Abstract
Both musculoskeletal pain and sleep disturbances are major health problems worldwide. Literature suggests that the two are reciprocally related and both may be associated with changes in C-reactive protein (CRP) levels. However, the relationships among musculoskeletal pain, sleep duration, and CRP remain unclear.
In this cross-sectional study, we investigated the relationship between acute and chronic musculoskeletal pain, sleep, and inflammation using the data from the initial visit of the UK Biobank. 17,642 individuals with chronic musculoskeletal pain, 11,962 individuals with acute musculoskeletal pain, and 29,604 pain-free controls were included in the analysis. In addition, we validated the findings using data from the second visit assessment of the UK Biobank.
We found that 1) chronic pain was associated with higher CRP levels compared to both acute pain and the pain-free controls; 2) chronic pain was associated with a lower sleep score (a measurement of sleep patterns), compared to acute pain and the pain-free controls; and acute pain was associated with lower sleep scores compared to the controls; 3) there was a significant negative association between the sleep score and CRP; 4) CRP may partially mediate the association between chronic pain and decreased sleep score. However, the effect size of the mediation was very small, and the pathophysiological significance remains uncertain. Further validation is needed. These findings were partly replicated in the UK Biobank second visit assessment cohort with a smaller sample size. Our findings, which are based on the large UK Biobank dataset, support the interplay between musculoskeletal pain, sleep patterns, and the potential mediating role of CRP on this reciprocal relationship.
Keywords: musculoskeletal pain, sleep disturbance, C-reactive protein, acute pain, chronic pain
INTRODUCTION
Both musculoskeletal pain and sleep disturbances are major health problems worldwide, with profound negative impact on quality of life, general health, and socio-economic well-being1–3.
Literature suggests that sleep and pain are reciprocally related and interact with each other. Pain causes sleep disruption, and sleep loss exacerbates pain4,5. Sleep disturbance may play an important role in pain modulation processes. Numerous studies have shown that elevation of spontaneous and evoked pain is promoted by sleep deprivation5–12. Epidemiological studies have shown that sleep disturbance, e.g., insomnia, is highly prevalent among people with chronic pain13, and individuals with insomnia are more likely to report chronic low back pain14.
Additionally, inflammatory processes are dysregulated in people with sleep disturbances and contribute to pain sensitivity15. Inflammation may play an important role in the development and maintenance of musculoskeletal pain as well as sleep disturbance5. For example, people with increased levels of inflammation are more likely to report chronic low back pain14. Inflammation is typically measured by inflammatory markers such as C-reactive protein (CRP), tumor necrosis factor alpha (TNFα), and interleukin 6 (IL-6). Conceptual models of relationships between insomnia, pain, and inflammation suggest that insomnia could increase basal inflammation, which could then lead to increased pain5,16,17. Self-reported poor sleep quality similarly is associated with elevated inflammatory markers11,18,19.
Musculoskeletal pain is typically categorized in two states: acute pain, lasting less than three months, and chronic pain, lasting three months or longer20,21. While there is growing interest in understanding how acute pain develops into chronic pain, the role of inflammation and sleep in the transition from acute to chronic pain remains unclear. Although there are studies about the relationship between inflammatory markers and different states of pain, sample sizes of those studies are relatively small, which could largely affect the reliability of conclusions that were drawn from them22.
The UK Biobank is a large-scale biomedical database, which includes many measurements and self-reported features for over 500,000 adult participants from the Great Britain area. Recently, investigators have started using the UK Biobank to investigate chronic pain, including the contributions of genetic factors, and have produced some interesting findings23–25.
Leveraging the rich data collected by the UK Biobank26, the cross-sectional study reported here aims to better understand the relationship among acute and chronic musculoskeletal pain, sleep, and inflammation. We specifically focus on the associations between the presence of self-reported acute and chronic musculoskeletal pain, self-reported sleep behaviors, and CRP serum levels. We examined data from age- and sex-matched participants with no pain as controls. We first established that there were differences in CRP levels and high-risk sleep patterns between each group. We then performed a mediation analysis to investigate the potential role of CRP as a mediator of bi-directional associations between pain and sleep patterns. We hypothesize that 1) both chronic and acute musculoskeletal pain are associated with increased sleep disturbances and CRP levels, and 2) CRP mediates the association between musculoskeletal pain and sleep disturbances.
METHODS
UK Biobank
The data used in this study were obtained from the UK Biobank26. The UK Biobank has ethics approval from the North West Multi-Centre Research Ethics Committee (MREC). Further details on the UK Biobank Ethics and Governance framework are available at: https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics. Data used in this study were obtained under application #32568.
Briefly, the UK Biobank recruited over 500,000 participants aged 40–70 years across Wales, England, and Scotland from 2006–2010. People who were identified from NHS patient registers according to being aged 40–69 and living within a reasonable travelling distance of an assessment centre were invited to participate. Around 5 million invitations were sent to recruit roughly 500,000 participants (https://www.ukbiobank.ac.uk/media/0xsbmfmw/egf.pdf). All participants provided informed consent for access to their medical records and health information for research purposes. In the initial assessment, participants provided detailed information on lifestyle, health, physical measures, and demographics, as well as blood, urine, and saliva samples. We focused primarily on three measures, including sleep behavior, CRP levels, and musculoskeletal pain. We repeated this examination using second visit data collected between 2012 and 2013 in a sample of approximately 20,000 individuals; this examination served to validate findings from the larger set of initial visit data. For more detailed information about the UK Biobank, please refer to the study website at www.ukbiobank.ac.uk.
Inclusion/exclusion criteria
We excluded participants with any psychological or neurological disorder captured by ICD-10 or ICD-9 diagnosis, a diagnosis of diabetes from a physician, or other self-reported long-standing illness, disability, or infirmity. Next, we excluded individuals who had: 1) missing data for any of the variables we analyzed or used as covariates, or 2) uninterpretable responses (e.g. “do not know” and “prefer not to answer”) to any of the variables we analyzed or used as covariates. After these exclusions and quality control checks, we created the musculoskeletal pain and pain-free control groups.
Group assignment – musculoskeletal pain classification
The present study examined individuals with chronic musculoskeletal pain, acute musculoskeletal pain, and pain-free controls. Chronic musculoskeletal pain is defined as pain that persists or recurs for more than 3 months21. To define these groups, pain-related questions in the UK Biobank touchscreen questionnaire were used. Question 1: “In the last month have you experienced any of the following that interfered with your usual activites? (You can select more than one answer)”. The choices included headache, facial pain, neck or shoulder pain, back pain, stomach or abdominal pain, hip pain, knee pain, pain all over the body, none, or prefer not to answer. Question 2: for each type of pain selected, participants were then asked if they “have had that pain for more than 3 months”. We included participants in the chronic pain group if they indicated having “neck or shoulder pain”, “back pain”, “hip pain”, or “knee pain” for at least three months and selected no additional sites of pain. We included participants in the acute pain group if they reported having “neck or shoulder pain”, “back pain”, “hip pain”, or “knee pain” at the time of the initial assessment but indicated the pain was not present for three or more months. A sample of individuals who self-reported no pain was used to create a 1-to-1 control group that was matched on the sex and age of each individual in the acute pain group and then the chronic pain group, both of which were combined to create one control group. A summary of the sample size and demographics for each group can be found in Table 1.
Table 1.
Group Characteristics. (mean ±SD)
| Group | n | Age | Sex (female) | CRP (mg/L) | Sleep score |
|---|---|---|---|---|---|
| Chronic pain -initial assessment cohort | 17642 | 56.24 ± 8.04 | 8586 (48.7%) | 2.18 ± 3.73 | 3.23 ± 0.94 |
| Acute pain -initial assessment cohort | 11962 | 55.08 ± 8.21 | 5348 (44.7%) | 2.04 ± 3.45 | 3.32 ± 0.95 |
| Pain-free controls -- initial assessment cohort | 29604 | 55.77 ± 8.13 | 13934 (47.1%) | 1.95 ± 3.42 | 3.39 ± 0.94 |
| Chronic pain – second visit assessment cohort | 669 | 61.10 ± 7.40 | 303 (45.3%) | 2.26 ± 4.98 | 3.25 ± 0.98 |
| Acute pain – second visit assessment cohort | 494 | 60.08 ± 7.84 | 207 (41.9%) | 2.07 ± 4.56 | 3.30 ± 0.91 |
| Pain-free controls – second visit assessment cohort | 1163 | 60.67 ± 7.60 | 510 (43.9%) | 1.85 ± 3.99 | 3.38 ± 0.91 |
Variables of Interest and Covariates
CRP (mg/L) was measured by immunoturbidimetric - high sensitivity analysis on a Beckman Coulter AU5800 using blood that collected from the UK Biobank initial visit.
Sleep behaviors, including sleep duration, chronotype, insomnia, snoring, and daytime sleepiness, were self-reported in a sleep-related questionnaire. The questions were as follows: “About how many hours sleep do you get in every 24 hours? (please include naps), “Do you consider yourself to be (choices of ‘Definitely a morning person’, ‘More a morning than evening person’, ‘More an evening than a morning person’, ‘Definitely an evening person’, ‘Do not know’, ‘Prefer not to answer’)?”, “Do you have trouble falling asleep at night or do you wake up in the middle of the night?”, “Does your partner or a close relative or friend complain about your snoring?”, and “How likely are you to doze off or fall asleep during the daytime when you don’t mean to? (e.g. when working, reading or driving)”. Based on previous studies27–29, we used sleep scores to quantify healthy sleep patterns. Low-risk sleep behaviors were defined as sleeping 7–8 h/day, early chronotype (“morning” or “morning than evening”), report of never or rarely having insomnia symptoms, no self-reported snoring, and no excessive daytime sleepiness (“never/rarely” or “sometimes”). For each sleep factor, low risk was coded as 1 and high risk as 0; a five-point scale were used to generate a sleep score ranging from 0 to 5, with higher scores corresponding to a healthier sleep pattern. Participants who indicated “do not know” or “prefer not to answer” were excluded from the analysis.
Sex, age, physical activity, body mass index (BMI), smoking status, and income were included as potential confounding variables; these factors have been shown to affect CRP levels and sleep behaviors14. Sex and age were acquired from the central registry at recruitment, but in some cases were updated by the participant. Physical activity was measured using summed Metabolic Equivalent Task minutes per week for all activity, derived from Metabolic Equivalent Task scores data based on IPAQ (International Physical Activity Questionnaire) guidelines. BMI was calculated from body composition estimated by impedance measurements. Smoking status and average total household income before tax were self-reported.
Statistical Analysis
Descriptive statistics were used to identify the mean and standard deviation of CRP and sleep score for each group. Hedges’ g was used to calculate effect size. Differences across groups in CRP levels and sleep scores were assessed using analysis of covariance (ANCOVA) with type II sum of squares, adjusting for sex, age, physical activity, body mass index (BMI), smoking status, and income. A post-hoc Tukey’s HSD test was used to assess differences between specific groups. Finally, we examined the relationship between sleep scores and CRP levels across the groups, and within each group using Pearson’s correlation. In addition, we repeated these analyses in the second visit assessment cohort.
To further explore the relationships between pain, sleep quality, and CRP levels, we performed a mediation analysis30 with 1000 bootstrapping iterations to determine whether CRP mediates the association between musculoskeletal pain and sleep score when comparing chronic pain vs. controls and comparing acute pain vs. controls. Since our data is cross-sectional, we performed the analysis first using pain as the independent variable and sleep score as the response variable, and then with sleep score as the independent variable and pain as the responses, and compared these results31,32.
We performed the mediation analysis only when the association between the independent and dependent variable, and the association between the mediator and the independent and dependent variables were significant based on the ANCOVA post-hoc analyses. The schematic representation of the mediation analyses is shown in Figure 1. We also repeated the same mediation analyses using only age and sex as covariates, as the other covariates may vary over time.
Figure 1.
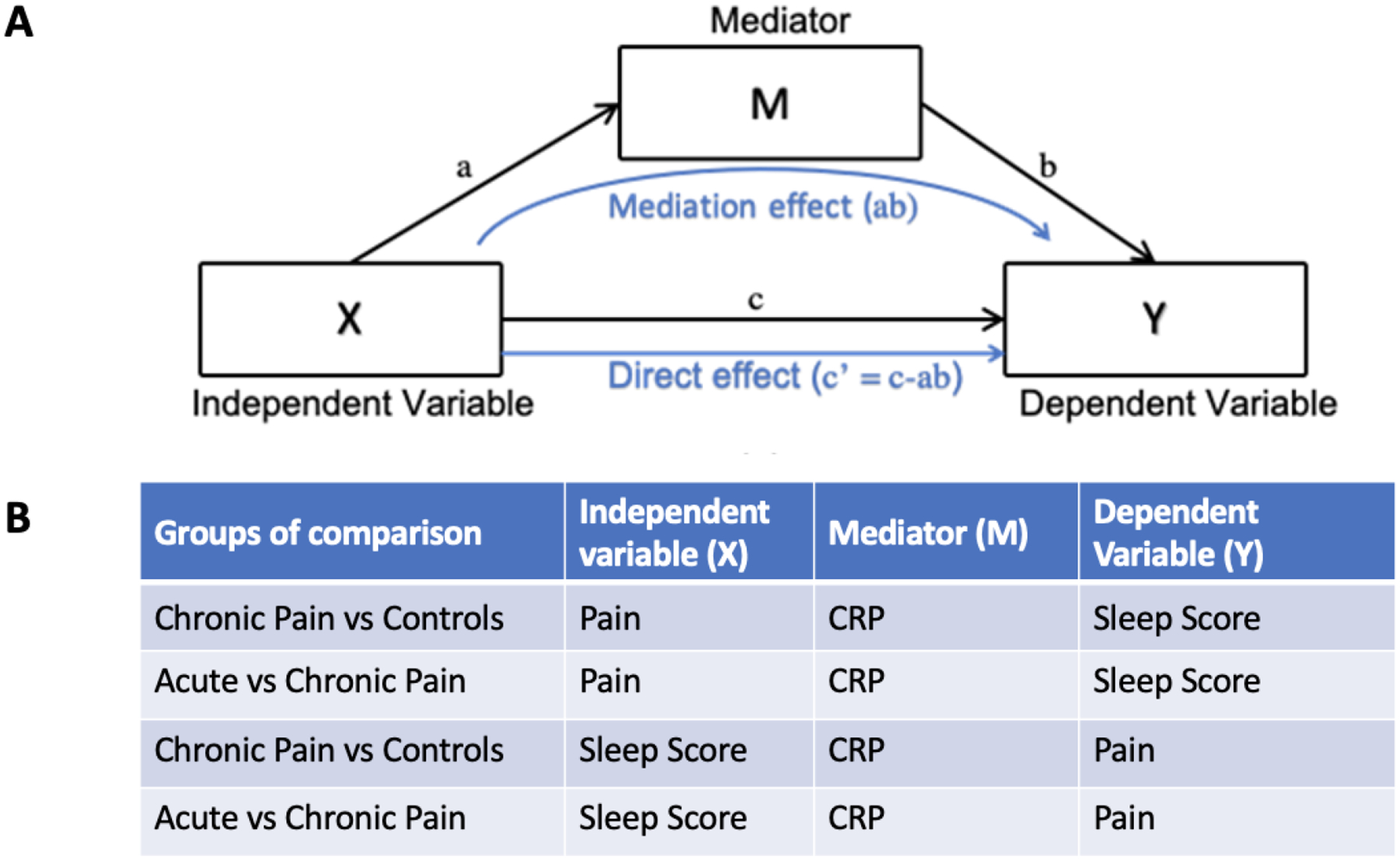
The schematic representation of mediation analysis (A), and the four mediation analyses we performed on sleep, pain, and CRP variables (B).
In all analyses, p values less than or equal to 0.05 (p≤0.05) were considered statistically significant. We used the programming language R for data analysis and performed statistical analyses using R studio with packages ‘car’, ‘psych’, ‘mediation’, ‘ggpubr’, and ‘ggplot2’33.
RESULTS
Participants in each group
Among 240,768 participants in the initial assessment, 17,642 individuals reported chronic musculoskeletal pain, 11,962 individuals reported acute musculoskeletal pain, and 29,604 pain-free individuals matched to the two pain groups reported no pain (Table 1). Thus, 59,208 individuals comprised the total sample with 47.1% female and a mean age of 55.77 years. Age and sex characteristics of each group can be found in Table 1. More information about the number of participants excluded can be found in Figure 2.
Figure 2.
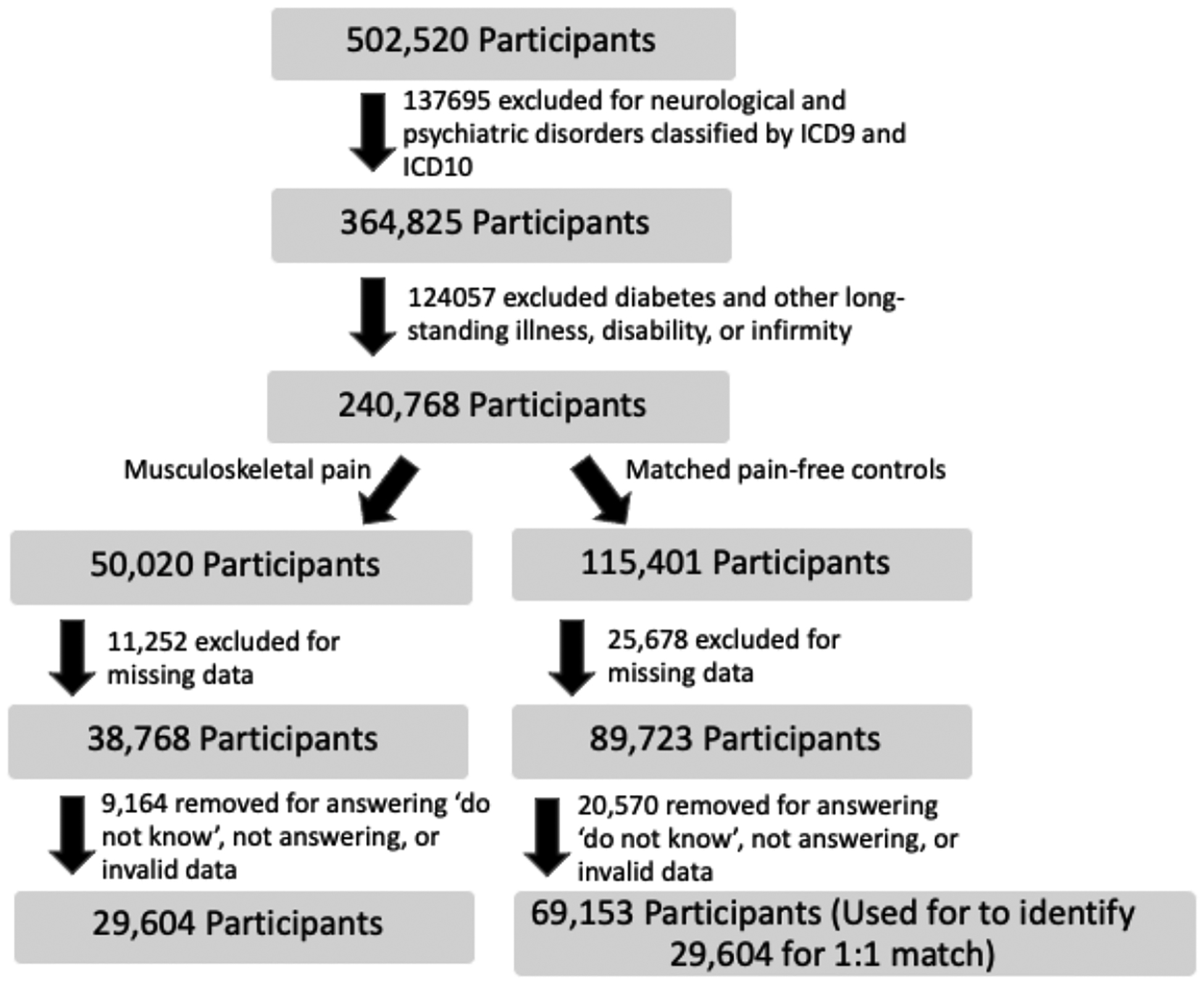
Flow Chart of dropout and exclusion numbers
In the validation analysis using second visit data, 669 individuals reported chronic pain, 494 individuals reported acute pain, and the matched pain-free control group contained 1,163 individuals. The entire cohort consisted of 2,326 individuals with 43.9% female and a mean age of 60.67 years. 887 of these participants were in the initial assessment cohort, of which 44 had their acute pain in the initial assessment transition to chronic pain in the second visit.
Associations between musculoskeletal pain, CRP, and sleep patterns
Musculoskeletal pain and CRP
The data showed a significant association between pain groups and CRP levels when adjusting for sex, age, physical activity, body mass index (BMI), smoking status, and income (p = 0.013). Post-hoc analysis revealed that chronic pain was associated with higher CRP levels, compared to the acute pain group and the pain-free control group (p ≤ 0.001, effect size: 0.04 and 0.07, respectively). There was no significant difference in mean CRP levels between the acute pain group and the pain-free controls (p = 0.062, effect size: 0.03). The mean and standard deviation of CRP levels for each group are summarized in Table 1. The CRP levels (mean and standard error of the mean) for each group are also shown in Figure 3.
Figure 3.
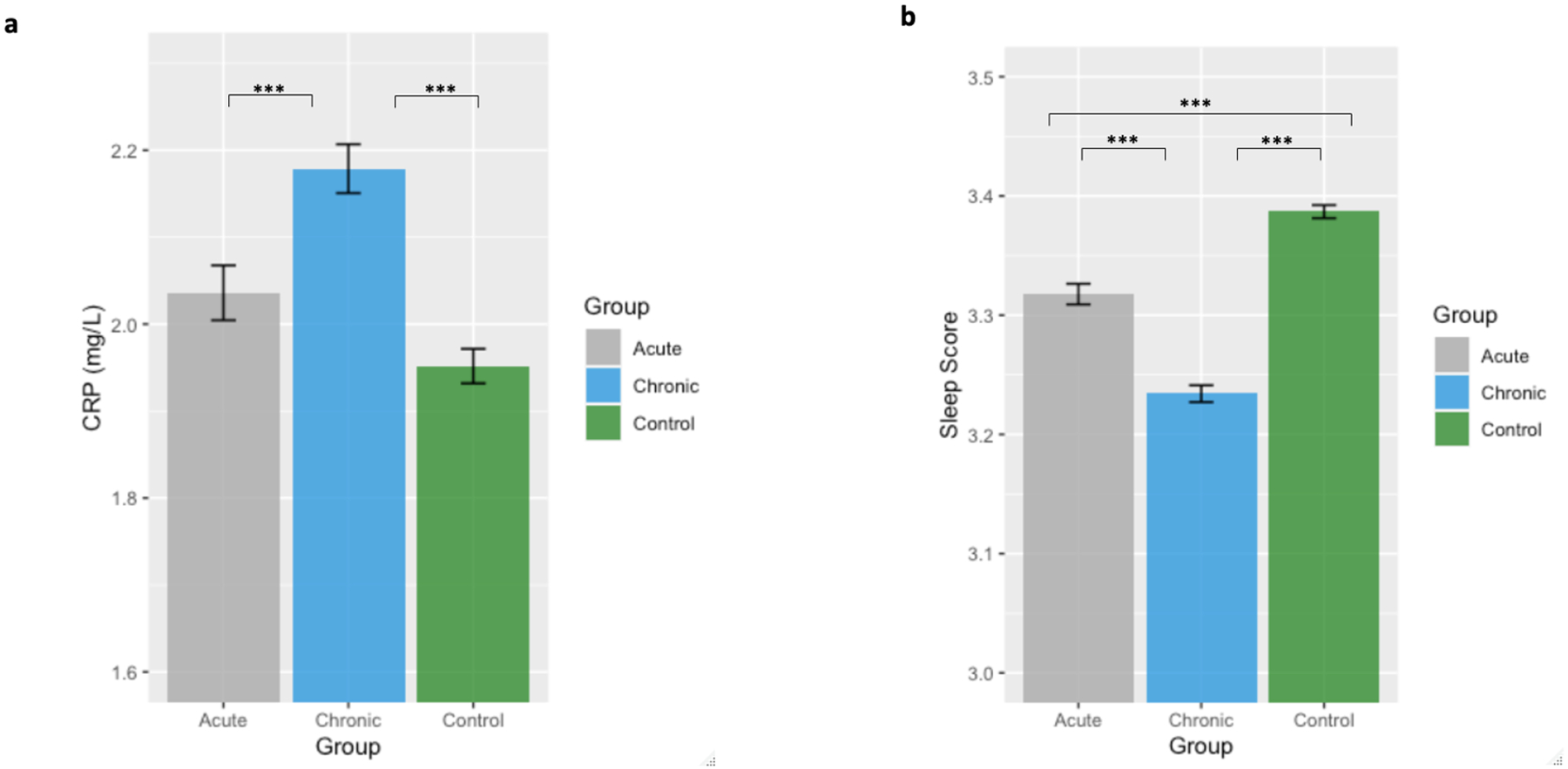
Mean and standard errors of the mean for CRP (a) and sleep score (b) values for each group. Pain and control groups in each plot represent participants with the corresponding type of pain and their matched controls, respectively. Significance codes are: ‘***’ ≤ 0.001; ‘**’ ≤ 0.01; ‘*’ ≤ 0.05.
In second visit cohort, the association between pain groups and CRP levels was not significant (p = 0.426). However, the trends and effect sizes were similar to that of the initial assessment results for the larger cohort. The effect size was 0.04 when comparing chronic vs. acute pain, 0.09 when comparing chronic pain vs. pain-free controls, and 0.05 when comparing acute pain vs. pain-free controls. The mean and standard deviation of CRP levels are presented in Table 1.
Musculoskeletal pain and sleep score
In the multivariable adjusted analysis, there was a significant group effect for sleep scores when adjusting for sex, age, physical activity, body mass index (BMI), smoking status, and income (p < 0.001). Tukey’s tests indicated that chronic pain was associated with lower sleep scores compared to the acute pain group and the pain-free control group (p < 0.001, effect size: 0.10 and 0.17, respectively). Acute pain was also associated with lower sleep scores, compared to the controls (p < 0.001, effect size: 0.03). The mean and standard deviation of the sleep scores for each group are summarized in Table 1. The sleep scores (mean and standard error of the mean) for each group is also shown in Figure 3.
Analyses of the second visit assessment cohort confirmed a significant group effect for sleep scores when adjusting for sex, age, physical activity, body mass index (BMI), smoking status, and income (p = 0.021). Tukey’s tests revealed that the chronic pain group had higher risk sleep patterns, as indicated by sleep scores, compared to pain-free controls (p = 0.007, effect size: 0.14); however, there was no significant difference between the acute pain group and the chronic pain and pain-free control groups, (p=0.655, effect size: 0.05; p = 0.185, effect size: 0.09, respectively). The mean sleep scores and standard deviations are presented in Table 1.
Association between Sleep score and CRP
Lower sleep scores were associated with higher CRP levels across groups and within each group (all groups: p < 0.001, r = −0.05; chronic pain: p < 0.001, r = −0.04; acute pain: p < 0.001, r = −0.06; controls: p < 0.001, r = −0.04), indicating that higher risk sleep patterns are associated with more severe inflammation.
The validation analysis using second visit cohort confirmed that higher CRP levels were associated with higher risk sleep patterns (p = 0.037, r = −0.04) across groups. Although correlations were not significant within the groups, the trend of the effect remained consistent (chronic pain: p = 0.107, f = −0.06, acute pain: p = 0.269, r = −0.05, controls: p = 0.493, r = −0.02).
Mediation analysis
We investigated if CRP was a mediator between pain and sleep score by comparing the chronic musculoskeletal pain group vs pain-free controls, as well as chronic pain vs acute pain groups, adjusting for sex, age, BMI, physical activity, smoking status, and income. The analysis indicated that CRP partially mediated the association between chronic pain (independent) and decreased sleep score (dependent) (p = 0.04). This partial mediation was also significant in the reverse direction (with chronic pain as dependent and decreased sleep score as independent) (p = 0.02). However, the mediation effect sizes were small (of the order of 1/1000) compared to the direct effect. When comparing the chronic and acute pain groups, CRP did not significantly mediate the relationship between sleep score and pain (p = 0.27). The total association, direct association, and mediation effects are presented in Table 2. Note that we did not investigate the mediation effect between acute pain and pain free controls as there was no significant difference between the acute pain and pain-free controls for CRP level (p = 0.062).
Table 2.
Mediation Analysis Results adjusting for sex, age, BMI, physical activity, smoking status, and income
| Groups of comparison | Independent variable (X) | Mediator (M) | Dependent variable (Y) | Total effect (c) | Direct effect (c’) | Mediation effect (ab) |
|---|---|---|---|---|---|---|
| Chronic Pain vs Controls | Pain | CRP | Sleep Score | 0.129 [0.112, 0.15] *** | 0.128 [0.111, 0.15] *** | 2.65e-4 [9.31e-6, 0] * |
| Chronic Pain vs Controls | Sleep Score | CRP | Pain | 0.035 [0.03, 0.04] *** | 0.035 [0.03, 0.04] *** | 7.23e-5 [6.63e-6,0] * |
| Acute vs Chronic Pain | Pain | CRP | Sleep Score | −0.074 [−0.095, −0.05] *** | −0.074 [−0.095, −0.05] *** | 0.000 [−0.001, 0] |
| Acute vs Chronic Pain | Sleep Score | CRP | Pain | −0.021 [−0.003, −0.01] *** | −0.020 [−0.003, −0.01] *** | −5.25e-5 [−1.7e-4, 0] |
Significance codes are:
< 0.001;
< 0.01;
< 0.05.
The results of mediation analyses adjusting for sex and age only achieved similar results. We found that CRP partially mediates the association between chronic pain (independent) and decreased sleep score (dependent) (p < 0.001). This partial mediation was also significant in the reverse direction (with chronic pain as dependent and decreased sleep score as independent) (p < 0.001). When comparing chronic and acute pain, CRP partially mediated the association between pain (independent) and decreased sleep score (dependent) (p = 0.008), as well as in the reverse direction (with pain as dependent and decreased sleep score as independent) (p = 0.028). The total association, direct association, and mediation effects are presented in Table 3.
Table 3.
Mediation Analysis Results adjusting for sex and age
| Groups of comparison | Independent variable (X) | Mediator (M) | Dependent variable (Y) | Total effect (c) | Direct effect (c’) | Mediation effect (ab) |
|---|---|---|---|---|---|---|
| Chronic Pain vs Controls | Pain | CRP | Sleep Score | 0.153 [0.136, 0.17] *** | 0.151 [0.133, 0.17] *** | 0.002 [0.001, 0] *** |
| Chronic Pain vs Controls | Sleep Score | CRP | Pain | 0.040 [0.036, 0.04] *** | 0.04 [0.035, 0.04] *** | 5.52e-4 [3.37e-4,0] |
| Acute vs Chronic Pain | Pain | CRP | Sleep Score | −0.084 [−0.107, −0.06] *** | −0.083 [−0.105, −0.06] *** | −0.001 [−0.002, 0] ** |
| Acute vs Chronic Pain | Sleep Score | CRP | Pain | −0.023 [−0.028, −0.02] *** | −0.022 [−0.028, −0.02] *** | −2.98e-4 [−5.7e-4, 0] * |
Significance codes are:
< 0.001;
< 0.01;
< 0.05.
Discussion
In this study, we investigated associations among musculoskeletal pain, CRP, and sleep patterns. We found that: 1) the chronic pain group showed higher CRP levels, compared to both the acute pain and the pain-free control groups. There was no significant difference in mean CRP levels between the acute pain and the pain-free control groups; 2) the chronic pain group produced lower sleep scores, compared to acute pain and the pain-free controls. The acute pain group also produced lower sleep scores, compared to pain-free controls; 3) the mediation analysis indicated that CRP partially mediated the association between chronic pain (independent) and decreased sleep score (dependent). Examination of the second visit cohort assessments showed similar effect sizes and similar but non-significant differences, likely due to reduced statistical power in a smaller sample size. Our findings support the hypothesized associations among musculoskeletal pain, CRP, and sleep patterns.
Musculoskeletal pain and CRP
Recently, CRP changes in patients with musculoskeletal pain has drawn increased attention22. In a recent review paper22, the authors summarized three studies that compared CRP levels in people with acute and chronic low back pain (LBP) vs. controls and found mixed results. In a study of 99 individuals having an onset of acute LBP within the past two weeks and 55 pain-free controls, the authors observed significantly higher baseline CRP levels34. In addition, they detected significantly higher median CRP levels in patients with high pain intensities (visual analog scale, VAS ≥ 4), compared to those with low pain intensities (VAS < 4) and pain-free controls34.
In a subsequent study from the same group35, the authors assessed individuals within two weeks of the onset of acute LBP (n = 109) and pain-free controls (n = 55). Levels of CRP, TNFα, IL-6 and interleukin-1β (IL-1β) as well as questionnaires related to pain, disability, sleep, and psychological status were collected. Participants repeated measurements throughout six months. Results showed that high inflammation (CRP/IL-6) was associated with good recovery, but specific elevation of TNFα along with depressive symptoms was associated with poor recovery. The early role of CRP (and perhaps IL-6) in the control of inflammation and recovery were further supported by a recent 12-month follow-up study of this sample36.
Sample sizes of the above studies are relatively small, which significantly influences one’s confidence in the reliability of findings22. In the present study which used a substantially larger cohort, we found that chronic pain was associated with higher CRP levels, compared to both the acute pain and the pain-free control groups. There was no significant difference in mean CRP levels between the acute pain group and the pain-free controls. However, the time since onset of pain in the acute pain group was unknown, which may contribute to the inconsistency with results from previous studies. In addition, it is worth noting that the effect size of the increased CRP level in chronic pain compared to the other groups is rather small (chronic pain compared to acute pain: 0.04, chronic pain compared to pain-free controls: 0.07).
Musculoskeletal pain and sleep
The reciprocal association / interaction between pain and sleep disturbance has been known for a long time37. For example, studies have shown that insomnia symptoms are associated with reduced pain thresholds38–40; sleep deficiency may be involved in the transition from acute to chronic pain41. Chronic pain has been found to be associated with greater sleep disturbance, reduced sleep duration and sleep quality, increased time taken to fall asleep, poor daytime functioning, and greater sleep dissatisfaction and distress42. Improved sleep quality can reduce the pain intensity and daytime symptoms associated back and neck pain43–45, and facilitate the treatment of chronic LBP46.
Additional studies suggest that sleep disturbance may contribute to pain development by impacting physiological and psychosocial functioning4,45. For example, sleep loss triggers inflammation47, impairs adaptive immune function48, disturbs emotion regulation49,50 and interferes with cognitive function51. These processes produce a range of physiological and psychological consequences52 that can exacerbate pain and make its management more difficult53.
With a large sample size, we found that individuals with acute and chronic pain produce lower sleep scores, compared to pain-free controls; chronic pain was associated with a lower sleep score than acute pain. These results are consistent with previous findings37,42.
Sleep, CRP, and musculoskeletal pain
We also found that lower sleep scores were associated with higher CRP levels in all the groups, combined and when individually examined, indicating that higher risk sleep patterns correlate with greater inflammation. These results are consistent with previous studies indicating that poor sleep behaviors, including excessive sleep duration, poor sleep quality, and sleep deprivation, are significantly associated with elevated CRP levels11,54,55.
Recently, the relationship between sleeplessness and inflammation on the risk of musculoskeletal pain has drawn research attention. A recent population-based prospective study of 3,214 women and 3,142 men56 detected an association between sleeplessness and CRP on the increased risk of any form of chronic musculoskeletal pain. In a subsequent study57, investigators found that preventing or reducing sleep problems among patients with chronic low back pain, including those with several additional pain sites, may have the potential to improve long-term prognosis of this condition.
To further explore the role of CRP in the interaction between musculoskeletal pain and sleep, we performed a mediation analysis. Our results showed that when comparing musculoskeletal pain and control groups, CRP partially mediated the association between chronic pain and decreased sleep score. This result suggests that CRP may be involved in the interaction between chronic pain and poor sleep patterns, but the mediation effect is rather mild. This partial mediation remained significant in both directions (pain as the dependent variable and sleep score as the independent variable; pain as the independent variable and sleep score as the dependent variable), consistent with the bi-directional relationship of pain and sleep disturbances. It is worth noting that the interpretation of mediation analyses should be performed with caution in cross-sectional data31,32, so further studies are required to validate our findings. In addition, the small effect size of the mediation may be driven by the large number of participants, so we cannot conclude pathophysiological significance. Further validation is needed.
To confirm our findings, we performed an identical set of analyses using second visit assessment data of the UK Biobank cohort. As only a portion of the individuals from the initial visit participated in the second visit, the sample size was relatively small. As a result, we could only partially replicate our findings. Nevertheless, the effect sizes and trends observed for the initial and second visit cohorts are similar.
In this study, we excluded individuals with psychiatric comorbidities because 1) depression can also increase CRP levels58,59; and 2) studies60 suggests that chronic pain alone and chronic pain comorbid with depression may be associated with different mechanisms. Thus, our findings may not be generalizable, i.e. not reflect the sleep and CRP alteration associated with musculoskeletal pain in the general population, but we believe it is reasonable to study a homogeneous subpopulation in order to obtain more readily interpretable results. In addition, for those who cannot obtain an adequate number of musculoskeletal pain patients without psychiatric comorbidities, an alternative method may be to include the psychiatric measurements in the model to adjust for their effects. Nevertheless, the interpretation of regression or other similar statistical analyses are conditional on the model being correct, and any statistical model is an imperfect reflection of reality. Therefore, even if we include these covariates in the analysis, we still would not fully account for the effects of these potential confounders. The UK Biobank is sufficiently large for enough subjects to remain after excluding these patients. Thus, we believe our decision to impose inclusion criteria is reasonable.
In this study, we used BMI calculated by bioelectrical impedance analysis (BIA). BIA is a non-invasive technique to measure body composition suitable in epidemiological research61 but can be influenced by many factors that make it less accurate for certain subpopulations62. Both BMI measurements, calculated through BIA or traditionally using height and weight measurements, are widely used, but neither measure body fat directly. We chose to use BIA because it has been shown to be closely correlated with and as valuable as traditional BMI in regard to detection of obesity in groups61,63.
Limitations
There are several limitations in this study. As a cross-sectional study, we cannot investigate the causal relationships amongst pain, sleep, and CRP. The UK Biobank did not collect pain intensity ratings at the same time blood samples were collected. Therefore, we cannot explore the relationships amongst pain intensity, sleep score, and CRP. Previous studies on the association between pain intensity and CRP levels were not consistent. For example, a prospective longitudinal study with a follow-up of 6 months64 found no statistically significant differences in mean CRP levels between patients with chronic low back pain and those in a control group. CRP remained approximately constant throughout the study period; there was no correlation with pain or function. Furthermore, our sample of musculoskeletal pain is heterogeneous due to the impossibility of defining the exact cause and subtype of musculoskeletal pain for each patient. In addition, the only inflammation marker the UK Biobank collected was CRP, and thus, we could not analyze other inflammation markers such as TNFα, IL-6, and IL-1β to have a more comprehensive picture of the role of inflammation in chronic pain and sleep disturbances. We were also not able to consider the diurnal fluctuation pattern of CRP. We did not include the depression, anxiety, and other mental measurement as covariate in this study, as we have excluded participants who were diagnosed with mental disorders. Another limitation that could introduce bias to the results is that we don’t know whether the participants were receiving therapy for their pain that might affect inflammation levels and sleep behaviors.
Summary
In this large-scale cross-sectional study, we used data from the UK Biobank to investigate the association among pain, sleep, and the inflammation marker CRP. Our findings suggest that compared with pain-free controls: 1) chronic musculoskeletal pain, but not acute musculoskeletal pain, was associated with higher CRP levels; and 2) both chronic and acute musculoskeletal pain were associated with lower sleep scores (with chronic pain producing a lower score than acute pain). CRP may partially mediate the interaction between chronic pain and decreased sleep score. However, the effect size of the mediation was very small, and the pathophysiological significance remains uncertain. Further validation is needed. Our study may provide further evidence about the interaction between sleep and musculoskeletal pain, and the involvement of CRP in their interplay.
Highlight.
We investigated the association among acute and chronic musculoskeletal pain, sleep, and inflammation
Chronic pain was associated with higher CRP levels compared to both acute pain and the pain-free controls
Both acute and chronic pain was associated with a lower sleep score
CRP may partially mediate the association between chronic pain and decreased sleep score.
Acknowledgements:
Jian Kong is supported by R01 AT008563, R33 AT009310, R33AT009341, R34DA046635 (through the NIH HEAL Initiative), and R01AG063975 from NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement:
Financial Disclosure: none
Non-financial Disclosure: none
Credit author statement
Conceiving idea: JK, SG, VS
Data curation: SG, VS
Data analysis: VS, SG, MV, SH
Data interpretation and manuscript preparation: SH, JK, SG, SO, EPS, YW, TG
References
- 1.Zammit GK, Weiner J, Damato N, Sillup GP, McMillan CA. Quality of life in people with insomnia. Sleep. 1999;22(SUPPL. 2). [PubMed] [Google Scholar]
- 2.Skevington SM, Carse MS, de C Williams AC. Validation of the WHOQOL-100: Pain management improves quality of life for chronic pain patients. Clinical Journal of Pain. 2001;17(3). doi: 10.1097/00002508-200109000-00013 [DOI] [PubMed] [Google Scholar]
- 3.Elliott AM, Smith BH, Penny KI, Smith WC, Chambers WA. The epidemiology of chronic pain in the community. Lancet. 1999;354(9186). doi: 10.1016/S0140-6736(99)03057-3 [DOI] [PubMed] [Google Scholar]
- 4.Campanini MZ, González AD, Andrade SM, et al. Bidirectional associations between chronic low back pain and sleep quality: A cohort study with schoolteachers. Physiol Behav. 2022;254. 10.1016/j.physbeh.2022.113880. Accessed June 23, 2022. [DOI] [PubMed] [Google Scholar]
- 5.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. Journal of Pain. 2013;14(12). doi: 10.1016/j.jpain.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith MT, Edwards RR, McCann UD, Haythomthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4). doi: 10.1093/sleep/30.4.494 [DOI] [PubMed] [Google Scholar]
- 7.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29(2). doi: 10.1093/sleep/29.2.145 [DOI] [PubMed] [Google Scholar]
- 8.Simpson NS, Scott-Sutherland J, Gautam S, Sethna N, Haack M. Chronic exposure to insufficient sleep alters processes of pain habituation and sensitization. Pain. 2018;159(1). doi: 10.1097/j.pain.0000000000001053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haack M, Scott-Sutherland J, Sethna N, Mullington JM Mechanisms of sleep loss-pain interactions. Sleep Med Dent A Pract overview. 2009:155–160. [Google Scholar]
- 10.Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66(6). doi: 10.1097/01.psy.0000145912.24553.c0 [DOI] [PubMed] [Google Scholar]
- 11.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1). doi: 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10(5). doi: 10.1016/j.smrv.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 13.Menefee LA, Cohen MJM, Anderson WR, Doghramji K, Frank ED, Lee H. Sleep Disturbance and Nonmalignant Chronic Pain: A Comprehensive Review of the Literature. Pain Medicine. 2000;1(2). doi: 10.1046/j.1526-4637.2000.00022.x [DOI] [PubMed] [Google Scholar]
- 14.Ho KKN, Simic M, Cvancarova Småstuen M, et al. The association between insomnia, c-reactive protein, and chronic low back pain: Cross-sectional analysis of the HUNT study, Norway. Scand J Pain. 2019. doi: 10.1515/sjpain-2019-0033 [DOI] [PubMed] [Google Scholar]
- 15.Afari N, Mostoufi S, Noonan C, et al. C-reactive protein and pain sensitivity: Findings from female twins. Annals of Behavioral Medicine. 2011;42(2). doi: 10.1007/s12160-011-9297-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9). doi: 10.1093/sleep/30.9.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: A conceptual model. Curr Pain Headache Rep. 2009;13(6). doi: 10.1007/s11916-009-0073-2 [DOI] [PubMed] [Google Scholar]
- 18.Slavish DC, Graham-Engeland JE, Engeland CG, Taylor DJ, Buxton OM. Insomnia symptoms are associated with elevated C-reactive protein in young adults. Psychol Health. 2018;33(11). doi: 10.1080/08870446.2018.1500577 [DOI] [PubMed] [Google Scholar]
- 19.Prather AA, Vogelzangs N, Penninx BWJH. Sleep duration, insomnia, and markers of systemic inflammation: Results from the Netherlands Study of Depression and Anxiety (NESDA). J Psychiatr Res. 2015;60. doi: 10.1016/j.jpsychires.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derry S, Wiffen PJ, Kalso EA, et al. Topical analgesics for acute and chronic pain in adults - an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews. 2017;2017(5). doi: 10.1002/14651858.CD008609.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1). doi: 10.1097/j.pain.0000000000001384 [DOI] [PubMed] [Google Scholar]
- 22.Morris P, Ali K, Merritt M, Pelletier J, Macedo LG. A systematic review of the role of inflammatory biomarkers in acute, subacute and chronic non-specific low back pain. BMC Musculoskelet Disord. 2020;21(1). doi: 10.1186/s12891-020-3154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parisien M, Khoury S, Chabot-Doré AJ, et al. Effect of Human Genetic Variability on Gene Expression in Dorsal Root Ganglia and Association with Pain Phenotypes. Cell Rep. 2017;19(9). doi: 10.1016/j.celrep.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman MS, Winsvold BS, Chavez Chavez SO, et al. Genome-wide association study identifies RNF123 locus as associated with chronic widespread musculoskeletal pain. Ann Rheum Dis. 2021;80(9). doi: 10.1136/annrheumdis-2020-219624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khoury S, Parisien M, Thompson SJ, et al. Genome-wide analysis identifies impaired axonogenesis in chronic overlapping pain conditions. Brain. 2022;145(3):1111–1123. doi: 10.1093/brain/awab359 [DOI] [PubMed] [Google Scholar]
- 26.Sudlow C et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015;12(3):1–10. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Zhou T, Li X, et al. Baseline vitamin d status, sleep patterns, and the risk of incident type 2 diabetes in data from the uk biobank study. Diabetes Care. 2020;43(11). doi: 10.2337/dc20-1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan M, Sun D, Zhou T, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: A prospective study of 385 292 UK biobank participants. Eur Heart J. 2020;41(11). doi: 10.1093/eurheartj/ehz849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Wang B, Chen C, et al. Sleep Patterns, Genetic Susceptibility, and Incident Chronic Kidney Disease: A Prospective Study of 370 671 Participants. Front Neurosci. 2022;16. doi: 10.3389/fnins.2022.725478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackinnon DP. Introduction to Statistical Mediation Analysis.; 2012. doi: 10.4324/9780203809556 [DOI] [Google Scholar]
- 31.Fairchild AJ, McDaniel HL. Best (but oft-forgotten) practices: Mediation analysis. American Journal of Clinical Nutrition. 2017;105(6). doi: 10.3945/ajcn.117.152546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxwell SE, Cole DA, Mitchell MA. Bias in cross-sectional analyses of longitudinal mediation: Partial and complete mediation under an autoregressive model. Multivariate Behav Res. 2011;46(5). doi: 10.1080/00273171.2011.606716 [DOI] [PubMed] [Google Scholar]
- 33.Team RC. R language definition. Vienna, Austria: R foundation for statistical computing. Published online 2000. 2000. [Google Scholar]
- 34.Klyne DM, Barbe MF, Hodges PW. Systemic inflammatory profiles and their relationships with demographic, behavioural and clinical features in acute low back pain. Brain Behav Immun. 2017;60. doi: 10.1016/j.bbi.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 35.Klyne DM, Barbe MF, van den Hoorn W, Hodges PW. ISSLS PRIZE IN CLINICAL SCIENCE 2018: longitudinal analysis of inflammatory, psychological, and sleep-related factors following an acute low back pain episode—the good, the bad, and the ugly. European Spine Journal. 2018;27(4). doi: 10.1007/s00586-018-5490-7 [DOI] [PubMed] [Google Scholar]
- 36.Klyne DM, Barbe MF, Hodges PW. Relationship between systemic inflammation and recovery over 12 months after an acute episode of low back pain. Spine Journal. 2022;22(2). doi: 10.1016/j.spinee.2021.09.006 [DOI] [PubMed] [Google Scholar]
- 37.O’Brien EM, Waxenberg LB, Atchison JW, et al. Intraindividual variability in daily sleep and pain ratings among chronic pain patients: Bidirectional association and the role of negative mood. Clinical Journal of Pain. 2011;27(5). doi: 10.1097/AJP.0b013e318208c8e4 [DOI] [PubMed] [Google Scholar]
- 38.Schrimpf M, Liegl G, Boeckle M, Leitner A, Geisler P, Pieh C. The effect of sleep deprivation on pain perception in healthy subjects: A meta-analysis. Sleep Med. 2015;16(11). doi: 10.1016/j.sleep.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 39.Chan AS, Ho YC, Cheung MC, Albert MS, Chiu HFK, Lam LCW. Association between mind-body and cardiovascular exercises and memory in older adults. J Am Geriatr Soc. 2005;53(10). doi: 10.1111/j.1532-5415.2005.53513.x [DOI] [PubMed] [Google Scholar]
- 40.Haack M, Scott-Sutherland J, Santangelo G, Simpson NS, Sethna N, Mullington JM. Pain sensitivity and modulation in primary insomnia. Eur J Pain. 2012;16(4). doi: 10.1016/j.ejpain.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andreucci A, Groenewald CB, Rathleff MS, Palermo TM. The role of sleep in the transition from acute to chronic musculoskeletal pain in youth—a narrative review. Children. 2021;8(3). doi: 10.3390/children8030241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly GA, Blake C, Power CK, Okeeffe D, Fullen BM. The association between chronic low back pain and sleep: A systematic review. Clinical Journal of Pain. 2011;27(2). doi: 10.1097/AJP.0b013e3181f3bdd5 [DOI] [PubMed] [Google Scholar]
- 43.Jungquist CR, Flannery M, Perlis ML, Grace JT. Relationship of Chronic Pain and Opioid Use with Respiratory Disturbance during Sleep. Pain Management Nursing. 2012;13(2). doi: 10.1016/j.pmn.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 44.Jungquist CR, O’Brien C, Matteson-Rusby S, et al. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. 2010;11(3). doi: 10.1016/j.sleep.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jungquist CR, Tra Y, Smith MT, et al. The Durability of Cognitive Behavioral Therapy for Insomnia in Patients with Chronic Pain. Sleep Disord. 2012;2012. doi: 10.1155/2012/679648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broberg M, Karjalainen J, Ollila HM. Mendelian randomization highlights insomnia as a risk factor for pain diagnoses. Sleep. 2021;44(7). doi: 10.1093/sleep/zsab025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bower JE, Irwin MR. Mind–body therapies and control of inflammatory biology: A descriptive review. Brain Behav Immun. 2016;51. doi: 10.1016/j.bbi.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haack M, Lee E, Cohen DA, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: Potential mediator of increased spontaneous pain. Pain. 2009;145(1–2). doi: 10.1016/j.pain.2009.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krause AJ, ben Simon E, Mander BA, et al. The sleep-deprived human brain. Nat Rev Neurosci. 2017;18(7). doi: 10.1038/nrn.2017.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol. 2014;10. doi: 10.1146/annurev-clinpsy-032813-153716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ader R, Mercurio MG, Walton J, et al. Conditioned pharmacotherapeutic effects: A preliminary study. Psychosom Med. 2010;72(2). doi: 10.1097/PSY.0b013e3181cbd38b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2). doi: 10.1093/sleep/26.2.117 [DOI] [PubMed] [Google Scholar]
- 53.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119(1–3). doi: 10.1016/j.pain.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 54.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-Reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4). doi: 10.1016/j.jacc.2003.07.050 [DOI] [PubMed] [Google Scholar]
- 55.Lee HW, Yoon HS, Yang JJ, et al. Association of sleep duration and quality with elevated hs-CRP among healthy Korean adults. PLoS One. 2020;15(8 August). doi: 10.1371/journal.pone.0238053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ettore E, Bakardjian H, Solé M, et al. Relationships between objectives sleep parameters and brain amyloid load in subjects at risk for Alzheimer’s disease: The INSIGHT-preAD Study. Sleep. 2019;42(9). doi: 10.1093/sleep/zsz137 [DOI] [PubMed] [Google Scholar]
- 57.Skarpsno ES, Mork PJ, Nilsen TIL, Nordstoga AL. Influence of sleep problems and co-occurring musculoskeletal pain on long-Term prognosis of chronic low back pain: The HUNT Study. J Epidemiol Community Health (1978). 2020;74(3). doi: 10.1136/jech-2019-212734 [DOI] [PubMed] [Google Scholar]
- 58.Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: A meta-analysis. J Affect Disord. 2020;277. doi: 10.1016/j.jad.2020.09.025 [DOI] [PubMed] [Google Scholar]
- 59.Wium-Andersen MK, Ørsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73131 individuals. JAMA Psychiatry. 2013;70(2). doi: 10.1001/2013.jamapsychiatry.102 [DOI] [PubMed] [Google Scholar]
- 60.Zhou W, Jin Y, Meng Q, et al. Publisher Correction: A neural circuit for comorbid depressive symptoms in chronic pain (Nature Neuroscience, (2019), 22, 10, (1649–1658), 10.1038/s41593-019-0468-2). Nat Neurosci. 2019;22(11). doi: 10.1038/s41593-019-0522-0 [DOI] [PubMed] [Google Scholar]
- 61.Böhm A, Heitmann BL. The use of bioelectrical impedance analysis for body composition in epidemiological studies. Eur J Clin Nutr. 2013;67. doi: 10.1038/ejcn.2012.168 [DOI] [PubMed] [Google Scholar]
- 62.Dehghan M, Merchant AT. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J. 2008;7(1). doi: 10.1186/1475-2891-7-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heydari ST, Ayatollahi SMT, Zare N. Diagnostic value of bioelectrical impedance analysis versus body mass index for detection of obesity among students. Asian J Sports Med. 2011;2(2). doi: 10.5812/asjsm.34777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gebhardt K, Brenner H, Stürmer T, et al. The course of high-sensitive C-reactive protein in correlation with pain and clinical function in patients with acute lumbosciatic pain and chronic low back pain - A 6 months prospective longitudinal study. European Journal of Pain. 2006;10(8). doi: 10.1016/j.ejpain.2005.11.005 [DOI] [PubMed] [Google Scholar]


