Abstract
Abstract
Glycogen particles are situated in key areas of the muscle cell in the vicinity of the main energy‐consumption sites and may be utilised heterogeneously dependent on the nature of the metabolic demands. The present study aimed to investigate the time course of fibre type‐specific utilisation of muscle glycogen in three distinct subcellular fractions (intermyofibrillar, IMF; intramyofibrillar, Intra; and subsarcolemmal, SS) during repeated high‐intensity intermittent exercise. Eighteen moderately to well‐trained male participants performed three periods of 10 × 45 s cycling at ∼105% watt max (EX1–EX3) coupled with 5 × 6 s maximal sprints at baseline and after each period. Muscle biopsies were sampled at baseline and after EX1 and EX3. A higher glycogen breakdown rate in type 2 compared to type 1 fibres was found during EX1 for the Intra (−72 vs. −45%) and IMF (−59 vs. −35%) glycogen fractions (P < 0.001) but with no differences for SS glycogen (−52 vs. −40%). In contrast, no fibre type differences were observed during EX2–EX3, where the utilisation of Intra and IMF glycogen in type 2 fibres was reduced, resulting in depletion of all three subcellular fractions to very low levels post‐exercise within both fibre types. Importantly, large heterogeneity in single‐fibre glycogen utilisation was present with an early depletion of especially Intra glycogen in individual type 2 fibres. In conclusion, there is a clear fibre type‐ and localisation‐specific glycogen utilisation during high‐intensity intermittent exercise, which varies with time course of exercise and is characterised by exacerbated pool‐specific glycogen depletion at the single‐fibre level.
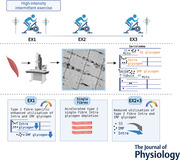
Key points
Muscle glycogen is the major fuel during high‐intensity exercise and is stored in distinct subcellular areas of the muscle cell in close vicinity to the main energy consumption sites.
In the present study quantitative electron microscopy imaging was used to investigate the utilisation pattern of three distinct subcellular muscle glycogen fractions during repeated high‐intensity intermittent exercise.
It is shown that the utilisation differs dependent on fibre type, subcellular localisation and time course of exercise and with large single‐fibre heterogeneity.
These findings expand on our understanding of subcellular muscle glycogen metabolism during exercise and may help us explain how reductions in muscle glycogen can attenuate muscle function even at only moderately lowered whole‐muscle glycogen concentrations.
Keywords: carbohydrate, excitation–contraction coupling, fatigue, metabolism, performance, subcellular
Abstract figure legend Subcellular muscle glycogen utilisation in three distinct fractions was investigated during repeated high‐intensity intermittent exercise using transmission electron microscopy imaging of m. vastus lateralis muscle samples. Muscle fibres were imaged, fibre typed based on Z‐disk width and subcellular glycogen volume fractions estimated based on point counting and direct glycogen particle diameter measurements. Enhanced type 2 fibre‐specific utilisation of intramyofibrillar (Intra) and in part intermyofibrillar (IMF) glycogen was observed during an initial but not during a second series of high‐intensity intermittent exercise, as the overall glycogen degradation rate was lowered and very low post‐exercise levels reached. Notably, the early enhanced depletion mainly of Intra glycogen was associated with pronounced depletion of a substantial proportion of single fibres of this specific fraction already at moderately lowered whole‐muscle glycogen levels.

Introduction
High‐intensity exercise relies heavily on muscle glycogen metabolism and reduced muscle glycogen content has been linked to impaired exercise tolerance (Balsom et al., 1999; Gaitanos et al., 1993). During high‐intensity exercise the ATP turnover of the muscle cell can increase more than 100‐fold, challenging the intracellular energy homeostasis (Sahlin et al., 1998). A tight association between energy utilisation and energy provision is therefore critical and has been shown to be controlled by instant feedforward and feedback regulation (Ørtenblad et al., 2009). Hence, the organisation and distribution of glycogen and glycolytic enzymes in distinct subcellular compartments situates glycogen particles in the close vicinity of the main energy consumption sites (myosin‐, Ca2+‐ and Na+–K+‐ATPases), which have been clearly elucidated using transmission electron microscopy (TEM) imaging (Friden et al., 1985; Marchand et al., 2007; Nielsen et al., 2011; Sjöström et al., 1982).
Three distinct subcellular fractions have been defined with glycogen particles stored between the sarcolemma and the most peripheral myofibrils (subsarcolemmal (SS) glycogen), between the myofibrils (intermyofibrillar (IMF) glycogen) and within the myofibrils in close association with the contractile apparatus (intramyofibrillar (Intra) glycogen). This distribution provides instant access to substrate for ATP regeneration in key areas of the muscle cell, which is characterised by a highly compartmentalised internal structure and a protein‐rich cytoplasm limiting free diffusion (Saks et al., 2008). This may be of particular importance during high‐intensity repeated exercise where a high and fluctuating ATP turnover necessitates an elevated muscle glycogen metabolism near the main energy‐requiring processes (Sahlin et al., 1998; Saks et al., 2008). Moreover, the adenine nucleotide concentrations in specific cell areas have been shown not to be in free equilibrium with the surrounding medium, for example in the restricted area of the triadic junctions formed by the transverse tubular (T‐tubular) system and sarcoplasmic reticulum (SR) membrane (Han et al., 1992; Korge & Campbell, 1994). Accordingly, a direct link between distinct subcellular glycogen fractions and key steps in excitation–contraction coupling has been proposed, which may compose an important connection between reduced glycogen content and muscle fatigue. Thus, depletion of Intra glycogen has been linked with SR Ca2+ release (Nielsen et al., 2014; Ørtenblad et al., 2011) and Na+–K+‐ATPase activity conceivably in the T‐tubules (Nielsen et al., 2009), whereas IMF glycogen has been associated with Ca2+ uptake (Nielsen et al., 2009). Moreover, SS glycogen has been proposed to supply energy consumption along the sarcolemma such as for Na+–K+‐ATPases bound at the surface membrane (Friden et al., 1985).
Interestingly, an uneven utilisation of subcellular glycogen has been demonstrated in previous studies, pointing to distinctions in local energy turnover and/or preferential utilisation of specific subfractions, dependent on the task‐specific requirements (Friden et al., 1985, 1989; Gejl et al., 2017; Jensen et al., 2020; Marchand et al., 2007; Nielsen et al., 2011; Sjöström et al., 1982). However, the findings of early studies were conflicting, with different subcellular depletion patterns reported following prolonged exercise (specific depletion of SS and IMF glycogen (Sjöström et al., 1982) or contrarily no depletion of SS glycogen (Friden et al., 1985)), as well as high‐intensity exercise (specific depletion of SS (Friden et al., 1985) or Intra glycogen (Friden et al., 1989)). This discrepancy may relate to the mainly qualitative (Friden et al., 1989; Sjöström et al., 1982) or semi‐quantitative initial approaches (Friden et al., 1985) coupled with restricted sample sizes (3–6 participants) (Friden et al., 1985, 1989; Sjöström et al., 1982). Later studies employing quantitative approaches have reported enhanced depletion of specifically the Intra glycogen fraction as a main feature of continuous exhaustive cycling (thigh muscle, with no distinction between fibre types) (Marchand et al., 2007), prolonged skiing (in type 1 and 2 fibres of thigh and arm muscle) (Nielsen et al., 2011) and in continuous high‐intensity skiing (in type 1 but not type 2 fibres from the arm muscle) (Gejl et al., 2017). In contrast, Jensen et al. (2020) found no clear distinction in subcellular depletion patterns during moderate intensity cycling, when the initial muscle glycogen content was manipulated in three different dietary conditions.
Taken together, increased relative depletion of the Intra glycogen fraction has been demonstrated, but not without inconsistencies. Accordingly, the utilisation of each glycogen fraction may be dependent on muscle group, fibre type, exercise intensity and availability of other substrates, and may vary across repetitions/duration of exercise. Notably, only one previous study using quantitative assessments has investigated the utilisation of subcellular glycogen employing high‐intensity exercise (skiing) performed as continuous intense exercise bouts (∼4 min) with the majority of the energy contribution being from oxidative phosphorylation (Gejl et al., 2017). The main finding was that Intra glycogen was utilised preferentially in type 1 fibres of the arm during an initial but not in a later repeated bout, suggesting that the Intra glycogen fraction is mainly used in the early period of exercise with a subsequent levelling off (Gejl et al., 2017). However, no study has investigated the subcellular glycogen utilisation during repeated short‐duration (<1 min) high‐intensity exercise with an exponential increase in glycogen breakdown rate and marked perturbations of the intracellular environment (Greenhaff et al., 1994; Karatzaferi et al., 2001). Specifically, the glycogen utilisation in type 2 fibres has been shown to be markedly increased at high intensities, which could stress the energy turnover in key cell areas and induce a distinct subcellular glycogen utilisation pattern (Greenhaff et al., 1994; Vøllestad et al., 1992). In line with this, fibre type‐specific fatigue patterns have been demonstrated during repeated high‐intensity exercise due to delayed type 2 fibre restoration of high‐energy phosphates, potentially altering the fibre recruitment pattern and substrate metabolism during repeated work with limited recovery (Casey et al., 1996). Moreover, the prior study on high‐intensity exercise utilised arm biopsies with distinct metabolic properties of type 1 and 2 fibres compared to the thigh musculature (Ørtenblad et al., 2018).
In addition to potential fibre type‐specific alterations in subcellular glycogen utilisation during exercise, the depletion pattern may also differ at the single‐fibre level since dissimilarities in maximal ATPase activity and metabolic phenotype have been reported even between individual fibres of the same myosin heavy chain composition (Bottinelli & Reggiani, 2000). Thus, the muscle glycogen utilisation during high‐intensity exercise appears to be characterised by large heterogeneity in single‐fibre glycogen storage and depletion patterns resulting in a substantial amount of fibres with near‐depleted levels prior to exhaustion of the whole‐muscle stores (Essen, 1977; Vigh‐Larsen et al., 2020; Vøllestad et al., 1992). This heterogeneity in single‐fibre glycogen metabolism could be exacerbated at the subcellular level and comprise a further link between reduced muscle glycogen and impaired high‐intensity exercise tolerance.
The aim of the present study was therefore to investigate the time course of fibre type‐specific utilisation of subcellular muscle glycogen in the m. vastus lateralis applying a high‐intensity intermittent exercise protocol. A secondary aim was to investigate the single‐fibre depletion patterns in each subcellular fraction. We hypothesised that specific depletion of Intra glycogen would occur in type 1 and 2 fibres, and that this would be most pronounced during the initial phase of exercise coupled with the most highly elevated muscle glycogen turnover. Moreover, we hypothesised that exacerbated early depletion of a substantial proportion of individual muscle fibres at the subcellular level would be present prior to exhaustion of the whole‐muscle glycogen stores.
Methods
Ethical approval
The project was approved by the Central Denmark Region Committees on Health Research (application number 1‐10‐72‐15‐20) and conformed to the standards of the Declaration of Helsinki, except for registration in a database. All participants were fully informed of any risks and discomforts associated with the experiments before entering the study and giving their informed, written consent to participate. The present study is a part of a larger project where further results are presented based on the muscle tissue and blood sampling (Vigh‐Larsen et al., 2022).
Participants
Twenty young, healthy and moderately to well‐trained male participants volunteered to participate in the study. Of these, two were excluded during the data collection because of technical errors, resulting in 18 participants being included in the data processing (mean ± SD age: 25 ± 2 years; body mass: 78 ± 9 kg; body fat: 9.4 ± 2.5%; and : 57 ± 5 (range: 51−66 ml kg−1 min−1). The fibre type distribution of the participants was 51 ± 6% type 1 fibres, 45 ± 5% type 2a fibres and 5 ± 4% type 2x fibres. All participants exercised 3−5 times weekly, mainly engaged in different intermittent team sport activities, but none were competitive elite athletes. For inclusion in the study, a above 50 ml kg−1 min−1 was mandatory and the participants needed to be familiar with exercising on a bike. Exclusion criteria were any injuries or conditions preventing full participation in the experiments.
Experimental design
The participants visited the laboratory on three different occasions within a 2‐week period. First, was determined using an incremental cycling protocol to exhaustion (6–10 min duration). Second, a repeated sprint test and one period of a high‐intensity intermittent exercise protocol was completed as familiarisation and intensity adjustment for the main experiments. The third visit was for the main experimental day, where muscle biopsies and blood samples were obtained prior to and during three periods of high‐intensity intermittent exercise (exercise period 1−3: EX1–EX3) in conjunction with repeated sprint testing, heart rate recording and collection of perceptual responses (ratings of perceived exertion, RPE). Prior to the main experimental day, the participants refrained from strenuous exercise for 48 h and from tobacco, alcohol or caffeine intake for 24 h.
Main experimental day
On the main experimental day, the participants arrived at the laboratory in the morning following the consumption of a standardised breakfast consisting of 1.8 g carbohydrate kg−1 body mass (oats, skimmed milk, sugar, raisins, juice and water), corresponding to ∼22 kJ kg−1 body mass. The participants were instructed to consume the breakfast ∼1.5 h prior to arrival. After a 30‐min rest period, a blood sample was drawn from a catheter inserted in an antecubital vein and a muscle biopsy obtained under local anaesthesia (∼5–10 ml 1% xylocaine) from the m. vastus lateralis through a small (∼1 cm) incision in the skin using the Bergström needle with suction. Preparations for the second biopsy, which was to be sampled instantly after EX1, were also performed at baseline and the incision covered with sterile strips and band aid. Following this procedure, a 10‐min warm‐up period at low to moderate intensity was completed before the first repeated sprint test prior to EX1, as described in the subsequent section (for an overview see Fig. 1). The participants then performed a 2‐min cycling test at ∼90% watt max (W max) before performing four brief maximal knee‐extensor contractions and four electrically induced 0.6 s contractions (results presented elsewhere). The warm‐up and assessments described above are expected to induce a negligible glycogen degradation but need to be considered as part of the protocol. After these initial preparations, the main high‐intensity work of EX1 was performed as described in detail below. Immediately after EX1, the second muscle biopsy and blood sample were obtained within ∼30 s after cessation of exercise with the participant quickly lying down on a bed placed next to the bike. Subsequently, the participants were repositioned on the bike and performed another repeated sprint test (∼150 s after termination of EX1) before resting passively for 10 min prior to initiating the main body of EX2. After EX2, no biopsy was obtained, but the repeated sprint test was performed. During the following 10‐min rest period prior to EX3, a last incision in the skin was made under local anaesthesia in preparation for the third muscle biopsy, which was obtained directly (∼30 s) after EX3 followed by the last repeated sprint test. During the experiments, heart rate was recorded continuously (Polar H10, Kempele, Finland), while RPE were collected instantly after each exercise bout using the RPE CR10 scale (Borg et al., 2010).
Figure 1. Overview of the main experimental day.
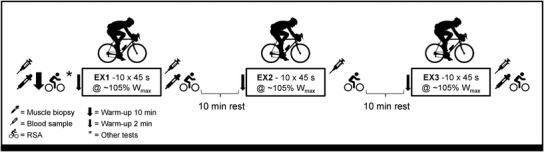
The main experimental day included three periods of high‐intensity intermittent exercise (EX1‐EX3), as well as muscle and blood sampling and tests of repeated sprint ability (RSA). Other tests included a brief series of isometric knee‐extensor contractions and 2 min of cycling at 90% W max.
Repeated sprint ability
The repeated sprint ability test consisted of 5 × 6 s maximal sprints separated by 24 s of passive recovery performed on an electronically braked cycle ergometer (Schoberer Rad Messtechnik (SRM), Jülich, Germany). These tests were incorporated to assess the deterioration of exercise tolerance throughout the protocol, as well as to introduce a further stress to increase the glycogen break down rate. Recordings were sampled at 3 Hz and processed using SRM software (version 6.41.04). The sprints were performed seated with standardised strong verbal encouragement. Peak power output was obtained from each sprint across the highest mean 3‐s period in order to avoid any noise in the power readings during the initiation and end of each sprint (Gejl et al., 2014).
High‐intensity intermittent exercise protocol
The high‐intensity intermittent exercise protocol consisted of three separated periods (EX1–EX3) of 10 × 45 s cycling at ∼105% W max with 135 s of passive recovery between bouts and 10‐min intermissions between periods. The participants were allowed to cycle at a self‐paced low intensity for 2 min prior to initiating each period. The protocol was performed on a Monark 894E Peakbike (Monark Exercise AB, Vansbro, Sweden) where the individual resistance was fixed and workload altered by increasing or decreasing the pedalling frequency. The workload was sampled at 1 Hz and stored and processed using Monark Anaerobic Test Software (Version 3.3.0.0). The individual resistance was determined based on the W max obtained through the incremental test performed prior to the main experimental day, and corresponded to 100% W max at a cadence of 90 r.p.m. with small adjustments in load based on the familiarisation trial. This was intended to enable the completion of the high‐intensity intermittent exercise protocol with no or only minor load adjustments throughout. Each 45‐s bout consisted of a fluctuating workload of 10 s at 108 r.p.m. (∼120% W max), 10 s at 90 r.p.m. (∼100% W max), 15 s at 101 r.p.m. (∼112% W max) and 10 s at 90 r.p.m. (∼100% W max) with continuous guidance and verbal encouragement to reach the prescribed intensities.
Muscle sampling and analyses
Muscle biopsies were sampled from the right leg at baseline and from the left leg after EX1 and EX3. The biopsies were separated by ∼3–5 cm to avoid repeated biopsy effects, with the last biopsy obtained from a more proximal site, and with previous studies reporting no differences in resting glycogen content or breakdown during cycling exercise between the dominant and non‐dominant leg (Costill et al., 1988; Hultman, 1967). Each muscle sample (∼150–200 mg WW) was dissected free of visible blood, fat and connective tissue, and divided into smaller separate pieces. For analyses included in the present study, one piece was frozen instantly in liquid nitrogen for assessments of whole‐muscle glycogen content biochemically and muscle metabolite concentrations, and stored at −80°C until further processing. Another small piece was fixed for TEM, as previously described in detail (Jensen et al., 2022). In brief, the sample was stored in a solution of 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.3) until the next day before a washout procedure was performed by rinsing the sample four times in 0.1 M sodium cacodylate buffer.
Muscle glycogen assay
The biochemical determination of whole‐muscle glycogen content was performed using the method described by Passonneau and Lowry (1993) and modified by Ørtenblad et al. (2009). Freeze‐dried muscle (1.5–2 mg) was boiled in 0.5 ml 1 M HCL for 150 min before being cooled quickly, whirl‐mixed and centrifuged at 3500 g for 10 min at 4°C. The supernatant (40 μl) was then mixed with 1 ml of reagent solution (Tris buffer (1 M), distilled water, ATP (100 mM), MgCl2 (1 M), NADP+ (100 mM) and glucose‐6‐phosphate dehydrogenase) before initiating the reaction by the addition of hexokinase. The absorbance was recorded spectrophotometrically before and after 60 min of reaction (Beckman DU 650, Beckman Instruments Inc., California, USA) before calculating the muscle glycogen content.
Transmission electron microcopy analyses
Muscle specimens for TEM analyses were post‐fixed and stained with 1% osmium tetroxide (OsO4) and 1.5% potassium ferrocyanide (K4Fe(CN)6) in 0.1 M sodium cacodylate buffer for 90 min at 4°C. After this, the fibre segments were rinsed in 0.1 M sodium cacodylate buffer at 4°C and dehydrated in graded series of alcohol at 4−20°C before infiltration using graded mixtures of propylene oxide and Epon at 20°C prior to embedment in 100% Epon at 30°C. A Leica Ultracut UCT ultramicrotome (Leica Microsystems, Wetzlar, Germany) was used to cut ultrathin (∼60 nm) longitudinal sections, which were contrasted with uranyl acetate and lead citrate. Ten muscle fibres were photographed using a Philips electron microscope (CM100) (Philips/FEI Corporation, Eindhoven, Holland) and an Olympus Veleta camera (Olympus Soft Imaging Solutions GmbH, Münster, Germany) at a magnification of ×13,500 in an unbiased systematic order. Accordingly, 24 images were obtained: firstly, six along the sarcolemma on one side of the fibre and six along the sarcolemma on the opposite section, then six images in the superficial myofibrillar area (three on each side of the fibre) and six in the central myofibrillar area (see Fig. 2 for sampling overview and representative images with more files in supplementary section). The fibres were fibre‐typed based on Z‐disk width, as previously described (Jensen et al., 2020). The three fibres with the most broad and narrow Z‐disks were defined as types 1 and 2 fibres, respectively, and included in the analysis for each biopsy sample, with the four remaining fibres with intermediate Z‐disk widths discarded. The glycogen volume fraction (V V) for each subcellular fraction was estimated as proposed by Weibel (1980): V V = A A − t{(1/π)B A − N A[(t × H)/(t + H)]}. A A is the glycogen area fraction (μm2/μm2), t is the section thickness (∼0.06 μm), B A is the glycogen boundary length density (μm/μm2), N A is the number of particles per area (n/μm2), and H is the average glycogen profile diameter (μm). With this approach, the section thickness was taken into account. Glycogen area fraction was determined by point‐counting and glycogen diameter by direct measurements, as previously described in detail (Jensen et al., 2020). IMF glycogen content was expressed relative to the myofibrillar space (estimated by point counting), Intra glycogen relative to the intramyofibrillar space (estimated by point counting), and SS glycogen relative to the fibre surface (estimated by direct length measurements) in accordance with the previously established methodology (Gejl et al., 2017; Jensen et al., 2020; Nielsen et al., 2011). Each fibre was analysed (point‐counting) by one of six blinded investigators after practicing until achieving a coefficient of variation <10%. All images were saved and screened continuously by the similarly blinded principal investigator for quality control. The coefficient of error for images within each fibre was 0.16, 0.17 and 0.22 for IMF, Intra and SS glycogen, respectively. Unfortunately, two samples from one participant were ruined and therefore this participant was completely excluded from the TEM analyses yielding a total of 17 subjects with pre, post‐EX1 and post‐EX3 TEM samples.
Figure 2. Representative transmission electron microscopy images.
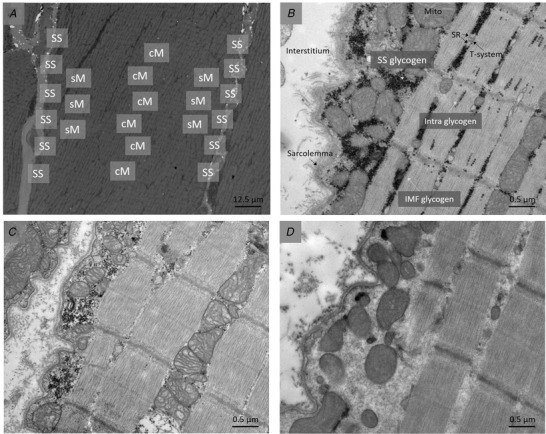
(A) Overview of micrograph sampling areas obtained at a magnification of x550, (B) an subsarcolemmal sampling area at baseline, (C) post‐exercise period 1 and (D) post‐exercise period 3, all obtained at x13,500 magnification. In D, note the almost complete lack of glycogen granules. All images are from type 2 fibres (fibre typed based on Z‐disc width) from the same participant. The black dots are glycogen particles. cM, central myofibrillar; IMF, intermyofibrillar; Intra, intramyofibrillar; Mito, mitochondria; sM, superficial myofibrillar; SR, sarcoplasmic reticulum; SS, subsarcolemmal.
Muscle metabolites
Freeze‐dried muscle (∼10 mg DW) was extracted with 0.5 M HClO4 and analysed for muscle lactate, ATP, ADP, AMP, PCr, Cr and pyruvate using enzymatic methods, as previously described (Sahlin et al., 1976). All values were normalised with total creatine content (PCr+Cr) by dividing by the total amount of creatine for the individual sample and multiplying by the mean total creatine content of the whole sample for each time point (to account for an overshoot in PCr from pre‐exercise to post‐exercise) in order to adjust for weighing variability and differences in non‐muscle constituents of the sample. Muscle pH was calculated based on the equation: muscle pH = 7.06 − 0.00532 × (lactate + pyruvate) (Sahlin et al., 1976). The total adenine nucleotide pool (TAN) was reported as the sum of ATP, AMP and ADP.
Myosin heavy chain composition
Myosin heavy chain (MHC) composition was determined from homogenate using gel electrophoresis and quantified densitometrically, as previously described (Jensen et al., 2020). In brief, muscle homogenate (20 μl) and sample buffer (100 μl, 10% glycerol, 5% 2‐mercaptoethanol, 2.3% SDS, 62.5 mM Tris, 0.2% bromophenolblue at pH 6.8) were mixed, boiled in water for 3 min and loaded on a SDS‐PAGE gel (6% polyacrylmide (100:1 acrylmid:bis‐acrylmid), 30% glycerol, 67.5 mM Tris‐base, 0.4% SDS and 0.1 M glycine) using three different protein quantities (25−40 μg). Gels were run at 4°C at 80 V for a minimum of 42 h and MHC bands made visible by Coomassie staining. The MHC composition for each participant was determined as an average from two biopsies (one from each leg) from three separate lanes for each biopsy.
Blood sampling and analyses
Blood samples were drawn in lithium–heparin tubes and serum‐tubes, and divided into separate portions for different analyses after centrifugation. The lithium–heparin tubes were kept cold and centrifuged immediately at 4°C; plasma was transferred to new tubes and stored at −20°C; while the serum samples rested for 1 h at room temperature before centrifugation. All samples were subsequently stored at −80°C. Capillary blood samples from a fingertip were obtained for measurement of blood lactate and glucose. Glucose was measured using the glucose dehydrogenase method and photometric detection (HemoCue 201 RT analyzer, Hemocue AB, Ängelholm, Sweden), while lactate was measured using the enzymatic‐amperometric method and chip‐sensor technology (Biosen, EKF Diagnostics GmbH, Barleben, Germany). One drop (∼50 μl) serum was used for lactate and glucose cuvette analyses. Plasma free fatty acids (FFA) were analysed using an enzymatic kit (Wako Chemicals, Neuss, Germany) on the Pentra 400 C clinical chemistry analyzer (Horiba ABS, Montpellier, France). Serum insulin was analysed by a sandwich enzyme immunoassay and electrochemiluminescence (Cobas 8000, e602, Roche Diagnostics, Rotkreuz, Switzerland). Plasma ammonium was measured using the enzyme glutamate dehydrogenase (and the coenzyme NADPH), which catalyses the conversion of ammonium and α‐ketoglutarate to glutamate and H2O (as well as the conversion of NADPH to NADP). The reaction rate of NAPH to NADP is proportionate to the concentration of ammonium in the sample and measured by absorbance photometry (Siemens Advia Chemistry XPT equipment, Erlangen, Germany).
Statistical analyses
Changes in absolute and relative subcellular volume fractions were determined using a linear mixed‐effects model with participant and time as random effects and time and fibre type as fixed effects. Data normality and heteroscedasticity were assessed by inspection of the distribution of residuals and normal probability plots. Data with skewed distributions were log‐transformed prior to analyses. One‐way ANOVA with repeated measures was applied for determination of changes over time in muscle metabolites, blood responses, repeated sprint ability, heart rate, etc. using the Holm–Šidák post hoc test when a significant interaction was detected. Pearson correlation coefficients were calculated and interpreted as: r ≤ 0.1 (trivial), 0.1–0.3 (small), 0.3−0.5 (moderate), 0.5–0.70 (large), 0.7–0.9 (very large) and ≥0.9 (almost perfect) (Hopkins et al., 2009). Absolute subcellular volume fractions in each of the three compartments are presented as geometric means and 95% confidence interval (CI). All other data are presented as means ± SD. Statistical analyses were performed using Stata/IC16 (StataCorp, College Station, TX, USA) and figures created in GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA). The significance level was set at P ≤ 0.05.
Results
Work rate, exertion and repeated sprint ability
During the repeated high‐intensity intermittent exercise periods (EX1–EX3), the average work rate was respectively 108 ± 5, 106 ± 4 and 105 ± 5% of W max, with a slight but significant reduction (∼3%) during the last exercise period (P = 0.002) (Fig. 3). In contrast, RPE increased progressively (P < 0.001), reaching maximal or near‐maximal levels at the end of EX3, whereas mean heart rate expressed without the recovery intervals was not significantly different between periods (∼85 ± 3%HRmax, P > 0.05). Average power output in the repeated sprints performed after EX1 and EX2 decreased similarly to 91 and 89% of baseline (P < 0.001), and was further reduced to 83% following EX3 (P < 0.001 vs. post‐EX1 and P = 0.053 vs. post‐EX2).
Figure 3. Workload, ratings of perceived exertion and repeated sprint ability during the repeated high‐intensity intermittent exercise protocol.
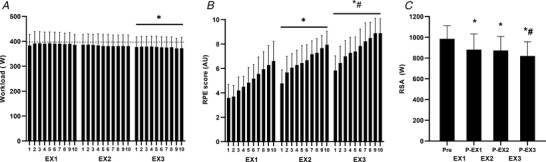
A and B, workload (A) and ratings of perceived exertion (RPE) (B) throughout the three periods (EX1–EX3) of high‐intensity intermittent exercise (10 × 45 s at ∼105% W max interspersed by 135 s of recovery between bouts and 10 min between periods. C, repeated sprint ability (RSA) (5 × 6 s sprints interspersed by 24 s of recovery) pre and post EX1–EX3. n = 18. Data are presented as means ± SD. *Significant difference compared to EX1 for workload and RPE scores and significant difference compared to pre for RSA. #Significant difference compared to EX2 for workload and compared to the post EX1 and EX2 values for RSA (P‐EX1 and P‐EX2). The dotted line in A represents the prescribed average work load in the 45‐s intervals.
Subcellular distribution of glycogen in type 1 and 2 fibres pre‐exercise
Whole‐muscle glycogen content decreased from 490 ± 94 mmol kg−1 DW pre‐exercise to 293 ± 136 and 76 ± 60 mmol kg−1 DW (P < 0.001) after EX1 and EX3, respectively. There was a strong overall correlation between biochemically determined whole‐muscle glycogen content and glycogen volume fractions determined from TEM (average of all six fibres from each sample) (r 2 = 0.91, P < 0.001) (Fig. 4). At baseline, total muscle glycogen content estimated from TEM was 16% (7, 26%) higher in type 2 compared to type 1 fibres (P < 0.001). This was mediated by a 23% (13, 34%) higher content of IMF glycogen (P < 0.001) in type 2 fibres, whereas the subcellular content of Intra and SS glycogen was similar in both main fibre types (P = 0.499 and P = 0.376, respectively). The relative distribution of glycogen in each of the three subcellular fractions was 77% (75, 78%) in the IMF region, 12% (10, 13%) in the Intra region and 12% (11, 14%) in the SS region in type 1 fibres, and 81% (79, 82%), 10% (9, 11%) and 9% (8, 10%) in type 2 fibres (see Fig. 5 for relative distribution of subcellular glycogen across time points). Accordingly, the relative content of IMF glycogen at rest was higher (P = 0.004), and Intra and SS glycogen lower, in type 2 compared to type 1 fibres (P = 0.084 and P = 0.005, respectively). Representative TEM images are shown in Fig. 2B–D .
Figure 4. Relationship between muscle glycogen determined biochemically (whole‐muscle) and by transmission electron microscopy (TEM).
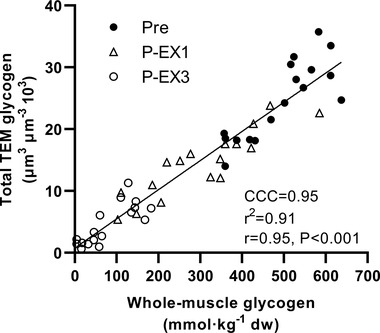
Data represents muscle biopsies obtained pre‐exercise (Pre), after exercise period 1 (P‐EX1) and after exercise period 3 (P‐EX3) during repeated high‐intensity intermittent exercise. Each data point represents muscle glycogen determined biochemically from each biopsy and TEM total muscle glycogen per biopsy determined as an average of all three type 1 and three type 2 fibres from each sample. CCC, Lin's concordance correlation coefficient.
Figure 5. Relative distribution of subcellular muscle glycogen content in type 1 and type 2 fibres during repeated high‐intensity intermittent exercise.
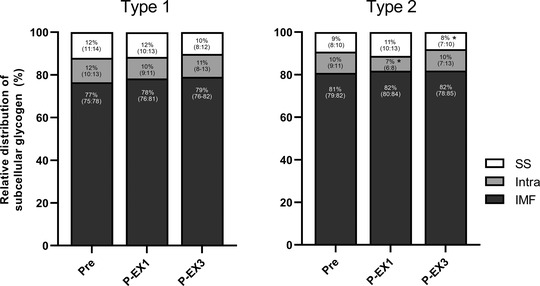
Data represents subsarcolemmal (SS), intramyofibrillar (Intra) and intermyofibrillar (IMF) glycogen determined pre‐exercise (Pre) and after exercise period 1 (P‐EX1) and 3 (P‐EX3). n = 17. Data are presented as means and 95% CI. *Significant effect of time within fibre type, P ≤ 0.05.
Utilisation of subcellular glycogen in type 1 and 2 fibres during EX1
During EX1, a fibre type‐specific utilisation of IMF glycogen was present with a larger degradation in type 2 [−59% (−72, −40%)] compared to type 1 fibres [−35% (−55, −5%); P < 0.001; Fig. 6]. The same was evident for Intra glycogen, as the content in type 2 fibres was lowered substantially more [−72% (−80, −61%)] than in type 1 fibres [−45% (−61, −23%); P < 0.001]. In contrast, no difference was observed between type 1 and 2 fibres for utilisation of SS glycogen [−40% (−61, −8%) and −52% (−69, −26%); P = 0.347]. In type 1 fibres, the relative distribution of glycogen in all three fractions was similar after EX1, suggesting a homogeneous within‐fibre subcellular utilisation pattern. However, in type 2 fibres there was a decrease in the relative distribution of Intra glycogen (P = 0.017), suggesting a higher preferential depletion of this particular subcellular fraction, as well as a trend towards a higher relative distribution of SS glycogen (P = 0.119), but no difference in the distribution of IMF glycogen and no time × type interactions (P > 0.05).
Figure 6. Utilisation of subcellular glycogen in type 1 and type 2 fibres during repeated high‐intensity intermittent exercise.
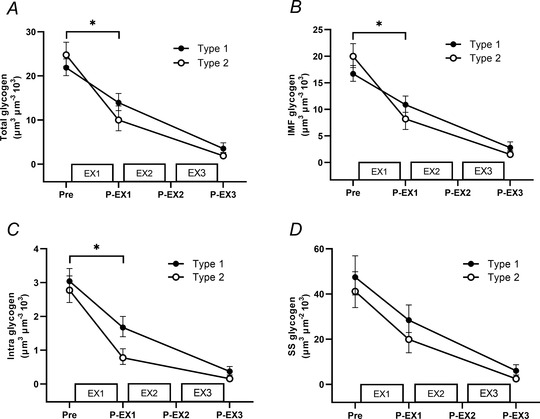
Total glycogen based on all subcellular pools (A), intermyofibrillar glycogen (IMF) (B), intramyofibrillar glycogen (Intra) (C), subsarcolemmal glycogen (SS) determined pre‐exercise (PRE) and after exercise period 1 (P‐EX1) and after exercise period 3 (P‐EX3) (D). n = 17. Data are presented as geometric means and 95% CI. *Significant difference in glycogen utilisation between fibre types from pre to post EX1, P ≤ 0.05. Main effects of time were present for all subcellular glycogen pools in each fibre type for baseline to EX1 and for EX1 to EX2+EX3 (P ≤ 0.05).
Utilisation of subcellular glycogen in type 1 and 2 fibres during EX2 and EX3
After EX1 and prior to EX2, the Intra and SS glycogen content in type 2 fibres was lower compared to type 1 fibres (P < 0.001 and P = 0.027, respectively) and numerically, but not significantly, lower levels were seen for type 2 vs. type 1 fibres in IMF glycogen (P = 0.099). Contrasting the utilisation pattern observed during EX1, no difference was found in subcellular glycogen degradation in any of the three fractions in type 1 compared to type 2 fibres during the last two exercise periods (P > 0.05) as the pools were depleted to very low levels. In type 1 fibres there was a total reduction of IMF of −83% (−89, −76%), Intra of −88% (−91, −83%) and SS of −87% (−92, −81%) following additional reductions of 48, 43 and 47%, respectively, in relation to the initial baseline level. In type 2 fibres the total reduction for IMF was −92 (−95, −89)%, for Intra was −94% (−96, −92%) and for SS was −94% (−96, −91%) corresponding to additional reductions of 33, 22 and 42%, respectively, from the baseline level. Thus, the utilisation rate of IMF and Intra glycogen in type 2 fibres was reduced compared to the utilisation rate of SS glycogen resulting in restoration of the pre‐exercise relative distribution of subcellular glycogen after all three exercise periods. Moreover, in all three subcellular fractions, the post‐exercise absolute levels of muscle glycogen were reduced to lower levels in type 2 compared to type 1 fibres (P < 0.05, Fig. 6).
The extent of muscle glycogen depletion at the whole‐muscle level and in individual fibres
The complete protocol of repeated high‐intensity intermittent exercise substantially depleted the whole‐muscle glycogen content to a mean level of 76 ± 60 mmol kg−1 DW (P < 0.001), as previously described and with very low individual values (<5 mmol kg−1 DW). This coincided with a marked depletion of several individual muscle fibres, as determined from TEM, including depleted or near‐depleted levels in all three subcellular fractions in some fibres (see example in Fig. 2). To quantify the occurrence of subcellular glycogen depletion, we employed a cut‐off level at 20% of the baseline geometric mean as an arbitrary reference point below which a subcellular fraction was determined as depleted or near‐depleted (Fig. 7). At pre‐exercise, no individual fibres were present with glycogen content below this level. After EX1, 2%, 8% and 0% of the type 1 fibres were depleted below this level for the IMF, Intra and SS subfractions, respectively, whereas in contrast the corresponding values were 8%, 31% and 14% for IMF, Intra and SS glycogen, respectively, in type 2 fibres. After EX2 and EX3, the percentage of depleted or near‐depleted type 1 fibres was increased to 51, 57 and 61% for IMF, Intra and SS glycogen, respectively, while the corresponding values for type 2 fibres were 71, 92 and 88% for the IMF, Intra and SS subfractions, respectively. To further elucidate the point of depletion of subcellular glycogen fractions at the single‐fibre level and due to the variation in whole‐muscle glycogen content at each time point between participants, the relative single‐fibre values were plotted against the whole‐muscle glycogen content rather than in relation to the given time point in Fig. 7. As can be seen from this plot, the exacerbated depletion of Intra glycogen in individual fibres occurred already at glycogen concentrations of 200−400 mmol kg−1 DW with ∼21% of the type 2 fibres depleted or near‐depleted in comparison with 8% of the type 1 fibres and no or almost no (<3%) IMF or SS glycogen subfractions depleted below this level in either fibre type.
Figure 7. Single‐fibre subcellular glycogen heterogeneity in relation to whole‐muscle glycogen content.
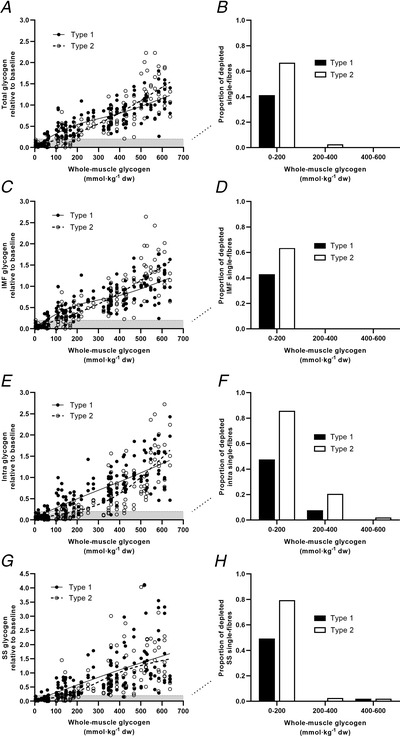
A, C, E and G, transmission electron microscopy determined single‐fibre subcellular glycogen in type 1 and type 2 fibres plotted relative to the whole‐muscle glycogen concentrations determined biochemically. (A) Total single‐fibre glycogen based on all subcellular pools, (C) intermyofibrillar glycogen (IMF), (E) intramyofibrillar glycogen (Intra) and (G) subsarcolemmal glycogen (SS) in type 1 and 2 single fibres expressed relative to the baseline geometric mean of all fibres in each pool (1 = baseline geometric mean). B, D, F and H, proportion of type 1 and 2 single fibres in each glycogen fraction depleted below 20% of the baseline level grouped at different whole‐muscle glycogen concentrations for (B) total single‐fibre glycogen based on all subcellular pools, (D) IMF glycogen, (F) Intra glycogen and (H) SS glycogen. n = 3 type 1 and 3 type 2 fibres from each of n = 17 participants. Data are presented as individual values and best fit lines (type 1 fibres, continuous line; type 2 fibres, dashed line). The grey‐shaded area below the dashed line denotes the depleted or near‐depleted level of <20% of baseline geometric mean for reference.
Muscle metabolites
Muscle lactate increased (P < 0.001) 8‐fold compared to baseline during EX1, but was only 4‐fold increased from baseline following EX3 (P < 0.001) (Table 1). In accordance, calculated muscle pH following EX1 was lower (P < 0.001) compared to after EX3. Notably, the muscle ATP concentration decreased progressively from baseline to post EX1 and EX3 (both P < 0.001), whereas increases in AMP and ADP were much smaller in absolute values indicating that the ATP had been metabolised to IMP or ammonium (not measured in muscle, but an increase in plasma ammonium was noted – see section below). Consequently, the muscle ATP concentration after EX1 and EX3 correlated almost perfectly (r = 0.99, P < 0.001) with the total adenine nucleotide pool (TAN). Interestingly, this decrease in ATP and/or TAN was moderately correlated with the absolute muscle glycogen concentrations after EX3 (r = 0.69, P = 0.002) but not after EX1 (r = 0.22, P = 0.406) (Fig. 8 A). Finally, PCr concentrations were reduced from 68.3 ± 6.7 to 47.3 ± 12.9 mmol kg−1 DW after EX1 (P < 0.001), which was a greater reduction (P = 0.005) than after EX3. However, the post‐exercise PCr content after EX1 or EX3 was not significantly related to the whole‐muscle glycogen content (r = 0.15, P = 0.566 and r = 0.14, P = 0.584) (Fig. 8 B).
Table 1.
Muscle metabolites during exercise
| Metabolite | Pre | P‐EX1 | P‐EX3 |
|---|---|---|---|
| ATP | 20.8 ± 1.7 | 18.8 ± 2.1* | 17.4 ± 2.3* , † |
| ADP | 2.7 ± 0.2 | 3.0 ± 0.3* | 3.0 ± 0.3* |
| AMP | 0.07 ± 0.04 | 0.10 ± 0.03 | 0.08 ± 0.03 |
| TAN | 23.5 ± 1.9 | 21.9 ± 2.2* | 20.5 ± 2.6* , † |
| PCr | 68.3 ± 6.7 | 47.3 ± 12.9* | 58.5 ± 11.8* , † |
| Cr | 39.4 ± 6.7 | 62.5 ± 12.9* | 55.7 ± 11.8* , † |
| TCr | 107.7 ± 7.5 | 109.8 ± 7.1 | 114.2 ± 8.1* |
| Lactate | 9.6 ± 3.6 | 77.7 ± 32.2* | 43.2 ± 23.2* , † |
| Pyruvate | 0.2 ± 0.1 | 0.4 ± 0.2* | 0.3 ± 0.1 |
| Muscle pH | 7.01 ± 0.02 | 6.66 ± 0.17* | 6.84 ± 0.10* , † |
Data are presented as means ± SD. n = 18. Values are given as mmol kg−1 DW for all metabolites and –log H+ for pH.
Significant difference comapred to baseline.
Significant difference compared to post EX1 (P‐EX1).
Figure 8. Correlations between muscle metabolites and whole‐muscle glycogen concentrations.
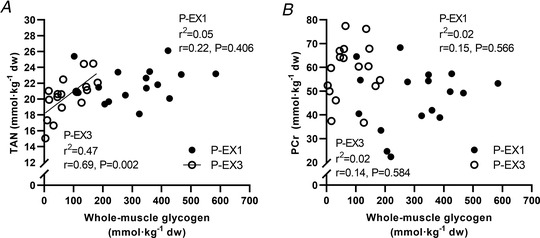
(A) total adenine nucleotide pool (TAN) and whole‐muscle glycogen, (B) phosphocreatine (PCr) and whole‐muscle glycogen during repeated high‐intensity intermittent exercise following exercise period 1 (P‐EX1) and following exercise period 3 (P‐EX3). n = 18. Data are presented as individual values and regressions.
Blood responses
Blood lactate concentrations were 6‐ to 7‐fold higher after EX1 (P < 0.001) compared to baseline and remained elevated at this level across EX2–EX3 (Table 2) despite the reductions observed in muscle lactate concentrations. The blood glucose levels decreased during exercise (P < 0.001), with no differences between the values post EX1 to EX3. Plasma FFA was unchanged following EX1, but increased 2‐fold from EX1 to EX2 (P = 0.026) and 6‐fold from EX1 to EX3 (P < 0.001), which was higher (<0.001) than all other time points. Serum insulin was progressively reduced during exercise (P < 0.001), whereas plasma ammonium concentrations increased progressively from EX1 to EX3 (P < 0.001).
Table 2.
Blood responses during exercise
| Blood variable | Pre | P‐EX1 | P‐EX2 | P‐EX3 |
|---|---|---|---|---|
| Blood lactate (mmol l−1) | 2.0 ± 0.5 | 12.7 ± 2.7* | 13.8 ± 3.2* | 11.3 ± 2.6* |
| Blood glucose (mmol l−1) | 5.9 ± 0.5 | 5.0 ± 1.1* | 4.7 ± 1.3* | 4.7 ± 0.6* |
| Plasma FFA (μmol l−1) | 71 ± 57 | 109 ± 91 | 197 ± 68* , † | 621 ± 211* , † , ‡ |
| Serum insulin (pmol l−1) | 241 ± 167 | 80 ± 68* | 47 ± 37* | 21 ± 14*(†) |
| Plasma ammonium (μmol l−1) | 24 ± 8 | 66 ± 30* | 112 ± 40* , † | 144 ± 65* , † , ‡ |
Data are presented as means ± SD. n = 18.
Significant difference compared to baseline.
Significant difference compared to P‐EX1.
Significant difference compared to P‐EX2.
Discussion
The principal findings of the present study were that the utilisation of subcellular glycogen was dependent on fibre type, localisation and time course of the repeated exercise. Thus: (1) pronounced fibre type‐specific glycogen degradation was present during the initial exercise period, with a greater utilisation of the Intra and IMF but not SS subfraction in type 2 compared to type 1 fibres; (2) accordingly, a preferential relative utilisation of Intra glycogen and, secondarily, IMF glycogen was present within type 2 fibres during the first exercise period, while conversely the relative utilisation of these two subfractions was reduced during the remaining two periods; (3) in contrast, type 1 fibres utilised all three subfractions homogeneously across all three exercise periods; and (4) the storage and utilisation of subcellular glycogen were characterised by pronounced single‐fibre heterogeneity and with appearance of near‐depleted glycogen subfractions in individual fibres when the whole‐muscle glycogen content was reduced to a moderate level, which appeared to occur earlier in the Intra glycogen fraction of type 2 fibres. Collectively, this demonstrates that high‐intensity intermittent exercise provokes an uneven breakdown of muscle glycogen with an initial enhanced utilisation of Intra and in part IMF glycogen in type 2 fibres followed by a subsequent levelling off. This occurs in association with an early exacerbated depletion mainly of Intra glycogen in a substantial proportion of type 2 single fibres prior to exhaustion of the whole‐muscle glycogen stores.
Fibre type differences
The pronounced fibre type‐specific degradation of muscle glycogen in the initial stage of exercise is in line with studies applying biochemical and histochemical assessments of single fibres demonstrating an elevated type 2 fibre glycogen metabolism during high‐intensity exercise (Gollnick et al., 1973; Greenhaff et al., 1994; Vøllestad et al., 1992). In the present study, the initial reduction in glycogen was nearly twice as high in type 2 as in type 1 fibres, which is in line with the literature where ∼50–100% higher degradation rates have been reported during different types of high‐intensity activities of short duration (Greenhaff et al., 1994; Nordheim & Vøllestad, 1990; Vøllestad et al., 1992). This disparity likely reflects the higher glycolytic potential of type 2 fibres characterised by approximately double the rates of maximal glycogen phosphorylase and phosphofructokinase activity, as well as a higher maximal ATPase activity (Bottinelli & Reggiani, 2000; Casey et al., 1996; Essen et al., 1975; Harris et al., 1976). However, as exercise progressed during the final two exercise periods, no fibre type difference in glycogen breakdown was apparent as the overall degradation rate was substantially lowered (∼50%). This was likely related to an increase in oxidative phosphorylation and a reduced glycolytic flux coupled with potential fatigue development in type 2 fibres either directly related to the pronounced early glycogen depletion or as a result of other simultaneous intracellular perturbations. In this regard, Casey et al. (1996) reported incomplete ATP and PCr restoration specifically in type 2 fibres during the recovery period between repeated bouts of high‐intensity exercise resulting in attenuated subsequent high‐energy phosphate utilisation and work capacity.
Subcellular depletion pattern during EX1
Interestingly, the initial difference in fibre type‐specific glycogen degradation was only present for the Intra and IMF glycogen fractions, while SS glycogen was utilised similarly between fibre types. Hence, within type 2 fibres Intra glycogen and secondarily IMF glycogen was preferentially utilised in relative terms during the initial bout. It should be highlighted that due to the markedly larger size of the IMF glycogen store (constituting ∼75–85% of the total glycogen content), at all time points this fraction was preferentially used in absolute terms in comparison with the smaller contribution to the total glycogen stores from SS and Intra glycogen (∼5–15% each). The enhanced relative depletion of the myofibrillar glycogen fractions could entail disproportionately elevated initial metabolic demands in the specific myofibrillar regions of type 2 fibres, an inability of other substrates (e.g. glucose) to support metabolism in these areas at an adequate rate to maintain a balanced intramuscular glycogen metabolism or differences in the metabolism of subcellular glycogen fractions through oxidative and substrate level phosphorylation. Alternately, it could be speculated that the tight co‐localisation between Intra glycogen and the major energy consuming process of the cell (cross‐bridge cycling) favours the use of this particular fraction when the energy turnover is highly elevated and the relative contribution from other nearby glycogen stores is limited due to diffusion limitations.
Specific depletion of Intra glycogen
The preferential utilisation mainly of Intra glycogen is in line with our hypothesis, though no such specific depletion pattern was detected in type 1 fibres. Our hypothesis was based on previous findings during prolonged and high‐intensity skiing, as well as during exhaustive prolonged cycling, where specific depletion of Intra glycogen in one or both main fibre types had been demonstrated (Gejl et al., 2017; Marchand et al., 2007; Nielsen et al., 2011). Quantitatively, the drop in relative distribution of Intra glycogen in percentage points was comparable with these previous results, meaning that the use of a repeated high‐intensity exercise protocol did not accentuate this depletion pattern despite marked elevations in muscle glycogen breakdown associated with exercise at such high intensities. Notably, the present study is the first to report increased relative utilisation of Intra glycogen in type 2 fibres only, which could be related to the fibre type‐specific highly elevated energy turnover during the initial stages as discussed above.
Subcellular depletion pattern during EX2+EX3
Contrasting the initial response, the utilisation rate of Intra and IMF glycogen in type 2 fibres was reduced compared to the utilisation of the SS fraction during periods 2 and 3. Consequently, all three fractions were depleted to comparable low relative levels post‐exercise, resulting in restoration of the pre‐exercise subcellular distribution. Thus, over the entire exercise protocol (EX1–EX3), the relative utilisation of each subcellular fraction was equal within both type 1 and type 2 fibres despite the perturbations observed in type 2 fibres during the initial stage. This is in line with the results obtained by Gejl et al. (2017), suggesting that the overall glycogen breakdown pattern is regulated in a way that allows utilisation of other intracellular stores when the preferred fraction is approaching complete depletion, which could be facilitated by channelling of glycolytic intermediates into depleted subcellular areas (Han et al., 1992). Thus, the pronounced specific early depletion of mainly Intra (∼72% reduction) and in part IMF (59% reduction) glycogen resulted in low absolute concentrations already at the onset of the subsequent exercise bouts compared to the ∼52% initial reduction of SS glycogen in type 2 fibres. Accordingly, differences in glycogen concentration have been shown to be an important mediator of glycogen phosphorylase activity as well as muscle glucose uptake during exercise (Hespel & Richter, 1990, 1992), which conceivably also regulates metabolism at the subcellular level. In contrast, others have proposed that muscle glycogen is degraded irrespective of initial levels during maximal exercise when the triggers of anaerobic metabolism are highly elevated coupled with the low K m of glycogen phosphorylase for glycogen (<10 mmol glycosyl units kg−1 DW; Katz, 2022). Nevertheless, it is conceivable that critically low glycogen levels were reached in local cell areas as exercise progressed or at least at the final stages when each fraction in type 2 fibres was severely depleted.
Of note, a large variation in muscle glycogen content was present between the participants at the onset of exercise (∼357–637 mmol kg−1 DW). To investigate whether the initial glycogen level influenced the subcellular depletion pattern, additional analyses were performed by separating the participants into tertiles with high and low initial glycogen levels (see Supplementary Fig. S1). At baseline the distribution of subcellular glycogen in type 1 and 2 fibres between tertiles was similar, and during exercise the utilisation of each glycogen subfraction during EX1 and during EX2+EX3 followed the same overall pattern irrespective of the initial glycogen concentration, for example a high initial breakdown followed by a subsequent levelling off, mainly in type 2 fibre IMF and Intra glycogen in both tertiles. However, within each period, the absolute breakdown rate was higher in the high compared to the low tertile, particularly in type 2 fibres. These differences were not statistically significant, however, most likely due to a low statistical power for these comparisons (6 vs. 6). It is therefore conceivable that the initial glycogen content to some degree regulates the absolute net glycogen breakdown rate. Thus, it is likely that the breakdown rates of the various glycogen pools could have been higher if more glycogen was available. We did not attempt to maximise glycogen stores prior to the experiments through any feeding regime, but it would be interesting to investigate whether prior glycogen maximisation could alter the glycogen breakdown and/or performance during this type of high‐intensity intermittent exercise.
Heterogeneous single‐fibre depletion patterns
Interestingly, near‐depleted or depleted subcellular glycogen concentrations in individual fibres were reached at an early stage of exercise when the whole‐muscle glycogen concentration was reduced below a certain moderate level. While this started to occur at whole‐muscle glycogen concentrations below ∼200 mmol kg−1 DW for IMF and SS glycogen in a sub‐group of fibres, this was the case considerably above this level for the Intra glycogen fraction in a substantial proportion of individual fibres as a consequence of the initial enhanced depletion (Fig. 7 E and F). As a result, nearly one‐third of the individual type 2 fibres had Intra glycogen contents below 20% of the pre‐exercise level, after the first exercise period, while this was the case only for a minor proportion of the individual fibres for the IMF and SS subfractions (∼10–15% of the fibres), suggesting a potential for glycogen‐dependent fatigue due to single‐fibre depletion of Intra glycogen. Importantly, it is not clearly established whether these reductions in single‐fibre levels represent physiologically relevant critically low levels and if a given relative reduction is directly comparable across subfractions characterised by largely different absolute levels. However, specific depletion of the Intra glycogen store has been linked with altered Ca2+ release in mouse single fibres (Nielsen et al., 2014) and in homogenate from human biopsies (Duhamel et al., 2006; Gejl et al., 2014; Ørtenblad et al., 2011), as well as with impaired fatigue resistance during tetanic contractions in single fibres (Nielsen et al., 2009) and in prolonged cycling (Jensen et al., 2020). Notably, as previously highlighted, this has been demonstrated despite the Intra fraction constituting only ∼10% of the total glycogen content and being present in low concentrations spread across the myofibres, mainly at the I‐band and M‐band, suggesting that the specific localisation of Intra glycogen may be critical despite the low absolute concentrations. Of note, Intra glycogen is not the only rapid ATP regenerative source in the vicinity of the contractile filaments as creatine kinase is co‐localised in these subcellular sites meaning that an increased reliance on PCr degradation potentially could counter, at least partly, the local depletion of Intra glycogen coupled with a possible augmented utilisation of the nearby IMF glycogen stores. As such, the myofibrillar creatine kinases are functionally coupled with mitochondrial creatine kinases, and substrate channelling from sites of production (e.g. at the mitochondria) to sites of consumption (e.g. at the myofibrils) has been proposed (Saks et al., 2008). In this regard, a PCr overshoot (∼6%) was present after the final exercise period, which has been shown previously following fatiguing exercise (Sahlin et al., 1997), resulting in a potential for a higher PCr contribution to the energy yield. Nevertheless, the depletion of individual fibre subfractions around a threshold of ∼200–250 mmol kg−1 DW fits well with previous observations demonstrating reduced high‐intensity exercise performance (Vigh‐Larsen et al., 2021), impaired calcium kinetics (Duhamel et al., 2006; Gejl et al., 2014; Ørtenblad et al., 2011) and increased cell signalling responses (Impey et al., 2018) when glycogen concentrations are reduced below this approximate level (∼200–300 mmol kg−1 DW) and could provide a causal link between reductions in muscle glycogen content and impaired muscle function.
Fuel metabolism
An additional finding was that fully depleted individual fibres (<1% baseline levels) and almost fully depleted individual whole‐muscle glycogen concentrations (<5 mmol kg−1 DW) were present post‐exercise (see Figs 7 A and 2 D). This supports previous findings by Essén and Henriksson (1974) showing fully depleted single fibres using biochemical assessments, thus confirming that severe exercise can result in complete degradation of the muscle glycogen stores. It should be noted that very small granules (<10 nm in diameter) could go undetected by the present microscopy technique. However, these would contribute insignificantly to the volume fraction estimations as confirmed by the very strong association between whole‐muscle and subcellular glycogen content also at the lowest glycogen concentrations. Despite these severe reductions, the participants were able to complete the intermittent exercise protocol with only minor load reductions (<5%), although RPE increased progressively to maximal or near‐maximal levels, whereas during the maximal repeated sprint tests, power output was substantially reduced (∼16% reduction post‐exercise). A somewhat intriguing observation was that the four most severely glycogen‐depleted participants, with whole‐muscle glycogen reduced to ∼4–15 mmol kg−1 DW, were able to complete the repeated sprint test (performed 150 s post‐exercise) at ∼83% of baseline levels, on par with the remaining participants, indicative of a remarkable ability to adapt metabolism. In line with this, Gaitanos et al. (1993) reported a decrease in glycolytic ATP production from 39 mmol kg−1 DW in an initial 6‐s sprint to ∼7 mmol kg−1 DW in the last of 10 × 6 s sprints performed with 30‐s rest periods, coupled with a 27% reduction in mean power. In that study, the ATP production from PCr degradation was relatively well maintained, resulting in an increased relative contribution from PCr hydrolysis from 50 to 80% of the anaerobic ATP production, coupled with a greater reliance on oxidative phosphorylation. Indeed, given the pronounced decline in muscle glycogen utilisation and reductions in post‐exercise muscle lactate concentrations, energy provision for the work in the final two exercise periods was clearly supported by processes other than glycolysis, which is in line with previous studies showing an increase in the aerobic contribution to the energy yield when high‐intensity exercise is repeated in association with increased availability of other substrates (Gaitanos et al., 1993; Gejl et al., 2017; Parolin et al., 1999).
Muscle energy homeostasis
Accompanying these low muscle glycogen concentrations, augmented reductions in the muscle ATP concentrations (∼13%) were observed immediately after the last exercise period, despite the slight reduction in work rate (<5%). Importantly, this most likely reflects a reduction in adenine nucleotides, since it is improbable that the ATP concentrations were sustained at a low level after our ∼30 s sampling delay. Interestingly, this apparent loss of adenine nucleotides seemed to be exacerbated below the proposed critical glycogen threshold (∼200 mmol kg−1 DW), possibly due to activation of the myokinase reaction and subsequent deamination of AMP in order to maintain energy balance (see Fig. 8 A). This conception is in line with some (Broberg & Sahlin, 1988; Norman et al., 1988; Spencer & Katz, 1991) but not all (Baldwin et al., 2003; Febbraio & Dancey, 1999) previous findings. In support, increased IMP formation in glycogen‐depleted single fibres was demonstrated by Norman et al. (1988) indicative of accelerated nucleotide degradation due to energy deficiency, whereas Krustrup et al. (2004) demonstrated a direct association between single‐fibre glycogen depletion and increased PCr degradation. Despite this, no acceleration in muscle PCr breakdown was evident in the present study, which speaks against the conception of glycogen depletion‐induced energy deficiency, at least at the whole‐muscle level. Nevertheless, given our sampling delay and whole‐muscle metabolite analyses it is probable that alterations in PCr degradation in association with reduced glycogen content could be present and go undetected.
Limitations
It should be noted that the study design did not account for potential preferential resynthesis of specific subcellular fractions that could occur during rest intervals. However, such effects should be minor since, for example, Hermansen and Vaage (1977) observed no resynthesis of muscle glycogen during the first 10 min of recovery after 3 × 1 min maximal cycling exercise. Moreover, we did not distinguish between type 2a and 2x fibres, but given the low (∼5%) share of type 2x fibres in the participants, the impact of this should be minor. In line with that, it has been shown that even within the same fibre type the maximal ATPase activity can vary substantially between individual fibres, which likely constitutes another aspect of the within‐fibre heterogeneity in muscle glycogen metabolism observed (Bottinelli & Reggiani, 2000).
Conclusions
In conclusion, we demonstrate substantial heterogeneity in the initial storage and utilisation of subcellular glycogen during repeated high‐intensity intermittent exercise dependent on fibre type, localisation and time course of exercise. Thus, Intra glycogen and, in part, IMF glycogen was preferentially utilised in type 2 fibres in relative terms during the initial parts of exercise and at higher rates compared to that of type 1 fibres. In contrast, no differences occurred between fibre types for the remaining exercise duration as the relative utilisation rate of type 2 fibre Intra and IMF glycogen was reduced, resulting in restoration of the pre‐exercise subcellular glycogen distribution. Moreover, the overall large degradation of muscle glycogen resulted in depletion or near‐depletion of a large proportion of single‐fibre subcellular fractions already at moderately lowered whole‐muscle glycogen levels. This emptying of muscle glycogen appeared to occur earlier for the Intra glycogen fraction in single type 2 fibres pointing to large distinctions in single‐cell glycogen distribution and metabolism.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
J.F.V.‐L., M.M., K.O. and N.Ø. were responsible for the conceptualisation of the study. J.F.V.‐L., M.M., K.O., N.Ø., J.N., O.E.A., H.T., T.H.K., S.B. and M.S.M. contributed to the acquisition, analysis and interpretation of the work. J.F.V.‐L. drafted the manuscript while all authors provided critical revisions for important intellectual content. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The work was funded by the Danish Ministry of Culture, the Danish elite sports organisation (Team Danmark), the Faroese Research Council, the A.P. Møller Foundation for the Advancement of Medical Science and by the Novo Nordisk Foundation Grant to Team Danmark (PRoKIT network).
Supporting information
Statistical Summary Document
Peer Review History
Figure S1
Representative images
Acknowledgements
We thank the highly committed efforts of all the participants involved in the study and the skilful assistance of Janni Mosgaard Jensen, Gitte Kaiser Hartvigsen, Simon Vester, Frederik Daugaard Andersen, Christian Bak Knudsen and Chris Christensen. We acknowledge the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen, where all transmission electron microscopy samples were processed and images obtained.
Biography
Jeppe F. Vigh‐Larsen has recently completed his PhD in exercise biology at the Department of Public Health, Aarhus University and is about to start in a postdoc position at the Department of Sports Science and Clinical Biomechanics, University of Southern Denmark. His current research areas include muscle metabolism and fatigue during high‐intensity intermittent exercise combining basic and translational approaches in relation to human performance and health using both in vivo and in vitro research experiments.

Handling Editors: Michael Hogan & Paul Greenhaff
The peer review history is available in the Supporting information section of this article (https://doi.org/10.1113/JP283225#support‐information‐section).
Data availability statement
The data that support the findings of the present study are available from the corresponding author upon reasonable request.
References
- Baldwin, J. , Snow, R. J. , Gibala, M. J. , Garnham, A. , Howarth, K. , & Febbraio, M. A. (2003). Glycogen availability does not affect the TCA cycle or TAN pools during prolonged, fatiguing exercise. Journal of Applied Physiology, 94(6), 2181–2187. [DOI] [PubMed] [Google Scholar]
- Balsom, P. D. , Gaitanos, G. C. , Søderlund, K. , & Ekblom, B. (1999). High‐intensity exercise and muscle glycogen availability in humans. Acta Physiologica Scandinavica, 165(4), 337–345. [DOI] [PubMed] [Google Scholar]
- Borg, E. , Borg, G. , Larsson, K. , Letzter, M. , & Sundblad, B. M. (2010). An index for breathlessness and leg fatigue. Scandinavian Journal of Medicine & Science in Sports, 20(4), 644–650. [DOI] [PubMed] [Google Scholar]
- Bottinelli, R. , & Reggiani, C. (2000). Human skeletal muscle fibres: Molecular and functional diversity. Progress in Biophysics and Molecular Biology, 73(2–4), 195–262. [DOI] [PubMed] [Google Scholar]
- Broberg, S. , & Sahlin, K. (1988). Hyperammoniemia during prolonged exercise: An effect of glycogen depletion? Journal of Applied Physiology, 65(6), 2475–2477. [DOI] [PubMed] [Google Scholar]
- Casey, A. , Constantin‐Teodosiu, D. , Howell, S. , Hultman, E. , & Greenhaff, P. L. (1996). Metabolic response of type I and II muscle fibers during repeated bouts of maximal exercise in humans. American Journal of Physiology, 271, E38–E43. [DOI] [PubMed] [Google Scholar]
- Costill, D. L. , Pearson, D. R. , & Fink, W. J. (1988). Impaired muscle glycogen storage after muscle biopsy. Journal of Applied Physiology, 64(5), 2245–2248. [DOI] [PubMed] [Google Scholar]
- Duhamel, T. A. , Perco, J. G. , & Green, H. J. (2006). Manipulation of dietary carbohydrates after prolonged effort modifies muscle sarcoplasmic reticulum responses in exercising males. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 291(4), R1100–R1110. [DOI] [PubMed] [Google Scholar]
- Essen, B. (1977). Intramuscular substrate utilization during prolonged exercise. Annals of the New York Academy of Sciences, 301(1), 30–44. [DOI] [PubMed] [Google Scholar]
- Essen, B. , & Henriksson, J. (1974). Glycogen content of individual muscle fibres in man. Acta Physiologica Scandinavica, 90(3), 645–647. [DOI] [PubMed] [Google Scholar]
- Essen, B. , Jansson, E. , Henriksson, J. , Taylor, A. W. , & Saltin, B. (1975). Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiologica Scandinavica, 95(2), 153–165. [DOI] [PubMed] [Google Scholar]
- Febbraio, M. A. , & Dancey, J. (1999). Skeletal muscle energy metabolism during prolonged, fatiguing exercise. Journal of Applied Physiology, 87(6), 2341–2347. [DOI] [PubMed] [Google Scholar]
- Friden, J. , Seger, J. , & Ekblom, B. (1985). Implementation of periodic acid‐thiosemicarbazide‐silver proteinate staining for ultrastructural assessment of muscle glycogen utilization during exercise. Cell and Tissue Research, 242(1), 229–232. [DOI] [PubMed] [Google Scholar]
- Friden, J. , Seger, J. , & Ekblom, B. (1989). Topographical localization of muscle glycogen: An ultrahistochemical study in the human vastus lateralis. Acta Physiologica Scandinavica, 135(3), 381–391. [DOI] [PubMed] [Google Scholar]
- Gaitanos, G. C. , Williams, C. , Boobis, L. H. , & Brooks, S. (1993). Human muscle metabolism during intermittent maximal exercise. Journal of Applied Physiology, 75(2), 712–719. [DOI] [PubMed] [Google Scholar]
- Gejl, K. D. , Hvid, L. G. , Frandsen, U. , Jensen, K. , Sahlin, K. , & Ørtenblad, N. (2014). Muscle glycogen content modifies SR Ca2+ release rate in elite endurance athletes. Medicine and Science in Sports and Exercise, 46(3), 496–505. [DOI] [PubMed] [Google Scholar]
- Gejl, K. D. , Ørtenblad, N. , Andersson, E. , Plomgaard, P. , Holmberg, H. C. , & Nielsen, J. (2017). Local depletion of glycogen with supramaximal exercise in human skeletal muscle fibres. Journal of Physiology, 595(9), 2809–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick, P. D. , Armstrong, R. B. , Sembrowich, W. L. , Shepherd, R. E. , & Saltin, B. (1973). Glycogen depletion pattern in human skeletal muscle fibers after heavy exercise. Journal of Applied Physiology, 34(5), 615–618. [DOI] [PubMed] [Google Scholar]
- Greenhaff, P. L. , Nevill, M. E. , Søderlund, K. , Bodin, K. , Boobis, L. H. , Williams, C. , & Hultman, E. (1994). The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. Journal of Physiology, 478(1), 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. W. , Thieleczek, R. , Varsanyi, M. , & Heilmeyer, L. M., Jr (1992). Compartmentalized ATP synthesis in skeletal muscle triads. Biochemistry, 31(2), 377–384. [DOI] [PubMed] [Google Scholar]
- Harris, R. C. , Essen, B. , & Hultman, E. (1976). Glycogen phosphorylase activity in biopsy samples and single muscle fibres of musculus quadriceps femoris of man at rest. Scandinavian Journal of Clinical and Laboratory Investigation, 36(6), 521–526. [DOI] [PubMed] [Google Scholar]
- Hermansen, L. , & Vaage, O. (1977). Lactate disappearance and glycogen synthesis in human muscle after maximal exercise. American Journal of Physiology, 233, E422–E429. [DOI] [PubMed] [Google Scholar]
- Hespel, P. , & Richter, E. A. (1990). Glucose uptake and transport in contracting, perfused rat muscle with different pre‐contraction glycogen concentrations. Journal of Physiology, 427(1), 347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespel, P. , & Richter, E. A. (1992). Mechanism linking glycogen concentration and glycogenolytic rate in perfused contracting rat skeletal muscle. Biochemical Journal, 284(3), 777–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, W. G. , Marshall, S. W. , Batterham, A. M. , & Hanin, J. (2009). Progressive statistics for studies in sports medicine and exercise science. Medicine and Science in Sports and Exercise, 41(1), 3–12. [DOI] [PubMed] [Google Scholar]
- Hultman, E. (1967). Muscle glycogen in man determined in needle biopsy specimens: Method and normal values. Scandinavian Journal of Clinical and Laboratory Investigation, 19(3), 209–217. [DOI] [PubMed] [Google Scholar]
- Impey, S. G. , Hearris, M. A. , Hammond, K. M. , Bartlett, J. D. , Louis, J. , Close, G. L. , & Morton, J. P. (2018). Fuel for the work required: A theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Medicine, 48(5), 1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, R. , Ørtenblad, N. , di Benedetto, C. , Qvortrup, K. , & Nielsen, J. (2022). Quantification of subcellular glycogen distribution in skeletal muscle fibers using transmission electron microscopy. Journal of Visualized Experiments, (180), 10.3791/63347. [DOI] [PubMed] [Google Scholar]
- Jensen, R. , Ørtenblad, N. , Stausholm, M. H. , Skjaerbaek, M. C. , Larsen, D. N. , Hansen, M. , Holmberg, H. C. , Plomgaard, P. , & Nielsen, J. (2020). Heterogeneity in subcellular muscle glycogen utilisation during exercise impacts endurance capacity in men. Journal of Physiology, 598(19), 4271–4292. [DOI] [PubMed] [Google Scholar]
- Karatzaferi, C. , de Haan, A. , Ferguson, R. A. , van Mechelen, W. , & Sargeant, A. J. (2001). Phosphocreatine and ATP content in human single muscle fibres before and after maximum dynamic exercise. Pflugers Archiv: European Journal of Physiology, 442(3), 467–474. [DOI] [PubMed] [Google Scholar]
- Katz, A. (2022). A century of exercise physiology: Key concepts in regulation of glycogen metabolism in skeletal muscle. European Journal of Applied Physiology, 122(8), 1751–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge, P. , & Campbell, K. B. (1994). Local ATP regeneration is important for sarcoplasmic reticulum Ca2+ pump function. American Journal of Physiology, 267(2), C357–C366. [DOI] [PubMed] [Google Scholar]
- Krustrup, P. , Søderlund, K. , Mohr, M. , & Bangsbo, J. (2004). Slow‐twitch fiber glycogen depletion elevates moderate‐exercise fast‐twitch fiber activity and O2 uptake. Medicine and Science in Sports and Exercise, 36(6), 973–982. [DOI] [PubMed] [Google Scholar]
- Marchand, I. , Tarnopolsky, M. , Adamo, K. B. , Bourgeois, J. M. , Chorneyko, K. , & Graham, T. E. (2007). Quantitative assessment of human muscle glycogen granules size and number in subcellular locations during recovery from prolonged exercise. Journal of Physiology, 580(2), 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, J. , Cheng, A. J. , Ørtenblad, N. , & Westerblad, H. (2014). Subcellular distribution of glycogen and decreased tetanic Ca2+ in fatigued single intact mouse muscle fibres. Journal of Physiology, 592(9), 2003–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, J. , Holmberg, H. C. , Schrøder, H. D. , Saltin, B. , & Ørtenblad, N. (2011). Human skeletal muscle glycogen utilization in exhaustive exercise: Role of subcellular localization and fibre type. Journal of Physiology, 589(11), 2871–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, J. , Schrøder, H. D. , Rix, C. G. , & Ørtenblad, N. (2009). Distinct effects of subcellular glycogen localization on tetanic relaxation time and endurance in mechanically skinned rat skeletal muscle fibres. Journal of Physiology, 587(14), 3679–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim, K. , & Vøllestad, N. K. (1990). Glycogen and lactate metabolism during low‐intensity exercise in man. Acta Physiologica Scandinavica, 139(3), 475–484. [DOI] [PubMed] [Google Scholar]
- Norman, B. , Sollevi, A. , & Jansson, E. (1988). Increased IMP content in glycogen‐depleted muscle fibres during submaximal exercise in man. Acta Physiologica Scandinavica, 133(1), 97–100. [DOI] [PubMed] [Google Scholar]
- Ørtenblad, N. , Macdonald, W. A. , & Sahlin, K. (2009). Glycolysis in contracting rat skeletal muscle is controlled by factors related to energy state. Biochemical Journal, 420(2), 161–168. [DOI] [PubMed] [Google Scholar]
- Ørtenblad, N. , Nielsen, J. , Boushel, R. , Søderlund, K. , Saltin, B. , & Holmberg, H. C. (2018). The muscle fiber profiles, mitochondrial content, and enzyme activities of the exceptionally well‐trained arm and leg muscles of elite cross‐country skiers. Frontiers in Physiology, 9, 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørtenblad, N. , Nielsen, J. , Saltin, B. , & Holmberg, H. C. (2011). Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. Journal of Physiology, 589(3), 711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolin, M. L. , Chesley, A. , Matsos, M. P. , Spriet, L. L. , Jones, N. L. , & Heigenhauser, G. J. (1999). Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. American Journal of Physiology, 277, E890–E900. [DOI] [PubMed] [Google Scholar]
- Passonneau, J. L. , & Lowry, O. H. (1993). Enzymatic analysis: A practical guide. Humana Press Inc. [Google Scholar]
- Sahlin, K. , Harris, R. C. , Nylind, B. , & Hultman, E. (1976). Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Archiv: European Journal of Physiology, 367(2), 143–149. [DOI] [PubMed] [Google Scholar]
- Sahlin, K. , Søderlund, K. , Tonkonogi, M. , & Hirakoba, K. (1997). Phosphocreatine content in single fibers of human muscle after sustained submaximal exercise. American Journal of Physiology, 273(1), C172–C178. [DOI] [PubMed] [Google Scholar]
- Sahlin, K. , Tonkonogi, M. , & Søderlund, K. (1998). Energy supply and muscle fatigue in humans. Acta Physiologica Scandinavica, 162(3), 261–266. [DOI] [PubMed] [Google Scholar]
- Saks, V. , Beraud, N. , & Wallimann, T. (2008). Metabolic compartmentation – A system level property of muscle cells: real problems of diffusion in living cells. International Journal of Molecular Sciences, 9(5), 751–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström, M. , Friden, J. , & Ekblom, B. (1982). Fine structural details of human muscle fibres after fibre type specific glycogen depletion. Histochemistry, 76(4), 425–438. [DOI] [PubMed] [Google Scholar]
- Spencer, M. K. , & Katz, A. (1991). Role of glycogen in control of glycolysis and IMP formation in human muscle during exercise. American Journal of Physiology, 260, E859–E864. [DOI] [PubMed] [Google Scholar]
- Vigh‐Larsen, J. F. , Ermidis, G. , Rago, V. , Randers, M. B. , Fransson, D. , Nielsen, J. L. , Gliemann, L. , Piil, J. F. , Morris, N. B. , DEP F. V., Overgaard, K. , Andersen, T. B. , Nybo, L. , Krustrup, P. , & Mohr, M. (2020). Muscle metabolism and fatigue during simulated ice hockey match‐play in elite players. Medicine and Science in Sports and Exercise, 52(10), 2162–2171. [DOI] [PubMed] [Google Scholar]
- Vigh‐Larsen, J. F. , Ørtenblad, N. , Nielsen, J. , Andersen, O. E. , Overgaard, K. , & Mohr, M. (2022). The Role of Muscle Glycogen Content and Localization in High‐Intensity Exercise Performance: A Placebo‐Controlled Trial. Medicine & Science in Sports & Exercise. Advance online publication. 10.1249/mss.0000000000003002 [DOI] [PubMed] [Google Scholar]
- Vigh‐Larsen, J. F. , Ørtenblad, N. , Spriet, L. L. , Overgaard, K. , & Mohr, M. (2021). Muscle glycogen metabolism and high‐intensity exercise performance: A narrative review. Sports Medicine, 51(9), 1855–1874. [DOI] [PubMed] [Google Scholar]
- Vøllestad, N. K. , Tabata, I. , & Medbo, J. I. (1992). Glycogen breakdown in different human muscle fibre types during exhaustive exercise of short duration. Acta Physiologica Scandinavica, 144(2), 135–141. [DOI] [PubMed] [Google Scholar]
- Weibel, E. (1980). Stereological methods (vol. 2). Theoretical Foundations Academic Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Summary Document
Peer Review History
Figure S1
Representative images
Data Availability Statement
The data that support the findings of the present study are available from the corresponding author upon reasonable request.


