Abstract
This case highlights the successful use of pembrolizumab for neoadjuvant treatment of MMR‐deficient sebaceous carcinoma of bilateral eyelids to reduce tumour burden allowing smaller defect post‐Mohs surgery and better reconstructive outcome. Microsatellite stability, tumour mutational burden and PD‐L1 expression are important prognostic factors to be considered for the use of neoadjuvant pembrolizumab. Further studies are needed to determine if neoadjuvant pembrolizumab consistently improves surgical and cosmetic outcomes and reduces local recurrence and metastasis.
Keywords: checkpoint inhibitors, immunotherapy, Mohs surgery, Muir‐Torre syndrome, neoadjuvant therapy, PD‐1 inhibitors, pembrolizumab, sebaceous carcinoma, sebaceous gland neoplasms
BACKGROUND
Sebaceous Carcinoma (SC) is a rare, aggressive skin cancer arising from sebaceous glands. It most commonly occurs on the eyelids, followed by the head and neck region, but it can occur anywhere on the body in the scope of Muir‐Torre syndrome (MTS). 1 , 2 SC is an aggressive cancer that is often locally invasive and associated with reoccurrence and regional and distant metastasis. 1 It can arise sporadically, but it may also occur secondary to germline mutations in DNA mismatch repair genes. 2
MTS is a rare variant of Lynch syndrome characterized by sebaceous neoplasms. It is most commonly caused by a germline mutation in one of the DNA mismatch repair (MMR) proteins (MSH2, MLH1, MSH6, PMS2, MLH3, MSH3 and PMS1) resulting in microsatellite instability. 2 MTS type I (Classic MTS, MTS) is an autosomal dominant disorder, that besides multiple sebaceous neoplasms, is also associated with keratoacanthomas and visceral malignancies—most commonly colorectal adenocarcinoma. 2 Due to SC's strong association with MTS type I, risk stratification can be calculated using the Mayo MTS risk score (range 0–5), and this can guide the need to conduct further germline genetic testing. A score of two or more has a sensitivity of 100% and specificity of 81% for predicting germline mutation in MMR genes. 2
Primary SC is often treated with Mohs surgery or wide local excision with frozen section margin evaluation. 1 However, local recurrence or metastasis post‐surgical removal often occurs. 1 This can be attributed to the delays in diagnosis of SC which may mimic other benign eyelid lesions (chalazion, blepharitis) and/or the multifocal nature of the tumour with discontinuous intraepithelial involvement and pagetoid spread of malignant sebocytes along conjunctiva. 1 In addition to surgical excision, intraoperative cryotherapy and postoperative topical mitomycin‐C have been considered for focally positive margins. 1 Successful neoadjuvant use of systemic chemotherapies 5‐fluorouracil and carboplatin/cisplatin has been reported not only just to reduce initial tumour size and make surgery more amenable but also to manage widespread disease and reduce tumour burden in the lymph nodes and metastases. 3
More recently, there have been a few reports regarding the off‐label use of the programmed cell death protein‐1 (PD‐1) inhibitor pembrolizumab for advanced SC. 4 , 5 , 6 We present a case of a patient with confirmed MTS who experienced significant tumour regression after two doses of neoadjuvant pembrolizumab rendering Mohs micrographic surgery less complex with smaller postoperative defect and better cosmetic reconstructive outcome.
CASE PRESENTATION
A 58‐year‐old Hispanic male with suspected MTS and history of colorectal adenocarcinoma (status‐post right hemicolectomy 2003 and left ileocolectomy 2021) as well as clear cell renal carcinoma (status‐post left nephrectomy 2021) was referred by ophthalmology for a rapidly growing mass on his right medial inferior eyelid over the past 6 months. On examination, there was a large necrotic and ulcerated exophytic nodule (20 × 9 mm) on the medial part of the right inferior internal eyelid with ciliary margin involvement (Figure 1a). He also had another small 3 × 3 mm yellowish papule on the left superior medial eyelid and a 4.3 × 2.2 cm large pink bluish dermal subcutaneous non‐tender nodule on the left buttock (Figure 1b,c). Biopsy of all three lesions were identical and showed numerous atypical sebocytes extending from the epidermis to the dermis or subcutis (buttock lesion), with extensive necrosis, mitotic figures and focal stromal invasion (Figure 1d). Scouting biopsies on the adjacent conjunctiva and palpebral skin were negative.
FIGURE 1.
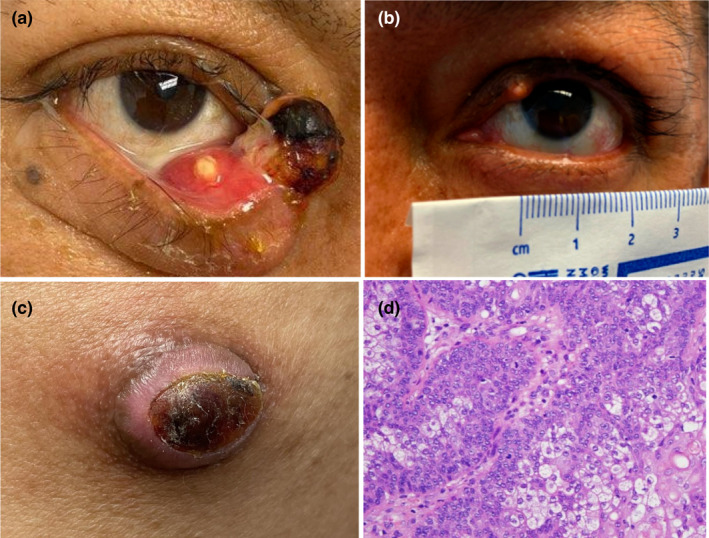
(a) Large ulcerated and necrotic nodule on the medial canthus of the right inferior eyelid. (b) A 3 × 3 mm yellow papule on the left superior medial eyelid. (c) A large pink nodule with central dry serous crust on the left superior lateral buttock. (d) At high magnification (400X), numerous atypical sebocytes show multivacuolated clear cytoplasm and indented nuclei extending into the dermis with extensive necrosis, mitotic figures and focal stromal invasion.
Immunohistochemistry for microsatellite instability demonstrated loss of expression of MLH‐1 and PMS‐2, consistent with MTS. Due to the patient's Mayo score of 3 (personal history of visceral malignancy and 2 or more sebaceous tumours) genomic testing of a blood sample was performed and revealed the presence of germline MLH1 heterozygosity with a loss‐of‐function mutation from exon 13, codon 1459 (C>T mutation creating a premature translational stop signal), confirming MTS. CT scan of the head and neck did not reveal any orbital extension, disseminated disease or enlargement of the regional lymph nodes (stage IIIb AJCC 8th). The tumour continued to grow over the next several weeks, measuring 40 × 12 mm on the right and 5 × 5 mm on the left eyelid respectively (Figure 2a). The buttock lesion was excised completely with 10 mm margins and medical oncology team was consulted for possible neoadjuvant treatment due to quickly enlarging right eyelid tumour, and to decrease postsurgical defect and morbidity. Decision was made to administer two doses of pembrolizumab 400 mg 6 weeks apart as neoadjuvant prior to Mohs surgical resection.
FIGURE 2.
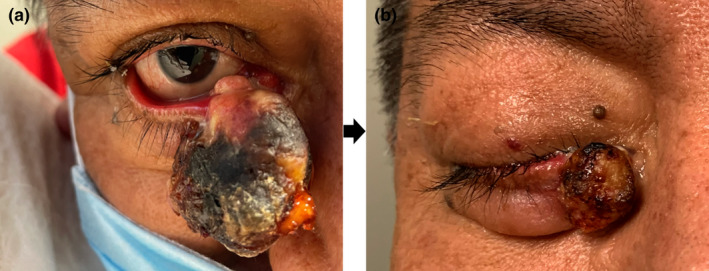
(a) Large necrotic, ulcerated, exophytic tumour on medial canthus of the right inferior eyelid with ciliary margin involvement before pembrolizumab treatment. (b) Regressed necrotic exophytic tumour of the medial lower right eyelid with involvement of the ciliary margin.
The patient tolerated pembrolizumab well and both tumours shrank to less than 1/3 of their original size (T2b) (Figure 2b). Mohs surgery requiring two stages was performed on both masses with clear margins, but with marked inflammation noted on frozen sections due to pembrolizumab treatment (Figure 3a, b). The absence of the tumour was further confirmed by permanent sections and consultation pathology evaluation. The reconstruction was performed by advancement flap on the right lower eyelid, and pentagonal repair on the left upper eyelid, with unremarkable postoperative course (Figure 3c, d). Patient continued receiving adjuvant pembrolizumab 400 mg every 6 weeks postoperatively and has been without disease progression for the follow‐up period of 12 months.
FIGURE 3.

Mohs surgical defects for right eye (a) and left eye (b). Two‐week post reconstruction with advancement flap of right lower eyelid (c) and pentagonal closure of left upper eyelid (d).
DISCUSSION
Neoadjuvant therapy can be used before surgery with the purpose of shrinking the tumour, stopping its spread, and making resection less invasive and more effective. In the case of SC, neoadjuvant therapy was mostly considered for large (T3bN0M0 or T3bN1M0), metastatic or inoperable tumours with orbital invasion. 3 The recommended chemotherapeutic agents (5‐FU, cisplatin/carboplatin) do not show benefit in MMR‐deficient tumours, and hence could not be considered in our patient. 7 Several previous case reports have highlighted the effective off‐label use of pembrolizumab monotherapy or pembrolizumab and carboplatin combination therapy for recurrent, inoperable widespread metastatic SC—including complete regression of unresectable chest wall SC with microsatellite instability. 4 , 5 , 6
Pembrolizumab, a humanized monoclonal antibody that inhibits PD‐1 receptors on T cells, presents an intriguing treatment modality for SC for numerous reasons. Due to periocular SC often being diagnosed late, with an average diagnostic delay of 14.7 months, the extent of SC at the time of diagnosis may make tissue and functional preservation challenging. 1 Mohs micrographic surgery and conjunctival mapping tissue biopsies may be utilized to preserve as much tissue as possible; however, significant morbidity is often still incurred due to the pagetoid spread and the often multifocal nature of SC. For cases of large periocular or eyelid SC, neoadjuvant therapy with pembrolizumab presents a new therapeutic option to decrease disease extent, preserve tissue and improve surgical outcomes. By blocking tumour‐induced immune system downregulation from overexpression of programmed death‐ligand 1 (PD‐L1), PD‐1 inhibitors promote immunological recognition and destruction of malignant cells. This is especially true for SC with impaired MMR mechanisms and high levels of microsatellite instability, as these tumours are associated with increased tumour mutational burden and higher PD‐L1 expression. 8 Furthermore, periocular SC have demonstrated high expression of PD‐L1 and PD‐1 in malignant sebocytes and tumour infiltrating lymphocytes rendering these tumours potentially sensitive to PD‐1 inhibitors. 9 For these reasons, assessment of MMR status and microsatellite instability of SC may have utility beyond diagnosis by helping predict likelihood of treatment response. 8 Of note, successful pembrolizumab use has been demonstrated in microsatellite‐stable SC as well, so use may not be limited to SC with deficient MMR mechanisms. 4 , 5 In microsatellite‐stable SC patients, tumour mutational burden and PD‐L1 expression may further help guide likelihood of treatment response. 8
Neoadjuvant therapy in Mohs surgery of eyelid tumours is a relatively new approach with the majority of reports considering the use of vismodegib for extensive periocular basal cell carcinoma. 10 Our case presents the first published report where neoadjuvant pembrolizumab was used to significantly reduce the tumour size, making the surgical defect significantly smaller than it would have occurred with initial untreated lesion. While this report offers insight into the promising use of pembrolizumab as neoadjuvant therapy for locally advanced SC, further research needs to be conducted. Larger studies need to further evaluate the efficacy of pembrolizumab in SC for use as neoadjuvant and adjuvant therapy. In addition, the ideal neoadjuvant regimen of pembrolizumab regarding dosing and timing prior to excisional surgery needs to be elucidated—as our patient had a notable response after two doses, it is unclear the extent of the response subsequent doses would produce. Furthermore, other regimens like retinoic‐acid receptor (RAR)‐β agonists or androgen receptor agonists could be considered as potential neoadjuvant agents with or without pembrolizumab to decrease SC tumour burden and postoperative morbidity since both receptors are abundantly present in these neoplasms. 11
CONCLUSION
SC is a rare skin cancer that often affects the eyelids and can be associated with Muir‐Torre syndrome. Definitive primary treatment is with surgical excision, although preservation of tissue and function is a concern especially when affecting the eyelids. This case highlights the use of pembrolizumab for neoadjuvant treatment of MMR‐deficient SC to reduce tumour burden prior to surgical excision. Further studies need to be conducted to determine if neoadjuvant pembrolizumab consistently improves surgical and cosmetic outcomes and reduces local reoccurrence and metastasis.
CONFLICT OF INTEREST
The authors have no conflicts to disclose, and received no funding for this manuscript.
ACKNOWLEDGEMENTS
The patient in this manuscript has given written informed consent to publication of their case details.
Woods AD, Grushchak S, Williams KM, Tan A, Krunic AL. Combination treatment of bilateral periocular sebaceous carcinomas with microsatellite instability with neoadjuvant pembrolizumab and Mohs surgery. Australas J Dermatol. 2022;63:e345–e349. 10.1111/ajd.13919
REFERENCES
- 1. Desiato VM, Byun YJ, Nguyen SA, Thiers BH, Day TA. Sebaceous carcinoma of the eyelid: a systematic review and meta‐analysis. Dermatol Surg. 2021;47(1):104–10. 10.1097/DSS.0000000000002660 [DOI] [PubMed] [Google Scholar]
- 2. Dany M. The DNA mismatch repair system in sebaceous tumors: an update on the genetics and workup of Muir‐Torre syndrome. Cutis. 2020;105(3):E34–7. [PubMed] [Google Scholar]
- 3. Kaliki S, Ayyar A, Nair AG, Mishra DK, Reddy VAP, Naik MN. Neoadjuvant systemic chemotherapy in the management of extensive eyelid sebaceous gland carcinoma: a study of 10 cases. Ophthal Plast Reconstr Surg. 2016;32(1):35–9. 10.1097/IOP.0000000000000398 [DOI] [PubMed] [Google Scholar]
- 4. Kodali S, Tipirneni E, Gibson PC, Cook D, Verschraegen C, Lane KA. Carboplatin and pembrolizumab chemoimmunotherapy achieves remission in recurrent, metastatic sebaceous carcinoma. Ophthal Plast Reconstr Surg. 2018;34(5):e149–51. 10.1097/IOP.0000000000001164 [DOI] [PubMed] [Google Scholar]
- 5. Domingo‐Musibay E, Murugan P, Giubellino A, Sharma S, Steinberger D, Yuan J, et al. Near complete response to pembrolizumab in microsatellite‐stable metastatic sebaceous carcinoma. J Immunother Cancer. 2018;6(1):58. 10.1186/s40425-018-0357-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banerjee N, Hossain F, Wirtschafter E, Fathizadeh P. Pembrolizumab in the treatment of microsatellite instability–high sebaceous carcinoma: a case report with review of the literature. JCO Precis Oncol. 2020;4:61–5. 10.1200/PO.19.00302 [DOI] [PubMed] [Google Scholar]
- 7. Sinicrope FA. DNA mismatch repair and adjuvant chemotherapy in sporadic colon cancer. Nat Rev Clin Oncol. 2010;7(3):174–7. 10.1038/nrclinonc.2009.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability‐high as a predictor for anti‐PD‐1/PD‐L1 immunotherapy efficacy. J Hematol Oncol. 2019;12(1):54. 10.1186/s13045-019-0738-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kandl TJ, Sagiv O, Curry JL, Ning J, Ma J, Hudgens CW, et al. High expression of PD‐1 and PD‐L1 in ocular adnexal sebaceous carcinoma. Oncoimmunology. 2018;7(9):e1475874. 10.1080/2162402X.2018.1475874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. González AR, Etchichury D, Gil ME, Del Aguila R. Neoadjuvant Vismodegib and Mohs micrographic surgery for locally advanced periocular basal cell carcinoma. Ophthal Plast Reconstr Surg. 2019;35(1):56–61. 10.1097/IOP.0000000000001166 [DOI] [PubMed] [Google Scholar]
- 11. Kibbi N, Worley B, Owen JL, Kelm RC, Bichakjian CK, Chandra S, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312(1):25–31. 10.1007/s00403-019-01971-4 [DOI] [PubMed] [Google Scholar]


