Abstract
Background
It is unclear whether low-level viremia (LLV), defined as repeatedly detectable viral load (VL) of <200 copies/mL, and/or transient viremic episodes (blips) during antiretroviral therapy (ART), predict future virologic failure. We investigated the association between LLV, blips, and virologic failure (VF) in a multicenter European cohort.
Methods
People with HIV-1 who started ART in 2005 or later were identified from the EuResist Integrated Database. We analyzed the incidence of VF (≥200 copies/mL) depending on viremia exposure, starting 12 months after ART initiation (grouped as suppression [≤50 copies/mL], blips [isolated VL of 51–999 copies/mL], and LLV [repeated VLs of 51–199 copies/mL]) using Cox proportional hazard models adjusted for age, sex, injecting drug use, pre-ART VL, CD4 count, HIV-1 subtype, type of ART, and treatment experience. We queried the database for drug-resistance mutations (DRM) related to episodes of LLV and VF and compared those with baseline resistance data.
Results
During 81 837 person-years of follow-up, we observed 1424 events of VF in 22 523 participants. Both blips (adjusted subhazard ratio [aHR], 1.7; 95% confidence interval [CI], 1.3–2.2) and LLV (aHR, 2.2; 95% CI, 1.6–3.0) were associated with VF, compared with virologic suppression. These associations remained statistically significant in subanalyses restricted to people with VL <200 copies/mL and those starting ART 2014 or later. Among people with LLV and genotype data available within 90 days following LLV, 49/140 (35%) had at least 1 DRM.
Conclusions
Both blips and LLV during ART are associated with increased risk of subsequent VF.
Keywords: HIV-1, low-level viremia, treatment failure, viral blips
Retrospective analysis of 22 523 people with HIV-1 receiving antiretroviral therapy indicates that both viral blips and low-level viremia of 51 to 199 copies/mL in repeated measurements are independent predictors of subsequent virologic failure.
Plasma human immunodeficiency virus-1 (HIV-1) RNA (viral load, VL) is the primary marker of response to antiretroviral therapy (ART), and most people on ART achieve continuous viral suppression. Still, other viremia patterns are found in some ART recipients, a phenomenon that has become more apparent as assays with a limit of quantification of <50 copies/mL are increasingly used in high-income settings. For example, a recent US study reported that 46% of ART recipients had ≥1 detectable VL without meeting criteria for virologic failure (VF), including both transient viremia (“blips”) and sustained low-level viremia (LLV) [1].
The underlying mechanisms for these viremia patterns remain incompletely understood, and data regarding the clinical significance of lack of complete and persistent viral suppression are limited. Increased risk of subsequent VF [1–6] and all-cause mortality [7] has been reported for different amplitudes of LLV, and blips have been associated with VF in some [8, 9], but not all, studies [5, 10]. The association between LLV and drug-resistance mutations (DRMs) also remains uncertain, especially because most studies on LLV and DRM have included participants with VL exceeding 200 copies/mL [11–18].
LLV is usually defined as detectable viremia below the threshold for VF, which varies between guidelines. Globally, the World Health Organization defines VF as repeated VL ≥1000 copies/mL [19], whereas a threshold of 200 copies/mL is commonly used in high-income settings [20–22]. The European AIDS Clinical Society defines VF as VL ≥50 copies/mL [23], but solid evidence for this lower threshold is lacking. Considering these discordances, we decided to perform a retrospective analysis of a large European cohort, with the aim to determine if LLV in the range of 51 to 199 copies/mL, as well as viral blips, are associated with increased risk of subsequent VF. In addition, we analyzed patterns of DRM in relation to LLV and VF.
METHODS
Setting and Participants
This study is based on the EuResist Integrated Database (EIDB, www.euresist.org/eidb), which includes >100 000 people with HIV-1 (PWH). EIDB contains demographic data, VL measurements, CD4 counts, data on ART regimens, as well as HIV-1 sequences (for subtype classification and genotypic drug-resistance analysis). For this study, the EIDB was queried in May 2021, and the last recorded VL was from 26 December 2020. Ethical approval was granted in the host countries of the respective origin databases contributing data to EIDB. The researchers only had access to anonymized data.
We included PWH who started ART 1 January 2005, or later from the following origin databases: CoRIS and IrsiCaixa (Spain), ARCA (Italy), Karolinska Institute (Sweden), AREVIR (Germany), Laboratoire de Rétrovirologie of CRP-Santé (Luxembourg), Instituto de Higiene e Medicina (Portugal), and Rega Institute (Belgium). ART was defined as ≥3 antiretroviral drugs (apart from booster agents) representing >1 drug class (including dolutegravir + lamivudine 2-drug regimens). Individuals with 2 discordant detectable VL results recorded on the same day were excluded, as were those with VLs measured with an assay with a lower limit of quantification >50 copies/mL. Participants could only be included once and were followed from the date of the first VL >12 months after ART initiation until incident VF, loss to follow-up (>365 days between VL measurements), or administrative censoring 31 December 2020.
Definition of Exposure and Outcome Variables
The main exposure of interest was the pattern of viremia during ART. Participants were classified into 3 groups: (1) virologic suppression ≤50 copies/mL; (2) blips (defined as 1 VL of 51–999 copies/mL preceded and followed by VL of ≤50 copies/mL; this group also included persons with several VLs of 51–999 copies/mL within ≤30 days); and (3) LLV (defined as ≥2 consecutive VLs of 51–199 copies/mL ≥30 days apart). Isolated detectable VLs of 51 to 999 copies/mL for which the criteria for blips or LLV could not be confirmed (eg, measurements followed by VF or loss to follow-up) were classified in the suppression group. Episodes with one VL of 51 to 199 copies/mL together with one VL of 200 to 999 copies/mL, followed by <200 copies/mL (consequently not meeting the definition of VF), were categorized as LLV for the main analysis. We also performed a sensitivity analysis where the categories blips and LLV were restricted to participants with VLs of 51 to 199 copies/mL by right-censoring participants who had subsequent VL of ≥200 copies/mL without meeting the definition of VF. Viremia was included as a time-varying covariate and reclassification was only possible to a higher group; consequently, the variable viremia category reflected the highest historical viremia exposure for each person (Supplementary Table 1). We also performed a separate analysis in which persons with detectable viremia during ART were separated into 2 subgroups (VL of ≥51 copies/mL without meeting criteria for VF in <25% and ≥25% of VL measurements, respectively), and compared with participants with suppression.
The study outcome was VF, which was defined as 2 consecutive VLs of ≥200 copies/mL or a single VL of ≥1000 copies/mL while on ART. We queried EIDB for DRM from reverse transcriptase (RT), protease, and integrase regions obtained in connection to LLV (date of first VL during a LLV episode and 90 days thereafter), as well as pre-ART DRM in the same individuals. Furthermore, we analyzed DRMs from participants who developed VF during the study period and compared the DRM profiles depending on previous viremia category. We considered major DRMs for protease inhibitors (PI), nucleoside/nucleotide reverse transcriptase inhibitors (NRTI), nonnucleoside reverse transcriptase inhibitors (NNRTI), and integrase strand transfer inhibitors (INSTI), as defined by the Stanford University HIV Drug Resistance Database [24].
Statistical Methods
To compare baseline characteristics across viremia categories, we used Pearson's χ2 tests for categorical and Kruskal–Wallis tests for continuous variables. Pearson's χ2 test was also used to compare the proportion of DRM between individuals of different viremia categories.
We fitted Cox regression models for the risk of VF by viremia category. The models were stratified by origin database, and the following variables were included to adjust for potential confounders: age (modelled linearly), sex (male/female), CD4 count (modelled linearly, time-updated), VL before start of ART (modelled logarithmically), transmission group (injecting drug use [IDU]/non-IDU), subtype (A/B/C/other), regimen type (NNRTI-based/PI-based/INSTI-based/other), and treatment experience. We tested the proportional hazard assumption by assessing Schoenfeld residuals. Because of missing data, we were not able to adjust for ethnicity; however, as a sensitivity analysis, we adjusted for this variable among those with complete data. In subanalyses, only participants starting ART 2014 or later and participants starting INSTI-based ART, respectively, were included. We handled missing data using a “complete case” approach, and after inspecting the pattern of missing data, we performed 2 separate regression models (not adjusting for subtype and CD4 counts, respectively), to check the robustness of our results.
RESULTS
Participant Characteristics
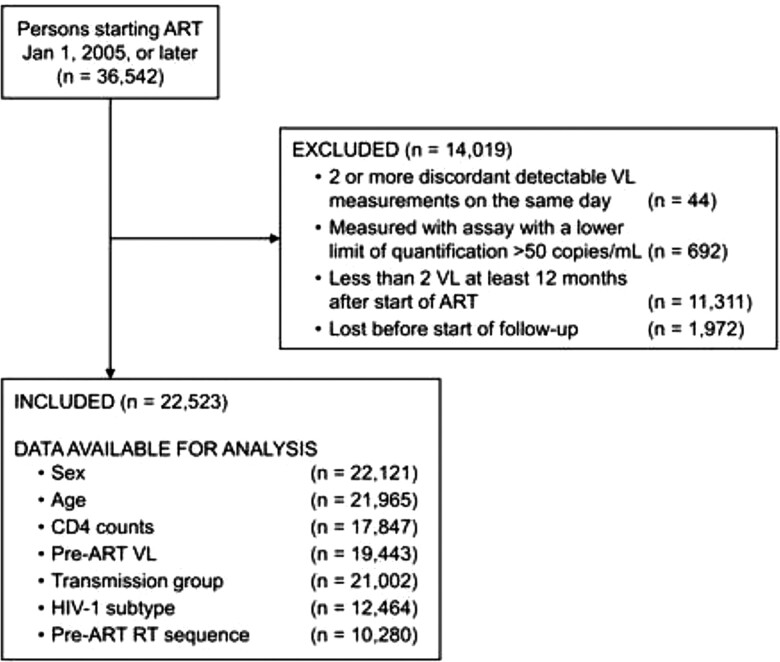
Of 36 542 persons registered in EIDB who started ART 2005 or later, 22 523 (62%) met our inclusion criteria (Figure 1). A majority were male (76%), and the median age was 38 years. Participants had a median number of VL measurements of 7 (interquartile range, 3–14), with a median of 2.6 measurements per year. At the end of follow-up, the distribution of viremia categories was: virologic suppression 77%, blips 16%, and LLV 7%. Participants with LLV had higher median VL and lower CD4 cell counts before starting ART (P < .001) (Table 1). Among 17 369 individuals classified as suppression, 1139 (7%) had recorded single detectable VLs of 51 to 999 copies/mL without meeting criteria for blips or LLV. Of 3500 individuals classified as blip at the end of follow-up, 67% only had 1 blip during follow-up and 23% had 2 separate blips.
Figure 1.
Exclusion Flowchart of Study Participants. Abbreviations: ART, antiretroviral therapy; RT, reverse transcriptase; VL, viral load.
Table 1.
Characteristics of Study Participants From EuResist Integrated Database
| Virologic Suppression (n = 17 369; 77%) | Viral Blips (n = 3500; 16%) | LLV 51–199 Copies/mL (n = 1654; 7%) | P a | |
|---|---|---|---|---|
| Sexb | .85 | |||
| ȃMale | 13 005 (76%) | 2616 (76%) | 1255 (76%) | |
| Age (y) | 38 (31–46) | 39 (32–47) | 40 (33–47) | <.001 |
| Region of origin | <.001 | |||
| ȃEuropean region | 10 560 (72%) | 2141 (71%) | 1000 (70%) | |
| ȃAfrican region | 2149 (15%) | 519 (17%) | 285 (20%) | |
| ȃRegion of the Americas | 1277 (9%) | 205 (7%) | 90 (6%) | |
| ȃSouth-East Asia region | 401 (3%) | 112 (4%) | 19 (1%) | |
| ȃEastern Mediterranean region | 126 (1%) | 21 (1%) | 24 (2%) | |
| ȃWestern Pacific region | 131 (1%) | 22 (1%) | 6 (0%) | |
| ȃUnknown | 39 (0%) | 1 (0%) | 4 (0%) | |
| Database of origin | <.001 | |||
| ȃCoRIS (Spain) | 5796 (33%) | 1085 (31%) | 459 (28%) | |
| ȃARCA (Italy) | 4521 (26%) | 820 (23%) | 429 (26%) | |
| ȃKarolinska Institute (Sweden) | 3723 (21%) | 889 (25%) | 389 (24%) | |
| ȃAREVIR (Germany) | 2609 (15%) | 613 (18%) | 289 (17%) | |
| ȃLaboratoire de Rétrovirologie of CRP-Santé (Luxembourg) | 335 (2%) | 42 (1%) | 22 (1%) | |
| ȃIrsiCaixa (Spain) | 179 (1%) | 30 (1%) | 12 (1%) | |
| ȃInstituto de Higiene e Medicina Tropical (Portugal) | 140 (1%) | 14 (0%) | 14 (1%) | |
| ȃRega Institute (Belgium) | 66 (0%) | 7 (0%) | 40 (2%) | |
| Transmission group | <.001 | |||
| ȃMale-to-male sex | 7585 (47%) | 1403 (42%) | 619 (40%) | |
| ȃHeterosexual contact | 5728 (36%) | 1312 (39%) | 571 (37%) | |
| ȃInjecting drug use | 1170 (7%) | 240 (7%) | 130 (8%) | |
| ȃOtherc | 518 (3%) | 145 (4%) | 87 (6%) | |
| ȃUnknown | 1114 (7%) | 244 (7%) | 139 (9%) | |
| Pre-ART CD4 cell counts (cells/µL) | 323 (206–467) | 250 (131–366) | 236 (110–367) | <.001 |
| Pre-ART VL (log10 copies/mL) | 4.6 (3.9–5.1) | 4.9 (4.4–5.4) | 5.2 (4.5–5.7) | <.001 |
| Median year of ART start | 2011 | 2009 | 2009 | <.001 |
| Initial ART regimen | <.001 | |||
| ȃNNRTI-based | 8272 (48%) | 1538 (44%) | 560 (34%) | |
| ȃPI-based | 4770 (27%) | 1456 (42%) | 796 (48%) | |
| ȃINSTI-based | 3896 (22%) | 377 (11%) | 210 (13%) | |
| ȃOther/combinations | 431 (2%) | 129 (4%) | 88 (5%) | |
| Use of antiretrovirals before the start of ART | 246 (1%) | 57 (2%) | 36 (2%) | .04 |
| HIV-1 subtype | <.001 | |||
| ȃA | 481 (5%) | 109 (6%) | 61 (6%) | |
| ȃB | 5301 (56%) | 1039 (54%) | 487 (45%) | |
| ȃC | 769 (8%) | 169 (9%) | 88 (8%) | |
| ȃOtherd | 2898 (31%) | 615 (32%) | 447 (41%) | |
| Any pre-ART NRTI resistance mutations | 681 (8%) | 79 (8%) | 57 (10%) | .21 |
Results are No. (%) or median (interquartile range). Participants are grouped by the last viremia category they belonged to during follow-up.
Abbreviations: ARCA, Antiretroviral Resistance Cohort Analysis; ART, antiretroviral therapy; CoRIS, Cohorte de la Red de Investigación en Sida; CRP-Santé, Centre de Recherche Public de la Santé; INSTI, integrase strand transfer inhibitor; LLV, low-level viremia; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; VL, viral load.
P values are the result of Kruskal–Wallis tests for continuous variables and Pearson χ2 tests for categorical variables.
In addition, 3 persons had undifferentiated sex and 26 had unknown sex.
Other (n = 476), mother–child (n = 184), and blood products (n = 90).
Including sequences with ambiguous result.
Association Between Viremia Category and Incident Virological Failure
During 81 837 person-years of follow-up (median, 2.8 years), 1424 events of VF were recorded; in 1025 (72%) cases, VF occurred in persons who previously had virologic suppression, 216 (15%) in persons with blips, and 183 (13%) with LLV. The overall incidence rate of VF was 17 per 1000 person-years. Following the first detectable VL, the absolute 2-year risk of VF was 12% among 987 participants with LLV and 5% among 2482 participants with blips.
In unadjusted Cox regression, LLV was associated with increased risk of VF (Table 2). This association remained significant after adjustment for potential confounders (adjusted hazard ratio [aHR], 2.2; 95% confidence interval [CI], 1.6–3.0). Blips were also associated with a statistically significant elevated risk of VF, although the effect size was smaller (aHR, 1.7; 95% CI, 1.3–2.2). Apart from viremia category, younger age, female sex, lower CD4 count, higher pre-ART VL, IDU, and treatment experience had statistically significant associations with VF. Compared with NNRTI-based ART, PI-based ART was associated with increased risk of VF (aHR, 1.5, 95% CI, 1.2–1.8), whereas INSTI-based ART was not (aHR, 1.0; 95% CI, .7–1.3) (Supplementary Table 2). For the fully adjusted model, 15 873 participants were excluded because of missing data. The 2 dominating patterns were missing only subtype (n = 7492) and missing only CD4 count (n = 3231); the adjusted model was rerun excluding subtype and CD4, respectively, with similar results as our main analysis (Supplementary Table 3). The associations between blips, LLV, and VF were statistically significant also in a subanalysis excluding participants starting ART before 2014 (Supplementary Table 4).
Table 2.
Cox Regression Models for Virological Failure Depending on Viremia Category, Stratified by Origin Database
| Unadjusted Model | Fully Adjusted Modela | |
|---|---|---|
| (n = 22 523) | (n = 6650) | |
| Virologic suppression | 1 (Ref.) | 1 (Ref.) |
| Viral blips | 1.4 (1.2–1.7) | 1.7 (1.3–2.2) |
| LLV 51–199 copies/mL | 2.6 (2.3–3.1) | 2.2 (1.6–3.0) |
Results are hazard ratio with 95% confidence interval.
Abbreviations: ART, antiretroviral therapy; IDU, injecting drug use; LLV, low-level viremia; Ref, reference category; VL, viral load.
Adjusted for age (at outcome event), sex (male/female), CD4 count (modelled linearly, time-updated), pre-ART VL (modelled logarithmically), HIV-1 subtype (B/non-B), transmission group (IDU/non-IDU), type of ART (time-updated) and treatment experience.
When restricting the definition of LLV by right-censoring individuals with VL of ≥200 copies/mL without meeting definitions of VF, the risk of VF remained statistically significant both for LLV (aHR, 2.0; 95% CI, 1.4–2.9) and blips (aHR, 1.5; 95% CI, 1.1–2.1). A separate analysis in which viremia was grouped as continuous suppression, LLV in <25% of measurements, and LLV in ≥25% of measurements, respectively, showed elevated risk of VF for both LLV subgroups (Supplementary Table 5). Among cases with recorded ethnicity, the associations between blips/LLV and VF remained after adjustment for ethnicity (Supplementary Tables 6 and 7). In a subanalysis of PWH with INSTI-based initial regimens, we observed no indication of increased risk of VF in relation to blips or LLV, although this analysis was limited by few outcome events (Supplementary Table 8).
Drug Resistance Mutation Patterns in Participants With low-level Viremia and Virologic Failure
Among 1654 persons who experienced LLV during the study period, 140 (8%) had a registered resistance test within 90 days of the first VL recorded during LLV. Of these, 49 (35%) had ≥1 DRM. Mutations associated with reduced susceptibility to NNRTI were most frequent (n = 33; 24%). Compared to pre-ART DRM data, which were available for 89/140, a new DRM was found in 16 individuals (18%); 7 of these met the strict definition of LLV, whereas 9 had single VLs of 200 to 999 copies/mL (Supplementary Table 9). None of these 16 individuals developed VF during the first 90 days following LLV, indicating that these mutations emerged during LLV. Seven of these individuals (44%) developed VF later during follow-up. The most common mutation emerging was M184V/I, which was detected in 6 (38%) individuals. Thirteen individuals had new DRMs associated with reduced susceptibility to the drug class used at the time of sampling.
We also compared resistance profile among participants with VF depending on previous viremia exposure. Among 1424 cases of VF, 338 (24%) had a recorded DRM result obtained within the first year after incident VF. The median time between VF and HIV-1 genotyping was 77 days (interquartile range, 23–173). Among persons with VF who were classified as LLV, 42% had detectable DRMs; the corresponding proportions were 50% and 59% for viral suppression and blips, respectively (Table 3; P = .20). The most common mutations observed were M184V/I (n = 100; 31% of those with an RT sequence) and K103N/S (n = 37; 11%), with approximately similar occurrence across viremia categories (data not shown).
Table 3.
Drug Resistance Mutations Among Participants With Virologic Failure
| Any Drug Resistance Mutationsa | NRTI-resistance Mutationsb | NNRTI-resistance Mutationsb | PI-resistance Mutationsc | INSTI-resistance Mutationsd | |
|---|---|---|---|---|---|
| Virologic suppression | 113 (50%) | 82 (37%) | 74 (34%) | 18 (8%) | 10 (16%) |
| Viral blips | 33 (59%) | 23 (43%) | 21 (40%) | 4 (7%) | 5 (21%) |
| LLV 51–199 copies/mL | 24 (42%) | 18 (33%) | 13 (24%) | 4 (7%) | 3 (14%) |
Abbreviations: IN, integrase; INSTI, integrase strand transfer inhibitor; LLV, low-level viremia; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PR, protease; RT, reverse transcriptase; VF, virologic failure.
n = 338 had any recorded drug resistance testing within the first year after VF.
n = 327 had a recorded RT sequence within the first year after VF.
n = 330 had a recorded PR sequence within the first year after VF.
n = 107 had a recorded IN sequence within the first year after VF.
DISCUSSION
In this study, based on a large European cohort, people with LLV (defined as repeated VLs of 51–199 copies/mL), as well as those with viral blips, had increased risk of VF. In contrast to our findings, several previous studies did not report increased risk of failure in patients with LLV of <200 copies/mL [2–4]. Importantly, these investigators used less strict definitions of VF (repeated VLs of ≥500 and 1000 copies/mL, respectively), and participants who developed viremia of ≥200 copies/mL without meeting the study definition of VF were reclassified before reaching the endpoint. Thus, these studies might have underestimated the risk of failure for LLV of <200 copies/mL. Indeed, studies using 200 copies/mL to define VF have consistently shown LLV to be associated with increased risk of VF [1, 5, 25, 26]. We believe that the current study is the largest analysis hitherto from a high-income setting exploring virological outcomes of LLV and blips and adds to this body of literature, indicating the relevance of LLV of <200 copies/mL as a predictor of VF.
Whether episodes of transient viremia, commonly called blips, during ART are also related to subsequent VF is more controversial. Several studies did not observe increased risk of subsequent VF in people with blips [5, 10], although the CIs are relatively wide and do not exclude an association of the size reported in this study (aHR, 1.7). The magnitude of transient viremia might impact these associations; however, increased risk of VF has been demonstrated both for blips of ≥500 [9] and 50 to 500 copies/mL [8]. In our study, the association with VF remained statistically significant when the definition of blips was restricted to <200 copies/mL, which is, to our knowledge, a novel finding. Instead of separating transient and repeated VLs in the low-level range, Joya et al used proportions of VL measurements to categorize participants with regard to LLV persistence [1]. Intriguingly, they found the risk of VF to be lower for people with LLV in <25% of measurements compared to persons with persistent viral suppression (aHR, 0.33; 95% CI, .21–.52). In our study, a similar analysis showed an association with VF both for persons with LLV in <25% and ≥25% of measurements, with a dose–response relationship between proportion of VLs and VF. Conceivably, detectable viremia during ART exists on a spectrum where patients may show intermittent or persistent LLV depending on sampling frequency and assay variation; hence, it seems plausible that these categories could have similar relationships with VF.
Genotypic drug resistance results obtained in relation to episodes of LLV were only available from 8% of study participants. Thus, our data cannot reliably estimate to which extent the increased risk of VF is explained by selection of DRMs during LLV. Nevertheless, we observed emergence of new DRMs during LLV even when no VL of >200 copies/mL was recorded. Detection of new DRMs not present before ART have previously been reported in 4/23 (17%) of participants with LLV of 20 to 250 copies/mL in a study from Belgium [18]. Our findings thus provide support for European AIDS Clinical Society's recommendation to perform drug resistance testing in people with LLV and to modify ART accordingly, or, if no DRMs are detected, to maintain the current regimen, provided that it has a high barrier to resistance [23].
LLV is considered to arise either from ongoing viral replication and/or release of virions from latently infected cells [27]. The relative contribution of these mechanisms has only been investigated in small populations; in 1 study, none of 18 patients with persistent LLV of 20 to 250 copies/mL had signs of ongoing replication [28]. In our study, as well as in several previous reports, LLV during ART has been associated with higher pre-ART VL and lower CD4 count [4, 29, 30], suggesting that people with LLV have more advanced HIV infection before starting ART. In turn, this is consistent with a larger viral reservoir, which has been linked to both blips and LLV [31]. However, our observation of emergent DRMs during LLV episodes implies that LLV, even in this low range, could be associated with ongoing replication, at least in some individuals. In this context, suboptimal adherence could act as a confounder for the relationship between LLV and VF [32], as could pre-ART DRM [29]; the lack of adherence data is a limitation of our study in this respect. Considering the possibility of confounding, here exemplified by reservoir size, adherence, and pre-ART resistance, a causal relationship between LLV and VF cannot be established from our study. Notwithstanding, our data indicate that both transient viral blips and LLV are predictors for subsequent VF.
Except for the risk of residual confounding, some further limitations should be mentioned. First, even though we analyzed a large contemporary material, we have comparatively limited data on INSTI-based regimens, and although we found no indication that blips/LLVs were associated with increased risk of VF among INSTI recipients, this analysis was limited by few events of VF. As follow-up time with this drug class accumulates, future studies may determine the relevance of our results for PWH on INSTI-based ART. Similarly, because we only had genotype data for a small proportion of LLV cases, these results should mainly be considered as hypothesis generating. Second, although the size of our cohort is an important strength of our study, it only includes PWH living in Europe. Furthermore, it does not represent a population-based sample, and whether the study population is generalizable to the whole population of PWH might vary between origin databases. We were not able to adjust for ethnicity in our main analysis, although the associations between blips/LLV and VF remained in a subanalysis of complete cases. Third, the clinical management of HIV has changed considerably during the study period; still, our results were similar in a subanalysis of participants starting ART 2014 or later. Last, we lack data on clinical consequences and thus cannot analyze whether the observed increase in VF risk also leads to higher morbidity and mortality. Higher mortality has previously been reported for people with LLV 50 to 199 copies/mL; of note, this was likely not related to VF because participants with virologic progression were reclassified before death [7].
In conclusion, our data show that detectable viremia in the range 51 to 199 copies/mL, both viral blips and prolonged viremic episodes, is associated with subsequent virologic failure, implying that these patterns of viremia during ART should be considered as predictors of emerging treatment failure. Future studies should explore whether targeted interventions could mitigate the risk for subsequent VF for these patients.
Supplementary Material
Contributor Information
Olof Elvstam, Department of Translational Medicine, Lund University, Malmö, Sweden; Department of Infectious Diseases, Växjö Central Hospital, Växjö, Sweden.
Kasper Malmborn, Department of Translational Medicine, Lund University, Malmö, Sweden.
Sixten Elén, Department of Translational Medicine, Lund University, Malmö, Sweden.
Gaetano Marrone, Department of Infectious Diseases and Clinical Virology, Karolinska University Hospital, Stockholm, Sweden.
Federico García, Servicio de Microbiología, Hospital Clinico Universitario San Cecilio, Instituto de Investigacíon Ibs. Granada, Ciber de Enfermedades Infecciosas, CIBERINFEC, Granada, Spain.
Maurizio Zazzi, Department of Medical Biotechnologies, University of Siena, Siena, Italy.
Anders Sönnerborg, Division of Infectious Diseases, Department of Medicine Huddinge, Karolinska Institutet, Stockholm, Sweden; Department of Infecious Diseases, Karolinska University Hospital, Stockholm, Sweden.
Michael Böhm, Institute of Virology, Faculty of Medicine and University Hospital of Cologne, University of Cologne, Cologne, Germany.
Carole Seguin-Devaux, Department of Infection and Immunity, Luxembourg Institute of Health, Esch sur Alzette, Luxembourg.
Per Björkman, Department of Translational Medicine, Lund University, Malmö, Sweden; Department of Infectious Diseases, Skåne University Hospital, Malmö, Sweden.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the Department of Research and Development, Region Kronoberg, Växjö (0825-011 8298 to O. E.), the Swedish State under the agreement between the Swedish government and the county councils, the ALF agreement (ALFSUS-40103 to P. B.), Region Skåne (REG 821541 to P. B.), Skåne University Hospital donation funds (20200907 to P. B.). The funders had no impact on the study design, data collection, decision to publish, or preparation of the manuscript.
References
- 1. Joya C, Won SH, Schofield C, et al. Persistent low-level viremia while on antiretroviral therapy is an independent risk factor for virologic failure. Clin Infect Dis 2019; 69:2145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antiretroviral Therapy Cohort Collaboration, Vandenhende MA, Ingle S, et al. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS 2015; 29:373–83. [DOI] [PubMed] [Google Scholar]
- 3. Elvstam O, Medstrand P, Yilmaz A, et al. Virological failure and all-cause mortality in HIV-positive adults with low-level viremia during antiretroviral treatment. PLoS One 2017; 12:e0180761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernal E, Gómez JM, Jarrín I, et al. Low-level viremia is associated with clinical progression in HIV-infected patients receiving antiretroviral treatment. J Acquir Immune Defic Syndr 2018; 78:329–37. [DOI] [PubMed] [Google Scholar]
- 5. Fleming J, Mathews WC, Rutstein RM, et al. Low-level viremia and virologic failure in persons with HIV infection treated with antiretroviral therapy. AIDS 2019; 33:2005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hermans LE, Moorhouse M, Carmona S, et al. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis 2018; 18:188–97. [DOI] [PubMed] [Google Scholar]
- 7. Elvstam O, Marrone G, Medstrand P, et al. All-cause mortality and serious non-AIDS events in adults with low-level human immunodeficiency virus viremia during combination antiretroviral therapy: results from a Swedish Nationwide Observational Study. Clin Infect Dis 2021; 72:2079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sörstedt E, Nilsson S, Blaxhult A, et al. Viral blips during suppressive antiretroviral treatment are associated with high baseline HIV-1 RNA levels. BMC Infect Dis 2016; 16:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grennan JT, Loutfy MR, Su D, et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis 2012; 205:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Havlir DV, Bassett R, Levitan D, et al. Prevalence and predictive value of intermittent viremia with combination HIV therapy. J Am Med Assoc 2001; 286:171–9. [DOI] [PubMed] [Google Scholar]
- 11. Mackie NE, Phillips AN, Kaye S, Booth C, Geretti AM. Antiretroviral drug resistance in HIV-1-infected patients with low-level viremia. J Infect Dis 2010; 201:1303–7. [DOI] [PubMed] [Google Scholar]
- 12. Taiwo B, Gallien S, Aga E, et al. Antiretroviral drug resistance in HIV-1-infected patients experiencing persistent low-level viremia during first-line therapy. J Infect Dis 2011; 204:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delaugerre C, Gallien S, Flandre P, et al. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PLoS One 2012; 7:e36673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villalobos C, Ceballos ME, Ferres M, Palma C. Drug resistance mutations in proviral DNA of HIV-infected patients with low level of viremia. J Clin Virol 2020; 132:104657. [DOI] [PubMed] [Google Scholar]
- 15. Swenson LC, Min JE, Woods CK, et al. HIV drug resistance detected during low-level viraemia is associated with subsequent virologic failure. AIDS 2014; 28:1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kao S-W, Liu Z-H, Wu T-S, et al. Prevalence of drug resistance mutations in HIV-infected individuals with low-level viraemia under combination antiretroviral therapy: an observational study in a tertiary hospital in Northern Taiwan, 2017-19. J Antimicrob Chemother 2021; 76:722–8. [DOI] [PubMed] [Google Scholar]
- 17. Li JZ, Gallien S, Do TD, et al. Prevalence and significance of HIV-1 drug resistance mutations among patients on antiretroviral therapy with detectable low-level viremia. Antimicrob Agents Chemother 2012; 56:5998–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vancoillie L, Mortier V, Demecheleer E, et al. Drug resistance is rarely the cause or consequence of long-term persistent low-level viraemia in HIV-1-infected patients on ART. Antiviral Therapy 2015; 20:789–94. [DOI] [PubMed] [Google Scholar]
- 19. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva: World Health Organization; 2021. [PubMed] [Google Scholar]
- 20. Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at:https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv. Accessed 26 January 2022.
- 21. BHIVA Writing Committee . BHIVA guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015 (2016 interim update). 2016. Available at:https://www.bhiva.org/file/RVYKzFwyxpgiI/treatment-guidelines-2016-interim-update.pdf. Accessed 26 January 2022.
- 22. The Swedish Reference Group for Antiviral Therapy (RAV) . Antiretroviral behandling av HIV-infektion 2021 - Behandlingsrekommendation. Available athttps://www.sls.se/rav/rekommendationer/hiv/Antiretroviral-behandling-av-hivinfektion-2021/. Accessed 26 January 2022.
- 23. EACS Guidelines version 11.0, October 2021. Available at: https://www.eacsociety.org/guidelines/eacs-guidelines/. Accessed 26 January 2022.
- 24. Shafer RW. Rationale and uses of a public HIV drug-resistance database. J Infect Dis 2006; 194(Suppl 1):S51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis 2013; 57:1489–96. [DOI] [PubMed] [Google Scholar]
- 26. Vandenhende MA, Perrier A, Bonnet F, et al. Risk of virological failure in HIV-1-infected patients experiencing low-level viraemia under active antiretroviral therapy (ANRS C03 cohort study). Antiviral Ther 2015; 20:655–60. [DOI] [PubMed] [Google Scholar]
- 27. Bull ME, Mitchell C, Soria J, et al. Monotypic low-level HIV viremias during antiretroviral therapy are associated with disproportionate production of X4 virions and systemic immune activation. AIDS 2018; 32:1389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vancoillie L, Hebberecht L, Dauwe K, et al. Longitudinal sequencing of HIV-1 infected patients with low-level viremia for years while on ART shows no indications for genetic evolution of the virus. Virology 2017; 510:185–93. [DOI] [PubMed] [Google Scholar]
- 29. Wirden M, Todesco E, Valantin MA, et al. Low-level HIV-1 viraemia in patients on HAART: risk factors and management in clinical practice. J Antimicrob Chemother 2015; 70:2347–53. [DOI] [PubMed] [Google Scholar]
- 30. Brattgård H, Björkman P, Nowak P, et al. Factors associated with low-level viraemia in people with HIV starting antiretroviral therapy: a Swedish observational study. PLoS One 2022; 17:e0268540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bachmann N, von Siebenthal C, Vongrad V, et al. Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat Commun 2019; 10:3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Castillo-Mancilla JR, Morrow M, Coyle RP, et al. Low-level viremia is associated with cumulative adherence to antiretroviral therapy in persons with HIV. Open Forum Infect Dis 2021; 8:ofab463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.