Abstract
Background:
A symptom cluster is a group of two or more symptoms that occur together and are related to each other. Family caregivers of allogeneic hematopoietic stem cell transplant (HSCT) recipients experience multiple concurrent symptoms, but the majority of symptom research in this population has focused on assessing and managing individual symptoms.
Objectives:
The purpose of this analysis was to determine (1) if clusters of five highly prevalent symptoms (fatigue, sleep disturbance, depression, anxiety, and cognitive impairment) in allogeneic HSCT caregivers could be identified and (2) what caregiver and patient characteristics influence membership in the identified symptom cluster groups.
Study Design:
Baseline cross-sectional data were collected from allogeneic HSCT caregivers participating in a randomized controlled trial at the National Institutes of Health Clinical Center. Measures included: Caregiver Reaction Assessment (CRA), the Health-Promoting Lifestyle Profile II (HPLP-II), Fatigue Symptom Inventory (MFSI), Pittsburgh Sleep Quality Index (PSQI), and PROMIS® (depression, anxiety, applied cognition, and self-efficacy). Cluster analysis was used to identify symptom clusters, and univariate analyses and multiple logistic regression were conducted to identify factors that contribute to symptom clusters.
Results:
Caregivers’ (N=44) were on average 45.20 ± 15.05 years; primarily white (52.3%), female (88.6%), and spouse/partner of the patient (50.0%). Two symptom cluster groups were identified: low symptom burden (n=24, 54.5%) and high symptom burden (n= 20, 45.5%). Caregivers with higher levels of loneliness (OR = 1.12, CI = [1.04, 1.22], p = .004) were more likely to be in the high symptom burden group.
Conclusions:
This study provides evidence that five symptoms commonly found in family caregivers (fatigue, sleep disturbance, depression, anxiety, and cognitive impairment) tend to occur in clusters. Therefore, clinicians should be aware that caregivers experiencing one or more of these symptoms may be at higher risk for developing the others, and caregivers reporting high levels of loneliness may be at particular risk. Future research is needed to identify novel interventions that target multiple, co-occurring symptoms. Such interventions might also include components that decrease loneliness.
Keywords: Caregivers, Hematopoietic cell transplant, Symptom burden, Symptom clusters
INTRODUCTION
The number of hematopoietic stem cell transplantations (HSCT) performed in the United States has steadily increased, with 22,013 transplants reported in 2020 [1]. HSCT, especially allogeneic, is a risky and complex procedure that can lead to multiple complications (e.g., graft versus host disease, cytopenia, infection) requiring the transplant recipient to seek supportive care for their treatment including physical, emotional, and financial assistance [2]. Serving as a caregiver for someone undergoing an intense HSCT, has benefits, but it is also considered a physically, psychologically, and financially burdensome and stressful event for the caregiver [2–4].
Family caregivers of HSCT recipients often suffer from increased levels of stress and associated symptoms as they experience a heavy caregiving burden while taking on multiple tasks, including providing emotional support, coordinating medical appointments, and managing medications [3,5]. Highly prevalent symptoms in HSCT caregivers identified in previous studies include fatigue, sleep disturbance, depression, anxiety, and cognitive impairment [3,5–8]. Symptoms with an acute onset or pre-existing status, may worsen during caregiving, making it difficult for the HSCT caregiver to fulfill their role and may negatively impact health status and quality of life of themselves and the HSCT recipient [5]. Many of these symptoms tend to occur concurrently. Symptom clusters are defined as “two or more symptoms that are related to each other and that occur together” [9(p278)] and consist of relatively independent and stable clusters of symptoms that may have common underlying mechanisms [9,10].
Existing symptom research in family caregivers, including HSCT caregivers, has focused on investigating individual symptoms or bivariate associations between these symptoms. Only more recent studies have focused on multiple co-occurring symptoms or symptom clusters [7,11,12]. In a cross-sectional study by Alfheim et al. [11], family caregivers of patients admitted to intensive care units reported experiencing multiple symptoms (median, 9; range, 0 – 24) of varying severity and distress, with the most occurring symptoms as worrying, feeling sad, difficulty concentrating and sleeping. Another study involving family caregivers and head and neck cancer patients [12] identified two clusters (low, moderate-to-high) of caregiving task burden and caregiver psychological distress, respectively, with a strong association between caregiving task burden and caregiving psychological distress. Our own previous research [7] investigated symptom clusters using five key symptoms (fatigue, sleep disturbance, depression, anxiety, and impaired cognition) in 129 family caregivers of individuals with diverse cancer types (carcinoma, leukemia, sarcoma, lymphoma, and myeloma). They also found two symptom clusters (low symptom burden, high symptom burden). The latter two studies used a cluster analysis approach to distinguish individuals into relatively independent subgroups with similar symptom patterns [10], suggesting that family caregivers’ symptoms may be reciprocally associated and appear to cluster.
Investigating factors associated with individual symptoms or co-occurring symptoms in family caregivers is essential when establishing supportive strategies to relieve their symptom burden. In particular, loneliness has been recognized as a major risk factor for multiple symptoms experienced by caregiving populations [7,13]. Loneliness, defined as a perceived experience of isolation, alienation, and a lack of connection with others, can be problematic for family caregivers who spend a considerable amount of time and energy caring for patients and lose their social and relational contacts [14]. In our previous work using a sample of family caregivers of patients with multiple cancer types [7], caregivers who reported higher levels of loneliness were more likely to be in the high symptom burden group. A longitudinal study involving dementia patients and spousal caregivers indicated that loneliness was a critical contributor to the development of depression, pain, and fatigue symptom cluster over time [13]. Studies using non-caregiver samples have also suggested that loneliness is a common risk factor for the development of multiple concurrent symptoms [15,16]. In the Jaremka et al.’s [15] study of breast cancer survivors, those feeling lonely showed higher levels of the pain, depression, and fatigue symptom cluster compared to those feeling less lonely. A population-based study using the University of Michigan’s Health and Retirement Study data demonstrated that loneliness is strongly associated with the symptom cluster of pain, fatigue, and depression in older adults [16]. Assessing loneliness and its impact on physical and psychological symptoms during the illness trajectory is vital for identifying potential health consequences of both family caregivers and care recipients. Both groups commonly experience long-term disconnections from social relationships due to their caregiving and care recipient roles [14,17].
Research remains limited regarding the presence of symptom clusters and what factors associated with caregiving contribute to symptom clusters in caregiving populations. There were studies that have attempted to determine the presence of symptom clusters and contributing factors in heterogeneous samples in terms of patient primary disease or cancer treatment type. However, to our knowledge, no studies have focused on patterns of multiple concurrent symptoms in family caregivers of patients undergoing HSCT and the associations with caregiver and patient characteristics. The purpose of this analysis was to determine (1) if clusters of five highly prevalent symptoms (fatigue, sleep disturbance, depression, anxiety, and cognitive impairment) in allogeneic HSCT caregivers could be identified, and (2) what caregiver and patient characteristics influence membership in symptom cluster groups.
METHODS
Study Design and Participants
A cross-sectional secondary analysis was conducted to evaluate symptoms in family caregivers of adult patients who underwent an allogeneic HSCT at the National Institutes of Health (NIH) Clinical Center. This analysis was part of a randomized controlled trial (RCT) that examined the effectiveness of a six-week yoga-based stress reduction intervention [18]. Participants were recruited between January 2015 and February 2019. Caregivers were eligible to participate in the study if they (1) were at least 18 years old; (2) were an active family caregiver for a patient undergoing their 1st allogeneic HSCT; (3) were able to read and speak English; (4) were able to lift their arms over head without pain; and (5) were able to stand from a seated position unassisted. When more than one caregiver was planned for the transplant recipient during the transplant phase, only one caregiver was eligible to participate in the study. An active caregiver was defined as someone who lives with or provides care regularly for the HSCT recipient during the 6-week study period. Written informed consent was obtained from caregivers prior to initiating any study procedures. Care recipients were not enrolled in the study, but they granted access to their medical records in order for the study team to collect disease and treatment information. This study obtained institutional review board approval from the National Heart, Lung, and Blood Institute at the NIH.
Conceptual Framework
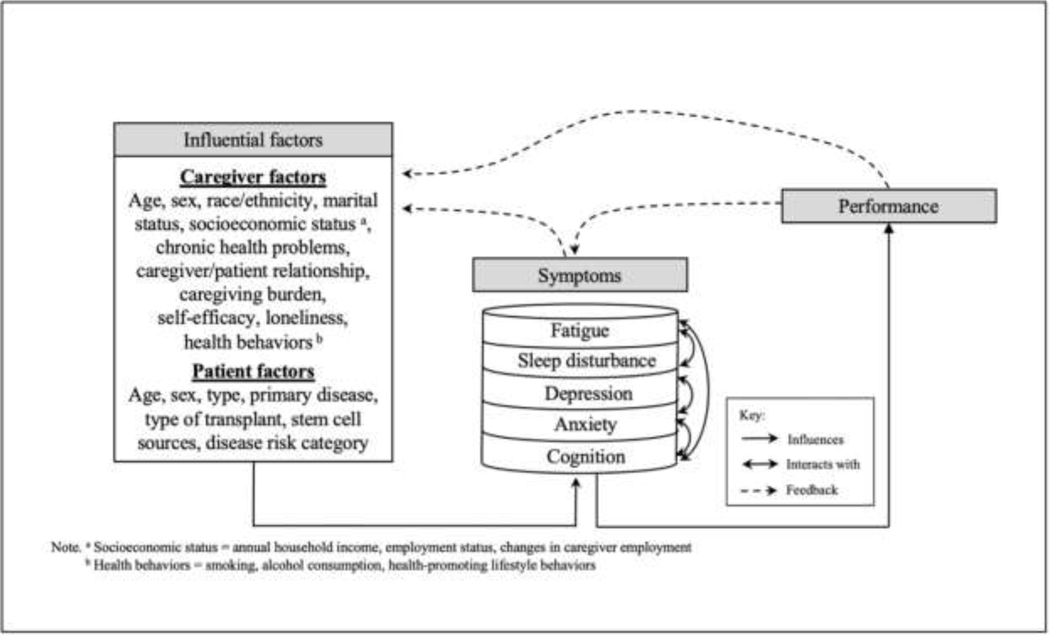
The analysis in this study was guided by the Theory of Unpleasant Symptoms (TOUS) [19]. The TOUS has three major components: influencing factors, symptoms, and performance. Influencing factors contributing to the symptom experience can be classified into physiological, psychological, and situational factors. The theory assumes that the symptom experience can be the occurrence of a single symptom or of multiple concurrent symptoms that interact with each other. The symptom experience can influence performance outcomes, such as an individual’s functional/cognitive status or health-related quality of life. Performance outcomes can then provide feedback on the symptom experience and influencing factors. The TOUS clearly illustrates the multidimensional and reciprocal aspects of an individual’s symptom experience [20].
Based on previous published research, we examined the relationship between the reported symptoms of HSCT caregivers and influencing factors, such as physiological, psychological, and situational factors. Physiological factors assumed to contribute to the symptoms experienced by HSCT caregivers included age, sex, race/ethnicity, and chronic health problems. Potential psychological factors included self-efficacy and loneliness. Situational factors included the following items: marital status, socioeconomic status (e.g., household income, employment status), health behaviors, caregiver burden, relationship with HSCT recipient, and HSCT recipient characteristics (e.g., age, sex, primary disease, type of transplant). Symptoms of HSCT caregivers that these influencing factors can contribute to included fatigue, sleep disturbance, depression, anxiety, and impaired cognition. The conceptual framework (Figure 1) shows the reciprocal relationships between these influencing factors and symptoms.
Figure 1.
The Theory of Unpleasant Symptoms* Adapted for the Analysis of Symptom Clusters in HSCT Caregivers
Measures
General Characteristics.
Demographic characteristics included age, sex, race/ethnicity, marital status (married, unmarried), annual household income, employment status, changes in caregiver employment, and presence of chronic health problems. Caregiving characteristics included relationship with HSCT recipient (spouse vs non-spouse), number of caregiving days per week, number of caregiving hours per day, and health risk behaviors (smoking, alcohol consumption). HSCT recipient characteristics included patient age, sex, primary disease, type of transplant, stem cell source, and disease risk category.
Multidimensional Fatigue Symptom Inventory- Short Form (MFSI-SF).
The MFSI-SF uses a 5-point Likert-scale to assess various dimensions of acute fatigue. The MFSI-SF was initially developed for cancer patients, but has been validated and consistently performed in other clinical populations and in healthy individuals [21]. The 30-item MFSI-SF asks respondents to rate their level of fatigue in the past week on a scale ranging from 0 (not at all) to 4 (extremely), with higher scores indicating greater or higher levels of fatigue. The MSFI-SF consists of five subscales including: general, physical, vigor, emotional, and mental. In this analysis, only general fatigue was calculated, and the Cronbach’s alpha for the general fatigue subscale was 0.95.
PROMIS® and NIH Toolbox Measures.
The Patient-Reported Outcomes Measurement Information System (PROMIS®) and NIH Toolbox are reliable and highly validated measures of self-reported health outcomes [22,23]. PROMIS® measures the areas of mental, physical, and social well-being, while the NIH toolbox measures emotional, cognitive, sensory, and motor functions. In this analysis, PROMIS® measures of depression, anxiety, and applied cognition were administered using a Computer Adaptive Testing (CAT) format. CAT uses validated algorithms to adapt a test based on the participant’s response. PROMIS® measures generate a raw score from which T-scores are calculated. Standardized scores are then normed to the general population with a mean of 50 and a standard deviation (SD) of 10. The NIH Toolbox was used to collect measures of self-efficacy (10-item fixed form) and loneliness (5-item fixed form), using a 4-point Likert scale ranging from 1 to 4 and a 5-point Likert scale ranging from 1 to 5, respectively, with higher scores indicative of greater or higher levels of the concept being measured.
The Pittsburgh Sleep Quality Index (PSQI).
The Pittsburgh Sleep Quality Index (PSQI) is an 18 item self-reported measure of sleep quality. Respondents rate their sleep quality over the past month and responses are collected using a 4-point scale, with 0 (no difficulty) and 3 (severe difficulty). The PSQI contains seven equally weighted subscales: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction [24]. The seven subscale scores can be summed to generate a total global score, which ranges from 0–21. Higher PSQI scores indicate poor sleep quality. In this study, the measure had a Cronbach’s alpha of 0.84.
Caregiver Reaction Assessment (CRA).
The Caregiver Reaction Assessment (CRA) is a 24-item self-administered survey measuring the positive and negative effects of caregiving in five domains: caregiver esteem (7 items), impact on schedule (5 items), lack of family support (5 items), impact on health (4 items), and impact on finances (3 items) [25]. This measure is a valid and reliable measure for use in caregivers of cancer patients. Responses are rated using a 5-point Likert scale from 1 (strongly disagree) to 5 (strongly agree). Scores are calculated as a total mean, and with the exception of caregiver esteem, higher total mean scores indicate greater overall caregiver burden. Mean-item scores range from 1.0 to 5.0. In this study, the measure had a Cronbach’s alpha of 0.69.
Health-Promoting Lifestyle Behaviors-II (HPLP-II).
The Health-Promoting Lifestyle Behaviors-II (HPLP-II) is a 52-item instrument that gathers self-reported information about participation in health-promoting behaviors [26]. This instrument contains six subscales and an overall health-promoting lifestyle mean score. The six subscales are stress management, spirituality, physical activity, nutrition, interpersonal support, and health responsibility (taking responsibility for one’s own health). Responses are reported along a 4-point Likert scale ranging from 1 (never) to 4 (routinely). Subscales are scored by calculating the mean of the items for each subscale, and the health-promoting lifestyle score is the mean of all questions in the scale. Subscale scores range from 0 to 4, and higher scores indicate greater participation in health-promoting behaviors. For our study, the Cronbach’s alpha was 0.87.
Statistical Analysis Cluster analysis was performed to identify subgroups of caregivers based on their responses to the five symptoms (fatigue, sleep disturbance, depression, anxiety, cognition impairment), using Stata version 15.0 [27]. An agglomerative, hierarchical cluster analysis was performed with squared Euclidean distance used in the proximities matrix and Ward’s method used as the clustering method. To determine the number of clusters, the Calinski and Harabasz pseudo-F stopping rule index and the Duda and Hart Je(2)/Je(1) index were used jointly [27]. A large Calinski and Harabaz pseudo-F statistic, combined with two measures from Duda and Hart (i.e., a large Je(2)/Je(1) index and its associated small pseudo-T squared value), resulted in two as the most appropriate number of clusters for the data.
Once clusters were identified, logistic regression was used to determine which factors predicted the symptom cluster membership. Caregiver factors (age, sex, race/ethnicity, marital status, annual household income, work status, work status change, chronic health problems, relationship with HSCT recipient, caregiving role, caregiver burden, self-efficacy, loneliness, health behaviors) and HSCT recipient factors (age, sex, type of transplant, primary disease) were included in the bivariate analyses. Factors were included in the multiple logistic regression model if they were significantly associated with the class membership in the bivariate analyses. All the statistical analyses were performed utilizing the IBM SPSS software package version 27.0 [28].
RESULTS
Two hundred twenty-six subjects were screened for participation and 108 were eligible. 50 subjects agreed to participate, enrolled, and completed the study. Six subjects with incomplete responses and were excluded from the study resulting in a total of 44 caregiver participants. Demographic and descriptive characteristics of the study participants and their mean symptom scores for fatigue, sleep disturbance, depression, anxiety, and cognition are shown in Table 1.
Table 1.
General Characteristics (N = 44)
| Variables | Category | N (%) Mean (SD), range |
|---|---|---|
| Caregiver characteristics | ||
|
| ||
| Age (years) | 45.20 (15.05), 18 – 74 | |
| Sex | Female | 39 (88.6) |
| Race/ethnicity | White/ Non-Hispanic | 23 (52.3) |
| Non-White/ Non-Hispanic | 13 (29.5) | |
| Hispanic | 8 (18.2) | |
| Marital status | Married/cohabiting | 36 (81.8) |
| Not marrieda | 18 (18.2) | |
| Annual household income | < $50,000 | 15 (43.6) |
| $50,000–$89,000 | 7 (17.9) | |
| > $89,000 | 15 (38.5) | |
| Employment status | Full-time | 15 (34.1) |
| Part-time | 9 (20.5) | |
| Not workingb | 20 (45.5) | |
| Changes in caregiver | Yes | 34 (77.3) |
| employment | No | 10 (22.7) |
| Chronic health problems | Yes | 31 (70.5) |
| No | 13 (29.5) | |
| Relationships with patient | Spouse | 22 (50.0) |
| Non-spouse (family member) | 22 (50.0) | |
| Caregiving days | 6.73 (1.09), 2 – 7 | |
| Caregiving hours | 14.59 (7.53), 2 – 24 | |
| Perceived stress | 54.24 (10.62), 30–78 – 74.78 | |
| Caregiving burden (CRA) | Total | 2.34 (0.41), 1.67 – 3.50 |
| Caregiver esteem | 4.29 (0.40), 3.43 – 5.00 | |
| Lack of family support | 1.96 (0.84), 1.00 – 4.20 | |
| Impact on finance | 2.70 (1.04), 1.00 – 5.00 | |
| Impact on schedule | 3.73 (0.63), 2.40 – 3.73 | |
| Impact on health | 1.97 (0.64), 1.00 – 3.75 | |
| Self-efficacy | 27.91 (4.85), 17 – 38 | |
| Loneliness | 11.09 (5.07), 5 – 23 | |
| Health behaviors (HPLP-II) | Total | 2.47 (0.34), 1.83 – 3.31 |
| Health responsibility | 2.13 (0.45), 1.44 – 3.56 | |
| Physical activity | 2.04 (0.63), 1.00 – 3.50 | |
| Nutrition | 2.62 (0.50), 1.89 – 3.67 | |
| Spiritual growth | 2.92 (0.43), 2.11 – 3.89 | |
| Interpersonal relations | 2.88 (0.45), 1.78 – 3.89 | |
| Stress management | 2.16 (0.49), 1.25 – 3.50 | |
| Symptoms | Fatigue | 7.73 (6.09), 0 – 22 |
| Sleep disturbance | 8.09 (3.93), 0 – 17 | |
| Depression | 51.29 (7.93), 34.17 – 71.37 | |
| Anxiety | 60.61 (6.56), 46.69 – 75.54 | |
| Cognition | 37.73 (10.68), 14.53 – 64.90 | |
| Smoking | Yes | 5 (11.4) |
| No | 39 (88.6) | |
| Alcohol consumption | Yes | 27 (61.4) |
| No | 17 (38.6) | |
| Care recipient characteristics | ||
| Age (years) | 35.25 (13.74), 18 – 66 | |
| Sex | Male | 31 (70.5) |
| Primary diseasec | Hematological malignancy | 22 (50.0) |
| Non-malignant hematologic disease | 22 (50.0) | |
| Type of transplant | Reduced intensity conditioning, (RIC) | 30 (68.2) |
| Myeloablative | 14 (31.8) | |
| Stem cell source | Peripheral blood | 35 (79.5) |
| Bone marrow | 8 (18.2) | |
| Cord | 1 ( 2.3) | |
| Disease risk category | Low | 28 (77.8) |
| Intermediate | 3 ( 8.3) | |
| High | 5 (13.9) | |
Note. Numbers may not sum to total due to missing data.
CRA = caregiver reaction assessment; HPLP-II = health promoting lifestyle profile II; RIC = reduced intensity conditioning
Not married = never married, divorced, separated, widowed
Not working = student, retired, disability, unemployed
Hematological malignancy = chronic myelogenous leukemia, acute lymphocytic leukemia, acute myelogenous leukemia, and chronic lymphocytic leukemia, Hodgkin’s and non-Hodgkin’s lymphoma; Non-malignant hematologic disease = aplastic anemia, sickle cell disease, inherited bone marrow failure disorders, primary immunodeficiency disease.
Identification of Symptom Clusters
Two distinct symptom cluster groups were identified, Cluster 1 and Cluster 2, that divided the caregivers into subgroups based upon the severity of their symptoms. Cluster 1 (n=24, 54.5%), was labeled “low symptom burden.” Cluster 2 (n= 20, 45.5%), labeled “high symptom burden” (Table 2).
Table 2.
Differences in Symptom Mean Scores among the Symptom Cluster Groups (N=44)
| Variables | Mean (SD) | p value | |
|---|---|---|---|
|
|
|||
| Cluster 1 | Cluster 2 | ||
|
| |||
| (n=24, 54.5%) | (n=20, 45.5%) | ||
| Low symptom burden | High symptom burden | ||
| Fatigue | 3.88 (2.71) | 12.35 (5.82) | < .001 |
| Sleep disturbance | 5.79 (3.02) | 10.85 (2.81) | < .001 |
| Depression | 45.72 (3.88) | 57.96 (6.18) | < .001 |
| Anxiety | 56.99 (4.95) | 64.94 (5.59) | < .001 |
| Impaired cognition | 32.00 (9.00) | 44.61 (8.29) | < .001 |
Note. Numbers may not sum to total due to missing data.
Factors associated with Symptom Cluster Groups
Two factors (self-efficacy and loneliness) were significantly associated with the symptom cluster membership in the bivariate analyses and were included in the multiple logistic regression model for symptom cluster membership. Table 3 displays final multiple logistic regression results with factors using Cluster 1 (low symptom burden) as the reference. Caregivers who reported higher levels of loneliness were more likely to be in the higher symptom burden group (odds ratio [OR] = 1.12; CI = [1.04, 1.22]).
Table 3.
Final Multiple Logistic Regression Model
| Variables | B (SE) | OR (95% CI) | p value |
|---|---|---|---|
| Self-efficacy | −0.08 (0.06) | 0.92 (0.83, 1.03) | .143 |
| Loneliness | 0.12 (0.04) | 1.12 (1.04, 1.22) | .004* |
Note. CI = confidence interval; OR = odds ratio
p < 0.05
DISCUSSION
This is the first known study to identify symptom clusters in family caregivers of HSCT recipients and to demonstrate the relationship between symptom clusters with the variables of interest. Our findings support the premise of the TOUS because multiple symptoms are experienced concurrently, and that these symptoms are affected by physiological, psychological, and situational factors [19]. In this study, two symptom cluster groups were identified among family caregivers of HSCT recipients: low symptom burden (54.5%) and high symptom burden (45.5%). Our analysis provides further evidence regarding who is at risk for experiencing symptom clusters; HSCT caregivers with high levels of loneliness were more likely to be in the high symptom burden group.
The findings from this study substantiated the findings of a previous cross-sectional study of family caregivers of individuals with cancer that found that caregivers experience clusters of five key symptoms (i.e., fatigue, sleep disturbance, depression, anxiety, impaired cognition). This current study further supported the previous work [7] in validating the presence of two symptom cluster subgroups, the low symptom burden (82.2%) and high symptom burden (17.8%) groups. Importantly, the percentage of HSCT caregivers in the high symptom burden group was about 28% higher than that of the previous study which included caregivers of cancer patients receiving diverse cancer treatment types (biotherapy/immunotherapy, HSCT, chemotherapy, surgery) [7]. The average perceived stress of the HSCT caregivers was 54.2 (SD = 10.6), somewhat higher than that of the cancer caregivers (Mean = 52.0, SD = 9.8) [7]. Although direct comparisons are difficult due to the heterogeneity of socio-demographic characteristics (e.g., sex, marital status) and clinical characteristics (e.g., chronic health problems) between the two groups, the results demonstrate that family caregivers of HSCT recipients are at particular risk for heightened symptom burden. HSCT is recognized as one of the most rigorous and exhausting treatments in oncology, for both the transplant recipient and the caregiver [2,6,29]. Patients typically undergo multiple rounds of chemotherapy before HSCT for disease control. In the majority of cases, caregivers are intimately involved in the recipient’s care and provide a critical role during the whole HSCT process [2]. Such an involvement represents a complete devotion of caregivers’ time to HSCT recipient, which impacts multiple areas of caregivers’ lives, including symptom burden. Clearly, the HSCT experience is clinically difficult for the recipient, but this study provides evidence that the experience may contribute to symptoms in the caregivers even more than other cancer treatments.
The most striking finding of this study was that loneliness was reported as a significant risk factor for the development of the symptom clusters among HSCT caregivers. In this study, lonelier caregivers were more likely to experience concurrent fatigue, sleep disturbance, depression, anxiety, and cognitive impairment than their less lonely counterparts. This study adds to a growing body of literature linking loneliness with the symptom clusters in diverse populations including family caregivers of individuals with cancer [7], cancer survivors [13,15], spousal caregivers of individuals with Alzheimer’s disease [13], and a general sample of older adults [16]. These studies found that loneliness was a common risk factor for the symptom clusters as well as changes in symptom clusters across these populations. Caregivers may be particularly susceptible to loneliness through their shrinking discretionary social interactions and changing social roles due to the fear of leaving the care recipient alone or the time demands associated with caregiving [14,30]. Little research, however, has examined loneliness and its consequences in caregiver populations. With loneliness and the symptom clusters on the rise [7,13,15,17], it is imperative to understand the impact of loneliness on symptom clusters present in HSCT caregivers. Furthermore, during the coronavirus disease 2019 (COVID-19) outbreak, social distancing and self-isolation have become a part of our daily routine, leading to an increased risk for individuals to experience loneliness [31]. Even before the pandemic, caregivers often isolated themselves from important social connections. Given COVID-19 restrictions are likely to be extended into the future, the caregiver’s risk of loneliness will likely increase. Therefore, studying the impact of COVID-19 on levels of loneliness and its sequelae in HSCT caregivers is warranted.
Loneliness is a multidimensional and complex emotional state linked to negative health consequences [32]. Hawkely and Cacioppo’s [33] model posited that loneliness serves as the social equivalent of physical pain, hunger, and thirst. It is an internal sensation that motivates the maintenance and formation of social connections necessary for the survival. When experienced temporarily, loneliness may activate similar stress responses in order to prepare the body to manage potential threats that may occur as a result of perceived loneliness and social disconnectedness. However, the chronically stressed state of loneliness may converge with the physiological responses that are seen in physical and psychological illness. This state may have deleterious effects on both sleep [34] and fatigue [13,15]. In addition, the breadth of emotional and cognitive processes is susceptible to the influence of loneliness. Studies have reported that loneliness is associated with increases in depressive symptoms [13,15,35], anxiety [36], impaired cognitive performance, and cognitive decline [37,38]. There is an increased prevalence in the symptom cluster of fatigue, sleep disturbance, depression, anxiety, and impaired cognition that is induced or exacerbated by loneliness and may be the mechanism underlying this relationship.
Immune system dysfunction may be a common physiological correlate of loneliness and symptom clusters and, thus, may partially explain the mechanism by which loneliness may lead to high symptom burden. People who were lonely had increased proinflammatory cytokines and glycoproteins, such as interleukin-6 (IL-6), tumor necrosis factor alpha (TNFα), interleukin-1 receptor antagonist (IL-1Ra), monocyte chemoattractant protein 1 (MCP-1), fibrinogen, and C-reactive protein (CRP) [39–41]. A growing body of research has proposed that acute or chronic inflammation may underlie the symptom clusters. Specifically, the co-occurrence of multiple symptoms, including fatigue, sleep disturbance, depression, anxiety, and impaired cognition, may present a type of “sickness behavior” induced by administration of proinflammatory cytokine [42,43]. The state of chronic stress caused by loneliness may also lead to dysregulation of sympathetic nervous system and the hypothalamic pituitary axis pathways that further elicit proinflammatory cytokine production [33]. Jaremka et al. [15] investigated the association between loneliness and the symptom cluster alongside immune dysfunction. They found that lonelier individuals had higher cytomegalovirus (CMV) antibody titers, which in turn, were associated with higher levels of the symptom cluster in breast cancer survivors. Expanding the scientific study of biological markers (e.g., cytokines, genomic DNA) would contribute to our understanding of the mechanisms explaining why and how loneliness positions individuals at risk for high symptom burden.
The findings of this study have implications for clinical practice. The approach focusing on symptom clusters may provide important insights leading to the development of a more effective and tailored intervention targeting multiple co-occurring symptoms within a cluster of HSCT caregivers. It is probable that the direct treatment of one symptom may indirectly affect another symptom within the cluster. However, few interventions specifically targeting symptom clusters have been tested and found to be effective in alleviating specific symptoms. A recent systematic review [44] provided preliminary evidence on interventions aimed at managing a symptom cluster consisting of pain, fatigue, and sleep disturbances among cancer survivors. In the 11 studies reviewed, three types of interventions (i.e., massage therapy, behavioral based therapy, and exercise- or movement-based therapy) were found to be effective in managing the symptoms for the entire cluster. Therefore, developing interventions to reduce symptom clusters should be considered. To promote symptom recognition, healthcare providers should provide baseline education explaining that symptoms may occur in clusters rather than in isolation and highlight strategies to facilitate better self-management, which could enable them to improve their health outcomes while meeting the needs of the care recipient.
Because loneliness was a significant risk factor differentiating the development of the symptom cluster among HSCT caregivers, healthcare providers could develop and implement interventions to increase social skills, provide social support, enhance opportunities for social interaction and social support, or address maladaptive social cognitions. A number of investigators have had preliminary efficacy mitigating feelings of loneliness, and the strategy of facilitating social interaction with peers or others as the most widespread approach to help address social isolation and loneliness [45–47]. Many of these social facilitation interventions involved group-based programs, such as friendship enriched programs [45], charity-funded friendship clubs [46], and peer-based interventions [47]. Cognitive-behavioral therapy (CBT) is another promising strategy to alleviate loneliness in caregivers. CBT-based psychoeducational interventions have been found to be effective at reducing levels of loneliness and related symptoms in heathy individuals experiencing loneliness [48] and lonely stroke survivors [49]. In addition, animal-assisted therapy (AAT) could alleviate loneliness by providing social support and companionship [50]. Banks et al. [51] found that elderly residents living in long-term care facilities with AAT, consisting of either a living or robotic dog, showed a significant reduction in loneliness compared to the control group. These strategies could help alleviate loneliness and, as a result, might decrease symptoms such as fatigue, sleep disturbance, depression, anxiety, and cognitive impairment. Future studies need to examine whether these or other interventions may be useful for decreasing loneliness in caregivers who are also experiencing symptom clusters. Notably, an array of technology-based interventions is increasingly being used as platforms to support family caregivers, including web-based interventions, smartphones with app-based technologies, videoconferencing technology, and virtual/augmented/mixed reality. Compared with face-to-face delivery interventions, an advantage of technology-based interventions is that they are always available and accessible, especially for caregivers with limited access to distant healthcare facilities with caregiver support services. The interventions can also be used at any time of day or night that the caregiver finds convenient [52]. Technology-based interventions allow individuals to facilitate online social support which allow participants to remain anonymous or identify themselves, customize their support services, and easily connect with other caregivers in similar situations [53]. Such interventions, or a combination of these diverse approaches might positively impact levels of loneliness and symptom burden among caregivers.
Limitations
Several limitations of this study should be considered. First, because this was cross-sectional a secondary analysis at a single point in time, we did not explore symptom cluster over time. A longitudinal design could further be conducted to identify the onset and rate of change in symptoms over time and to demonstrate how symptom clusters change at difference phases of the caregiving trajectory. The cross-sectional nature of the current study did not allow the study to determine the direction of the relationship between risk factors and symptom clusters. For example, the causal nature of the association between loneliness and symptom clusters may appear to be reciprocal. Future studies would need to examine this relationship longitudinally to better understand causality. The analysis also was part of the RCT that examined the efficacy of the stress reduction intervention, which limited the ability to assess other factors that may be important to symptom cluster membership. For example, patient outcomes, such as patient symptom burden and patient survival, could not be included in this analysis, although they might be associated with caregiver symptom burden. Second, the sample size for this study was relatively small, and that may have contributed to our inability to find a relationship between certain situational factors, such as patient treatment type or double-duty caregiving with symptom cluster membership. The study was limited by the lack of a comparison group. Further studies using large samples and those using comparison groups are needed to confirm the subgroup findings of the present study. Finally, this study recruited only caregivers of individual receiving HSCT at the NIH Clinical Center, a unique research hospital providing care to individuals enrolled on a clinical research protocol. Thus, the findings may not be generalizable to caregivers of patients receiving more traditional care in general hospitals or clinics.
Highlights.
Two symptom clusters were identified: low symptom burden and high symptom burden.
Loneliness was a significant predictor of the development of the symptom cluster.
Results may lead to the development of targeted interventions in HSCT caregivers.
Acknowledgements:
This research was supported by the Intramural Research Program at the National Institutes of Health, Clinical Center. The authors thank and to the caregivers who participated in this research study.
Funding:
This research was supported by the Intramural Research Program at the National Institutes of Health, Clinical Center.
Footnotes
Ethical Conduct of Research: The Office of Human Subjects Research Protections approved this study at the National Institutes of Health, Clinical Center (NCT#02257853).
Financial Disclosure Statement: There are no financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Health Resources and Services Administration. HRSA Blood Stem Cell - Donation and Transplantation Statistics, 2022. Available at: https://bloodstemcell.hrsa.gov/data/donationand-transplantation-statistics#:~:text=Registry%20Data%20page.-,Transplants%20Performed,19%25)%20were%20related%20transplants. Accessed September 15, 2022.
- 2.Applebaum AJ, Bevans M, Son T, et al. A scoping review of caregiver burden during allogeneic HSCT: lessons learned and future directions. Biol Bone Marrow Transplant. 2016;51(11):1416–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta V, Raj M, Hoodin F, Yahng L, Braun T, Choi SW. Electronic health record portal use by family caregivers of patients undergoing HSCT: United States national survey study. JMIR Cancer. 2021;7(1):e26509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie L, Shen C, Shi Y, Li H. Caregiver burden among primary family caregivers of patients undergoing HSCT: a cross-sectional study from Suzhou, China. Cancer Nurs. 2021;44(6):E556–E566. [DOI] [PubMed] [Google Scholar]
- 5.Ravyts SG, Sannes TS, Dzierzewski JM, et al. Check your sleep before you start: a secondary analysis of a stress management intervention for caregivers of stem cell transplant patients. Psycho-Oncology. 2021;30(6):936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamani K, Onstad LE, Bar M, et al. Quality of life of caregivers of hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2018;24(11):2271–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee LJ, Wehrlen L, Wallen GR, Ding Y, Ross A. Symptom clusters and influencing factors in family caregivers of individuals with cancer. Cancer Nurs. 2021;44(6):E547–E555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross A, Yang L, Klagholz SD, Wehrlen L, Bevans MF. The relationship of health behaviors with sleep and fatigue in transplant caregivers. Psycho-Oncology. 2016;25(5):506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28(4):270–282. [DOI] [PubMed] [Google Scholar]
- 10.Miaskowski C, Barsevick A, Berger A, et al. Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst. 2017;109(4):djw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alfheim HB, Rosseland LA, Hofsø K, Småstuen MC, Rustøen T. Multiple symptoms in family caregivers of intensive care unit patients. J Pain Symptom Manage. 2017;55(2):387–394. [DOI] [PubMed] [Google Scholar]
- 12.Castellanos EH, Dietrich MS, Bond SM, et al. Impact of patient symptoms and caregiving tasks on psychological distress in caregivers for head and neck cancer. Psycho-Oncology. 2019;28(3):511–517. [DOI] [PubMed] [Google Scholar]
- 13.Jaremka LM, Andridge RR, Fagundes CP, et al. Pain, depression, and fatigue: loneliness as a longitudinal risk factor. Health Psychol. 2014;33(9):948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray TF, Azizoddin DR, Nersesian PV. Loneliness among cancer caregivers: a narrative review. Palliat Support Care. 2020;18(3):359–367. [DOI] [PubMed] [Google Scholar]
- 15.Jaremka LM, Fagundes CP, Glaser R, Bennett JM, Malarkey WB, Kiecolt-Glaser JK. Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38(8):1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell VD, Abedini NC, Galecki AT, Kabeto M, Kumar N, Silveira MJ. Unwelcome companions: loneliness associates with the cluster of pain, fatigue, and depression in older adults. Gerontol Geriatr Med. 2021;7:2333721421997620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasileiou K, Barnett J, Barreto M, et al. Experiences of loneliness associated with being an informal caregiver: a qualitative investigation. Front Psychol. 2017;8:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee LJ, Shamburek RD, Son H, et al. Effects of a yoga-based stress reduction intervention on stress, psychological outcomes and cardiometabolic biomarkers in cancer caregivers: a randomized controlled trial. 2022; Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: an update. ANS Adv Nurs Sci. 1997;19(3):14–27. [DOI] [PubMed] [Google Scholar]
- 20.Lee SE, Vincent C, Finnegan L. An analysis and evaluation of the theory of unpleasant symptoms. ANS Adv Nurs Sci. 2017;40(1):E16–E39. [DOI] [PubMed] [Google Scholar]
- 21.Donovan KA, Stein KD, Lee M, Leach CR, Ilozumba O, Jacobsen PB. Systematic review of the multidimensional fatigue symptom inventory-short form. Support Care Cancer. 2015; 23(1):191–212. [DOI] [PubMed] [Google Scholar]
- 22.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010; 63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology 2013;80(11 Suppl 3):S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 25.Nijboer C, Triemstra M, Tempelaar R, Sanderman R, van den Bos GA. Measuring both negative and positive reactions to giving care to cancer patients: psychometric qualities of the Caregiver Reaction Assessment (CRA). Soc Sci Med. 1999;48(9):1259–1269. [DOI] [PubMed] [Google Scholar]
- 26.Walker SN, Sechrist KR, Pender NJ. The Health-Promoting Lifestyle Profile: development and psychometric characteristics. Nurs Res. 1987;36(2):76–81. [PubMed] [Google Scholar]
- 27.Corp Stata. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 28.Corp IBM. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp; 2020. [Google Scholar]
- 29.Khaddour K, Hana CK, Mewawalla P. Hematopoietic Stem Cell Transplantation. [Updated 2022 Jun 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK536951/ [PubMed] [Google Scholar]
- 30.Gibbons SW, Ross A, Wehrlen L, Klagholz S, Bevans M. Enhancing the cancer caregiving experience: building resilience through role adjustment and mutuality. Eur J Oncol Nurs. 2019;43:101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sannes TS, Yeh IM, Gray TF. Caring for loved ones with cancer during the COVID-19 pandemic: a double hit risk for social isolation and need for action. Psycho-Oncology. 2020;29(9):1418–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanguas J, Pinazo-Henandis S, Tarazona-Santabalbina FJ. The complexity of loneliness. Acta Biomed. 2018;89(2):302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawkley L, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40(2):218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffin SC, Mladen SN, Williams AB, et al. Sleep disturbance mediates the association between loneliness and health in older Americans. Int J Behav Med. 2021;28(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saadi JP, Carr E, Fleischmann M, et al. The role of loneliness in the development of depressive symptoms among partnered dementia caregivers: evidence from the English Longitudinal Study of Aging. Eur Psychiatry. 2021;64(1):e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park C, Majeed A, Gill H, et al. The effect of loneliness on distinct health outcomes: a comprehensive review and meta-analysis. Psychiatry Res. 2020;294:113514. [DOI] [PubMed] [Google Scholar]
- 37.Luo Y, Hawkley LC, Waite LJ, Cacioppo JT. Loneliness, health, and morality in old age: a national longitudinal study. Soc Sci Med. 2012;74(6):907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theeke LA, Mallow JA, Moore J, et al. Using gene expression analysis to examine changes in loneliness, depression and systemic inflammation in lonely chronically ill older adults. Open J Nurs. 2016;6(8):620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hackett RA, Hamer M, Endrighi R, Brydon L, Steptoe A. Loneliness and stress-related inflammatory and neuroendocrine responses in older men and women. Psychoneuroendocrinology. 2012;37(11):1801–1809. [DOI] [PubMed] [Google Scholar]
- 40.Jaremka LM, Fagundes CP, Peng J, et al. Loneliness promotes inflammation during acute stress. Psychol Sci. 2013;24(7):1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nersesian PV, Han HR, Yenokyan G, et al. Loneliness in middle age and biomarkers of systemic inflammation: findings from Midlife in the United States. Soc Sci Med. 2018;209:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwekkeboom KL, Tostrud L, Costanzo E, et al. The role of inflammation in the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. J Pain Symptom Manage. 2018;55(5):1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheikh-Wu SF, Downs CA, Anglade D. Interventions for managing a symptom cluster of pain, fatigue, and sleep disturbances during cancer survivorship: a systematic review. Oncol Nurs Forum. 2020;47(4):E107–E119. [DOI] [PubMed] [Google Scholar]
- 45.Bouwman TE, Aartsen MJ, van Tilburg TG, Stevens NL. Does stimulating various coping strategies alleviate loneliness? Results from an online friendship enrichment program. J Soc Pers Relat. 2017;34(6):793–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hemingway A, Jack E. Reducing social isolation and promoting well-being in older people. QAOA. 2013;14(1):25–35. [Google Scholar]
- 47.Lai DWL, Li J, Ou X, Li CYP. Effectiveness of a peer-based intervention on loneliness and social isolation of older Chinese immigrants in Canada: a randomized controlled trial. BMC Geriatr. 2020;20(1):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Käll A, Jägholm S, Hesser H, et al. Internet-based cognitive behavior therapy for loneliness: a pilot randomized controlled trial. Behav Ther. 2019;51(1):54–68. [DOI] [PubMed] [Google Scholar]
- 49.Theeke LA, Mallow JA, Theeke E. A pilot one group feasibility, acceptability, and initial efficacy trial of LISTEN for loneliness in lonely stroke survivors. SAGE Open Nurs. 2021;7: 23779608211015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krause-Parello CA. Pet ownership and older women: the relationships among loneliness, pet attachment support, human social support, and depressed mood. Geriatr Nurs. 2012;33(3):194–203. [DOI] [PubMed] [Google Scholar]
- 51.Banks MR, Willoughby LM, Banks WA. Animal-assisted therapy and loneliness in nursing homes: use of robotic versus living dogs. J Am Med Dir Assoc. 2008;9(3):173–177. [DOI] [PubMed] [Google Scholar]
- 52.Lindeman DA, Kim KK, Gladstone C, Apesoa-Varano EC. Technology and caregiving: emerging interventions and directions for research. Gerontologist. 2020;60(Suppl 1):S41–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colvin J, Chenoweth L, Bold M, Harding C. Caregivers of older adults: advantages and disadvantages of internet-based social support. Fam Relat. 2004;53(1):49–57. [Google Scholar]