Abstract
RASopathies are a set of clinical syndromes that have molecular and clinical overlap. Genetically, these syndromes are defined by germline pathogenic variants in RAS/MAPK pathway genes resulting in activation of this pathway. Clinically, their common molecular signature leads to comparable phenotypes, including cardiac anomalies, neurologic disorders and notably, elevated cancer risk. Cancer risk in individuals with RASopathies has been estimated from retrospective reviews and cohort studies and have found clear associations with some RASopathies and increased cancer incidence. For example, in Costello syndrome, cancer incidence is significantly elevated over the general population, largely due to solid tumors. In some forms of Noonan syndrome, cancer risk is also elevated over the general population and is enriched for hematologic malignancies. Thus, cancer surveillance guidelines have been developed to monitor for the occurrence of such cancers in individuals with some RASopathies. These include abdominal ultrasound and urinalyses for individuals with Costello syndrome, while complete blood counts and splenic examination are recommended in Noonan syndrome. Improved cancer risk estimates and refinement of surveillance recommendations will improve the care of individuals with RASopathies.
Keywords: RASopathy, cancer predisposition, screening
The importance of the RAS pathway in cancer
RAS/MAPK signaling is involved in nearly every facet of cell biology, and it is therefore not surprising that pathogenic variants disrupting these vital cellular processes have potent effects on disease development. Mutations in the RAS/MAPK pathway are among the most common oncogenic variants detected across a range of both adult and pediatric cancers, in some cases approaching 90% (Kamisawa, Wood et al. 2016, Grobner, Worst et al. 2018, Ma, Liu et al. 2018). Germline pathogenic variants in the RAS/MAPK pathway give rise to a spectrum of clinical syndromes called RASopathies, which can affect multiple organ systems, including cardiac, skeletal and neurologic. Given the importance of the RAS pathway in somatic cancer development, germline RAS signaling pathway hyperactivation predisposes some of these individuals to cancer. Care of these high-risk individuals is challenging, and one could consider tumor surveillance as part of their routine care. However, recommendations for screening vary across professional opinions and depends not only on the specific RASopathy but also the specific variant of that individual. Thus, careful consideration is needed and is likely unique for each patient. Successful treatment of individuals with a RASopathy and cancer is likely dependent on the ability to identify them early, as has been shown in other tumor predisposition syndromes, such as Li-Fraumeni (Ballinger, Best et al. 2017). Therefore, having detailed knowledge of the incidence of cancer, and cancer type, in each of the RASopathies to inform recommended screening protocols is crucial for the optimal clinical management of these patients.
In this article, we undertook a librarian-informed comprehensive literature review of what is known, and remains unknown, about cancer incidence in the non-neurofibromatosis type 1 (NF1) RASopathies and discuss current recommendations for cancer surveillance.
Methods
A biomedical librarian conducted a literature search in the PubMed (US National Library of Medicine) and Embase (Elsevier) citation and abstract databases in August 2022. Two search strategies were created with input from the authors to find articles on 1) clinical management of all RASopathies and 2) cancer incidence of non-NF1 RASopathies in humans. The search strategies incorporated both keywords and controlled vocabulary terms (i.e., MeSH (PubMed) and EMTREE (Embase)) for the specified RASopathies and concepts of interest (i.e., clinical management and cancer incidence). Key articles identified by the authors were reviewed to further identify keywords for the searches. The searches were not limited by language nor publication year but were limited to exclude animal studies and specific article types (e.g., conference abstracts, conference proceedings, letters, editorials, commentary, case reports, case series, corrigenda, errata, protocols). See Supplemental Materials for final search strategies used. All search results from each database were exported to EndNote 20 (Clarivate Analytics) and duplicates identified and removed. Two reviews in Covidence (Veritas Health Innovations, Ltd.) were setup and used to screen and select relevant articles from the unique search results.
A total of 1289 unique articles were retrieved on clinical management of all RASopathies and 375 on cancer incidence of non-NF1 RASopathies. One author screened each article to determine its relevance to the specific questions. Each reviewer assessed the title and abstract returned during the search to determine appropriateness for inclusion. After abstract review, a final list of references was obtained and a second examination by the same reviewer was conducted using full-text to determine relevance to manuscript content.
Costello Syndrome
Costello syndrome is a RASopathy with a reported prevalence of 1:380,000 live births (Gripp and Rauen 1993). The majority of individuals with Costello syndrome carry an HRAS p.G12S variant, and therefore represent a large percentage (~80%) of this population (Gripp, Morse et al. 2019). Given the identification of the causative gene for Costello syndrome, genotype-phenotype correlations have been noted, including HRAS p.G12D, HRAS p.G12V, HRAS p.G12C and HRAS p.G12E, which often have a severe presentation early in life, most often cardiac in origin (Gripp, Morse et al. 2019). Although rare, less severely affected genotype-phenotypes have been associated with other HRAS variants, such as HRAS p.G60D (Gripp, Sol-Church et al. 2015).
Cancer Incidence
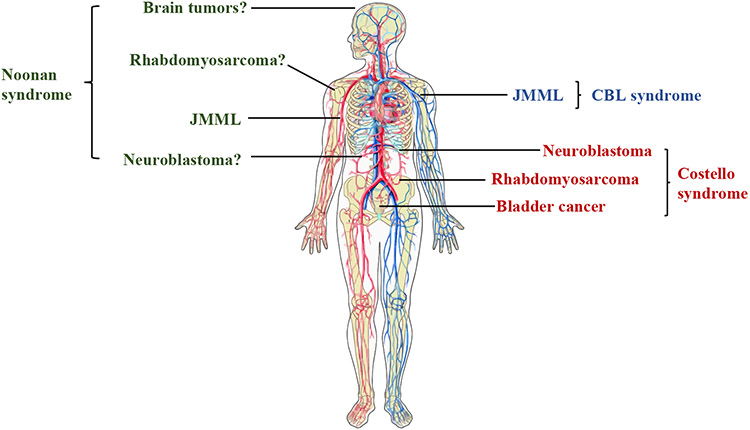
Despite its low prevalence, there are clear data indicating that individuals with Costello syndrome have increased cancer risk. Previous analyses of cancer cases among 735 individuals with a germline variant in a RASopathy gene showed a cancer incidence roughly 40-fold higher in Costello syndrome than in the general population (Kratz, Franke et al. 2015) and represents an estimate of cancer incidence based on existing data. For specific HRAS variants, such as HRAS p.G12A, cancer incidence may be even higher (Gripp, Morse et al. 2019) (Table 1). These more recent observations remain consistent with the earliest cancer estimates in Costello syndrome, where the solid tumor incidence was initially 17% (Gripp, Scott et al. 2002) and add further observations regarding potential genotype-phenotype associations. The cancers reported in Costello syndrome are largely early-onset solid tumors, such as rhabdomyosarcoma (highest risk until age 6), neuroblastoma (also in early childhood) and bladder cancer in childhood and adolescence (Gripp, Scott et al. 2002, Villani, Greer et al. 2017) (Figure 1). In a study of pediatric patients enrolled in Children’s Oncology Group trials for rhabdomyosarcoma, about 7% had an underlying cancer predisposition syndrome (Li, Sisoudiya et al. 2021). Of those, 11% had a pathogenic/likely pathogenic germline variant in HRAS, representing 1.4% of all individuals with embryonal rhabdomyosarcoma (Li, Sisoudiya et al. 2021).
Table 1:
Incidence of Specific Cancer Types in RASopathies. ALL: Acute lymphocytic leukemia; AML: Acute myeloid leukemia; JMML: Juvenile myelomonocytic leukemia
| RASopathy | Most Common Cancer Type(s) |
Approximate Incidence |
|---|---|---|
| Costello syndrome (HRAS) | General | ~40-fold increase over general population |
| Bladder cancer | ~1.5% | |
| Neuroblastoma | ~2% | |
| Rhabdomyosarcoma | ~7% | |
| Noonan syndrome (PTPN11; e.g., codon 61 or T73I), (KRAS; e.g., T58I) | JMML, rhabdomyosarcoma, ALL, neuroblastoma, brain tumors | ~8-fold increase over general population |
| Noonan syndrome (SOS1, RAF1, RIT1, SOS2, RRAS, LZTR1, BRAF) | Case reports of ALL, neuroblastoma, rhabdomyosarcoma | Unclear risk |
| Noonan syndrome with multiple lentigines (PTPN11, RAF1, BRAF, MAP2K1) | Case reports of AML, ALL, neuroblastoma, melanoma | Possibly mildly elevated, although unclear how much so |
| Noonan syndrome with loose anagen hair (PPPC1B, SHOC2) | Case reports of neuroblastoma, myelofibrosis, t cell lymphoma | Possibly mildly elevated, although unclear how much so |
| CBL syndrome (CBL) | JMML | Elevated, but unclear how much so |
| Cardiofaciocutaneous syndrome (BRAF, MAP2K1, MAP2K2, KRAS) | Case reports of rhabdomyosarcoma, ALL, lymphoma | Possibly mildly elevated, although unclear how much so |
| Legius syndrome (SPRED1) | Case reports of pediatric leukemias | Not believed to be elevated |
Figure 1:
Common cancer associations across the RASopathies. JMML: Juvenile myelomonocytic leukemia. Blue= CBL syndrome; Green= Noonan syndrome; Red= Costello syndrome. Image created with ChemDraw.
In addition to rhabdomyosarcoma, individuals with Costello syndrome are at high risk for a range of other solid tumors. In childhood, individuals with Costello syndrome are at increased risk of neuroblastoma, with an incidence close to 1% (Kratz, Franke et al. 2015). Similar to rhabdomyosarcoma, risk for neuroblastoma tends to occur early in infancy and young childhood, consistent with non-syndromic neuroblastoma diagnoses (Kratz, Rapisuwon et al. 2011). In parallel to the high frequency of somatic HRAS mutations reported in bladder cancer, individuals with germline HRAS variants are also at elevated risk (Kompier, Lurkin et al. 2010, Kratz, Franke et al. 2015, Yee, Zheng et al. 2019). Notably, unlike non-syndromic bladder cancer, which often occurs after age 60, increased bladder cancer risk begins at age 10 years in Costello syndrome (Gripp 2005, Beukers, Hercegovac et al. 2014, Villani, Greer et al. 2017, Gripp, Morse et al. 2019). Furthermore, while the majority of those diagnosed with bladder cancer are > 10 years of age, bladder cancer in CS has been detected in much younger patients (Yu, Luk et al. 2019). Despite the well-characterized increased solid tumor risk, hematologic malignancies in Costello syndrome are likely rare, although myeloproliferative disorders have recently been newly described (Pabari, Chun et al. 2022)
Surveillance Guidelines
Given the high risk of cancers in these younger populations and well-defined cancer types, tumor surveillance recommendations exist for Costello syndrome. The most recent consensus recommendations for screening were detailed by Villani et al in 2017 and include physical exam and abdominal ultrasound with or without a chest radiograph every 3-4 months for patients under 10 years old, and annual urinalysis for those ≥ 10 years (Table 1). The recommendation to include a chest radiograph for the surveillance of neuroblastoma was added by Villani, et al to align with other recommendations for individuals at high risk for developing neuroblastoma (Kamihara, Bourdeaut et al. 2017). Given the frequency with which chest radiographs are recommended and exposure to radiation, the interval between chest radiographs and their completion should be a shared decision between caregivers and providers. The first set of tumor surveillance recommendations for Costello syndrome were proposed by Gripp et al in 2002 and included: 1) routine ultrasounds of the abdomen and pelvis through 10 years of age to screen for rhabdomyosarcoma and abdominal neuroblastoma, 2) urine catecholamines and metabolites every 6-12 months until 5 years old for neuroblastoma and 3) urinalysis for assessment of hematuria annually starting at 10 years old (Gripp, Scott et al. 2002). Subsequent to this proposal, it was found that patients with Costello syndrome have a baseline increase in urinary catecholamines without the presence of neuroblastoma and therefore the recommendation for routine urinary catecholamine screening was rescinded (Gripp 2005).
In addition to the consensus recommendations by Villani et al, several single center cohort studies have led to additional surveillance considerations in these patients. These are not consensus recommendations and should therefore be utilized and considered on a case-by-case basis. In 2010, Ahmadi et al recommended nasal endoscopy and ear examination including tympanography every 4-6 months to screen for nasopharyngeal rhabdomyosarcoma (Ahmadi and Harley 2010). In 2022, Leoni et al reported that 10 of 13 asymptomatic patients were found by cystoscopy to have bladder lesions that were not identified by ultrasound or urinalysis, and therefore recommended that cystoscopy every 12-24 months be added as a routine screening in patients with Costello syndrome > 10 years old (Leoni, Paradiso et al. 2022). Given the invasiveness and unclear benefit of these procedures, additional studies are needed to determine if these screening methodologies will provide clinically meaningful improvement in outcomes for these patients.
Noonan Syndrome
Noonan syndrome, similar to other RASopathies, is characterized by multi-system involvement with characteristic cardiac defects and short stature (Roberts, Allanson et al. 2013). The estimated incidence of Noonan syndrome is around 1:2500 live births, however, it is thought that the incidence may be higher due to under- or unrecognized mild phenotypes. About half of all individuals with a diagnosis of Noonan syndrome have a pathogenic or likely pathogenic variant in PTPN11, which leads to increased cancer risk, as discussed below.
Cancer Incidence
Studies evaluating cancer risk in Noonan syndrome are overwhelmingly derived from PTPN11-positive individuals, while cancer predisposition is less often reported in rarer Noonan-associated genes. For example, a recent Noonan syndrome study showed that individuals with a PTPN11 variant have a high risk of malignancy (~10%), however this is likely an overestimate as about half of those individuals developed juvenile myelomonocytic leukemia (JMML)-like myeloproliferation and not frank JMML (Li, Yao et al. 2019). Importantly, specific variants have been associated with a higher risk of myeloproliferative disorders and JMML, notably variants at codon 61 or T73I in PTPN11 and T58I in KRAS (Kratz, Niemeyer et al. 2005, Schubbert, Zenker et al. 2006, Strullu, Caye et al. 2014). However, this list is likely not exhaustive and, as more is learned about cancer risk and genetics of individuals with RASopathies, new associations with high-risk or low-risk features will likely be uncovered. Overall, when excluding patients diagnosed with Noonan syndrome because of a JMML-like myeloproliferative condition, childhood cancer risk in individuals with Noonan syndrome due to any pathogenic/likely pathogenic variant is roughly 8-fold increased when compared to the general population (Kratz, Franke et al. 2015). Notably, literature-reported cases of Noonan syndrome with cancer have been examined, with cancer incidence estimated at 4% by age 20 (Kratz, Rapisuwon et al. 2011). However, this may be an overestimate of true cancer risk as ascertainment bias is likely when evaluating clinical phenotypes reported rather than examining the entire population that carries a genetic diagnosis.
Dysregulation of the RAS/MAPK pathway is common in pediatric hematologic malignancies and is often associated with somatic or germline variants in the pathway (Tartaglia, Niemeyer et al. 2003, Bolouri, Farrar et al. 2018, Ma, Liu et al. 2018). Within the hematopoietic stem cell compartment, hyperactive RAS signaling leads to clonal expansion and hematopoietic progenitor survival, most often skewing towards a myeloid phenotype (Li, Bohin et al. 2013, Ney, Yang et al. 2021). Thus, the elevated risk for development of hematologic cancers and myeloproliferative neoplasms is plausible. In this vein, studies of infantile cancers over a 14-year period in France observed 50 individuals with JMML, of whom 10 were in individuals with Noonan syndrome (Desandes, Faure et al. 2020). Additionally, JMML-like myeloproliferation occurs in a high percentage of individuals with germline PTPN11 variants and can be self-limited (Niemeyer 2014). However, a small percentage (~10%) of these individuals can progress to frank JMML and connects the increased risk of JMML to Noonan syndrome (Tartaglia, Niemeyer et al. 2003, Niemeyer 2014, Locatelli and Niemeyer 2015). Although spontaneous regression of JMML and JMML-like myeloproliferation in Noonan syndrome are common and the majority of myeloid disorders occur in pediatric patients with Noonan syndrome, adults with acute myeloid leukemia (AML) and germline variants in PTPN11 have been reported (Yang, Long et al. 2022). Cancer risk in adults with Noonan syndrome remains largely unknown. Finally, while dysregulated RAS signaling within the hematopoietic system favors clonal expansion within the myeloid compartment, hyperdiploid acute lymphoblastic leukemia (ALL) has been reported in 0.5% and 2% of patients with a germline pathogenic/likely pathogenic variant in PTPN11 and SOS1, respectively (Cave, Caye et al. 2016)
While the highest risk of cancer in Noonan syndrome is for hematologic malignancies, cases of solid tumors have also been commonly described (Jongmans, van der Burgt et al. 2011, Kratz, Franke et al. 2015). Similar to Costello syndrome, cases of neuroblastoma and rhabdomyosarcoma have been reported in Noonan syndrome, typically in younger children (Jung, Bechthold et al. 2003, Li, Yao et al. 2019). An analysis of the German Childhood Cancer Registry showed an approximately 8-fold increased cancer risk in patients with Noonan syndrome, of which there were three Noonan children with a germline PTPN11 variant and a solid tumor: one neuroblastoma, one pilocytic astrocytoma and one dysembryoplastic neuroepithelial tumour (Kratz, Franke et al. 2015) (Figure 1). Furthermore, multiple literature cases exist describing individuals with Noonan syndrome and solid tumors including rhabdomyosarcoma, brain tumors and neuroblastoma (Khan, McDowell et al. 1995, Lopez-Miranda, Westra et al. 1997, Jung, Bechthold et al. 2003, Sherman, Ali-Nazir et al. 2009, Hastings, Newbury-Ecob et al. 2010, Jongmans, Hoogerbrugge et al. 2010, Rankin, Short et al. 2013, Garavelli, Cordeddu et al. 2015, Harms, Alawi et al. 2018, Boonyawat, Charoenpitakchai et al. 2019, El-Ayadi, Ansari et al. 2019, Garren, Stephan et al. 2020). While these reports suggest a possible association, a definitive, quantitative analysis of solid tumor risk is still needed to understand its true incidence in Noonan syndrome.
Surveillance guidelines
The risk of hematologic disorders in Noonan syndrome is most notably increased in those patients with specific PTPN11 (e.g., variants at codon 61 or T73I) and KRAS (e.g., T58I) variants (Kratz, Niemeyer et al. 2005, Schubbert, Zenker et al. 2006). Therefore, the most recent consensus by Villani et al recommends those patients with higher risk germline pathogenic variants undergo a physical exam (with assessment of spleen) and complete blood count (CBC) with a differential every 3–6 months until 5 years of age (Villani, Greer et al. 2017) (Table 2). Of note, although this group did not recommend specific surveillance for those with other germline variants causing Noonan syndrome (e.g., SOS1, NRAS, RAF1, BRAF, SHOC2), several other groups have recommended that these patients get a baseline CBC as part of their initial RASopathy evaluation and, if diagnosed in infancy, at least one additional CBC after one year of age. In addition, routine physical exams should include evaluation for hepatosplenomegaly and a low threshold for additional work-up, such as a bone marrow aspirate/biopsy if any abnormalities are found on CBC (Roberts, Allanson et al. 2013, Porter, Druley et al. 2017). As expected, recommendations are concentrated on the well-defined hematologic malignancy risk in Noonan syndrome, but strategies for solid tumor surveillance do not exist because of anecdotal and descriptive associations reported in the literature. Thus, screening individuals with Noonan syndrome for solid tumors relies on joint decision-making between the primary physician and families.
Table 2:
Summary of Published Surveillance Guidelines in RASopathies
| RASopathy | Cancer Surveillance Recommendations |
Source | Source Details |
|---|---|---|---|
| Costello syndrome (HRAS) | 0 to 8–10 yrs: Physical exam and abdominal ultrasound +/− Chest radiograph every 3–4 months Age 10+: Annual urinalysis |
(Villani, Greer et al. 2017) | Consensus RecommendationsA (American Association for Cancer Research) |
| 0 to 8–10 yrs: Physical examination plus abdominal and pelvic ultrasounds are suggested every 3 months Age 10+: Annual urinalysis |
(Gripp, Morse et al. 2019) | Expert opinionB | |
| Nasal endoscopy and ear examination including tympanography every 4-6 months | (Ahmadi and Harley 2010) | Expert opinionB | |
| Age 10+: Cystoscopy every 12-24 months | (Leoni, Paradiso et al. 2022) | Expert opinionB | |
| Cardiofaciocutaneous syndrome (BRAF, MAP2K1, MAP2K2, KRAS) | No routine surveillance* | (Rauen, Adam et al. 1993, Pierpont, Magoulas et al. 2014, Villani, Greer et al. 2017) | Consensus RecommendationsA (American Association for Cancer Research) & Expert opinions |
| Noonan syndrome with specific high-risk mutations (PTPN11; e.g., codon 61 or T73I), (KRAS; e.g., T58I) | 0 to 5 years: Physical exam (with assessment of spleen) and CBC with differential every 3–6 months | (Villani, Greer et al. 2017) | Consensus RecommendationsA (American Association for Cancer Research) |
| CBC with differential at baseline evaluation and then as clinically indicated; physical exam with evaluation for hepatosplenomegaly | (Porter, Druley et al. 2017) | Consensus RecommendationsA (American Association for Cancer Research) | |
| Noonan syndrome; no high risk variant (SOS1, RAF1, RIT1, SOS2, RRAS, LZTR1, BRAF, non high-risk PTPN11, KRAS) | No routine surveillance* | (Villani, Greer et al. 2017) | Consensus RecommendationsA (American Association for Cancer Research) |
| CBC with differential at diagnosis and after 6–12 months of age if initial screen performed in infancy | (Romano, Allanson et al. 2010) | Interdisciplinary Expert PanelA (Noonan Syndrome Support Group) | |
| CBC with differential at diagnosis and repeat at least once after >1 year old, then as clinically indicated; physical exam with evaluation for hepatosplenomegaly | (Roberts, Allanson et al. 2013) | Expert opinionB | |
| CBL syndrome (CBL) | 0 to 5 years: Physical exam (with assessment of spleen) and CBC with differential every 3–6 months | (Villani, Greer et al. 2017) | Consensus RecommendationsA (American Association for Cancer Research) |
| CBC with differential at baseline evaluation and then as clinically indicated; physical exam with evaluation for hepatosplenomegaly | (Porter, Druley et al. 2017) | Consensus RecommendationsA (American Association for Cancer Research) | |
| CBC with differential at baseline evaluation and at least once after > 1 year old, then as clinically indicated; physical exam with evaluation for hepatosplenomegaly | (Roberts, Allanson et al. 2013) | Expert opinionB | |
| Noonan syndrome with multiple lentigines (PTPN11, RAF1, BRAF, MAP2K1) | No routine surveillance* | (Villani, Greer et al. 2017) | Consensus RecommendationsA (American Association for Cancer Research) |
| Noonan syndrome with loose anagen hair (PPPC1B, SHOC2) | No routine surveillance* | (Villani, Greer et al. 2017) | Consensus RecommendationsA (American Association for Cancer Research) |
| Legius Syndrome | No routine surveillance* | (Villani, Greer et al. 2017) | Consensus RecommendationsA (American Association for Cancer Research) |
For patients with these conditions, there should still be increased awareness and low threshold for investigating new potential tumor-related symptoms (Villani, Greer et al. 2017).
Consensus recommendations are defined as widely accepted guidelines from disease-specific experts in the field.
Expert opinion is defined as a recommendation based on individual subspecialist or single institution study. These opinions are not widely accepted in the RASopathy community but are included in this review for completeness.
Noonan syndrome with multiple lentigines (NSML) and Noonan syndrome with loose anagen hair (NSLH)
Noonan-like spectrum disorders, NSML and NSLH, have overlapping features with classic Noonan syndrome such as cardiac anomalies but also distinctive characteristics (e.g., many lentigines in NSML and slow-growing hair in NSLH). NSML is a Noonan-like disorder due to pathogenic germline variants in BRAF, MAP2K1, PTPN11, and RAF1 (Gelb and Tartaglia 1993) and can be mistaken for classic Noonan syndrome in childhood if lentigines are not present and molecular testing is unknown or not pursued (Sarkozy, Digilio et al. 2008). NSLH, also a rare Noonan-like spectrum disorder, is caused by pathogenic germline variants in SHOC2 (most common) and PPP1CB (Cordeddu, Di Schiavi et al. 2009, Gripp, Aldinger et al. 2016).
Cancer incidence
The majority of cancers described in NSML and NSLH have been case reports and therefore specific estimates of cancer incidence are not well described. Rare hematologic malignancies, such as myelofibrosis and T-cell lymphoma, have been reported in NSLH (Gripp, Zand et al. 2013, Avery, Metcalf et al. 2022). Additionally, there have been cases of neuroblastoma in individuals with SHOC2 variants (Garavelli, Cordeddu et al. 2015). In NSML, five cases of cancer were observed in 296 individuals with NSML in a literature review, estimating cancer incidence just above 1% (Kratz, Rapisuwon et al. 2011). Several cases of leukemia (ALL and AML) have been reported in NSML (Ucar, Calyskan et al. 2006, Laux, Kratz et al. 2008) Additional case reports in NSML include melanoma, which is perhaps not surprising given the skin lesions associated with NSML (Garcia-Gil, Alvarez-Salafranca et al. 2020). These reports suggest a possible association between Noonan-like syndromes and cancer, but the lack of epidemiologic data in these disorders remains problematic to conclusively define cancer risk and propose surveillance guidelines.
Road to discovery: LZTR1 and SPRED2 in Noonan Syndrome
With improving and evolving genetic sequencing efforts, new genes are being discovered as causative for RASopathies, including Noonan syndrome. LZTR1 and SPRED2 are two such examples (Johnston, van der Smagt et al. 2018, Motta, Fasano et al. 2021). LZTR1, most often associated with autosomal dominant schwannomatosis (Piotrowski, Xie et al. 2014), has been identified as causative for both autosomal dominant and autosomal recessive Noonan syndrome (Roberts, Adam et al. 1993, Johnston, van der Smagt et al. 2018). Given the known association with schwannomatosis risk, individuals with LZTR1-associated Noonan syndrome may also be at increased risk for cancer. Case reports in LZTR1-Noonan syndrome have described Grade II-III oligoastrocytoma and ALL (Johnston, van der Smagt et al. 2018, Jacquinet, Bonnard et al. 2020), however the relative novelty of this gene/syndrome association means there is uncertainty regarding cancer risk. In SPRED2-associated Noonan syndrome, first described in 2021, even less is known about clinical phenotype (Motta, Fasano et al. 2021). In functional laboratory studies, similar to SPRED1, SPRED2 acts as a tumor suppressor gene (Kachroo, Valencia et al. 2013, Jiang, Liu et al. 2016). Thus, one could postulate an increased cancer risk for individuals who carry a pathogenic/likely pathogenic germline variant. However, given the redundancy and nuances of the RAS pathway, prospective studies must be done to define true cancer incidence in these individuals.
CBL Syndrome
CBL syndrome, first described in 2010 as a disorder with similarities to Noonan syndrome, is due to germline pathogenic/likely pathogenic variants in CBL, a negative regulator of receptor tyrosine kinase activity (Niemeyer, Kang et al. 2010). Thus, CBL works upstream of the Ras protein to impact its downstream signaling cascades.
Cancer incidence
Individuals with germline variants in CBL are enriched in cohorts of JMML patients, suggesting there is an increased risk for JMML development (Niemeyer, Kang et al. 2010, Perez, Mechinaud et al. 2010). Importantly, loss of heterozygosity was often observed in individuals with CBL germline variants, suggesting it is directly involved in tumorigenesis (Locatelli and Niemeyer 2015) (Figure 1). Initially, and similar to individuals with PTPN11 variants, germline CBL variants detected in JMML samples were believed to be associated with a less aggressive clinical course (Stieglitz, Mazor et al. 2017). However, recent data points to a more unpredictable course in CBL-associated JMML (Hecht, Meyer et al. 2022). Thus, given the recent description of CBL syndrome, additional data will be needed to fully ascertain cancer risks and genotype-phenotype correlations (Martinelli, Stellacci et al. 2015).
Even less clear than the role of CBL in hematologic malignancies is its role in solid tumors. Of children with pediatric rhabdomyosarcoma enrolled in Children’s Oncology Group studies with a cancer predisposition syndrome (~7% of all cases), 4% harbored a pathogenic/likely pathogenic germline variant in CBL, suggesting there is an additional solid tumor risk (Li, Sisoudiya et al. 2021). Therefore, longitudinal studies and systematic retrospective reviews are needed to better define these solid tumor risks for patients with germline CBL variants (Ji, Navid et al. 2019, Kim, Light et al. 2021).
Surveillance guidelines
Patients with CBL syndrome have an increased risk of JMML/myelodysplastic syndrome (MDS) that is similar to patients with high-risk Noonan genotypes (such as variants at codon 61 or T73I in PTPN11 or T58I in KRAS) (Roberts, Allanson et al. 2013). Therefore, similar screening guidelines are recommended, including physical exams (with assessment of spleen) and CBC with differential every 3–6 months until 5 years of age (Villani, Greer et al. 2017), when the risk for JMML is diminished (Table 2).
Cardiofaciocutaneous syndrome (CFC)
Cardiofaciocutaneous syndrome (CFC) is a rare condition, with the best estimates placing prevalence around 1:810,000 individuals in Japan (Abe, Aoki et al. 2012), although confirmatory national and international epidemiologic studies are lacking. CFC syndrome, like many of the other RASopathies, is characterized by distinct cardiac and facial anomalies, neurocognitive/developmental disorders, and skin manifestations due to germline variants in BRAF, MAP2K1, MAP2K2 and KRAS (Rauen, Adam et al. 1993).
Cancer incidence
Assessing true cancer risk in CFC syndrome is challenging due to the limited number of individuals reported with this condition (Table 1). Nonetheless, individual cases of ALL, non-Hodgkin lymphoma, B-cell lymphoma and hepatoblastoma have been reported in patients with CFC (Pierpont, Magoulas et al. 2014). A literature-based review identified eight cases of cancer among 226 individuals with CFC (3.5%) which was close to the estimated incidence of cancer in Noonan syndrome (Kratz, Rapisuwon et al. 2011). However, more recent retrospective oncology studies did not identify individuals with a concurrent diagnosis of CFC and cancer (Kratz, Franke et al. 2015), making it unclear if clinical (rather than genetic) diagnoses of CFC contributed to this discrepancy (Bisogno, Murgia et al. 1999). Additional support for non-elevated cancer risk in CFC come from ALL and rhabdomyosarcoma studies, where individuals with concurrent cancer and CFC diagnosis were not identified (Cave, Caye et al. 2016, Li, Sisoudiya et al. 2021). Although not malignant per se, pathogenic/likely pathogenic germline variants in BRAF, MAP2K1, MAP2K2 are associated with a higher incidence of melanocytic nevi in CFC patients and could serve as an initiating event in melanoma tumorigenesis, although melanoma risk remains unknown (Kiuru, Urban et al. 2020).
Surveillance guidelines
A recent 2016 consensus conference of the American Association for Cancer Research recommended that screening guidelines should be established for syndromes when known cancer risk exceeds 5% in the first 20 years of life (Brodeur, Nichols et al. 2017). Thus, given the uncertainty of cancer incidence in CFC syndrome, tumor surveillance guidelines do not currently exist, and further longitudinal and genetic studies are needed to precisely define the genotype-cancer phenotypes.
Legius syndrome
Legius syndrome, a RASopathy with phenotypic overlap with neurofibromatosis type 1, is due to SPRED1 variants (Legius, Messiaen et al. 2021). Pathogenic germline variants in SPRED1 activate RAS/MAPK signaling through effects on neurofibromin (Yan, Markegard et al. 2020). However, despite the clinical similarities with NF1, individuals with Legius syndrome have a cancer incidence similar to the general population (Legius, Stevenson et al. 1993, Yan, Markegard et al. 2020) and thus diverges from NF1 in this very important phenotype. Hence, tumor surveillance is not recommended at this time.
Future of Screening and Surveillance in RASopathies
Early tumor detection is key to improved outcomes. In RASopathies, there are multiple potential avenues to achieve this goal. Annual whole-body MRI screening has been effective in Li-Fraumeni syndrome but remains unstudied in the RASopathies (Ballinger, Best et al. 2017). Improvements in clinical phenotyping and pre-natal screening may permit earlier RASopathy testing and thus identification of these patients and better surveillance starting at birth (Sinajon, Chitayat et al. 2020). Assay of circulating tumor DNA to perform “liquid biopsies” from blood samples as a screening or surveillance tool for cancers may soon be a reality (Sundby, Pan et al. 2022). For example, in NF1, circulating tumor DNA can distinguish between the malignant and benign peripheral nerve sheath tumors that can develop (Szymanski, Sundby et al. 2021, Mattox, Douville et al. 2022). If similar results could be achieved in other RASopathies, it could address ongoing concerns about limited availability of advanced imaging modalities and, in some cases, radiation associated with CT and/or radiograph. Though additional validation is needed, and evaluation of its broad clinical use is unknown, there may be significant promise in this type of technology.
Knowledge of cancer incidence and best practices for cancer surveillance in RASopathy patients remains relatively limited. Thus, prospective natural history studies are needed to help further quantify cancer incidence in these conditions. These studies will provide the longitudinal data needed to understand acute and long-term cancer risk and help inform surveillance guidelines and improve outcomes in the future.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Supplementary Material
Acknowledgments:
This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics and the Center for Cancer Research of the National Cancer Institute, Bethesda, MD. CPK has been supported by the German Childhood Cancer Foundation (DKS 2021.02).
References:
- Abe Y, Aoki Y, Kuriyama S, Kawame H, Okamoto N, Kurosawa K, Ohashi H, Mizuno S, Ogata T, Kure S, Niihori T, Matsubara Y, Costello and C. F. C. s. s. g. i. Japan (2012). "Prevalence and clinical features of Costello syndrome and cardio-facio-cutaneous syndrome in Japan: findings from a nationwide epidemiological survey." Am J Med Genet A 158A(5): 1083–1094. [DOI] [PubMed] [Google Scholar]
- Ahmadi N and Harley E (2010). "Costello syndrome and the importance of cancer screening." Archives of Otolaryngology - Head and Neck Surgery 136(10): 1028–1029. [DOI] [PubMed] [Google Scholar]
- Avery A, Metcalf JS, Maize JC and Swanson LA (2022). "Cutaneous T-cell lymphoma in SHOC2 mutation-associated Noonan-like syndrome with loose anagen hair." JAAD Case Rep 24: 52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger ML, Best A, Mai PL, Khincha PP, Loud JT, Peters JA, Achatz MI, Chojniak R, Balieiro da Costa A, Santiago KM, Garber J, O'Neill AF, Eeles RA, Evans DG, Bleiker E, Sonke GS, Ruijs M, Loo C, Schiffman J, Naumer A, Kohlmann W, Strong LC, Bojadzieva J, Malkin D, Rednam SP, Stoffel EM, Koeppe E, Weitzel JN, Slavin TP, Nehoray B, Robson M, Walsh M, Manelli L, Villani A, Thomas DM and Savage SA (2017). "Baseline Surveillance in Li-Fraumeni Syndrome Using Whole-Body Magnetic Resonance Imaging: A Meta-analysis." JAMA Oncol 3(12): 1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukers W, Hercegovac A and Zwarthoff EC (2014). "HRAS mutations in bladder cancer at an early age and the possible association with the Costello Syndrome." Eur J Hum Genet 22(6): 837–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno G, Murgia A, Mammi I, Strafella MS and Carli M (1999). "Rhabdomyosarcoma in a patient with cardio-facio-cutaneous syndrome." J Pediatr Hematol Oncol 21(5): 424–427. [DOI] [PubMed] [Google Scholar]
- Bolouri H, Farrar JE, Triche T Jr., Ries RE, Lim EL, Alonzo TA, Ma Y, Moore R, Mungall AJ, Marra MA, Zhang J, Ma X, Liu Y, Liu Y, Auvil JMG, Davidsen TM, Gesuwan P, Hermida LC, Salhia B, Capone S, Ramsingh G, Zwaan CM, Noort S, Piccolo SR, Kolb EA, Gamis AS, Smith MA, Gerhard DS and Meshinchi S (2018). "The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions." Nat Med 24(1): 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyawat B, Charoenpitakchai M and Suwanpakdee P (2019). "A First Case Report of Subependymoma in PTPN11 Mutation-Associated Noonan Syndrome." Case Rep Neurol Med 2019: 6091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur GM, Nichols KE, Plon SE, Schiffman JD and Malkin D (2017). "Pediatric Cancer Predisposition and Surveillance: An Overview, and a Tribute to Alfred G. Knudson Jr." Clinical Cancer Research 23(11): e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave H, Caye A, Strullu M, Aladjidi N, Vignal C, Ferster A, Mechinaud F, Domenech C, Pierri F, Contet A, Cacheux V, Irving J, Kratz C, Clavel J and Verloes A (2016). "Acute lymphoblastic leukemia in the context of RASopathies." Eur J Med Genet 59(3): 173–178. [DOI] [PubMed] [Google Scholar]
- Cordeddu V, Di Schiavi E, Pennacchio LA, Ma'ayan A, Sarkozy A, Fodale V, Cecchetti S, Cardinale A, Martin J, Schackwitz W, Lipzen A, Zampino G, Mazzanti L, Digilio MC, Martinelli S, Flex E, Lepri F, Bartholdi D, Kutsche K, Ferrero GB, Anichini C, Selicorni A, Rossi C, Tenconi R, Zenker M, Merlo D, Dallapiccola B, Iyengar R, Bazzicalupo P, Gelb BD and Tartaglia M (2009). "Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair." Nat Genet 41(9): 1022–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desandes E, Faure L, Guissou S, Goujon S, Berger C, Minard-Colin V, Petit A, Schleiermacher G, Poulalhon C, Lacour B and Clavel J (2020). "Infant cancers in France: Incidence and survival (2000-2014)." Cancer Epidemiol 65: 101697. [DOI] [PubMed] [Google Scholar]
- El-Ayadi M, Ansari M, Kuhnol CD, Bendel A, Sturm D, Pietsch T, Kramm CM and von Bueren AO (2019). "Occurrence of high-grade glioma in Noonan syndrome: Report of two cases." Pediatr Blood Cancer 66(5): e27625. [DOI] [PubMed] [Google Scholar]
- Garavelli L, Cordeddu V, Errico S, Bertolini P, Street ME, Rosato S, Pollazzon M, Wischmeijer A, Ivanovski I, Daniele P, Bacchini E, Lombardi AA, Izzi G, Biasucci G, Del Rossi C, Corradi D, Cazzaniga G, Dominici C, Rossi C, De Luca A, Bernasconi S, Riccardi R, Legius E and Tartaglia M (2015). "Noonan syndrome-like disorder with loose anagen hair: a second case with neuroblastoma." Am J Med Genet A 167A(8): 1902–1907. [DOI] [PubMed] [Google Scholar]
- Garcia-Gil MF, Alvarez-Salafranca M, Valero-Torres A and Ara-Martin M (2020). "Melanoma in Noonan Syndrome With Multiple Lentigines (Leopard Syndrome): A New Case." Actas Dermosifiliogr (Engl Ed) 111(7): 619–621. [DOI] [PubMed] [Google Scholar]
- Garren B, Stephan M and Hogue JS (2020). "NRAS associated RASopathy and embryonal rhabdomyosarcoma." Am J Med Genet A 182(1): 195–200. [DOI] [PubMed] [Google Scholar]
- Gelb BD and Tartaglia M (1993). Noonan Syndrome with Multiple Lentigines. GeneReviews((R)). Adam MP, Everman DB, Mirzaa GM et al. Seattle (WA). [PubMed] [Google Scholar]
- Gripp KW (2005). "Tumor predisposition in Costello syndrome." Am J Med Genet C Semin Med Genet 137c(1): 72–77. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Aldinger KA, Bennett JT, Baker L, Tusi J, Powell-Hamilton N, Stabley D, Sol-Church K, Timms AE and Dobyns WB (2016). "A novel rasopathy caused by recurrent de novo missense mutations in PPP1CB closely resembles Noonan syndrome with loose anagen hair." Am J Med Genet A 170(9): 2237–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Morse LA, Axelrad M, Chatfield KC, Chidekel A, Dobyns W, Doyle D, Kerr B, Lin AE, Schwartz DD, Sibbles BJ, Siegel D, Shankar SP, Stevenson DA, Thacker MM, Weaver KN, White SM and Rauen KA (2019). "Costello syndrome: Clinical phenotype, genotype, and management guidelines." Am J Med Genet A 179(9): 1725–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW and Rauen KA (1993). Costello Syndrome. GeneReviews((R)). Adam MP, Everman DB, Mirzaa GM et al. Seattle (WA). [Google Scholar]
- Gripp KW, Scott CI Jr., Nicholson L, McDonald-McGinn DM, Ozeran JD, Jones MC, Lin AE and Zackai EH (2002). "Five additional Costello syndrome patients with rhabdomyosarcoma: proposal for a tumor screening protocol." Am J Med Genet 108(1): 80–87. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Sol-Church K, Smpokou P, Graham GE, Stevenson DA, Hanson H, Viskochil DH, Baker LC, Russo B, Gardner N, Stabley DL, Kolbe V and Rosenberger G (2015). "An attenuated phenotype of Costello syndrome in three unrelated individuals with a HRAS c.179G>A (p.Gly60Asp) mutation correlates with uncommon functional consequences." Am J Med Genet A 167A(9): 2085–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Zand DJ, Demmer L, Anderson CE, Dobyns WB, Zackai EH, Denenberg E, Jenny K, Stabley DL and Sol-Church K (2013). "Expanding the SHOC2 mutation associated phenotype of Noonan syndrome with loose anagen hair: structural brain anomalies and myelofibrosis." Am J Med Genet A 161A(10): 2420–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobner SN, Worst BC, Weischenfeldt J, Buchhalter I, Kleinheinz K, Rudneva VA, Johann PD, Balasubramanian GP, Segura-Wang M, Brabetz S, Bender S, Hutter B, Sturm D, Pfaff E, Hubschmann D, Zipprich G, Heinold M, Eils J, Lawerenz C, Erkek S, Lambo S, Waszak S, Blattmann C, Borkhardt A, Kuhlen M, Eggert A, Fulda S, Gessler M, Wegert J, Kappler R, Baumhoer D, Burdach S, Kirschner-Schwabe R, Kontny U, Kulozik AE, Lohmann D, Hettmer S, Eckert C, Bielack S, Nathrath M, Niemeyer C, Richter GH, Schulte J, Siebert R, Westermann F, Molenaar JJ, Vassal G, Witt H, Project IP-S, Project IM-S, Burkhardt B, Kratz CP, Witt O, van Tilburg CM, Kramm CM, Fleischhack G, Dirksen U, Rutkowski S, Fruhwald M, von Hoff K, Wolf S, Klingebiel T, Koscielniak E, Landgraf P, Koster J, Resnick AC, Zhang J, Liu Y, Zhou X, Waanders AJ, Zwijnenburg DA, Raman P, Brors B, Weber UD, Northcott PA, Pajtler KW, Kool M, Piro RM, Korbel JO, Schlesner M, Eils R, Jones DTW, Lichter P, Chavez L, Zapatka M and Pfister SM (2018). "The landscape of genomic alterations across childhood cancers." Nature 555(7696): 321–327. [DOI] [PubMed] [Google Scholar]
- Harms FL, Alawi M, Amor DJ, Tan TY, Cuturilo G, Lissewski C, Brinkmann J, Schanze D, Kutsche K and Zenker M (2018). "The novel RAF1 mutation p.(Gly361Ala) located outside the kinase domain of the CR3 region in two patients with Noonan syndrome, including one with a rare brain tumor." Am J Med Genet A 176(2): 470–476. [DOI] [PubMed] [Google Scholar]
- Hastings R, Newbury-Ecob R, Ng A and Taylor R (2010). "A further patient with Noonan syndrome due to a SOS1 mutation and rhabdomyosarcoma." Genes Chromosomes Cancer 49(10): 967–968. [DOI] [PubMed] [Google Scholar]
- Hecht A, Meyer JA, Behnert A, Wong E, Chehab F, Olshen A, Hechmer A, Aftandilian C, Bhat R, Choi SW, Chonat S, Farrar JE, Fluchel M, Frangoul H, Han JH, Kolb EA, Kuo DJ, MacMillan ML, Maese L, Maloney KW, Narendran A, Oshrine B, Schultz KR, Sulis ML, Van Mater D, Tasian SK, Hofmann WK, Loh ML and Stieglitz E (2022). "Molecular and phenotypic diversity of CBL-mutated juvenile myelomonocytic leukemia." Haematologica 107(1): 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquinet A, Bonnard A, Capri Y, Martin D, Sadzot B, Bianchi E, Servais L, Sacre JP, Cave H and Verloes A (2020). "Oligo-astrocytoma in LZTR1-related Noonan syndrome." Eur J Med Genet 63(1): 103617. [DOI] [PubMed] [Google Scholar]
- Ji J, Navid F, Hiemenz MC, Kaneko M, Zhou S, Saitta SC and Biegel JA (2019). "Embryonal rhabdomyosarcoma in a patient with a germline CBL pathogenic variant." Cancer Genet 231-232: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Liu M, Lin G, Mao B, Cheng W, Liu H, Gal J, Zhu H, Yuan Z, Deng W, Liu Q, Gong P, Bi X and Meng S (2016). "Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death." Oncotarget 7(18): 25652–25667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JJ, van der Smagt JJ, Rosenfeld JA, Pagnamenta AT, Alswaid A, Baker EH, Blair E, Borck G, Brinkmann J, Craigen W, Dung VC, Emrick L, Everman DB, van Gassen KL, Gulsuner S, Harr MH, Jain M, Kuechler A, Leppig KA, McDonald-McGinn DM, Can NTB, Peleg A, Roeder ER, Rogers RC, Sagi-Dain L, Sapp JC, Schaffer AA, Schanze D, Stewart H, Taylor JC, Verbeek NE, Walkiewicz MA, Zackai EH, Zweier C, N. Members of the Undiagnosed Diseases, Zenker M, Lee B and Biesecker LG (2018). "Autosomal recessive Noonan syndrome associated with biallelic LZTR1 variants." Genet Med 20(10): 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongmans MC, Hoogerbrugge PM, Hilkens L, Flucke U, van der Burgt I, Noordam K, Ruiterkamp-Versteeg M, Yntema HG, Nillesen WM, Ligtenberg MJ, van Kessel AG, Kuiper RP and Hoogerbrugge N (2010). "Noonan syndrome, the SOS1 gene and embryonal rhabdomyosarcoma." Genes Chromosomes Cancer 49(7): 635–641. [DOI] [PubMed] [Google Scholar]
- Jongmans MC, van der Burgt I, Hoogerbrugge PM, Noordam K, Yntema HG, Nillesen WM, Kuiper RP, Ligtenberg MJ, van Kessel AG, van Krieken JH, Kiemeney LA and Hoogerbrugge N (2011). "Cancer risk in patients with Noonan syndrome carrying a PTPN11 mutation." Eur J Hum Genet 19(8): 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung A, Bechthold S, Pfluger T, Renner C and Ehrt O (2003). "Orbital rhabdomyosarcoma in Noonan syndrome." J Pediatr Hematol Oncol 25(4): 330–332. [DOI] [PubMed] [Google Scholar]
- Kachroo N, Valencia T, Warren AY and Gnanapragasam VJ (2013). "Evidence for downregulation of the negative regulator SPRED2 in clinical prostate cancer." Br J Cancer 108(3): 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamihara J, Bourdeaut F, Foulkes WD, Molenaar JJ, Mosse YP, Nakagawara A, Parareda A, Scollon SR, Schneider KW, Skalet AH, States LJ, Walsh MF, Diller LR and Brodeur GM (2017). "Retinoblastoma and Neuroblastoma Predisposition and Surveillance." Clin Cancer Res 23(13): e98–e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisawa T, Wood LD, Itoi T and Takaori K (2016). "Pancreatic cancer." Lancet 388(10039): 73–85. [DOI] [PubMed] [Google Scholar]
- Khan S, McDowell H, Upadhyaya M and Fryer A (1995). "Vaginal rhabdomyosarcoma in a patient with Noonan syndrome." J Med Genet 32(9): 743–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Light N, Subasri V, Young EL, Wegman-Ostrosky T, Barkauskas DA, Hall D, Lupo PJ, Patidar R, Maese LD, Jones K, Wang M, Tavtigian SV, Wu D, Shlien A, Telfer F, Goldenberg A, Skapek SX, Wei JS, Wen X, Catchpoole D, Hawkins DS, Schiffman JD, Khan J, Malkin D and Stewart DR (2021). "Pathogenic Germline Variants in Cancer Susceptibility Genes in Children and Young Adults With Rhabdomyosarcoma." JCO Precis Oncol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuru M, Urban J, Zhu G, Rybak I, Terrell JR, Qi L, McPherson JD, Marghoob AA and Rauen KA (2020). "RAS pathway influences the number of melanocytic nevi in cardiofaciocutaneous and Costello syndromes." J Am Acad Dermatol 82(5): 1091–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompier LC, Lurkin I, van der Aa MN, van Rhijn BW, van der Kwast TH and Zwarthoff EC (2010). "FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy." PLoS One 5(11): e13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz CP, Franke L, Peters H, Kohlschmidt N, Kazmierczak B, Finckh U, Bier A, Eichhorn B, Blank C, Kraus C, Kohlhase J, Pauli S, Wildhardt G, Kutsche K, Auber B, Christmann A, Bachmann N, Mitter D, Cremer FW, Mayer K, Daumer-Haas C, Nevinny-Stickel-Hinzpeter C, Oeffner F, Schluter G, Gencik M, Uberlacker B, Lissewski C, Schanze I, Greene MH, Spix C and Zenker M (2015). "Cancer spectrum and frequency among children with Noonan, Costello, and cardio-facio-cutaneous syndromes." Br J Cancer 112(8): 1392–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz CP, Niemeyer CM, Castleberry RP, Cetin M, Bergstrasser E, Emanuel PD, Hasle H, Kardos G, Klein C, Kojima S, Stary J, Trebo M, Zecca M, Gelb BD, Tartaglia M and Loh ML (2005). "The mutational spectrum of PTPN11 in juvenile myelomonocytic leukemia and Noonan syndrome/myeloproliferative disease." Blood 106(6): 2183–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz CP, Rapisuwon S, Reed H, Hasle H and Rosenberg PS (2011). "Cancer in Noonan, Costello, cardiofaciocutaneous and LEOPARD syndromes." Am J Med Genet C Semin Med Genet 157C(2): 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux D, Kratz C and Sauerbrey A (2008). "Common acute lymphoblastic leukemia in a girl with genetically confirmed LEOPARD syndrome." J Pediatr Hematol Oncol 30(8): 602–604. [DOI] [PubMed] [Google Scholar]
- Legius E, Messiaen L, Wolkenstein P, Pancza P, Avery RA, Berman Y, Blakeley J, Babovic-Vuksanovic D, Cunha KS, Ferner R, Fisher MJ, Friedman JM, Gutmann DH, Kehrer-Sawatzki H, Korf BR, Mautner VF, Peltonen S, Rauen KA, Riccardi V, Schorry E, Stemmer-Rachamimov A, Stevenson DA, Tadini G, Ullrich NJ, Viskochil D, Wimmer K, Yohay K, C. International Consensus Group on Neurofibromatosis Diagnostic, Huson SM, Evans DG and Plotkin SR (2021). "Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: an international consensus recommendation." Genet Med 23(8): 1506–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legius E, Stevenson D, Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW and Amemiya A (1993). "Legius Syndrome." GeneReviews(®). [PubMed] [Google Scholar]
- Leoni C, Paradiso FV, Foschi N, Tedesco M, Pierconti F, Silvaroli S, Diego MD, Birritella L, Pantaleoni F, Rendeli C, Onesimo R, Viscogliosi G, Bassi P, Nanni L, Genuardi M, Tartaglia M and Zampino G (2022). "Prevalence of bladder cancer in Costello syndrome: New insights to drive clinical decision-making." Clin Genet 101(4): 454–458. [DOI] [PubMed] [Google Scholar]
- Li H, Sisoudiya SD, Martin-Giacalone BA, Khayat MM, Dugan-Perez S, Marquez-Do DA, Scheurer ME, Muzny D, Boerwinkle E, Gibbs RA, Chi YY, Barkauskas DA, Lo T, Hall D, Stewart DR, Schiffman JD, Skapek SX, Hawkins DS, Plon SE, Sabo A and Lupo PJ (2021). "Germline Cancer Predisposition Variants in Pediatric Rhabdomyosarcoma: A Report From the Children's Oncology Group." J Natl Cancer Inst 113(7): 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Bohin N, Wen T, Ng V, Magee J, Chen SC, Shannon K and Morrison SJ (2013). "Oncogenic Nras has bimodal effects on stem cells that sustainably increase competitiveness." Nature 504(7478): 143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yao R, Tan X, Li N, Ding Y, Li J, Chang G, Chen Y, Ma L, Wang J, Fu L and Wang X (2019). "Molecular and phenotypic spectrum of Noonan syndrome in Chinese patients." Clin Genet 96(4): 290–299. [DOI] [PubMed] [Google Scholar]
- Locatelli F and Niemeyer CM (2015). "How I treat juvenile myelomonocytic leukemia." Blood 125(7): 1083–1090. [DOI] [PubMed] [Google Scholar]
- Lopez-Miranda B, Westra SJ, Yazdani S and Boechat MI (1997). "Noonan syndrome associated with neuroblastoma: a case report." Pediatr Radiol 27(4): 324–326. [DOI] [PubMed] [Google Scholar]
- Ma X, Liu Y, Liu Y, Alexandrov LB, Edmonson MN, Gawad C, Zhou X, Li Y, Rusch MC, Easton J, Huether R, Gonzalez-Pena V, Wilkinson MR, Hermida LC, Davis S, Sioson E, Pounds S, Cao X, Ries RE, Wang Z, Chen X, Dong L, Diskin SJ, Smith MA, Guidry Auvil JM, Meltzer PS, Lau CC, Perlman EJ, Maris JM, Meshinchi S, Hunger SP, Gerhard DS and Zhang J (2018). "Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours." Nature 555(7696): 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli S, Stellacci E, Pannone L, D'Agostino D, Consoli F, Lissewski C, Silvano M, Cencelli G, Lepri F, Maitz S, Pauli S, Rauch A, Zampino G, Selicorni A, Melancon S, Digilio MC, Gelb BD, De Luca A, Dallapiccola B, Zenker M and Tartaglia M (2015). "Molecular Diversity and Associated Phenotypic Spectrum of Germline CBL Mutations." Hum Mutat 36(8): 787–796. [DOI] [PubMed] [Google Scholar]
- Mattox AK, Douville C, Silliman N, Ptak J, Dobbyn L, Schaefer J, Popoli M, Blair C, Judge K, Pollard K, Pratilas C, Blakeley J, Rodriguez F, Papadopoulos N, Belzberg A and Bettegowda C (2022). "Detection of malignant peripheral nerve sheath tumors in patients with neurofibromatosis using aneuploidy and mutation identification in plasma." eLife 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta M, Fasano G, Gredy S, Brinkmann J, Bonnard AA, Simsek-Kiper PO, Gulec EY, Essaddam L, Utine GE, Guarnetti Prandi I, Venditti M, Pantaleoni F, Radio FC, Ciolfi A, Petrini S, Consoli F, Vignal C, Hepbasli D, Ullrich M, de Boer E, Vissers L, Gritli S, Rossi C, De Luca A, Ben Becher S, Gelb BD, Dallapiccola B, Lauri A, Chillemi G, Schuh K, Cave H, Zenker M and Tartaglia M (2021). "SPRED2 loss-of-function causes a recessive Noonan syndrome-like phenotype." Am J Hum Genet 108(11): 2112–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney GM, Yang KB, Ng V, Liu L, Zhao M, Kuk W, Alaka L, Sampang L, Ross A, Jones MA, Jin X, McKay LM, Evarts H and Li Q (2021). "Oncogenic N-Ras Mitigates Oxidative Stress-Induced Apoptosis of Hematopoietic Stem Cells." Cancer Res 81(5): 1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer CM (2014). "RAS diseases in children." Haematologica 99(11): 1653–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer CM, Kang MW, Shin DH, Furlan I, Erlacher M, Bunin NJ, Bunda S, Finklestein JZ, Gorr TA, Mehta P, Schmid I, Kropshofer G, Corbacioglu S, Lang PJ, Klein C, Schlegel PG, Heinzmann A, Schneider M, Stary J, van den Heuvel-Eibrink MM, Hasle H, Locatelli F, Sakai D, Archambeault S, Chen L, Russell RC, Sybingco SS, Ohh M, Braun BS, Flotho C and Loh ML (2010). "Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia." Nat Genet 42(9): 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabari R, Chun K and Naqvi A (2022). "The Clinical Landscape of NRAS-mutated Juvenile Myelomonocytic Leukemia-like Myeloproliferation Includes Children With Costello Syndrome." J Pediatr Hematol Oncol. [DOI] [PubMed] [Google Scholar]
- Perez B, Mechinaud F, Galambrun C, Ben Romdhane N, Isidor B, Philip N, Derain-Court J, Cassinat B, Lachenaud J, Kaltenbach S, Salmon A, Desiree C, Pereira S, Menot ML, Royer N, Fenneteau O, Baruchel A, Chomienne C, Verloes A and Cave H (2010). "Germline mutations of the CBL gene define a new genetic syndrome with predisposition to juvenile myelomonocytic leukaemia." J Med Genet 47(10): 686–691. [DOI] [PubMed] [Google Scholar]
- Pierpont ME, Magoulas PL, Adi S, Kavamura MI, Neri G, Noonan J, Pierpont EI, Reinker K, Roberts AE, Shankar S, Sullivan J, Wolford M, Conger B, Santa Cruz M and Rauen KA (2014). "Cardio-facio-cutaneous syndrome: clinical features, diagnosis, and management guidelines." Pediatrics 134(4): e1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski A, Xie J, Liu YF, Poplawski AB, Gomes AR, Madanecki P, Fu C, Crowley MR, Crossman DK, Armstrong L, Babovic-Vuksanovic D, Bergner A, Blakeley JO, Blumenthal AL, Daniels MS, Feit H, Gardner K, Hurst S, Kobelka C, Lee C, Nagy R, Rauen KA, Slopis JM, Suwannarat P, Westman JA, Zanko A, Korf BR and Messiaen LM (2014). "Germline loss-of-function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas." Nat Genet 46(2): 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter CC, Druley TE, Erez A, Kuiper RP, Onel K, Schiffman JD, Schneider KW, Scollon SR, Scott HS, Strong LC, Walsh MF and Nichols KE (2017). "Recommendations for surveillance for children with leukemia-predisposing conditions." Clinical Cancer Research 23(11): e14–e22. [DOI] [PubMed] [Google Scholar]
- Rankin J, Short J, Turnpenny P, Castle B and Hanemann CO (2013). "Medulloblastoma in a patient with the PTPN11 p.Thr468Met mutation." Am J Med Genet A 161A(8): 2027–2029. [DOI] [PubMed] [Google Scholar]
- Rauen KA, Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW and Amemiya A (1993). "Cardiofaciocutaneous Syndrome." GeneReviews(®). [Google Scholar]
- Roberts AE, Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW and Amemiya A (1993). "Noonan Syndrome." GeneReviews(®). [PubMed] [Google Scholar]
- Roberts AE, Allanson JE, Tartaglia M and Gelb BD (2013). "Noonan syndrome." Lancet 381(9863): 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano AA, Allanson JE, Dahlgren J, Gelb BD, Hall B, Pierpont ME, Roberts AE, Robinson W, Takemoto CM and Noonan JA (2010). "Noonan syndrome: clinical features, diagnosis, and management guidelines." Pediatrics 126(4): 746–759. [DOI] [PubMed] [Google Scholar]
- Sarkozy A, Digilio MC and Dallapiccola B (2008). "Leopard syndrome." Orphanet J Rare Dis 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubbert S, Zenker M, Rowe SL, Böll S, Klein C, Bollag G, van der Burgt I, Musante L, Kalscheuer V, Wehner LE, Nguyen H, West B, Zhang KY, Sistermans E, Rauch A, Niemeyer CM, Shannon K and Kratz CP (2006). "Germline KRAS mutations cause Noonan syndrome." Nat Genet 38(3): 331–336. [DOI] [PubMed] [Google Scholar]
- Sherman CB, Ali-Nazir A, Gonzales-Gomez I, Finlay JL and Dhall G (2009). "Primary mixed glioneuronal tumor of the central nervous system in a patient with noonan syndrome: a case report and review of the literature." J Pediatr Hematol Oncol 31(1): 61–64. [DOI] [PubMed] [Google Scholar]
- Sinajon P, Chitayat D, Roifman M, Wasim S, Carmona S, Ryan G, Noor A, Kolomietz E and Chong K (2020). "Microarray and RASopathy-disorder testing in fetuses with increased nuchal translucency." Ultrasound Obstet Gynecol 55(3): 383–390. [DOI] [PubMed] [Google Scholar]
- Stieglitz E, Mazor T, Olshen AB, Geng H, Gelston LC, Akutagawa J, Lipka DB, Plass C, Flotho C, Chehab FF, Braun BS, Costello JF and Loh ML (2017). "Genome-wide DNA methylation is predictive of outcome in juvenile myelomonocytic leukemia." Nat Commun 8(1): 2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strullu M, Caye A, Lachenaud J, Cassinat B, Gazal S, Fenneteau O, Pouvreau N, Pereira S, Baumann C, Contet A, Sirvent N, Mechinaud F, Guellec I, Adjaoud D, Paillard C, Alberti C, Zenker M, Chomienne C, Bertrand Y, Baruchel A, Verloes A and Cave H (2014). "Juvenile myelomonocytic leukaemia and Noonan syndrome." J Med Genet 51(10): 689–697. [DOI] [PubMed] [Google Scholar]
- Sundby RT, Pan A and Shern JF (2022). "Liquid biopsies in pediatric oncology: opportunities and obstacles." Curr Opin Pediatr 34(1): 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski JJ, Sundby RT, Jones PA, Srihari D, Earland N, Harris PK, Feng W, Qaium F, Lei H, Roberts D, Landeau M, Bell J, Huang Y, Hoffman L, Spencer M, Spraker MB, Ding L, Widemann BC, Shern JF, Hirbe AC and Chaudhuri AA (2021). "Cell-free DNA ultra-low-pass whole genome sequencing to distinguish malignant peripheral nerve sheath tumor (MPNST) from its benign precursor lesion: A cross-sectional study." PLOS Medicine 18(8): e1003734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Niemeyer CM, Fragale A, Song X, Buechner J, Jung A, Hahlen K, Hasle H, Licht JD and Gelb BD (2003). "Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia." Nat Genet 34(2): 148–150. [DOI] [PubMed] [Google Scholar]
- Ucar C, Calyskan U, Martini S and Heinritz W (2006). "Acute myelomonocytic leukemia in a boy with LEOPARD syndrome (PTPN11 gene mutation positive)." J Pediatr Hematol Oncol 28(3): 123–125. [DOI] [PubMed] [Google Scholar]
- Villani A, Greer MC, Kalish JM, Nakagawara A, Nathanson KL, Pajtler KW, Pfister SM, Walsh MF, Wasserman JD, Zelley K and Kratz CP (2017). "Recommendations for Cancer Surveillance in Individuals with RASopathies and Other Rare Genetic Conditions with Increased Cancer Risk." Clin Cancer Res 23(12): e83–e90. [DOI] [PubMed] [Google Scholar]
- Yan W, Markegard E, Dharmaiah S, Urisman A, Drew M, Esposito D, Scheffzek K, Nissley DV, McCormick F and Simanshu DK (2020). "Structural Insights into the SPRED1-Neurofibromin-KRAS Complex and Disruption of SPRED1-Neurofibromin Interaction by Oncogenic EGFR." Cell Rep 32(3): 107909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Long N, Anekpuritanang T, Bottomly D, Savage JC, Lee T, Solis-Ruiz J, Borate U, Wilmot B, Tognon C, Bock AM, Pollyea DA, Radhakrishnan S, Radhakrishnan S, Patel P, Collins RH, Tantravahi S, Deininger MW, Fan G, Druker B, Shinde U, Tyner JW, Press RD, McWeeney S and Agarwal A (2022). "Identification and prioritization of myeloid malignancy germline variants in a large cohort of adult patients with AML." Blood 139(8): 1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CH, Zheng Z, Shuman L, Yamashita H, Warrick JI, Wu XR, Raman JD and DeGraff DJ (2019). "Maintenance of the bladder cancer precursor urothelial hyperplasia requires FOXA1 and persistent expression of oncogenic HRAS." Sci Rep 9(1): 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KPT, Luk HM, Leung GKC, Mak CCY, Cheng SSW, Hau EWL, Chan DKH, Lam STS, Tong TMF, Chung BHY and Lo IFM (2019). "Genetic landscape of RASopathies in Chinese: Three decades' experience in Hong Kong." Am J Med Genet C Semin Med Genet 181(2): 208–217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.