Abstract
Compression spinal cord injuries are a common cause of morbidity in people who experience a spinal cord injury (SCI). Either as a by‐product of a traumatic injury or due to nontraumatic conditions such as cervical myelitis, compression injuries are growing in prevalence clinically and many attempts of animal replication have been described within the literature. These models, however, often focus on the traumatic side of injury or mimic short‐term injuries that are not representative of the majority of compression SCI. Of this, nontraumatic spinal cord injuries are severely understudied and have an increased prevalence in elderly populations, adults, and children. Therefore, there is a need to critically evaluate the current animal models of compression SCI and their suitability as a method for clinically relevant data that can help reduce morbidity and mortality of SCI. In this review, we reviewed the established and emerging methods of animal models of compression SCI. These models are the clip, balloon, solid spacer, expanding polymer, remote, weight drop, calibrated forceps, screw, and strap methods. These methods showed that there is a large reliance on the use of laminectomy to induce injury. Furthermore, the age range of many studies does not reflect the elderly and young populations that commonly suffer from compression injuries. It is therefore important to have techniques and methods that are able to minimize secondary effects of the surgeries, and are representative of the clinical cases seen so that treatments and interventions can be developed that are specific.
Keywords: age related, compression, elderly, nontraumatic, pediatric, rodent
Significance.
While the majority of spinal cord injuries (SCIs) are caused by acute mechanical trauma there are also a proportion caused by chronic spinal cord compression. The current trend of SCI shows an increase in compression SCI in all ages, but especially in the very young and the elderly. There is relatively little research undertaken in animals for compression SCI compared to trauma‐related SCI. In this review paper, we described current animal models of compressive SCI and evaluated their broader applications. Models that are reproducible, uncomplicated, and adaptable across species and ages have the most potential for translational research.
1. INTRODUCTION
The global incidence of spinal cord injuries (SCIs) is estimated to be between 40 and 80 cases per million, with an average of between 250 and 500 thousand new cases per year (WHO, 2013). In the adult population, a substantial proportion of SCI is due to traumatic causes such as motor‐related injury, sporting accidents, falls, or violence, while a smaller yet growing proportion (incidence of 26.3 cases per million) of spinal damage is due to nontraumatic causes (Müller‐Jensen et al., 2021; New, 2019; New et al., 2014; WHO, 2013). There are also significant age‐related differences in the causes of SCI, with the young and the elderly more likely to have a higher proportion of nontraumatic spinal cord injury (NTSCI) (Smith et al., 2017).
NTSCI can further be classified as compressive (tumors (benign/malignant), metastases, malformations, abscess, bleeding, spondylitis, and disk herniation/spinal stenosis) or non‐compressive inflammatory/infections, immunological (e.g., multiple sclerosis), vascular (e.g., infarction), metabolic (e.g., vitamin deficiency), toxins, physical (e.g., postradiation myelopathy) (Adams & Salam‐Adams, 1991; Müller‐Jensen et al., 2021). It is important to acknowledge that the terminology for describing these types of SCI is not standardized. For the purpose of this review the term “NTSCI” will be used to differentiate from animal models that rely on a mechanical impact to produce a traumatic injury and “compression” to describe the primary mode of injury where the spinal cord is deformed due to the application of sustained pressure of varying duration rather than an acute impact of varying force. Other descriptive terms used clinically may include spinal cord dysfunction (SCDys) or compressive myelopathy. NTSCI has received relatively little attention compared to traumatic SCI, with considerable variation in approaches to research. As a result, there is a lack of robust animal models to investigate causes, pathophysiology, and intervention strategies.
Most current NTSCI research focuses on compressive type injuries as they have the highest incidence, and many animal models of NTSCI focus on acute compression injuries as a confounding factor of traumatic injury (De Martino et al., 2019; New et al., 2017). In a 2017 review of all animal models of SCI, 94.5% were modeled using mechanical traumatic injury while the remaining 5.5% were modeled using nonmechanical injury (Sharif‐Alhoseini et al., 2017). Currently, only about 17% of all SCI publications include “compression” and <5% of those refer to animal models. There is a limited number of methods to reproduce compressive SCI in animals. The most common method used is clip compression, making up over 50% of all compression models (Sharif‐Alhoseini et al., 2017). This is followed by balloon compression models and then several less utilized methods such as screw compression, solid spacer compression, weight drop compression, remote compression, expanding polymers, and spinal cord strapping (Cheriyan et al., 2014; Muir & Webb, 2000; New, 2019; Sharif‐Alhoseini et al., 2017; Zhang et al., 2014). These models can be used to investigate specific aspects of spinal cord compression but may not entirely cover the broad range of compressive NTSCI seen clinically (Kang et al., 2018; New, 2019).
In this review, each of these animal models of spinal cord compression will be evaluated for their use in modeling human spinal cord compression injury and their future potential for accurate and useful intervention techniques in NTSCI.
2. TYPES OF COMPRESSION MODELING
2.1. Models requiring laminectomy
2.1.1. The limitation of laminectomy
All the techniques described below have the same limited consideration. They all require at least a partial laminectomy to be undertaken to allow access to the spinal cord. The main limitations of these methods are the impedance and removal of spinal processes and lamina and alterations to the fluid dynamics of the spinal cord (Cheriyan et al., 2014; Do Espírito Santo et al., 2019; Xu et al., 2008; Zhang et al., 2014). By removing any part of the lamina whether extensive or minimal, the fluid dynamics of the spinal cord are changed. Laminectomies may also cause alterations to the degree of spinal cord compression due to the increased space at or near the injury site. The location of the laminectomy compared to the injury site may change the biomechanics of the injury, especially if in close proximity. The damaged cord may then “bulge” into the space created. Laminectomy and laminoplasty are in fact treatment options for spinal compression caused by cervical myelopathy (Liu et al., 2016). However, there are two methods of spinal cord compression that overcome this limitation by avoiding the necessity for a laminectomy which will be described in the following section.
2.1.2. Clip compression
Clip compression (Figure 1) is an acute compression that reflects a sudden and short compression of the spinal cord such as an injury caused after a vertebral bone fracture. Developed by Rivlin and Tator, this method used a repurposed aneurysm clip to compress the spinal cord of rats using 180 g of force for varying times (1978). This study aimed to understand the importance of time and decompression from the incidence of the injury. This method has since been developed and used to induce varying levels of acute compressive SCI in rats and mice. Most commonly the procedure includes thoracic laminectomy (Chamankhah et al., 2013; Lanza et al., 2018; Sajad et al., 2021) followed by the application of a clip to exert extradural compression of the spinal cord. Typically, clips are applied for 30 s to 1 min before release and use 20–180 g of force for compression (Chamankhah et al., 2013; Lanza et al., 2018; Sajad et al., 2021). The clips used for compression are often an aneurysm or vascular clips but may also be custom designed (Do Espírito Santo et al., 2019; Gao et al., 2019; Wang et al., 2021; Zhang et al., 2021).
FIGURE 1.
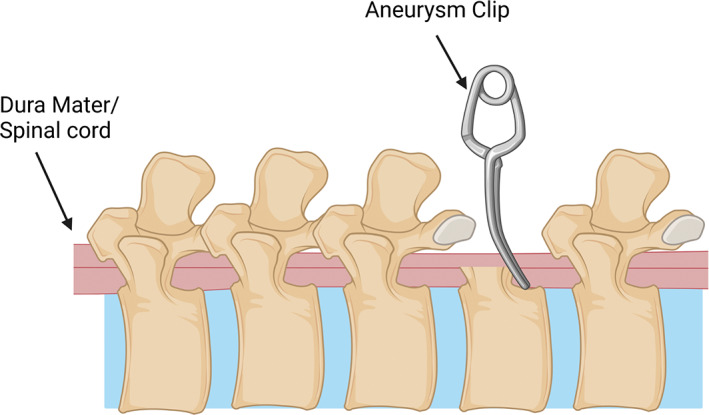
Schematic of spinal cord injury compression model using an aneurysm clip on the exposed spinal cord post‐laminectomy. The spinal cord sits between the two prongs of the aneurysm clip and compresses the spinal cord laterally for a short time before been removed. Created with BioRender.com.
Clip compression is a simple and effective method that results in an acute compression SCI. By altering the time and force of the clip this model also produces a graded injury (Do Espírito Santo et al., 2019; Rivlin & Tator, 1978; Sajad et al., 2021; Wang et al., 2021; Zhang et al., 2021). This model further provides an excellent histological example of injury to the spinal cord (Hassannejad et al., 2019; Zhang et al., 2021). This is important as it allows quantification and visualization of the damage in the spinal cord, aiding discussion about the corresponding motor deficits. Using a clip also allows for changes in compression angle so that both the anterior–posterior and lateral spinal cord compression injuries can be replicated (Wang et al., 2021). These all make for a relatively simple reproducible procedure that creates good histological outcomes.
However, clip compression is limited in its use as an accurate NTSCI model in a few ways. Clip compression may be better defined as an acute traumatic injury using compression rather than a model of NTSCI due to compression. This is reflected in the literature as it is sometimes referred to as a traumatic injury (Gao et al., 2019; Hassannejad et al., 2019). While this is not a poor characteristic of the procedure, it makes it unsuitable for modeling chronic compression SCI such as those caused by tumors or spondylitis (Müller‐Jensen et al., 2021). Moreover, clip compressions of spinal cord have been described only for adult animals and may not be suitable for use in young or old animals. This limits the potential to transfer accurate data about NTSCI to humans, as they most commonly occur in the very young and the elderly populations (Müller‐Jensen et al., 2021; New et al., 2013).
2.1.3. Balloon compression
Balloon compression (Figure 2) is a short‐term compressive injury that increases pressure significantly for a short time above the dura mater of the spinal cord and below the spinal processes. Balloon compression is a popular method among scientists as it mimics a compression injury and decompression of the spinal cord, simulating the removal of a tumor, cervical myelitis, and the like. Tarlov first described this method in 1957 when he inserted an inflatable catheter into the vertebral canal above the spinal cords and inflated it for varying amounts of time (1957). Since the initial study, this method of spinal cord compression has been used sporadically to induce spinal cord compression in animals such as dogs, rabbits, and rodents (Krupa et al., 2020; Lim et al., 2007; Murgoci et al., 2019; Ruzicka et al., 2017; Yang et al., 2017). Most studies now use either a rabbit or rodent model when inducing a compression injury. The standard procedure uses a “2‐French Fogarty” catheter which has a balloon‐like tip, which is then inserted into the spinal canal and above the dura as the means for compression injury (Krupa et al., 2020). A laminectomy is needed to insert the catheter but does not need to be positioned directly over the intended injury site. Instead, it may be several segments away and the catheter is then introduced until it reaches the required spinal level before inflation (Krupa et al., 2020; Lim et al., 2007; Ruzicka et al., 2017). A graded injury can be obtained by varying the volume of filling solution from 12 to 50 μl saline solution and by varying the inflation time before decompression. This is most commonly 5–10 min (Krupa et al., 2020; Lim et al., 2007; Ruzicka et al., 2017) but inflated “balloons” may be left in post‐surgery for up to 4 weeks (Yang et al., 2017). It has been suggested that the balloon method is not actually best for rodents due to their small subarachnoid space (Vanický et al., 2001) but nevertheless it has been used quite extensively in both rats and mice and has also been employed in several larger species including rabbits (Yang et al., 2017), dogs (Lim et al., 2007; Tarlov, 1957), and goats (Jiang et al., 2016).
FIGURE 2.
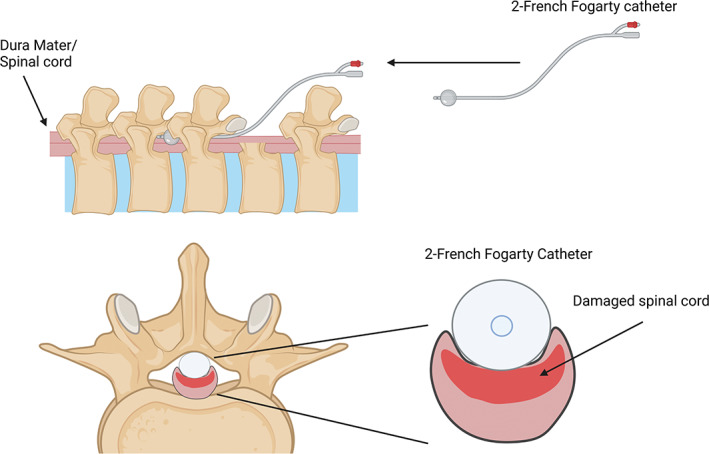
Schematic of balloon compression model with a 2‐French Fogarty catheter and the inflatable “balloon” tip. The catheter is maneuvered under the spinal column and above the dura upstream from the laminectomy, before being inflated. Created with BioRender.com.
The balloon method is also relatively simple and easy to implement, and this model has the advantage of allowing for the decompression of the injury after time which is clinically relevant.
Nevertheless, the balloon model causes more of an acute compressive injury (Cheriyan et al., 2014). While the compressive injury does occur, followed by loss of function in the animals, it does not accurately reflect the changes in compression over a prolonged period. Almost all the balloon models describe a short compressive injury time of 5 min before removal of the balloon. Therefore, while this model helps research clinical acute compression injuries, its use in NTSCI is limited. Additionally, all reported balloon compressions have been undertaken in adult animals only. Even the smallest balloon catheters used for this method, are too large for use on younger rodents as they are too large for their spinal column.
2.1.4. Solid spacer compression
A solid spacer compression (Figure 3) is used to mimic compression injuries by inserting a solid wedge‐shaped object into the epidural space. This process is done by performing a laminectomy above the level of the desired injury and then moving the spacer into position above the dura and below the intact lamina. This procedure can occlude the spinal column at varying levels depending on the dimensions of the spacer, where 50% occlusion is the first instance of visual motor deficit (Dimar et al., 1999). After insertion of the spacer, it can be left in the epidural space for a prolonged period and has the potential to be removed via surgery if desired (Batchelor et al., 2010). The initial use for the spacer was to test the impact of contusion injury and compression using a Teflon spacer in a rat model of SCI (Dimar et al., 1999). This has since been modified to either a poly (methyl methacrylate) plastic or polycarbonate rod spacer in rats (Batchelor et al., 2010; Schiaveto‐de‐Souza et al., 2013) and in mice (Kubota et al., 2012).
FIGURE 3.
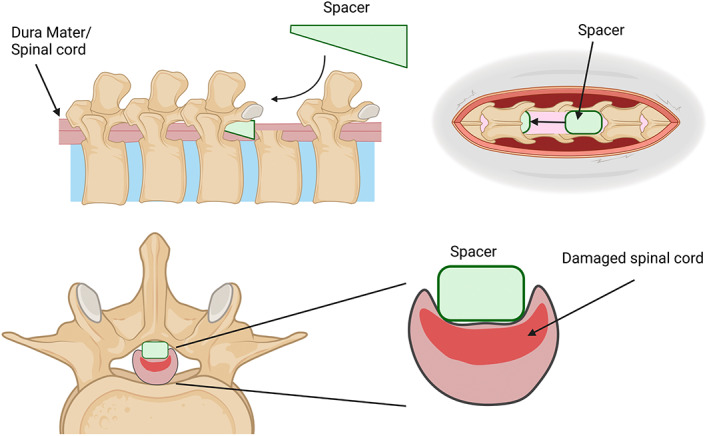
Schematic of solid spacer model using a wedged shape spacer which is inserted between the dorsal spinal column and dura of the spinal cord after a laminectomy. The spacer can be left in for days–weeks. Created with BioRender.com.
2.1.5. Expanding polymer models
Similarly, an expanding polymer (Figure 4) inserted underneath the cervical spinal cord was first described by Kim et al. (2004). This method used a sheet of polymer that would expand and sit in situ for 25 weeks between the spinal cord and spinal column. This reproduces a chronic compressive injury that mimics delayed cervical myelopathy. Since then, this method has been enhanced by using different sized polymers, and several types of polymers to try and create a more accurate and repeatable experiment. One such method used a water‐absorbing polymer that hooks around the spinal cord at the C5/6 region and absorbs water over the desired time of up to 24 h (Ijima et al., 2017; Long et al., 2013). Additionally, Karadimas et al. have used a different type of polymer that absorbs phosphate anions in situ and encourages the formation of new bone in the area to replicate the same chronic compression seen clinically in myelopathy (2013, 2015). These polymers require a laminectomy to implant and are left in for days to weeks depending on the study. They have been able to show that there is a variable loss of large motor neurons using this method, but with a range of spinal cord occlusion that is hard to replicate (Karadimas et al., 2015). This method currently lacks the ability and definitive measurements to be a succinct reproducible model that can accurately replicate injury at the same level each time (Ijima et al., 2017). It is unclear whether this model could be a reliable model for spinal cord compression, even though it can accurately mimic the chronic increase and sustained compression that is missing from other models.
FIGURE 4.
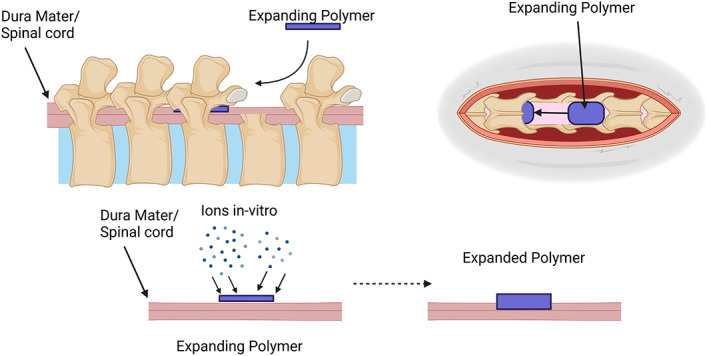
Schematic of expanding polymer with a thin strip of polymer inserted between the posterior spinal column and dura of the spinal cord after a laminectomy. The spacer expands in situ over time to create a growing spinal cord injury that slowly compresses the spinal cord. Created with BioRender.com.
2.1.6. Weight drop compression
The weight drop model of SCI (Figure 5) is commonly used to induce a contusion injury that mimics a traumatic injury (Sharif‐Alhoseini et al., 2017). However, some researchers have suggested its use as a compression model in conjunction with the contusion model to replicate the compression created from edema after injury (Dimar et al., 1999; Kim et al., 2019; Lau et al., 2013). This model uses multiple animal models, such as rodents and pigs, commonly at adult age (Cheriyan et al., 2014; Sharif‐Alhoseini et al., 2017). This procedure opens the animal's spinal column via laminectomy and drops a weighted (30–50 g) rod from a fixed height onto the exposed spinal cord (Gruner, 1992; Stokes, 1992). To induce compression, the rod is additionally held down with an increased mass (50–100 g) for up to 15 min on the exposed cord (Gruner, 1992; Scheff et al., 2003; Stokes, 1992). This model may also skip the weight drop and compress the exposed spinal cord with the weighted rod from the start (Scheff et al., 2003). After the injury, the animal is sutured and allowed to recover while motor deficits are observed (Gruner, 1992; Scheff et al., 2003; Stokes, 1992). There are many models of weight drop, most commonly the Ohio state method, The NYU method, and the Infinite Horizons impact method (Cheriyan et al., 2014; Gruner, 1992; Scheff et al., 2003; Stokes, 1992). These methods were all created to model contusion injuries. Still, they may have the potential to be converted into compression models, mainly when controlled by a consistent force for a prolonged period that does not involve the weight to “drop.”
FIGURE 5.
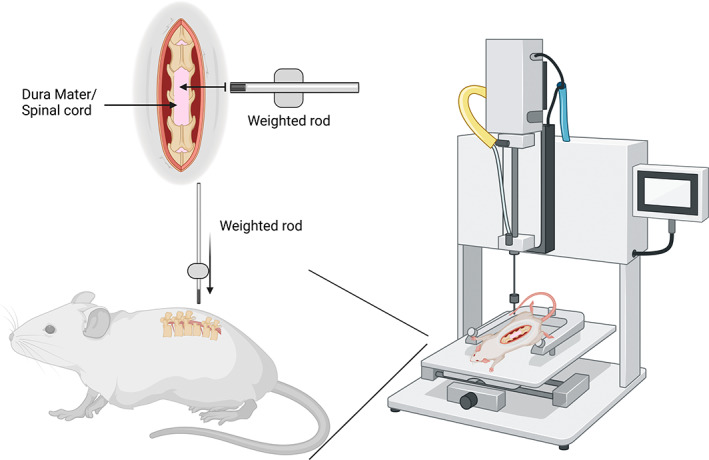
Schematic of the weight drop method using a mechanical impactor. The weighted rod drops on the exposed spinal cord from a set height and will displace the cord a varying degree and time depending on the model of weight drop used. Created with BioRender.com.
2.1.7. Forceps compression
Other models of compression injury include forceps compression (Figure 6) (Blight, 1991). Following a laminectomy, forceps compression involves using calibrated forceps separated by a spacer at different lengths (0.25 mm (mild), 0.4 mm (intermediate), 0.55 mm (severe)) to compress the spinal cord for 15 s and induce a compressive SCI (Blight, 1991; Luo et al., 2002; McDonough et al., 2015; Plemel et al., 2008; Vaughn et al., 2013). This method was initially designed to mimic mild compression injuries but has evolved to induce a graded level of injury that can be measured (Blight, 1991; McDonough et al., 2015) and might also be better described as an acute traumatic injury using compression. While this method can provide a consistent injury, some discrepancy in the actual ability for this model to record the injury parameters accurately has been reported (Cheriyan et al., 2014). Therefore, while forceps compression is an inexpensive and straightforward method of NTSCI, variability in its accuracy, reliability for accurate replication and modeling needs to be considered.
FIGURE 6.
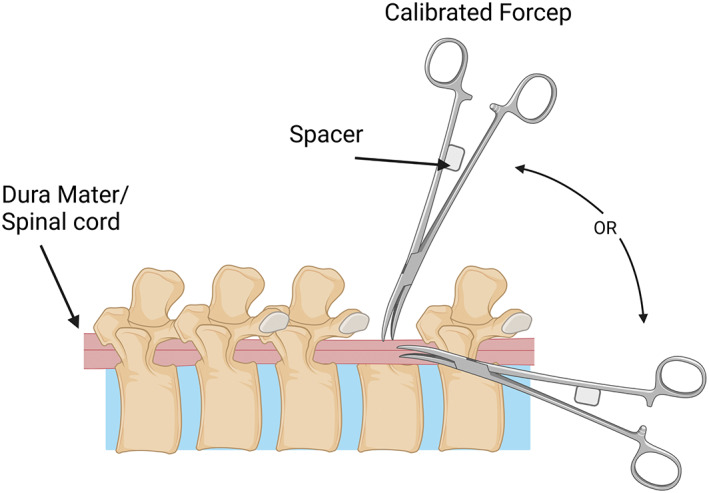
Schematic of calibrated forceps model for compression spinal cord injury. The forceps are modified to have a spacer between the handles so that the compression of the spinal cord is consistent. This procedure can be done from the posterior plane or lateral plane of the spinal cord post‐laminectomy. Created with BioRender.com.
2.2. Models not requiring laminectomy
2.2.1. Screw compression
The screw compression model (Figure 7) is a relatively new chronic compression model, with its first reference being in 2008 by Xu and associates (2008). This model uses a small plastic, or titanium screw with a flat bottom (pitch of 0.5 mm, a diameter of 3 mm, and a height of 9 mm) drilled through the lamina at the desired region after removing the spinous process (Sun et al., 2016). This screw is then left slightly exposed or sutured over until the following procedures (Lee et al., 2012; Sun et al., 2017; Xu et al., 2008). At each time interval, the screw is turned to increase compression between 0.1 and 0.5 mm each turn, commonly occurring every 7 to 14 days for up to 2 months (Jiang et al., 2016; Lee et al., 2012; Sun et al., 2016, 2017; Xin et al., 2020; Xu et al., 2008). This incrementally increases compression over time to mimic injuries such as tumors or cervical myelitis (Lee et al., 2012). Over the several weeks of study with the screw‐in, measurements of spinal deficits are recorded before sacrifice. After sacrifice, histological and pathological measurements are done to assess the extent of the injury (Sun et al., 2017; Xin et al., 2020). In conjunction with motor deficits observed before sacrifice, this measurement measures the extent of the injury and how compression affects the spinal cord. Intervention can be done after removing the screw to test further recovery after such an injury but has yet to be explored in literature.
FIGURE 7.
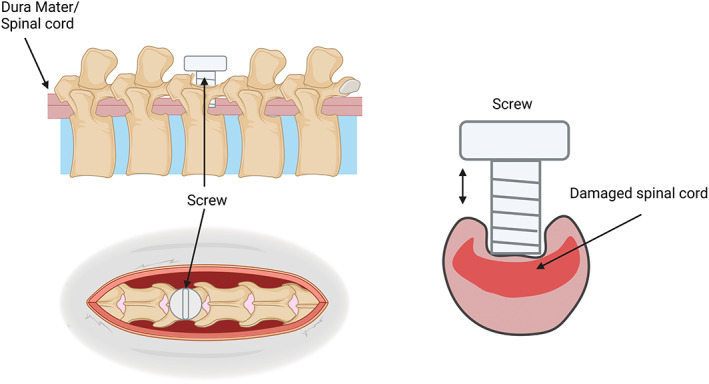
Schematic of the screw method of NTSCI where a screw goes through the lamina of the spinal column and compresses the spinal cord. The screw can be adjusted incrementally over a period of days to weeks. Created with BioRender.com.
There are many benefits of the screw compression model in replicating and reproducing NTSCI and compression. This model allows for sustained and increasing compression over a prolonged period that accurately reflects the morphology of a compression growth such as a tumor, spondylitis, metastasis, and many more (Lee et al., 2012; Sun et al., 2016). This model further improves upon other methods as it does not remove the lamina as part of the operation, meaning that the fluid dynamics of the spinal cord are not altered in a way that may affect results as seen in other methods of spinal cord compression (Xu et al., 2008). Even with the removal of the screw, compression can be relived while leaving in a small part of the screw to block off the opening in the lamina of the spinal cord (Xin et al., 2020).
There is a concern that the exposed screw or recurrent opening of the wound to tighten concealed screws has the potential for increased infection compared to most of the other models. None of the published articles describing the screw method addressed the morbidity and mortality rates due to infection and while this does not detract from the potential of this model there may be ethical implications that should be taken into account. Additionally, due to the underdevelopment and softness of the spinal bone in neonatal rodents, it is unlikely that this method would be suitable for use on younger aged rodents, limiting its potential for use in mimicking child NTSCI.
2.2.2. Spinal cord strapping
Spinal cord strapping (Figure 8) is a minimally invasive spinal cord compression injury that replicated NTSCI due to increasing pressure in the spinal column (da Costa et al., 2008). This model is sparse in the literature, most likely due to this method's surgical specificity and complexity. This technique requires a suture to pass through the dermal layers and between the gap of the spinal column. The suture attached to a hooked needle must then wrap under the spinal cord/dura mater and exit the derma without damaging the dura mater and accompanying blood vessels to begin the compression (da Costa et al., 2008). The suture is then attached to a pulley system device developed specifically in the laboratory of the creators Costa et al., to be used for graded injury using different weights to compress the spinal cord against the posterior spinal column (da Costa et al., 2008). This model allows for a minimally invasive and safe surgery when done right. It can create a graded injury that represents clinical NTSCI of mild, intermediate, and severe cases while minimizing the risk of undesired side effects due to surgery (Cheriyan et al., 2014; da Costa et al., 2008). However, this model is limited in its ability to create a consistent injury and its accessibility for reproduction is an unvalidated method of NTSCI replication (Cheriyan et al., 2014).
FIGURE 8.
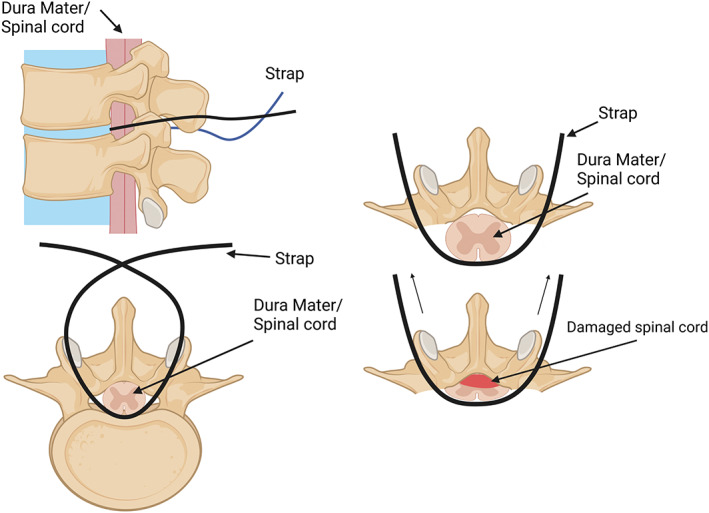
Schematic of the strap method of compressive spinal cord injuries. The thread passed through the dermal layers and wraps under the spinal cord before exiting the derma. The straps are then attached to weights that pull the spinal cord up and compress it against the posterior spinal column. Created with BioRender.com.
2.2.3. Remote compression model
A more recent compression model that can be operated remotely has been established by Li and colleagues (2022). In this model, a Bluetooth‐controlled device (Figure 9) comprised of an in‐situ component to compress the spinal cord, and a sub‐cutaneous part to receive transmissions is implanted between the C2/3 region of adult male Han sheep. While the sheep lay in a supine position, the compressive device was screwed into the front or anterior side of the vertebral column such that the rod sat between the C2 and C3 intravertebral disk space. In this position, the rod is used to compress the spinal cord positioned above it. After the procedure, the spinal cord was compressed 0.1 mm every 2 days for 10 or 20 weeks, with one 20‐week group receiving decompression after the experimental time. This is the first described method of its kind which provided clinically relevant information about chronic cervical compressive myelopathy, that not only reproduced chronic compression in situ over a prolonged time but was also less invasive and can be decompressed at any time (Li et al., 2022). However, while this model shows exciting potential, an animal model of this experiment that would allow for greater replication more efficiently would be far more beneficial to the broader research community, as sheep are harder and more expensive to house than other animals such as dogs or rodents.
FIGURE 9.
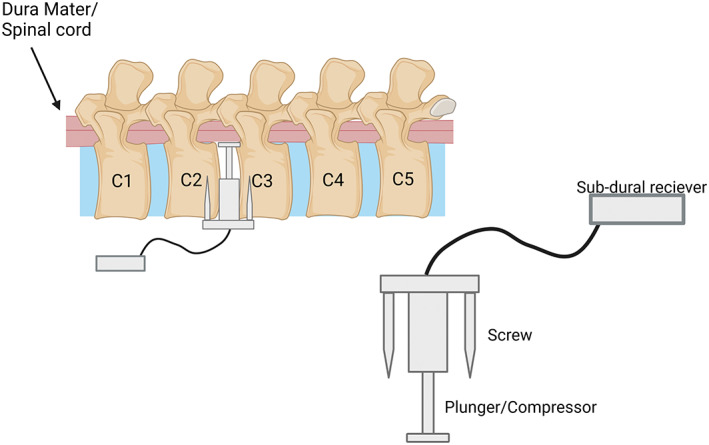
Schematic of Bluetooth compression model in sheep. The compression device is screwed into the anterior C2–C3 spinal column after removal of the intervertebral disk. The device then increases compression on the spinal cord using a Bluetooth‐controlled plunger that can be incrementally increased. Created with BioRender.com.
3. DISCUSSION
3.1. Age‐related NTSCI
NTSCIs occur in all age groups due to many varied reasons. The most common age brackets that experience NTSCI are the very young and the elderly (Smith et al., 2017, 2022). While SCI in children represents <12% of all SCI globally, in Australia, 66% of SCI in children are due to a nontraumatic cause (Galvin et al., 2013; Smith et al., 2017). This is important as children with NTSCI have a high morbidity rate, including motor deficits and lower quality of life when compared to other illnesses (Stettler et al., 2013). This further leads to lower life expectancy and an increased risk of other health complications for those affected (WHO, 2013). In addition, Stettler et al. (2013) found that in acute ischemic incidences in children, there were similar symptoms, recovery and overall outcomes compared to adults (Stettler et al., 2013).
It is essential to note the rising mean age for all SCI and the increasing prevalence of NTSCI in all age demographics (Noonan et al., 2012; Shepherd et al., 2021). While NTSCI does occur at all ages, most of the literature only reflects NTSCI models on adult‐aged animals. As mentioned, the increasing age and high prevalence of NTSCI in both elderly and child populations are not reflected currently in animal modeling of SCI (Shepherd et al., 2021; Smith et al., 2017, 2022). There is an importance in reflecting the clinical symptoms and age groups that NTSCI occurs so that appropriate intervention strategy can be developed due to the difference in how the body adapts and responds at different ages to SCI (Sezer et al., 2015).
3.2. Summary of models
The compressive SCI models discussed here are primarily well‐established and clinically relevant models that mimic acute spinal cord compression (Table 1). However, the application of animal models for chronic NTSCI is not as prevalent. Screw compression and solid spacer compression provide a long‐term compression injury that mimics what is seen clinically in tumors, spinal cord stenosis, spondylitis, and metastasis but are not yet well‐established. These two robust approaches provide the most clinically relevant models for the investigation and intervention of long‐term NTSCI. The weight drop compression model further provides good modeling of a compressive injury after a traumatic insult.
TABLE 1.
Summary table of all described compression spinal cord injury models and their most common usages
| Method | Level of injury | Injury duration | Common occlusion | Animal models | Pros | Cons |
|---|---|---|---|---|---|---|
| Clip | Cervical/thoracic | Seconds–minutes | 30–90 g force | Rodent | Accurate, reproducible, graded injury, simple set up, decompression | Suitability for different ages, laminectomy, no prolonged compression model |
| Balloon | Thoracic | Seconds–minutes | 15 μl saline | Rodent/rabbit/dog/goat | Graded injury, usable on many animals, simple set up, decompression | Short compression model, suitability on small/young rodents, laminectomy |
| Spacer | Thoracic | Days–weeks | 50% spinal cord | Rodent | Prolonged compression, graded injuries, simple, many devices can be used, used on all ages | Laminectomy, static pressure (no increase/decrease) |
| Expanding polymer | Cervical/thoracic | Weeks | Variable | Rodent | Slow compression model in situ, gradually increases compression | Laminectomy, minimal control of compression size, reproducibility |
| Weight drop | Thoracic | Seconds–minutes | 30/40/50 g | Rodent/pig | Can readily change parameters of injury force/duration/pressure | Variability in the actual force applied, laminectomy, pressure instantly released |
| Calibrated forceps | Cervical/thoracic | Seconds–minutes | 0.25–0.55 mm | Rodent | Inexpensive, graded injury, easy to perform | Laminectomy, discrepancy in ability to record injury parameters |
| Screw | Cervical/thoracic | Weeks | 3.5–5.0 mm | Rodent/goat | Sustained and increasing compression injury, representative of many types of SCI | No reports of morbidity or mortality due to screw, possible risk of infection due to external exposure and repeated opening |
| Spinal cord strap | Thoracic | Seconds–minutes | Variable | Rodent | Minimally invasive, graded injury | Highly technical procedure, unvalidated method of SCI replication |
| Remote compression | Cervical | Weeks | 7–14 mm | Sheep | In‐vivo control of compression for prolonged periods of time and can be readily changed | Requires major surgery, only been done on large animals, not easily accessible to broader research community |
4. CONCLUSION
Animal models of compressive SCI vary in their approach and in their complexity. While acute spinal cord compression is relatively easily modeled it is more difficult to establish a clinically relevant chronic spinal cord compression and even harder to model one that does not require a laminectomy or an extensively invasive technique to access the cord. In addition, most models of compression SCI have been trialed in adult animals which is not reflective of the rising population of NTSCI patients. Therefore, it is recommended that animal models of compression SCI are clearly identified as acute or chronic and that the emerging models with good potential be further tested and developed with the intent to minimize invasiveness to the animals and the spinal column, as well as be reflective of the age demographics most common in NTSCI.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
AUTHOR CONTRIBUTIONS
The authors confirm contributions to this paper as follows: Conceptualisation, R.R., K.M., and C.G.; Investigation, R.R. and C.G.; Writing – Original Draft, R.R.; Writing – Reviewing & Editing, C.G. and K.M., Supervision, C.G. and K.M.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jnr.25120.
1. OPEN RESEARCH BADGE
Is the author interested in applying for an Open Research Badge?: If yes, look for the completed Open Research Disclosure Form within the package of exported files.
Supporting information
Transparent Science Questionnaire for Authors
ACKNOWLEDGMENT
Open access publishing facilitated by University of Technology Sydney, as part of the Wiley ‐ University of Technology Sydney agreement via the Council of Australian University Librarians.
Ridlen, R. , McGrath, K. , & Gorrie, C. A. (2022). Animal models of compression spinal cord injury. Journal of Neuroscience Research, 100, 2201–2212. 10.1002/jnr.25120
Edited by Cristina Antonella Ghiani and John A. Kessler. Reviewed by Mahdi Sharif‐Alhoseini and Antje Kroner.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- Adams, R. D. , & Salam‐Adams, M. (1991). Chronic nontraumatic diseases of the spinal cord. Neurologic Clinics, 9(3), 605–623. 10.1016/S0733-8619(18)30270-6 [DOI] [PubMed] [Google Scholar]
- Batchelor, P. E. , Kerr, N. F. , Gatt, A. M. , Aleksoska, E. , Cox, S. F. , Ghasem‐Zadeh, A. , Wills, T. E. , & Howells, D. W. (2010). Hypothermia prior to decompression: Buying time for treatment of acute spinal cord injury. Journal of Neurotrauma, 27(8), 1357–1368. 10.1089/neu.2010.1360 [DOI] [PubMed] [Google Scholar]
- Blight, A. R. (1991). Morphometric analysis of a model of spinal cord injury in Guinea pigs, with behavioral evidence of delayed secondary pathology. Journal of the Neurological Sciences, 103(2), 156–171. 10.1016/0022-510X(91)90159-5 [DOI] [PubMed] [Google Scholar]
- Chamankhah, M. , Eftekharpour, E. , Karimi‐Abdolrezaee, S. , Boutros, P. C. , San‐Marina, S. , & Fehlings, M. G. (2013). Genome‐wide gene expression profiling of stress response in a spinal cord clip compression injury model. BMC Genomics, 14(1), 583. 10.1186/1471-2164-14-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyan, T. , Ryan, D. J. , Weinreb, J. H. , Cheriyan, J. , Paul, J. C. , Lafage, V. , Kirsch, T. , & Errico, T. J. (2014). Spinal cord injury models: A review. Spinal Cord, 52(8), 588–595. 10.1038/sc.2014.91 [DOI] [PubMed] [Google Scholar]
- da Costa, E. S. A. , Carvalho, A. L. , Martinez, A. M. B. , De‐Ary‐Pires, B. , Pires‐Neto, M. A. , & de Ary‐Pires, R. (2008). Strapping the spinal cord: An innovative experimental model of CNS injury in rats. Journal of Neuroscience Methods, 170(1), 130–139. 10.1016/j.jneumeth.2008.01.004 [DOI] [PubMed] [Google Scholar]
- De Martino, L. , Spennato, P. , Vetrella, S. , Capasso, M. , Porfito, C. , Ruotolo, S. , Abate, M. E. , Cinalli, G. , & Quaglietta, L. (2019). Symptomatic malignant spinal cord compression in children: A single‐center experience. Italian Journal of Pediatrics, 45(1), 80. 10.1186/s13052-019-0671-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimar, J. R. , Glassman, S. D. , Raque, G. H. , Zhang, Y. P. , & Shields, C. B. (1999). The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine, 24(16), 1623–1633. 10.1097/00007632-199908150-00002 [DOI] [PubMed] [Google Scholar]
- Do Espírito Santo, C. C. , da Silva Fiorin, F. , Ilha, J. , Duarte, M. M. M. F. , Duarte, T. , & Santos, A. R. S. (2019). Spinal cord injury by clip‐compression induces anxiety and depression‐like behaviours in female rats: The role of the inflammatory response. Brain, Behavior, and Immunity, 78, 91–104. 10.1016/j.bbi.2019.01.012 [DOI] [PubMed] [Google Scholar]
- Galvin, J. , Scheinberg, A. , & New, P. W. (2013). A retrospective case series of pediatric spinal cord injury and disease in Victoria, Australia. Spine, 38(14), E878–E882. 10.1097/BRS.0b013e318294e839 [DOI] [PubMed] [Google Scholar]
- Gao, L. , Zhang, Z. , Xu, W. , Li, T. , Ying, G. , Qin, B. , Li, J. , Zheng, J. , Zhao, T. , Yan, F. , Zhu, Y. , & Chen, G. (2019). Natrium benzoate alleviates neuronal apoptosis via the DJ‐1‐related anti‐oxidative stress pathway involving Akt phosphorylation in a rat model of traumatic spinal cord injury. Frontiers in Molecular Neuroscience, 12, 42. 10.3389/fnmol.2019.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner, J. A. (1992). A monitored contusion model of spinal cord injury in the rat. Journal of Neurotrauma, 9(2), 123–128. 10.1089/neu.1992.9.123 [DOI] [PubMed] [Google Scholar]
- Hassannejad, Z. , Zadegan, S. A. , Vaccaro, A. R. , Rahimi‐Movaghar, V. , & Sabzevari, O. (2019). Biofunctionalized peptide‐based hydrogel as an injectable scaffold for BDNF delivery can improve regeneration after spinal cord injury. Injury, 50(2), 278–285. 10.1016/j.injury.2018.12.027 [DOI] [PubMed] [Google Scholar]
- Ijima, Y. , Furuya, T. , Koda, M. , Matsuura, Y. , Saito, J. , Kitamura, M. , Miyamoto, T. , Orita, S. , Inage, K. , Suzuki, T. , Yamazaki, M. , & Ohtori, S. (2017). Experimental rat model for cervical compressive myelopathy. Neuroreport, 28(18), 1239–1245. https://journals.lww.com/neuroreport/Fulltext/2017/12030/Experimental_rat_model_for_cervical_compressive.9.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H. , Wang, J. , Xu, B. , Yang, H. , & Zhu, Q. (2016). A model of acute central cervical spinal cord injury syndrome combined with chronic injury in goats. European Spine Journal, 26(1), 56–63. 10.1007/s00586-016-4573-6 [DOI] [PubMed] [Google Scholar]
- Kang, Y. , Ding, H. , Zhou, H. X. , Wei, Z. J. , Liu, L. , Pan, D. Y. , & Feng, S. Q. (2018). Epidemiology of worldwide spinal cord injury: A literature review. Journal of Neurorestoratology, 6, 1–9. [Google Scholar]
- Karadimas, S. K. , Gatzounis, G. , & Fehlings, M. G. (2015). Pathobiology of cervical spondylotic myelopathy. European Spine Journal, 24(2), 132–138. 10.1007/s00586-014-3264-4 [DOI] [PubMed] [Google Scholar]
- Karadimas, S. K. , Moon, E. S. , Yu, W.‐R. , Satkunendrarajah, K. , Kallitsis, J. K. , Gatzounis, G. , & Fehlings, M. G. (2013). A novel experimental model of cervical spondylotic myelopathy (CSM) to facilitate translational research. Neurobiology of Disease, 54, 43–58. 10.1016/j.nbd.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Kim, K.‐T. , Streijger, F. , So, K. , Manouchehri, N. , Shortt, K. , Okon, E. B. , Tigchelaar, S. , Fong, A. , Morrison, C. , Keung, M. , Sun, J. , Liu, E. , Cripton, P. A. , & Kwon, B. K. (2019). Differences in morphometric measures of the uninjured porcine spinal cord and dural sac predict histological and behavioral outcomes after traumatic spinal cord injury. Journal of Neurotrauma, 36(21), 35–3017. 10.1089/neu.2018.5930 [DOI] [PubMed] [Google Scholar]
- Kim, P. , Haisa, T. , Kawamoto, T. , Kirino, T. , & Wakai, S. (2004). Delayed myelopathy induced by chronic compression in the rat spinal cord. Annals of Neurology, 55(4), 503–511. 10.1002/ana.20018 [DOI] [PubMed] [Google Scholar]
- Krupa, P. , Stepankova, K. , Kwok, J. C. F. , Fawcett, J. W. , Cimermanova, V. , Jendelova, P. , & Urdzikova, L. M. (2020). New model of ventral spinal cord lesion induced by balloon compression in rats. Biomedicine, 8(11), 1–18. 10.3390/biomedicines8110477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota, K. , Saiwai, H. , Kumamaru, H. , Kobayakawa, K. , Maeda, T. , Matsumoto, Y. , Harimaya, K. , Iwamoto, Y. , & Okada, S. (2012). Neurological recovery is impaired by concurrent but not by asymptomatic pre‐existing spinal cord compression after traumatic spinal cord injury. Spine, 37(17), 1448–1455. 10.1097/BRS.0b013e31824ffda5 [DOI] [PubMed] [Google Scholar]
- Lanza, M. , Campolo, M. , Casili, G. , Filippone, A. , Paterniti, I. , Cuzzocrea, S. , & Esposito, E. (2018). Sodium butyrate exerts neuroprotective effects in spinal cord injury. Molecular Neurobiology, 56(6), 3937–3947. 10.1007/s12035-018-1347-7 [DOI] [PubMed] [Google Scholar]
- Lau, N.‐S. S. , Gorrie, C. A. , Chia, J. Y. , Bilston, L. E. , & Clarke, E. C. (2013). Severity of spinal cord injury in adult and infant rats after vertebral dislocation depends upon displacement but not speed. Journal of Neurotrauma, 30(15), 1361–1373. 10.1089/neu.2012.2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Satkunendrarajah, K. , & Fehlings, M. G. (2012). Development and characterization of a novel rat model of cervical spondylotic myelopathy: The impact of chronic cord compression on clinical, neuroanatomical, and neurophysiological outcomes. Journal of Neurotrauma, 29(5), 112–1027. 10.1089/neu.2010.1709 [DOI] [PubMed] [Google Scholar]
- Li, Z. , Zhai, S. , Liu, S. , Chen, C. , Guo, X. , Hu, P. , Wang, B. , Zhang, Y. , Wei, F. , & Liu, Z. (2022). A sheep model of chronic cervical compressive myelopathy via an implantable wireless compression device. European Spine Journal, 31(5), 1219–1227. 10.1007/s00586-022-07138-6 [DOI] [PubMed] [Google Scholar]
- Lim, J.‐H. , Jung, C.‐S. , Byeon, Y.‐E. , Kim, W. H. , Yoon, J.‐H. , Kang, K.‐S. , & Kweon, O.‐K. (2007). Establishment of a canine spinal cord injury model induced by epidural balloon compression. Journal of Veterinary Science, 8(3), 311. 10.4142/jvs.2007.8.3.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F.‐Y. , Yang, S.‐D. , Huo, L.‐S. , Wang, T. , Yang, D.‐L. , & Ding, W.‐Y. (2016). Laminoplasty versus laminectomy and fusion for multilevel cervical compressive myelopathy: A meta‐analysis. Medicine, 95(23), e3588. 10.1097/MD.0000000000003588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, H. Q. , Li, G. S. , Lin, E. J. , Xie, W. H. , Chen, W. L. , Luk, K. D. , & Hu, Y. (2013). Is the speed of chronic compression an important factor for chronic spinal cord injury rat model? Neuroscience Letters, 545, 75–80. 10.1016/j.neulet.2013.04.024 [DOI] [PubMed] [Google Scholar]
- Luo, J. , Li, N. , Robinson, J. P. , & Shi, R. (2002). The increase of reactive oxygen species and their inhibition in an isolated guinea pig spinal cord compression model. Spinal Cord, 40(12), 656–665. 10.1038/sj.sc.3101363 [DOI] [PubMed] [Google Scholar]
- McDonough, A. , Monterrubio, A. , Ariza, J. , & Martínez‐Cerdeño, V. (2015). Calibrated forceps model of spinal cord compression injury. Journal of Visualized Experiments, 98, e52318. 10.3791/52318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir, G. D. , & Webb, A. A. (2000). Mini‐review: Assessment of behavioural recovery following spinal cord injury in rats. European Journal of Neuroscience, 12(9), 3079–3086. [DOI] [PubMed] [Google Scholar]
- Müller‐Jensen, L. , Ploner, C. J. , Kroneberg, D. , & Schmidt, W. U. (2021). Clinical presentation and causes of non‐traumatic spinal cord injury: An observational study in emergency patients. Frontiers in Neurology, 12, 701927. 10.3389/fneur.2021.701927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgoci, A.‐N. , Baciak, L. , Cubinkova, V. , Smolek, T. , Tvrdik, T. , Juranek, I. , Kafka, J. , & Cizkova, D. (2019). Diffusion tensor imaging: Tool for tracking injured spinal cord fibres in rat. Neurochemical Research, 45(1), 180–187. 10.1007/s11064-019-02801-9 [DOI] [PubMed] [Google Scholar]
- New, P. W. (2019). A narrative review of pediatric nontraumatic spinal cord dysfunction. Topics in Spinal Cord Injury Rehabilitation, 25(2), 112–120. 10.1310/sci2502-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- New, P. W. , Cripps, R. A. , & Bonne Lee, B. (2014). Global maps of non‐traumatic spinal cord injury epidemiology: Towards a living data repository. Spinal Cord, 52(2), 97–109. 10.1038/sc.2012.165 [DOI] [PubMed] [Google Scholar]
- New, P. W. , Farry, A. , Baxter, D. , & Noonan, V. K. (2013). Prevalence of non‐traumatic spinal cord injury in Victoria, Australia. Spinal Cord, 51(2), 99–102. 10.1038/sc.2012.61 [DOI] [PubMed] [Google Scholar]
- New, P. W. , Guilcher, S. J. T. , Jaglal, S. B. , Biering‐Sørensen, F. , Noonan, V. K. , & Ho, C. (2017). Trends, challenges, and opportunities regarding research in non‐traumatic spinal cord dysfunction. Topics in Spinal Cord Injury Rehabilitation, 23(4), 313–323. 10.1310/sci2304-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan, V. K. , Fingas, M. , Farry, A. , Baxter, D. , Singh, A. , Fehlings, M. G. , & Dvorak, M. F. (2012). Incidence and prevalence of spinal cord injury in Canada: A national perspective. Neuroepidemiology, 38(4), 219–226. 10.1159/000336014 [DOI] [PubMed] [Google Scholar]
- Plemel, J. R. , Duncan, G. , Chen, K.‐W. K. , Shannon, C. , Park, S. , Sparling, J. S. , & Tetzlaff, W. (2008). A graded forceps crush spinal cord injury model in mice. Journal of Neurotrauma, 25(4), 350–370. 10.1089/neu.2007.0426 [DOI] [PubMed] [Google Scholar]
- Rivlin, A. S. , & Tator, C. H. (1978). Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surgical Neurology, 10(1), 38–43. [PubMed] [Google Scholar]
- Ruzicka, J. , Machova‐Urdzikova, L. , Gillick, J. , Amemori, T. , Romanyuk, N. , Karova, K. , Zaviskova, K. , Dubisova, J. , Kubinova, S. , Murali, R. , Sykova, E. , Jhanwar‐Uniyal, M. , & Jendelova, P. (2017). A comparative study of three different types of stem cells for treatment of rat spinal cord injury. Cell Transplantation, 26(4), 585–603. 10.3727/096368916X693671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajad, F. , Amir, K. , Cyrus, J. , Fatemeh, A. , Sana, P. , Mohammad, H. F. , Mohsen, R.‐P. , Ehsan, M.‐N. , & Haroon, K. (2021). Intrathecal administration of melatonin ameliorates the neuroinflammation‐ mediated sensory and motor dysfunction in a rat model of compression spinal cord injury. Current Molecular Pharmacology, 14(4), 646–657. 10.2174/1874467213666201230101811 [DOI] [PubMed] [Google Scholar]
- Scheff, S. W. , Rabchevsky, A. G. , Fugaccia, I. , Main, J. A. , & Lumpp, J. E. (2003). Experimental modeling of spinal cord injury: Characterization of a force‐defined injury device. Journal of Neurotrauma, 20(2), 179–193. 10.1089/08977150360547099 [DOI] [PubMed] [Google Scholar]
- Schiaveto‐de‐Souza, A. , Da‐Silva, C. A. , Defino, H. L. A. , & Bel, E. A. D. (2013). Effect of melatonin on the functional recovery from experimental traumatic compression of the spinal cord. Brazilian Journal of Medical and Biological Research, 46(4), 348–358. 10.1590/1414-431X20132322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezer, N. , Akkuş, S. , & Uğurlu, F. G. (2015). Chronic complications of spinal cord injury. World Journal of Orthopedics, 6(1), 24–33. 10.5312/wjo.v6.i1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif‐Alhoseini, M. , Khormali, M. , Rezaei, M. , Safdarian, M. , Hajighadery, A. , Khalatbari, M. M. , Safdarian, M. , Meknatkhah, S. , Rezvan, M. , Chalangari, M. , Derakhshan, P. , & Rahimi‐Movaghar, V. (2017). Animal models of spinal cord injury: A systematic review. Spinal Cord, 55(8), 714–721. 10.1038/sc.2016.187 [DOI] [PubMed] [Google Scholar]
- Shepherd, J. , Tu, K. , Young, J. , Chishtie, J. , Craven, B. C. , Moineddin, R. , & Jaglal, S. (2021). Identifying cases of spinal cord injury or disease in a primary care electronic medical record database. Journal of Spinal Cord Medicine, 44(Suppl. 1), S28–S39. 10.1080/10790268.2021.1971357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, E. , Finn, S. , & Fitzpatrick, P. (2017). Epidemiology of pediatric traumatic and acquired nontraumatic spinal cord injury in Ireland. Topics in Spinal Cord Injury Rehabilitation, 23(3), 279–284. 10.1310/sci16-00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, É. , Fitzpatrick, P. , Lyons, F. , Morris, S. , & Synnott, K. (2022). Epidemiology of non‐traumatic spinal cord injury in Ireland—A prospective population‐based study. Journal of Spinal Cord Medicine, 45(1), 76–81. 10.1080/10790268.2020.1762829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler, S. , El‐Koussy, M. , Ritter, B. , Boltshauser, E. , Jeannet, P.‐Y. , Kolditz, P. , Meyer‐Heim, A. , & Steinlin, M. (2013). Non‐traumatic spinal cord ischaemia in childhood—Clinical manifestation, neuroimaging and outcome. European Journal of Paediatric Neurology, 17(2), 176–184. 10.1016/j.ejpn.2012.09.002 [DOI] [PubMed] [Google Scholar]
- Stokes, B. T. (1992). Experimental spinal cord injury: A dynamic and verifiable injury device. Journal of Neurotrauma, 9(2), 129–134. 10.1089/neu.1992.9.129 [DOI] [PubMed] [Google Scholar]
- Sun, G.‐D. , Chen, Y. , Zhou, Z.‐G. , Yang, S.‐X. , Zhong, C. , & Li, Z.‐Z. (2017). A progressive compression model of thoracic spinal cord injury in mice: Function assessment and pathological changes in spinal cord. Neural Regeneration Research, 12(8), 1365–1374. 10.4103/1673-5374.213693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , Zhang, L.‐H. , Fu, Y.‐M. , Li, Z.‐R. , Liu, J.‐H. , Peng, J. , Liu, B. , & Tang, P.‐F. (2016). Establishment of a rat model of chronic thoracolumbar cord compression with a flat plastic screw. Neural Regeneration Research, 11(6), 963–970. 10.4103/1673-5374.184496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlov, I. M. (1957). Spinal cord compression: Mechanism of paralysis and treatment. Charles C. Thomas, Publisher. [Google Scholar]
- Vanický, I. , Urdzíková, L. , Saganová, K. , Cízková, D. , & Gálik, J. (2001). A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. Journal of Neurotrauma, 18(12), 1399–1407. 10.1089/08977150152725687 [DOI] [PubMed] [Google Scholar]
- Vaughn, C. N. , Iafrate, J. L. , Henley, J. B. , Stevenson, E. K. , Shlifer, I. G. , & Jones, T. B. (2013). Cellular neuroinflammation in a lateral forceps compression model of spinal cord injury. Anatomical Record, 296(8), 1229–1246. 10.1002/ar.22730 [DOI] [PubMed] [Google Scholar]
- Wang, X.‐H. , Jiang, C. , Zhang, Y.‐Y. , Chen, Z. , Wang, Z.‐Y. , Yang, H. , & Hao, D.‐J. (2021). Analysis and comparison of a spinal cord injury model with a single‐axle‐lever clip or a parallel‐moving clip compression in rats. Spinal Cord, 60(4), 332–338. 10.1038/s41393-021-00720-7 [DOI] [PubMed] [Google Scholar]
- WHO . (2013). Spinal cord injury. https://www.who.int/news‐room/fact‐sheets/detail/spinal‐cord‐injury [Google Scholar]
- Xin, S. , Xing‐Zhen, L. , Jia, W. , Hai‐Rong, T. , Tong, Z. , Wen‐Jie, J. , & Kang‐Ping, S. (2020). Changes in neurological and pathological outcomes in a modified rat spinal cord injury model with closed canal. Neural Regeneration Research, 15(4), 697–704. 10.4103/1673-5374.266919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, P. , Gong, W. M. , Li, Y. , Zhang, T. , Zhang, K. , Yin, D. Z. , & Jia, T. H. (2008). Destructive pathological changes in the rat spinal cord due to chronic mechanical compression. Laboratory investigation. Journal of Neurosurgery: Spine, 8(3), 279–285. 10.3171/spi/2008/8/3/279 [DOI] [PubMed] [Google Scholar]
- Yang, C. , Yu, B. , Ma, F. , Lu, H. , Huang, J. , You, Q. , Yu, B. , Qiao, J. , & Feng, J. (2017). What is the optimal sequence of decompression for multilevel noncontinuous spinal cord compression injuries in rabbits? BMC Neurology, 17(1), 44. 10.1186/s12883-017-0824-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Kong, X. , Liu, C. , Liang, Z. , Zhao, H. , Tong, W. , Ning, G. , Shen, W. , Yao, L. , & Feng, S. (2014). ERK2 small interfering RNAs prevent epidural fibrosis via the efficient inhibition of collagen expression and inflammation in laminectomy rats. Biochemical and Biophysical Research Communications, 444(3), 395–400. 10.1016/j.bbrc.2014.01.070 [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Younsi, A. , Zheng, G. , Tail, M. , Harms, A.‐K. , Roth, J. , Hatami, M. , Skutella, T. , Unterberg, A. , & Zweckberger, K. (2021). Sonic hedgehog modulates the inflammatory response and improves functional recovery after spinal cord injury in a thoracic contusion–compression model. European Spine Journal, 30(6), 1509–1520. 10.1007/s00586-021-06796-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparent Science Questionnaire for Authors
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


