Abstract
Background
Sulopenem is a thiopenem antibiotic being developed for the treatment of multidrug-resistant infections. The availability of both intravenous (IV) and oral formulations will facilitate earlier hospital discharge.
Methods
Hospitalized adults with pyuria, bacteriuria, and signs and symptoms of complicated urinary tract infection (cUTI) were randomized to 5 days of IV sulopenem followed by oral sulopenem etzadroxil/probenecid or 5 days of IV ertapenem followed by oral ciprofloxacin or amoxicillin-clavulanate, depending on uropathogen susceptibility. The primary end point was overall combined clinical and microbiologic response at the test-of-cure visit (day 21).
Results
Of 1392 treated patients, 444 and 440 treated with sulopenem and ertapenem, respectively, had a positive baseline urine culture and were eligible for the primary efficacy analyses. Extended-spectrum β-lactamase-producing organisms were identified in 26.6% of patients and fluoroquinolone-nonsusceptible pathogens in 38.6%. For the primary end point, noninferiority of sulopenem to the comparator regimen was not demonstrated, 67.8% vs 73.9% (difference, −6.1%; 95% confidence interval, −12.0 to −.1%). The difference was driven by a lower rate of asymptomatic bacteriuria in the subgroup of ertapenem-treated patients who stepped down to ciprofloxacin. No substantial difference in overall response was observed at any other time point. Both IV and oral formulations of sulopenem were well-tolerated and compared favorably to the comparator.
Conclusions
Sulopenem followed by oral sulopenem-etzadroxil/probenecid was not noninferior to ertapenem followed by oral step-down therapy for the treatment of cUTIs, driven by a lower rate of asymptomatic bacteriuria in those who received ciprofloxacin. Both formulations of sulopenem were well-tolerated.
Clinical Trial Registration
Keywords: sulopenem, complicated urinary tract infection, acute pyelonephritis
In this phase 3, double-blind, double-dummy study comparing intravenous (IV) sulopenem followed by oral sulopenem to IV ertapenem followed by oral ciprofloxacin or amoxicillin-clavulanate in patients with complicated urinary tract infections, sulopenem was not noninferior to the comparator regimen for overall combined clinical and microbiologic response.
The prevalence of infections caused by extended–spectrum β–lactamase (ESBL)–producing Enterobacterales has been increasing worldwide in both hospital- and community-acquired settings. The Centers for Disease Control and Prevention’s analysis of data reported in 2011–2014 to the National Healthcare Safety Network revealed that the proportion of Escherichia coli resistant to extended–spectrum cephalosporins that caused hospital–acquired infections was 13.4% nationally, with rates as high as 24% in some states [1]. That analysis also demonstrated that more than one-third of E. coli isolates in 2014 were resistant to fluoroquinolones. In 2017, ESBL-producing Enterobacterales infections occurred in 197 400 hospitalized patients in the United States, causing 9100 deaths; the estimated attributable healthcare costs for those infections that year was $1.2 billion [2]. Escherichia coli and Klebsiella pneumoniae are both important causes of complicated urinary tract infections (cUTIs) and pyelonephritis. A 2019 report by the European Antimicrobial Resistance Surveillance Network noted that in 2018, more than half of E. coli isolates were resistant to at least 1 of the antimicrobial groups, including the aminopenicillins (57.4%), fluoroquinolones (25.3%), third–generation cephalosporins (15.1%), and aminoglycosides (11.1%) [3]. More than 30% of K. pneumoniae isolates were resistant to third-generation cephalosporins (31.7%) and fluoroquinolones (31.6%). Coresistance rates among E. coli that are resistant to either fluoroquinolones or trimethoprim-sulfamethoxazole (TMP-SMX) have exceeded 45% [4].
Sulopenem, a broad-spectrum thiopenem β–lactam antibiotic being developed for treatment of infections caused by multidrug-resistant bacteria, is active against species of Enterobacterales that encode ESBLs or AmpC–type β–lactamases that confer resistance to third–generation cephalosporins, similar to ertapenem [5]. In addition to an intravenous (IV) formulation, sulopenem is available as an oral prodrug, sulopenem etzadroxil. Probenecid, coformulated with sulopenem etzadroxil as a bilayer tablet, reduces renal clearance and increases systemic exposure to sulopenem. This oral formulation offers the option of treatment in outpatient settings, as well as IV to oral switch therapy for early discharge of hospitalized patients.
In this study, we compared the efficacy and safety of sulopenem with that of ertapenem in patients with cUTIs, including acute pyelonephritis.
METHODS
Objectives
The primary objective was to demonstrate noninferiority of IV sulopenem followed by oral sulopenem etzadroxil/probenecid to IV ertapenem followed by oral ciprofloxacin or amoxicillin–clavulanate in adults with cUTIs. The primary end point was overall success, defined as clinical cure and microbiologic eradication, in the microbiologic modified intent-to-treat (mMITT) population at the test-of-cure (TOC) visit (day 21). Secondary objectives were to compare the per–patient microbiologic response rates, compare efficacy outcomes at relevant time points, and assess the safety profile of each regimen.
Study Design and Participants
This was a multicenter, randomized, comparative, double-blind phase 3 trial conducted at 131 sites in 13 countries. All patients, or their legal representatives, provided written informed consent. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the sites’ independent ethics committees and/or institutional review boards. An independent data monitoring committee reviewed blinded, pooled overall response data from an interim analysis.
The Supplementary Materials list full inclusion/exclusion criteria. Eligible patients were aged ≥18 years with a urine specimen positive for both nitrite and pyuria and signs and symptoms of acute pyelonephritis or cUTI. Qualifying signs and symptoms included rigors, chills, or fever/hypothermia; flank or pelvic pain; nausea or vomiting; dysuria, urinary urgency, or frequency; and costovertebral angle tenderness on physical examination. Complicating factors that qualified a patient for enrollment included indwelling catheters, neurogenic bladder, obstructive uropathy due to nephrolithiasis, tumor or fibrosis, and surgically modified or abnormal urinary tract anatomy. Patients could be enrolled before cultures were available.
Key exclusion criteria included the presence of chronic indwelling catheters, infections likely due to complete obstruction, emphysematous pyelonephritis, known or expected renal or perinephric abscess, or otherwise expected to require surgical intervention to achieve cure; ileal loops or vesicoureteral reflux; or a history of renal transplantation.
Randomization and Blinding
Eligible patients were randomized 1:1 to receive either IV sulopenem 1000 mg once daily followed by sulopenem etzadroxil 500 mg/probenecid 500 mg (referred to as oral sulopenem) twice daily or IV ertapenem 1000 mg once daily followed by oral ciprofloxacin 500 mg or amoxicillin–clavulanate 875 mg twice daily; duration of treatment was to be 7–10 days. Treatment could be extended to 14 days for patients with bacteremia at baseline. After at least 5 days of IV therapy, patients could be switched to oral therapy if they could tolerate oral medications, if signs and symptoms of infection were improving, and if the baseline pathogen was susceptible to the oral study drugs. Study drug doses could be adjusted for patients with severe renal impairment (creatinine clearance <30 mL/min) who were not on regular hemodialysis (Supplementary Materials).
Randomization was done using an interactive web randomization system and was stratified by type of infection (pyelonephritis vs cUTI without pyelonephritis). No more than 25% of patients could have received prior antibiotic therapy, and at least 30%, but no more than 70%, of patients could have acute pyelonephritis.
The study was a double-blind study. All oral study drugs and placebos were matched for blinding. The sponsor, site personnel, and patients were blinded. The site pharmacist was unblinded in order to prepare the IV study medications and to select the appropriate oral follow–on therapy for patients randomized to ertapenem.
Study Procedures
The schedule of procedures (Supplementary Materials) included urine collection for quantitative culture as well as blood cultures at baseline and as clinically indicated. Isolates from baseline urine cultures growing ≥105 colony-forming units (CFU)/mL and any positive blood cultures were sent to the central laboratory for identification, quantification, susceptibility testing, and further characterization of the organism(s). Sulopenem minimum inhibitory concentrations (MICs) were determined at a central laboratory using the agar dilution reference method.
Assessments (Supplementary Materials) included patient-reported symptomatic responses derived programmatically from a patient symptom assessment questionnaire as resolution, persistence, or indeterminate. Microbiological outcomes were classified as eradication, persistence, persistence with increasing MIC, or indeterminate. Per-patient and per-pathogen microbiological responses were assessed as favorable (ie, eradication), unfavorable (ie, persistence or persistence with increasing MIC), or indeterminate. Investigator-determined clinical responses were assessed as cure, failure, or indeterminate.
Efficacy End Points
The primary end point, overall response at TOC, was a composite of clinical and microbiologic outcomes. A patient was considered a success if their baseline signs and symptoms of cUTI had resolved and they had no new symptoms and if the bacterial pathogen found at ≥105 CFU/mL in the baseline urine culture was reduced to <103 CFU/mL in the TOC urine culture and blood cultures, if positive at baseline, had cleared. Patients were assigned an outcome of indeterminate if any data needed to determine whether the outcome was a success or failure were missing.
The secondary end point was the microbiologic response per patient at TOC. Additional efficacy analyses included the patient-determined clinical response at TOC, the overall response at time points other than TOC, and the clinical response in the intention-to-treat and MITT populations.
Safety
Safety end points included assessment of treatment-emergent adverse events and serious adverse events and evaluation of changes from baseline in laboratory test results, and vital signs. Adverse events were coded using version 21.0 of the Medical Dictionary for Regulatory Activities.
Statistical Analyses
The safety population included all patients who received any study therapy. The primary efficacy population, mMITT, comprised all randomized patients with the disease under study and a qualifying urine culture obtained before the first dose of study therapy, defined as ≥105 CFU/mL of a uropathogen (Enterobacterales only), susceptible to sulopenem (MIC ≤1 μg/mL) and ertapenem (MIC ≤0.5 μg/mL). All analysis populations, including microbiologically evaluable and clinically evaluable, are defined in the Supplementary Materials.
The proposed sample size of 578 patients per treatment regimen was based on a 10% non-inferiority margin, a predicted evaluability rate of 80%, a 70% overall success rate in both treatment groups, 90% power, and 1-sided α = 0.025. Following a blinded interim analysis of the pooled overall success rate and based on prespecified sample size adjustment criteria, the sample size was increased from 1156 to 1356 patients. The number and percentage of patients in each treatment group with an overall outcome of success, failure, and indeterminate was determined in the mMITT population. A 2-sided 95% confidence interval (CI) for the observed difference in the response rates of sulopenem minus ertapenem was calculated using a Z statistic. Noninferiority of sulopenem to ertapenem was to be concluded if the lower bound of the 95% CI was greater than −10%.
Sensitivity and subgroup analyses of the primary efficacy variable were also conducted (Protocol, Statistical Analysis Plan). Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
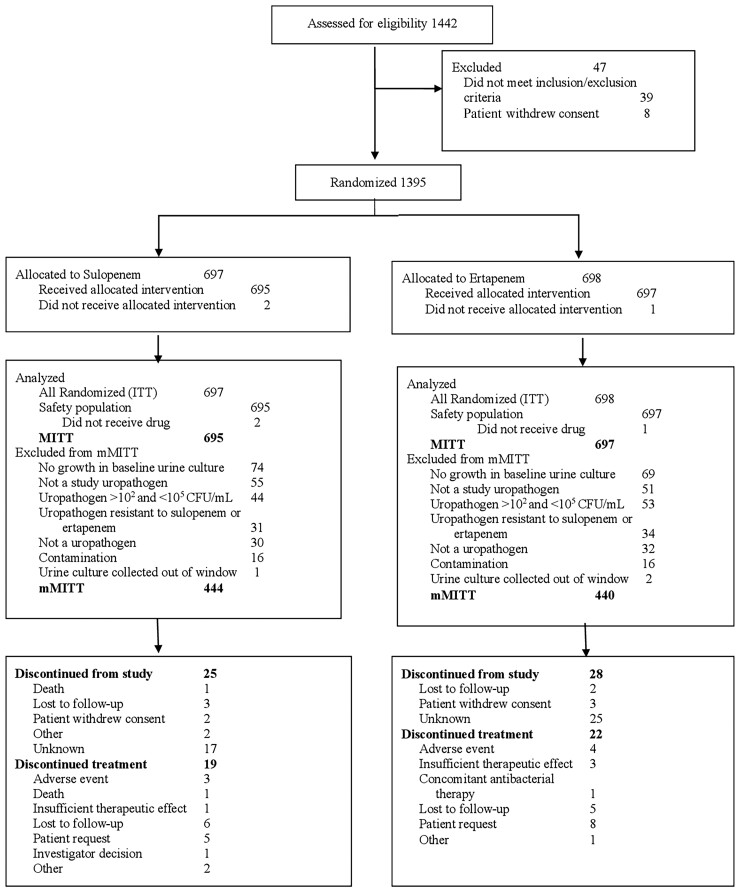
The study was initiated in October 2018 and completed in December 2019. A total of 1395 patients were randomized, 1392 of whom received at least 1 dose of the study drug (Figure 1). Patient demographics and baseline characteristics were well matched between treatment groups (Table 1). The majority of patients enrolled were in Eastern Europe (Supplementary Table 1). The mMITT population comprised 884 patients, of whom 366 (41.4%) had cUTIs without pyelonephritis and 518 (58.6%) had acute pyelonephritis (Table 1). Most patients (845 of 884, 95.6%) had a single baseline uropathogen. Escherichia coli was the most frequently isolated pathogen from urine and blood. A total of 235 (27%) patients had at least 1 baseline ESBL-positive Enterobacterales pathogen, as determined by a ceftriaxone MIC >1 μg/mL. A total of 341 (38.6%) and 315 (35.6%) study patients had at least 1 baseline Enterobacterales pathogen that was nonsusceptible to fluoroquinolones and TMP-SMX, respectively; 22% of patients had baseline organisms nonsusceptible to both ceftriaxone and fluoroquinolones; and 15% had baseline organisms nonsusceptible to ceftriaxone, fluoroquinolones, and TMP-SMX.
Figure 1.
Analysis population disposition. Abbreviations: CFU, colony-forming unit; ITT, intent-to-treat; MITT, modified intent-to-treat; mMITT, microbiologic modified intent-to-treat.
Table 1.
Patient Demographics: Primary Analysis Population: Microbiologic Modified Intent-to-Treat
| Parameter | Sulopenem (N = 444), n (%) |
Ertapenem (N = 440), n (%) |
|---|---|---|
| Age, mean (SD), years | 57.4 (18.4) | 59.5 (17.9) |
| Gender | ||
| ȃMale | 174 (39.2) | 189 (43.0) |
| ȃFemale | 270 (60.8) | 251 (57.0) |
| Geographic region | ||
| ȃUnited States | 25 (5.6) | 16 (3.6) |
| ȃNot the United States | 419 (94.4) | 424 (96.4) |
| Race | ||
| ȃBlack or African American | 0 (0.0) | 1 (0.2) |
| ȃAsian | 1 (0.2) | 2 (0.5) |
| ȃWhite | 441 (99.3) | 437 (99.3) |
| ȃNative Hawaiian or Pacific Islander | 1 (0.2) | 0 (0.0) |
| ȃOther | 1 (0.2) | 0 (0.0) |
| Body mass index, kg/m2 | ||
| ȃMean (SD) | 27.4 (6.0) | 27.5 (5.7) |
| Creatinine clearance, mL/mina | ||
| ȃMean (SD) | 73.0 (30.3) | 71.3 (31.5) |
| ȃ>60 | 276 (62.2) | 262 (59.5) |
| ȃ30–60 | 149 (33.6) | 152 (34.5) |
| ȃ<30 | 19 (4.3) | 26 (5.9) |
| Type of infection | ||
| ȃPyelonephritis | 261 (58.8) | 257 (58.4) |
| ȃcUTI | 183 (41.2) | 183 (41.6) |
| cUTI factors | ||
| ȃPresence of an indwelling urethral catheter | 21 (4.7) | 23 (5.2) |
| ȃ>100 mL of residual urine after voiding | 82 (18.5) | 86 (19.5) |
| ȃNeurogenic bladder | 13 (2.9) | 11 (2.5) |
| ȃObstructive uropathy due to nephrolithiasis, tumor, or fibrosis | 50 (11.3) | 38 (8.6) |
| ȃAzotemia | 3 (0.7) | 4 (0.9) |
| ȃUrinary retention in men possibly due to benign prostatic hypertrophy | 68 (15.3) | 80 (18.2) |
| ȃSurgically modified or abnormal urinary tract anatomy | 30 (6.8) | 42 (9.5) |
| Bacteremia | 44 (9.9) | 43 (9.8) |
| Baseline pathogen from urine or blood cultureb | ||
| ȃEscherichia coli | 338/444 (76.1) | 346/440 (78.6) |
| ȃKlebsiella pneumoniae | 56/444 (12.6) | 47/440 (10.7) |
| ȃProteus mirabilis | 26/444 (5.9) | 14/440 (3.2) |
| ȃEnterobacter cloacae complex | 9/444 (2.0) | 15/440 (3.4) |
| ȃKlebsiella oxytoca | 7/444 (1.6) | 7/440 (1.6) |
| ȃOtherc | 28/444 (6.3) | 30/440 (6.8) |
| ESBL-positive Enterobacterales | 110/444 (24.8) | 125/440 (28.4) |
| FQ-nonsusceptible Enterobacterales | 162/444 (36.5) | 179/440 (40.7) |
| TMP-SMX–nonsusceptible Enterobacterales | 154/444 (34.7) | 161/440 (36.6) |
| ESBL-positive/FQ-nonsusceptible | 91/444 (20.5) | 99/440 (22.5) |
| ESBL-positive/FQ-nonsusceptible/TMP-SMX–nonsusceptible | 58/444 (13.1) | 75/440 (17.0) |
Data are presented as number (%) unless otherwise indicated.
Abbreviations: cUTI, complicated urinary tract infection; ESBL, extended-spectrum β-lactamase; FQ, fluoroquinolone; SD, standard deviation; TMP-SMX, trimethoprim-sulfamethoxazole.
As reported by the site using the Cockcroft-Gault method based on local laboratory data.
Includes pathogens reported with a combined frequency of ≥10 patients. Patients could have >1 pathogen. Multiple isolates of the same species from the same patient are counted only once.
Other pathogens isolated in <10 patients were Citrobacter freundii (n = 9 overall), Morganella morganii (n = 9), Klebsiella variicola (n = 6), Serratia marcescens (n = 6), Citrobacter koseri (n = 5), Klebsiella aerogenes (n = 4), Proteus vulgaris (n = 3), Providencia rettgeri (n = 3), Raoultella ornithinolytica (n = 3), Proteus hauseri (n = 2), Providencia stuartii (n = 2), Enterobacter nonspeciated (n = 1), Klebsiella nonspeciated (n = 1), Leclercia adecarboxylata (n = 1), Raoultella planticola (n = 1), Serratia liquefaciens (n = 1), and Serratia nonspeciated (n = 1).
Patients whose baseline pathogens were ciprofloxacin-resistant were older, more likely to be diabetic, and had diminished renal function compared with those who had ciprofloxacin-susceptible pathogens (Table 2).
Table 2.
Patient Demographics by Ciprofloxacin Susceptibility: Microbiologic Modified Intent-to-Treat Population
| Parameter | Ciprofloxacin-Susceptible (N = 542), n (%) |
Ciprofloxacin-Resistant (N = 341), n (%) |
P Valuea |
|---|---|---|---|
| Age, mean (SD), years | 55.3 (19.1) | 63.4 (15.3) | <.0001 |
| ȃMin, max | 18, 94 | 20, 89 | |
| Gender | <.001 | ||
| ȃMale | 188 (34.7) | 175 (51.3) | |
| ȃFemale | 354 (65.3) | 166 (48.7) | |
| Ethnicity | .192 | ||
| ȃHispanic or Latinx | 20 (3.7) | 10 (2.9) | |
| ȃNot Hispanic or Latinx | 518 (95.6) | 330 (96.8) | |
| ȃNot reported | 4 (0.7) | 0 | |
| ȃUnknown | 0 | 1 (0.3) | |
| Geographic region | .032 | ||
| ȃUnited States | 32 (5.9) | 9 (2.6) | |
| ȃNot the United States | 510 (94.1) | 332 (97.4) | |
| Race | .644 | ||
| ȃBlack or African American | 1/542 (0.2) | 0 | |
| ȃAsian | 3/542 (0.6) | 0 | |
| ȃWhite | 536/542 (98.9) | 341/341 (100.0) | |
| ȃNative Hawaiian or Pacific Islander | 1/542 (0.2) | 0 | |
| ȃOther | 1/542 (0.2) | 0 | |
| Diabetes mellitus | 71/542 (13.1) | 80/341 (23.5) | <.001 |
| Body mass index, mean (SD), kg/m2 | 26.9 (6.0) | 28.1 (5.5) | <.001 |
| Creatinine clearance, mean (SD), mL/minb | 75.9 (32.0) | 66.3 (28.0) | <.0001 |
One patient in the ertapenem arm did not have ciprofloxacin susceptibility testing done.
Abbreviation: SD, standard deviation.
Differences between treatment groups were analyzed using the Fisher exact test for categorical variables and the Wilcoxon rank sum test for continuous variables.
As reported by the site using the Cockcroft-Gault method based on local laboratory data.
Treatment
The median duration of IV therapy was 5 days for sulopenem and 6 days for ertapenem. The median duration of oral therapy was 4 days in both arms. More patients on sulopenem (603 of 695, 86.8%) were able to step down from IV to oral therapy than were able to do so in the ertapenem arm (463 of 697, 66.4%) due largely to the resistance of baseline pathogens to both fluoroquinolones and β–lactams.
Efficacy Outcomes
In the mMITT population, overall success was demonstrated in 67.8% of sulopenem patients and 73.9% of ertapenem patients (treatment difference −6.1%; 95% CI, −12.0 to −.1); the noninferiority of sulopenem to ertapenem was not established (Table 3). An analysis of overall success in an adjusted mMITT population, which included patients with baseline urine culture colony counts between 103 and 105 CFU/mL but had ≥105 copies/mL of a uropathogen by polymerase chain reaction (PCR), demonstrated a smaller treatment difference (Supplementary Table 2).
Table 3.
Primary and Additional Key Efficacy End Points
| Outcome | Sulopenem (N = 444), n (%) |
Ertapenem (N = 440), n (%) |
Difference, % (95% Confidence Interval) |
|---|---|---|---|
| Microbiologic modified intent-to-treat population | |||
| Overall response at TOC (primary end point) | |||
| ȃOverall responder | 301 (67.8) | 325 (73.9) | −6.1 (−12.0 to −.1) |
| ȃOverall nonresponder | 126 (28.4) | 93 (21.1) | |
| ȃIndeterminate | 17 (3.8) | 22 (5.0) | |
| Clinical response at TOC | |||
| ȃSuccess | 397 (89.4) | 389 (88.4) | 1.0 (−3.1 to 5.1) |
| ȃFailure | 33 (7.4) | 34 (7.7) | |
| ȃIndeterminate | 14 (3.2) | 17 (3.9) | |
| Microbiologic response per patient at TOC | |||
| ȃSuccess | 316 (71.2) | 343 (78.0) | −6.8 (−12.5 to −1.1) |
| ȃFailure | 111 (25.0) | 74 (16.8) | |
| ȃIndeterminate | 17 (3.8) | 23 (5.2) | |
| Overall success at TOC by baseline infection type | |||
| ȃPyelonephritis | 179/261 (68.6) | 186/257 (72.4) | −3.8 (−11.6 to 4.1) |
| ȃComplicated urinary tract infection | 122/183 (66.7) | 139/183 (76.0) | −9.3 (−18.5 to −.1) |
| Overall success at day 5 | |||
| ȃȃCure | 198 (44.6) | 193 (43.9) | 0.7 (−5.8 to 7.3) |
| ȃȃCure + Improveda | 360 (81.1) | 352 (80.0) | 1.1 (−4.2 to 6.3) |
| ȃClinical success | |||
| ȃȃCure | 203 (45.7) | 196 (44.5) | 1.2 (−5.4 to 7.7) |
| ȃȃCure + Improveda | 369 (83.1) | 362 (82.3) | 0.8 (−4.2 to 5.9) |
| ȃMicrobiologic success | 427 (96.2) | 419 (95.2) | 0.9 (−1.7 to 3.6) |
| Overall success at end of treatment (day 10) | 385 (86.7) | 391 (88.9) | −2.2 (−6.5 to 2.2) |
| ȃClinical success | 399 (89.9) | 399 (90.7) | −0.8 (−4.7 to 3.1) |
| ȃMicrobiologic success | 418 (94.1) | 421 (95.7) | −1.5 (−4.4 to 1.4) |
| Clinical success at final visit (day 28) | 386 (86.9) | 383 (87.0) | −0.1 (−4.5 to 4.3) |
| Clinical response at TOC (intention-to-treat population) | 615/697 (88.2) | 603/698 (86.4) | 1.8 (−1.6 to 5.3) |
| Clinical response at TOC ( modified intent-to-treat population) | 615/695 (88.5) | 603/697 (86.5) | 2.0 (−1.5 to 5.4) |
Abbreviation: TOC, test of cure.
The Improved category includes patients whose clinical signs and symptoms, while not resolved, had decreased in severity from baseline.
The difference between treatment groups was driven by microbiologic outcomes (Supplementary Table 3) . While patient-determined clinical success rates were high and similar in both treatment groups at TOC (89.4% for sulopenem, 88.4% for ertapenem; Table 3, Figure 2), microbiologic success rates were lower in the sulopenem group (71.2% for sulopenem vs 78.0% for ertapenem). This difference was due to the higher incidence of asymptomatic bacteriuria (ASB) in patients who received sulopenem (Table 4). This difference was observed primarily among the patients who had ciprofloxacin-susceptible uropathogens and, per protocol, received ciprofloxacin as step-down treatment. Of those who received IV sulopenem followed by oral sulopenem, 21.8% failed at TOC due to ASB compared with just 4.7% of those who received IV ertapenem followed by oral ciprofloxacin. Of the patients with ciprofloxacin-nonsusceptible uropathogens, those in the ertapenem group, depending on their clinical status, either received their entire treatment course intravenously or were stepped down to amoxicillin-clavulanate. Among these patients, ASB was the reason for failure in 21.8%, similar to the 19.8% in sulopenem patients. Asymptomatic bacteriuria at TOC did not contribute to higher rates of clinical failure at the final visit on day 28; 86.9% and 87.0% of sulopenem and ertapenem patients, respectively, remained clinical successes (Table 3). Success rates among patients with pyelonephritis or cUTIs were similar to the overall response of the combined group (Table 3). In assessing the efficacy of IV therapy at day 5, whether overall response, clinical response, or microbiologic response, the outcomes for sulopenem and ertapenem were similar (Table 3).
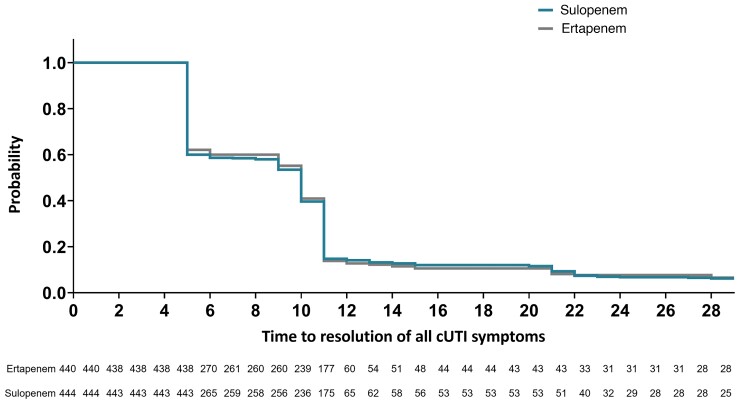
Figure 2.
Time to resolution (days) of all cUTI symptoms, survival and without nonstudy antibiotic use. Patients who received rescue antibiotic prior to resolution or who died without resolution were censored at day 29. Abbreviation: cUTI, complicated urinary tract infection.
Table 4.
Overall Response at Test of Cure by Step-Down Category: Microbiologic Modified Intent-to-Treat Population
| Outcome | Sulopenem, n/N (%) |
Ertapenem, n/N (%) |
Difference, % (95% Confidence Interval) |
|---|---|---|---|
| All patients | |||
| ȃPrimary end point: overall success (test of cure) | 301/444 (67.8) | 325/440 (73.9) | −6.1 (−12.0 to −.1) |
| ȃReason for failure: asymptomatic bacteriuria | 93 (20.9) | 59 (13.4) | |
| Patients with ciprofloxacin-susceptible isolates by treatment regimen | |||
| Sulopenem IV/oral sulopenem, n/N (%) | Ertapenem IV/oral ciprofloxacin, n/N (%) | ||
| ȃOverall success | 168/248 (67.7) | 186/215 (86.5) | −18.8 (−26.1 to −11.0) |
| ȃReason for failure: asymptomatic bacteriuria | 54 (21.8) | 10 (4.7) | |
| Sulopenem IV only | Ertapenem IV only or ertapenem IV/oral A/C, | ||
| ȃOverall success | 19/34 (55.9) | 17/32 (53.1) | 2.8 (−20.9 to 26.2) |
| ȃReason for failure: asymptomatic bacteriuria | 7 (20.6) | 7 (21.9) | |
| Patients with ciprofloxacin-nonsusceptible isolates by treatment regimen | |||
| Sulopenem IV only or sulopenem IV/oral sulopenem | Ertapenem IV or ertapenem IV/oral A/Ca | ||
| ȃOverall success | 114/162 (70.4) | 122/193 (63.2) | 7.2 (−2.7 to 16.8) |
| ȃReason for failure: asymptomatic bacteriuria | 32 (19.8) | 42 (21.8) | |
Abbreviations: A/C, amoxicillin-clavulanate; IV, intravenous.
Includes 5 patients for whom ciprofloxacin susceptibility testing was not performed.
In patients treated with sulopenem, the overall response at TOC was consistent across resistance classes (Table 5) and was not impacted by ESBL activity. Response rates among patients with fluoroquinolone-susceptible pathogens were higher in the ertapenem arm, as these individuals received oral ciprofloxacin for step-down therapy and had lower rates of ASB. Among those with pathogens that were resistant to either fluoroquinolones or TMP-SMX or resistant to all 3 antibiotic classes, sulopenem demonstrated efficacy comparable to ertapenem.
Table 5.
Overall Response at Test of Cure by Resistance Class: Microbiologic Modified Intent-to-Treat Population
| Outcome | Sulopenem, n/N (%) |
Ertapenem, n/N (%) |
Difference, % (95% Confidence Interval) |
|---|---|---|---|
| Overall success at test of cure (primary end point) | 301/444 (67.8) | 325/440 (73.9) | −6.1 (−12.0 to −.1) |
| ESBL-negative | 219/329 (66.6) | 238/311 (76.5) | −10.0 (−16.9 to −3.0) |
| ȃQuinolone-resistant | 47/69 (68.1) | 51/79 (64.6) | 3.6 (−12.0 to 18.6) |
| ȃQuinolone-susceptible | 172/260 (66.2) | 187/232 (80.6) | −14.4 (−22.0 to −6.7) |
| ESBL-positive | 79/110 (71.8) | 85/125 (68.0) | 3.8 (−7.9 to 15.5) |
| ȃQuinolone-resistant | 64/92 (69.6) | 64/99 (64.6) | 4.9 (−8.5 to 18.1) |
| ȃQuinolone-susceptible | 15/18 (83.3) | 21/26 (80.8) | 2.6 (−23.0 to 25.3) |
| Quinolone-resistant | 112/162 (69.1) | 116/179 (64.8) | 4.3 (−5.6 to 14.3) |
| Quinolone-susceptible | 189/282 (67.0) | 209/260 (80.4) | −13.4 (−20.7 to −6.1) |
| TMP-SMX–resistant | 106/154 (68.8) | 111/161 (68.9) | −0.1 (−10.3 to 10.1) |
| TMP-SMX–susceptible | 192/285 (67.4) | 212/275 (77.1) | −9.7 (−17.1 to −2.4) |
| ESBL-positive, quinolone-resistant, TMP-SMX–resistant | 43/59 (72.9) | 47/75 (62.7) | 10.2 (−5.5 to 26.0) |
ESBL testing was not performed on 5 sulopenem patients and 4 ertapenem patients.
Abbreviations: ESBL, extended-spectrum beta-lactamase; TMP-SMX, trimethoprim-sulfamethoxazole.
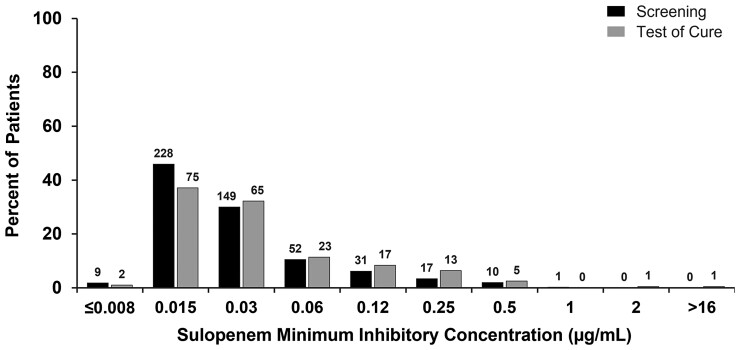
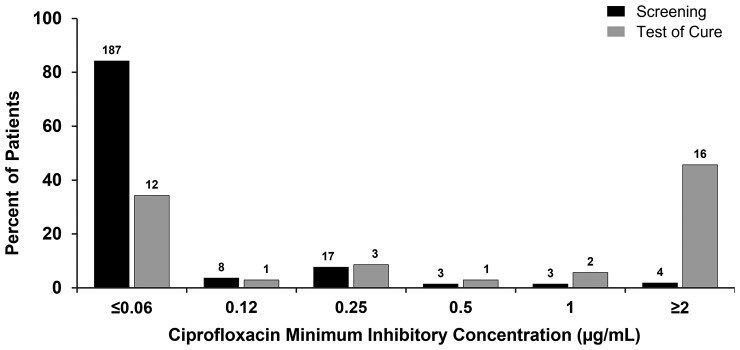
In order to explore the impact of each regimen on the distribution of in vitro susceptibility of organisms post-treatment (Supplemental Figures 1 and 2), an additional analysis was performed in which all Enterobacterales identified at any colony-forming units per milliliter in urine cultures, not just recurrent baseline pathogens, were examined before and after treatment. The distribution of sulopenem MICs did not differ after treatment with sulopenem (Figure 3). In the ertapenem group, however, step-down treatment with ciprofloxacin in patients with a ciprofloxacin-susceptible uropathogen at baseline selected for a ciprofloxacin-resistant organism in 16 of 35 (45.7%) patients with isolates identified at TOC (Table 6, Figure 4). The demographics of these patients were similar to those for patients who had ciprofloxacin-resistant pathogens at baseline (Table 2). PCR testing of the initial urine sample for these 16 patients did not reveal any evidence of a gene associated with quinolone resistance in any of the 15 evaluable specimens, confirming that a resistant clone was not present in the urine at baseline.
Figure 3.
Distribution of sulopenem minimum inhibitory concentrations in the sulopenem treatment group.
Table 6.
Patients With Ciprofloxacin-Susceptible Organisms at Baseline and a Ciprofloxacin-Resistant Organism at Test of Cure: Microbiologic Modified Intent-to-Treat Population
| Parameter | Ertapenem/Ciprofloxacin, n (%) |
|---|---|
| Total number of positive TOC cultures obtained from patients with ciprofloxacin-susceptible isolates at baseline (N) | 35 |
| Total number (%) of isolates that were ciprofloxacin-resistant in TOC cultures | 16 (45.7) |
| Age, years | |
| ȃMean (SD) | 63.9 (19.5) |
| ȃMin, max | 24.0, 87.0 |
| Gender | |
| ȃMale | 2 (12.5%) |
| ȃFemale | 14 (87.5%) |
| Ethnicity | |
| ȃHispanic or Latinx | 1 (6.3) |
| ȃNot Hispanic or Latinx | 14 (87.5) |
| ȃNot reported | 1 (6.3) |
| Geographic region | |
| ȃNot the United States | 16 (100.0) |
| Race | |
| ȃWhite | 16 (100.0) |
| Diabetes mellitus | 2 (12.5) |
| Body mass index, mean (SD), kg/m2 | 25.9 (4.6) |
| Creatinine clearance,a mean (SD), mL/min | 66.9 (25.5) |
Abbreviations: SD, standard deviation; TOC, test of cure.aCalculated by Cockroft-Gault method.
Figure 4.
Distribution of ciprofloxacin minimum inhibitory concentrations in ertapenem patients with ciprofloxacin-susceptible organisms at baseline.
Eighty-seven (9.8%) patients had baseline bacteremia associated with their cUTI, with similar numbers in each treatment regimen (Table 7). Overall response rates at day 5 were similar, implying that initial control of the infection occurred with each IV regimen and all patients cleared their bacteremia. Overall response rates at TOC for patients with bacteremia were higher for patients in the ertapenem arm, again driven by a lower rate of ASB in patients who received oral ciprofloxacin.
Table 7.
Patients With Baseline Bacteremia: Microbiologic Modified Intent-to-Treat Population
| Population/Response |
Sulopenem, n/N (%) |
Ertapenem, n/N (%) |
P Value | Difference, % (95% Confidence Interval) |
|---|---|---|---|---|
| Patients with uropathogen in baseline blood culture | 44 | 43 | .9454 | |
| ȃEscherichia coli | 39 | 39 | ||
| ȃKlebsiella pneumoniae | 1 | 4 | ||
| ȃProteus mirabilis | 2 | 0 | ||
| ȃMorganella morganii | 1 | 0 | ||
| ȃEnterobacter cloacae complex | 1 | 0 | ||
| Same bloodstream uropathogen present in baseline urine culture | ||||
| ȃPresent ≥1000 CFU/mL | 42 (95.5) | 40 (93.0) | ||
| ȃPresent ≥100 000 CFU/mL | 36 (81.8) | 37 (86.0) | ||
| ȃBaseline bloodstream uropathogen not present in screening urine culture | 2 (4.5) | 3 (7.0) | ||
| Time to clearance of bacteremia after first does of study drug | ||||
| ȃMean (standard deviation), h:min | 85:45 (62:53) | 75:40a (55:15) | .5700 | |
| ȃMedian, h:min | 66:15 | 68:46a | ||
| ȃRange, h:min | 17:50–256:00 | 16:45–260:00a | ||
| ȃCleared between day 0 and day5 | 38 | 38 | ||
| ȃCleared between day 5 and day 11 | 6 | 5 | ||
| Overall success at day 5 | 18/44 (40.9) | 16/43 (37.2) | 3.7 (−16.7 to 23.8) | |
| Overall success at test of cure | 25/44 (56.8) | 28/43 (65.1) | −8.3 (−28.1 to 12.2) | |
| ȃQuinolone-resistant | 5/10 (50.0) | 5/8 (62.5) | −12.5 (−52.5 to 2.7) | |
| ȃQuinolone-susceptible | 20/34 (58.8) | 23/35 (65.7) | −6.9 (−29.1 to 15.9) | |
| ȃESBL-negative | 20/36 (55.6) | 24/37 (64.9) | −9.3 (−30.9 to 13.1) | |
| ȃESBL-positive | 5/8 (62.5) | 4/6 (66.7) | −4.2 (−49.4 to 44.9) | |
| Reasons for overall nonresponse | ||||
| ȃUrine culture at the follow-up visit demonstrates ≥103 CFU/mL of the baseline uropathogen; all symptoms had resolved | 15/44 (34.1) | 5/43 (11.6) | ||
| ȃNo resolution or worsening of symptoms of cUTI present at trial entry and/or new cUTI symptoms | 2/44 (4.5) | 2/43 (4.7) | ||
| ȃReceipt of nonstudy antibacterial therapy for cUTI | 1/44 (2.3) | 0 | ||
| ȃUrine culture ≥103 and at least 1 symptom not resolved (both clinical and micro failures) | 0 | 2/43 (4.7) | ||
| ȃDeath due to cUTI | 0 | 0 | ||
Abbreviations: CFU, colony-forming unit; cUTI, complicated urinary tract infection; ESBL, extended-spectrum beta-lactamase.
Excluding 1 patient who cleared bacteremia before first dose of study drug (patient had received single dose of ceftriaxone before first dose of study drug). P value for the number of patients with bacteremia derived using the Cochran-Mantel-Haenszel test. P value for the time to clearance of bacteremia derived using the log-rank test.
Safety
At least 1 adverse event occurred in 15.1% and 16.4% of sulopenem and ertapenem recipients, respectively (Table 8). Adverse events were predominantly mild or moderate in intensity and generally balanced across groups. Fourteen (2.0%) and 6 (0.9%) patients treated with sulopenem and ertapenem, respectively, had ≥1 serious adverse event, none of which were felt to be related to study therapy. There were 2 deaths in the sulopenem treatment arm, both due to malignancy (salivary gland tumor, renal cell carcinoma). Very few patients discontinued the study drug because of adverse events. Adverse events reported in ≥2% of patients included headache and diarrhea, and there were no cases of Clostridioides difficile colitis. No clinically meaningful trends in laboratory values, or vital signs were identified.
Table 8.
Safety Evaluation Through Final Visit: Safety Population
| AE Category | Sulopenem (N = 695), n (%) |
Ertapenem (N = 697), n (%) |
|---|---|---|
| Any AE | 105 (15.1) | 114 (16.4) |
| Any drug-related AE | 42 (6.0) | 64 (9.2) |
| Any AE with an outcome of death | 2 (0.3) | 0 (0.0) |
| Any serious AE | 14 (2.0) | 6 (0.9) |
| Any AE leading to discontinuation of study drug | 3 (0.4) | 4 (0.6) |
| Any AE of severe intensity | 5 (0.7) | 5 (0.7) |
| AEs reported in ≥2% of patients in either treatment group by system organ class and preferred terma | ||
| Nervous system disorders | ||
| ȃHeadache | 21 (3.0) | 16 (2.3) |
| Gastrointestinal disorders | ||
| ȃDiarrhea | 19 (2.7) | 21 (3.0) |
Abbreviation: AE, adverse event.
Medical Dictionary for Regulatory Activities (version 16.1) classification. Patients with multiple AEs were counted once for each category or system organ class and/or preferred term. Patients with AEs in >1 category were counted once in each of those categories.
DISCUSSION
Using the US Food and Drug Administration’s (FDA) current definition of a successful response to treatment, that is, one that requires both clinical and microbiologic success, sulopenem was not noninferior to the comparator regimen. The difference in outcome rates was driven by a lower rate of ASB among patients who received ciprofloxacin as oral step-down therapy. The overall responses at the end of IV therapy (day 5) and at the end of treatment (EOT, day 10), as well as the clinical response at the final visit (day 28), were similar on each regimen. Among the approximately 40% of patients who had fluoroquinolone-resistant organisms, outcomes were also similar in the 2 treatment arms.
The prespecified statistical analysis plan clearly leads to the conclusion that sulopenem is not noninferior to ertapenem in the treatment of cUTIs. The clinical relevance of this conclusion, however, rests solely on whether or not ASB 11 days after completion of therapy increases the risk of near-term treatment failure. Based on the results of this study, with an identical clinical response 18 days after completion of treatment, that risk would appear to be low.
Why the rate of ASB was lowest after treatment with ciprofloxacin cannot be fully explained by the results from this study, but it is likely related to tissue concentrations of ciprofloxacin and their effect on recolonization of vaginal flora and, in turn, bladder mucosa [6–10]. While the rate of ASB was lower, the use of ciprofloxacin selected for Enterobacterales resistant to quinolones, possibly also a consequence of tissue penetration. Similar findings were observed in an uncomplicated UTI study comparing oral sulopenem to oral ciprofloxacin [11].
The rates of ASB differ by antibiotic class. For sulopenem, the ASB rate appears consistent with the treatment outcomes for penem antibiotics in every registrational study of cUTI performed in the last 10 years (Supplementary Table 4), including a recent study that compared the efficacy of the oral carbapenem, tebipenem, to that of IV ertapenem, which resulted in similar ASB rates at TOC (154 of 449, 34.3% for tebipenem and 134 of 419, 32.0% for ertapenem) [12], rates of ASB even higher than that observed for sulopenem in this trial (Table 4). In the current study, while 2 IV carbapenems were compared, ciprofloxacin was not provided to both arms as oral step-down, as in previous registrational studies; thus, for the first time, the differential effect of a fluoroquinolone on the incidence of post-treatment bacteriuria could be observed in this patient population.
Per current FDA guidance, the presence of ASB at TOC qualifies the patient as a microbiologic failure. While not prespecified in the analysis plan for this study, we explored alternative criteria for microbiologic failure. If, to qualify as a microbiologic failure, a patient needed to have ongoing bacteriuria at both the EOT and TOC visits, the overall success rates would be very similar in the 2 arms and the confidence interval much tighter (Supplementary Table 5). This analysis relies on the patient’s clinical outcome to define success yet allows for the possibility that truly persistent bacteriuria could pose a significant risk for future clinical failure.
Sulopenem appears to have comparable activity to that of ertapenem in patients with quinolone-resistant organisms, and oral sulopenem may provide an important option for step-down therapy. Until further clarity is provided on the relative importance of ASB, future studies to define sulopenem’s activity in this quinolone-resistant patient population should be considered.
Supplementary Material
Contributor Information
Michael W Dunne, Iterum Therapeutics, Old Saybrook, Connecticut, USA.
Steven I Aronin, Iterum Therapeutics, Old Saybrook, Connecticut, USA.
Anita F Das, Das Statistical Consulting, Guerneville, California, USA.
Karthik Akinapelli, Takeda Pharmaceuticals, Cambridge, MA, USA.
Jeanne Breen, Iterum Therapeutics, Old Saybrook, Connecticut, USA.
Michael T Zelasky, Johnson & Johnson, Cambridge, Massachusetts, USA.
Sailaja Puttagunta, Iterum Therapeutics, Old Saybrook, Connecticut, USA.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. Funding for this study was provided by Iterum Therapeutics.
References
- 1. Weiner LM, Webb AK, Limbago B, et al. . Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 2016; 37:1288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. US Department of Health and Human Services . Extended-spectrum beta-lactamase (ESBL) producing Enterobacteriaceae. In: Antibiotic resistance threats in the United States 2019. Washington, DC: Centers for Disease Control and Prevention, 2019: 83–4.
- 3. European Centre for Disease Prevention and Control . Surveillance Report. Surveillance of antimicrobial resistance in Europe 2018. Stockholm: European Centre for Disease Prevention and Control, 2019.
- 4. Critchley IA, Cotroneo N, Pucci MJ, Mendes R. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PLoS One 2019; 14:e0220265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karlowsky JA, Adam HJ, Baxter MR, et al. . In vitro activity of sulopenem, an oral penem, against urinary isolates of Escherichia coli. Antimicrob Agents Chemother 2018; 63:e01832–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hooton TM, Roberts PL, Stapleton AE. Cefpodoxime vs ciprofloxacin for short-course treatment of acute uncomplicated cystitis. A randomized trial. JAMA 2012; 307:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hooton TM, Scholes D, Gupta K, Stapleton AE, Roberts PL, Stamm WE. Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women. A randomized trial. JAMA 2005; 293:949–55. [DOI] [PubMed] [Google Scholar]
- 8. Brannon JR, Dunigan TL, Beebout CJ, et al. . Invasion of vaginal epithelial cells by uropathogenic Escherichia coli. Nat Commun 2020; 11:2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Forrest A, Nix DE, Ballow CH, Goss TF, Birmingham MC, Schentag JJ. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother 1993; 37:1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas-White K, Forster SC, Kumar N, et al. . Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat Commun 2018; 9:1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunne MW, Aronin SI, Das AF, et al. Sulopenem or ciprofloxacin for the treatment of uncomplicated urinary tract infections in women: a phase 3 randomized trial. Clin Infect Dis 2023; 76:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eckburg PB, Muir L, Critchley IA, et al. . Oral tebipenem pivoxil hydrobromide in complicated urinary tract infection. N Engl J Med 2022; 386:1327–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.