Abstract
Background
There is insufficient evidence in children and adolescents with human immunodeficiency virus (CAHIV) to guide the timing of antiretroviral treatment (ART) initiation after starting treatment for pulmonary tuberculosis (pTB). To address this knowledge gap, we evaluated the risk of mortality associated with timing of ART initiation in ART-naive CAHIV treated for pTB.
Methods
Data were extracted from electronic medical records of ART-naive patients, aged 0–19 years, who were treated for HIV-associated pTB at Baylor Centers of Excellence in Botswana, Eswatini, Malawi, Lesotho, Tanzania, or Uganda between 2013 and 2020. Data were analyzed against a primary outcome of all-cause mortality with unadjusted Kaplan-Meier curves and Cox proportional hazard models.
Results
The study population included 774 CAHIV with variable intervals to ART initiation after starting TB treatment: <2 weeks (n = 266), 2 weeks to 2 months (n = 398), >2 months (n = 66), and no ART initiated (n = 44). Adjusted Cox proportional hazards models demonstrated increased mortality 1 year from TB treatment initiation in children never starting ART (adjusted HR [aHR]: 2.67; 95% CI: 1.03, 6.94) versus children initiating ART between 2 weeks and 2 months from TB treatment initiation. Mortality risk did not differ for the <2-weeks group (aHR: 1.02; 95% CI: .55, 1.89) versus the group initiating ART between 2 weeks and 2 months.
Conclusions
This retrospective study demonstrated no increase in mortality among CAHIV initiating ART <2 weeks from TB treatment initiation. Given the broad health benefits of ART, this evidence supports the recent WHO recommendation for CAHIV to initiate ART within 2 weeks of initiating TB treatment.
Keywords: tuberculosis, HIV, children, ART
This retrospective study evaluates mortality risk in ART-naive children and adolescents categorized by the timing of ART initiation following tuberculosis (TB) treatment initiation. Our analysis demonstrates no increased mortality when starting ART within 2 weeks of TB treatment initiation.
People with human immunodeficiency virus (PHIV) are 18 times (uncertainty interval: 15 to 21 times) more likely than human immunodeficiency virus (HIV)–negative people to develop tuberculosis (TB) [1]. Children and adolescents with HIV (CAHIV) also experience increased risk of TB following TB exposure, with increased risk in infancy and adolescence [2–5]. Antiretroviral therapy (ART) reduces TB incidence in CAHIV, but the risk remains elevated above that of HIV-negative children [5, 6].
Children and adolescents with HIV also have a higher risk of death due to TB than their HIV-negative peers. Recent programmatic data from cohorts in Kenya and South Africa suggest that the adjusted hazard ratio (aHR) for mortality ranges from 4.84 to 7.99 in ART-naive CAHIV and 3.69 to 5.11 in CAHIV treated with ART [7, 8]. Children and adolescents with HIV with preserved immune function are at reduced risk of mortality secondary to HIV-associated TB as compared with children with advanced HIV [5].
Universal and rapid initiation of ART in CAHIV has clear benefits for mortality reduction and improved quality of life [9]; however, concerns about immune reconstitution syndrome (IRIS) and drug interactions have led to uncertainty about the timing of ART initiation relative to TB treatment initiation in CAHIV who are ART naive.
A systematic review of studies in adults was conducted to inform the 2021 World Health Organization (WHO) HIV Guidelines and found that ART initiation within 2 weeks of TB treatment as compared with within 8 weeks resulted in no increase in mortality regardless of CD4 count, and with no difference in viral suppression or AIDS defining events [10]. These data led to a WHO recommendation to initiate ART as soon as possible and within 2 weeks of TB treatment initiation in all PHIV, including CAHIV, given the other known benefits of rapid ART initiation.
There is a scarcity of evidence in CAHIV to guide the timing of ART initiation from within 2 weeks compared with within 8 weeks of initiating TB treatment. Further, only limited data suggest that ART initiation within 8 weeks of TB treatment improves HIV-associated TB treatment outcomes [11]. To address this knowledge gap, we evaluated the risk of mortality associated with the timing of ART initiation in ART-naive CAHIV treated for pulmonary TB (pTB) in a large, multinational retrospective cohort from TB/HIV high-burden settings throughout sub-Saharan Africa.
METHODS
STROBE Statement
Study implementation was aligned with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [12]. The primary objective was to evaluate if the interval from initiation of treatment for pTB to ART initiation is associated with the risk of mortality in CAHIV.
Participants
The study population included CAHIV initiating treatment for pTB and HIV at 7 Centers of Excellence (COEs) within the Baylor International Pediatric AIDS Initiative (BIPAI) network across 6 countries, including Botswana, Eswatini, Lesotho, Malawi, Tanzania (Mbeya and Mwanza), and Uganda. To reduce bias, all ART-naive CAHIV who were aged 0 to 19 years at treatment initiation for pTB were included in the analysis.
Outcomes of Interest
Mortality was the primary outcome. However, considering the high risk of death among CAHIV who are lost to follow-up (LTFU) [13, 14], a planned sensitivity analysis was performed, assuming death as the outcome among those LTFU. Children and adolescents with HIV who transferred out were censored at the time of transfer.
Data Extraction
Patient data were extracted and analyzed from the electronic medical record (EMR) as previously reported [5]. In brief, the database allows extraction of patient-level data, supporting longitudinal individual patient-level analysis. The period of analysis differed among the COEs based on the timing of the introduction of standardized TB-specific EMR fields to optimize data validity and homogeneity. All COEs introduced TB-specific EMR fields in 2013, except for Malawi, which began in 2016, and data were extracted through September of 2020. Covariates, thought to be potential confounders, were identified a priori, including sex, age, COE location, body mass index (BMI) z score (defined by WHO growth charts), TB confirmation status (confirmed by a positive acid-fast smear, Xpert [MTB/RIF or Ultra] test (Cepheid, Sunnyvale, California), or Mycobacterium tuberculosis culture vs unconfirmed), CD4 percent, and a composite variable categorizing immunosuppression as preserved (CD4 of >350 cells/μL in children aged ≥5 years or >25% in children <5 years) or suppressed (defined as the inverse of the definition for preserved). Time period for anti-tuberculosis therapy (ATT) initiation was stratified by into 3 periods—2013–2015, 2016–2017, and 2018–2020—to evaluate changes in the cohort composition over time.
Statistical Approach
Descriptive statistics were performed on the cohort, stratified by the primary exposure. Chi-square tests were performed for categorical covariates and analysis of variance for continuous variables. Kaplan–Meier curves were developed, with time to ART initiation as a fixed variable, with death and death or LTFU as the outcome (R package “survival”; R Foundation for Statistical Computing). Unadjusted and adjusted Cox regression models, with timing of ART initiation as a time-dependent variable, were developed and adjusted for covariates found to vary by the primary exposure (P < .2). For the regression models, all patients were classified as untreated until ART initiation to limit immortal time bias [15]. The adjusted models included age, BMI z score, time period of TB treatment initiation, TB certainty, and immune status as defined by CD4 category. Models were developed for the primary outcome of mortality and a composite outcome of death and LTFU. The duration of follow-up was evaluated at 2-year intervals (1 year, 3 years, 5 years, and current). All statistical analysis was performed in R (version 1.3.959) using the packages described above for each analysis. An adjusted complete case analysis was performed for the primary analysis. To assess whether the data supporting the complete case analysis were representative of the full cohort, an unadjusted complete case analysis was compared with the unadjusted analysis in the full dataset. Further, imputation of missing data was performed using multiple imputation by chained equations (MICE) with polytomous regression for categorical variables and predictive mean matching for continuous variables (R package: Mice) [16].
All clinical investigation supporting the data handling, analysis, and reporting of these findings was conducted according to the principles of the Declaration of Helsinki. Approval was obtained from the national ethical bodies in each country and the Baylor College of Medicine Institutional Review Board.
RESULTS
The analytic study population included all 774 CAHIV who were ART naive at the time of pTB treatment initiation. This cohort was divided across 4 exposure categories: those who (1) never initiated ART (no ART, n = 44), (2) started ART within 2 weeks of TB treatment (<2 weeks, n = 266), (3) started ART at least 2 weeks after but less than 2 months from TB treatment (2 weeks–2 months, n = 398), and (4) started ART more than 2 months after TB treatment (>2 months, n = 66).
Patient characteristics were compared across the different ART initiation timing groups (Table 1). Sex proportions were balanced across the 4 groups (P = .516). Although the no-ART group had the lowest median age (2.76 years) at ATT initiation, the confidence intervals (CIs) for all groups were overlapping (P = .072), suggesting no difference in age composition. The proportion of patients within the ART initiation groups varied by study sites (COEs) (P < .001); however, at all sites, the greatest proportion of patients initiated ART in the less-than-2-week or 2-week to 2-month time period.
Table 1.
Baseline Characteristics Per Exposure Category
| ATT to ART Interval: Exposure Categories | |||||
|---|---|---|---|---|---|
| No ART (n = 44) | <2 Weeks (n = 266) | 2 Weeks–2 Months (n = 398) | >2 Months (n = 66) | P | |
| Gender (female) | 19 (43%) | 140 (53%) | 198 (50%) | 37 (56%) | .516 |
| Age in years | 2.76 (1.10, 7.30) | 3.16 (1.40, 8.55) | 4.71 (1.37, 10.36) | 4.59 (1.32, 9.14) | .072 |
| Age category | .398 | ||||
| ȃ<1 year | 9 (20%) | 45 (17%) | 61 (15%) | 11 (17%) | |
| ȃ1–4 years | 20 (45%) | 108 (41%) | 143 (36%) | 23 (35%) | |
| ȃ5–9 years | 10 (23%) | 61 (23%) | 87`` (22%) | 17 (26%) | |
| ȃ10–19 years | 5 (11%) | 52 (20%) | 107 (27%) | 15 (23%) | |
| COE | <.001 | ||||
| ȃBotswana | 0 | 20 (8%) | 7 (2%) | 2 (3%) | |
| ȃEswatini | 5 (11%) | 18 (7%) | 70 (18%) | 27 (41%) | |
| ȃLesotho | 4 (9%) | 34 (13%) | 127 (32%) | 22 (33%) | |
| ȃMalawi | 0 | 13 (5%) | 3 (1%) | 1 (2%) | |
| ȃMbeya | 4 (9%) | 37 (14%) | 27 (7%) | 2 (3%) | |
| ȃMwanza | 15 (34%) | 84 (32%) | 73 (18%) | 6 (9%) | |
| ȃUganda | 16 (36%) | 60 (23%) | 91 (23%) | 6 (9%) | |
| BMI z score | −1.99 (−3.2, −0.6) | −1.75 (−3.1, −0.6) | −1.62 (−2.8, −0.3) | −1.45 (−2.7, −0.3) | .206 |
| ȃMissing | 8 (18%) | 23 (9%) | 39 (10%) | 16 (24%) | |
| TB certainty | .006 | ||||
| ȃConfirmed | 3 (7%) | 90 (34%) | 110 (28%) | 19 (29%) | |
| ȃUnconfirmed | 36 (82%) | 172 (65%) | 288 (72%) | 46 (70%) | |
| ȃMissing | 5 (11%) | 4 (2%) | 0 | 1 (2%) | |
| ȃCD4 percent | 11 (8.3, 20.3) | 14 (7, 21.3) | 13.8 (6.9, 21) | 16 (8, 19) | .970 |
| ȃMissing | 13 (30%) | 52 (20%) | 74 (19%) | 18 (27%) | |
| CD4 category | .130 | ||||
| ȃPreserved | 18 (41%) | 120 (45%) | 190 (48%) | 35 (53%) | |
| ȃSuppressed | 14 (32%) | 107 (40%) | 149 (37%) | 14 (21%) | |
| ȃMissing | 12 (27%) | 39 (15%) | 59 (15%) | 17 (26%) | |
| Year of ATT start | <.001 | ||||
| ȃ2013–2015 | 12 (27%) | 89 (33%) | 206 (52%) | 47 (71%) | |
| ȃ2016–2017 | 17 (39%) | 88 (33%) | 124 (31%) | 14 (21%) | |
| ȃ2018–2020 | 15 (34%) | 89 (33%) | 68 (17%) | 5 (8%) | |
Continuous variables are summarized with median and IQR, while categorical variables are summarized with counts and percentages. Statistical differences among exposure groups were assessed with ANOVA for continuous variables and chi-square for categorical variables.
Abbreviations: ANOVA, analysis of variance; ART, antiretroviral therapy; ATT, anti-tuberculosis therapy; BMI, body mass index; COE, Center of Excellence; IQR, interquartile range; TB, tuberculosis.
Although BMI z score medians were broadly similar across groups, the no-ART group had the lowest z score (−1.99; 95% CI: −3.2 to −.6) compared with the other 3 groups, which had z score values approximating −1.6 (P = .206). The no-ART group was also quite different with respect to TB certainty; most patients had unconfirmed TB (82%). In contrast, 34% of the less-than-2-week group had confirmed TB (P = .006). Baseline CD4 percentage was lowest in the no-ART group (11%; 95% CI: 8.3–20.3%) but was not different from the other groups (P = .970). The more-than-2-month group had the highest CD4 percentage (16%; 95% CI: 8.0–19.0%) and also the highest rate of preserved immune function (53%). Overall, the other groups were evenly split by categorical immune status, with just over half the cohort being defined as having preserved immune function. The interval from ATT to ART initiation was also associated with the time period of ART initiation. Given the prolonged analytic period and the association with the ATT to ART interval, we evaluated the cohort by time period (Table 2). While largely similar over time, children initiated on ATT in later time periods were slightly younger (P = .026) and had increased rates of immune preservation (P < .001) and malnutrition (P = .008). Children in later time periods were also more likely to be initiated on ART less than 2 weeks from ATT initiation (P < .001).
Table 2.
Baseline Characteristics by Time Period of Tuberculosis Treatment Initiation
| ATT to ART Interval: TB Treatment Initiation Time Period | ||||
|---|---|---|---|---|
| 2013–2015 (n = 354) | 2016–2017 (n = 243) | 2018–2020 (n = 177) | P | |
| Gender (female) | 192 (54%) | 125 (51%) | 77 (44%) | .065 |
| Age in years | 5.06 (1.53, 10.18) | 2.95 (1.31, 8.72) | 3.92 (1.23, 8.12) | .026 |
| Age category | .084 | |||
| ȃ<1 year | 124 (35%) | 104 (43%) | 66 (37%) | |
| ȃ1–4 years | 52 (15%) | 40 (16%) | 34 (19%) | |
| ȃ5–9 years | 85 (24%) | 44 (18%) | 46 (26%) | |
| ȃ10–19 years | 93 (26%) | 55 (23%) | 31 (18%) | |
| COE | .172 | |||
| ȃBotswana | 10 (3%) | 15 (6%) | 4 (2%) | |
| ȃEswatini | 89 (25%) | 23 (9%) | 8 (5%) | |
| ȃLesotho | 111 (31%) | 57 (23%) | 19 (11%) | |
| ȃMalawi | 1 (0.3%) | 10 (4%) | 6 (3%) | |
| ȃMbeya | 22 (6%) | 28 (12%) | 20 (11%) | |
| ȃMwanza | 75 (21%) | 55 (23%) | 48 (27%) | |
| ȃUganda | 46 (13%) | 55 (23%) | 72 (41%) | |
| BMI z score | −1.47 (−2.6, 0.3) | −1.81 (−3.3, −0.5) | −1.91 (−3.2, −0.7) | .005 |
| ȃMissing | 49 (14%) | 11 (4.5%) | 26 (15%) | |
| TB certainty | .275 | |||
| ȃConfirmed | 93 (26%) | 76 (31%) | 53 (30%) | |
| ȃUnconfirmed | 261 (74%) | 161 (66%) | 120 (68%) | |
| ȃMissing | 0 | 6 (2%) | 4 (2%) | |
| CD4 percent | 14 (6, 20) | 13 (8, 21) | 14.3 (8, 22.6) | .227 |
| ȃMissing | 37 (10%) | 61 (25%) | 59 (33%) | |
| CD4 category | <.001 | |||
| ȃPreserved | 204 (58%) | 104 (43%) | 55 (31%) | |
| ȃSuppressed | 123 (35%) | 86 (35%) | 75 (42%) | |
| ȃMissing | 27 (8%) | 53 (22%) | 47 (27%) | |
| ART timing | <.001 | |||
| ȃNo ART | 12 (3%) | 17 (7%) | 15 (8%) | |
| ȃ<2 weeks | 89 (25%) | 88 (36%) | 89 (50%) | |
| ȃ2 weeks to 2 months | 206 (58%) | 124 (51%) | 68 (38%) | |
| ȃ>2 months | 47 (13%) | 14 (6%) | 5 (3%) | |
Continuous variables are summarized with median and IQR, while categorical variables are summarized with counts and percentages. Statistical differences among exposure groups were assessed with ANOVA for continuous variables and chi-square for categorical variables.
Abbreviations: ANOVA, analysis of variance; ART, antiretroviral therapy; ATT, anti-tuberculosis therapy; BMI, body mass index; COE, Center of Excellence; IQR, interquartile range; TB, tuberculosis.
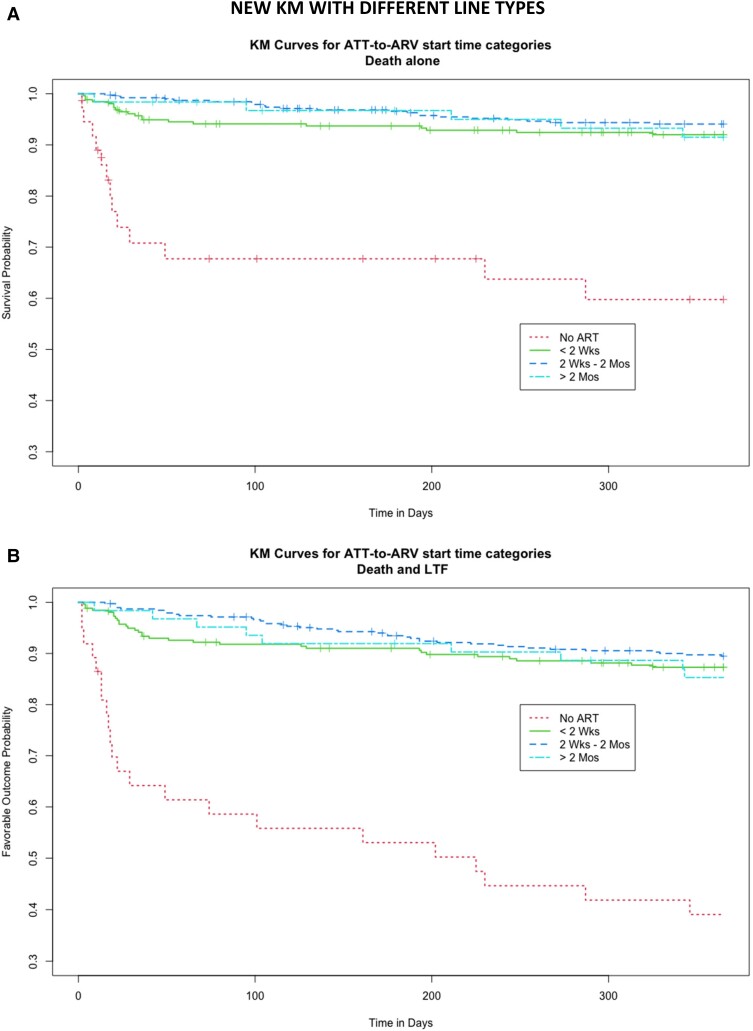
Kaplan–Meier curves demonstrate survival after initiation of ART or TB treatment in the case of the no-ART group (Figure 1A ) and survival and retention in care (Figure 1B ). The no-ART group experienced high rates of early mortality, with just under 70% of the population surviving to 100 days post–ATT initiation. This group continued to experience higher rates of LTFU throughout the year following ATT initiation, and less than half of patients remained active in care at the end of 1 year. The unadjusted mortality and mortality and retention in care were broadly similar in the remaining groups. Analysis was repeated in the cohort stratified by age (<1 year, 1–4 years, and ≥ 5 years) (Supplementary Figure 1). The Kaplan–Meier curves demonstrated overall higher rates of mortality in the younger populations, but the relationship between the ATT to ART initiation interval groups remained similar.
Figure 1.
Kaplan–Meier curves demonstrating the unadjusted risk of survival (A) and a favorable outcome as evidenced by retention in care without death or lost to follow up (B). Timing of ART initiation is defined by line pattern in the figure legend. Abbreviations: ART, antiretroviral therapy; ATT, anti–tuberculosis treatment; Mos, months; TB, tuberculosis; Wks, weeks.
Cox proportional hazards models were generated to control for potential confounders and covariates that were included in the model based on evidence of differences between ART initiation groups (Table 3). The complete case analysis, controlling for age, BMI z score, COE location, TB certainty, and immune status, included 71% of the total cohort and 2178 years of patient follow-up time. At 1-year follow-up, the no-ART group had an increased risk of mortality in the adjusted analysis (aHR: 2.67; 95% CI: 1.03–6.94) and death and LTFU (aHR: 3.29; 95% CI: 1.49–7.25) compared with the reference group of ART initiation at 2 weeks to 2 months. There were no differences in the aHR for death or death and LTFU for the other ART initiation groups. To evaluate the effect of the primary exposure (timing of ART initiation) on death at defined intervals, this analysis was repeated with an endpoint of 3 years, 5 years, and indefinite from treatment initiation; results of this analysis were similar to those of analysis with unlimited follow-up time (Supplementary Table 1). The Cox regression model was also performed on the imputed dataset, which did not reveal any differences in survival between groups (Supplementary Table 2).
Table 3.
Survival Analysis 1 Year From Tuberculosis Treatment Initiation Expressed With Unadjusted and Adjusted Hazard Ratios and 95% Confidence Intervals
| Outcome and Model | No ART | <2 Weeks | 2 Weeks–2 Months | >2 Months |
|---|---|---|---|---|
| Death and LTFU | ||||
| Unadjusted (n = 743a) | 2.09 (1.17, 3.74) | 1.11 (.69, 1.79) | 1 | .95 (.29, 3.11) |
| Unadjusted CCA (n = 552) | 3.11 (1.42, 6.78) | 1.13 (.62, 2.07) | 1 | 1.88 (.56, 6.31) |
| Adjustedb (n = 552) | 3.29 (1.49, 7.25) | .85 (.44, 1.64) | 1 | 2.70 (.77, 9.54) |
| Death alone | ||||
| Unadjusted (n = 743a) | 1.76 (0.90, 3.46) | 1.21 (.76, 1.93) | 1 | .48 (.11, 1.98) |
| Unadjusted CCA (n = 552) | 2.82 (1.07, 7.38) | 1.25 (.70, 2.23) | 1 | .46 (.06, 3.39) |
| Adjustedb (n = 552) | 2.67 (1.03, 6.94) | 1.02 (.55, 1.89) | 1 | .81 (.10, 6.31) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CCA, complete case analysis; COE, Center of Excellence; LTFU, lost to follow-up; TB, tuberculosis.
Reduced from total cohort due to poorly defined outcomes (n = 31).
Models adjusted for the following covariates: age, BMI z score, COE, TB certainty, CD4 category, and year of TB treatment initiation.
DISCUSSION
In this multinational observational cohort of 774 CAHIV treated for TB prior to ART initiation, over 2632 person-years of follow-up we found no increase in death or death and LTFU in patients initiating ART in the first 2 weeks following TB treatment initiation compared with patients initiating ART later (up to 2 months following TB treatment initiation). These data are derived from 7 clinics across 6 high-burden TB/HIV countries in sub-Saharan Africa and are among the most generalizable data available for CAHIV in the region.
These data provide evidence that rapid initiation of ART, both in children who are being evaluated for presumptive pTB and in children diagnosed with HIV-associated TB, will not increase the risk of death. These data provide evidence to support the recommendation from the WHO to initiate ART as soon as possible and within 2 weeks of pTB treatment initiation in CAHIV, which was informed purely from adult data. This evidence in adults suggests that initiation of ART within 2 weeks of TB treatment initiation as compared with 2 to 8 weeks does not increase mortality (risk difference= −0.01; 95% CI: −.06 to .04) or result in differences in viral load suppression or AIDS defining events [10, 17].
A randomized controlled trial of hospitalized CAHIV [18], 15% of whom were admitted with TB, demonstrated no difference in survival between children randomized to urgent initiation of ART within 48 hours of admission or 7 to 14 days following admission (HR: 1.26; 95% CI: .67–2.37). Further, there was no increase in the risk of IRIS in the urgent-initiation group (HR: .96; 95% CI: .41–2.23) [18]. While this was a very specific population of very ill CAHIV, with an overall mortality of 22%, it provides reassurance that rapid initiation of ART in medically unstable children does not result in harm. Another study among CAHIV with severe malnutrition, a large proportion of whom had TB, also suggested that early compared with deferred initiation of ART resulted in similar outcomes. These data among extremely ill cohorts of CAHIV with a high TB prevalence also suggest that early ART is unlikely to cause harm but may not result in dramatic reductions in mortality.
Our study, and others in children less acutely ill, also suggests that mortality is predominantly attributable to whether ART is initiated at all [19] or initiated within 2 months of TB treatment in children with severe immunocompromise [20], rather than the rapidity of ART initiation in all children with HIV-associated TB. While a benefit of initiation of ART within 2 weeks has not been shown in any study of HIV-associated TB in CAHIV, a large, prospective, observational cohort of ART-naive children, 59% of whom had TB, suggested that initiation of ART within 1 month of follow-up reduced mortality (aHR: .08; 95% CI: .01–.67) [21]. Collectively, these data suggest that initiation of ART within 1 to 2 months of TB treatment initiation is likely to be beneficial. Importantly, our data demonstrate that initiation of ART within 2 weeks of TB treatment initiation is unlikely to be harmful in CAHIV, a population at high risk of death and with much to gain from ART initiation.
Even without dramatic mortality reductions, rapid initiation of ART is important for PHIV of all ages but may be most critical in CAHIV [9]. In infants and young children diagnosed with HIV, rapid initiation of ART improves immune recovery [22] and neurocognitive development and growth [23, 24] and reduces HIV reservoirs and immune activation [25]. In older children and in adolescents, rapid initiation of ART enhances immune recovery [22, 24], normalizes pubertal development [26], and reduces HIV-related cardiac and pulmonary complications [27, 28]. Therefore, in the absence of harm related to rapid ART initiation following a diagnosis of HIV-associated pTB, it is imperative that CAHIV experience the other benefits of rapid ART initiation.
This study is subject to limitations. Most notably, the data were derived from routine sources and, therefore, the analysis was complicated by missing data. We addressed this both through careful comparison of the complete case cohort and the full cohort, as well as through imputation. Results were consistent across all methods. However, routine viral load and CD4 data had too much missingness to be evaluated as outcomes following ART initiation. Observational studies evaluating treatment initiation strategies are also subject to immortal time bias, which we addressed through statistical methods, but we cannot exclude residual confounding. Certainly, the high rates of mortality observed in the Kaplan–Meier curves are not attributable solely to the absence of ART initiation. The reduction in the unadjusted and adjusted HRs in the Cox models using a time-dependent variable for timing of ART initiation suggests that we effectively addressed this bias. The clinical settings described in this study, while established as public–private partnerships, are each part of and predominantly supported by the Ministry of Health in each country. The settings are representative of care provided nationally, but with the benefit of caring for a higher volume of CAHIV due to referrals. The CAHIV described may also have more advanced disease due to referral bias but, in general, had immune status that aligned with CAHIV in other studies in other high-burden settings [29]. Due to the prolonged analytic period, there were changes in health policy, and in general, children were initiated on ART after a shorter interval during the most recent study period. This was adjusted for through inclusion of treatment time period in the model. The decreased age, increased malnutrition, and immune suppression among children treated during the most recent time period may reflect a survival bias present among the children treated between 2013 and 2015 but also indicates the ongoing need for improved case finding of CAHIV. Finally, with observational retrospective studies, it is difficult to exclude misattribution errors through inaccurate data entry, although steps were taken to limit this risk through manual data checks. Further, prospective randomized studies are unlikely to be performed to address this question in CAHIV.
The age of initiation and the low rate of disease confirmation highlight the ongoing challenge with TB diagnosis in children and indicate that infants with HIV-associated TB continue to go undiagnosed. These diagnostic challenges speak to the importance of data-driven clinical diagnostic algorithms in CAHIV [30], particularly in the absence of large immunologic or microbiologic diagnostic advances. It is also possible that benefits of early ART were not fully realized due to the limited ART options available for infants and children with HIV-associated TB. Triple nucleoside reverse transcriptase inhibitors, nevirapine, and lopinavir-ritonavir all had limitations. The introduction of pediatric dolutegravir marks the first time that many CAHIV less than 3 years of age have had access to effective and child-friendly ART regimens while receiving TB treatment. This treatment option, when dosed twice daily, is likely to be more effective than the previously available ART treatment regimens and has a lower risk of toxicity. With reduced concerns about drug interactions and toxicity, clinicians may now also feel more comfortable rapidly initiating ART in children with TB.
CONCLUSIONS
This study provides critical data indicating that rapid initiation of ART, within 2 weeks following initiation of TB treatment, is not associated with an increased risk of death or death and LTFU. The data support existing recommendations from the WHO to initiate ART within 2 weeks in CAHIV who are being evaluated for presumptive TB or who are being treated for HIV-associated TB.
Supplementary Material
Contributor Information
Alexander Kay, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA; Baylor College of Medicine Children's Foundation–Swaziland, Mbabane, Eswatini.
Jose Mendez-Reyes, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA.
Tara Devezin, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA.
Meenakshi Bakaya, Baylor College of Medicine Children's Foundation–Lesotho, Maseru, Lesotho.
Teresa Steffy, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA; Baylor College of Medicine Children's Foundation–Lesotho, Maseru, Lesotho.
Sandile Dlamini, Baylor College of Medicine Children's Foundation–Swaziland, Mbabane, Eswatini.
Amos Msekandiana, Baylor College of Medicine Children's Foundation, Lilongwe, Malawi.
Tara Ness, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA.
Jason Bacha, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA; Baylor College of Medicine Children’s Foundation–Tanzania, Mbeya, Tanzania.
Pauline Amuge, Baylor College of Medicine Children's Foundation–Uganda, Kampala, Uganda.
Mogomotsi Matshaba, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA; Botswana-Baylor Children's Clinical Centre of Excellence, Gaborone, Botswana.
Moses Chodota, Baylor College of Medicine Children’s Foundation–Tanzania, Mbeya, Tanzania.
Phoebe Nyasulu, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA; Baylor College of Medicine Children's Foundation, Lilongwe, Malawi.
Lineo Thahane, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA; Baylor College of Medicine Children's Foundation–Lesotho, Maseru, Lesotho.
Lumumbwa Mwita, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA; Baylor College of Medicine Children's Foundation–Tanzania, Mwanza, Tanzania.
Adeodata Kekitiinwa, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA; Baylor College of Medicine Children's Foundation–Uganda, Kampala, Uganda.
Andrew DiNardo, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA.
Bhekumusa Lukhele, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA; Baylor College of Medicine Children's Foundation–Swaziland, Mbabane, Eswatini.
H Lester Kirchner, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Population Health Sciences, Geisinger, Danville, Pennsylvania, USA.
Anna Mandalakas, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Department of Pediatrics, Texas Children's Hospital, Houston, Texas, USA; Research Center Borstel, Clinical Infectious Diseases, Borstel, Germany.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support . This work was supported by the Fogarty International Center at the National Institutes of Health (grant number: 1K01TW011482-01A1 to A. Kay).
References
- 1. World Health Organization . Global tuberculosis report. Geneva, Switzerland: World Health Organization, 2021.
- 2. Nduba V, Kaguthi G, Van’t Hoog AH, Mitchell EMH, Borgdorff M. The incidence of tuberculosis in infants, Siaya district, Western Kenya. Pediatr Infect Dis J 2020; 39:591–7. [DOI] [PubMed] [Google Scholar]
- 3. Frigati LJ, Wilkinson KA, le Roux S, et al. Tuberculosis infection and disease in South African adolescents with perinatally acquired HIV on antiretroviral therapy: a cohort study. J Int AIDS Soc 2021; 24:e25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinez L, Cords O, Horsburgh CR, Andrews JR; Pediatric TB Contact Studies Consortium . The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet 2020; 395:973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mandalakas AM, Kay AW, Bacha JM, et al. Tuberculosis among children and adolescents at HIV treatment centers in sub-Saharan Africa. Emerg Infect Dis 2020; 26:2933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dodd PJ, Prendergast AJ, Beecroft Cet al. The impact of HIV and antiretroviral therapy on TB risk in children: a systematic review and meta-analysis. Thorax 2017; 72:559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osman M, du Preez K, Seddon JA, et al. Mortality in South African children and adolescents routinely treated for tuberculosis. Pediatrics 2021; 147:e2020032490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Onyango DO, Yuen CM, Masini E, Borgdorff MW. Epidemiology of pediatric tuberculosis in Kenya and risk factors for mortality during treatment: a national retrospective cohort study. J Pediatr 2018; 201:115–21. [DOI] [PubMed] [Google Scholar]
- 9. Barlow-Mosha L, Musiime V, Davies M-A, et al. Universal antiretroviral therapy for HIV-infected children: a review of the benefits and risks to consider during implementation. J Int AIDS Soc 2017; 20:21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva, Switzerland: World Health Organization, 2021. [PubMed] [Google Scholar]
- 11. Carlucci JG, Blevins Peratikos M, Kipp AM, et al. Tuberculosis treatment outcomes among HIV/TB-coinfected children in the International Epidemiology Databases to Evaluate AIDS (IeDEA) network. J Acquir Immune Defic Syndr 2017; 75:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370:1453–7. [DOI] [PubMed] [Google Scholar]
- 13. Ardura-Garcia C, Feldacker C, Tweya H, et al. Implementation and operational research: early tracing of children lost to follow-up from antiretroviral treatment: true outcomes and future risks. J Acquir Immune Defic Syndr 2015; 70:e160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osman M, Meehan S-A, von Delft A, et al. Early mortality in tuberculosis patients initially lost to follow up following diagnosis in provincial hospitals and primary health care facilities in Western Cape, South Africa. PLoS One 2021; 16:e0252084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol 2005; 162:1016–23. [DOI] [PubMed] [Google Scholar]
- 16. Van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. Available at: https://www.jstatsoft.org/article/view/v045i03. Accessed 26 May 2022.
- 17. Burke RM, Rickman HM, Singh V, et al. What is the optimum time to start antiretroviral therapy in people with HIV and tuberculosis coinfection? A systematic review and meta-analysis. J Int AIDS Soc 2021; 24:e25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Njuguna IN, Cranmer LM, Otieno VO, et al. Urgent versus post-stabilisation antiretroviral treatment in hospitalised HIV-infected children in Kenya (PUSH): a randomised controlled trial. Lancet HIV 2018; 5:e12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buck WC, Olson D, Kabue MM, et al. Risk factors for mortality in Malawian children with human immunodeficiency virus and tuberculosis co-infection. Int J Tuberc Lung Dis 2013; 17:1389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yotebieng M, Edmonds A, Lelo P, et al. High completion of isoniazid preventive therapy among HIV-infected children and adults in Kinshasa, Democratic Republic of Congo. AIDS 2015; 29:2055–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marcy O, Tejiokem M, Msellati P, et al. Mortality and its determinants in antiretroviral treatment-naive HIV-infected children with suspected tuberculosis: an observational cohort study. Lancet HIV 2018; 5:e87–95. [DOI] [PubMed] [Google Scholar]
- 22. Musoke PM, Mudiope P, Barlow-Mosha LN, et al. Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort study. BMC Pediatr 2010; 10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brahmbhatt H, Boivin M, Ssempijja V, et al. Neurodevelopmental benefits of anti-retroviral therapy in Ugandan children 0–6 years of age with HIV. J Acquir Immune Defic Syndr 2014; 67:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGrath CJ, Chung MH, Richardson BA, Benki-Nugent S, Warui D, John-Stewart GC. Younger age at HAART initiation is associated with more rapid growth reconstitution. AIDS 2011; 25:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pierson T, McArthur J, Siliciano RF. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Ann Rev Immunol 2000; 18:665–708. [DOI] [PubMed] [Google Scholar]
- 26. Szubert AJ, Musiime V, Bwakura-Dangarembizi M, et al. Pubertal development in HIV-infected African children on first-line antiretroviral therapy. AIDS 2015; 29:609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lipshultz SE, Williams PL, Wilkinson JD, et al. Cardiac status of children infected with human immunodeficiency virus who are receiving long-term combination antiretroviral therapy: results from the adolescent master protocol of the multicenter Pediatric HIV/AIDS cohort study. JAMA Pediatr 2013; 167:520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrand RA, Desai SR, Hopkins C, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis 2012; 55:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peacock-Villada E, Richardson BA, John-Stewart GC. Post-HAART outcomes in pediatric populations: comparison of resource-limited and developed countries. Pediatrics 2011; 127:e423–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marcy O, Borand L, Ung V, et al. A treatment-decision score for HIV-infected children with suspected tuberculosis. Pediatrics 2019; 144:e20182065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.