Abstract
Aim
Risk factors for vitamin B12 deficiency in infants are not fully understood. The aim of the study was to assess predictors of total homocysteine and methylmalonic acid analysed in newborn screening dried blood spots.
Methods
In a Norwegian case control study, we analysed total homocysteine and methylmalonic acid in newborn screening dried blood spots of 86 infants clinically diagnosed with vitamin B12 deficiency during 2012–2018. Results were compared to 252 healthy infants and 400 dried blood spot controls. Medical records were reviewed, and mothers completed questionnaires.
Results
Both total homocysteine and methylmalonic acid were significantly higher on newborn screening dried blood spots in infants later clinically diagnosed with vitamin B12 deficiency than controls. Multiple regression analysis showed that the dose of nitrous oxide during labour was the strongest predictor for total homocysteine level in newborn screening dried blood spots for all infants, with larger effect in infants later clinically diagnosed with vitamin B12 deficiency than controls.
Conclusion
Nitrous oxide dose during labour was a predictor for total homocysteine and may impact the interpretation of total homocysteine analysis in newborn screening. Nitrous oxide is suggested as a contributing risk factor for infants prone to develop vitamin B12 deficiency.
Keywords: homocysteine, newborn screening, nitrous oxide, risk factor, second‐tier, vitamin b12 deficiency
Abbreviations
- B12
vitamin B12
- CI
confidence interval
- DBS
dried blood spot
- IQR
interquartile interval range
- MMA
methylmalonic acid
- NBS
newborn screening
- SD
standard deviation
- tHcy
total homocysteine
Key Notes.
Total homocysteine and methylmalonic acid were significantly increased at newborn screening in infants later clinically diagnosed with vitamin B12 deficiency compared to healthy controls.
The dose of nitrous oxide used in labour was the strongest predictor for the total homocysteine level in newborn screening.
Nitrous oxide is suggested as a contributing risk factor for infants prone to develop vitamin B12 deficiency.
1. INTRODUCTION
Analyses of total homocysteine (tHcy) and methylmalonic acid (MMA) are used in newborn screening (NBS) programs as second‐tier tests for cystathionine‐β‐synthase deficiency, remethylation diseases, and methylmalonic and propionic acidaemia. 1 More recently, they have also been recommended for detection of vitamin B12 (B12) deficiency in newborn infants, 2 , 3 , 4 tHcy being regarded as the best marker of infant B12 deficiency. 5 Maternal–foetal transfer of B12 results in a higher concentration of B12 in the newborn infant than in the mother, consistent with active transport of the vitamin. 6 In B12‐replete mothers, B12 stores are gradually accrued in the foetal liver during gestation, achieving 25–30 μg at term, compared to 2–5 μg in newborn infants of B12‐deficient mothers. 6 A B12‐deficient mother is also the strongest predictor for B12 deficiency in a breastfed infant. 3 , 5 However, maternal B12 deficiency is not always evident in B12‐deficient newborn infants detected by NBS. 3 Vegetarianism is only an exceptional cause of maternal B12 deficiency in high‐income countries 2 , 3 , 7 and thus other risk factors for infant B12 deficiency need to be considered. Nitrous oxide is extensively used for analgesia during labour. 8 , 9 It oxidises the methionine synthase bound cob(I)alamin to cob(II)alamin, thereby irreversibly inhibiting this enzyme, which leads to accumulation of Hcy and lack of S‐adenosyl‐methionine. 10 , 11 , 12 Nitrous oxide does not affect methyl malonyl‐CoA‐mutase activity. 12 tHcy increases significantly after nitrous oxide has been given to children during anaesthesia for surgery, with dose–response kinetics. 13 Furthermore, already 30 years ago, nitrous oxide given as pain relief during labour was shown to inhibit methionine synthase in the placenta in a dose‐responsive manner. 14 Nitrous oxide is distributed to and accumulates in the foetus when provided to the mother prenatally. 15 Only short‐term safety for obstetric use has been documented, 8 , 9 but the longer‐term effect of the inhibition of methionine synthase has not been evaluated. In a previous publication, we found that nitrous oxide correlates with both infant tHcy and MMA levels several months after birth in infants with clinically diagnosed B12 deficiency, suggesting nitrous oxide as a possible risk factor for early infant B12 deficiency. 16
The aims of this retrospective case–control study were to explore predictors for tHcy and MMA levels, analysed in dried blood spots (DBS) obtained from NBS, and to analyse the frequency distribution of tHcy and MMA levels for infants later diagnosed with B12 deficiency, compared to controls.
2. MATERIALS AND METHODS
2.1. Study population
We performed a retrospective case–control study. We included infants below 1 year of age, born between 2012 and 2018, who were treated for clinical B12 deficiency, designated as cases. They were identified after search in medical record databases of two hospitals in South‐East Norway. As controls, we used a cohort of healthy, age‐matched infants (Figure S1), referred to as clinical controls, since they were recruited for postnatal clinical follow‐up in 2018–2019 from the Postnatal and Neonatal Unit at Vestfold Hospital Trust, Norway. Details on inclusion, background characteristics, and clinical and biochemical findings have previously been published. 16 , 17 , 18 , 19 We also included DBS controls, matched for date of birth, age in days, sex, hospital, birth weight, and gestational age of the cases and clinical controls. Data on pregnancy, delivery, and clinical follow‐up were not available for the DBS controls. The study was approved by the Regional Committee for Medical Research Ethics Northern Norway (179/2018) and was conducted according to the Declaration of Helsinki. Written informed consent was collected for all participants.
2.2. Background data
We collected obstetric data from hospital records. Mothers completed non‐standardised questionnaires on vitamin‐supplementation and self‐reported health. We retrieved information on the use of nitrous oxide during labour from the mothers' obstetric files and included time for start and stop of intermittent administration of nitrous oxide and its concentration in percentage in the nitrous oxide/oxygen blend. We calculated the total dose of nitrous oxide as the concentration of nitrous oxide multiplied by the time for intermittent administration in minutes. The selection of covariates, that are suggested risk factors for infant B12 deficiency, was based on previous reports. 5 , 16 , 17 , 20 We also calculated the storage time of DBS card from birth until tHcy and MMA analyses were performed since storage possibly could influence the levels.
2.3. Newborn screening analyses
Blood samples were collected on filter cards 48–72 h after birth and sent by prioritised mail to the Norwegian National NBS laboratory at Oslo University Hospital. 21 After the standard NBS analyses were performed, filter cards were first collected in a fridge at 2–4°C for up to some weeks before being stored in a biobank at −20°C until they were retrieved for second‐tier tHcy and MMA analysis in 2020–2021. A combined second‐tier method for tHcy, MMA, and 2‐methylcitric acid was established in DBS by LC–MS/MS, partially adapted from Fu et al. 22 tHcy was introduced in 2020 as second‐tier analysis for cystathionine β‐synthase deficiency and MMA for methylmalonic aciduria and propionic aciduria (Appendix S1). Only NBS filter cards obtained after the expansion of the NBS program in Norway, on 1 March 2012, were available for second‐tier analysis.
2.4. Statistical analysis
Data were registered in EpiData version 4.4 (EpiData Association, Odense, Denmark). Continuous variables were presented as mean and standard deviation or if skewed, as median and interquartile range (IQR). Categorical variables were given as proportions and percentages and compared between groups using the chi‐square test of proportions or Fisher's exact test for small samples. Differences between independent groups were quantified with the Mann–Whitney U test because of skewness in the data. All statistical tests were two‐sided, and a p value <0.05 was considered statistically significant. All regression models were significant with p < 0.001. Linear regression analyses were performed to identify predictors for DBS tHcy and MMA. A forward method with criterion probability of F to enter ≤0.05 was used to calculate significant variables. Variables entered in regressions of tHcy and MMA to identify risk factors were maternal Norwegian origin, smoking during the last 2 years before pregnancy, meat‐consumer, known self‐reported B12 deficiency, B12 supplements during pregnancy, diabetes in pregnancy, metformin use, self‐reported nausea in pregnancy, age, body mass index at pregnancy start, primiparity, hospital‐diagnosed celiac disease, folate supplement, nitrous oxide dose during labour, prematurity, growth restriction, gender, and vaginal delivery. Significant variables were re‐analysed by the enter method. Analyses were performed in IBM SPSS Statistics version 28 (IBM Corp, New York, USA), and graphs were created in NCSS 2021 Statistical Software (NCSS LLC, Utah, USA).
3. RESULTS
3.1. Characteristics of population
We included 85 clinically diagnosed B12‐deficient infant cases, 252 clinical controls (Table S1‐S2), and 400 DBS controls. DBS tHcy and MMA were analysed in 79/85 (93%) cases. Six filter cards for children born prior to 1 March 2012 had been destroyed according to Norwegian NBS regulations. tHcy and MMA were analysed in all clinical and DBS controls (Figure S1).
Storage time of DBS (age of DBS) before second‐tier analyses [IQR, total range] for cases was median 3.5 years [2.8–5.4, 7] and for all the 652 control median 2.0 years [1.8–2.4, 7]. Mean (SD) birth weight for cases was 3375 g (671) and for all controls 3293 g (668). The median [IQR, total range] case gestational week was 39 [38–41, 15] and for all controls 39 [38–41, 13]. The median [IQR, total range] case age in hours at collection of blood for NBS DBS was 58 h [51–66, 110] and for all controls 58 h [51–67, 113].
3.2. DBS tHcy and MMA
For the 79 clinical cases, the median [IQR] tHcy was 6.29 μmol/L [5.18–8.23] (Figure S2) and MMA 0.043 μmol/L [0.00–0.31] (Figure S3). For the 652 controls, the median [IQR] tHcy was 5.04 μmol/L [3.82–6.66] (Figure S4) and MMA 0.00 μmol/L [0.00–0.039] (Figure S5). Both DBS tHcy and MMA were significantly higher in cases than in controls (Mann–Whitney U test, p < 0.001) (Figures 1 and 2).
FIGURE 1.
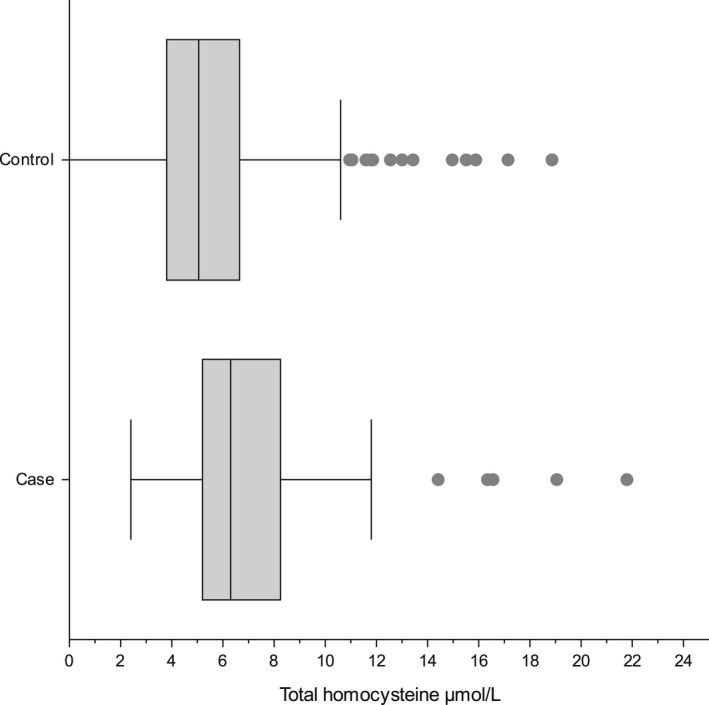
Comparison of frequency distribution for total homocysteine (tHcy) between the 79 clinical cases and the 652 controls.
FIGURE 2.
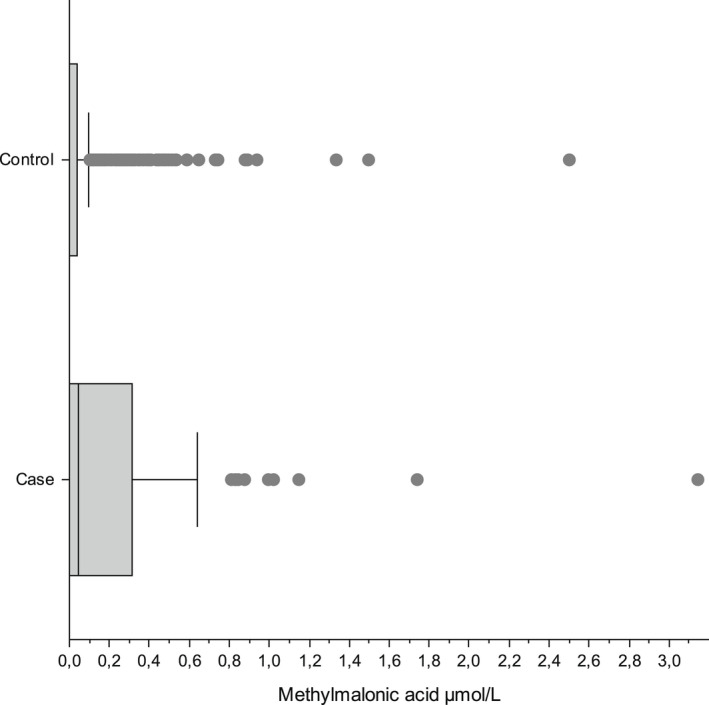
Comparison of frequency distribution for methylmalonic acid (MMA) between the 79 clinical cases and the 652 controls.
3.3. Associations between predictors and DBS tHcy
A multiple linear regression was run with DBS tHcy in μmol/L as the dependent variable, storage time of DBS in years, and DBS case/control as the independent variables, in total n = 730. Both the storage time of DBS (beta = 0.350, 95% CI 0.239–0.460, p < 0.001, standardised beta = 0.229) and DBS case versus control (beta = 1.178, 95% CI 0.576–1.780, p < 0.001, standardised beta = 0.142) predicted DBS tHcy significantly. Multiple linear regression analyses were run separately to identify predictors for DBS tHcy for clinical cases and clinical controls (Table S2 and Table 1). The dose of nitrous oxide given to the mother during labour was the strongest predictor for tHcy for the clinical cases (standardised beta 0.413, p < 0.001) and the only significant predictor for the clinical controls (standardised beta 0.240, p < 0.001). For the clinical cases, nausea in pregnancy was associated with DBS tHcy (standardised beta 0.301, p = 0.003) (Table 1).
TABLE 1.
Linear model coefficients of predictors for dried blood spot (DBS) total homocysteine for clinical cases and controls separately
| Clinical cases (n = 76) | Clinical controls (n = 243) | |||||
|---|---|---|---|---|---|---|
| Beta (95% CI) | Std beta | p | Beta (95% CI) | Std beta | p | |
| Dose N2O a | 0.017 (0.009–0.025) | 0.413 | <0.001 | 0.006 (0.003–0.008) | 0.240 | <0.001 |
| Nausea in pregnancy | 2.19 (0.751–3.62) | 0.301 | 0.003 | |||
| Storage of DBS (years) | −0.538 (−0.898 to −0.178) | −0.297 | 0.004 | |||
Dose of nitrous oxide (N2O) is the product of concentration of N2O and the administration time in minutes.
3.4. Associations between predictors and DBS MMA
A multiple linear regression was run with DBS MMA in μmol/L as the dependent variable, storage time DBS in years, and DBS case/control as the independent variables, n = 731. DBS case/control predicted DBS MMA significantly (beta = 0.173, 95% CI 0.117–0.228, p < 0.001, standardised beta = 0.229), while the storage time of DBS (beta = 0.006, 95% CI ‐0.004 to 0.016, p = 0.23, standardised beta = 0.045) did not. Multiple linear regression analyses were run to identify predictors for DBS MMA for all clinical infants (Table 2). Later clinical infant B12 deficiency was associated with increased DBS MMA (standardised beta 0.284, p < 0.001). Celiac disease and nausea in pregnancy predicted MMA for all clinical infants (standardised betas 0.157 and 0.122, p = 0.003 and 0.019, respectively) (Table 2).
TABLE 2.
Linear model coefficients of predictors for dried blood spot methylmalonic acid, all 326 clinical infants
| Beta (95% CI) | Std beta | p | |
|---|---|---|---|
| Infant B12 deficiency | 0.180 (0.115–0.246) | 0.283 | <0.001 |
| Celiac disease | 0.211 (0.074–0.348) | 0.157 | 0.003 |
| Nausea in pregnancy | 0.073 (0.022–1.52) | 0.122 | 0.019 |
4. DISCUSSION
This case–control study investigated predictors for tHcy and MMA, analysed in DBS obtained from newborn screening for healthy infants and infants with known B12 deficiency, clinically diagnosed during the first year of life. We showed that the strongest predictor for tHcy was the dose of nitrous oxide given to the mother during labour followed by self‐reported nausea in pregnancy. Celiac disease and nausea in pregnancy predicted MMA.
We have previously published an association between dose of nitrous oxide to the mother in labour and both serum tHcy and MMA retrieved several months later in life in clinically diagnosed B12‐deficient infants, hypothesizing that the more nitrous oxide delivered to the mother in labour, the less B12 remains in her infant months later. 16 Accordingly, when we in the present study analysed the DBS collected on the third day of life from the same, clinically presenting B12‐deficient infants and their controls, only tHcy but not MMA, was associated with dose of nitrous oxide, indicating decreased methionine synthase activity. This was evident for both cases and controls. In contrast, both the tHcy and the MMA–levels were higher on the third day of life in later, clinically presenting B12‐deficient cases compared to controls. Since nitrous oxide has been shown to affect methionine synthase only, not methylmalonyl‐CoA‐mutase, 12 , 23 this finding indicates a lower B12 status in the cases rendering them more prone to later B12 deficiency. Furthermore, the higher infant MMA level at NBS could be explained by insufficient maternal B12 status, a well‐known risk factor for infant B12 deficiency. 5
Nitrous oxide chemically inactivates B12 through irreversible oxidation of its coenzyme form, methyl cobalamin, at the active site of the B12‐dependent methionine synthase reaction. 10 , 11 , 12 The nitrous oxide‐induced homocysteine response depends on the cobalamin status of the individual exposed to the gas and will be higher with lower cobalamin status. 5 , 14 The irreversible inactivation requires re‐synthesis of methionine synthase and B12 stores are consumed. Hence, nitrous oxide given during labour will decrease B12 stores in both the mother and the newborn infant, the effect being relatively larger if the mother is B12‐deficient or have a suboptimal B12 status during pregnancy. The exclusively breastfed infant is at risk to develop symptomatic B12 deficiency since breastmilk B12 content is accordingly reduced. 5 Consequently, our results propose nitrous oxide to be an unrecognised contributor for B12 deficiency in vulnerable infants with lower B12 status. This is both in line with results recently reported by us, 16 and with findings by Landon et al. over 30 years ago, in that nitrous oxide inactivated placental methionine synthase in a dose‐responsive manner and more so if maternal B12 was low. 14 Low maternal B12 status, nitrous oxide in labour, and breastfeeding may reinforce the risk of infant B12 deficiency. If the mother's B12 status is sufficient though, or if the infant is formula fed, the risk for B12 deficiency is low. Nitrous oxide during labour should also be considered when interpreting increased tHcy in NBS. Transient elevation of tHcy in the newborn infant may be one of the factors explaining why a subset of mothers are not diagnosed with B12 deficiency following detection of her infant at NBS. 3 , 7 , 24 , 25
We also found associations between MMA and plausible risk factors for maternal B12 deficiency such as self‐reported nausea in pregnancy and celiac disease, both potentially impacting on the pregnant women's B12 stores. 5 We assume, like others before us, that this may be explained by decreased intake or uptake of B12 from the food in the pregnant woman with nausea or celiac disease. 5 This is also in accordance with a previous study of infants with confirmed B12 deficiency suggested by NBS, in which nausea and food aversion were reported in 28% and gastrointestinal disorder in 8% of 19 mothers as a cause for maternal B12 deficiency. 24 We did not find associations between self‐reported maternal B12 deficiency and DBS tHcy or MMA in the infants, presumably because this variable was inaccurate since we did not collect temporal information on when the mothers were B12‐deficient. In the light of our findings, we encourage to screen and treat mothers for B12 deficiency early in pregnancy to reduce risk of infant B12 deficiency.
Maternal B12 status, recognised as the most important determinant of neonatal B12 status, 20 was not available and was a limitation to our study. B12 status is not included as part of standard pregnancy blood tests in Norway and could only have been accessed through a planned prospective study. Since this was a retrospective case–control study, our associations were mainly found in linear regression models and causality was not proven. We showed that storage time of DBS was associated with an increase of 0.35 μmol/L per year for tHcy, but not for MMA. Therefore, we could not infer the differences we found in tHcy between cases and controls for tHcy directly without correction for storage time of DBS since the time elapsed was longer for cases than for controls. Our finding showing increased concentration with time for tHcy for DBS stored in a cold environment has not been reported before. A decrease in tHcy has been observed for DBS stored in dry, sealed plastic bags. 26 , 27 The reason suggested for the latter situation is that whole blood with erythrocytes contains less homocysteine than plasma. It has previously been shown that tHcy increases in plasma if whole blood is stored uncentrifuged after sampling, explained by the release of homocysteine from the erythrocytes even at storage at 4°C, but we do not know if this applies for whole blood sampled on filter paper. 28 However, preanalytical factors, such as collection devices, humidity, and temperature may all potentially influence long‐term stability of the analytes. 29 We consequently chose to include storage time of DBS in all regressions for tHcy to correct for this systematic error. Our study was not designed to analyse the relation between storage time of DBS and tHcy and this association should therefore be interpreted with caution. We measured time between the use of nitrous oxide started and stopped. The use was intermittent, and since we did not measure the volume used, the measure is inexact.
5. CONCLUSION
In conclusion, nitrous oxide dose during labour was a predictor for tHcy at NBS and is suggested as a risk factor for infant B12 deficiency. We recommend to routinely analyse B12 status of mothers prior to use of nitrous oxide in labour and that mothers should be informed of the potential risks to their infants.
FUNDING INFORMATION
This work was funded by Vestfold Hospital Trust.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Appendix S1
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Table S1‐S2
ACKNOWLEDGEMENTS
We thank the children and families who participated in the study. We also thank Carlos Sagredo who developed the method for analysing tHcy and MMA simultaneously on DBS. The co‐author Trine Tangeraas is a health care representative on behalf of Oslo University Hospital, member of the European Reference Network for Rare Hereditary Metabolic Disorders (MetabERN).
Ljungblad UW, Lindberg M, Eklund EA, Saeves I, Bjørke‐Monsen A‐L, Tangeraas T. Nitrous oxide in labour predicted newborn screening total homocysteine and is a potential risk factor for infant vitamin B12 deficiency. Acta Paediatr. 2022;111:2315–2321. 10.1111/apa.16530
Trine Tangeraas Member of the European Reference Network for Rare Hereditary Metabolic Disorders (MetabERN).
REFERENCES
- 1. Turgeon CT, Magera MJ, Cuthbert CD, et al. Determination of total homocysteine, methylmalonic acid, and 2‐methylcitric acid in dried blood spots by tandem mass spectrometry. Clin Chem. 2010;56(11):1686‐1695. doi: 10.1373/clinchem.2010.148957 [DOI] [PubMed] [Google Scholar]
- 2. Pajares S, Arranz JA, Ormazabal A, et al. Implementation of second‐tier tests in newborn screening for the detection of vitamin B12 related acquired and genetic disorders: results on 258,637 newborns. Orphanet J Rare Dis. 2021;16(1):195. doi: 10.1186/s13023-021-01784-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gramer G, Fang‐Hoffmann J, Feyh P, et al. Newborn screening for vitamin B12 deficiency in Germany—strategies, results, and public health implications. J Pediatr. 2020;216:165‐172.e4. doi: 10.1016/j.jpeds.2019.07.052 [DOI] [PubMed] [Google Scholar]
- 4. Rozmarič T, Mitulović G, Konstantopoulou V, et al. Elevated homocysteine after elevated propionylcarnitine or low methionine in newborn screening is highly predictive for low vitamin B12 and holo‐transcobalamin levels in newborns. Diagnostics. 2020;10(9):626. doi: 10.3390/diagnostics10090626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Green R, Allen LH, Bjørke‐Monsen AL, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3:17040. doi: 10.1038/nrdp.2017.40 [DOI] [PubMed] [Google Scholar]
- 6. Baker SJ, Jacob E, Rajan KT, Swaminathan SP. Vitamin‐B12 deficiency in pregnancy and the puerperium. Br Med J. 1962;1(5293):1658‐1661. doi: 10.1136/BMJ.1.5293.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reinson K, Künnapas K, Kriisa A, Vals MA, Muru K, Õunap K. High incidence of low vitamin B12 levels in Estonian newborns. Mol Genet Metab Rep. 2018;15:1‐5. doi: 10.1016/j.ymgmr.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain. Anesth Analg. 2014;118(1):153‐167. doi: 10.1213/ANE.0b013e3182a7f73c [DOI] [PubMed] [Google Scholar]
- 9. Vallejo MC, Zakowski MI. Pro‐con debate: nitrous oxide for labor analgesia. Biomed Res Int. 2019;2019:1‐12. doi: 10.1155/2019/4618798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Banerjee RV, Matthews RG. Cobalamin‐dependent methionine synthase. FASEB J. 1990;4(5):1450‐1459. [DOI] [PubMed] [Google Scholar]
- 11. Sanders RD, Weimann J, Maze M. Biologic effects of nitrous oxide: a mechanistic and toxicologic review. Anesthesiology. 2008;109(4):707‐722. doi: 10.1097/ALN.0b013e3181870a17 [DOI] [PubMed] [Google Scholar]
- 12. Chanarin I. Cobalamins and nitrous oxide: a review. J Clin Pathol. 1980;33(10):909–916. Accessed December 18, 2017. http://www.ncbi.nlm.nih.gov/pubmed/6107306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pichardo D, Luginbuehl IA, Shakur Y, Wales PW, el‐Sohemy A, O'Connor DL. Effect of nitrous oxide exposure during surgery on the homocysteine concentrations of children. Anesthesiology. 2012;117(1):15‐21. doi: 10.1097/ALN.0b013e318259a8cc [DOI] [PubMed] [Google Scholar]
- 14. Landon MJ, Creagh‐Barry P, McArthur S, Charlett A. Influence of vitamin B12 status on the inactivation of methionine synthase by nitrous oxide. Br J Anaesth. 1992;69(1):81‐86. doi: 10.1093/bja/69.1.81 [DOI] [PubMed] [Google Scholar]
- 15. Marx G, Joshi CW, Orkin LR. Placental transmission of nitrous oxide. Anesthesiology 1970;32(5):429–432. Accessed December 25, 2018. http://anesthesiology.pubs.asahq.org/article.aspx?articleid=1963746 [DOI] [PubMed] [Google Scholar]
- 16. Ljungblad UW, Astrup H, Mørkrid L, et al. Breastfed infants with spells, tremor, or irritability: rule out vitamin B12 deficiency. Pediatr Neurol. 2022;131:4‐12. doi: 10.1016/j.pediatrneurol.2022.03.003 [DOI] [PubMed] [Google Scholar]
- 17. Ljungblad UW, Paulsen H, Mørkrid L, et al. The prevalence and clinical relevance of hyperhomocysteinemia suggesting vitamin B12 deficiency in presumed healthy infants. Eur J Paediatr Neurol. 2021;35:137‐146. doi: 10.1016/j.ejpn.2021.10.008 [DOI] [PubMed] [Google Scholar]
- 18. Ljungblad UW, Paulsen H, Tangeraas T, Evensen KAI. Reference material for hammersmith infant neurologic examination scores based on healthy, term infants age 3‐7 months. J Pediatr. 2022;244:79‐85.e12. doi: 10.1016/j.jpeds.2022.01.032 [DOI] [PubMed] [Google Scholar]
- 19. Ljungblad UW, Tangeraas T, Paulsen H, Lindberg M. Lower iron stores were associated with suboptimal gross motor scores in infants at 3‐7 months. Acta Paediatrica. 2022. doi: 10.1111/apa.16469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith AD, Warren MJ, Refsum H. Vitamin B12. In: Michael Eskin NA, ed. Advances in Food and Nutrition Research. Vol 83. National Library of Medicine (US); 2018:215‐279. doi: 10.1016/bs.afnr.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 21. Tangeraas T, Sæves I, Klingenberg C, et al. Performance of expanded newborn screening in norway supported by post‐analytical bioinformatics tools and rapid second‐tier DNA analyses. Int J Neonatal Screen. 2020;6(3):51. doi: 10.3390/ijns6030051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fu X, Xu YK, Chan P, Pattengale PK. Simple, fast, and simultaneous detection of plasma total homocysteine, methylmalonic acid, methionine, and 2‐methylcitric acid using Liquid Chromatography and Mass Spectrometry (LC/MS/MS). JIMD Rep. 2013;10:69‐78. doi: 10.1007/8904_2012_205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nunn JF, NunnJ F. Clinical aspects of the interaction between nitrous oxide and vitamin B12. Br J Anaesth. 1987;59(1):3‐13. doi: 10.1093/bja/59.1.3 [DOI] [PubMed] [Google Scholar]
- 24. Mütze U, Walter M, Keller M, et al. Health outcomes of infants with vitamin B12 deficiency identified by newborn screening and early treated. J Pediatr. 2021;235:42‐48. doi: 10.1016/j.jpeds.2021.02.009 [DOI] [PubMed] [Google Scholar]
- 25. Held PK, Singh E, Schwoerer JS. Screening for methylmalonic and propionic acidemia: clinical outcomes and follow‐up recommendations. Int J Neonatal Screen. 2022;8(1). doi: 10.3390/IJNS8010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alodaib AN, Carpenter K, Wiley V, Wotton T, Christodoulou J, Wilcken B. Homocysteine measurement in dried blood spot for neonatal detection of homocystinurias. JIMD Rep. 2012;5:1‐6. doi: 10.1007/8904_2011_109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCann SJ, Gillingwater S, Keevil BG, Cooper DP, Morris MR. Measurement of total homocysteine in plasma and blood spots using liquid chromatography‐tandem mass spectrometry: comparison with the plasma Abbott IMx method. Ann Clin Biochem. 2003;40(Pt 2):161‐165. doi: 10.1258/000456303763046094 [DOI] [PubMed] [Google Scholar]
- 28. Andersson A, Isaksson A, Hultberg B. Homocysteine export from erythrocytes and its implication for plasma sampling. Clin Chem. 1992;38(7):1311‐1315. doi: 10.1093/clinchem/38.7.1311 [DOI] [PubMed] [Google Scholar]
- 29. Moat SJ, George RS, Carling RS. Use of dried blood spot specimens to monitor patients with inherited metabolic disorders. Int J Neonatal Screen. 2020;6(2). doi: 10.3390/IJNS6020026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Table S1‐S2


