Abstract
C57BL/6 mice were vaccinated with plasmid DNA encoding Ag85 from Mycobacterium tuberculosis, with Ag85 protein in adjuvant, or with a combined DNA prime-protein boost regimen. While DNA immunization, as previously described, induced robust Th1-type cytokine responses, protein-in-adjuvant vaccination elicited very poor cytokine responses, which were 10-fold lower than those observed with DNA immunization alone. Injection of Ag85 DNA-primed mice with 30 to 100 μg of purified Ag85 protein in adjuvant increased the interleukin-2 and gamma interferon (IFN-γ) response in spleen two- to fourfold. Further, intracellular cytokine analysis by flow cytometry also showed an increase in IFN-γ-producing CD4+ T cells in DNA-primed–protein-boosted animals, compared to those that received only the DNA vaccination. Moreover, these responses appeared to be better sustained over time. Antibodies were readily produced by all three methods of immunization but were exclusively of the immunoglobulin G1 (IgG1) isotype following protein immunization in adjuvant and preferentially of the IgG2a isotype following DNA and DNA prime-protein boost vaccination. Finally, protein boosting increased the protective efficacy of the DNA vaccine against an intravenous M. tuberculosis H37Rv challenge infection, as measured by CFU or relative light unit counts in lungs 1 and 2 months after infection. The capacity of exogenously given protein to boost the DNA-primed vaccination effect underlines the dominant role of Th1-type CD4+ helper T cells in mediating protection.
Tuberculosis (TB) remains a major health problem affecting millions of people worldwide. The only TB vaccine presently available is an attenuated strain of Mycobacterium bovis termed M. bovis BCG. The efficacy of BCG remains controversial, particularly against pulmonary TB in young adults (5), and development of a better vaccine is urgently needed to counter the global threat of this disease (22).
Secreted and surface-exposed cell wall proteins are major antigens recognized by the protective immune response against TB and immunization with whole-culture filtrate, a rich source of these extracellular proteins, can protect mice and guinea pigs to some extent against subsequent challenge with the tubercle bacillus (1, 14, 15). A major portion of the secreted proteins in Mycobacterium tuberculosis and BCG culture filtrate is formed by the Ag85 complex, a 30- to 32-kDa family of three proteins (Ag85A, Ag85B, and Ag85C) (38) which all possess a mycoloyltransferase enzyme activity required for the biogenesis of cord factor (4), a dominant structure necessary for maintaining cell wall integrity (19, 29). Ag85 complex induces strong T-cell proliferation and gamma interferon (IFN-γ) production in most healthy individuals infected with M. tuberculosis and/or Mycobacterium leprae (24) and in BCG-vaccinated mice (16), making it a promising candidate as a protective antigen. Vaccination with naked plasmid DNA encoding Ag85A and Ag85B can stimulate strong humoral and cell-mediated immune responses and confer significant protection to C57BL/6 (B6) mice challenged by the aerosol or intravenous route with live M. tuberculosis H37Rv (17, 20). Only intramuscular (i.m.) needle injection but not epidermal gene gun bombardment is capable of inducing a protective, Th1-biased immune response with this vaccine (36). In experimental mouse models, Ag85A DNA vaccine so far is effective only during the first weeks after M. tuberculosis challenge, and subsequently its protection, as measured by reduced CFU counts in lungs, wanes (37).
Here we report on an attempt to improve the immunogenicity and protective efficacy of this Ag85 DNA TB vaccine by a DNA prime-protein boost immunization regimen. Indeed, i.m. DNA vaccination is particularly effective in priming a Th1-type immune response, but the low amount of actual protein antigen synthesized in the host is a serious limitation of this type of immunization. Prime-boost strategies of consecutive DNA priming followed by boosting with purified proteins or with attenuated poxviruses have the potential to improve dramatically these DNA-based vaccines through preferential amplification of CD4+ or CD8+ effectors, respectively (27, 30).Whereas a number of studies have reported on the effect of protein boosting of DNA vaccines encoding viral (3, 25, 26, 28, 31, 35) and protozoal (12, 21) antigens, little is known with respect to mycobacterial infections. Here we demonstrate that protein boosting of B6 mice vaccinated with plasmid DNA encoding Ag85A and Ag85B from M. tuberculosis is capable of increasing the immunogenicity and (to a lesser extent) protective efficacy of this experimental TB DNA vaccine.
MATERIALS AND METHODS
Plasmid construction.
Plasmid DNAs encoding a mature or secreted form of Ag85A and Ag85B from M. tuberculosis were prepared as described previously (2).
Mice.
B6 mice were bred in the Animal Facilities of the Pasteur Institute of Brussels. Only female mice 6 to 8 weeks old at the start of vaccination were used.
Protein, DNA, and BCG vaccination.
For protein immunization, mice were injected subcutaneously (s.c.) in the back with 100 μg of Ag85A purified by sequential chromatography from BCG culture filtrate (7) and emulsified in monophosphoryl lipid A (MPL-A) from Salmonella enterica serovar Minnesota (Ribi ImmunoChem Research, Hamilton, Mont.) solubilized in triethanolamine. The amino acid sequences of Ag85A from M. tuberculosis and of BCG are 100% identical (8). For DNA vaccination, mice were anesthetized by intraperitoneal injection of ketamine and xylazine (100 and 10 mg/kg of body weight, respectively) and injected i.m. in both quadriceps with 2 × 50 μg of plasmid DNA either in saline (Ag85A DNA) or complexed in the cationic lipid vaxfectin (Ag85B DNA) (13). For the DNA prime-protein boost, mice were immunized i.m. with Ag85 DNA and s.c. with 1, 10, 30, 50, or 100 μg of purified native Ag85A protein in MPL-A or with 50 μg of purified recombinant Ag85B protein (11) in SBAS2A adjuvant (SmithKline Beecham). All mice received three immunizations at 3-week intervals. For BCG vaccination, mice were injected intravenously (i.v.) in a lateral tail vein with 106 CFU of freshly prepared BCG (strain GL2) grown as a surface pellicle on synthetic Sauton medium (16) on the same day as the third immunization.
ELISA.
Sera from immunized mice were collected by retro-orbital bleeding 2 months after the third vaccination. Levels of total anti-Ag85A Igκ antibodies (Abs) were determined by enzyme-linked immunosorbent assay (ELISA) in sera from individual mice (five/group). The serum titer was converted to Ab concentration (nanograms per milliliter) by comparison with a standard monoclonal Ab, and mean Ab concentration was calculated from at least three points of the linear portion of the titration curve. Concentrations were converted to log10 values. For isotype analysis, peroxidase-labeled rat anti-mouse immunoglobulin G1 (IgG1) and IgG2a (Experimental Immunology Unit, Université Catholique de Louvain, Brussels, Belgium) were used. Equal amounts of the five sera in each group were pooled, and isotype titers were determined and converted to arbitrary units by comparison with the titer of a standard serum pool from Ag85A DNA-immunized mice, arbitrarily assigned a titer of 1,000 for both isotypes.
Cytokine production.
Vaccinated mice were sacrificed 3 weeks (dose-response experiment) or 2 months (peptide mapping) after the third immunization, and spleens were removed aseptically. Spleen cells from three mice per group were tested individually for cytokine response to whole Ag85A (5 μg/ml) or p25 (10 μg/ml) (dose-response experiment) and as a pool for peptide mapping (18). Supernatants were harvested after 24 h (interleukin-2 [IL-2]) and 72 h (IFN-γ), when peak values of the respective cytokines could be measured. Supernatants from at least three separate wells were pooled and stored frozen at −20°C until the assay. Analysis was performed twice, and data from one experiment are reported.
IL-2 assay.
IL-2 activity was measured using a bioassay, as described previously (16). Each sample was tested in duplicate. IL-2 levels are expressed in mean counts per minute. The standard deviation (SD) was below 10%. In this assay, a standard IL-2 preparation (18) at 600 pg/ml corresponded to ±15,000 cpm and the detection limit was 30 pg/ml.
IFN-γ assay
IFN-γ activity was quantified by sandwich ELISA using coating Ab and biotinylated detection Ab XMG1.2 (both from PharMingen, Erembodegem, Belgium). The sensitivity of ELISA was 10 pg/ml.
Intracellular IFN-γ measurement using flow cytometry.
Splenocytes from vaccinated mice were cultured at 2.5 × 106/ml in 48-well tissue culture plates (Nunclon, Roskilde, Denmark) in the presence of 5 μg of Ag85A protein/ml for 1 or 3 days. Brefeldin A (Sigma, St. Louis, Mo.) was added to the cultures for the last 5 h to prevent secretion of the intracellular cytokine. One million cells from each group were first incubated with fluorescein isothiocyanate-conjugated anti-CD4 Ab (clone RM4 to 4 PharMingen) for 30 min at 4°C. Cells were then washed, fixed with 4% paraformaldehyde, and permeabilized with phosphate-buffered saline containing 0.1% saponin. To label intracellular IFN-γ, cells were incubated with phycoerythrin-conjugated anti-IFN-γ Ab (clone XMG1.2; PharMingen) for 30 min at 4°C, washed, and acquired on a cytofluorometer (FACSCALIBUR; BD, Mountain View, Calif.). Lymphocytes were gated by their forward and side light scattering properties, and 100,000 cells were acquired in the lymphocyte gate. Analysis was done using Cell Quest software.
M. tuberculosis challenge.
B6 mice were rested for 2 months after the third immunization and were challenged i.v. in a lateral tail vein with 106 CFU of M. tuberculosis H37Rv (37) (Ag85A DNA) or with 106 CFU of recombinant luciferase reporter M. tuberculosis H37Rv (34) (Ag85B DNA). Mice vaccinated with Ag85A DNA were sacrificed 30, 60, or 90 days after challenge, and serial threefold total lung homogenate dilutions were plated on 7H11 Middlebrook agar supplemented with oleic acid-albumin-dextrose-catalase (OADC). Colonies were counted visually after 4 weeks. CFU counts obtained from two or three dilutions were used to calculate the total number of CFU/lung/mouse. For statistical analysis (Student's t test), these data were converted to log10 values and log10 (mean ± SD) values for CFU/lung/mouse were calculated for each experimental group, which consisted of 3 to 10 animals tested individually (as indicated in Table 3). Mice vaccinated with Ag85B DNA were sacrificed 30 days after challenge, and the number of bacteria per lung was determined by classical CFU counting on Middlebrook 7H11 agar and in a bioluminescence assay using a Turner Design 20/20 luminometer and 1% n-decylaldehyde in ethanol as the substrate (34).
TABLE 3.
Bacterial replication in lungs from B6 mice vaccinated with Ag85A protein, Ag85A DNA, or a DNA prime-protein boost regimen and challenged with M. tuberculosis H37Rv 2 months after the last immunization
| Vaccine used | Level of replication (no. of CFU/lung) after i.v. M. tuberculosis challengea (no. of mice)
|
||
|---|---|---|---|
| 30 days | 60 days | 90 days | |
| MPL-A-control DNA | 5.27 ± 0.20 (10) | 5.06 ± 0.23 (8) | 5.20 ± 0.22 (8) |
| BCG | 3.70 ± 0.22b (3) | NDc | 4.22 ± 0.11b (3) |
| Ag85A protein | 5.15 ± 0.26 (5) | 4.87 ± 0.13 (5) | 4.93 ± 0.20 (4) |
| Ag85A DNA | 4.82 ± 0.27b (5) | 4.93 ± 0.26 (5) | 5.18 ± 0.22 (4) |
| DNA-protein | 4.54 ± 0.16b (5) | 4.56 ± 0.25b (5) | 5.06 ± 0.25 (3) |
Mean ± SD (log10 values) is given for numbers of CFU/lung.
P < 0.005.
ND, not done.
RESULTS
Ag85A-specific IL-2 and IFN-γ production in spleen cell cultures from B6 mice vaccinated with Ag85A DNA can be improved by Ag85A protein boosting.
As shown in Table 1, the production of specific IL-2 or IFN-γ in response to purified Ag85A protein or to the immunodominant p25 peptide (amino acids 241 to 260), respectively, was significantly higher in spleen cell cultures from mice that had been immunized with a DNA prime-protein boost regimen than in spleen cell cultures from mice that had been vaccinated with Ag85A DNA only. Boosting with a dose of 30 or 50 μg of purified native Ag85A protein increased the Th1 cytokine response to the same extent, whereas doses of 1 or 10 μg of protein resulted in cytokine levels comparable to those observed with an immunization protocol of three DNA injections.
TABLE 1.
Ag85A-specific IL-2 and IFN-γ production in spleen cell cultures from B6 mice vaccinated with Ag85A DNA and boosted with Ag85A protein
| Vaccine used (μg of protein) | IL-2 (cpm)a | IFN-γ (pg/ml)b |
|---|---|---|
| DNA 3× | 16,393 ± 6,716 | 1,118 ± 915 |
| DNA 2×-protein (1) | 20,836 ± 8,414 | 787 ± 367 |
| DNA 2×-protein (10) | 14,501 ± 3,294 | 992 ± 374 |
| DNA 2×-protein (30) | 127,494 ± 57,549 | 5,521 ± 1,486 |
| DNA 2×-protein (50) | 84,841 ± 22,793 | 3,950 ± 1,327 |
IL-2 levels (counts per minute [mean ± SD] for three mice tested individually) in spleen cell cultures stimulated for 24 h with purified Ag85A (5 μg/ml) 3 weeks after the last immunization.
IFN-γ levels (picograms per milliliter [mean ± SD] for three mice tested individually) in spleen cell cultures stimulated for 72 h with p25 (amino acids 241 to 260, 10 μg/ml) 3 weeks after the last immunization.
IFN-γ production is more sustained in Ag85A DNA-primed–protein-boosted mice.
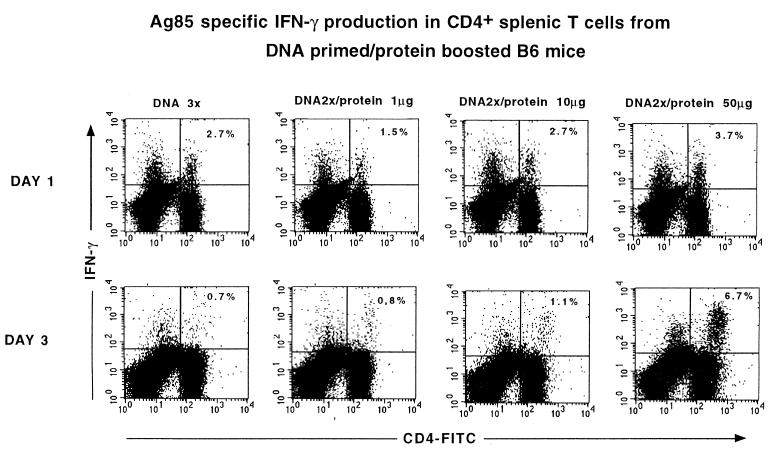
Using flow cytometry for intracellular IFN-γ measurement in CD4+ T cells, it was found that the increased IFN-γ production measured in vitro in the protein-boosted animals was the result of more sustained cytokine production. Whereas 2.7% of CD4+ T cells from mice vaccinated with Ag85A DNA were positive for intracellular IFN-γ at day 1 after stimulation with Ag85A protein, their cytokine production had ceased at day 3. In contrast, DNA-primed mice boosted with 50 μg of protein showed a slightly higher intracellular IFN-γ response at day 1 but also showed a remarkable increase at day 3, with about a 10-fold-higher percentage of IFN-γ-producing CD4+ T cells than in mice immunized with DNA only (Fig. 1).
FIG. 1.
Flow cytometry analysis of Ag85A-specific IFN-γ production on days 1 and 3 by CD4+ spleen T cells from B6 mice that were vaccinated with plasmid DNA encoding a mature form of Ag85A and boosted with increasing doses of purified Ag85A protein in MPL-A. FITC, fluorescein isothiocyanate.
Spleen cell IFN-γ production in B6 mice vaccinated with Ag85A protein, Ag85A DNA, or a DNA prime-protein boost regimen.
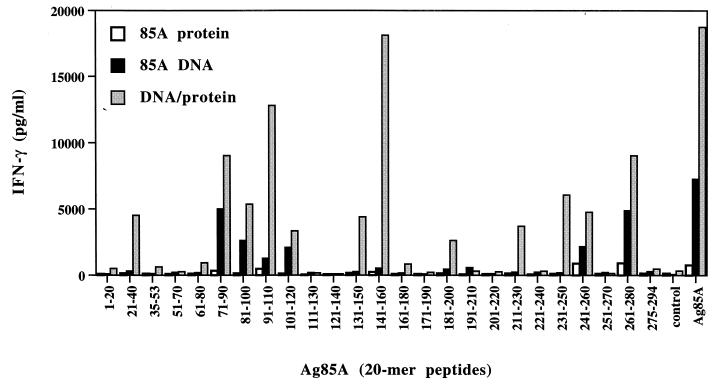
Three vaccinations with 100 μg of purified Ag85A protein in MPL-A induced only a weak IFN-γ response to whole native Ag85A protein (768 ± 253 pg/ml) when animals were tested 2 months after the last immunization (Fig. 2). T-cell epitope mapping using synthetic 20-mer peptides spanning the entire mature Ag85A sequence from M. tuberculosis showed that following protein immunization, IFN-γ responses were the strongest against two peptide regions that have previously been identified as immunodominant in B6 mice vaccinated with live BCG (18) or infected with M. tuberculosis (data not shown), i.e., p27 (amino acids 261 to 280) and p25 (amino acids 241 to 260) (see also the earlier description of the dose-response experiment). Additional but weaker reactivity was detected in protein-immunized mice in response to p10 (amino acids 91 to 110), which is a region not recognized following live mycobacterial infection. Three vaccinations with 2 × 50 μg of plasmid DNA encoding a secreted form of Ag85A (signal sequence of human tissue plasminogen activator preceding the mature Ag85A gene) induced a 10-fold-higher spleen cell IFN-γ response following stimulation with native purified Ag85A (7,282 ± 253 pg/ml) and its synthetic peptides. IFN-γ responses in B6 mice vaccinated with Ag85A DNA were directed against peptides p25 and p27 but also against a peptide region spanning amino acids 71 to 120 (p8-p9-p10-p11). Whereas BCG-vaccinated B6 mice reacted more strongly against p25 than against p27 (18), this hierarchy was changed by DNA vaccination, resulting in stronger responses to p27 and p8 than to p25. Finally, DNA immunization followed by a protein boost dramatically increased the IFN-γ response to whole Ag (18,726 ± 4,622 pg/ml) and to the various peptides identified by DNA immunization. Responses were boosted against peptides strongly recognized following DNA vaccination but also against peptides that were only weakly recognized by DNA vaccination: amino acids 21 to 40, 131 to 160, 181 to 200, and 211 to 230.
FIG. 2.
Spleen cell IFN-γ response to whole Ag85A and its synthetic peptides in mice vaccinated with Ag85A protein or Ag85A DNA or in B6 mice vaccinated with Ag85A DNA and a protein boost 2 months after the third immunization.
Spleen cell IL-2 production in B6 mice vaccinated with Ag85A protein, Ag85A DNA, or a DNA prime-protein boost regimen.
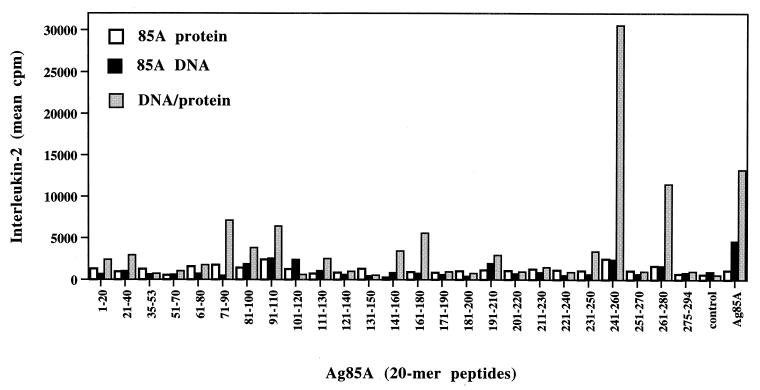
IL-2 responses towards Ag85A and its synthetic peptides could also be significantly increased by protein boosting (Fig. 3). Protein immunization induced only weak IL-2 responses in response to purified Ag85A protein (1,088 ± 271 cpm). The response in DNA-vaccinated mice was about fivefold higher (4,636 ± 1,021 cpm), and protein boosting resulted in a further threefold increase (13,217 ± 5,879 cpm). IL-2 levels in DNA-vaccinated mice were lower than those reported previously or in the dose-response experiment described above (Table 1), probably because of the later time point tested (2 months in this study versus 3 weeks after the last DNA immunization) (17, 37). DNA vaccination and the prime-boost regimen resulted in a broader IL-2-inducing epitopic repertoire than that of BCG vaccination. The strongest boost of IL-2 responses was observed in response to the immunodominant epitope identified following BCG vaccination, i.e., p25; the other epitopes elicited a weaker boost.
FIG. 3.
Spleen cell IL-2 response to whole Ag85A and its synthetic peptides in mice vaccinated with Ag85A protein or Ag85A DNA or in B6 mice vaccinated with Ag85A DNA and a protein boost 2 months after the third immunization.
Ab production in B6 mice vaccinated with purified Ag85A protein, Ag85A DNA, or a DNA prime-protein boost regimen.
All three immunization protocols resulted in significant Ag85A-specific Ab production, compared to mice immunized with MPL-A or the empty vector only (data not shown) (Table 2). However, profound differences were observed in Ab isotypes. Protein in MPL-A induced an exclusive IgG1 Ab response which was 10-fold higher than that in DNA-vaccinated mice. In contrast, DNA vaccination induced a strong IgG2a response which was 150-fold higher than that in protein-vaccinated mice. DNA prime-protein boost resulted in a doubling of the IgG1 and the IgG2a response, leading to IgG2a levels 350 times higher and IgG1 levels still 6 times lower than in protein-immunized mice. Confirming previous findings (17, 36), Ag85-specific IL-4 levels were very low (<50 pg/ml) in spleen cell culture supernatant from DNA-immunized mice but also in supernatant from protein- and prime-boosted mice (data not shown).
TABLE 2.
Ag85A-specific Ab production in B6 mice vaccinated with DNA and boosted with purified protein
| Vaccine used | Concn (ng/ml) of total Iga | IgG1 (arbitrary units/ml)b | IgG2a (arbitrary units/ml)b |
|---|---|---|---|
| MPL-A | 2.34 ± 0.08 | 1 ± 0.1 | 1 ± 0.1 |
| 85A protein | 3.70 ± 0.16 | 404 ± 17 | 1 ± 0.1 |
| 85A DNA | 4.40 ± 0.13 | 44 ± 1.26 | 151 ± 38 |
| DNA-protein | 4.71 ± 0.26 | 72 ± 4 | 350 ± 61 |
Ag85A-specific IGκ concentration (mean ± SD of five sera tested individually) expressed in log10 values.
Antibody isotype titers (determined on pooled sera) compared to a standard serum with an assigned titer of 1,000 for both isotypes.
Protein boosting of Ag85A and Ag85B DNA vaccine increases its protective efficacy.
Immunization with purified Ag85A protein alone was not protective against an i.v. challenge with M. tuberculosis H37Rv (Table 3). This was not unexpected in light of the low cellular Th1-type immune response induced by this immunization strategy. In contrast, and confirming previous findings (17, 37), vaccination with Ag85A DNA could significantly reduce the number of CFU in lungs at day 30 compared to the number of CFU in lungs of control mice injected with the MPL-A adjuvant or control DNA alone (Δlog10 = 0.45). Protein boosting of DNA-vaccinated mice increased the protection further (Δlog10 = 0.73, as compared to CFU in the control group; P < 0.05, as compared to Ag85A DNA only). Whereas mice vaccinated with Ag85A DNA showed a reduced CFU count in lungs only at day 30 after TB challenge, this reduction in CFU counts persisted at least up to day 60 of challenge in DNA-primed–protein-boosted mice (Δlog10, 0.50; 0.025 < P < 0.05, as compared to Ag85A DNA-vaccinated mice). At day 90 postchallenge, only BCG-vaccinated mice demonstrated a statistically significant reduced CFU count in lungs. Lower CFU counts in lungs were not the result of a redistribution of the bacteria to other organs, as lowest CFU counts in spleen were also observed in the DNA prime-protein boost group (control DNA count, 5.23 ± 0.18; Ag85A DNA count, 5.21 ± 0.14; and DNA prime-protein boost count, 4.76 ± 0.3 [five mice in each group]; 0.01 < P < 0.025, as compared to the control DNA group). Similarly, boosting with recombinant Ag85B protein of mice vaccinated with plasmid DNA encoding Ag85B was also effective in increasing the protective efficacy against M. tuberculosis, as compared to vaccination with DNA alone, as measured by CFU and relative light unit counting in lungs 4 weeks after challenge with a bioluminescent strain of M. tuberculosis (Table 4).
TABLE 4.
Bacterial replication in lungs from B6 mice vaccinated with Ag85B DNA, boosted with recombinant Ag85B protein in SBAS2A adjuvant, and challenged with bioluminescent M. tuberculosis H37Rv
| Vaccine used | No. of CFU/lunga (no. of mice) | No. of RLU/lungb (no. of mice) |
|---|---|---|
| Control DNA | 5.09 ± 0.21 (6) | 4.02 ± 0.28 (6) |
| BCG | 3.98 ± 0.39 (5) | 3.15 ± 0.24 (5) |
| Ag85B DNA | 4.50 ± 0.14 (4) | 3.43 ± 0.20 (4) |
| DNA-protein | 3.94 ± 0.26 (5) | 3.13 ± 0.11 (5) |
Mean number of CFU ± SD (log10 values) as determined by plating on Middlebrook 7H11 agar.
Mean number of relative light units (RLU) ± SD (log10 values) as determined in a Turner Design luminometer (15-s integration time).
DISCUSSION
Although DNA prime-protein boost immunization protocols are well known for their capacity to increase Ab production, much less is known concerning their effects on cell-mediated immune responses, essential in protection against intracellular pathogens such as M. tuberculosis. Here we have shown that a boost injection of protein in mice that were given a DNA vaccine encoding the mycoloyltransferase Ag85 from M. tuberculosis is capable of dramatically enhancing the Th1-type immune response primed with this DNA vaccine. Antigen-specific IL-2 and IFN-γ production in spleen cell cultures was augmented two- to fourfold by the protein boost compared to that for DNA vaccination, whereas immunization with protein in MPL-A induced only marginal levels of these Th1 cytokines, highlighting the power of DNA vaccines as priming agents for Th1-biased immune responses. Furthermore, flow cytometry analysis demonstrated that IFN-γ response was more sustained in spleen cell cultures from DNA-primed–protein-boosted mice: significant intracellular cytokine staining could be visualized up to 3 days after the onset of in vitro antigenic stimulation of CD4+ T cells from protein-boosted mice, whereas CD4+ T cells from DNA-immunized mice stained positive for IFN-γ only on day 1 of culture. Whether this sustained IFN-γ production is a mere consequence of a quantitative increase in effector cells in the protein-boosted group or the result of a qualitative difference in susceptibility to apoptosis is not clear for the moment. Analysis of Ab isotypes also showed that protein boosting following DNA priming preferentially stimulated the Th1 arm of the immune response. In complete agreement with our findings, H. M. Vordermeier et al. have recently reported on enhanced and fine-tuned immune responses by recombinant protein boosting in cattle immunized with a DNA vaccine encoding another mycobacterial antigen, i.e., HSP65 (H. M. Vordermeier, D. Lowrie, M. Singh, and R. G. Hewinson, submitted for publication).
It has previously been demonstrated that vaccination of BALB/c mice with Ag85A DNA stimulates a broader T-cell epitopic repertoire than does vaccination with live BCG or infection with M. tuberculosis (6). As shown here, Ag85A DNA vaccination of B6 mice also increased this Th1-type epitopic repertoire and besides a response to the carboxy-terminal peptides p25 and p27 immunodominant in mycobacterial infection (18), an additional peptide region spanning amino acids 71 to 120 could be identified following DNA vaccination. Interestingly, the hierarchy of immunodominance was changed by DNA vaccination, the response to p25 being clearly weaker than the response to p27 and the peptide region spanning amino acids 71 to 120. Protein boosting increased Th1 cytokine responses both to the immunodominant and the newly defined epitopes. The reason for this broadening of the epitope repertoire is not clear. However, we speculate that antigenic processing may be partially different in DNA-immunized and mycobacterially infected mice: upon infection only the complete and folded Ag85A protein would be available for processing (following secretion by the live bacillus), resulting in a preferential generation of p25-specific CD4+ T cells. Upon DNA vaccination, on the other hand, both complete but also truncated or unfolded forms of the protein might be processed by antigen-presenting cells.
Whereas in BALB/c mice, part of the broadening of the IFN-γ repertoire by DNA vaccination is related to the induction of Ag85A-specific major histocompatibility complex class I (MHC-I)-restricted CD8+ cytotoxic T lymphocytes (CTL) (9), in Ag85A DNA-vaccinated B6 mice, cellular immune responses appear to be exclusively mediated by MHC-II-restricted CD4+ T cells. So far, we have been unable to visualize any Ag85A- or, for that matter, Ag85B-specific CD8+ responses in H-2b haplotype mice (10), most likely because Ag85 lacks the correct epitopes that could be presented by Kb or Db molecules. With progressive infection, M. tuberculosis is thought to escape from the phagosome to the cytoplasm of the infected macrophage, which may then be recognized by MHC-I-restricted CD8+ T cells. We hypothesize that waning of protective efficacy of the Ag85 DNA vaccine in B6 mice is related to this lack of available MHC-I-restricted CTL epitopes. This could explain why the prime-boost immunization had a strong enhancing effect on CD4+-mediated IFN-γ production, whereas the effects on reducing bacterial burden in lungs could be demonstrated only at early time points after challenge.
Moreover, the question remains whether mycobacterial infection overall induces a murine Ag85-specific CD8+ T-cell response; we were unable to detect any Ag85A-specific CTL response following BCG vaccination or M. tuberculosis infection even in BALB/c mice (9), although three CTL epitopes could be defined in this mouse strain following DNA vaccination. It must be mentioned that the situation may be different in humans from that in mice, as Ag85A- and Ag85B-specific CD8+ T cells have been identified in BCG-vaccinated donors, using target cells infected with recombinant vaccinia virus expressing the mycobacterial antigens (32, 33). Moreover, we have recently been able to identify Ag85B-specific HLA-A*0201-restricted CD8+ epitopes using Ag85B DNA vaccination in HLA-transgenic mice, and these epitopes were also recognized in BCG-vaccinated individuals (11).
In contrast to infections with viral and protozoal pathogens, infection with M. tuberculosis remains largely confined to an intracellular localization, mostly in the lung macrophage phagosomes, and extracellular multiplication occurs only in advanced disease. Therefore, it is generally accepted that cell-mediated immunity leading to activation of bactericidal capacity of these macrophages rather than of Abs is essential for control of the infection. Nevertheless, it cannot be excluded that Abs, particularly those present in the lung mucosa, could play some role at very early stages of infection through mechanisms of macrophage- and natural killer cell-mediated Ab-dependent cytotoxicity. Daffé and Etienne have shown that Ag85 is present in the capsule of M. tuberculosis (6) and that it is possible that antibody-dependent cell-mediated cytotoxicity mediated through Ag85-specific IgG2a immunoglobulins, preferentially induced by DNA vaccination and known for their high affinity for FcγR (23), could play some role in the initial control of TB infection. In vitro experiments are needed to confirm this hypothesis.
In conclusion, our results show that DNA priming followed by exogenous protein boosting is an effective way to increase the immunogenicity and protective efficacy of an experimental TB DNA vaccine encoding Ag85 and that this technique underlines the essential role of MHC-II-restricted Th1-type CD4+ helper T cells in the protection mediated by this vaccine.
ACKNOWLEDGMENTS
We thank Donna Montgomery at Merck Research Laboratories, West Point, Pa. The excellent technical assistance of Fabienne Jurion, Nathalie De Smet, Albert Vanonckelen, and Kamiel Palfliet is gratefully acknowledged. We thank K. Franken (LUMC) for the recAg85B.
This work was partially supported by grant G.0266.00 from the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, by EEC (TB Vaccine Cluster QLK2-CT-1999–01093), by “La Région de Bruxelles-Capitale,” by the Nederlandse Lepra Stichting, and by the Damiaanaktie Belgium.
REFERENCES
- 1.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin S L, D'Souza C D, Orme I M, Liu M A, Huygen K, Denis O, Tang A, Zhu L, Montgomery D, Ulmer J B. Immunogenicity and protective efficacy of DNA vaccines encoding secreted and non-secreted forms of Mycobacterium tuberculosis Ag85A. Tuber Lung Dis. 1999;79:251–259. doi: 10.1054/tuld.1998.0196. [DOI] [PubMed] [Google Scholar]
- 3.Barnett S W, Rajasekar S, Legg H, Doe B, Fuller D H, Haynes J R, Walker C M, Steimer K S. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine. 1997;15:869–873. doi: 10.1016/s0264-410x(96)00264-2. [DOI] [PubMed] [Google Scholar]
- 4.Belisle J T, Vissa V D, Sievert T, Takayama K, Brennan P J, Besra G S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 5.Bloom B R, Fine P E M. The BCG experience: implications for future vaccines against tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: ASM Press; 1994. pp. 531–557. [Google Scholar]
- 6.Daffé M, Etienne G. The capsule of Mycobacterium tuberculosis and its implications for pathogenicity. Tuber Lung Dis. 1999;79:153–169. doi: 10.1054/tuld.1998.0200. [DOI] [PubMed] [Google Scholar]
- 7.De Bruyn J, Bosmans R, Turneer M, Weckx M, Nyabenda J, Van Vooren J-P, Falmagne P, Wiker H G, Harboe M. Purification, partial characterization, and identification of a skin-reactive protein antigen of Mycobacterium bovis BCG. Infect Immun. 1987;55:245–252. doi: 10.1128/iai.55.1.245-252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Bruyn J, Huygen K, Van Vooren J P, Content J, Turneer M. The 32 kDa protein antigen of M. bovis B.C.G. and M. tuberculosis H37Rv. Trop Med Parasitol. 1990;41:331–332. [PubMed] [Google Scholar]
- 9.Denis O, Tanghe A, Palfliet K, Jurion F, van den Berg T P, Vanonckelen A, Ooms J, Saman E, Ulmer J B, Content J, Huygen K. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998;66:1527–1533. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Souza S, Denis O, Scorza T, Nzabintwali F, Verschueren R, Huygen K. CD4+ T cells contain Mycobacterium tuberculosis infection in the absence of CD8+ T cells, in mice vaccinated with DNA encoding Ag85A. Eur J Immunol. 2000;30:2455–2459. doi: 10.1002/1521-4141(200009)30:9<2455::AID-IMMU2455>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Geluk A, van Meijgaarden K E, Franken K L M C, Drijfhout J W, D'Souza S, Necker A, Huygen K, Ottenhoff T H M. Identification of major epitopes of Mycobacterium tuberculosis Ag85B that are recognized by HLA-A*0201 restricted CD8+ T cells in HLA-transgenic mice and humans. J Immunol. 2000;165:6463–6471. doi: 10.4049/jimmunol.165.11.6463. [DOI] [PubMed] [Google Scholar]
- 12.Haddad D, Liljeqvist S, Stahl S, Hansson M, Perlmann P, Ahlborg N, Berzins K. Characterization of antibody responses to a Plasmodium falciparum blood-stage antigen induced by a DNA prime/protein boost immunization protocol. Scand J Immunol. 1999;49:506–514. doi: 10.1046/j.1365-3083.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 13.Hartikka J, Bozoukova V, Ferrari M, Sukhu L, Enas J, Sawdey M, Wloch M K, Tonsky K, Norman J, Manthorpe M, Wheeler C J. Vaxfectin enhances the humoral response to plasmid DNA-encoded antigens. Vaccine. 2001;19:1911–1923. doi: 10.1016/s0264-410x(00)00445-x. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz A M, Lee B W E, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubbard R D, Flory C M, Collins F M. Immunization of mice with mycobacterial culture filtrate proteins. Clin Exp Immunol. 1992;87:94–98. doi: 10.1111/j.1365-2249.1992.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huygen K, Abramowicz D, Vandenbussche P, Jacobs F, De Bruyn J, Kentos A, Drowart A, Van Vooren J-P, Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992;60:2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J-P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 18.Huygen K, Lozes E, Gilles B, Drowart A, Palfliet K, Jurion F, Roland I, Art M, Dufaux M, Nyabenda J, De Bruyn J, Van Vooren J-P, Deleys R. Mapping of Th1 helper T-cell epitopes on major secreted mycobacterial antigen 85A in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1994;62:363–370. doi: 10.1128/iai.62.2.363-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson M, Raynaud C, Lanéelle M-A, Guilhot C, Laurent-Winter C, Ensergueix D, Gicquel B, Daffé M. Inactivation of the antigen 85C gene profoundly affects the mycolate content and alters the permeability of the Mycobacterium tuberculosis cell envelope. Mol Microbiol. 1999;31:1573–1587. doi: 10.1046/j.1365-2958.1999.01310.x. [DOI] [PubMed] [Google Scholar]
- 20.Kamath A T, Feng C G, MacDonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang Y, Calvo P A, Daly T M, Long C A. Comparison of humoral immune responses elicited by DNA and protein vaccines based on merozoite surface protein-1 from Plasmodium yoelii, a rodent malaria parasite. J Immunol. 1998;161:4211–4219. [PubMed] [Google Scholar]
- 22.Kaufmann S H E. Is the development of a new tuberculosis vaccine possible? Nat Med. 2000;6:955–960. doi: 10.1038/79631. [DOI] [PubMed] [Google Scholar]
- 23.Kipps T J, Parham P, Punt J, Herzenberg L A. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antobodies. J Exp Med. 1985;161:1–17. doi: 10.1084/jem.161.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Launois P, DeLeys R, Niang M, Drowart A, Andrien M, Dierckx P, Cartel J-L, Sarthou J-L, Van Vooren J-P, Huygen K. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun. 1994;62:3679–3687. doi: 10.1128/iai.62.9.3679-3687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letvin N L. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putkonen P, Quesada-Rolander M, Leandersson A-C, Schwartz S, Thorstensson R, Okuda K, Wahren B, Hinkula J. Immune responses but no protection against SHIV by gene-gun delivery of HIV-1 DNA followed by recombinant protein boosts. Virology. 1998;250:293–301. doi: 10.1006/viro.1998.9379. [DOI] [PubMed] [Google Scholar]
- 27.Ramshaw I A, Ramsay A J. The prime-boost strategy: exciting prospects for improved vaccination. Immunol Today. 2000;21:163–165. doi: 10.1016/s0167-5699(00)01612-1. [DOI] [PubMed] [Google Scholar]
- 28.Richmond J F L, Lu S, Santoro J C, Weng J, Hu S-L, Montefiori D C, Robinson H L. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J Virol. 1998;72:9092–9100. doi: 10.1128/jvi.72.11.9092-9100.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronning D R, Klabunde T, Besra G S, Vissa V D, Belisle J, Sacchettini J C. Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat Struct Biol. 2000;7:141–146. doi: 10.1038/72413. [DOI] [PubMed] [Google Scholar]
- 30.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V S. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 31.Sin J-I, Bagarazzi M, Pachuk C, Weiner D B. DNA priming-protein boosting enhances both antigen-specific antibody and Th1-type cellular immune responses in a murine herpes simplex virus-2 gD vaccine model. DNA Cell Biol. 1999;18:771–779. doi: 10.1089/104454999314917. [DOI] [PubMed] [Google Scholar]
- 32.Smith S M, Brooks R, Klein M R, Malin A S, Lukey P T, King A S, Ogg G S, Hill A V S, Dockrell H M. Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J Immunol. 2000;165:7088–7095. doi: 10.4049/jimmunol.165.12.7088. [DOI] [PubMed] [Google Scholar]
- 33.Smith S M, Malin A S, Lukey P T, Atkinson S E, Content J, Huygen K, Dockrell H M. Characterization of human Mycobacterium bovis bacille Calmette-Guérin reactive CD8+ T cells. Infect Immun. 1999;67:5223–5230. doi: 10.1128/iai.67.10.5223-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snewin V A, Gares M-P, Gaora P O, Hasan Z, Brown I, Young D B. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect Immun. 1999;67:4586–4593. doi: 10.1128/iai.67.9.4586-4593.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song K M, Lee S W, Suh Y S, Lee K J, Sung Y C. Enhancement of immunoglobulin G2a and cytotoxic T-lymphocyte responses by a booster immunization with recombinant hepatitis C virus E2 protein in E2 DNA-primed mice. J Virol. 2000;74:2920–2925. doi: 10.1128/jvi.74.6.2920-2925.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanghe A, Denis O, Lambrecht B, Motte V, van den Berg T, Huygen K. Tuberculosis DNA vaccine encoding Ag85A is immunogenic and protective when administered by intramuscular needle injection but not by epidermal gene gun bombardment. Infect Immun. 2000;68:3854–3860. doi: 10.1128/iai.68.7.3854-3860.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanghe A, Lefèvre P, Denis O, D'Souza S, Braibant M, Lozes E, Singh M, Montgomery D, Content J, Huygen K. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J Immunol. 1999;162:1113–1119. [PubMed] [Google Scholar]
- 38.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]