Abstract
Objectives
To analyse the prevalence of respiratory tract infection (RTI) episodes with and without antibiotic prescriptions in adult patients in Norwegian general practice during the period 2012–2019.
Methods
Observational study linking data from the Norwegian Control and Payment for Health Reimbursements Database and the Norwegian Prescription Database. Episodes of acute RTIs in patients aged 18 years or older were identified and linked to antibiotic prescriptions dispensed within 7 days after diagnosis. We analysed annual infection rates and antibiotic prescription rates and antibiotics prescribed for the different RTI conditions.
Results
RTI episode rate per 1000 inhabitants was 312 in 2012 and 277 in 2019, but showed no linear trend of change during the study period (P = 0.205). Antibiotic prescription rate decreased from 37% of RTI episodes in 2012 to 23% in 2019 (P < 0.001). The reduction in prescribing was most pronounced for episodes coded with ICPC-2 symptom diagnoses, as well as upper RTIs, influenza, acute bronchitis and sinusitis. Prescriptions for phenoxymethylpenicillin decreased from 178 746 in 2012 to 143 095 in 2019, but increased as proportion of total antibiotic prescriptions from 40% in 2012 to 53% in 2019 (P < 0.001).
Conclusions
This study demonstrates stable RTI episode rates and reduced antibiotic prescription rates for RTIs for adults in Norwegian general practice 2012–2019. We also observed a shift towards relatively more use of phenoxymethylpenicillin and less broad-spectrum antibiotics. These changes are in line with the aims of the Norwegian strategy against antibiotic resistance.
Introduction
Antimicrobial resistance (AMR) is a global threat to public health.1 Excessive consumption of antibiotics is a main driver for antibiotic resistance development at both individual and community levels.2–4 Antibiotic stewardship aims to reduce inappropriate and unnecessary use of antibiotics to reduce AMR and preserve the therapeutic effect of antibiotics. Norwegian health authorities launched a National Strategy for Antibiotic Resistance in 2015, aiming to reduce the national antibiotic consumption by 30% expressed as defined daily dose (DDD) per 1000 inhabitants per day (DID) and reduce antibiotic prescriptions for respiratory tract infections (RTIs) by 20% by year 2020 compared to 2012 levels.5 Following the national strategy, the health authorities facilitated several voluntary quality improvement projects aiming for more appropriate prescribing in Norwegian hospitals and general practice. Among these projects, an audit-based course for primary care doctors was implemented in 2018,6 based on methods proven to be effective in a primary care setting.7,8
In Norway, 84% of all antibiotics for human use are prescribed outside hospitals and nursing homes.9 Most of these prescriptions are usually attributed to general practitioners (GPs).9 Thus, GPs have a key role for the appropriateness of antibiotic use. RTIs account for approximately half of all antibiotic prescriptions in Norway.9 However, many of the RTIs seen in general practice are viral or self-limiting bacterial infections, and antibiotic treatment for mild infections, such as sinusitis and sore throat, have no or only a marginal effect.10,11 As well as prescribing an antibiotic when this is not warranted, a suboptimal choice of antibiotic is also considered as inappropriate antibiotic prescribing. The current national guidelines for Norwegian primary care recommend phenoxymethylpenicillin (PcV) as a first-line antibiotic for almost all acute RTIs,12 and beta-lactamase sensitive penicillin as percentage of the total antibiotic consumption is a national and European quality indicator.13,14 Studies on overprescribing in general practice have identified high proportions of inappropriate prescribing.15–17
From 2012 to 2019, Norway had achieved a 22% decrease in total human antibiotic consumption based on sales statistics from primary care, hospitals and nursing homes.9 In 2019, the human antibiotic consumption in the community was 13.6 DID, while the European mean was 18.0 DID.18 Norway has a low ratio of broad- to narrow-spectrum antibiotics at 0.1, compared with the European mean ratio at 2.8.18
To inform clinicians, policy makers and stewardship programmes, and to better understand the changes in antibiotic use, it is essential to monitor both the number of patients seeking their GP for infections, and the corresponding antibiotic prescriptions. In this study we aimed to analyse the prevalence of RTI episodes in adult patients treated in Norwegian general practice and the rate of dispensed antibiotic prescriptions, DDD per prescription and type of antibiotic for RTI treatment during the period 2012–2019.
Materials and methods
Ethics
The study was approved by the Regional Committee for Medical and Health Research Ethics, REC South East (ref. 2016/2283), and the Norwegian Data Protection Authority (ref. 282558). The study was conducted in accordance with the Declaration of Helsinki and institutional standards.
Design and setting
We conducted an observational study linking data from nationwide health registries. The study period was from 2012 to 2019. All Norwegian residents have access to public healthcare through the National Insurance Scheme and are assigned to a regular GP. In 2015, 99% of the population was assigned to a regular GP list, and most of the Norwegian population use their GP or the municipal GP-staffed out-of-hours (OOH) services when seeking medical help.19 In Norway, antibiotics are available through prescription only.
Data sources
The Norwegian Control and Payment for Health Reimbursements (KUHR) Database receives compensation claims from GPs and OOH services.20 For each claim, the database contains ID for patient and physician, date of contact and one or two diagnoses per contact according to the International Classification of Primary Care (ICPC-2).21 We obtained claims for all registered contacts (consultations by attendance, telephone contacts and e-consultations) from GPs and OOH services with an infection related ICPC-2 diagnosis. The Norwegian Prescription database (NorPD) includes detailed information on all prescription drugs dispensed at Norwegian pharmacies.22 We obtained the following data from NorPD: encrypted IDs for patients and prescribers, prescription date, prescribed drug item categorized according to the Anatomical Therapeutic Chemical Classification (ATC) system, and DDD of prescription in accordance with the ATC/DDD index of 2019.23 Statistics Norway routinely collects demographic and geographic data on the Norwegian population.24 We extracted data on patient sex, and month and year of birth and death (if applicable). Data from the registries were linked using the unique personal identification number (encrypted by the NorPD algorithm) assigned to all Norwegian residents. All databases cover the entire Norwegian population.
Population
We included all patients aged ≥18 years with a relevant diagnosis (see Table S1, available as Supplementary data at JAC-AMR online) recorded during 2012–2019. We selected adult patients as both treatment guidelines and clinical decision-making in paediatric patients differ from adults. Patients were included from 1 January 2012. Further patients were included each month after their 18th birthday, along with new registrants in the KUHR database, and follow-up ended at study completion (31 December 2019) or the month of the patient’s death.
RTI episode definition and antibiotic prescriptions
As each patient could have more than one GP contact during the course of an infection, we investigated episodes of acute RTIs rather than consultations. The episode start date (index date) was defined as the date of diagnosis if no RTI diagnoses had been recorded within the previous 30 days. If there were less than 30 days between diagnoses, the latter diagnosis was defined as a re-consultation and assigned to the initial episode. The 30-day period was based on previous comparable studies on RTI episodes.25–28 For each acute RTI episode, follow-up ended 90 days after the index date. If an episode had more than one of the included diagnoses recorded during the follow-up, we selected the diagnosis most likely to receive an antibiotic prescription as main diagnosis (Table S1). Episodes were defined as symptom-related or infection-specific according to the ICPC-2 classification system, where codes R01-29 are used for respiratory symptoms/complaints, and R71-81 and R83 are used for particular RTIs. Episodes with infectious diagnoses not related to the respiratory tract but which may indicate antibiotic treatment (e.g. urinary tract infections, skin- and soft tissue infections), were excluded.
We included antibacterial agents for oral use (ATC code J01) excluding methenamine, nitrofurantoin, (piv)mecillinam and trimethoprim, because in Norway, these antibiotics are exclusively prescribed for urinary tract infections. For each RTI episode, we analysed the first antibiotic prescription dispensed within 7 days following a GP consultation. Antibiotic classes were grouped as tetracyclines (ATC code J01AA), PcV (J01CE02), other penicillins (J01C except J01CE02), macrolides (J01FA) and other antibiotics.
Statistical analyses
Descriptive statistics were calculated with means and proportions for each year and for the entire study period. Age was analysed in groups from 18–24 years, then 10-year age groups up to >85 years. Annual RTI episode rates were calculated by dividing the number of episodes by number of inhabitants ≥18 years in the year of index date. To account for changes in the population, rates were age-and-sex-standardized using direct standardization using the Norwegian 2012 population as reference. The study mean RTI episode rates presented are the mean of yearly episode rates for the 8 year study period. Prescription rate was calculated as the proportion of episodes resulting in at least one dispensed antibiotic prescription. Relative proportions of the different antibiotic classes were calculated.
For annual trends, we used linear regression with standardized yearly RTI episode rates over year for all diagnoses individually, and for total RTI episodes. Linear regression over year was also conducted for yearly prescription rate, DDD per prescription and proportion of each antibiotic class. Coefficients from the regressions are presented as mean yearly change with 95% CI.
To test for differences in mean contacts per episode, we used the Mann–Whitney U-test to compare sexes and non-parametric ANOVA (Kruskal–Wallis test) to compare age groups. Chi-squared tests were used to compare differences between sexes and between age groups in prescription rates and penicillin proportion. As we observed different patterns in episode rates for age groups between men and women, we ran a linear regression with yearly episode rate over age group for each sex to test for linear trends.
The significance level was set to 0.05. Means are presented with standard deviation (SD). STATA/SE v.16.1 (StataCorp LLC) was used for all calculations.
Results
RTI episodes
During the study period, 2 931 421 adult patients were diagnosed with at least one RTI in general practice. At first consultation, 54% were women, mean age was 45.5 years (SD 19.5) (Table 1). Sex and age distribution did not change substantially over time. In total, 14 209 411 RTI contacts were registered during the study period. According to our definition, this amounted to 9 181 118 acute RTI episodes. Seven out of ten episodes, 70% (6 397 673) had only one consultation, 18% (1 692 388) had two, while 12% (1 091 057) had three or more consultations. Mean number of contacts per RTI episode was 1.5 (SD 1.1) for men and 1.6 (SD 1.2) for women (P < 0.001), and did not change noticeably during the study period.
Table 1.
Acute RTI episodes in adult patients treated in Norwegian general practice 2012–2019
| Total | Females | Males | |
|---|---|---|---|
| Patients | 2 931 421 | 1 591 663 | 1 339 758 |
| % | 54% | 46% | |
| Mean age (SD) | 45.5 (19.5) | 45.6 (19.8) | 45.3 (19.2) |
| RTI consultations | 14 209 411 | 8 541 093 | 5 668 318 |
| RTI episodes | 9 181 118 | 5 422 569 | 3 758 549 |
| Mean number of consultations per episode (SD) | 1.5 (1.1) | 1.6 (1.2) | 1.5 (1.1) |
| Mean annual RTI episodes per 1000 inhabitants | 284 | 335 | 232 |
| By age group | |||
| 18–24 | 326 | 395 | 258 |
| 25–34 | 286 | 353 | 218 |
| 35–44 | 278 | 337 | 218 |
| 45–54 | 263 | 317 | 208 |
| 55–64 | 283 | 340 | 226 |
| 65–74 | 276 | 307 | 245 |
| 75–84 | 300 | 306 | 293 |
| 85+ | 311 | 277 | 344 |
| By infection (six most common diagnoses) | |||
| URTI (R74)a | 65 | 80 | 52 |
| Sinusitis (R75)a | 26 | 37 | 16 |
| Tonsillitis (R72 + R76)a | 12 | 15 | 10 |
| Acute bronchitis (R78)a | 27 | 32 | 22 |
| Pneumonia (R81)a | 21 | 22 | 21 |
| Influenza (R80)a | 19 | 21 | 17 |
ICPC-2 codes.
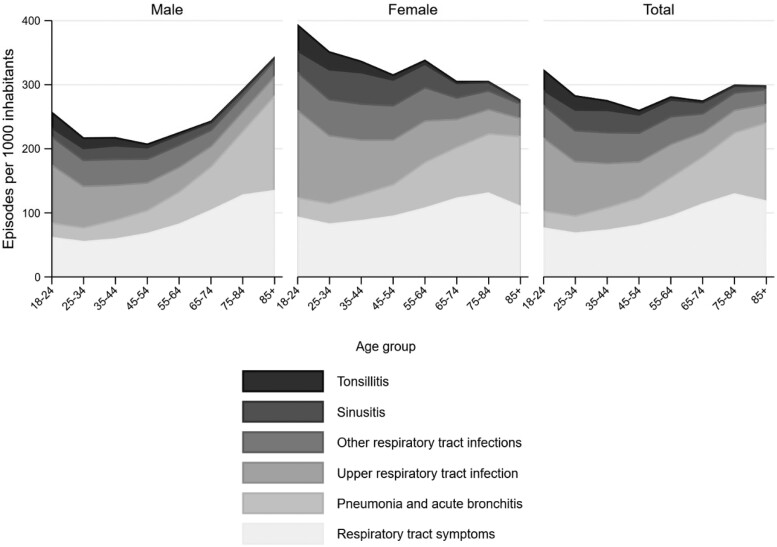
Women had more episodes than men (Table 1), and the number of yearly RTI episodes per 1000 inhabitants varied between age groups for both men and women, with episode rates increasing by age for men (P of linear trend <0.001) and decreasing by age for women (P of linear trend <0.001) (Figure 1). The number of consultations per episode increased with age, from 1.4 (SD 0.9) consultations per episode in the youngest age group, to 1.7 (SD 1.4) in the oldest age group (P for difference <0.001). Mean age of patients with pneumonia was 61.7 years (SD 19.3), for sinusitis 43.8 years (SD 15.4), for upper respiratory tract infection (URTI) 41.1 years (SD 17.4), and for tonsillitis 33.4 years (SD 13.1).
Figure 1.
Age and sex distribution of mean annual acute respiratory tract episodes per 1000 adult inhabitants per year in Norwegian general practice 2012–2019.
There were 1 205 782 RTI episodes in 2012, and 1 165 545 in 2019. Accounting for population changes, the age- and sex-standardized yearly RTI episode rates per 1000 adult inhabitants were 312 in 2012 and 277 in 2019. However, we did not observe a significant linear decrease (P = 0.205) (Table 2). URTI (ICPC code R74) was the most commonly used diagnosis, and the use of this diagnosis increased during the study period (P of linear trend = 0.003). The use of all other infection-specific ICPC-2 codes except acute bronchitis (R78), influenza (R80) and other RTI (R83) decreased (Table S1). Symptom-related diagnoses (R1–29) accounted for 30% of all episodes in 2012 and 33% of all episodes in 2019.
Table 2.
Respiratory tract infection episode rates and corresponding antibiotic prescription rates for the six most common RTI diagnoses and the corresponding symptoms in adult patients in Norwegian general practice 2012–2019
| Study mean | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Mean annual changea (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|
| All RTIs | ||||||||||
| Episode rateb | 284 | 312 | 285 | 266 | 284 | 284 | 281 | 280 | 277 | −2.6 (−7.1 to 1.9) |
| Antibiotic prescription ratec | 29 | 37 | 34 | 33 | 30 | 28 | 25 | 23 | 23 | −2.1 (−2.4 to −1.7)d |
| PcV proportion (%)e | 47 | 40 | 44 | 45 | 46 | 48 | 51 | 52 | 53 | 1.8 (1.5 to 2.1)d |
| Diagnosis-specific ICPC-2 codes | ||||||||||
| Episode rate | 193 | 218 | 197 | 179 | 193 | 193 | 191 | 192 | 187 | −2.6 (−6.4 to 1.3) |
| Antibiotic prescription rate | 37 | 45 | 42 | 42 | 38 | 36 | 33 | 30 | 31 | −2.3 (−2.8 to −1.9)d |
| PcV proportion (%) | 48 | 42 | 46 | 46 | 47 | 50 | 53 | 54 | 55 | 1.8 (1.5 to 2.2)d |
| Symptom-related ICPC-2 codes | ||||||||||
| Episode rate | 90 | 93 | 87 | 87 | 91 | 91 | 90 | 88 | 91 | −0.1 (−0.9 to 0.8) |
| Antibiotic prescription rate | 12 | 17 | 14 | 13 | 12 | 11 | 9 | 9 | 8 | −1.2 (−1.4 to −1.0)d |
| PcV proportion (%) | 35 | 30 | 33 | 35 | 35 | 36 | 37 | 38 | 39 | 1.2 (0.9 to 1.4)d |
| URTI (R74) | ||||||||||
| Episode rate | 65 | 63 | 59 | 58 | 63 | 65 | 70 | 71 | 75 | 2.2 (1.1 to 3.3)d |
| Antibiotic prescription rate | 18 | 24 | 22 | 21 | 19 | 17 | 15 | 14 | 14 | −1.6 (−1.9 to −1.3)d |
| PcV proportion (%) | 56 | 50 | 54 | 54 | 55 | 57 | 60 | 62 | 62 | 1.7 (1.3 to 2.1)d |
| Sinusitis (R75) | ||||||||||
| Episode rate | 26 | 32 | 30 | 27 | 27 | 25 | 24 | 22 | 22 | −1.5 (−1.7 to −1.2)d |
| Antibiotic prescription rate | 57 | 65 | 64 | 61 | 59 | 55 | 51 | 49 | 49 | −2.7 (−3.1 to −2.3)d |
| PcV proportion (%) | 50 | 46 | 47 | 48 | 49 | 51 | 55 | 56 | 57 | 1.7 (1.3 to 2.2)d |
| Sinus symptoms (R09) | ||||||||||
| Episode rate | 5 | 5 | 5 | 5 | 5 | 6 | 6 | 5 | 6 | 0.2 (0.1 to 0.3)d |
| Antibiotic prescription rate | 24 | 32 | 31 | 28 | 27 | 24 | 20 | 19 | 19 | −2.1 (−2.5 to −1.8)d |
| PcV proportion (%) | 44 | 41 | 42 | 42 | 43 | 43 | 46 | 47 | 48 | 1.0 (0.7 to 1.4)d |
| Tonsillitis (R72 + R76) | ||||||||||
| Episode rate | 12 | 15 | 13 | 13 | 12 | 12 | 12 | 11 | 11 | −0.5 (−0.7 to −0.3)d |
| Antibiotic prescription rate | 76 | 76 | 76 | 76 | 77 | 76 | 75 | 74 | 76 | −0.2 (−0.4 to 0.0) |
| PcV proportion (%) | 83 | 80 | 81 | 81 | 82 | 84 | 86 | 86 | 87 | 1.1 (0.9 to 1.3)d |
| Throat symptoms (R21) | ||||||||||
| Episode rate | 15 | 16 | 15 | 15 | 15 | 15 | 15 | 14 | 15 | −0.1 (−0.2 to 0.1) |
| Antibiotic prescription rate | 15 | 20 | 19 | 18 | 17 | 15 | 13 | 12 | 11 | −1.3 (−1.5 to −1.2)d |
| PcV proportion (%) | 66 | 64 | 64 | 66 | 66 | 67 | 69 | 69 | 70 | 0.8 (0.7 to 1.0)d |
| Acute bronchitis (R78) | ||||||||||
| Episode rate | 27 | 31 | 26 | 26 | 30 | 30 | 28 | 24 | 22 | −0.8 (−1.8 to 0.2) |
| Antibiotic prescription rate | 44 | 57 | 53 | 50 | 45 | 41 | 36 | 34 | 36 | −3.4 (−4.1 to −2.6)d |
| PcV proportion (%) | 25 | 20 | 23 | 24 | 25 | 27 | 30 | 30 | 29 | 1.5 (1.0 to 2.0)d |
| Pneumonia (R81) | ||||||||||
| Episode rate | 21 | 29 | 23 | 21 | 22 | 21 | 21 | 19 | 17 | −1.2 (−1.9 to −0.5)d |
| Antibiotic prescription rate | 66 | 68 | 66 | 67 | 67 | 67 | 66 | 64 | 65 | −0.4 (−0.6 to −0.1)d |
| PcV proportion (%) | 40 | 34 | 38 | 38 | 40 | 42 | 44 | 45 | 45 | 1.6 (1.1 to 2.0)d |
| Cough (R05) | ||||||||||
| Episode rate | 37 | 41 | 37 | 35 | 37 | 37 | 37 | 35 | 34 | −0.6 (−1.2 to 0.0) |
| Antibiotic prescription rate | 13 | 20 | 16 | 15 | 14 | 12 | 10 | 9 | 9 | −1.5 (−1.8 to −1.2)d |
| PcV proportion (%) | 20 | 16 | 19 | 19 | 20 | 21 | 23 | 24 | 24 | 1.1 (0.9 to 1.3)d |
| Influenza (R80) | ||||||||||
| Episode rate | 19 | 19 | 22 | 13 | 20 | 20 | 18 | 26 | 16 | 0.2 (−1.3 to 1.7) |
| Antibiotic prescription rate | 7 | 9 | 8 | 8 | 7 | 7 | 6 | 5 | 5 | −0.6 (−0.7 to −0.5)d |
| PcV proportion (%) | 41 | 35 | 39 | 39 | 39 | 43 | 43 | 45 | 44 | 1.2 (0.7 to 1.7)d |
Mean annual change represents coefficient from linear regression with 95% CI.
Episode rate: episodes per 1000 inhabitants adjusted for age and sex.
Antibiotic prescription rate: proportion of episodes receiving ≥1 prescription within 7 days.
P value <0.05.
PcV proportion (%): phenoxymethylpenicillin as proportion of all first prescriptions.
Antibiotic prescriptions
Antibiotics were prescribed for 29% (2 659 088) of all RTI episodes. Most prescriptions (72%; 1 922 967) were dispensed after the first GP contact, and 17% (461 404) after the second contact of the episode. Prescriptions dispensed the same day as the GP consultation comprised 83% (2 201 251) of all, whereas 9% (245 619) were dispensed on day two and 8% (212 218) between day 3 and 7. The overall prescription rates varied between age groups, with the least prescribing (27.3% of episodes) in 45–54-year-olds and the most (30.7% of episodes) in 65–64-year-olds during the study period (P for difference of 0.001).
The antibiotic prescription rate declined from 37% (443 633/1 205 782) in 2012 to 23% (271 149/1 165 545) in 2019, corresponding to a 37% decrease throughout the study period (P of linear trend <0.001) (Table 2). Reduced yearly prescription rates were observed in all age groups for both men and women.
PcV accounted for 47% of the antibiotic prescriptions during the study period; followed by macrolides (22%) and tetracyclines (17%) (Table 3). Although the absolute number of episodes receiving PcV prescriptions decreased from 178 746 in 2012 to 143 095 in 2019, PcV as a proportion of all antibiotic prescriptions increased from 40% in 2012 to 53% in 2019 (P of linear trend <0.001) (Table 3). Macrolide use declined throughout the study period from 28% of all in 2012 to 15% in 2019 (P of linear trend <0.001).
Table 3.
Dispensed prescriptions for acute RTIs adult patients in Norwegian general practice 2012–2019
| Total | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Mean annual changea (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|
| RTI episodes with ≥1 dispensed AB prescription | 2 659 088 | 443 633 | 376 136 | 345 585 | 344 393 | 320 045 | 292 444 | 265 703 | 271 149 | |
| Mean DDD per prescription (SD) | 12.1 (5.3) | 11.6 (5.1) | 11.7 (5.2) | 11.9 (5.3) | 12.1 (5.3) | 12.2 (5.3) | 12.4 (5.5) | 12.5 (5.5) | 12.5 (5.4) | 0.1 (0.1 to 0.1)b |
| Phenoxymethylpenicillin J01CE02 | ||||||||||
| Proportion of first prescriptions (%) | 47 | 40 | 44 | 45 | 46 | 48 | 51 | 52 | 53 | 1.8 (1.5 to 2.1)b |
| Mean DDD per prescription (SD) | 14.1 (4.5) | 13.7 (4.3) | 13.7 (4.3) | 13.9 (4.5) | 14.3 (4.6) | 14.2 (4.6) | 14.2 (4.6) | 14.3 (4.7) | 14.3 (4.6) | 0.1 (0.1 to 0.1)b |
| Other penicillins J01C (−CE02) | ||||||||||
| Proportion of first prescriptions (%) | 11 | 9 | 10 | 10 | 11 | 11 | 11 | 12 | 12 | 0.5 (0.4 to 0.6)b |
| Mean DDD per prescription (SD) | 9.1 (2.7) | 9.0 (3.1) | 9.1 (2.7) | 9.2 (2.5) | 9.1 (2.5) | 9.1 (2.6) | 9.2 (2.6) | 9.2 (2.5) | 9.4 (2.7) | 0.0 (0.0 to 0.0)b |
| Macrolides J01FA | ||||||||||
| Proportion of first prescriptions (%) | 22 | 28 | 24 | 24 | 22 | 20 | 18 | 16 | 15 | −1.8 (−2.0 to −1.5)b |
| Mean DDD per prescription (SD) | 8.2 (2.5) | 8.4 (3.3) | 8.2 (3.5) | 8.2 (3.5) | 8.2 (3.3) | 8.1 (3.1) | 8.2 (4.2) | 8.2 (3.6) | 8.4 (3.8) | −0.0 (−0.0 to −0.0)b |
| Tetracyclines J01A | ||||||||||
| Proportion of first prescriptions (%) | 17 | 19 | 18 | 18 | 18 | 17 | 16 | 16 | 16 | −0.5 (−0.7 to −0.3)b |
| Mean DDD per prescription (SD) | 14.1 (6.4) | 14.1 (6.1) | 14.0 (6.3) | 14.2 (6.5) | 14.1 (6.3) | 14.2 (6.4) | 14.2 (6.6) | 14.3 (6.8) | 14.1 (6.9) | 0.0 (0.0 to 0.0)b |
| Other antibiotics | ||||||||||
| Proportion of first prescriptions (%) | 4 | 3 | 4 | 4 | 4 | 4 | 3 | 4 | 4 | 0.0 (−0.1 to 0.1) |
| Mean DDD per prescription (SD) | 7.5 (4.0) | 7.5 (4.0) | 7.5 (3.8) | 7.6 (4.1) | 7.6 (4.0) | 7.6 (3.8) | 7.6 (4.0) | 7.6 (4.5) | 7.6 (4.3) | 0.0 (0.0 to 0.0)b |
Mean annual change represents coefficient from linear regression with 95% CI.
P value <0.05.
The mean DDD per prescription for all antibiotics was 12.1 (SD 5.3) during the study period. Overall, mean DDD per prescription increased from 11.6 DDD (SD 5.1) in 2012 to 12.5 DDD (SD 5.4) in 2019 (P of linear trend <0.001). Mean DDD per prescription increased for PcV from 13.7 (SD 4.3) in 2012 to 14.3 (SD 4.6) in 2019 (P < 0.001) and for other penicillins from 9.0 (SD 3.1) in 2012 to 9.4 (SD 2.7) in 2019 (P < 0.001) (Table 3).
Younger patients were more frequently treated with PcV. Patients aged 18–24 years received PcV in 64% of first prescriptions, whereas corresponding figures for patients aged 75–84 years and older than 85 years were 35% and 39%, respectively (P for difference <0.001). Men received a slightly higher proportion of PcV compared with women, with 47% versus 46% of all prescriptions (P for difference <0.001).
The highest prescription rates were seen for tonsillitis (76%), pneumonia (66%) and otitis media (65%). From 2012 to 2019, the prescription rates decreased significantly for all infection-specific diagnoses except for tonsillitis and whooping cough (Table S1). The largest relative reduction was seen for URTI, influenza, acute bronchitis and sinusitis (Table 2).
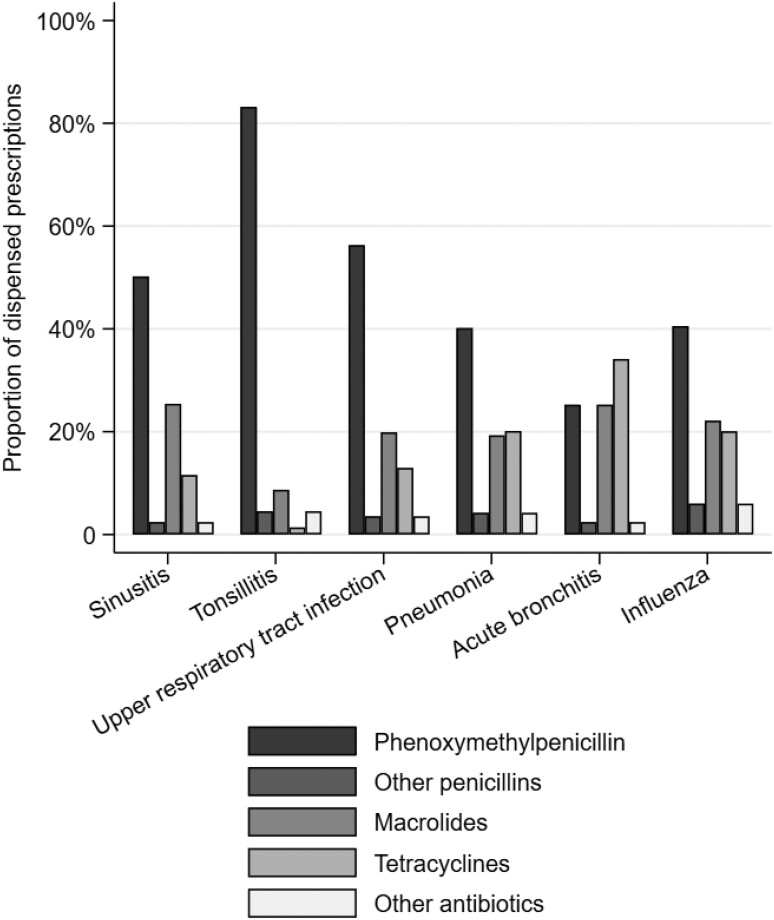
Figure 2 illustrates differences in use of antibiotic classes between the six most common infection-specific diagnoses. Acute tonsillitis was almost exclusively treated with PcV (83% of prescriptions), macrolides were issued in one out of four patients with sinusitis or acute bronchitis, while tetracyclines were prescribed in 34% and 20% of prescriptions for acute bronchitis and pneumonia, respectively. PcV as a proportion of all prescriptions increased significantly for all infection-specific diagnoses except whooping cough and laryngitis/tracheitis (Table S1).
Figure 2.
Antibiotic groups of dispensed prescriptions for the six most common RTI diagnoses in Norwegian general practice during 2012–2019.
Discussion
Main findings
This registry-based study demonstrated a stable rate of acute RTI episodes and a decreased antibiotic prescription rate for RTIs in adults in Norwegian general practice between 2012 and 2019. The antibiotic prescription rate decreased by almost 40% in 2019 compared with 2012. Reduced prescribing rates were seen for all RTI diagnoses except tonsillitis and whooping cough. PcV was the most frequently prescribed antibiotic for RTIs, and a relative increase in PcV use was seen compared to other antibiotics.
Comparison with other studies
Although we did not find a linear decrease in RTI episodes, we observed a change from 312 episodes per 1000 inhabitants in 2012 to 277 per 1000 in 2019. Norway experienced an epidemic of mycoplasma pneumoniae during the winter of 2011–2012,29 which explains a high episode rate and a relatively high proportion of macrolides issued in 2012. A recent Norwegian study covering 2006–2015 reported an increased number of consultations in primary care, but a decreased proportion of RTI consultations.30 Studies from Swedish general practice from the same decade reported decreased consultation rates for RTIs and a corresponding decrease in antibiotic prescription rates.26,31 Also, both Danish and English primary care research have reported decreased antibiotic prescription rates.32,33
Women had more RTI episodes and more contacts per episode than men, which is in line with findings from Swedish general practice.34 Interestingly, we observed that RTI episode rates increased with age for men, while decreasing with age for women. For both men and women, we observed relatively high RTI episode rates in the youngest age group mostly made up by patients aged 18 and 19 years, especially from 2016. This may reflect an absentee regulation for high school students implemented in 2016, urging students to see their GP to get a certificate of absence when unable to attend class.35 This may potentially contribute to antibiotic overuse, due to the positive association between RTI consultation rates and antibiotic prescription rates.36,37
Older patients had on average more consultations per episode, and were diagnosed with more severe infections than younger patients. However, we did not find higher antibiotic prescription rates in the oldest age groups, consistent with previous studies on antibiotic use in the community.38 An explanation for this is that our dataset did not include data from either hospitals or nursing homes. Elderly patients with more severe RTIs are more commonly treated in hospitals and about 40 000, mostly frail, are living in nursing homes.
We observed a relative increase in the use of symptom-related diagnoses (ICPC-2 R01-29) compared with infection-specific diagnoses (ICPC-2 R71-83) as well as increased use of the diagnosis URTI (R74). We assume that physicians tend to use more symptom-related diagnostic codes when deciding not to prescribe antibiotics and vice versa, on the basis of studies showing that the intention to prescribe influences the diagnostic labelling.39 However, although the reduction in antibiotic prescription rate was larger for symptom-related episodes, we saw a significant decrease in prescriptions for the infection-specific diagnoses.
The increased use of the URTI diagnosis could partly explain the reduction in sinusitis episodes, as URTI and sinusitis have overlapping symptoms and accurate diagnosing of bacterial sinusitis in general practice is challenging.40 We observed a decrease in sinusitis episode rates of 32%, combined with a reduction in antibiotic prescription rate of 26% while the proportion of PcV increased by 24%. Similar changes were observed for acute bronchitis. Both sinusitis and acute bronchitis have been specifically targeted by the Norwegian stewardship interventions in general practice.
Our finding that PcV was the most frequently prescribed antibiotic for RTIs is in line with studies from general practice in Sweden and Denmark.26,41 This is also reflected in the high proportions of narrow-spectrum penicillins out of the total antibiotic consumption in Scandinavia, with 21% in Norway, 27% in Denmark and 28% in Sweden.14 Although macrolide use decreased, it was still the second most commonly used antibiotic for RTIs accounting for 22% of prescriptions in the study period. This corresponds well with figures from Danish and British general practice.41,42 According to the Norwegian guidelines for antibiotic treatment of RTIs in primary care, macrolides are only recommended as first choice empirical treatment for atypical pneumonia, whooping cough and as an alternative to PcV in patients with penicillin allergy.12 Penicillin allergy is frequently self-reported by patients on the basis rashes and/or gastrointestinal symptoms, and although 8% of the population in the USA carries a history of penicillin allergy, <1 in 20 were really allergic when tested.43 Unverified penicillin allergy could represent a public health problem leading to use of unnecessary broad-spectrum antibiotics. Our results support the fact that there is still potential to further reduce macrolide prescribing in Norway.
Strengths and limitations
The major strength of our study is the size of the dataset covering the entire Norwegian population. Linking data from different national registries by individual personal identification number gives the opportunity to obtain information across the public health system. However, the KUHR database does not receive any compensation claims from hospitals, nursing homes or commercial health care providers. There are currently no available statistics regarding the use of commercial primary care providers in Norway, but it is so far considered to be of negligible size.
To include all acute RTI episodes, we obtained all registered contacts, including phone and electronic communication. The diagnoses were obtained from the national reimbursement scheme that contain only one or two recorded diagnoses per claim, which in turn relies on the coding practices of the GPs. The ICPC-2 coding is necessary to generate compensation claims, and is not intended for research. A limitation of the KUHR database, compared to data from patient records, is the lack of information on clinical presentation and patient comorbidity. However, a reasonably good correspondence between medical record text and diagnosis coding in Norwegian general practice has been reported.44
NorPD data includes dispensed antibiotics only, and the number of prescriptions not dispensed therefore remains unknown. That 8% of the prescriptions were dispensed between day 3 and 7 may represent delayed prescriptions. Considering that only about 60% of delayed prescriptions are filled at pharmacies, compared to 92% of ordinary antibiotic prescriptions,7 the overall reduction in antibiotic prescriptions for RTIs may partly be attributed to more use of delayed prescribing.
By only including antibiotics dispensed the first week after a GP-diagnosed RTI, we probably reduced the risk of including antibiotics given for other indications. However, the NorPD does not contain diagnoses for each prescription. That we only included the first prescription per RTI episode, may have led to an overestimation of narrow-spectrum penicillin if patients were prescribed a second antibiotic in cases of treatment failure.
The NorPD only contains information on strength and size of the dispensed package of antibiotic, and no information about the prescribed dose or duration of the antibiotic course. We have, therefore, reported DDD per prescription as a standardized estimate of treatment dose and duration for each prescription. For penicillins, we observed increased mean DDDs per prescription during the study period. However, the DDD value defined by the WHO does not necessarily reflect the prescribed daily doses, as the dose vary according to diagnosis and patient characteristics.45 The definition of one DDD for PcV is 2 g,23 whereas the Norwegian primary care guidelines for treatment of pneumonia in adults recommend 4 g for 7 days, resulting in a prescription of 14 DDD.12 The DDD definition for macrolides and tetracyclines correspond better to the guideline recommendations. Thus, a shift towards more use of PcV would lead to increased mean DDD per prescription without necessarily reflecting enlarged prescribed doses.
Implications for policy and practice
The decreased rate of prescriptions per episode indicates that GPs’ prescribing practices contribute to the overall reduction in antibiotic consumption reported by the national surveillance programme.9
So far, efforts to improve prescribing in Norwegian general practice have been time limited and project based. Our findings may reflect effects of these interventions, as reduced prescribing per RTI episode and increased use of PcV indicate an increased adherence to the national guidelines.8,12 The reduction in RTI antibiotic prescribing was substantially larger than the overall reduction in antibiotic use reported in Norway during the period,9 demonstrating that the potential for change in antibiotic use for RTIs is larger than for other infections. To ensure further improvement of antibiotic use, it is important to establish a permanent system for quality improvement of antibiotic prescribing in general practice. It is also crucial to assess whether reducing antibiotic treatment too much may have undesirable effects such as increased rates of complications.
Conclusion
This study demonstrates stable RTI episode rates and decreased antibiotic prescription rates for RTIs for adults in Norwegian general practice during 2012–2019. We also observed a shift towards relatively more use of phenoxymethylpenicillin and less use of broad-spectrum antibiotics. These changes are in line with the aims of the Norwegian AMR strategy.
Supplementary Material
Contributor Information
Marius Skow, Department of General Practice, The Antibiotic Centre for Primary Care, Institute of Health and Society, University of Oslo, Oslo, Norway; Department of General Practice, Institute of Health and Society, University of Oslo, Oslo, Norway.
Guro H Fossum, Department of General Practice, The Antibiotic Centre for Primary Care, Institute of Health and Society, University of Oslo, Oslo, Norway; Department of General Practice, General Practice Research Unit (AFE), Institute of Health and Society, University of Oslo, Oslo, Norway.
Sigurd Høye, Department of General Practice, The Antibiotic Centre for Primary Care, Institute of Health and Society, University of Oslo, Oslo, Norway.
Jørund Straand, Department of General Practice, General Practice Research Unit (AFE), Institute of Health and Society, University of Oslo, Oslo, Norway.
Louise Emilsson, Department of General Practice, Institute of Health and Society, University of Oslo, Oslo, Norway; Vårdcentralen Värmlands Nysäter and Centre for Clinical Research, County Council of Värmland, Varmlands Nysater, 661 95 Karlstad, Sweden; Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; School of Medical Science, University of Örebro, Örebro, Sweden.
Anja Maria Brænd, Department of General Practice, Institute of Health and Society, University of Oslo, Oslo, Norway.
Funding
Norwegian Research Council (grant number 288165) as a part of the BASIC project (Better Treatment for Acute Sinusitis in Primary Care).
Transparency declarations
The authors declare that they have no competing interests. The data were provided by the Norwegian Directorate of Health, the Norwegian Institute of Public Health (NIPH), and Statistics Norway by permission. NIPH linked and anonymized the data, which cannot be shared publicly due to restrictions by the Norwegian Data Protection Authority.
Supplementary data
Table S1 is available as Supplementary data at JAC-AMR online.
References
- 1. World Health Organization . Antimicrobial Resistance: Global Report on Surveillance. Geneva: World Health Organization, 2014. https://www.who.int/publications/i/item/9789241564748 [Google Scholar]
- 2. Bell BG, Schellevis F, Stobberingh Eet al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014; 14: 13. doi: 10.1186/1471-2334-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goossens H, Ferech M, Vander Stichele Ret al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005; 365: 579–87. doi: 10.1016/S0140-6736(05)17907-0 [DOI] [PubMed] [Google Scholar]
- 4. Costelloe C, Metcalfe C, Lovering Aet al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340: c2096. doi: 10.1136/bmj.c2096 [DOI] [PubMed] [Google Scholar]
- 5. Norwegian Ministry of Health and Care Services. National Strategy against Antibiotic Resistance 2015–2020 . https://www.regjeringen.no/contentassets/5eaf66ac392143b3b2054aed90b85210/antibiotic-resistance-engelsk-lavopploslig-versjon-for-nett-10-09-15.pdf
- 6. The Antibiotic Centre for Primary Health Care . The ENORM study. https://www.riktigantibiotika.no/the-enorm-study
- 7. Høye S, Gjelstad S, Lindbæk M. Effects on antibiotic dispensing rates of interventions to promote delayed prescribing for respiratory tract infections in primary care. Br J Gen Pract 2013; 63: e777–86. doi: 10.3399/bjgp13X674468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gjelstad S, Høye S, Straand Jet al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing (Rx-PAD) study). BMJ Brit Med J 2013; 347: f4403. doi: 10.1136/bmj.f4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NORM/NORM-VET . Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. 2019. https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2020/norm-norm-vet-rapport/norm-norm-vet-2019_komplett.pdf.
- 10. Lemiengre MB, van Driel ML, Merenstein D, et al. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst Rev 2018; 9: CD006089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev 2013; 11: CD000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norwegian Directorate of Health. Nasjonal faglig retningslinje for antibiotikabruk i primærhelsetjenesten. [National guidelines for antibiotics, primary care]. https://www.helsedirektoratet.no/retningslinjer/antibiotika-i-primaerhelsetjenesten.
- 13. Norwegian Directorate of Health . Antibiotikabehandling ved luftveisinfeksjon hos befolkningen mellom 10-79 år [Antibiotic treatment for respiratory tract infection in the population between 10–79 years of age]. https://www.helsedirektoratet.no/statistikk/kvalitetsindikatorer/legemidler/antibiotikabehandling-ved-luftveisinfeksjon-hos-befolkningen-mellom-10-79-%C3%A5r
- 14. Adriaenssens N, Bruyndonckx R, Versporten Aet al. Quality appraisal of antibiotic consumption in the community, European Union/European Economic Area, 2009 and 2017. J Antimicrob Chemoth 2021; 76: ii60–7. doi: 10.1093/jac/dkab178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smieszek T, Pouwels KB, Dolk FCK, et al. Potential for reducing inappropriate antibiotic prescribing in English primary care. J Antimicrob Chemoth 2018; 73: ii36–43. doi: 10.1093/jac/dkx500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleming-Dutra KE, Hersh AL, Shapiro DJet al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315: 1864–73. doi: 10.1001/jama.2016.4151 [DOI] [PubMed] [Google Scholar]
- 17. Gjelstad S, Dalen I, Lindbaek M. GPs’ antibiotic prescription patterns for respiratory tract infections–still room for improvement. Scand J Prim Health 2009; 27: 208–15. doi: 10.3109/02813430903438718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. European Centre for Disease Prevention and Control (ECDC) . Antimicrobial consumption in the EU/EEA—Annual Epidemiological Report 2019. 2020.https://www.ecdc.europa.eu/sites/default/files/documents/Antimicrobial-consumption-in-the-EU-Annual-Epidemiological-Report-2019.pdf
- 19. Norwegian Health Network . The right to a doctor. https://www.helsenorge.no/en/gp/om/the-right-to-a-doctor/#General-Practitioner-assistance-in-the-event-of-acute-illness
- 20. Norwegian Directorate of Health . HELFO database. https://www.helsedirektoratet.no/kuhr-databasen
- 21. WHO International Classification of Primary Care, Second edition (ICPC-2) . https://www.who.int/standards/classifications/other-classifications/international-classification-of-primary-care
- 22. Norwegian Institute of Public Health . The Norwegian Prescription Database (NorPD). www.norpd.no/
- 23. World Health Organization . WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index. https://www.whocc.no/atc_ddd_index/
- 24. Statistics Norway. https://www.ssb.no/en
- 25. Pallon J, Sundqvist M, Rööst Met al. Association between bacterial finding, antibiotic treatment and clinical course in patients with pharyngotonsillitis: a registry-based study in primary healthcare in Sweden. BMC Infect Dis 2021; 21: 779. doi: 10.1186/s12879-021-06511-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cars T, Eriksson I, Granath Aet al. Antibiotic use and bacterial complications following upper respiratory tract infections: a population-based study. BMJ Open 2017; 7: e016221. doi: 10.1136/bmjopen-2017-016221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Venekamp RP, Rovers MM, Verheij TJMet al. Treatment of acute rhinosinusitis: discrepancy between guideline recommendations and clinical practice. Fam Pract 2012; 29: 706–12. doi: 10.1093/fampra/cms022 [DOI] [PubMed] [Google Scholar]
- 28. Peng Z, Hayen A, Liu B. Practice- and individual-level antibiotic prescribing associated with antibiotic treatment non-response in respiratory tract infections: a national retrospective observational study. J Antimicrob Chemoth 2021; 76: 804–12. doi: 10.1093/jac/dkaa509 [DOI] [PubMed] [Google Scholar]
- 29. Blix HS, Vestrheim DF, Hjellvik Vet al. Antibiotic prescriptions and cycles of Mycoplasma pneumoniae infections in Norway: can a nationwide prescription register be used for surveillance? Epidemiol Infect 2015; 143: 1884–92. doi: 10.1017/S0950268814002908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larsen L, Wensaas K-A, Emberland KEet al. Respiratory tract infections in Norwegian primary care 2006–2015: a registry-based study. Scand J Prim Health 2022; 40: 173–80. doi: 10.1080/02813432.2022.2069711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tyrstrup M, Beckman A, Mölstad Set al. Reduction in antibiotic prescribing for respiratory tract infections in Swedish primary care—a retrospective study of electronic patient records. BMC Infect Dis 2016; 16: 709. doi: 10.1186/s12879-016-2018-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kristensen PK, Johnsen SP, Thomsen RW. Decreasing trends, and geographical variation in outpatient antibiotic use: a population-based study in Central Denmark. BMC Infect Dis 2019; 19: 337. doi: 10.1186/s12879-019-3964-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bou-Antoun S, Costelloe C, Honeyford Ket al. Age-related decline in antibiotic prescribing for uncomplicated respiratory tract infections in primary care in England following the introduction of a national financial incentive (the quality premium) for health commissioners to reduce use of antibiotics in the community: an interrupted time series analysis. J Antimicrob Chemoth 2018; 73: 2883–92. [DOI] [PubMed] [Google Scholar]
- 34. Cronberg O, Tyrstrup M, Ekblom Ket al. Diagnosis-linked antibiotic prescribing in Swedish primary care—a comparison between in-hours and out-of-hours. BMC Infect Dis 2020; 20: 616. doi: 10.1186/s12879-020-05334-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bakken IJ, Wensaas KA, Furu Ket al. General practice consultations and use of prescription drugs after changes to school absence policy. Tidsskr Nor Laegeforen 2017; 137: 1178–84. doi: 10.4045/tidsskr.17.0427 [DOI] [PubMed] [Google Scholar]
- 36. Walle-Hansen MM, Høye S. Geographic variation in antibiotic consumption—is it due to doctors’ prescribing or patients’ consulting? Antibiotics 2018; 7: 26. doi: 10.3390/antibiotics7010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ashworth M, Latinovic R, Charlton Jet al. Why has antibiotic prescribing for respiratory illness declined in primary care? A longitudinal study using the general practice research database. J Public Health (Oxf) 2004; 26: 268–74. doi: 10.1093/pubmed/fdh160 [DOI] [PubMed] [Google Scholar]
- 38. Akkerman AE, van der Wouden JC, Kuyvenhoven MMet al. Antibiotic prescribing for respiratory tract infections in Dutch primary care in relation to patient age and clinical entities. J Antimicrob Chemoth 2004; 54: 1116–21. doi: 10.1093/jac/dkh480 [DOI] [PubMed] [Google Scholar]
- 39. van Duijn HJ, Kuyvenhoven MM, Tiebosch HMet al. Diagnostic labelling as determinant of antibiotic prescribing for acute respiratory tract episodes in general practice. BMC Fam Pract 2007; 8: 55. doi: 10.1186/1471-2296-8-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ebell MH, McKay B, Dale Aet al. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann Fam Med 2019; 17: 164–72. doi: 10.1370/afm.2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aabenhus R, Hansen MP, Saust LTet al. Characterisation of antibiotic prescriptions for acute respiratory tract infections in Danish general practice: a retrospective registry based cohort study. NPJ Prim Care Respir Med 2017; 27: 37. doi: 10.1038/s41533-017-0037-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun X, Gulliford MC. Reducing antibiotic prescribing in primary care in England from 2014 to 2017: population-based cohort study. BMJ Open 2019; 9: e023989. doi: 10.1136/bmjopen-2018-023989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Macy E. Penicillin and beta-lactam allergy: epidemiology and diagnosis. Curr Allergy Asthma Rep 2014; 14: 476. doi: 10.1007/s11882-014-0476-y [DOI] [PubMed] [Google Scholar]
- 44. Sporaland GL, Mouland G, Bratland Bet al. General practitioners’ use of ICPC diagnoses and their correspondence with patient record notes. Tidsskr Nor Laegeforen 2019; 139: 1468–72. doi: 10.4045/tidsskr.18.0440 [DOI] [PubMed] [Google Scholar]
- 45. Muller A, Monnet DL, Talon Det al. Discrepancies between prescribed daily doses and WHO defined daily doses of antibacterials at a university hospital. Br J Clin Pharmacol 2006; 61: 585–91. doi: 10.1111/j.1365-2125.2006.02605.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.