Abstract
Previous studies have demonstrated an adjuvant effect for the C3d fragment of complement C3 when coupled to T-dependent protein antigens. In this study, we examined the antibody response to covalent conjugates of C3d and a T-independent antigen, the capsular polysaccharide of serotype 14 Streptococcus pneumoniae (PPS14). We prepared a conjugate of mouse C3d and PPS14 and compared its immunogenicity with that of a conjugate of PPS14 and ovalbumin (OVA). When BALB/c mice were immunized with PPS14-C3d, there was a significant increase in serum anti-PPS14 concentrations compared with either native PPS14 or control PPS14-glycine conjugates. This was accompanied by a switch in anti-PPS14 from predominantly immunoglobulin M (IgM) to IgG1 by day 25 following primary immunization. Following secondary immunization with PPS14-C3d, there was a marked booster response and a further increase in the ratio of IgG1 to IgM anti-PPS14. Although the primary antibody response to the PPS14-OVA conjugate exceeded that induced by immunization with PPS14-C3d, serum anti-PPS14 concentrations after a second injection of PPS14-C3d were nearly identical to those induced by secondary immunization with PPS14-OVA. Experiments with athymic nude mice suggested that T cells were not required for the adjuvant effect of C3d on the primary immune response to PPS14 but were necessary for enhancement of the memory response after a second injection of PPS14-C3d. These studies show that the adjuvant effects of C3d extend to T-independent antigens as well as T-dependent antigens. As a means of harnessing the adjuvant potential of the innate immune system, C3d conjugates may prove useful as a component of vaccines against encapsulated bacteria.
Protective immunity to encapsulated bacterial pathogens is principally mediated by the reaction between antibody and capsular polysaccharide epitopes. In encapsulated gram-negative bacteria, protection results primarily from a direct complement-mediated bactericidal effect (28), whereas the cell wall of gram-positive encapsulated bacteria prevents their lysis by complement (2, 28). Instead, fixation of complement leads indirectly to death by opsonizing the bacteria for ingestion and killing by phagocytic cells. Vaccines have been prepared from the capsular polysaccharides of Haemophilus influenzae type b, Neisseria meningitidis (groups A, C, W135, and Y), Salmonella enterica serovar Typhi, and Streptococcus pneumoniae (23 serotypes) (6, 35). These and other polysaccharides have been classified as T cell-independent type 2 (TI-2) antigens based on their inability to stimulate an immune response in CBA/N mice that carry an X-linked immune B-cell defect (xid) (25). TI-2 antigens tend to be characterized by high molecular weight, multiple repeat epitopes, slow degradation in vivo, and a failure to stimulate major histocompatibility complex (MHC) type II-mediated T-cell help (6, 25). TI-2 antigens generally are incapable of stimulating an immune response in neonatal mice or in humans under 18 months of age (6, 25). This has spurred attempts to modify the capsular polysaccharides such that vaccines protective for all at-risk groups will result. To date, the most successful approach has been to covalently bind carrier proteins to the polysaccharides, thus engendering a vaccine capable of invoking a T-dependent response (6, 35). In the United States, use of glycoconjugate vaccines has nearly eliminated severe clinical disease resulting from infection with H. influenzae type b.
S. pneumoniae, the pneumococcus, presents a unique challenge for those attempting to develop an effective vaccine (39). Worldwide, over one million children die of pneumococcal infections each year, primarily in developing countries (16, 39, 49). In the United States, S. pneumoniae is a major cause of pneumonia in the elderly and of meningitis and bacteremia in children age 6 to 15 months (16). About 90 different serotypes have been identified based on differences in the chemical composition of the pneumococcal capsular polysaccharide. Many different serotypes are associated with clinical disease and 11 serotypes are responsible for about 75% of invasive infection worldwide (12). Therefore, the use of multivalent vaccines is required to provide adequate protection against infection with pneumococcus. The currently licensed 23-valent vaccine has an overall protective efficacy of 60 to 70%, with children under 2 years of age and patients with immunodeficiencies of various causes failing to consistently mount a protective response (49). Thus, the development of more effective vaccines against this organism has become a high priority.
To attain this goal, protein carriers that have been used in conjugate vaccines to H. influenzae type b have been employed in the synthesis of vaccines for immunization against S. pneumoniae. The critical difference between the two vaccines is the requirement that a pneumococcal conjugate vaccine contain conjugates of several different capsular serotypes. Conjugate vaccines against S. pneumoniae containing from 7 to 11 polysaccharide-protein conjugates are currently in clinical trial (38), and a 7-valent vaccine has recently been licensed for clinical use. The presence of several different polysaccharide-protein conjugates in a single vaccine introduces a variety of potential problems (reviewed in references 16 and 39). For example, the presence of several different antigens can lead to high total concentrations of polysaccharide or carrier protein, which may decrease the antibody response to any individual component (16). An additional unknown is the possibility that there will be a change in the most prevalent serotypes encountered in clinical practice as these newer vaccines come into widespread use. Thus, it is imperative that research continue to determine methods of improving the antibody response to the individual pneumococcal polysaccharide components of a multivalent vaccine. Approaches which engage the adjuvant capabilities of the innate immune system are demonstrating great promise when used in vaccine design. These include the use of oligodeoxynucleotides containing CpG motifs (20), cytokines (3), and synthetic antigen constructs containing fragments of complement component C3 (8).
The critical role of the complement system in the humoral immune response to both T-dependent and T-independent antigens was first reported in studies performed over a quarter of a century ago (31, 32). Complement's potential use as an adjuvant in vaccines was suggested when Dempsey et al. demonstrated that anti-hen egg lysozyme (HEL) transgenic mice immunized with 0.05 pmol of a genetically engineered construct containing three copies of mouse C3d fused to HEL had an immunoglobulin G1 (IgG1) anti-HEL primary antibody response equivalent to that of mice immunized with 500 pmol of unmodified HEL (5). The HEL-C3d3 construct was shown to lower the threshold for HEL-induced transgenic B-cell activation by 1,000-fold but did not activate B cells from nontransgenic mice (5). Subsequent studies have shown an adjuvant effect for C3 fragments in nontransgenic mice immunized with HEL having a single molecule of C3b covalently attached (46), in mice immunized with an anti-idiotype vaccine incorporating a C3d peptide (22), and in mice immunized with a DNA vaccine consisting of soluble influenza virus hemagglutinin fused to three copies of C3d (37). In mice and humans, the primary receptor for C3d is CR2 (CD21), which is found primarily on B cells and follicular dendritic cells (14).
Serotype 14 pneumococcus is one of the three most prevalent serotypes causing invasive pneumococcal disease worldwide, and its capsular polysaccharide is included in all conjugate vaccines currently under development or evaluation (38). The serotype 14 pneumococcus capsular polysaccharide (PPS14) is able to activate the alternative pathway of complement (11), and its ability to induce an antibody response in BALB/c mice is complement dependent (24). Thus, PPS14-C3d conjugates would have the potential to enhance the PPS14-specific antibody response, but whether this would be an improvement over that which results from natural fixation of C3 by native PPS14 following immunization remained to be seen. In the studies presented here, we examined the effects of C3d conjugation on the immunogenicity of PPS14 in BALB/c mice. The antibody response to PPS14-C3d conjugates was compared with that resulting from immunization with conjugates of PPS14 and ovalbumin (OVA), a T-dependent protein carrier. Our results show that conjugation of C3d to PPS14 resulted both in a significant enhancement of the serum anti-PPS14 antibody response in immunized mice and in switching of the anti-PPS14 response from IgM to primarily IgG1. These effects were comparable to those induced by immunization with a PPS14-OVA conjugate.
MATERIALS AND METHODS
Mouse C3.
C3 was purified from EDTA-anticoagulated mouse plasma (Harlan Bioproducts for Science, Indianapolis, Ind.) by using published methods (34) with a modification based on the method of Van den Berg et al. (44). Mouse plasma (150 ml) was precipitated with 5% polyethylene glycol 3350 (J. T. Baker, Phillipsburg, N.J.), and then the supernatant was precipitated with 10% polyethylene glycol. The precipitate was suspended in 0.02 M Tris, pH 8.7, containing 6.5 mM EDTA, 33 mM ɛ-amino-n-caproic acid, 6.5 mM benzamidine HCl, and 1 mM phenylmethylsulfonyl fluoride (all from Sigma, St. Louis, Mo.) and was applied to a 2.5- by 10-cm column of Q Sepharose Fast Flow (Amersham Pharmacia Biotech, Piscataway, N.J.). The column was eluted at 4°C at pH 8.7 with a linear NaCl gradient from 0 to 400 mM. Fractions containing C3 as identified by enzyme-linked immunosorbent assay (ELISA) were pooled and were concentrated on an Amicon PM10 membrane (Millipore Corp., Bedford, Mass.). The only major contaminant after ion-exchange chromatography was IgG, which was removed by passing the C3 preparation over a column of protein A-Sepharose CL4B (Amersham Pharmacia Biotech). The final yield of purified C3 was ∼50 mg from 150 ml of mouse plasma.
Mouse C3d.
Mouse C3d was generated by trypsinization of purified mouse C3 (34). Ten milligrams of C3 was incubated with 0.5 mg of TPCK-trypsin (Worthington Biochemical Corp., Lakewood, N.J.) in 5 ml of 100 mM Tris, pH 7.4, containing 100 mM NaCl and 1 mM EDTA for 1 h at 37°C. The reaction was terminated by the addition of 2.5 mg of soybean trypsin inhibitor (SBTI; Sigma) and a 200-fold molar excess of phenylmethylsulfonyl fluoride. The C3d was isolated by incubation with thiopropyl Sepharose 6B (Amersham Pharmacia Biotech) for 16 h at 4°C followed by elution with a stepwise gradient of 0, 3, and 5 mM 2-mercaptoethanol (Sigma). Fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels under reducing conditions; those fractions containing purified C3d were pooled and dialyzed into phosphate-buffered saline (PBS) containing 1 mM EDTA and were stored under N2 at −80°C. This procedure typically yielded from 700 to 1,000 μg of C3d from 10 mg of mouse C3. To exclude the presence of trypsin, trypsin fragments, or SBTI in the final C3d preparation, it was analyzed by SDS-PAGE and Western blotting using rabbit anti-trypsinogen (Biodesign International, Kennebunk, Maine) to detect trypsin and rabbit anti-SBTI (Biogenesis Inc., Sandown, N.H.) to detect SBTI, followed by alkaline phosphatase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, Pa.). Bands were visualized with a combination of nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indoylphosphate p-toluidine salt (AP Color Development Reagent; Bio-Rad Laboratories, Hercules, Calif.).
Preparation and characterization of PPS14 conjugate vaccines.
Mouse C3d was conjugated to PPS14 according to the method of Lees et al. (21). C3d was buffer exchanged into 0.1 M sodium borate by centrifugation over a Bio-Spin 6 chromatography column (Bio-Rad Laboratories). PPS14 (American Type Culture Collection, Rockville, Md.) was activated by incubation with the mild organic cyanylating reagent 1-cyano-4-dimethylaminopyridium tetrafluoroborate (CDAP; Research Organics, Cleveland, Ohio). Five hundred micrograms of PPS14 was gently stirred with 7.6 μl of CDAP (100 mg/ml in acetonitrile) at room temperature for 30 s, followed by the addition of 22 μl of 0.1 M sodium borate, pH 8.8, and incubation for an additional 2 min. Seven hundred fifty micrograms of mouse C3d was then added to 200 μg of activated PPS14 and was gently stirred for 4 h at room temperature. Glycine (20 μmol) was added to block any remaining activation sites on the PPS14, and the incubation was continued for 30 min. A PPS14-glycine conjugate control was prepared by incubating PPS14 under conditions identical to those described above except with the substitution of 0.1 M sodium borate, pH 8.8, at the step where mouse C3d would have been added. PPS14-C3d conjugate was isolated by chromatography on a 0.7- by 14-cm column of Bio-Gel P-150 (Bio-Rad Laboratories [no longer available]). Fractions were analyzed by SDS-PAGE on 10% gels under nonreducing conditions, followed by electrophoretic transfer to polyvinylidene fluoride membranes (Bio-Rad Laboratories). C3d was detected with a polyclonal rabbit anti-human C3d (Dako Corp., Carpinteria, Calif.) that cross-reacts with mouse C3d, followed by alkaline phosphatase-conjugated goat anti-rabbit IgG. PPS14 was detected with affinity-purified murine anti-PPS14 IgG, followed by alkaline phosphatase-conjugated goat anti-mouse IgG (Caltag Laboratories, South San Francisco, Calif.). Bands were visualized as described above for analysis of purified C3d. Fractions containing PPS14-C3d conjugate eluted at the void volume; only those fractions containing conjugate in the absence of free C3d were pooled for further use. The final PPS14-C3d preparation was sterilized by passage through a 0.2-μm-pore-size filter and stored at 4°C. The PPS14 concentration of the conjugate preparation was determined by a resorcinol sulfuric acid micromethod (27), and the protein concentration was determined with the bicinchoninic acid protein assay (Pierce, Rockford, Ill.). The final PPS14-C3d conjugate preparation used for immunizations had a C3d concentration of 439 μg/ml and a PPS14 concentration of 317 μg/ml, yielding a 4:1 molar ratio of C3d to PPS14, assuming a molecular mass of 100,000 Da for PPS14.
Subsequent to its use in these experiments, the PPS14-C3d conjugate was found by Western blotting to contain a trace of SBTI. The concentration of SBTI in the conjugate preparation was determined by ELISA using rabbit anti-SBTI as the capture antibody and biotinylated rabbit anti-SBTI followed by a streptavidin-alkaline phosphatase conjugate (Caltag Laboratories) to detect captured antigen and was found to be 55 ng/ml. Thus, the molar ratio of C3d to SBTI in the conjugate was ∼5,000:1. This degree of contamination was judged to be inconsequential, as immunizations performed with PPS14-C3d prepared using C3d isolated without the addition of SBTI gave results similar, if not identical, to those performed with the conjugate used in the experiments reported here.
Identical methods were used to conjugate OVA (Pierce) to PPS14 except that 1 mg of OVA was added to 200 μg of activated PPS14 during formation of the conjugate. The final filter-sterilized PPS14-OVA conjugate preparation had an OVA concentration of 176 μg/ml and a PPS14 concentration of 167 μg/ml, yielding an OVA/PPS14 molar ratio of 2.5:1.
To determine whether the PPS14 in our conjugate preparations retained its antigenicity, we tested the ability of the conjugates to inhibit the binding of a human serum antibody pool against PPS14 to radioiodinated PPS14 in a radioantigen binding assay (RABA). The procedure used for radioiodination of PPS14 and the RABA for determination of serum concentrations of antibodies to PPS14 are described in detail elsewhere (23). The PPS14 in both conjugate preparations and the PPS14-glycine control retained full antigenicity when compared with unmodified PPS14 (data not shown).
To ascertain that the PPS14-C3d conjugate could bind to CR2, we assessed binding of the conjugate to the Raji B lymphoblastic leukemia cell line by flow cytometry. Raji cells (American Type Culture Collection) were incubated for 45 min at 4°C with purified PPS14, purified mouse C3d, or PPS14-C3d conjugate. After being washed, the cells were incubated with mouse anti-PPS14 IgG, followed by fluorescein isothiocyanate-conjugated F(ab′)2 goat anti-mouse IgG, Fcγ fragment specific (Jackson ImmunoResearch Laboratories). After a final wash, the cells were analyzed by flow cytometry on a FACScan (BD Immunocytometry Systems, San Jose, Calif.). This combination of reagents would detect bound C3d only if it were coupled to PPS14. Binding to Raji cells was demonstrated for the PPS14-C3d conjugate but not for unmodified PPS14 or for purified mouse C3d, confirming that the conjugate was binding to the Raji cells via interactions between C3d and CR2 (data not shown). Binding of the PPS14-C3d to unfractionated BALB/c mouse splenocytes was assessed by using similar methods except that bound conjugate was detected with a monoclonotypic human anti-PPS14 antibody isolated from an individual immunized with polyvalent pneumococcal vaccine (23) followed by fluorescein isothiocyanate-conjugated F(ab′)2 donkey anti-human IgG (Jackson ImmunoResearch Laboratories). The conjugate bound to ∼50% of the splenocytes; these were determined to be B lymphocytes by simultaneous staining with rat anti-mouse CD45R/B220 (PharMingen International, San Diego, Calif.) followed by R-phycoerythrin-conjugated mouse anti-rat κ-light chain (Sigma). Binding of PPS14-C3d to either Raji cells or mouse splenocytes could be completely inhibited by preincubation of the cells with polymerized C3d (as C3d conjugated to the capsular polysaccharide of H. influenzae type b; see below), providing further confirmation that the conjugate was binding via C3d-CR2 interactions.
Mice and Immunizations.
Female BALB/c mice were obtained from Charles River Laboratories (Wilmington, Mass.) and were used at 9 to 11 weeks of age. Female BALB/c nu/nu mice were also from Charles River and were used at 7 weeks of age. Blood samples were obtained 1 to 3 days prior to immunization, and serum was stored at −80°C. Mice were immunized with 0.01, 0.1, or 1.0 μg of PPS14 as either unmodified PPS14, PPS14-glycine control, PPS14-C3d, or PPS14-OVA diluted in 200 μl of sterile PBS. Antigens were administered by subcutaneous injection, with the total antigen dose divided equally between two sites. A second immunization was performed at 47 or 70 days after the primary immunization. Blood samples were obtained at approximately 10 and 25 days following each injection. In experiments comparing PPS14-C3d and PPS14-OVA conjugates, blood samples were also obtained at days 45 and 63 following primary immunization and at day 42 postsecondary immunization. When these conjugates were compared in BALB/c nu/nu mice, blood samples were obtained at 14, 28, and 44 days after primary immunization and at days 15 and 29 after secondary immunization. As additional controls for the adjuvant effect of C3d, in some experiments mice were immunized simultaneously with native PPS14 and monomeric C3d or with native PPS14 plus polymerized C3d in the form of C3d conjugated to the capsular polysaccharide of H. influenzae type b (C3d/PS molar ratio, 8:1).
Measurement of anti-PPS14 antibody concentrations.
Serum concentrations of antibodies to PPS14 were determined by RABA as described previously (23). Because commercially available preparations of purified pneumococcal capsular polysaccharides are contaminated with cell wall polysaccharide, all serum samples were diluted in buffer containing 10 μg of pneumococcal cell wall polysaccharide (Statens Seruminstitut, Copenhagen, Denmark)/ml to neutralize any serum antibodies to this cell wall component.
Isotype analysis.
The Ig subclass composition of serum antibodies to PPS14 was determined by ELISA with the SBA Clonotyping System/AP (Southern Biotechnology Associates, Birmingham, Ala.). Serum samples were diluted in PBS containing 1% bovine serum albumin, 0.1% sodium azide, and 20 μg of pneumococcal cell wall polysaccharide/ml (dilution buffer) such that the total anti-PPS14 concentration was the same in each sample. A control for each serum sample consisted of diluted serum (or standard) plus an equal volume of PPS14 (20 μg/ml) in dilution buffer. The value for this control was subtracted from the corresponding value for diluted serum incubated with an equal volume of dilution buffer. PPS14-specific IgM, IgA, IgG1, IgG2a, IgG2b, and IgG3 were determined using alkaline phosphatase goat anti-mouse conjugates specific for each subclass. Results are expressed as the absorbance at 405 nm at 30 min for each serum sample.
Statistical analysis.
Serum anti-PPS14 concentrations were determined for individual mice of each immunization group, and the geometric mean and 95% confidence intervals (CIs) of the geometric mean were calculated. To eliminate the effects of mouse-to-mouse variability, statistical comparisons were made on log-transformed data. Comparisons between mice receiving control vaccinations (unmodified PPS14 or PPS14-glycine) and mice receiving conjugate vaccine preparations were made using Student's t test for unpaired samples. Statistical significance was set at P < 0.05.
RESULTS
Conjugation of C3d enhances the antibody response to PPS14.
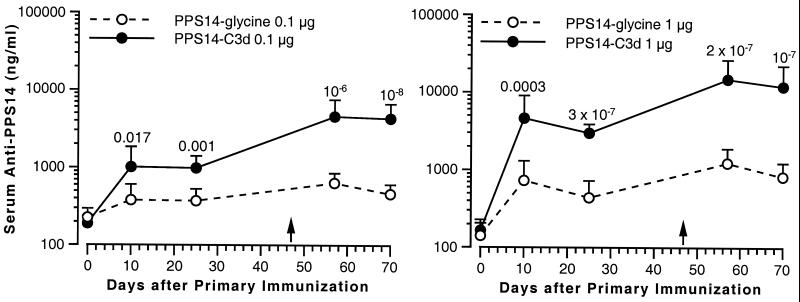
Groups of 15 mice were immunized with 0.01, 0.1, or 1.0 μg of PPS14 as either PPS14-glycine control or PPS14-C3d. The mice received a second subcutaneous injection of the same preparation 47 days after the first. Conjugation of C3d to PPS14 did not enhance the antibody response at the lowest dose tested (data not shown), but there was significant enhancement of anti-PPS14 antibody levels in mice immunized with either 0.1 or 1.0 μg of PPS14-C3d (Fig. 1). Maximum antibody levels generally were achieved 10 days after immunization, and the majority of mice showed a booster effect after secondary immunization. For example, mice immunized with 1 μg of PPS14-C3d had a serum anti-PPS14 geometric mean concentration (GMC) of 4,620 ng/ml 10 days after primary immunization, with a 95% CI of 2,362 to 9,035 ng/ml. Thirteen of fifteen mice had antibody levels greater than 1,000 ng/ml. By contrast, only 7 of 15 mice immunized with 1 μg of PPS14-glycine achieved a serum anti-PPS14 concentration greater than 1,000 ng/ml 10 days after primary immunization, with a GMC of 730 ng/ml (CI, 409 to 1,302 ng/ml). Following secondary immunization with 1 μg of PPS14-C3d, 15 of 15 mice had anti-PPS14 concentrations of >3,000 ng/ml at day 10, with a GMC of 14,808 ng/ml (CI, 8,343 to 26,282 ng/ml). Mice immunized with PPS14-glycine had a GMC of 1,234 ng/ml (CI, 804 to 1,893 ng/ml) following secondary immunization, with 2 of 15 mice having concentrations of >3,000 ng/ml. The differences were statistically significant at all time points in mice receiving both the 0.1-μg dose (P < 0.02) and the 1.0-μg dose (P ≤ 0.0003).
FIG. 1.
Enhancement of the PPS14 antibody response by conjugation of C3d to PPS14. BALB/c mice (15 animals per group) were immunized subcutaneously on day 0 with either 0.1 μg (left) or 1.0 μg (right) of PPS14 either as the PPS14-glycine control (open circles) or as PPS14-C3d conjugate (closed circles). A second immunization with the same PPS14 preparation given in the primary immunization was performed 47 days following the first injection (arrows). Serum samples were obtained 10 and 25 days after the primary immunization and 10 and 23 days after the secondary immunization. Serum anti-PPS14 immunoglobulin concentrations were determined by RABA. Data points represent the GMC for each group of mice. The error bars represent the 95% CI of the geometric mean for each group of sera. Data were analyzed by Student's t test on log-transformed data; the P values for the PPS14-C3d conjugate versus the PPS14-glycine conjugate are shown above the error bar at each time point.
The adjuvant effect of C3d was observed only when C3d was conjugated to PPS14. Compared with mice immunized with unmodified PPS14, there was no enhancement in serum anti-PPS14 when mice were immunized simultaneously with native PPS14 and either monomeric C3d or polymerized C3d in the form of C3d conjugated to the capsular polysaccharide of H. influenzae type b (data not shown).
Antibodies to C3d were measured by ELISA using plates coated with purified mouse C3d. Following incubation with mouse sera, antibodies were detected with alkaline phosphatase-conjugated goat anti-mouse Ig. As expected, the mice did not have a demonstrable antibody response to the mouse C3d portion of the PPS14-C3d conjugate (data not shown).
Comparison of the PPS14 antibody response to PPS14-C3d with that induced by a conjugate of PPS14 and OVA, a T-dependent protein carrier.
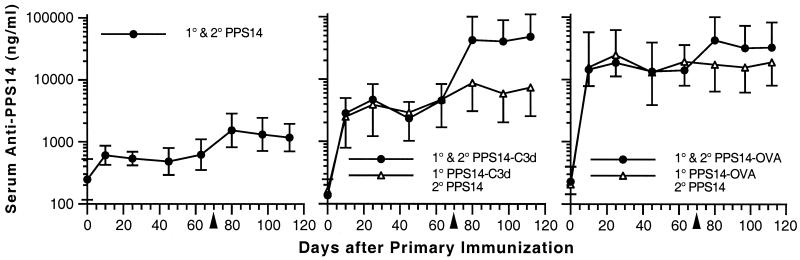
To compare the antibody response of the PPS14-C3d conjugate to that to a conjugate of PPS14 and a T-dependent protein carrier, we prepared conjugates of PPS14 and OVA. OVA was chosen because its molecular mass (43 kDa) is similar to that of C3d; therefore, we could prepare OVA conjugates using methods identical to those used for the preparation of C3d conjugates. Further, we wished to determine whether secondary immunization with unmodified PPS14 following primary immunization with PPS14 conjugates gave a boost in the anti-PPS14 response similar to that from reimmunization with the respective conjugate vaccine. We compared the anti-PPS14 response to those after control immunizations with both unmodified PPS14 and PPS14-glycine. To determine the longevity of the primary antibody response, we postponed the secondary immunization until 70 days after the original immunization. These experiments are summarized in Fig. 2. Mice immunized with unmodified PPS14 and PPS14-glycine had identical anti-PPS14 responses, and therefore we have shown the results for unmodified PPS14 controls only. Figure 2 shows that for mice receiving two 1-μg injections of conjugate vaccine, the primary response was greater for those receiving PPS14-OVA (GMC, 18,632 ng/ml; CI, 5,568 to 62,352 ng/ml at 25 days) than for those receiving PPS14-C3d (GMC, 4,702 ng/ml; CI, 2,659 to 8,314 ng/ml). Ten days following secondary immunization with either conjugate vaccine, a pronounced booster effect was observed. Anti-PPS14 values for the two groups were nearly identical, with the PPS14-OVA group having a serum anti-PPS14 GMC of 42,693 ng/ml (CI, 18,117 to 100,609 ng/ml) and the PPS14-C3d group having an anti-PPS14 GMC of 42,546 ng/ml (CI, 17,998 to 100,573 ng/ml). Serum anti-PPS14 concentrations were significantly greater for both conjugate preparations compared with those for PPS14 alone (P ≤ 0.02 at all time points). The data also show that serum anti-PPS14 levels remained at nearly constant levels from day 25 up to the time of secondary immunization and that reimmunization with native PPS14 instead of conjugate did not result in a boost in serum anti-PPS14 (Fig. 2).
FIG. 2.
Comparison of the PPS14 antibody response to PPS14-C3d with that induced by a conjugate of PPS14 and OVA, a T-dependent protein carrier. BALB/c mice (5 animals per group) were immunized subcutaneously on day 0 with 1 μg of PPS14 either as unmodified PPS14 (left graph) or as PPS14 conjugated to C3d (middle graph) or OVA (right graph). For mice receiving PPS14 conjugates (right two graphs), one group of mice received PPS14 conjugate on both day 0 and day 70 (closed circles) and another group of mice received PPS14 conjugate on day 0 followed by unmodified PPS14 on day 70 (open triangles). The mice were bled 10, 25, 45, and 63 days after the primary immunization and 10, 27, and 42 days after the secondary immunization. Serum anti-PPS14 immunoglobulin concentrations were determined by RABA. Data points represent the GMC for each group of mice. The error bars represent the 95% CI of the geometric mean for each group of sera. The arrowheads indicate the day of secondary immunization. Serum anti-PPS14 concentrations were significantly greater for mice receiving two injections of either conjugate preparation than for those receiving two injections of PPS14 (P ≤ 0.02 at all time points). They were also significantly increased for mice reimmunized with unmodified PPS14 after primary immunization with PPS14-C3d or PPS14-OVA (P ≤ 0.05 at all time points). Reimmunization with unmodified PPS14 after primary immunization with either conjugate did not cause a significant increase in serum anti-PPS14 compared with values 7 days prior to secondary immunization (P ≥ 0.1 at all time points after secondary immunization).
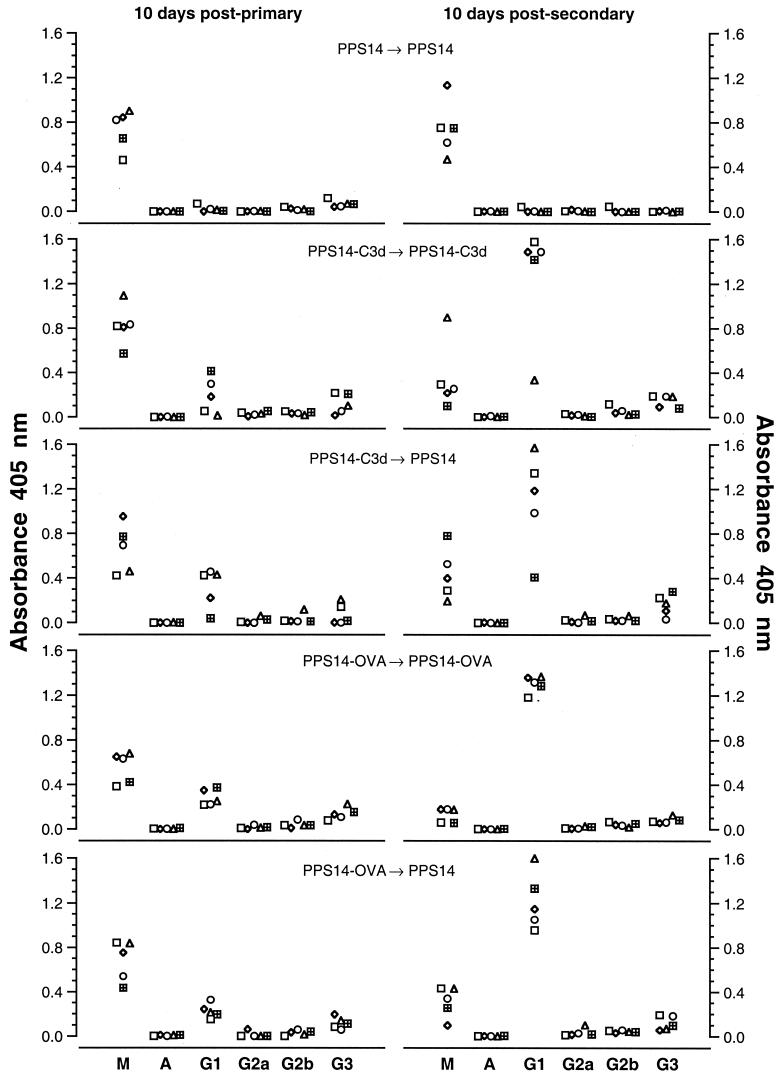
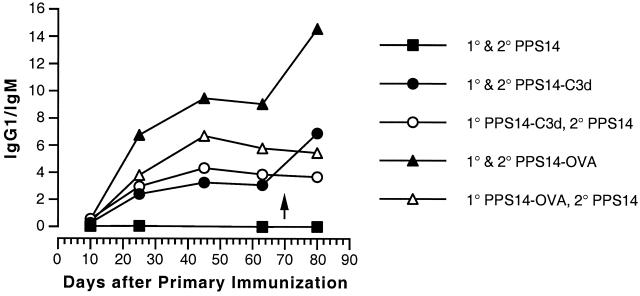
Figure 3 and 4 summarize the results of isotype analysis for each immunization protocol. Figure 3 shows the data for all of the mice in each group at day 10 following both primary and secondary immunization. At 10 days following primary immunization, mice immunized with either PPS14-C3d or PPS14-OVA responded with serum anti-PPS14 antibodies primarily of the IgM subclass, with smaller amounts of IgG1 and IgG3. Ten days following secondary immunization, anti-PPS14 IgG1 predominated in mice receiving either conjugate vaccine, but the switch from IgM to IgG1 was in general more complete for the mice that received PPS14-OVA conjugate (Fig. 3). The switch to IgG1 was also less marked in mice that received a secondary immunization with unmodified PPS14. These differences are especially apparent in Fig. 4, in which the data at several time points are expressed as the ratio of anti-PPS14 IgG1 to anti-PPS14 IgM. The antibody response to unmodified PPS14 consisted solely of IgM, with no evidence of subclass switching to IgG at later time points following primary immunization or after secondary immunization. By contrast, the response to both PPS14-C3d and PPS14-OVA showed a progressive switch from predominantly IgM to predominantly IgG1 up to 45 days following primary immunization. A further increase in the proportion of IgG1 accompanied the increase in total serum anti-PPS14 after secondary immunization with conjugate vaccines but not after reimmunization with unmodified PPS14. Switching to IgG1 was more complete at all time points for mice receiving PPS14-OVA conjugates than for those injected with PPS14-C3d.
FIG. 3.
ELISA heavy chain isotype analysis of anti-PPS14 antibodies in mice immunized with either PPS14, PPS14-C3d, or PPS14-OVA. BALB/c mice were immunized as described in the Fig. 2 legend, and serum samples obtained 10 days after primary and secondary immunization were analyzed for immunoglobulin isotype. For each assay, individual serum samples were diluted so that each had an equivalent concentration of total anti-PPS14 Ig. Isotype concentrations were determined by ELISA. Results are expressed as the absorbance at 405 nm for each sample. Within each horizontal pair of graphs, each symbol represents data for the same mouse; each pair of graphs displays data for a single group of five mice immunized as shown.
FIG. 4.
Kinetics of anti-PPS14 heavy chain switching in mice immunized with PPS14, PPS14-C3d, or PPS14-OVA. BALB/c mice were immunized as described in the Fig. 2 legend; the arrow indicates the time of secondary immunization at day 70 post-primary immunization. Serum samples from all time points were analyzed for immunoglobulin isotype as described in the Fig. 3 legend. The predominant isotypes at all time points were IgM and/or IgG1. The ratio of the A405 for IgG1 to the A405 for IgM was calculated for each mouse. Each point represents the mean ratio for each group of five mice. Error bars are omitted for the sake of clarity.
These results suggest that two injections of PPS14-C3d have immunogenicity equivalent to two injections of PPS14-OVA. The significant increase in total serum anti-PPS14 and the increase in anti-PPS14 IgG1 relative to the level of anti-PPS14 IgM following the second injection of either conjugate suggests that both PPS14-C3d and PPS14-OVA are capable of eliciting the development of memory B cells.
Comparison of the PPS14 antibody response to PPS14-C3d and PPS14-OVA in athymic nude mice.
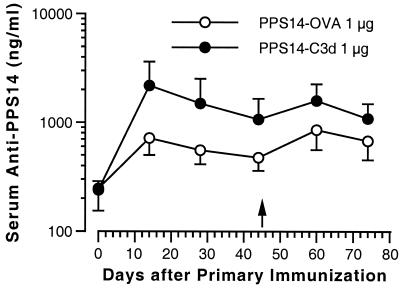
To determine the relative role of T cells in the antibody response to the two different conjugates, we immunized athymic nude BALB/c mice (Fig. 5). As expected, the primary anti-PPS14 response to the PPS14-OVA conjugate was severely blunted compared with the primary response in conventional BALB/c mice (see Fig. 2). Fourteen days after primary immunization, 0 of 7 mice receiving 1 μg of PPS14-OVA had a serum anti-PPS14 concentration of >2,000 ng/ml, with a GMC of 717 ng/ml (CI, 503 to 1,023 ng/ml). By contrast, mice injected with 1 μg of PPS14-C3d had a primary antibody response similar in magnitude to that seen in conventional BALB/c mice, with a GMC of 2,187 ng/ml (CI, 1,318 to 3,628 ng/ml) at 14 days post-primary immunization (P = 0.01 versus values for mice immunized with PPS14-OVA). Five of seven of these mice had a serum anti-PPS14 concentration of >2,000 ng/ml. Compared with the results for conventional BALB/c mice shown in Fig. 2, there was little or no booster response in serum anti-PPS14 levels after a second injection of either PPS14-OVA or PPS14-C3d. Fifteen days after secondary immunization, mice immunized with PPS14-OVA had a serum anti-PPS14 GMC of 857 ng/ml (CI, 562 to 1,307 ng/ml), while those immunized with PPS14-C3d had a serum anti-PPS14 GMC of 1,590 ng/ml (CI, 1,116 to 2,265 ng/ml). These values were not significantly different (P = 0.09). Thus, conjugation of C3d to PPS14 appears to have an adjuvant effect on the primary antibody response that is largely independent of T-cell help but has a substantially diminished effect on the anti-PPS14 memory response in the absence of T cells.
FIG. 5.
Comparison of the PPS14 antibody response to PPS14-C3d and PPS14-OVA in athymic nude mice. BALB/c nu/nu mice (seven animals per group) were immunized subcutaneously on day 0 with 1 μg of PPS14 either as PPS14 conjugated to OVA (open circles) or to C3d (closed circles). A second immunization with the same PPS14 preparation given in the primary immunization was performed 45 days following the first injection (arrow). The mice were bled 14, 28, and 44 days after the primary immunization and 15 and 29 days after the secondary immunization. Serum anti-PPS14 immunoglobulin concentrations were determined by RABA. Data points represent the GMC for each group of mice. Error bars represent the 95% CI of the geometric mean for each group of sera. Serum anti-PPS14 concentrations were significantly greater for mice receiving two injections of PPS14-C3d than for those receiving two injections of PPS14-OVA at day 14 (P = 0.01), day 28 (P = 0.02), and day 44 (P = 0.03) after primary immunization, but not at day 15 (P = 0.09) and day 29 (P = 0.15) after secondary immunization.
DISCUSSION
Conjugation of C3d to an immunogen could potentially influence every step of the humoral immune response subsequent to antigen administration. First, transport of antigen to secondary lymphoid tissues could be facilitated by interactions between C3d and CR1/CR2 on circulating B cells or the antigen-transporting cells described by Tew et al. (41). Second, coligation of CR2 and antigen receptors on B cells could have a number of effects resulting in an enhanced primary antibody response. These effects include a lowering of both the concentration threshold and the affinity threshold for B-cell activation (4, 26); increasing the level of B-cell activation for a given dose of antigen (4, 5, 43); increasing membrane expression of CD 80 and CD86 (B7.1 and B7.2), both of which are involved in cognate B-cell–T-cell interactions (18); and decreasing B-cell apoptosis (19, 36). Finally, interactions between C3d and CR2 on follicular dendritic cells could result in enhanced follicular trapping of antigen (7, 15, 45) and enhanced cognate interactions between follicular dendritic cells and antigen-specific B cells (33). This, coupled with the enhancing effects of CR2 ligation on germinal center formation (11, 18) and the survival of germinal center B cells (9), would promote the development and maintenance of memory B cells (15).
The ability of unmodified PPS14 to induce CR2-dependent immune enhancement would depend both on its ability to activate the alternative pathway of complement (APC) and on the nature of the C3 fragments bound to the polysaccharide as a consequence of APC activation. Prior studies have suggested that purified PPS14 is only a weak activator of the APC. PPS14 was unable to activate the APC when incubated with guinea pig serum (48). Incubation of PPS14 with human serum resulted in increased serum concentrations of C3d when incubated with serum from a patient with severe combined immunodeficiency but not when incubated with serum from two patients with C1q deficiency (11). In that study, binding of C3d to PPS14 was demonstrated after incubation of PPS14 with normal human serum (11), but the polyclonal antibody to human C3d used to detect C3d binding has also been shown to bind to C3b and iC3b (34). Thus, the relatively weak activation of the APC by PPS14 may result in binding of C3b and iC3b in addition to C3d. Indeed, incubation of intact serotype 14 pneumococcus with human serum has been shown to result in covalent binding of iC3b alone to the capsular surface (13). The nature of the C3 fragments bound to PPS14 would be important, because C3b and iC3b could bind to CR1 and CR3 and/or CR4, which are present on a variety of cells that would not be involved in the generation of an antibody response (14). By contrast, synthetic conjugates of C3d and PPS14 would be targeted specifically to B cells and follicular dendritic cells, both of which are critical components of the humoral immune response, and this would occur even in the absence of complement activation.
The results presented here suggest that synthetic conjugates of PPS14 and C3d are far more effective immunogens than unmodified PPS14, with an efficacy similar to that of conjugates of PPS14 and OVA, a T-dependent protein carrier. After two 1-μg injections of PPS14-C3d, there was a mean 411-fold increase in serum anti-PPS14 concentrations compared to preimmunization levels. By contrast, two injections of a PPS14-glycine control resulted in only a 16-fold increase in serum anti-PPS14 levels. In a subsequent experiment, two 1-μg injections of PPS14-C3d resulted in serum anti-PPS14 concentrations that were nearly identical to those observed after two injections of PPS14-OVA. This result could be fortuitous, since we did not attempt to optimize the OVA conjugate in terms of the ratio of polysaccharide to protein. This ratio has been shown to influence the efficacy of PPS conjugated to protein carriers (29). We also did not attempt to optimize our PPS14-C3d conjugate, although the ratio of ∼4 molecules of C3d per 100 kDa of PPS14 in our conjugate preparation would result in efficient cross-linking of CR2 while preserving the antigenicity of the polysaccharide moiety. The increased serum anti-PPS14 concentrations induced by two injections of PPS14-C3d in the experiment shown in Fig. 2 compared with that shown in Fig. 1 most likely resulted from the longer interval between primary and secondary immunization in the second experiment (70 versus 47 days), as extending the interval between the two injections has been shown to increase the secondary response to PPS conjugate vaccines (1).
Despite the similarities in the total serum antibody levels elicited by the C3d and OVA conjugates, the characteristics of the immune response to the two conjugates differed in several respects. The primary PPS14 antibody response was similar in both conventional and athymic nude BALB/c mice immunized with PPS14-C3d, whereas the primary response to PPS14-OVA was profoundly impaired in athymic nude mice. In conventional BALB/c mice, the response to primary immunization with the PPS14-OVA conjugate was about four times greater than that in mice immunized with the PPS14-C3d conjugate. Thus, the magnitude of the booster response was much greater in mice receiving two injections of the PPS14-C3d conjugate than in those receiving two injections of PPS14-OVA (Fig. 2). Isotype analysis showed that immunization with the OVA conjugate induced a greater degree of switching from IgM to IgG1 than did immunization with the C3d conjugate (Fig. 3 and 4). Although both conjugates induced a further increase in the proportion of anti-PPS14 IgG1 after secondary immunization, the increase was greater in mice receiving the PPS14-OVA conjugate. The switch in Ig isotype after immunization with the OVA conjugate is consistent with a mechanism involving T-cell help, while the relatively higher proportion of anti-PPS14 IgM after immunization with the PPS14-C3d conjugate may indicate that the C3d conjugate retained some features characteristic of a TI-2 antigen (25).
Differences in the immune response to the OVA and C3d conjugates could reflect differences in B-cell activation induced by interactions with T cells versus that induced by direct cross-linking of antigen receptors and the CD21/CD19 complex (40). However, the absence of a booster response to PPS14-C3d in athymic nude mice suggests that binding of the PPS14-C3d conjugate resulted in sufficient activation of B cells (and/or follicular dendritic cells) to enable interactions with T cells that were necessary for development of a full anti-PPS14 memory response. Recruitment of T-cell help by TI-2 antigens could occur by either MHC II-restricted (via display of idiotypic peptides) or non-MHC II-restricted mechanisms (reviewed in references 25 and 47). For example, increased expression of B7-1 and B7-2 on B cells following cross-linking of CR2 (18) by PPS14-C3d could lead to enhanced interactions with CD28/CTLA-4 on T cells.
Both the boost in total serum anti-PPS14 and the increase in the proportion of anti-PPS14 IgG1 relative to anti-PPS14 IgM following secondary immunization with PPS14-C3d suggested that a true anamnestic response was induced. The same was true for the PPS14-OVA conjugate. However, the failure of a second immunization with native PPS14, following primary immunization with either conjugate, to induce an increase in total and IgG-specific anti-PPS14 antibody concentrations (Fig. 4) appears to conflict with this evidence. Although native PPS has been shown to induce a memory response in humans following primary immunization with PPS conjugate vaccines (38), this does not consistently occur in mice. The response to secondary immunization with native PPS in mice could be influenced by the mouse strain, the dose of conjugate, the dose of native PPS, and the timing of the secondary immunization. Indeed, Peeters et al. showed that secondary immunization with 2.5 μg of PPS4 resulted in a memory response only after primary immunization with a PPS4-tetanus toxoid conjugate dose of <0.5 μg (30). Further experiments will be necessary to optimize immunization schedules combining the use of PPS14-C3d (or PPS14-OVA) conjugates and native PPS14 in BALB/c mice.
An obvious concern regarding the use of C3d conjugates is the possibility that they could bind to non-antigen-specific B cells and trigger an increase in nonspecific antibody production leading to autoimmune disease. However, in vitro studies suggest that C3d cross-linking in the absence of antigen receptor ligation does not lead to antibody production. Direct conjugates of HEL and C3d failed to activate non-antigen-specific B cells as measured by increases in intracellular calcium (5). In another study, keyhole limpet hemocyanin immune complexes that fixed iC3b/C3dg following classical pathway activation were able to induce increases in CD80 and activated LFA-1 expression on nonspecific B cells, but there was no stimulation of immunoglobulin production except by keyhole limpet hemocyanin-specific B cells (42). If the effects of C3d conjugation to PPS14 hold true for capsular polysaccharides from other pneumococcal serotypes and species of bacteria, conjugates of C3d and bacterial capsular polysaccharides will provide a potentially safe and effective alternative to conjugates utilizing T-dependent protein carriers. The potential for vaccines incorporating both types of conjugate to induce additive or synergistic increases in the immune response to capsular polysaccharides will depend on the degree of overlap in the mechanisms by which they increase polysaccharide immunogenicity.
ACKNOWLEDGMENTS
We thank Richard Quigg (University of Chicago) for advice on the purification of murine C3 and C3d and John Lambris (University of Pennsylvania) for helpful discussions.
This work was supported by National Institutes of Health grants AI-25008 and AI-45250 and by a grant from Children's Hospital Oakland Research Institute.
REFERENCES
- 1.Braley-Mullen H. Secondary IgG responses to type III pneumococcal polysaccharide. I. Kinetics and antigen requirements. J Immunol. 1975;115:1194–1198. [PubMed] [Google Scholar]
- 2.Brown E J. Interaction of gram-positive microorganisms with complement. Curr Top Microbiol Immunol. 1985;121:159–187. doi: 10.1007/978-3-642-45604-6_8. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan R M, Arulanandam B P, Metzger D W. IL-12 enhances the antibody responses to T-independent polysaccharide vaccines in the absence of T and NK cells. J Immunol. 1998;161:5525–5533. [PubMed] [Google Scholar]
- 4.Carter R H, Spycher M O, Ng Y C, Hoffman R, Fearon D T. Synergistic interaction between complement receptor 2 and membrane IgM on B lymphocytes. J Immunol. 1988;141:457–463. [PubMed] [Google Scholar]
- 5.Dempsey P W, Allison M E D, Akkaraju S, Goodnow C C, Fearon D T. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 6.Dick W E, Jr, Beurret M. Glycoconjugates of bacterial carbohydrate antigens. A survey and consideration of design and preparation factors. Contrib Microbiol Immunol. 1989;10:48–114. [PubMed] [Google Scholar]
- 7.Fang Y, Xu C, Fu Y-X, Holers V M, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160:5273–5279. [PubMed] [Google Scholar]
- 8.Fearon D T. Innate immunity—beginning to fulfill its promise? Nat Immunol. 2000;1:102–103. doi: 10.1038/77773. [DOI] [PubMed] [Google Scholar]
- 9.Fischer M B, Goerg S, Shen L, Prodeus A P, Goodnow C C, Kelsoe G, Carroll M C. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science. 1998;280:582–585. doi: 10.1126/science.280.5363.582. [DOI] [PubMed] [Google Scholar]
- 10.Fischer M B, Ma M, Goerg S, Zhou X, Xia J, Finco O, Han S, Kelsoe G, Howard R G, Rothstein T L, Kremmer E, Rosen F S, Carroll M C. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J Immunol. 1996;157:549–556. [PubMed] [Google Scholar]
- 11.Griffioen A W, Rijkers G T, Janssens-Korpela P, Zegers B J M. Pneumococcal polysaccharides complexed with C3d bind to human B lymphocytes via complement receptor type 2. Infect Immun. 1991;29:1839–1845. doi: 10.1128/iai.59.5.1839-1845.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausdorff W P, Bryant J, Paradiso P R, Siber G R. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. 2000;30:100–121. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 13.Hostetter M K. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J Infect Dis. 1986;153:682–693. doi: 10.1093/infdis/153.4.682. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita T. Complement receptors and regulation of the humoral immune response. In: Cruse J M, Lewis R E Jr, editors. Complement today. Complement profiles. Vol. 1. Basel, Switzerland: Karger; 1993. pp. 46–55. [Google Scholar]
- 15.Klaus G G B, Humphrey J H, Kunkl A, Dongworth D W. The follicular dendritic cell: its role in antigen presentation in the generation of immunological memory. Immunol Rev. 1980;53:3–29. doi: 10.1111/j.1600-065x.1980.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 16.Klein D L, Ellis R W. Conjugate vaccines against Streptococcus pneumoniae. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker, Inc; 1997. pp. 503–525. [Google Scholar]
- 17.Kopf M, Herren S, Wiles M V, Pepys M B, Kosco-Vilbois M H. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med. 1998;188:1895–1906. doi: 10.1084/jem.188.10.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozono Y, Abe R, Kozono H, Kelly R G, Azuma T, Holers V M. Cross-linking CD21/CD35 or CD19 increases both B7–1 and B7–2 expression on murine splenic B cells. J Immunol. 1998;160:1565–1572. [PubMed] [Google Scholar]
- 19.Kozono Y, Duke R C, Schleicher M S, Holers V M. Co-ligation of mouse complement receptors 1 and 2 with surface IgM rescues splenic B cells and WEHI-231 cells from anti-surface IgM-induced apoptosis. Eur J Immunol. 1995;25:1013–1017. doi: 10.1002/eji.1830250423. [DOI] [PubMed] [Google Scholar]
- 20.Krieg A M. The role of CpG motifs in innate immunity. Curr Opin Immunol. 2000;12:35–43. doi: 10.1016/s0952-7915(99)00048-5. [DOI] [PubMed] [Google Scholar]
- 21.Lees A, Nelson B L, Mond J J. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for use in protein-polysaccharide conjugate vaccines and immunological reagents. Vaccine. 1996;14:190–198. doi: 10.1016/0264-410x(95)00195-7. [DOI] [PubMed] [Google Scholar]
- 22.Lou D, Kohler H. Enhanced molecular mimicry of CEA using photoaffinity crosslinked C3d peptide. Nat Biotechnol. 1998;16:458–462. doi: 10.1038/nbt0598-458. [DOI] [PubMed] [Google Scholar]
- 23.Lucas A H, Granoff D M, Mandrell R E, Connolly C C, Shan A S, Powers D C. Oligoclonality of serum immunoglobulin G antibody responses to Streptococcus pneumoniae capsular polysaccharide serotypes 6B, 14, and 23F. Infect Immun. 1997;65:5103–5109. doi: 10.1128/iai.65.12.5103-5109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markham R B, Nicholson-Weller A, Schiffman G, Kasper D L. The presence of sialic acid on two related bacterial polysaccharides determines the site of the primary immune response and the effect of complement depletion on the response in mice. J Immunol. 1982;128:2731–2733. [PubMed] [Google Scholar]
- 25.Mond J J, Lees A, Snapper C M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 26.Mongini P K A, Vilensky M A, Highet P F, Inman J K. The affinity threshold for human B cell activation via the antigen receptor complex is reduced upon co-ligation of the antigen receptor with CD21 (CR2) J Immunol. 1997;159:3782–3791. [PubMed] [Google Scholar]
- 27.Monsigny M, Petit C, Roche A-C. Colorimetric determination of neutral sugars by a resorcinol sulfuric acid micromethod. Anal Biochem. 1988;175:525–530. doi: 10.1016/0003-2697(88)90578-7. [DOI] [PubMed] [Google Scholar]
- 28.Nahm M H, Apicella M A, Briles D E. Immunity to extracellular bacteria. In: Paul W E, editor. Fundamental immunology. 4th ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1999. pp. 1373–1386. [Google Scholar]
- 29.Peeters C C A M, Tenbergen-Meekes A-M, Evenberg D E, Poolman J T, Zegers B J M, Rijkers G T. A comparative study of the immunogenicity of pneumococcal type 4 polysaccharide and oligosaccharide tetanus toxoid conjugates in adult mice. J Immunol. 1991;146:4308–4314. [PubMed] [Google Scholar]
- 30.Peeters C C A M, Tenbergen-Meekes A-M J, Poolman J T, Zegers B J M, Rijkers G T. Immunogenicity of a Streptococcus pneumoniae type 4 polysaccharide-protein conjugate vaccine is decreased by admixture of high doses of free saccharide. Vaccine. 1992;10:833–840. doi: 10.1016/0264-410x(92)90046-m. [DOI] [PubMed] [Google Scholar]
- 31.Pepys M B. Role of complement in induction of antibody production in vivo. Effect of cobra venom factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med. 1974;140:126–145. doi: 10.1084/jem.140.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pryjma J, Humphrey J H. Prolonged C3 depletion by cobra venom factor in thymus-deprived mice and its implication for the role of C3 as an essential second signal for B-cell triggering. Immunology. 1975;28:569–576. [PMC free article] [PubMed] [Google Scholar]
- 33.Qin D, Wu J, Carroll M C, Burton G F, Szakal A K, Tew J G. Evidence for an important interaction between a complement-derived CD21 ligand on follicular dendritic cells and CD21 on B cells in the initiation of IgG responses. J Immunol. 1998;161:4549–4554. [PubMed] [Google Scholar]
- 34.Quigg R J, Alexander J J, Lo C F, Lim A, He C, Holers V M. Characterization of C3-binding proteins on mouse neutrophils and platelets. J Immunol. 1997;159:2438–2444. [PubMed] [Google Scholar]
- 35.Robbins J B, Schneerson R, Anderson P, Smith D H. The 1996 Albert Lasker Medical Research Awards. Prevention of systemic infections, especially meningitis, caused by Haemophilus influenzae type b. Impact on public health and implications for other polysaccharide-based vaccines. JAMA. 1996;276:1181–1185. doi: 10.1001/jama.276.14.1181. [DOI] [PubMed] [Google Scholar]
- 36.Roberts T, Snow E C. Cutting edge: recruitment of the CD19/CD21 coreceptor to B cell antigen receptor is required for antigen-mediated expression of Bcl-2 by resting and cycling hen egg lysozyme transgenic B cells. J Immunol. 1999;162:4377–4380. [PubMed] [Google Scholar]
- 37.Ross T M, Xu Y, Bright R A, Robinson H L. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat Immunol. 2000;1:127–131. doi: 10.1038/77802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin L G. Pneumococcal vaccine. Pediatr Clin North Am. 2000;47:269–285. doi: 10.1016/s0031-3955(05)70207-8. [DOI] [PubMed] [Google Scholar]
- 39.Siber G R. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 40.Tedder T F, Inaoki M, Sato S. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6:107–118. doi: 10.1016/s1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- 41.Tew J G, Wu J, Qin D, Helm S, Burton G F, Szakal A K. Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol Rev. 1997;156:39–52. doi: 10.1111/j.1600-065x.1997.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 42.Thornton B P, Vetvicka V, Ross G D. Function of C3 in a humoral response: iC3b/C3dg bound to an immune complex generated with natural antibody and a primary antigen promotes antigen uptake and the expression of co-stimulatory molecules by all B cells, but only stimulates immunoglobulin synthesis by antigen-specific B cells. Clin Exp Immunol. 1996;104:531–537. doi: 10.1046/j.1365-2249.1996.57761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsokos G C, Lambris J D, Finkelman F D, Anastassiou E D, June C H. Monovalent ligands of complement receptor 2 inhibit whereas polyvalent ligands enhance anti-Ig-induced human B cell intracytoplasmic free calcium concentration. J Immunol. 1990;144:1640–1645. [PubMed] [Google Scholar]
- 44.Van den Berg C W, Van Dijk H, Capel P J A. Rapid isolation and characterization of native mouse complement components C3 and C5. J Immunol Methods. 1989;122:73–78. doi: 10.1016/0022-1759(89)90336-0. [DOI] [PubMed] [Google Scholar]
- 45.Van den Eertwegh A J M, Laman J D, Schellekens M M, Boersma W J A, Claassen E. Complement-mediated follicular localization of T-independent type-2 antigens: the role of marginal zone macrophages revisited. Eur J Immunol. 1992;22:719–726. doi: 10.1002/eji.1830220315. [DOI] [PubMed] [Google Scholar]
- 46.Villiers M-B, Villiers C L, Laharie A-M, Marche P N. Amplification of the antibody response by C3b complexed to antigen through an ester link. J Immunol. 1999;162:3647–3652. [PubMed] [Google Scholar]
- 47.Vos Q, Lees A, Wu Z-Q, Snapper C M, Mond J J. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–170. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 48.Winkelstein J A, Bocchini J A, Jr, Schiffman G. The role of the capsular polysaccharide in the activation of the alternative pathway by the pneumococcus. J Immunol. 1976;116:367–370. [PubMed] [Google Scholar]
- 49.World Health Organization. Pneumococcal vaccines. WHO position paper. Wkly Epidemiol Rec. 1999;74:177–183. [PubMed] [Google Scholar]