Abstract
Importance
Recent changes in guidelines for managing Clostridioides difficile infections (CDI) have placed fidaxomicin as a first-line treatment.
Objective
To estimate the net cost of first-line fidaxomicin compared to vancomycin in the American and Canadian healthcare systems and to estimate the price points at which fidaxomicin would become cost saving for the prevention of recurrence.
Data sources and study selection
We identified randomized, placebo-controlled trials directly comparing fidaxomicin with vancomycin that reported on recurrence. Medication costs were obtained from the Veterans Affairs Federal Supply Schedule (US) and the Quebec drug formulary (Canada). The average cost of a CDI recurrence was established through a systematic review for each country.
Data extraction, synthesis and outcome measures
For efficacy, data on CDI recurrence at day 40 were pooled using a restricted maximal likelihood random effects model. For the cost review, the mean cost across identified studies was adjusted to reflect May 2022 dollars. These were used to estimate the net cost per recurrence prevented with fidaxomicin and the price point below which fidaxomicin would be cost saving.
Results
The estimated mean system costs of a CDI recurrence were $15 147USD and $8806CAD, respectively. Preventing one recurrence by using first-line fidaxomicin over vancomycin would cost $38 222USD (95%CI $30 577–$57 332) and $13 760CAD (95%CI $11 008–$20 640), respectively. The probability that fidaxomicin was cost saving exceeded 95% if priced below $1140USD or $860CAD, respectively.
Conclusions and Relevance
An increased drug expenditure on fidaxomicin may not be offset through recurrence prevention unless the fidaxomicin price is negotiated.
Introduction
Clostridioides difficile infection (CDI) is a major cause of healthcare-associated diarrhoea in North America. It is estimated that in 2017 there were nearly 462 000 cases in the USA and in 20121 there were approximately 37 900 cases in Canada.2 Of these, approximately 20%–25% represent recurrent infections.2,3 The prevention of incident and recurrent episodes of CDI is therefore an important public health goal. Several pharmacologic and nonpharmacologic interventions have been investigated as initial treatment, and more specifically, to reduce risk of recurrence. For much of the twenty-first century, the recommended initial treatment of CDI has been oral metronidazole or vancomycin. In 2011, fidaxomicin was first demonstrated to be non-inferior to oral vancomycin for clinical cure.4 This has ultimately been shown in two out of three double-blind, randomized, placebo-controlled trials,4,5 with all three providing evidence of a reduced risk of recurrence at day 40.4–6 However, recommendations for fidaxomicin as first-line therapy have lagged in guidelines and formulary uptake has been slow, presumably due to fidaxomicin’s higher cost. Issues surrounding affordability were highlighted in the 2017 Infectious Diseases and Healthcare Epidemiology Societies of America (IDSA-SHEA) guidelines7 and in the 2018 Association of Medical Microbiology and Infectious Diseases of Canada (AMMI) guidelines.8 Now more than a decade since the initial trial was published, the 2021 update to the IDSA-SHEA C. difficile guidelines recommended fidaxomicin as first-line therapy for all patients.9 At the current pricing, treating all American10 and Canadian11 patients with fidaxomicin would cost an estimated $2.06 billion US dollars (USD) and $60 million Canadian dollars (CAD) per year, respectively.
Due to higher drug costs, other groups have attempted to evaluate the costs associated with first-line fidaxomicin in the USA and Canada, with mixed results. In one of the earliest evaluations, Bartsch et al. estimated that fidaxomicin would be dominated by other available options.12 Reveles et al. suggested fidaxomicin had similar overall costs to compounded vancomycin and that it might be cost saving in some high-risk populations.13 By contrast, Rajasingham et al. estimated that fidaxomicin would be cost-effective below a willingness-to-pay threshold of $100 000 per quality-adjusted life year (QALY).14 In Canada, Wagner et al. estimated fidaxomicin use would be associated with an overall average cost increase of $13 202 per recurrence prevented, assuming a drug cost of $2200.15
Consequently, we believe that whether reductions of recurrent CDI will offset the higher up-front costs of fidaxomicin has not yet been determined. We therefore sought to estimate: (i) the net (added) cost of first-line use of fidaxomicin required to prevent a recurrence as compared to oral vancomycin and compare this with (ii) the estimated cost of a CDI recurrence so that we could determine (iii) the price point where a treatment course with fidaxomicin becomes unequivocally cost saving in the American and Canadian contexts.
Methods
To estimate the comparative efficacy of fidaxomicin versus vancomycin we conducted a meta-analysis of the three double-blind, placebo-controlled, randomized controlled trials identified by IDSA-SHEA9 wherein fidaxomicin was compared head-to-head with vancomycin.4–6 We excluded a fourth open-label trial that compared a longer total duration of fidaxomicin (30 versus 10 days in all other included studies).16 This trial was excluded because the open-label nature of the study could create bias in favour of the treatment group both in terms of patient reported symptoms and subsequent physician testing and treating behaviour. We examined the primary outcome of the first CDI recurrence by day 40, which was the longest common duration reported, and meta-analysed the risk ratio with 95% confidence intervals using a restricted maximum likelihood random effects model in STATA v.17 (StataCorp LP). Using the overall control event rate as the expected baseline rate of recurrence, we then estimated the absolute risk difference, number needed to treat, and associated 95% confidence intervals.
We obtained the US drug costs from the Veterans Affairs Federal Supply Schedule (FSS)10 by choosing the lowest FSS price. We obtained the Canadian drug costs from the Quebec formulary11 (the province with the highest rate of CDI). A 10-day course of fidaxomicin was estimated to cost $3845.44USD and $1584 CAD, and that of vancomycin at $23.28USD (capsules) and $208 CAD (capsules). While some jurisdictions use the IV formulation as a PO treatment with consequent lower costs, our comparison is based on commercial products. The difference in estimated costs and the NNT were used to calculate the additional cost per recurrence prevented.
We estimated the cost of a CDI recurrence in USD and CAD through a systematic review of the literature. Is the USA, we assumed cost would apply to an insurer and/or patient, and in Canada, to the public payer. We searched PubMed on 10 July 2022, with the search terms described in Appendix 1. We included studies that were primary research articles, contained a cost-analysis of CDI, included cases of recurrent CDI, and were calculated with cost parameters based on the American or Canadian healthcare systems. Studies were excluded if the population exclusively contained hospitalized patients, as the purpose of this analysis was to evaluate fidaxomicin use in all cases of CDI from mild outpatient to more severe inpatient cases and analyses based only on hospitalized patients might inflate costs and not be representative. Studies of paediatric populations were also excluded. References for all included studies were examined for additional applicable studies. Screening and data extraction was performed in duplicate (D.P., J.S. and T.C.L.) with disagreement resolved by consensus. All costs were converted to the May 2022 dollar rate using the Consumer Price Index Inflation Calculator17 (USD) and Bank of Canada Inflation Calculator18 (CAD), respectively. Across included studies, the average 2022 dollar cost was calculated and used for the analysis. We also extracted the cost perspective that was examined in each of the included studies (e.g. public payer, traditional insurers, patient, societal, Medicare, Medicaid or third-party payer).
Finally, we calculated the probability of various effect sizes from the baseline recurrence rate and 95% confidence interval associated with the relative risk. We then identified how probable it was, at a specified price for fidaxomicin (rounded down to nearest $10), that the total cost of treating all patients with fidaxomicin relative to vancomycin would be offset by the cost savings from preventing recurrences (probability of cost equivalence). We created scatter plots of the probability of cost equivalence as a function of fidaxomicin price. For visualization purposes, a smooth line of best fit was generated with curvefit19 for STATA using a rational estimator. A graphical summary of this analysis is shown in Figure S1 (available as Supplementary data at JAC-AMR online).
Results
Fidaxomicin effectiveness
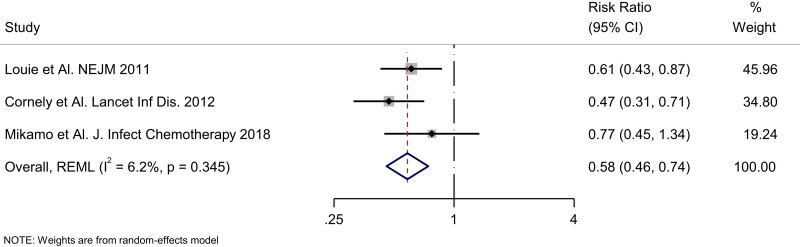
The overall relative risk for recurrence with 10 days of fidaxomicin versus 10 days of vancomycin was 0.58 (95% CI 0.46–0.74; Figure 1). This corresponds to an absolute risk reduction of 10.8% (95% CI 6.7%–14%) or a NNT of 10 (95% CI 8–15) using the pooled control event rate of 25.9%. At the current fidaxomicin and vancomycin prices, the estimated additional cost to prevent one recurrence in the USA was estimated as $38 222USD (95%CI $30 577–$57 332) and in Canada this was estimated at $13 760CAD (95%CI $11,008-$20 640).
Figure 1.
Forest plot of fidaxomicin randomized controlled trials risk of recurrent CDI.
Cost of recurrence
The results of the systematic literature review for the cost of a CDI recurrence in the American and Canadian healthcare systems are presented in Table 1. Additional descriptions of each included study are contained in Tables S1 and S2. For the USA, the initial search for the cost of a CDI recurrence yielded 786 results. Of these results, 110 articles were selected for further review. Of the 110 articles, 13 were reviews or meta-analyses, 36 included only hospitalized patients, 50 did not calculate the cost of a recurrent CDI episode, three included only a paediatric population, and one was not based in the USA. The seven remaining articles were retained for the final analysis.20–26 One article was subsequently excluded because it calculated the 12-month all-cause medical costs (as opposed to the attributable cost) of patients with recurrent CDI episodes.26 Additionally, Luo et al. calculated the cost of recurrent CDI based on differing treatment strategies; the cost of the treatment with fidaxomicin was excluded from the overall average.20
Table 1.
Summary of CDI recurrence cost by study
| Study | Recurrence cost | May 2022 dollars | Cost perspectives |
|---|---|---|---|
| USA | |||
| McFarland et al.25 | $1914.00 | $3405.08 | Healthcare perspective; costs obtained from medical billing records and laboratory charges |
| Desai et al.22 | $9501.74 | $11 722.81 | Healthcare perspective; study used societal perspective however indirect costs (productivity loss) were excluded from present analysis |
| Rodrigues et al.21 | $34 104.00 | $41 049.68 | Payer perspective; most cost values obtained from Centers for Medicare and Medicaid Services, with hospitalization cost from Healthcare Cost Utilization Project Nationwide Inpatient Sample (all-payer hospitalization database) |
| Zilberberg et al.24, 2017 | $12 043.00 | $14 495.70 | Payer perspective; costs measured as Medicare payments |
| Zhang et al.23 | $10 580.00 | $12 476.42 | Healthcare perspective; total healthcare costs were calculated as amount paid by primary and secondary insurers and by patients (i.e. copayment and deductibles) across all claims |
| Luo et al.20 | $6826.00 | $7734.25 | Modified third-party payer’s perspective (included costs of medications, hospitalizations and any procedures) |
| Average USA cost | $15 147.32 | ||
| Canada | |||
| Wagner et al.15 | $8250.05 | $9961.71 | Public payer perspective |
| Levy et al.2 | $8157.89 | $9765.04 | Public payer perspective; study used a societal perspective however indirect costs were excluded from present analysis |
| Lapointe-Shaw et al.27 | Metronidazole: $5386.00 Vancomycin: $5929.00 |
Metronidazole:$6351.97 Vancomycin:$6692.35 |
Public payer perspective, only vancomycin pathway cost considered |
| Average Canada cost | $8806.37 |
The search for the cost of a recurrence in Canada yielded 123 results, of which 18 articles were reviewed based on the title and abstract. Of these 18 studies, 14 studies were excluded: five studies did not include cases of recurrent CDI, four studies did not measure the cost of CDI, four studies were literature reviews, and one study measured the cost of readmission to hospital due to CDI without specifying whether it was for first episode or recurrence. Four remaining studies included cases of recurrent or relapsed CDI and their cost.2,15,27,28 One study that included cases of recurrent CDI was subsequently excluded as it presented the cost in median ($1812CAD) instead of mean.28 This left three studies that were included in the Canadian analysis for the cost of recurrence.
The estimated mean 2022 systemic costs for a recurrence of CDI in the American and Canadian healthcare systems, respectively, were $15 147USD and $8806CAD. In the USA, cost perspectives included payer and healthcare system perspectives, calculated using Medicare, third-party payers, and traditional insurers. In Canada, all studies reported costs based on a public payer perspective.
Cost equivalence
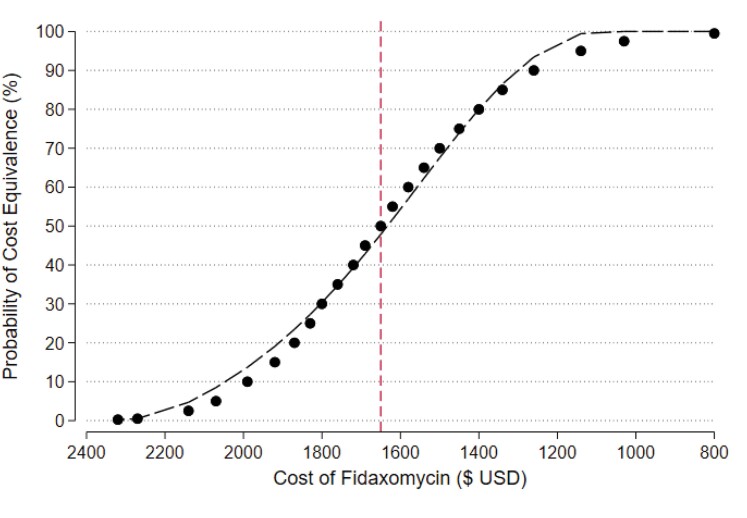
With respect to the USA, at the quoted price for 10 days of fidaxomicin and for 10 days of vancomycin capsules, there is a 0% chance that fidaxomicin will be cost equivalent by preventing the first CDI recurrence (Figure 2). At a price of approximately $1650 ($1630 more than the current cost of 10-day course of vancomycin) the probability of cost equivalence rises to 50% and at approximately $1140 ($1120 more than vancomycin) the probability rises to 95%. Therefore, fidaxomicin is very likely to be cost saving if priced below $1140 in the USA.
Figure 2.
Probability of fidaxomicin cost equivalence—USA.
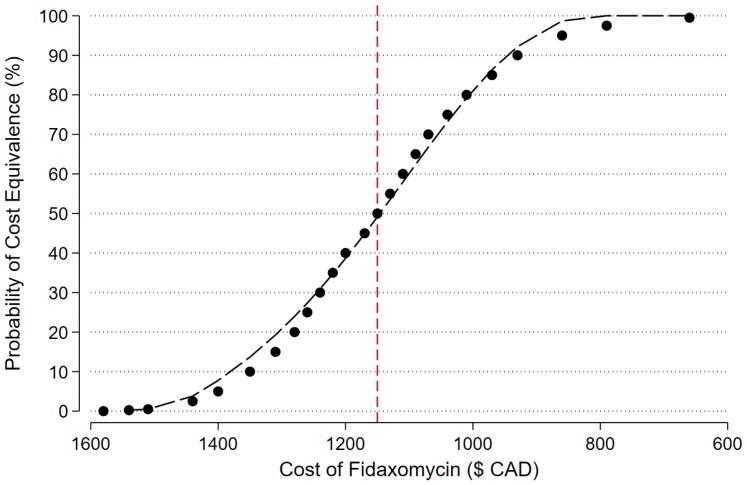
For Canada, at the current 10-day price of $1580 CAD for fidaxomicin and $208 CAD for vancomycin, there is less than a 0.25% chance that fidaxomicin will be cost equivalent by preventing the next CDI recurrence (Figure 3). At a fidaxomicin price of approximately $1150 CAD ($950 more than the current cost of a 10-day course of vancomycin) the probability of cost equivalence rises to 50% and at approximately $860 CAD ($660 more than vancomycin) the probability rises to 95%. In Canada, at any price below $860 CAD, fidaxomicin is likely to be cost saving.
Figure 3.
Probability of fidaxomicin cost equivalence—Canada.
Discussion
From our detailed review of the literature and associated calculations, we found that for both the USA and Canada, the use of fidaxomicin as first-line treatment for CDI will cost substantially more to both the public payer in Canada and to US payers compared with potential cost savings realized through recurrence reduction. We identified price points of approximately $1140 USD and $860 CAD at or below which the use of fidaxomicin is highly probable to be cost equivalent or cost saving. Despite double-blind, randomized, placebo-controlled trial evidence that 10 days of fidaxomicin is superior to 10 days of vancomycin for the secondary outcome of prevention of first recurrence at day 40, this efficacy has not translated into a meaningful uptake of fidaxomicin that we hypothesize is due to this very high financial impact. In Canada, individual provinces have their own drug plans, and negotiation with the manufacturer to obtain a more cost-equivalent price point is possible, which could facilitate a financially viable practice change. In the USA, such negotiations are generally not currently permitted by Medicare by law; however, negotiation of pricing could save the USA billions per year for all drugs, including fidaxomicin.29 Individual US insurance companies, particularly ones with large formulary budgets may have negotiating power to reduce costs.
Previous economic analyses in the North American context have yielded conflicting results regarding the cost-benefit of fidaxomicin. Bartsch et al. used a decision-analytic model to compare fidaxomicin versus no fidaxomicin and estimated that vancomycin would be dominated unless the cost was reduced to below $150 without strain typing (and $400 with strain typing).12 A decision-analytic model comparing fidaxomicin to vancomycin by Reveles et al. for hospitalized patients yielded similar overall treatment costs per patient ($14 442 for fidaxomicin versus $14 179 for vancomycin), using fidaxomicin cost of $2350.13 By contrast, Rajasingham used a Markov model approach and found fidaxomicin to be cost-effective with a cost of $31 751/QALY assuming a fidaxomicin cost of $1767.20.14 In Canada, Wagner et al. estimated fidaxomicin use would be associated with an overall average cost increase of $13 202 per recurrence prevented assuming a drug cost of $2200 and accounting for CDI-related admissions to hospital.15
In terms of observational studies with paired economic analyses, McDaniel et al.30 conducted a retrospective single-centre study using electronic medical record data. Comparing pre- versus post-implementation of a treatment pathway favouring fidaxomicin for first and second episodes of C. difficile, they found 30-day C. difficile recurrence rates fell from 18.0% to 6.3% with lower total costs post-intervention for index admissions ($2588.63) and 30-day readmissions ($4738.62). However, metronidazole was used in 48% of cases pre-implementation falling to 1.6% post, suggesting the results may reflect comparisons with metronidazole as much as they do vancomycin. Further, while fidaxomicin was independently associated with a sustained response in a multivariable model (odds ratio 1.96; 95% CI 1.03–3.72), this did not represent a direct comparison between vancomycin and fidaxomicin. Another retrospective analysis by Gallagher et al.31 evaluated a protocol which encouraged fidaxomicin for high-risk patients and compared those who received fidaxomicin and vancomycin. They found that 90-day readmission for C. difficile recurrence occurred in 20.4% and 41.3%, respectively, and that at a fidaxomicin cost of $1840, fidaxomicin use saved the hospital $3047 per patient based on lower readmission costs.
These observational studies have several key challenges. Principally, subsequent testing and treating behaviour could be biased by knowledge of fidaxomicin treatment in an open-label context. Second, confounding by indication can be challenging to eliminate, particularly in small studies. Finally, before–after designs may not adequately control for other temporal trends (e.g. changes in dominant strains) and time-series methods are generally preferred in terms of the hierarchy of evidence. Given large and granular enough data sets, a target trial emulation study with adjustment for temporal trends could be an important addition to the literature.
Our analysis has several limitations. At the current price of fidaxomicin, any strategy that increases the efficacy of vancomycin, for example, the use of an up-front decreasing dose taper to prevent recurrence (NCT04138706), would affect our results and would require recalculation. We have presented a best-case scenario for fidaxomicin by comparing it to a 10-day course of vancomycin. Furthermore, the efficacy of fidaxomicin to prevent recurrence at day 56 (the IDSA-SHEA definition of recurrence), or day 90, was not studied in the included randomized trials. Up to 31% of recurrences may occur after day 4232 and there are no RCT data to allow comparisons including delayed recurrences. Fidaxomicin treatment outcomes have not been properly studied in patients with multiple recurrences, but it is possible that preventing the first recurrence could reduce the risk of subsequent recurrences and therefore be more attractive. Such an evaluation would ideally be conducted with randomized data, which is currently limited. Also, US drug prices are not fully transparent, and the costs borne to different parts of the system (patient, insurance company, hospitals/facilities) are often unclear. We used publicly available data to estimate the costs, but these costs may not reflect the costs to each party. Finally, further reducing the cost of vancomycin through the compounding of generic IV vancomycin into liquid form or reducing the cost of other formulations would increase the break-even price of fidaxomicin, particularly in Canada.
The estimation of CDI recurrence cost through systematic review for each country also has some limitations. The articles from the USA had differing cost perspectives, with half the articles having a payer perspective while the other half had a healthcare perspective. The time frame of both Canadian and US studies also differed, ranging from within 6 weeks of a recurrence to up to 12 months from a recurrence, with some studies having an unspecified time frame. Another limitation stems from the cost of recurrence calculations in the studies retained. All the Canadian studies used decision models, with resource use and costs being derived from Canadian surveillance programmes, Canadian hospitals, and published literature. Out of the six US studies, four had real-world data, either from observational studies21,23,24 or a clinical trial.25 The remaining two studies used decision models.20,22
Finally, the aim of our study was to evaluate only direct medical costs from either a payer or healthcare perspective. This study did not look at broader indirect costs, such as costs related to patient time and lost productivity. Only two of the studies included evaluated costs from a broader societal perspective.2,22 Therefore, additional studies are needed to draw conclusions based on broader societal perspective costs.
A strength of our study is the use of a meta-analytic assessment of the effect size for fidaxomicin from all the placebo-controlled trials, coupled with a systematic estimate of recurrence costs to produce a practical and easily understood comparison. Comparing additional drug costs versus an estimate of the cost of a recurrence is a different analytic perspective than the cost per quality-adjusted life-year point of view. Previous cost-effectiveness studies have been conducted, most showing a fractional difference (e.g. 0.0233–0.0314) in QALYs. More fundamentally, cost-effectiveness is not the same as cost saving. Cost-effectiveness measures, including cost per QALY and cost per incremental cost-effectiveness ratio, assess added costs by a subjectively perceived threshold of value. Often this is contextualized against the historical price for a year of haemodialysis, which is lifesaving. However, hospitals, patients and governments do not have unlimited budgets and most treatments are not a crucially lifesaving as haemodialysis. Even if an intervention is perceived as valuable, if the cost is unsustainable, cost-effectiveness may be irrelevant whereas cost equivalence or cost saving compared to current effective therapies is always relevant.
CDI causes a major burden to health systems worldwide and reduction of recurrence has value. Yet, health system sustainability requires thoughtful assessment of both current and future costs and benefits. At current pricing, a switch to first-line fidaxomicin will cost billions of excess healthcare dollars to US and Canadian payers and, on the basis of this analysis, these costs will not be recouped through the reduction of recurrent CDI. Assuming vancomycin costs remain the same, and until additional placebo-controlled trials of novel vancomycin or fidaxomicin dosing strategies are available, a reduction of the cost of fidaxomicin to below $1140USD and $860CAD, respectively, would support a substantial change to fidaxomicin prescribing practices.
Supplementary Material
Contributor Information
Devangi Patel, Faculty of Medicine and Health Sciences, McGill University, Montréal, Canada.
Julien Senecal, Faculty of Medicine and Health Sciences, McGill University, Montréal, Canada.
Brad Spellberg, Los Angeles County and University of Southern California Medical Center, Los Angeles, CA, USA.
Andrew M Morris, Division of Infectious Diseases, Department of Medicine, Sinai Health, University Health Network, and University of Toronto, Toronto, Canada.
Lynora Saxinger, Division of Infectious Diseases, Department of Medicine, University of Alberta, Edmonton, Canada.
Brent W Footer, Providence Portland Medical Center, Portland, USA.
Emily G McDonald, Division of General Internal Medicine, Department of Medicine, McGill University Health Centre, Montréal, Canada; Clinical Practice Assessment Unit, Department of Medicine, McGill University Health Centre, Montréal, Canada.
Todd C Lee, Division of General Internal Medicine, Department of Medicine, McGill University Health Centre, Montréal, Canada; Clinical Practice Assessment Unit, Department of Medicine, McGill University Health Centre, Montréal, Canada; Division of Infectious Diseases, Department of Medicine, McGill University Health Centre, Montréal, Canada.
Funding
This study was carried out as part of routine work.
Transparency declarations
TCL and EGM receive research salary support from the Fonds de Recherche du Québec—Santé, unrelated to this work. All other authors: non to declare.
Conflicts of interest
EGM and TCL are principal investigators on a Canadian Institutes of Health Research Funded clinical trial looking at alternative vancomycin dosing strategies for the first episode of C. difficile.
CRediT author statement
Conceptualization—TCL, DP; methodology—TCL; validation—TCL, DP; formal analysis—TCL, DP; investigation—TCL, DP, JS; resources—TCL; data curation—TCL, DP, JS; writing—original draft—DP, EGM, TCL, JS; writing: review and editing—all authors; visualization—DP, TCL; supervision—TCL; project administration—TCL.
Supplementary data
Figure S1 and Tables S1 and 2 are available as Supplementary data at JAC-AMR online.
References
- 1. Guh AY, Mu Y, Winston LGet al. . Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med 2020; 382: 1320–30. 10.1056/NEJMoa1910215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levy AR, Szabo SM, Lozano-Ortega Get al. . Incidence and costs of Clostridium difficile infections in Canada. Open Forum Infect Dis 2015; 2: ofv076. 10.1093/ofid/ofv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finn E, Andersson FL, Madin-Warburton M. Burden of Clostridioides difficile infection (CDI)—a systematic review of the epidemiology of primary and recurrent CDI. BMC Infect Dis 2021; 21: 456. 10.1186/s12879-021-06147-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louie TJ, Miller MA, Mullane KMet al. . Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364: 422–31. 10.1056/NEJMoa0910812 [DOI] [PubMed] [Google Scholar]
- 5. Cornely OA, Crook DW, Esposito Ret al. . Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012; 12: 281–9. 10.1016/S1473-3099(11)70374-7 [DOI] [PubMed] [Google Scholar]
- 6. Mikamo H, Tateda K, Yanagihara Ket al. . Efficacy and safety of fidaxomicin for the treatment of Clostridioides (Clostridium) difficile infection in a randomized, double-blind, comparative phase III study in Japan. J Infect Chemother 2018; 24: 744–52. 10.1016/j.jiac.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 7. McDonald LC, Gerding DN, Johnson Set al. . Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66: e1–48. 10.1093/cid/cix1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loo VG, Davis I, Embil J, et al. . Association of Medical Microbiology and Infectious Disease Canada treatment practice guidelines for Clostridium difficile infection. J Assoc Med Microbiol Infect Dis Canada 2018; 3: 71–92. (https://jammi.utpjournals.press/doi/abs/10.3138/jammi.2018.02.13) [Google Scholar]
- 9. Johnson S, Lavergne V, Skinner AMet al. . Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 2021; 73: e1029–44. 10.1093/cid/ciab549 [DOI] [PubMed] [Google Scholar]
- 10. Veterans Affairs Office of Procurement, Acquisition and Logistics . Pharmaceutical prices. https://www.va.gov/opal/nac/fss/pharmprices.asp.
- 11. Régie de l’assurance maladie du Québec (RAMQ) . List of medications, May 26, 2022. 2022. https://www.ramq.gouv.qc.ca/en/media/12091.
- 12. Bartsch SM, Umscheid CA, Fishman Net al. . Is fidaxomicin worth the cost? An economic analysis. Clin Infect Dis 2013; 57: 555–61. 10.1093/cid/cit346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reveles KR, Backo JL, Corvino FAet al. . Fidaxomicin versus vancomycin as a first-line treatment for Clostridium difficile–associated diarrhea in specific patient populations: a pharmacoeconomic evaluation. Pharmacotherapy 2017; 37: 1489–97. 10.1002/phar.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rajasingham R, Enns EA, Khoruts Aet al. . Cost-effectiveness of treatment regimens for Clostridioides difficile infection: an evaluation of the 2018 infectious diseases society of America guidelines. Clin Infect Dis 2020; 70: 754–62. 10.1093/cid/ciz318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner M, Lavoie L, Goetghebeur M. Clinical and economic consequences of vancomycin and fidaxomicin for the treatment of Clostridium difficile infection in Canada. Can J Infect Dis Med Microbiol 2014; 25: 87–94. 10.1155/2014/793532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guery B, Menichetti F, Anttila V-Jet al. . Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis 2018; 18: 296–307. 10.1016/S1473-3099(17)30751-X [DOI] [PubMed] [Google Scholar]
- 17. U.S. Bureau of Labor Statistics . CPI Inflation Calculator. https://www.bls.gov/data/inflation_calculator.htm.
- 18. Bank of Canada . Inflation Calculator. https://www.bankofcanada.ca/rates/related/inflation-calculator/.
- 19. Wei L. CURVEFIT: stata module to produces curve estimation regression statistics and related plots between two variables for alternative curve estimation regression models. Statistical Software Components 2020. https://ideas.repec.org/c/boc/bocode/s457136.html. [Google Scholar]
- 20. Luo Y, Lucas AL, Grinspan AM. Fecal transplants by colonoscopy and capsules are cost-effective strategies for treating recurrent Clostridioides difficile infection. Dig Dis Sci 2020; 65: 1125–33. 10.1007/s10620-019-05821-1 [DOI] [PubMed] [Google Scholar]
- 21. Rodrigues R, Barber GE, Ananthakrishnan AN. A comprehensive study of costs associated with recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol 2017; 38: 196–202. 10.1017/ice.2016.246 [DOI] [PubMed] [Google Scholar]
- 22. Desai K, Gupta SB, Dubberke ERet al. . Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis 2016; 16: 303. 10.1186/s12879-016-1610-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang D, Prabhu VS, Marcella SW. Attributable healthcare resource utilization and costs for patients with primary and recurrent Clostridium difficile infection in the United States. Clin Infect Dis 2018; 66: 1326–32. 10.1093/cid/cix1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zilberberg MD, Shorr AF, Jesdale WMet al. . Recurrent Clostridium difficile infection among medicare patients in nursing homes: a population-based cohort study. Medicine (Baltimore) 2017; 96: e6231. 10.1097/MD.0000000000006231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McFarland LV, Surawicz CM, Rubin Met al. . Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol 1999; 20: 43–50. 10.1086/501553 [DOI] [PubMed] [Google Scholar]
- 26. Feuerstadt P, Stong L, Dahdal DNet al. . Healthcare resource utilization and direct medical costs associated with index and recurrent Clostridioides difficile infection: a real-world data analysis. J Med Econ 2020; 23: 603–9. 10.1080/13696998.2020.1724117 [DOI] [PubMed] [Google Scholar]
- 27. Lapointe-Shaw L, Tran KL, Coyte PCet al. . Cost-effectiveness analysis of six strategies to treat recurrent Clostridium difficile infection. PLoS ONE 2016; 11: e0149521. 10.1371/journal.pone.0149521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh H, Nugent Z, Walkty Aet al. . Direct cost of health care for individuals with community associated Clostridium difficile infections: a population-based cohort study. PLoS ONE 2019; 14: e0224609. 10.1371/journal.pone.0224609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mulcahy AW, Schwam D, Rao Pet al. . Estimated savings from international reference pricing for prescription drugs. JAMA 2021; 326: 1744–5. 10.1001/jama.2021.13338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McDaniel LF, White MN, Obi ENet al. . Clinical and economic outcomes after implementation of a fidaxomicin treatment optimization and access pathway at a US hospital system. Infect Dis Ther 2022. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gallagher JC, Reilly JP, Navalkele Bet al. . Clinical and economic benefits of fidaxomicin compared to vancomycin for Clostridium difficile infection. Antimicrob Agents Chemother 2015; 59: 7007–10. 10.1128/AAC.00939-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McDonald EG, Milligan J, Frenette Cet al. . Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med 2015; 175: 784–91. 10.1001/jamainternmed.2015.42 [DOI] [PubMed] [Google Scholar]
- 33. Cornely OA, Watt M, McCrea Cet al. . Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients aged ≥60 years (EXTEND): analysis of cost-effectiveness. J Antimicrob Chemother 2018; 73: 2529–39. 10.1093/jac/dky184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.