Abstract
The number of patients with coronary artery disease (CAD) who have persisting angina pectoris despite optimal medical treatment known as refractory angina pectoris (RAP) is growing. Current estimates indicate that 5–10% of patients with stable CAD have RAP. In absolute numbers, there are 50 000–100 000 new cases of RAP each year in the USA and 30 000–50 000 new cases each year in Europe. The term RAP was formulated in 2002. RAP is defined as a chronic disease (more than 3 months) characterized by diffuse CAD in the presence of proven ischaemia which is not amendable to a combination of medical therapy, angioplasty, or coronary bypass surgery. There are currently few treatment options for patients with RAP. One such last-resort treatment option is spinal cord stimulation (SCS) with a Class of recommendation IIB, level of evidence B in the 2019 European Society of Cardiology guidelines for the diagnosis and management of chronic coronary syndromes. The aim of this review is to give an overview of neuromodulation as treatment modality for patients with RAP. A comprehensive overview is given on the history, proposed mechanism of action, safety, efficacy, and current use of SCS.
Keywords: Refractory angina pectoris, Spinal cord stimulation, Coronary artery disease
Graphical Abstract
Graphical Abstract.
Introduction
Refractory angina pectoris (RAP), first defined in 2002 by Mannheimer et al.,1 is ‘a chronic condition characterized by the presence of angina caused by coronary insufficiency in the presence of coronary artery disease which cannot be controlled by a combination of medical therapy, angioplasty and coronary bypass surgery. The presence of reversible myocardial ischaemia should be clinically established to be the cause of symptoms. Chronic is defined as a duration of more than 3 months’. The number of patients with RAP has been growing in the last years due to a combination of more complex coronary artery disease (CAD), co-morbidities and the advancing age of the general population. It has been estimated that 5–10% of patients with stable CAD have RAP, with absolute numbers of up to 1.8 million people in the USA.2 On a yearly basis, 30 000–50 000 new cases of RAP are reported in Europe and 50 000–100 000 new cases in the USA.2 It has also been estimated that 6–14% of patients undergoing a diagnostic coronary angiogram (CAG) due to angina pectoris meet the definition of RAP with no intervention options.3 Confirming that a significant number of patients with CAD have RAP and that these numbers are continuing to grow.
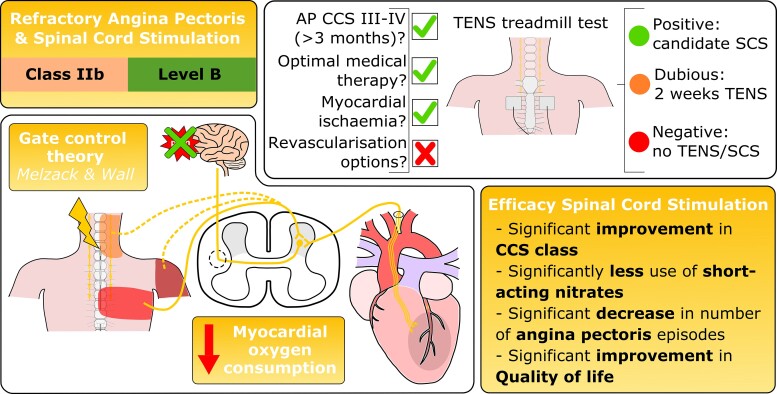
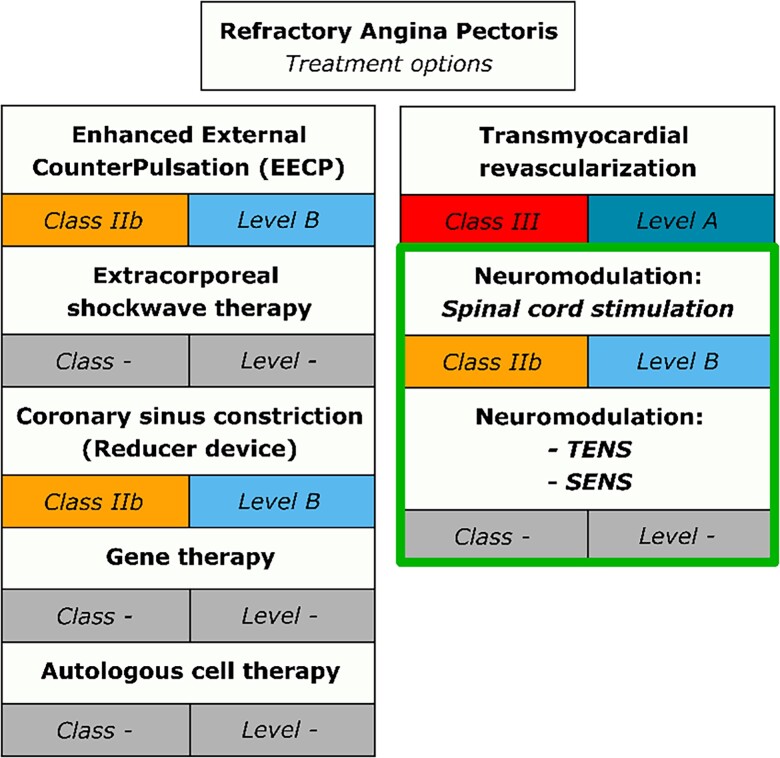
It has been acknowledged that this growing patient population have a ‘therapy resistant condition’, but that there are additional ‘last resort’ treatment possibilities. The 2019 European Society of Cardiology (ESC) guidelines for the diagnosis and management of chronic coronary syndromes describe a growing number of potential treatment options for RAP. The main concern outlined in the guidelines is the varying levels of evidence with regard to safety and efficacy of these potential treatment modalities, which ranges from non-existent to promising.4 The treatment options that are currently available for patients with RAP are: (i) enhanced external counterpulsation with class of recommendation IIb and level of evidence B, (ii) extracorporeal shockwave therapy (no class of recommendation), (iii) coronary sinus constriction (Reducer device) with class of recommendation IIb and level of evidence B, (iv) gene therapy (no class of recommendation), (v) autologous cell therapy (no class of recommendation), and (vi) neuromodulation (Figure 1). Previously transmyocardial revascularization was a therapy used in patients with RAP. However, this treatment is no longer recommended with a current class of recommendation III and level of evidence A.
Figure 1.
Current treatment options for refractory angina pectoris with class of recommendation and level of evidence in accordance with the 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes.4 TENS, transcutaneous electrical nerve stimulation; SENS, subcutaneous electrical nerve stimulation.
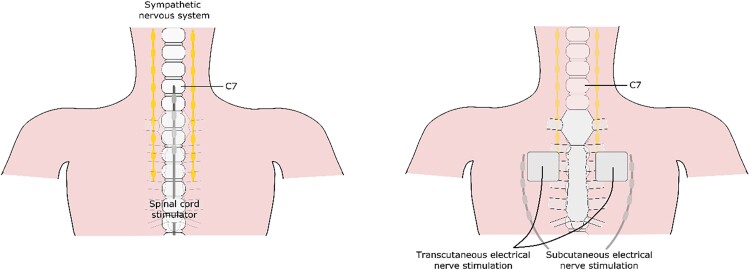
Neuromodulation is defined as ‘the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific sites in the body’.5 Neuromodulation includes transcutaneous electrical nerve stimulation (TENS), subcutaneous electrical nerve stimulation, and spinal cord stimulation (SCS) (Figure 2). In the current ESC guidelines, there is a class of recommendation IIb and level of evidence B for use of SCS in RAP patients with no class of recommendation given to the other forms of neuromodulation. The aim of this review is to give a comprehensive overview of neuromodulation as treatment modality for patients with RAP.
Figure 2.
Overview of the different forms of neuromodulation.
History of neuromodulation
The first form of neuromodulation was the surgical bilateral thoracic sympathectomy which was applied in the 1950s in patients with severe angina pectoris.6 This was in a time period when coronary artery bypass grafting was emerging as a treatment modality for patients with angina pectoris (first procedure 2 May 1960), whilst percutaneous transluminal coronary angioplasty(first procedure 16 September 1977) did not yet exist. In 1964, a study was done to determine the effectiveness of bilateral thoracic sympathectomy in eight patients with severe angina pectoris. It was shown that in 75% of the patients angina symptoms improved significantly, there was an increase in exercise tolerance and a delay in the onset of ischaemic changes, although the mechanism of action was not elucidated.6 An important concern raised at the time was that the warning signal of pain would no longer be present after patients had undergone a bilateral thoracic sympathectomy, even in the presence of ischaemia, leading to very little use of this treatment modality in patients with severe angina.
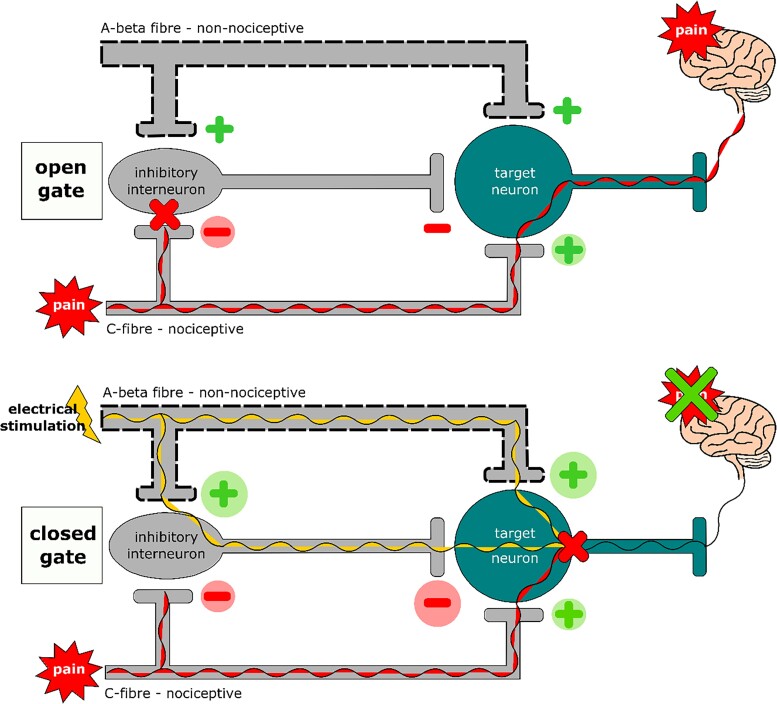
In 1965, a landmark study was published by Melzack and Wall7 in which the gate control theory of pain was presented. This theory postulated that when an electrical current is applied to the myelinated, fast-conducting, thicker, non-nociceptive A-beta fibres, pain processing in the non-myelinated, slower-conducting, nociceptive C-fibres is modulated through activation of the inhibitory interneurones in the dorsal horn of the spinal column, affecting pain.7 An ‘open’ gate will let the pain from the C-fibres through to the brain, whilst a ‘closed’ gate will not let the pain from the C-fibres through to the brain (Figure 3). With this new pain theory as background high frequency electrical stimulation was used to stimulate only the A-fibres leading to a reduction in pain sensation.8 In 1967, Shealy et al.9 was the first to implant a dorsal column stimulator (now known as a spinal cord stimulator) to apply electrical stimulation in a 70-year-old patient with metastasized lung cancer suffering from intractable pain in the chest and abdomen region. The treatment was a success with significant relief of the pain. These initial findings led to additional research with the aim of determining the effect of neuromodulation on pain relief in patients with ischaemic disorders. Cooke et al.10 was the first to study the effect of neuromodulation in a patient with lower extremity ischaemic ulcers in 1976. The treatment with SCS led to pain relieve and there was improved healing of the ulcers.10
Figure 3.
‘Open’ and ‘closed’ gate in accordance with the gate control theory of pain by Melzack and Wall.7
The first application of electrical neuromodulation, using TENS, in patients with severe angina pectoris was initiated by Mannheimer in 1982 and 1985. These patients had severe angina pectoris, had optimal medical therapy and no interventional treatment options. The aim of these studies was to evaluate if TENS could reduce the frequency of angina pectoris and increase working capacity both short- and long-term.11,12 The results were positive with less angina pectoris, an increase in maximal work capacity, less ST-segment depression during exercise and a decrease in time to recovery. However there are several limitations when using TENS: (i) the adhesive electrodes attached to the chest can cause contact dermatitis, (ii) the adhesive electrodes can come off due to transpiration, (iii) the adhesive electrodes can be difficult to place due to excess hair (in men) or large breasts (in women), (iv) cannot be applied for the full 24 h, and (v) performing sports such as swimming is not possible.13 With these limitations of TENS the next step was to use an implanted spinal cord stimulator. The first study to use an implanted spinal cord stimulator, then called dorsal column stimulator, in patients with severe angina pectoris was in 1987 by Murphy,14 closely followed by two additional studies in 1988 by Mannheimer and in 1992 by Sanderson.15,16 The aim of these first studies using SCS was to evaluate the clinical usefulness of SCS in reducing angina pectoris in patients with severe angina pectoris. All three studies showed a positive effect of SCS with less angina pectoris, less short-acting nitrate use, and an improved exercise capacity, and these positive effects remained present during long-term follow-up proving that SCS could be an effective treatment option for patients with RAP. These initial studies showed the possible clinical effectiveness of neuromodulation in patients with severe angina pectoris, but further studies were necessary to determine whether this treatment modality was safe to use and to clarify the underlying mechanism(s) of action of SCS were.
Mechanism(s) of action
The first studies regarding neuromodulation in severe angina pectoris have determined that SCS is an effective and safe treatment modality as mentioned previously.14–16 An important unanswered question remaining is which underlying mechanism(s) of action leads to the positive effect of neuromodulation. Several small studies have been performed with the aim of answering this question. The general conclusion is that the underlying mechanism of action remains unknown. There are multiple hypothetical mechanisms of action based on the relatively limited available evidence which will each be discussed in more detail. To better understand these mechanisms of action, a short overview of the neuro-humoral interaction between the heart and the central nervous system, including what happens when myocardial ischaemia occurs, will be given.
Neuro-humoral interaction heart and central nervous system
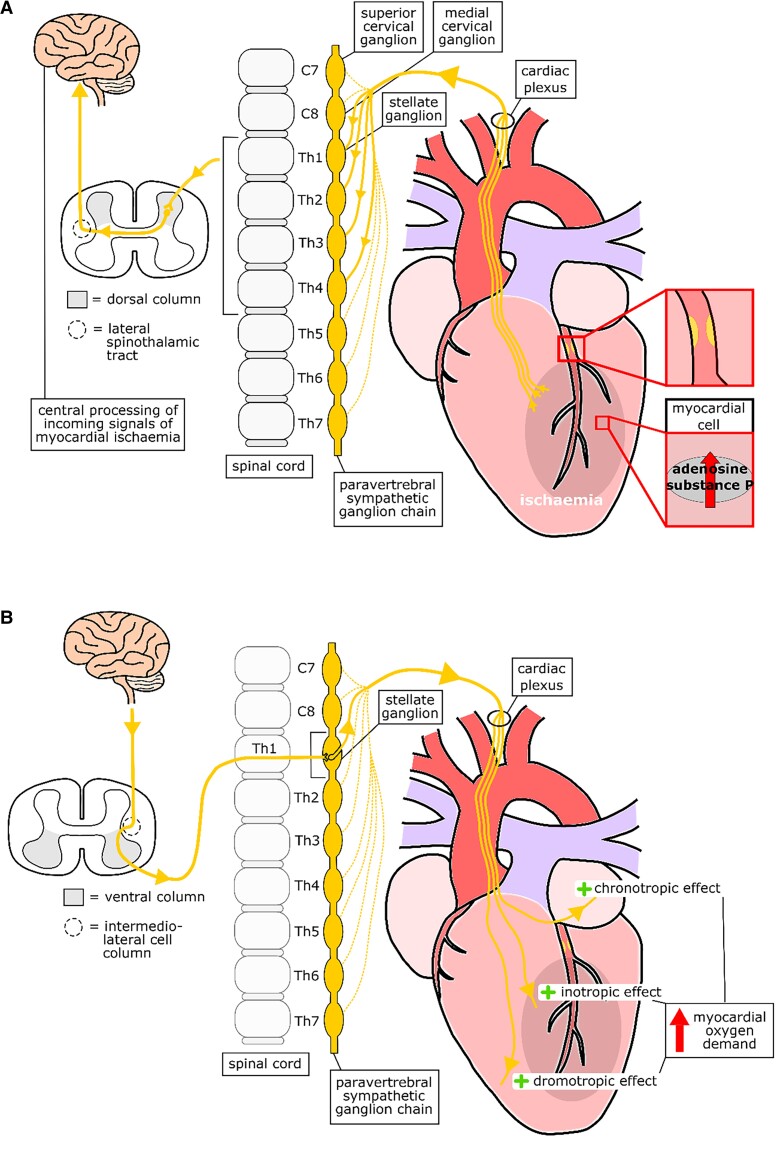
If myocardial ischaemia occurs, levels of adenosine and substance P in the myocardial cells will increase, which leads to stimulation of the afferent sympathetic cardiac neurones. The signal will pass through the superficial and deep cardiac plexus to the paravertebral sympathetic ganglion chain at the level of thoracic vertebrae Th1 through to Th4, although this range can vary per person from the upper cervical ganglion down to Th6 or Th7 and enter the dorsal column of the spinal cord. The neurones synapse in the dorsal horn; the signal subsequently passes through the lateral spinothalamic tract and enters the posterolateral and ventral nuclei of the thalamus.17 From the thalamus, the signal will be passed on to the areas of the cerebrum that are involved in the processing of nociception, and these include the peri-aqueductal grey matter, nucleus raphe magnus, insula, amygdalohippocampal apparatus, sensory cortex, and frontal cortex18 (Figure 4A).
Figure 4.
Schematic overview of the neuro-humoral interaction between the heart and the central nervous system. A, neuro-humoral interaction from the heart to the central nervous system. B, Neuro-humoral interaction from the central nervous system to the heart.
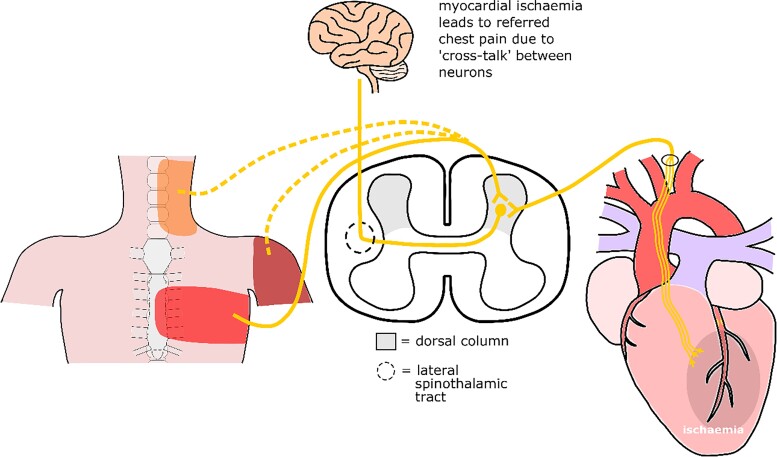
When the incoming cardiac neurone synapses in the dorsal horn, other sensory neurones, such as neurones from the different skin dermatomes, also enter the dorsal horn to synapse. Both types of neurone use secondary sensory fibres to transmit the incoming signals to the spinothalamic tract. It has been postulated that incoming signals from the skin and myocardium often share a single pain fibre in the spinothalamic tract. Due to the shared pain fibre, the distinction between the incoming information from the myocardium and from the skin cannot be made.19 This leads to the somatic expression of the incoming myocardial signal, known as angina pectoris17 (Figure 5). The location of where the pain is felt is dependent on which incoming fibre from the skin shares the incoming fibre from the myocardium.
Figure 5.
Schematic overview of referred pain in myocardial ischaemia.
The preganglionic sympathetic neurones innervating the heart originate from the intermediolateral cell column in the spinal cord. These neurones leave the spinal cord within the range of cervical vertebrae C8 down to thoracic vertebrae Th5 through the anterior roots and reach the paravertebral ganglia through the white rami communicans. In the paravertebral ganglia, the preganglionic sympathetic neurones synapse with the postganglionic sympathetic neurones. The stellate ganglion (fusion of the lower cervical ganglion and first thoracic ganglion) provides the cardiac postganglionic neurones. These postganglionic sympathetic neurones enter the heart following the pathway of the vasculature. The sympathetic neurones converge with the parasympathetic neurones (coming from the nervus vagus) on the surface of the myocardium to form the cardiac plexus (superficial and deep cardiac plexus) and innervate the heart itself20 (Figure 4B). When the sympathetic nervous system is stimulated this has a positive chronotropic, inotropic and dromotropic effect leading to an increase in myocardial oxygen demand. In patients with CAD this increase in myocardial oxygen demand will lead to the development of ischaemia.
Mechanisms of action of neuromodulation
In the previous decades, a large number of studies have been performed with the aim of elucidating the underlying mechanism(s) action of neuromodulation (specifically TENS and SCS). There are three proposed mechanisms of action:
Direct inhibition of pain
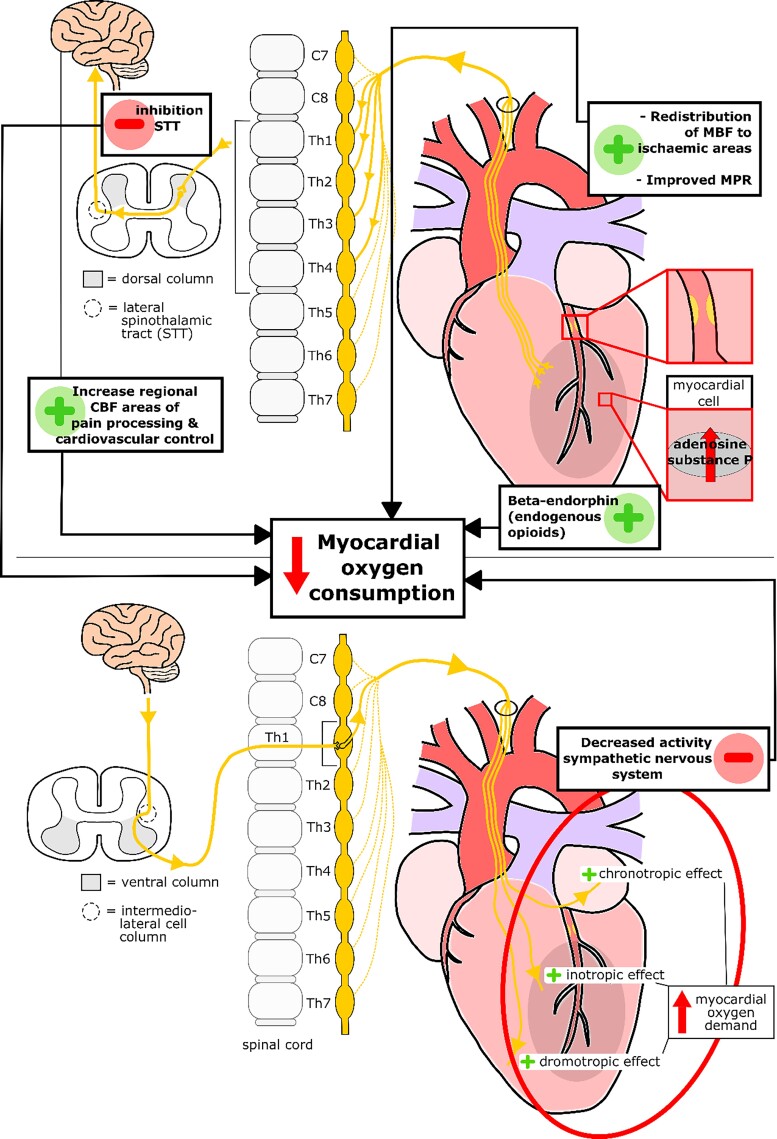
Neuromodulation leads to direct inhibition of pain through several pathways. Firstly, neuromodulation stimulates the dorsal column, leading to an inhibition of the activity of the lateral spinothalamic tract cells which pass the signals of the cardiac sympathetic neurones to the central nervous system.16,21,22 Secondly, changes are seen in the levels of endogenous opioids [beta-endorphins (BEs), enkephalins, and dynorphin] in the myocardium.23 During pacing-induced ischaemia, there is an increase in the release of BE, whilst SCS is applied.24 These endogenous opioids are thought to stimulate the delta-receptors on the presynaptic sympathetic nerve endings in the myocardium, inhibiting the norepinephrine (NE) release, thereby decreasing the sympathetic activity and leading to a decrease in the myocardial oxygen consumption.23 Thirdly, a significant increase has been found in the regional cerebral blood flow in those parts of the brain known to be associated with cardiovascular control and pain processing when neuromodulation is applied.25
Decreased sympathetic tone
To determine the effect of neuromodulation on the activity of the sympathetic nervous system, several studies invasively measured the catecholamine (epinephrine and/or NE) levels. In patients responding to neuromodulation (TENS) with abolishment of angina, a decrease is seen in the arterial levels of catecholamines suggesting a decreased sympathetic activity as a result of TENS.26 This effect has been determined to be a primary effect of neuromodulation because a decrease arterial epinephrine levels is still found in patients experiencing no angina whilst applying neuromodulation.27 Thereby ruling out a secondary effect due to pain inhibition. The decrease in sympathetic nerve activity is a general effect, no significant decrease has been found in the cardiac sympathetic nerve activity.28 An important side note to this finding is that neuromodulation was applied in patients with no angina in this specific study, whilst it is known that cardiac sympathetic nerve activity only increases during stress and/or ischaemia. Thus a possible decrease in cardiac sympathetic nerve activity during angina as a consequence of neuromodulation cannot be ruled out.
The influence of SCS on the cardiac nervous system has also been studied in animals. It has been shown that SCS prevents induction of atrial tachyarrhythmias (in dogs) induced by excessive activation of the cardiac neurones, confirming the inhibitory effect of neuromodulation on the sympathetic nervous system.22,29 In the animal studies of Foreman et al.30 and Armour et al.31 SCS suppressed activity generated by the cardiac neurones in rest, during ischaemia and during the reperfusion phase. These animal studies confirm that neuromodulation has an inhibitory effect on the sympathetic nervous system.22,32
Changes in myocardial blood flow
Initially, it was thought that neuromodulation would lead to an increase in myocardial blood flow (MBF). This assumption was based on earlier research in patients with peripheral vascular disease where an enhanced microvascular blood flow in the ischaemic limbs was seen after application of neuromodulation.33 In all studies performed in patients with RAP, no direct increase in MBF has been found when neuromodulation is applied.34–38 An explanation for this finding is that the coronary arteries in patients with RAP are severely diseased and thus not capable of vasodilation as would happen in normal coronary arteries ultimately leading to an increase in MBF.35
Whilst there is no increase in MBF, a decrease in MBF has been found using positron emission tomography (PET). There is a linear relation between MBF and myocardial oxygen consumption. If there is a decrease in the MBF, then there is also a decrease in the myocardial oxygen consumption.35,36 In addition the variation in flow decreased after SCS is applied, leading to homogenization of the MBF.36 This finding suggests that SCS leads to a redistribution of the MBF between ischaemic and non-ischaemic areas of the myocardium. Redistribution of the MBF has been confirmed in additional studies with MBF increasing in the ischaemic areas of the myocardium (low or normal basal MBF) and the MBF decreasing in the non-ischaemic areas of the myocardium (high basal MBF).39 Furthermore, the myocardial perfusion reserve (MPR), the ratio of MBF during maximal hyperaemia to MBF at rest, improved with a globally lower resting MBF and improved maximal hyperaemia MBF in the ischaemic areas of the myocardium.38
In conclusion, there is evidence that neuromodulation alleviates the severity of angina pectoris by (i) direct inhibition of pain through modulation of signals in both the spinal cord and specific regions of the brain, (ii) decreasing the activity of the overall sympathetic nervous system, and (iii) redistributing the MBF in favour of ischaemic areas and the combination of these three mechanisms of action ultimately lead to a decrease in myocardial oxygen consumption (Figure 6).
Figure 6.
Schematic overview of the mechanisms of action of neuromodulation in patients with refractory angina pectoris. CBF, cerebral blood flow; MBF, myocardial blood flow; MPR, myocardial perfusion reserve; STT, spinothalamic tract.
Safety of SCS
After the initial studies showing that SCS is an effective treatment modality for patients with severe angina pectoris, concerns were raised that SCS could mask the warning signal of myocardial ischaemia thereby creating silent myocardial ischaemia with potentially serious consequences.21,40 From 1994 onwards, multiple studies were undertaken to determine the safety of SCS both short- and long-term.40–43 One study used 24-hour ambulatory electrocardiogram monitoring to determine if the ischaemic burden (based on ST-segment depression) increased and if more arrhythmias occurred, which was not the case.41 Two studies looked at the long-term follow-up and occurrence of acute myocardial infarction (AMI) and concluded that there was no evidence that SCS concealed AMI.42,43 Additional studies were undertaken to determine if patients treated with SCS have similar mortality rates during follow-up compared to patients with RAP in general.44–46 Results showed that mortality rates of patients treated with SCS were indeed similar with the annual all-cause mortality rate ranging from 6.5% up to 8.6% for patients treated with SCS,45,47 compared to 3.9% up to 10% in patients with RAP.48,49 Based on these findings, the conclusion is that SCS is a safe treatment modality with no evidence that the warning signal of myocardial ischaemia is masked and the annual all-cause mortality rates are comparable to that of patients with RAP in general.
SCS and an implanted cardiac device
An important concern when implanting a spinal cord stimulator is a possible interaction with another implanted cardiac device such as an implantable cardiac defibrillator (ICD) or pacemaker (PM). Manufacturers of spinal cord stimulators have issued a warning of possible interference between these devices. In several recent studies, the safety of implanting and using a spinal cord stimulator due RAP or heart failure in patients with either an ICD or a PM has been proven.47,50–52 When considering spinal cord stimulator implantation in a patient with an implanted cardiac device, it is important to inform the patient of the possible risk of interference. When implanting it is important to have the PM technician present to test for interference. During testing, the output of the spinal cord stimulator should be increased to levels higher than necessary for treatment to ensure no interference occurs. After implantation, the cardiac implanted device should be checked for interference once yearly and if mode switches of the spinal cord stimulator occur. If clinicians adhere to this protocol SCS can be safely applied in patients with a cardiac implanted device.47
Efficacy of SCS
The first studies performed in patients with RAP and SCS were mainly aimed at determining the short-term effectiveness of neuromodulation in patients with severe angina pectoris, which was proven in these studies.14–16 There was a decrease in the number of angina pectoris episodes, less use of short-acting nitrates and an improvement in exercise duration. To determine the long-term efficacy additional studies were performed. The duration of follow-up varied from 12.1 months up to 31.4 months, and the number of patients included in the studies varied from 23 up to 121 patients.53–58 All studies confirmed the earlier findings and proved a long-term effect with a significant decrease in the number of weekly angina pectoris episodes, less use of short-acting nitrates, an improvement in Canadian Cardiovascular Society (CCS) class and a significant improvement in the quality of life. These studies were single-arm, open-label studies with no control group.
It is difficult to perform a placebo-controlled trial because patients feel paraesthesia in the chest region when the spinal cord stimulator is turned on, making it difficult to properly blind the patient. In 2007 the first placebo-controlled trial was performed by Eddicks et al.59 In this trial, 12 patients were included and a spinal cord stimulator was implanted in all patients. The duration of the study was 16 weeks divided into four phases with a different stimulation pattern in each phase: (A) three times 2 hours with conventional output, (B) 24 h a day with conventional output, (C) three times 2 hours with subthreshold output (defined as 85% of the minimum stimulation output causing paraesthesia), and (D) 24 h a day with 0.1 volt output (defined as placebo). It was postulated that the subthreshold phase could induce a therapeutic effect, thus there were three phases of active treatment (A, B, and C) and one control phase (D). The primary endpoint was the total walking distance using the 6-minute walking test which was significantly better in phases A, B, and C compared to phase D. Secondary endpoints revealed a better CCS class, a decrease in angina frequency and nitrate usage in phases A, B, and C compared to phase D confirming previous findings of the positive effect of SCS in patients with RAP. Due to the small study population additional research was deemed necessary for further confirmation of these results. Three additional placebo controlled trials were performed and all showed a significant reduction in the number of angina pectoris attacks.60–62 An important question raised by the study of Zipes et al.60 was the role of a possible placebo effect. This study included 68 patients and compared high stimulation (defined as conventional stimulation for a minimum of four times two hours a day) to low stimulation (defined as conventional stimulation for one minute once daily; placebo). In both arms there was a significant decrease in number of angina pectoris attacks, but no significant difference between the two arms was seen over a period of six months. Possible explanation for these findings is a placebo effect or a therapeutic effect of the low stimulation. The study by Eldabe et al.61 compared SCS treatment to usual care over a period of six months and found a significant improvement in exercise capacity and quality of life in favour of SCS. The study by Lanza et al.62 compared paresthesic stimulation (defined as continuous conventional stimulation), subliminal stimulation (defined as 75–80% of the minimum stimulation output causing paraesthesia) and sham stimulation (defined as one hour once daily of 0.1 mV stimulation; placebo) over a period of three months. Paresthesic stimulation was superior to sham stimulation with regard to improvement in angina symptoms and quality of life. No superiority of subliminal stimulation compared to sham stimulation could be demonstrated, although a trend of progressive improvement was seen. Combining the findings of the three studies is difficult due to the different study designs applied. All three studies included less patients than was initially calculated to be necessary and were terminated early. In conclusion, it is currently not clear what the role of the placebo effect is in patients with RAP being treated with SCS, but all studies showed a positive effect.
Two systematic reviews give an overview of the data available up to 2017 on the efficacy of SCS in patients with RAP (Table 1).63,64 The reviews show a significant improvement in exercise duration, CCS-class, Visual Analogue Scale score, daily angina episodes, daily nitrate consumption, angina frequency, disease perception, and treatment satisfaction. Confirming the positive effect of SCS in patients with RAP as reported previously. All studies performed up to date have had issues with blinding, including a sufficient number of patients and have a relatively short follow-up period, highlighting the deficiencies in methodology and data quality of these studies.65
Table 1.
Overview of results by Pan et al.63 and Imran et al.64 regarding the efficacy of spinal cord stimulation in patients with refractory angina pectoris
| Pan et al.63 | Imran et al.64 | |
|---|---|---|
| Number of studies (N) | 12 | 14 |
| Number of patients (N) | 476 | 518 |
| Follow-up duration (months) | 0.5–24 | 1–60 |
| Endpoints—mean difference | ||
| Exercise duration (min) | 0.49 (95% CI: 0.13–0.85); P = 0.008 | 1.68 (95% CI: 0.83–2.52); P < 0.0001 |
| Change in CCS class (≥2) | OR 2.12 (95% CI: 1.19–3.76); P = 0.01 | — |
| Rate pressure product | — | 0.54 (95% CI: −0.09 to 1.18); P = 0.09 |
| VAS score | −0.50 (95% CI: −0.80 to −0.20); P = 0.001 | — |
| Daily angina episodes | — | −1.55 (95%-CI: −1.75–−1.33); P < 0.0001 |
| Daily nitrate consumption | −0.64 (95% CI: −0.84 to −0.45); P < 0.00001 | −1.54 (95% CI: −1.81 to −1.26); P < 0.001 |
| Seattle Angina Quest.: | — | |
| Physical limitation | −2.69 (95% CI: −8.75 to −3.38); P = 0.39 | |
| Angina frequency | −9.03 (95% CI: −15.7 to −2.36); P = 0.008 | |
| Angina stability | −1.94 (95% CI: −7.55 to −3.67); P = 0.50 | |
| Treatment satisfaction | 6.87 (95% CI: 2.07–11.66); P = 0.005 | |
| Disease perception | −8.34 (95% CI: −14.45 to −2.23); P = 0.007 | |
| SF-36 angina frequency score | — | 21.78 (95% CI: 10.76–32.81); P < 0.0001 |
Green is a positive outcome/result, Red is a negative outcome/result.
CCS, Canadian Cardiovascular Society; OR, odds ratio; SF-36, Short Form 36 Health Survey; VAS, Visual Analogue Scale.
Current practice
Screening potential candidates
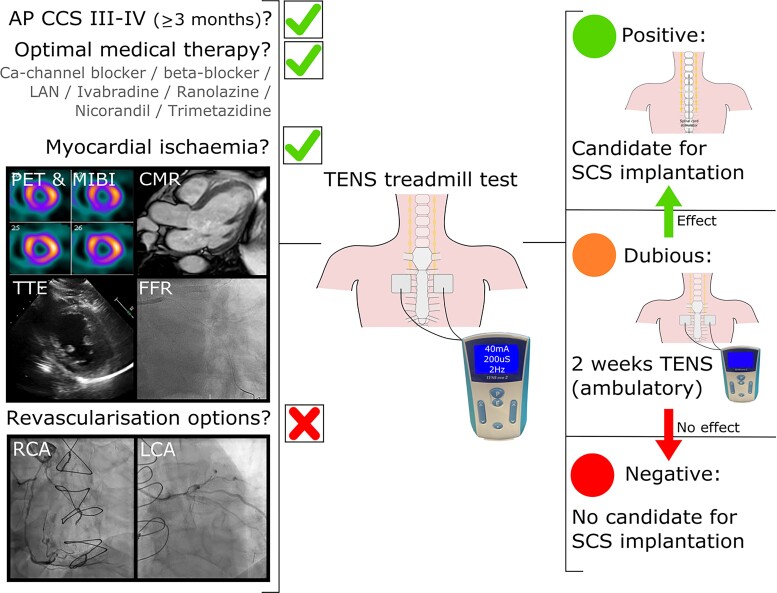
It is important to screen patients with RAP who are potential candidates for SCS to ensure that the patient has a beneficial effect. Currently, there is no uniform screening process for patients with RAP. We propose to use the following screening process, as applied in our centre, to ensure uniformity and good patient selection. Firstly patients have to meet the definition of RAP as previously described which is determined by one (or two) interventional cardiologist(s) using the referral letter and recent coronary angiogram (less than 12 months old) (Figure 7).1 Important in this is assessment is to confirm the presence of myocardial ischaemia which can be assessed using a variety of diagnostic tools as shown in Figure 7. If a patient meets the criteria of RAP a TENS treadmill test is performed. The aim of the TENS treadmill test is to determine the effect of neuromodulation on angina pectoris as advised by the ESC Joint Study Group on the Treatment of Refractory Angina.1 During the TENS treadmill test a multidisciplinary team is present to determine if the patient is suitable. The patient will be asked to start walking on the treadmill, the pace and angle of the treadmill will be steadily increased until angina is provoked, then TENS is initiated.47,58 Based on the patient response to the TENS test the possible outcomes are: (i) positive: the patient is eligible for SCS, (ii) dubious: trial of two weeks TENS, or (iii) negative: the patient is ineligible for SCS. In case of a dubious test outcome a second evaluation will take place after two weeks of ambulatory TENS which is either positive or negative. All patients who have a positive test are suitable candidates for SCS (Figure 8). By applying this method of using the TENS treadmill test as a screening tool uniformity and proper patient selection for definitive SCS is guaranteed.
Figure 7.
Schematic overview of the screening process for spinal cord stimulation. AP, angina pectoris; CCS, Canadian Cardiovascular Society; Ca-channel, calcium-channel; CMR, cardiac magnetic resonance imaging; FFR, fractional flow reserve; LAN, long-acting nitrates; LCA, left coronary artery; MIBI, myocardial perfusion imaging; PET, positron emission tomography; RCA, right coronary artery; SCS, spinal cord stimulation; TENS, transcutaneous electrical nerve stimulation; TTE, transthoracic echocardiography.
Figure 8.
Schematic overview of set-up spinal cord stimulator implantation.
Implantation procedure
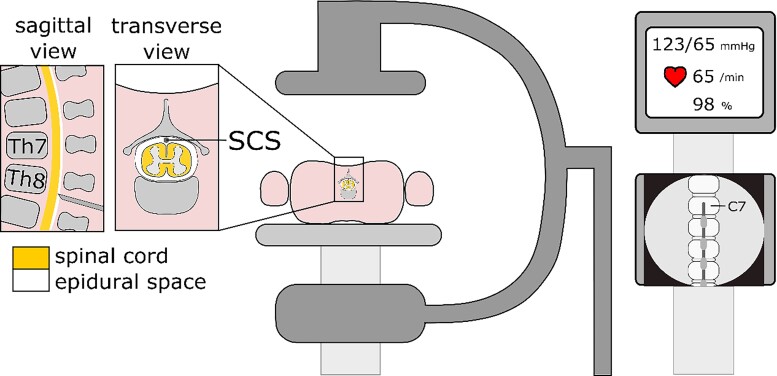
Prior to the spinal cord stimulator implantation a single dose of cefazolin 1 g is administered intravenously to reduce the risk of infection. The location of the implantable pulse generator (IPG) is determined, usually the left buttock. The implantation of the spinal cord stimulator is performed by an experienced anaesthesiologist-pain specialist (or neurosurgeon) under sterile conditions in an operating theatre. Throughout the implantation procedure, the patients’ vital signs are monitored. To ensure patient comfort during the implantation procedure sedation is achieved using a combination of propofol and remifentanil administered continuously using an intravenous access. Secondary field blocks and wound infiltration with local anaesthetics are used to provide analgesia. Prior to initiating sedation the patient is asked to lie in a prone position creating a thoracic kyphosis (Figure 8). A Tuohy needle is used to enter the epidural space at the level of thoracic vertebrae (Th) 7 or 8. The loss of resistance technique is used to confirm entry into the epidural space and checked using a through the needle guide wire and its position on fluoroscopic images. This is followed by a through the needle introduction of an eight contact electrode array. The type of electrode array used varies per centre. Using fluoroscopic guidance the cephalad tip of the electrode is positioned at the level of C7—Th1. Subsequently, test stimulation is performed using an external neurostimulator, whilst the sedation is temporarily lifted. The aim of the test is to determine if the electrode is in the optimal position with at least 80% of the target area covered. Subtle position modifications of the lead are performed to achieve the most ideal coverage. When the operator is satisfied with the lead position sedation is reinstated, the lead is fixated at the thoracodorsal fascia and subcutaneously tunnelled to the IPG pocket (left buttock). Once the implantation is completed an X-ray is made after the patient has returned to the ward to confirm the position of the lead and to rule out lead dislocation. The majority of patients are discharged from the hospital the same day. Patients receive instructions prior to discharge what to do if complications arise and how to handle their programmer.
The success rate of spinal cord stimulator implantation is high with reported successful implantation rates ranging from 93.3% to 100%.61,62 The most important factor leading to spinal cord stimulator implantation failure is the inability to achieve adequate paraesthesia coverage of the target area. If adequate paraesthesia coverage is not achieved the implantation will be terminated. Other factors that can influence the implantation success rate are (i) spinal cord abnormalities such as deformities of the spine or spinal stenosis and (ii) inability of the patient to lie in a prone position for a longer period of time.
Current use of SCS
Neuromodulation as treatment modality for patients with RAP is used infrequently nowadays due to a variety of reasons66–68:
Costs of SCS: The current cost of a spinal cord stimulator including the implantation is estimated to be approximately €16.050—and in the majority of cases, these costs are covered by the patients insurance. However, SCS is an expensive treatment modality, and it is of interest to know whether SCS is cost-effective. Comparison of costs prior to and after spinal cord stimulator implantation (including the costs of the device and the implantation) revealed that the cost of the SCS treatment was balanced after 16 months.69 After patients have received a spinal cord stimulator there is also a significant decline in the number of days patients are admitted to the hospital on a yearly basis from 8.3 days to 2.5 days with 63.2% (12 out of 19 patients) having no hospital admissions in the year following spinal cord stimulator implantation. This also leads to a reduction in health care costs.70 Patients who receive SCS will use the device long-term, with one study showing that the majority of patients (91%) were still using SCS after an average follow-up of 53.6 months.71 In comparison, only 9% of patients underwent an explantation, but the time between implantation and explantation is not known. Additionally patients still have a beneficial effect during long-term follow-up, ranging from 6,6 years up to 15 years, showing that SCS is cost-effective.72,73
IPG depletion: Initial IPGs used during the implantation procedure were non-rechargeable IPGs. This required additional procedures during the follow-up period due to IPG end of life (EOL). The longevity of the IPG is dependent on the type of stimulation and the frequency of use. For a non-rechargeable IPG the longevity can be up to five years, whilst the longevity for rechargeable IPGs ranges from 10 up to 25 years depending on the manufacturer.74 In current practice, the operator can opt to implant a rechargeable IPG to reduce the chance of having to perform a procedure to replace the IPG due to EOL.
Necessity to discontinue antithrombotic therapy: For the implantation of the spinal cord stimulator, the epidural space is entered to enable placement of the electrode. This is associated with an increased perioperative bleeding risk, necessitating temporary discontinuation of all antithrombotic therapy including acetylsalicylic acid, P2Y12-inhibitors, vitamin K antagonists, and direct oral anticoagulants. In general patients with RAP have stable CAD, and there is a low risk of complications occurring when antithrombotic therapy is temporarily discontinued, although this has not been investigated in previous studies.
Risks of implantation and follow-up at specialized centres: The are several adverse events that can occur in relation to the implanted spinal cord stimulator: (i) lead migration, (ii) lead fracture and/or malfunction, or (iii) infection. When looking at all indications for a spinal cord stimulator implantation since it’s use the risk of these adverse events are: (i) 15.49%, (ii) 6.37%, and (iii) 4.89%, respectively.75 Numbers are lower when looking specifically at spinal cord stimulator implantation in RAP. Reported rates of adverse events are 1% in case of infections and 7.8% in case of device related problems,76 with an additional study describing an adverse event rate of 2.3% after 1 year of follow-up.58 An important factor in the risk of adverse events occurring is operator experience. It has been proven in device-related treatment modalities that the experience of the operator significantly impacts the rate of adverse events.75 It is therefore important that implantation of spinal cord stimulators are performed at centres with ample experience to ensure a low rate of adverse events. The consequence is that SCS for patients with RAP is centred in selected, specialized centres for the implantation procedure and follow-up.
Not approved treatment modality for RAP: The European and American guidelines have given the use of SCS in patients with RAP a class of recommendation IIb with level of evidence B and C, respectively.4,77 Class of recommendation IIb refers to the fact that the treatment ‘may be considered’ and that the usefulness/efficacy is less well established by evidence/opinion. The results up to date have been variable, with the majority of studies proving a beneficial effect of SCS compared to a few studies suggesting that the efficacy of SCS is at least partly a placebo effect.57–62 The endpoints used in these studies have been patient centred endpoints such as quality of life and frequency of angina pectoris episodes. Additional difficulties in previous studies have been the inability to perform a blinded study due to paraesthesia felt by the patient and slow enrolment rates. This has led to persisting doubts as to the true effectiveness of SCS with a low level of evidence and weak strength of recommendation in the Dutch national anaesthesiologist recommendations using the Grading of Recommendations, Assessment, Development and Evaluation system (GRADE) classification.78 To provide more clarity on this point, a double-blind, cross-over, placebo-controlled, single-centre randomized controlled trial, the Efficacy of Spinal Cord Stimulation in patients with Refractory Angina Pectoris; a randomized controlled trial (SCRAP trial), is being undertaken (NCT04915157). The aim of this study is to determine if SCS leads to a significant reduction in myocardial ischaemia in patients with RAP using the PET perfusion scan whilst applying the high density mode of stimulation, instead of conventional stimulation, to ensure the patient does not experience paraesthesias. Secondary endpoints include patient centred outcomes, safety outcomes and a cost-effectiveness analysis will also be performed.
Conclusion
In conclusion, based on the currently available research, SCS is a safe and effective treatment modality for patients with RAP that has been available since the 80 s. The proposed working mechanisms of neuromodulation is a combination of (i) direct inhibition of pain through modulation of signals in both the spinal cord and specific regions of the brain, (ii) decreased activity of the overall sympathetic nervous system, and (iii) redistribution of the MBF to ischaemic areas. All three mechanisms of action ultimately lead to a decrease in myocardial oxygen consumption. Although current use of neuromodulation is sparse, it is an effective treatment modality when a good selection process, as described in this review, is used. To provide more data on the effectiveness of SCS in patients with RAP, the SCRAP trial is currently being undertaken.
Contributor Information
Fabienne Elvira Vervaat, Department of Cardiology, Catharina Hospital, Michelangelolaan 2, 5623 EJ Eindhoven, the Netherlands.
Antal van der Gaag, Department of Anaesthesiology, Catharina Hospital, Eindhoven, the Netherlands.
Koen Teeuwen, Department of Cardiology, Catharina Hospital, Michelangelolaan 2, 5623 EJ Eindhoven, the Netherlands.
Hans van Suijlekom, Department of Anaesthesiology, Catharina Hospital, Eindhoven, the Netherlands.
Inge Wijnbergen, Department of Cardiology, Catharina Hospital, Michelangelolaan 2, 5623 EJ Eindhoven, the Netherlands.
Lead author biography
 Fabienne Vervaat graduated from Maastricht University with a degree in medicine in 2014. She is currently working as a cardiology resident at Catharina hospital, Eindhoven, the Netherlands. In addition, she is working on a PhD regarding complex coronary artery disease, specifically refractory angina pectoris and the use of spinal cord stimulation.
Fabienne Vervaat graduated from Maastricht University with a degree in medicine in 2014. She is currently working as a cardiology resident at Catharina hospital, Eindhoven, the Netherlands. In addition, she is working on a PhD regarding complex coronary artery disease, specifically refractory angina pectoris and the use of spinal cord stimulation.
Data availability
No new data were generated in support of the article.
Funding
None declared.
References
- 1. Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, Follath F, Hellemans I, Herlitz J, Lüscher T, Pasic M, Thelle D. The problem of chronic refractory angina. Report from the ESC joint study group on the treatment of refractory angina. Eur Heart J 2002;23:355–370. [DOI] [PubMed] [Google Scholar]
- 2. Henry TD, Satran D, Jolicoeur EM. Treatment of refractory angina in patients not suitable for revascularization. Nat Rev Cardiol 2013;11:78–95. [DOI] [PubMed] [Google Scholar]
- 3. Riley RF, Kereiakes DJ, Henry TD. More data than options for the “no-option” refractory angina patient in the United States. Circ Res 2019;124:1689–1691. [DOI] [PubMed] [Google Scholar]
- 4. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, Group ESD, Neumann F-J, Sechtem U, Banning AP, Bonaros N, Bueno H, Bugiardini R, Chieffo A, Crea F, Czerny M, Delgado V, Dendale P, Flachskampf FA, Gohlke H, Grove EL, James S, Katritsis D, Landmesser U, Lettino M, Matter CM, Nathoe H, Niessner A, Patrono C, Petronio AS, Pettersen SE, Piccolo R, Piepoli MF, Popescu BA, Räber L, Richter DJ, Roffi M, Roithinger FX, Shlyakhto E, Sibbing D, Silber S, Simpson IA, Sousa-Uva M, Vardas P, Witkowski A, Zamorano JL, Achenbach S, Agewall S, Barbato E, Bax JJ, Capodanno D, Cuisset T, Deaton C, Dickstein K, Edvardsen T, Escaned J, Funck-Brentano C, Gersh BJ, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Prescott E, Saraste A, Storey RF, Svitil P, Valgimigli M, Windecker S, Aboyans V, Baigent C, Collet J-P, Dean V, Delgado V, Fitzsimons D, Gale CP, Grobbee D, Halvorsen S, Hindricks G, Iung B, Jüni P, Katus HA, Landmesser U, Leclercq C, Lettino M, Lewis BS, Merkely B, Mueller C, Petersen S, Petronio AS, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Touyz RM, Benkhedda S, Metzler B, Sujayeva V, Cosyns B, Kusljugic Z, Velchev V, Panayi G, Kala P, Haahr-Pedersen SA, Kabil H, Ainla T, Kaukonen T, Cayla G, Pagava Z, Woehrle J, Kanakakis J, Tóth K, Gudnason T, Peace A, Aronson D, Riccio C, Elezi S, Mirrakhimov E, Hansone S, Sarkis A, Babarskiene R, Beissel J, Maempel AJC, Revenco V, de Grooth GJ, Pejkov H, Juliebø V, Lipiec P, Santos J, Chioncel O, Duplyakov D, Bertelli L, Dikic AD, Studenčan M, Bunc M, Alfonso F, Bäck M, Zellweger M, Addad F, Yildirir A, Sirenko Y, Clapp B. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 5. Thomson S. Neuromodulation, or neuromodulatory effect 2013. https://www.neuromodulation.com/Neuromodulation-Defined
- 6. Apthorp GH, Chamberlain DA, Hayward GW. The effects of sympathectomy on the electrocardiogram and effort tolerance in angina pectoris. Br Heart J 1964;26:218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971–979. [DOI] [PubMed] [Google Scholar]
- 8. Sanderson JE. Electrical neurostimulators for pain relief in angina. Heart 1990;63:141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. 1967. [PubMed]
- 10. Cooke AW, Oygar A, Baggenstos P, Pacheco S, Keriga E. Vascular disease of extremities. Electric stimulation of spinal cord and posterior roots. NY State J Med 1976;76:366–378. [PubMed] [Google Scholar]
- 11. Mannheimer C, Carlsson C, Ericson K, Vedin A, Wilhelmsson C. Transcutaneous electrical nerve stimulation in severe angina pectoris. Eur Heart J 1982;3:297–302. [DOI] [PubMed] [Google Scholar]
- 12. Mannheimer C, Carlsson CA, Emanuelsson H, Vedin A, Waagstein F, Wilhelmsson C. The effects of transcutaneous electrical nerve stimulation in patients with severe angina pectoris. Circulation 1985;71:308–316. [DOI] [PubMed] [Google Scholar]
- 13. DeJongste MJL. Electrical neuromodulation and the heart with special emphasis on myocardial ischemia. In: Carrillo-Ruiz JD, ed., Topics in Neuromodulation Treatment. Croatia: InTech; 2012. p143–166. [Google Scholar]
- 14. Murphy DF, Giles KE. Dorsal column stimulation for pain relief from intractable angina pectoris. Pain 1987;28:365–368. [DOI] [PubMed] [Google Scholar]
- 15. Mannheimer C, Augustinsson LE, Carlsson CA, Manhem K, Wilhelmsson C. Epidural spinal electrical stimulation in severe angina pectoris. Heart 1988;59:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanderson J, Brooksby P, Waterhouse D, Palmer R, Neubauer K. Epidural spinal electrical stimulation for severe angina: a study of its effects on symptoms, exercise tolerance and degree of ischaemia. Eur Heart J 1992;13:628–633. [DOI] [PubMed] [Google Scholar]
- 17. White J. Cardiac pain: anatomic pathways and physiologic mechanisms. Circulation 1957;16:404–410. [DOI] [PubMed] [Google Scholar]
- 18. Sainsbury PA, Fisher M, de Silva R. Alternative interventions for refractory angina. Heart 2017;103:1911–1922. [DOI] [PubMed] [Google Scholar]
- 19. Gorlin R. Pathophysiology of cardiac pain. Circulation 1965;32:138–148. [DOI] [PubMed] [Google Scholar]
- 20. Wink J, van Delft R, Notenboom RGE, Wouters PF, DeRuiter MC, Plevier JWM, Jongbloed MRM. Human adult cardiac autonomic innervation: controversies in anatomical knowledge and relevance for cardiac neuromodulation. Auton Neurosci 2020;227:102674. [DOI] [PubMed] [Google Scholar]
- 21. Chandler M, Brennan T, Garrison D, Kim K, Schwartz P, Foreman R. A mechanism of cardiac pain suppression by spinal cord stimulation: implications for patients with angina pectoris. Eur Heart J 1993;14:96–105. [DOI] [PubMed] [Google Scholar]
- 22. Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci 2008;138:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mannheimer C, Emanuelsson H, Larsson G, Waagstein F, Augustinsson L-E, Eliasson T. Myocardial release of endogenous opioids in the human heart and the effects of epidural spinal electrical stimulation (ESES) in pacing-induced angina pectoris. J Am Coll Cardiol 1991;17:A107. [Google Scholar]
- 24. Eliasson T, Mannheimer C, Waagstein F, Andersson B, Bergh C-H, Augustinsson L-E, Hedner T, Larson G. Myocardial turnover of endogenous opioids and calcitonin-gene-related peptide in the human heart and the effects of spinal cord stimulation on pacing-induced angina pectoris. Cardiology 1998;89:170–177. [DOI] [PubMed] [Google Scholar]
- 25. Hautvast RWM, ter Horst GJ, DeJong BM, DeJongste MJL, Blanksma PK, Paans AMJ, Korf J. Relative changes in regional cerebral blood flow during spinal cord stimulation in patients with refractory angina pectoris. Eur J Neurosci 1997;9:1178–1183. [DOI] [PubMed] [Google Scholar]
- 26. Emanuelsson H, Mannheimer C, Waagstein F, Wilhelmsson C. Catecholamine metabolism during pacing-induced angina pectoris and the effect of transcutaneous electrical nerve stimulation. Am Heart J 1987;114:1360–1366. [DOI] [PubMed] [Google Scholar]
- 27. Chauhan A, Mullins PA, Thuraisingham SI, Taylor G, Petch MC, Schofield PM. Effect of transcutaneous electrical nerve stimulation on coronary blood flow. Circulation 1994;89:694–702. [DOI] [PubMed] [Google Scholar]
- 28. Norrsell H, Eliasson T, Mannheimer C, Augustinsson L-E, Bergh C-H, Andersson B, Waagstein F, Friberg P. Effects of pacing-induced myocardial stress and spinal cord stimulation on whole body and cardiac norepinephrine spillover. Eur Heart J 1997;18:1890–1896. [DOI] [PubMed] [Google Scholar]
- 29. Cardinal R, Pagé P, Vermeulen M, Bouchard C, Ardell JL, Foreman RD, Armour JA, Bou-Chard C, Andrew J. Spinal cord stimulation suppresses bradycardias and atrial tachyarrhythmias induced by mediastinal nerve stimulation in dogs. Am J Physiol Regul Integr Comp Physiol 2006;291:1369–1375. [DOI] [PubMed] [Google Scholar]
- 30. Foreman RD, Linderoth B, Ardell JL, Barron KW, Chandler MJ, Hull SS, TerHorst GJ, DeJongste MJL, Armour JA. Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res 2000;47:367–375. [DOI] [PubMed] [Google Scholar]
- 31. Armour JA, Linderoth B, Arora RC, DeJongste MJL, Ardell JL, Kingma JG, Hill M, Foreman RD. Long-term modulation of the intrinsic cardiac nervous system by spinal cord neurons in normal and ischaemic hearts. Auton Neurosci 2002;95:71–79. [DOI] [PubMed] [Google Scholar]
- 32. Linderoth B, Foreman R. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Medicine 2006;7:S14–S26. [Google Scholar]
- 33. Jacobs MJ, Jörning PJ, Joshi SR, Kitslaar PJ, Slaaf DW, Reneman RS. Epidural spinal cord electrical stimulation improves microvascular blood flow in severe limb ischemia. Ann Surg 1988;207:179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Landsheere C, Mannheimer C, Habets A, Guillaume M, Bourgeois I, Augustinsson LE, Eliasson T, Lamotte D, Kulbertus H, Rigo P. Effect of spinal cord stimulation on regional myocardial perfusion assessed by positron emission tomography. Am J Cardiol 1992;69:1143–1149. [DOI] [PubMed] [Google Scholar]
- 35. Mannheimer C, Eliasson T, Andersson B, Bergh CH, Augustinsson LE, Emanuelsson H, Waagstein F. Effects of spinal cord stimulation in angina pectoris induced by pacing and possible mechanisms of action. BMJ 1993;307:477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hautvast RWM, Blanksma PK, DeJongste MJL, Pruim J, van der Wall EE, Vaalburg W, Lie KI. Effect of spinal cord stimulation on myocardial blood flow assessed by positron emission tomography in patients with refractory angina pectoris. Am J Cardiol 1996;77:462–467. [DOI] [PubMed] [Google Scholar]
- 37. Norrsell H, Eliasson T, Albertsson P, Augustinsson L, Emanuelsson H, Eriksson P, Mannheimer C. Effects of spinal cord stimulation on coronary blood flow velocity. Coron Artery Dis 1998;9:273–278. [DOI] [PubMed] [Google Scholar]
- 38. Saraste A, Ukkonen H, Varis A, Vasankari T, Tunturi S, Taittonen M, Rautakorpi P, Luotolahti M, Airaksinen KEJ, Knuuti J. Effect of spinal cord stimulation on myocardial perfusion reserve in patients with refractory angina pectoris. Eur Heart J Cardiovasc Imaging 2015;16:449–455. [DOI] [PubMed] [Google Scholar]
- 39. Mobilia G, Zuin G, Zanco P, di Pede F, Pinato G, Neri G, Cargnel S, Raviele A, Ferlin G, Buchberger R. Effects of spinal cord stimulation on regional myocardial blood flow in patients with refractory angina. A positron emission tomography study. G Ital Cardiol 1998;28:1113–1119. [PubMed] [Google Scholar]
- 40. Eliasson T, Augustinsson LE, Mannheimer C. Spinal cord stimulation in severe angina pectoris—presentation of current studies, indications and clinical experience. Pain 1996;65:169–179. [DOI] [PubMed] [Google Scholar]
- 41. Eliasson T, Jern S, Augustinsson L, Mannheimer C. Safety aspects of spinal cord stimulation in severe angina pectoris. Coron Artery Dis 1994;5:845–850. [PubMed] [Google Scholar]
- 42. Sanderson J, Ibrahim B, Waterhouse D, Palmer R. Spinal electrical stimulation for intractable angina—long-term clinical outcome and safety. Eur Heart J 1994;15:810–814. [DOI] [PubMed] [Google Scholar]
- 43. Andersen C, Hole P, Oxhøj H. Does pain relief with spinal cord stimulation for angina conceal myocardial infarction? Heart 1994;71:419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. TenVaarwerk IAM, Jessurun GAJ, DeJongste MJL, Andersen C, Mannheimer C, Eliasson T, Tadema W, Staal MJ. Clinical outcome of patients treated with spinal cord stimulation for therapeutically refractory angina pectoris. Heart 1999;82:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jessurun G, ten Vaarwerk I, DeJongste M, Tio R, Staal M. Sequelae of spinal cord stimulation for refractory angina pectoris. Reliability and safety profile of long-term clinical application. Coron Artery Dis 1997;8:33–38. [DOI] [PubMed] [Google Scholar]
- 46. Ekre O, Eliasson T, Norrsell H, Währborg P, Mannheimer C. Long-term effects of spinal cord stimulation and coronary artery bypass grafting on quality of life and survival in the ESBY study. Eur Heart J 2002;23:1938–1945. [DOI] [PubMed] [Google Scholar]
- 47. Vervaat FE, van Suijlekom H, Wijnbergen I. Feasibility of spinal cord stimulation in patients with refractory angina pectoris and a cardiac implanted electronic device. Neuromodulation 2021. 10.1111/ner.13411 (online ahead of print). [DOI] [PubMed] [Google Scholar]
- 48. Henry TD, Satran D, Hodges JS, Johnson RK, Poulose AK, Campbell AR, Garberich RF, Bart BA, Olson RE, Boisjolie CR, Harvey KL, Arndt TL, Traverse JH. Long-term survival in patients with refractory angina. Eur Heart J 2013;34:2683–2688. [DOI] [PubMed] [Google Scholar]
- 49. Andréll P, Ekre O, Grip L, Währborg P, Albertsson P, Eliasson T, Jeppsson A, Mannheimer C. Fatality, morbidity and quality of life in patients with refractory angina pectoris. Int J Cardiol 2011;147:377–382. [DOI] [PubMed] [Google Scholar]
- 50. Torre-Amione G, Alo K, Estep JD, Valderrabano M, Khalil N, Farazi TG, Rosenberg SP, Ness L, Gill J. Spinal cord stimulation is safe and feasible in patients with advanced heart failure: early clinical experience. Eur J Heart Fail 2014;16:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tse HF, Turner S, Sanders P, Okuyama Y, Fujiu K, Cheung CW, Russo M, Green MDS, Yiu KH, Chen P, Shuto C, Lau EOY, Siu CW. Thoracic spinal cord stimulation for heart failure as a restorative treatment (SCS HEART study): first-in-man experience. Heart Rhythm 2015;12:588–595. [DOI] [PubMed] [Google Scholar]
- 52. Zipes DP, Neuzil P, Theres H, Caraway D, Mann DL, Mannheimer C, van Buren P, Linde C, Linderoth B, Kueffer F, Sarazin SA, DeJongste MJL. Determining the feasibility of spinal cord neuromodulation for the treatment of chronic systolic heart failure: the DEFEAT-HF study. JACC Heart Fail 2016;4:129–136. [DOI] [PubMed] [Google Scholar]
- 53. Greco S, Auriti A, Fiume D, Gazzeri G, Gentilucci G, Antonini L, Santini M. Spinal cord stimulation for the treatment of refractory angina pectoris: a two-year follow-up. Pacing Clin Electrophysiol 1999;22:26–32. [DOI] [PubMed] [Google Scholar]
- 54. Romanò M, Auriti A, Cazzin R, Chiarandà G, Circo A, de Luca A, di Pede F, Fiume D, Greco S, Grieco A, Mangiameli S, Maritano M, Mazzarino F, Pinato G, Raciti S, Raviele A, Santini M, Zucco F, Zuin G. Epidural spinal stimulation in the treatment of refractory angina pectoris. Its clinical efficacy, complications and long-term mortality. An Italian multicenter retrospective study. Ital Heart J Suppl 2000;1:97–102. [PubMed] [Google Scholar]
- 55. di Pede F, Lanza GA, Zuin G, Alfieri O, Rapati M, Romanò M, Circo A, Cardano P, Bellocci F, Santini M, Maseri A. Immediate and long-term clinical outcome after spinal cord stimulation for refractory stable angina pectoris. Am J Cardiol 2003;91:951–955. [DOI] [PubMed] [Google Scholar]
- 56. Lapenna E, Rapati D, Cardano P, de Bonis M, Lullo F, Zangrillo A, Alfieri O. Spinal cord stimulation for patients with refractory angina and previous coronary surgery. Ann Thorac Surg 2006;82:1704–1708. [DOI] [PubMed] [Google Scholar]
- 57. Andréll P, Yu W, Gersbach P, Gillberg L, Pehrsson K, Hardy I, Ståhle A, Andersen C, Mannheimer C. Long-term effects of spinal cord stimulation on angina symptoms and quality of life in patients with refractory angina pectoris—results from the European Angina Registry Link Study (EARL). Heart 2010;96:1132–1136. [DOI] [PubMed] [Google Scholar]
- 58. Vervaat FE, van der Gaag A, van Suijlekom H, Botman CJ, Teeuwen K, Wijnbergen I. Improvement in quality of life and angina pectoris: 1-year follow-up of patients with refractory angina pectoris and spinal cord stimulation. Neth Heart J 2020;28:478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eddicks S, Maier-Hauff K, Schenk M, Müller A, Baumann G, Theres H. Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: the first placebo-controlled randomised study. Heart 2007;93:585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zipes DP, Svorkdal N, Berman D, Boortz-Marx R, Henry T, Lerman A, Ross E, Turner M, Irwin C. Spinal cord stimulation therapy for patients with refractory angina who are not candidates for revascularization. Neuromodulation 2012;15:550–559. [DOI] [PubMed] [Google Scholar]
- 61. Eldabe S, Thomson S, Duarte R, Brookes M, deBelder M, Raphael J, Davies E, Taylor R. The effectiveness and cost-effectiveness of spinal cord stimulation for refractory angina (RASCAL study): a pilot randomized controlled trial. Neuromodulation 2016;19:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lanza GA, Grimaldi R, Greco S, Ghio S, Sarullo F, Zuin G, de Luca A, Allegri M, di Pede F, Castagno D, Turco A, Sapio M, Pinato G, Cioni B, Trevi G, Crea F. Spinal cord stimulation for the treatment of refractory angina pectoris: a multicenter randomized single-blind study (the SCS-ITA trial). Pain 2011;152:45–52. [DOI] [PubMed] [Google Scholar]
- 63. Pan X, Bao H, Si Y, Xu C, Chen H, Gao X, Xie X, Xu Y, Sun F, Zeng L. Spinal cord stimulation for refractory angina pectoris: a systematic review and meta-analysis. Clin J Pain 2017;33:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Imran TF, Malapero R, Qavi AH, Hasan Z, de la Torre B, Patel YR, Yong RJ, Djousse L, Gaziano JM, Gerhard-Herman M-D. Efficacy of spinal cord stimulation as an adjunct therapy for chronic refractory angina pectoris. Int J Cardiol 2017;227:535–542. [DOI] [PubMed] [Google Scholar]
- 65. McNicol E, Ferguson M, Bungay K, Rowe EL, Eldabe S, Gewandter JS, Hayek SM, Katz N, Kopell BH, Markman J, Rezai A, Taylor RS, Turk DC, Dworkin RH, North RB, Thomson S. Systematic review of research methods and reporting quality of randomized clinical trials of spinal cord stimulation for pain. J Pain 2021;22:127–142. [DOI] [PubMed] [Google Scholar]
- 66. Povsic TJ, Henry TD, Ohman EM. Therapeutic approaches for the no-option refractory angina patient. Circ Cardiovasc Interv 2021;14:218–232. [DOI] [PubMed] [Google Scholar]
- 67. Gallone G, Baldetti L, Tzanis G, Gramegna M, Latib A, Colombo A, Henry TD, Giannini F. Refractory angina: from pathophysiology to new therapeutic nonpharmacological technologies. JACC Cardiovasc Interv 2020;13:1–19. [DOI] [PubMed] [Google Scholar]
- 68. Davies A, Fox K, Galassi AR, Banai S, Ylä-Herttuala S, Lüscher TF. Management of refractory angina: an update. Eur Heart J 2021;42:269–283. [DOI] [PubMed] [Google Scholar]
- 69. Yu W, Maru F, Edner M, Hellströmm K, Kahan T, Persson H. Spinal cord stimulation for refractory angina pectoris: a retrospective analysis of efficacy and cost-benefit. Coron Artery Dis 2004;15:31–37. [DOI] [PubMed] [Google Scholar]
- 70. Murray S, Carson K, Ewings P, Collins P, James M. Spinal cord stimulation significantly decreases the need for acute hospital admission for chest pain in patients with refractory angina pectoris. Heart 1999;82:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gomes B, Valchanov K, Davies W, Brown A, Schofield P. Spinal cord stimulation for refractory angina: 100 case-experience from the national refractory angina service. Br J Cardiol 2016;23:106–109. [Google Scholar]
- 72. Andersen C, Enggaard TP, Scherer C. Spinal cord stimulation has proven benefit on pain and quality of life in patients with angina pectoris when less invasive therapies have failed. Neuromodulation 2006;9:314–319. [DOI] [PubMed] [Google Scholar]
- 73. de Vries J, DeJongste MJL, Zijlstra F, Staal M. Long-Term effects of electrical neurostimulation in patients with unstable angina: refractory to conventional therapies. Neuromodulation 2007;10:345–348. [DOI] [PubMed] [Google Scholar]
- 74. Thomson S. Fact sheet spinal cord stimulation 2019. 2019. https://www.neuromodulation.com/assets/documents/Fact_Sheets/fact_sheet_spinal_cord_stimulation.pdf (Accessed 26 August 2021).
- 75. Eldabe S, Buchser E, Duarte RV. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literature. Pain Med 2016;17:325–336. [DOI] [PubMed] [Google Scholar]
- 76. Taylor RS, de Vries J, Buchser E, DeJongste MJ. Spinal cord stimulation in the treatment of refractory angina: systematic review and meta-analysis of randomised controlled trials. BMC Cardiovasc Disord 2009;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fihn S, Gardin J, Abrams J, Berra K, Blankenship J, Dallas A, Douglas P, Foody J, Gerber T, Hinderliter A, King S, Kligfield P, Krumholz H, Kwong R, Lim M, Linderbaum J, Mack M, Munger M, Prager R, Sabik J, Shaw L, Sikkema J, Smith C, Smith S, Spertus J, Williams S. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126:3097–3137. [DOI] [PubMed] [Google Scholar]
- 78. Sectie Pijn- en Palliatieve Geneeskunde of the Nederlandse Vereniging voor Anesthesiologie. Recommendations Evidence based interventional pain practice: according to clinical diagnoses. https://www.anesthesiologie.nl/uploads/files/Evidence_based_interventional_pain_practice_def.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated in support of the article.