.
Abbreviations:
- ATS
American Thoracic Society
- BD
bronchodilator
- GLI
Global Lung Index reference values
- BMI
Body Mass Index
- CAMP
the Childhood Asthma Management Program
- COPD
chronic obstructive pulmonary disease
- ERS
European Respiratory Society
- ETS
environmental tobacco smoke
- FeNO
fractional exhaled nitric oxide
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- ICS
Inhaled corticosteroids
- IgE
immunoglobulin E
- SARP 3
Severe Asthma Research Program 3
- SD
standard deviation
- tdFEV1
Triamcinolone Acetate (TA) - induced difference from baseline FEV1 (post-TA FEV1 % predicted – pre-TA FEV1% predicted)
- VC
vital capacity
Footnotes
ClinicalTrials.gov identifier NCT01606826
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinez FD. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N Engl J Med. 2016/09/01. 2016;375:871–8. [DOI] [PubMed] [Google Scholar]
- 2.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018/04/10. 2018;6:535–44. [DOI] [PubMed] [Google Scholar]
- 3.Berry CE, Billheimer D, Jenkins IC, Lu ZJ, Stern DA, Gerald LB, et al. A Distinct Low Lung Function Trajectory from Childhood to the Fourth Decade of Life. Am J Respir Crit Care Med [Internet]. 2016/09/02. 2016 [cited 2020 Sep 30];194:607–12. Available from: /pmc/articles/PMC5027213/?report=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belgrave DCM, Granell R, Turner SW, Curtin JA, Buchan IE, Le Souef PN, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med [Internet]. 2018/04/10. 2018;6:526–34. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29628377 [DOI] [PubMed] [Google Scholar]
- 5.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med [Internet]. 2005;172:1253–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16109980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai A, Tran H, Roberts M, Clarke N, Gibson AM, Vidmar S, et al. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol. 2014/02/06. 2014;133:1572–8.e3. [DOI] [PubMed] [Google Scholar]
- 7.Ödling M, Wang G, Andersson N, Hallberg J, Janson C, Bergström A, et al. Characterization of asthma trajectories from infancy to young adulthood. J allergy Clin Immunol Pract. 2021; [DOI] [PubMed] [Google Scholar]
- 8.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of Growth and Decline in Lung Function in Persistent Childhood Asthma. N Engl J Med [Internet]. 2016;374:1842–52. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27168434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J [Internet]. 2013/12/18. 2014;43:343–73. Available from: https://www.ncbi.nlm.nih.gov/pubmed/24337046 [DOI] [PubMed] [Google Scholar]
- 10.Denlinger LC, Phillips BR, Sorkness RL, Bleecker ER, Castro M, DeBoer MD, et al. Responsiveness to Parenteral Corticosteroids and Lung Function Trajectory in Adults with Moderate to Severe Asthma. Am J Respir Crit Care Med. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almqvist C, Worm M, Leynaert B. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63:47–57. [DOI] [PubMed] [Google Scholar]
- 12.Yung JA, Fuseini H, Newcomb DC. Hormones, sex, and asthma. Ann allergy, asthma Immunol Off Publ Am Coll Allergy, Asthma, Immunol. 2018;120:488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross KR, Gupta R, DeBoer MD, Zein J, Phillips BR, Mauger DT, et al. Severe asthma during childhood and adolescence: A longitudinal study. J Allergy Clin Immunol. 2020;145:140–146.e9. [DOI] [PubMed] [Google Scholar]
- 14.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline Features of the Severe Asthma Research Program (SARP III) Cohort: Differences with Age. J allergy Clin Immunol Pract [Internet]. 2018;6:545–554.e4. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28866107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, et al. Effects of Age and Disease Severity on Systemic Corticosteroid Responses in Asthma. Am J Respir Crit Care Med [Internet]. 2017;195:1439–48. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27967215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J [Internet]. 2012/06/30. 2012;40:1324–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22743675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J [Internet]. 2005;26:319–38. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16055882 [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal M, Bain SH, Cramer D, Helms P, Denison D, Bush A, et al. Lung function in white children aged 4 to 19 years: I--Spirometry. Thorax. 1993;48:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, et al. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: a longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med. 2002/06/05. 2002;165:1480–8. [DOI] [PubMed] [Google Scholar]
- 20.Covar RA, Spahn JD, Murphy JR, Szefler SJ. Progression of asthma measured by lung function in the childhood asthma management program. Am J Respir Crit Care Med. 2004; [DOI] [PubMed] [Google Scholar]
- 21.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd Editio. Wiley; 2011. [Google Scholar]
- 22.Bui DS, Walters HE, Burgess JA, Perret JL, Bui MQ, Bowatte G, et al. Childhood Respiratory Risk Factor Profiles and Middle-Age Lung Function: A Prospective Cohort Study from the First to Sixth Decade. Ann Am Thorac Soc. 2018;15:1057–66. [DOI] [PubMed] [Google Scholar]
- 23.Gold DR, Wypij D, Wang X, Speizer FE, Pugh M, Ware JH, et al. Gender- and race-specific effects of asthma and wheeze on level and growth of lung function in children in six U.S. cities. Am J Respir Crit Care Med. 1994;149:1198–208. [DOI] [PubMed] [Google Scholar]
- 24.DeBoer MD, Phillips BR, Mauger DT, Zein J, Erzurum SC, Fitzpatrick AM, et al. Effects of endogenous sex hormones on lung function and symptom control in adolescents with asthma. BMC Pulm Med [Internet]. 2018/04/11. 2018;18:58. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29631584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaston B, Marozkina N, Newcomb DC, Sharifi N, Zein J. Asthma Risk Among Individuals With Androgen Receptor Deficiency. JAMA Pediatr. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zein JG, McManus JM, Sharifi N, Erzurum SC, Marozkina N, Lahm T, et al. Benefits of Airway Androgen Receptor Expression in Human Asthma. Am J Respir Crit Care Med. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ, et al. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol [Internet]. 2006;118:1040–7. Available from: https://www.ncbi.nlm.nih.gov/pubmed/17088127 [DOI] [PubMed] [Google Scholar]
- 28.2022. GOLD Reports - Global Initiative for Chronic Obstructive Lung Disease - GOLD [Internet]. [cited 2022 May 11]. Available from: https://goldcopd.org/2022-gold-reports-2/
- 29.Tai A, Tran H, Roberts M, Clarke N, Wilson J, Robertson CF. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax [Internet]. 2014/03/22. 2014;69:805–10. Available from: https://www.ncbi.nlm.nih.gov/pubmed/24646659 [DOI] [PubMed] [Google Scholar]
- 30.National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol [Internet]. 2007;120:S94–138. Available from: https://www.ncbi.nlm.nih.gov/pubmed/17983880 [DOI] [PubMed] [Google Scholar]
- 31.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med [Internet]. 2006;354:1985–97. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16687711 [DOI] [PubMed] [Google Scholar]
- 32.Strunk RC, Sternberg AL, Szefler SJ, Zeiger RSB, Tonascia J. Long-term budesonide or nedocromil treatment, once discontinued, does not alter the course of mild to moderate asthma in children and adolescents. J Pediatr. 2009;154:682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.