Abstract
Objectives
Children with acute lymphoblastic leukemia (ALL) have a tendency to gain weight during treatment. As overweight and obesity associate with health problems, prophylactic interventions are warranted. Therefore, it is important to identify the children most prone to gain weight.
Methods
Patients aged 2.0–17.9 years at ALL diagnosis were identified from the NOPHO ALL2008 registry. Registry data was complemented with height and weight at the end of therapy from questionnaires. Body mass index (BMI) was classified according to international age‐ and sex‐adjusted International Obesity Task Force BMI cut‐offs. BMI values were transformed into standard deviation scores (SDS) to calculate the difference in BMISDS during treatment.
Results
Data on BMI change were available for 765 children. Overweight and obesity doubled during treatment: 9.7% were overweight and 2.1% obese at diagnosis and 21.8% and 5.4% at the end of therapy, respectively. The mean BMISDS change was +0.64. Younger (2.0–5.9 years) and healthy weight children were most prone to become overweight (mean change in BMI SDS +0.85 and + 0.65, respectively).
Conclusions
Younger children (2.0–5.9 years) with healthy weight at diagnosis were most prone to becoming overweight and therefore are an important group to target while considering interventions.
Keywords: acute lymphoblastic leukemia, body mass index, children, obesity, overweight, support care
1. BACKGROUND
Though the prevalence of overweight and obesity in the Nordic countries is lower than in many Western countries, childhood obesity is a growing problem. 1 , 2 In childhood acute lymphoblastic leukemia (ALL), obesity at diagnosis is associated with a higher risk of relapse and poorer overall survival, compared to being healthy weight. 3 , 4 , 5 , 6 , 7 , 8 Furthermore, weight gain is common in children during ALL treatment, 9 , 10 , 11 , 12 , 13 , 14 , 15 especially during induction therapy with glucocorticoids. 9 , 16
Body mass index (BMI) is modifiable and interventions to normalize BMI during ALL treatment could improve cure rates. Studies investigating the impact of changes in BMI on outcome in childhood ALL are scarce, and whether weight loss in patients with ALL and extreme body weight at diagnosis can improve survival rates remains unclear. However, Orgel et al. demonstrated potential benefit from caloric restriction via diet/exercise to augment chemotherapy efficacy and improve disease response. 17 Another study demonstrated an improved event‐free survival in children who normalized their weight during pre‐maintenance therapy. 8 On the contrary, Den Hoed et al. observed that decrease in BMI during the first 32 weeks of treatment led to poorer overall survival compared to patients without decreases in BMI. 18
Many children survive ALL and having a healthy BMI after treatment is desirable for future health and quality of life. By identifying children most prone to unfavorable weight development, appropriate interventions could be applied prophylactically during treatment.
2. METHODS
2.1. Study population
Children aged 2.0–17.9 years at ALL diagnosis with data on height and weight both at diagnosis and end of first‐line treatment (EOT) and enrolled in the NOPHO (Nordic Society of Paediatric Haematology and Oncology) ALL2008 protocol between 2008 and 2018 in Sweden, Norway, Denmark, Finland, Iceland, Estonia, and Lithuania were included. Data retrieved from the registry‐included information on patient demographics, height, and weight at diagnosis, immunophenotype, white blood cell count (WBC) and central nervous system (CNS) status. Data on the height and weight at EOT was obtained through questionnaires completed by healthcare providers. Patients with missing data on BMI, hematopoietic stem cell transplantation as first‐line treatment, or Down syndrome were excluded. Children <2 years old were excluded as the BMI cut‐offs used here are defined from 2 years of age. The study was approved by the Ethical Review Board in Stockholm (number 2018/1888–31).
2.2. Leukemia treatment protocol
The ALL2008 protocol has been described in detail earlier. 19 Stratification into risk groups, and subsequently into treatment arms, is based on prognostic factors. At diagnosis, the patients with T‐ALL and/or WBC ≥100 × 109/L received high‐risk induction therapy with dexamethasone. Other patients received standard risk induction with prednisolone. The second stratification, done after the induction, is based on the cytogenetics of the leukemic clone and the response to the early treatment, measured by minimal residual disease (MRD, proportion of leukemic blasts remaining in the bone marrow).
2.3. BMI definitions
BMI at diagnosis and EOT was classified according to international age‐ and sex‐adjusted International Obesity Task Force (IOTF) BMI cut‐offs, based on smoothed sex‐ and age‐specific IOTF curves, and correspond to BMI of <17 kg/m2 for thinness/underweight, BMI >25 kg/m2 for overweight, and ≥ 30 kg/m2 for obesity at 18 years of age. 20 , 21 Values for BMI, height and weight were also transformed into standard deviation scores (SDS) to calculate the difference between BMISDS, height SDS and weight SDS at diagnosis and at EOT. 22 , 23 , 24 , 25 , 26 For the purposes of this study, weight loss was defined as a decrease of >1 BMISDS.
2.4. Statistical analysis
The mean change in BMISDS between diagnosis and EOT was calculated for different subgroups with paired t tests and compared within the groups by using independent t tests and one‐way ANOVA. Chi‐square tests were used to describe associations between different categorical variables, such as BMI at diagnosis and at EOT, age group (younger children 2.0–9.9 years and older children 10–17.9 years), risk group, ALL subtype, and sex. Uni‐ and bivariate linear regression analysis were used to test the association of BMISDS with other continuous variables. Two‐tailed p values <.05 were considered statistically significant. Statistical analyses were performed using SPSS version 28.0 for Windows (SPSS Inc., Illinois, USA).
3. RESULTS
3.1. Study group
In total 765 survivors after first‐line treatment were included. Data on BMI were missing from 801 patients at EOT who otherwise met the inclusion criteria. There were no differences in sex, age, risk group, immunophenotype, WBC at diagnosis, or CNS status between those with or without available data. Characteristics of the study group are shown in Table 1.
TABLE 1.
Change in body mass index standard deviation score (BMISDS) in different patients characteristics groups
| BMISDS change | ||||
|---|---|---|---|---|
| All patients | ||||
| No. (%) | Mean | Median | p value a | |
| Overall population | 765 | 0.64 | 0.61 | |
| Age group (years) | ||||
| Age 2–5.9 | 464 (60.7) | 0.85 | 0.83 | |
| Age 6–9.9 | 158 (20.7) | 0.46 | 0.48 | <.001 |
| Age 10–17.9 | 143 (18.7) | 0.15 | 0.10 | |
| Gender | ||||
| Male | 417 (54.5) | 0.62 | 0.59 | .48 |
| Female | 348 (45.5) | 0.67 | 0.64 | |
| Weight group at diagnosis | ||||
| Underweight | 35 (4.6) | 2.27 | 2.08 | |
| Healthy weight | 640 (83.7) | 0.65 | 0.65 | <.001 |
| Overweight | 74 (9.7) | −0.01 | 0.04 | |
| Obese | 16 (2.1) | −0.15 | −0.13 | |
| Risk group | ||||
| Standard risk treatment | 410 (53.6) | 0.69 | 0.64 | |
| Intermediate risk treatment | 287 (37.5) | 0.67 | 0.63 | <.001 |
| High risk treatment | 68 (8.9) | −0.09 | 0.13 | |
| Immunophenotype | ||||
| B‐cell ALL | 668 (87.3) | 0.67 | 0.63 | .03 |
| T‐cell ALL | 97 (12.7) | 0.43 | 0.49 | |
| Induction therapy | ||||
| Prednisolone induction | 621 (81.2) | 0.68 | 0.68 | .03 |
| Dexamethasone induction | 144 (18.8) | 0.46 | 0.47 | |
| WBC at diagnosis | ||||
| WBC <50 × 109/L | 602 (78.7) | 0.67 | 0.63 | |
| WBC 50–100 × 109/L | 73 (9.6) | 0.63 | 0.59 | .06 |
| WBC >100 × 109/L | 90 (11.8) | 0.41 | 0.51 | |
| CNS status at diagnosis | ||||
| CNS negative ALL | 741 (96.9 | 0.64 | 0.61 | .48 |
| CNS positive ALL | 24 (3.1) | 0.49 | 0.54 | |
Abbreviations: ALL, acute lymphoblastic leukemia; CNS, central nervous system; WBC, white blood cell count.
Qui‐square test, independent t tests, and one‐way ANOVA are based on mean.
3.2. Change in BMISDS during treatment
There were no significant differences between proportion of children in each BMI category at diagnosis and age group or risk protocol. The mean BMISDS at diagnosis was 0.04 increasing to 0.68 at EOT (mean difference + 0.64). When the BMISDS change was analyzed as mean paired change in each patient characteristic group (Table 1), only high‐risk patients (p = 0.17), children aged 10–17.9 years (p = 0.07) and those who were overweight (p = 0.72) or obese (p = 0.74) at diagnosis did not gain weight significantly during the treatment. To differentiate the effect of age and BMISD at diagnosis to the BMISDS change we compared age and BMISDS at diagnosis with BMISD change separately by linear regression; the coefficient of both variables were negatively significant (p value <.001), also after adjusting for age and BMISDS at diagnosis, respectively (Figure S1). Patients with pre B‐cell ALL and induction therapy with prednisolone had a greater increase in mean BMISDS than patients with T‐cell ALL or induction therapy with dexamethasone, but these differences were not significant when the comparisons were performed without high‐risk patients.
Underweight and normal weight children at diagnosis presented with the most noteworthy weight gain by EOT (mean BMISDS change of +2.27, +0.65, −0.03, and −0.15 for underweight, normal weight, overweight and obese children respectively, Figure 1). The increase in BMISDS for underweight and healthy weight remained significantly higher when stratifying for age group. Patients in the standard‐ and intermediate‐risk groups had a greater increase in mean BMISDS (+0.69 respectively +0.67) than patients in the high‐risk group (0.09; p value <.001) (Table 1).
FIGURE 1.
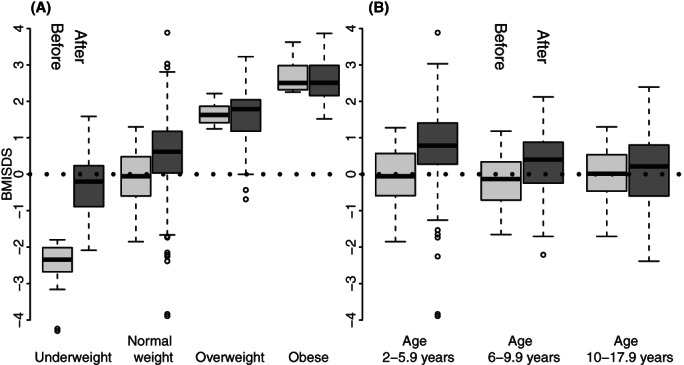
(A) The change in body mass index standard deviation scores (BMISDS) at diagnosis and the end of therapy in different BMI categories, and (B) The change in BMISDS at diagnosis and end of therapy in healthy weight children in different age categories
The prevalence of children decreasing in BMISDS (> −1 BMISDS) during treatment was 3.8% (29/765). Those who received high‐risk treatment were more prone to weight loss (10.3%; 7/68) compared to nonhigh‐risk treated children (3.2%; 22/697). Older children were most susceptible to weight loss; 2.2% (10/464) in the youngest age group and 9.8% (14/142) of the children aged 10–17.9 years decreased in BMISDS. The difference was not due to more older children having high‐risk treatment as 8.9% (10/117) of the nonhigh‐risk older children decreased in BMISDS during treatment. Of healthy weight children, 3.3% (21/640) decreased with more than 1 BMISDS compared to 8.9% (8/90) overweight or obese patients (Table 2).
TABLE 2.
Change in body mass index standard deviation scores (BMISDS) in different patient characteristic groups
| BMISDS change | ||||||
|---|---|---|---|---|---|---|
| No. (%) | > −1 BMISDS | <−1– < +1 BMISDS | > +1 BMISDS | > +2 BMISDS | p value | |
| Overall population | 765 | 29 (3.8) | 470 (61.4) | 212 (27.7) | 54 (7.1) | |
| Age 2–5.9 years | 464 (60.7) | 10 (2.2) | 256 (55.2) | 150 (32.2) | 48 (10.3) | <.001 |
| Age 6–9.9 years | 158 (20.7) | 5 (3.2) | 110 (69.6) | 40 (25.3) | 3 (1.9) | |
| Age 10–17.9 years | 143 (18.7) | 14 (9.8) | 104 (72.7) | 22 (15.4) | 3 (2.1) | |
| Male | 417 (54.5) | 16 (3.8) | 255 (61.2) | 117 (28.1) | 29 (7.0) | .99 |
| Female | 348 (45.5) | 13 (3.7) | 215 (61.8) | 95 (27.3) | 25 (7.2) | |
| BMI category at diagnosis | ||||||
| Underweight | 35 (4.6) | 0 | 3 (8.6) | 13 (37.1) | 19 (54.3) | <.001 |
| Healthy | 640 (83.7) | 21 (3.3) | 290 (60.9) | 194 (30.3) | 35 (5.5) | |
| Overweight | 74 (9.7) | 7 (9.5) | 62 (83.8) | 5 (6.8) | 0 | |
| Obese | 16 (2.1) | 1 (6.3) | 15 (93.8) | 0 | 0 | |
| Standard risk treatment | 410 (53.6) | 12 (2.9) | 257 (62.7) | 108 (26.3) | 33 (8.0) | .003 |
| Intermediate risk treatment | 287 (37.5) | 10 (3.5) | 165 (57.5) | 95 (33.1) | 16 (5.6) | |
| High risk treatment | 68 (8.9) | 7 (10.3) | 57 (69.1) | 9 (13.2) | 5 (7.4) | |
| B‐cell ALL | 668 (87.3) | 22 (3.3) | 412 (61.7) | 185 (27.7) | 49 (7.3) | .255 |
| T‐cell ALL | 97 (12.7) | 7 (7.2) | 58 (59.8) | 27 (27.9) | 5 (5.2) | |
| Prednisolone induction | 625 (81.7) | 18 (2.9) | 386 (62.2) | 172 (27.7) | 46 (7.4) | .015 |
| Dexamethasone induction | 140 (18.3) | 10 (7.9) | 75 (59.5) | 40 (27.8) | 8 (5.6) | |
| WBC a at diagnosis | ||||||
| <50 × 109/L | 602 (78.7) | 17 (2.8) | 371 (61.6) | 169 (28.1) | 45 (7.5) | .093 |
| 50–100 × 109/L | 73 (9.6) | 4 (5.5) | 48 (65.8) | 16 (21.9) | 5 (6.8) | |
| >100 × 109/L | 90 (11.8) | 8 (8.9) | 51 (56.7) | 27 (30.0) | 4 (4.4) | |
White blood cell count at diagnosis.
3.3. Change in height and weight during treatment
The mean height SDS at diagnosis was 0.63 and decreased to 0.12 at EOT (mean difference − 0.51). Mean weight SDS increased from 0.34 to 0.48 at EOT (mean difference + 0.14). The younger children aged 2–5.9 years decreased −0.59 SDS in height during treatment, significantly more compared to older children (height SDS −0.43 in age group 6–9.9 years and − 0.39 in age group 10–17.9 years, p value .001). Children treated with high‐risk protocol had a tendency to decrease more in height during treatment than nonhigh risk patients (change in height SDS at the EOT −0.64 and − 0.50 respectively, p value .074).
3.4. BMI category at diagnosis and EOT
The number of overweight and obese patients more than doubled after treatment (Table 3). At diagnosis 9.7% (n = 74) were overweight and 2.1% (n = 16) obese compared to 21.9% (n = 167) and 5.4% (n = 41) at EOT, respectively. Especially in the youngest age category (2.0–5.9 years, at diagnosis), the number of overweight and obese patients during treatment increased; 8.8% (n = 50) were overweight/obese at diagnosis and 33.4% (n = 154) at EOT. In children aged 6.0–9.9 years, 11.4% (n = 18) were overweight/obese at diagnosis, compared to 17.7% (n = 28) at EOT. The percentage of overweight and obese in the older children (10.0–17.9 years) did not significantly change before and after treatment; 15.3% (n = 22) at diagnosis and 18.2% (n = 26) at EOT, with some patients gaining, some losing weight.
TABLE 3.
Body mass index (BMI) category at diagnosis and at end of acute lymphoblastic leukemia (ALL) treatment
| BMI category at end of therapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI category at diagnosis | TOTAL | Underweight | Healthy weight | Overweight | Obese | |||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Underweight | 35 | 4.6 | 1 | 2.9 | 33 | 94.3 | 1 | 2.9 | 0 | 0 |
| Healthy weight | 640 | 83.7 | 6 | 0.9 | 494 | 77.2 | 124 | 19.4 | 16 | 2.5 |
| Overweight | 74 | 9.7 | 0 | 0 | 23 | 31.1 | 36 | 48.6 | 15 | 20.3 |
| Obese | 16 | 2.1 | 0 | 0 | 0 | 0 | 6 | 37.5 | 10 | 62.5 |
| Total | 7 | 550 | 167 | 41 | ||||||
| Aged 2.0–5.9 years | 464 | 60.7 | ||||||||
| Underweight | 26 | 4.6 | 0 | 0 | 25 | 96.2 | 1 | 3.8 | 0 | 0 |
| Healthy weight | 388 | 83.6 | 3 | 0.8 | 271 | 69.8 | 99 | 25.5 | 15 | 3.9 |
| Overweight | 40 | 8.6 | 0 | 0 | 11 | 27.5 | 21 | 52.5 | 8 | 20.0 |
| Obese | 10 | 2.2 | 0 | 0 | 0 | 0 | 6 | 60.0 | 4 | 40.0 |
| Aged 6.0–9.9 years | 158 | 20.6 | ||||||||
| Underweight | 4 | 2.5 | 0 | 0 | 4 | 100 | 0 | 0 | 0 | 0 |
| Healthy weight | 136 | 86.1 | 1 | 0.7 | 120 | 88.3 | 15 | 11.0 | 0 | 0 |
| Overweight | 14 | 8.9 | 0 | 0 | 5 | 35.7 | 7 | 50.0 | 2 | 14.3 |
| Obese | 4 | 2.5 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 100.0 |
| Aged 10–17.9 | 143 | 18.7 | ||||||||
| Underweight | 5 | 3.5 | 1 | 20 | 4 | 80 | 0 | 0 | 0 | 0 |
| Healthy weight | 116 | 81.1 | 2 | 1.7 | 103 | 88.8 | 10 | 8.6 | 1 | 0.9 |
| Overweight | 20 | 14.3 | 0 | 0 | 7 | 35.0 | 8 | 40.0 | 5 | 25.0 |
| Obese | 2 | 1.4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 100.0 |
Note: % of the number of patients in the BMI category at diagnosis.
The majority (94.3%; 33/35) of underweight patients at diagnosis had a healthy BMI at EOT. However, 22% (141/640) of patients with a healthy BMI at diagnosis were overweight or obese and 0.9% (6/649) were underweight at EOT. The younger children aged 2.0–5.9 year were most vulnerable to weight gain with almost every third (29.4%;114/388) normal weight child being overweight/obese at EOT, compared to 10.5% and 9.5% in the 6.0–9.9 years and 10.0–17.9 years age categories (Table 3).
4. DISCUSSION
In line with previous studies, we observed an increase in BMISDS during ALL treatment. The number of overweight/obese patients at EOT more than doubled from diagnosis. Younger children, especially those with a healthy BMI at diagnosis, nonhigh‐risk group patients, and underweight patients at diagnosis were most prone to gain weight during treatment.
The mean change in BMISDS in our study was +0.64. A meta‐analysis by Zhang et al. observed a mean increase of +0.83 in BMI z‐scores (BMISDS) in 1514 pediatric patients. 15 A greater increase in weight has been observed in patients receiving dexamethasone than in those receiving prednisolone, though the difference has been transient. 27 , 28 No such difference was observed herein. However, as we only have data on BMI at diagnosis and EOT, it's not possible to conclude whether a transient greater weight gain occurred in patients receiving dexamethasone.
Younger and healthy weight children are at greater risk of unhealthy weight gain than other children treated for ALL. A study by Browne et al. on 372 children aged 2–18 years at ALL diagnosis demonstrated that younger underweight or healthy weight children were at significantly higher risk of becoming overweight and obese during and after treatment compared to older children. 9 Similar to our study, Reilly et al. also showed that younger and underweight children at diagnosis were more likely to gain weight during treatment. 14
In our study we could conclude, that only 0.9% of the children with healthy weight at diagnosis were underweight at EOT. The results indicate that we probably are managing the nutritional challenges in the undernourished child with ALL sufficiently, but we are far from managing the same challenges in the supportive care, regarding excessive BMI gain during treatment. How do we inform the families? How do we manage tube feeding? In what ways do we support physical activity during treatment?
Overweight/obese children and adolescents have a higher prevalence of comorbidities, such as diabetes, hypertension, and dyslipidemia. 29 Obesity also affects quality of life and mental health, with obese children at higher risk for depression than nonobese children. 30 Studies on BMI years after end of leukemia treatment have shown that weight gain persists beyond treatment completion. 9 , 12 , 14 , 15 , 31 Considering that many patients become overweight/obese during treatment and that the weight gain is likely to persist, interventions to prevent weight gain early in treatment are warranted. Previous small life‐style intervention studies under and after childhood ALL treatment have shown promising result both on feasibility and efficacy. 17 , 32 , 33
Obesity has over the last decades become a large problem in the general pediatric population. Further, the prevalence of overweight and obesity increased significantly with age in Swedish school children. 34 The international growth curves are mainly constructed and based on children measured at the end of the last decennium, contributing to some uncertainty when analyzing standard deviations of BMI and BMI cut‐offs in different age groups. 20 , 35
Weight gain is closely related to growth in children; although there was an decrease in height SDS during treatment, the children increased in mean weight SDS. This is in line with Browne et al, who describe a decrease in height z‐score and increase in weight z score in 372 children with ALL during treatment. 9 At the follow up time (median 10.7 years) there was some improvement of height z score after EOT especially in the younger children. Bruzzi et al. showed that the height SDS of 162 survivors of childhood ALL decreased with 0.36 SDS during treatment, not followed by an appropriate catch‐up. 36
The limitations of the study include missing data on the pubertal status at diagnosis and EOT. Further, the results do not apply to patients under 2 years of age who were excluded due to the BMI cut‐offs applied. Also, we did not have detailed data on the body composition changes; although BMI is feasible and readily available at diagnosis and EOT, it does not differentiate between changes in muscle tissue and body fat or their distribution in the body. 37 , 38 Previous studies have also described loss of skeletal muscle mass during and after treatment in long term survivors of ALL. 39 , 40 Dual‐energy X‐ray absorptiometry (DXA) is often described as gold standard for direct measuring of body composition and body fat changes, which may not be feasible for large‐scale interventions. Other indirect methods to gather information on the body composition, such as the Bioelectrical Impedance Analysis (BIA) method, could be considered for future studies. 41 Lastly, we have no data after EOT and cannot describe the height development and the effect on BMISDS in that off treatment period.
In conclusion, this study demonstrated how BMI changes during ALL treatment in a large population treated for ALL according to NOPHO protocols. Younger children, and especially those aged 2–5.9 years, treated with nonhigh‐risk protocols and with a healthy weight at diagnosis, are prone to becoming overweight/obese during treatment. Therefore, they are an important group to target with multidisciplinary lifestyle interventions during and after treatment, in addition to children who are already overweight/obese at diagnosis. More research on how BMI change during therapy is associated with treatment outcome including toxicity is warranted to be able to target interventions to those patients who can benefit from them the most.
AUTHOR CONTRIBUTIONS
All authors have contributed in this manuscript according to ICMJE definition of authorship. Birgitte Klug Albertsen, Kristi Lepik, Riitta Niinimäki, Ólafur G. Jonsson, Niklas Stabell, Goda Vaitkeviciene, and Kjeld Schmiegelow participated in collecting data, were national NOPHO representatives, participated in the analytical framework for the study, and contributed to the writing of the manuscript. Christina Egnell, Arja Harila‐Saari, and Susanna Ranta supervised the design and execution of the study, and performed the final data analyses and writing of the manuscript. Hanna Närhinen collected data, prepared the statistical framework, did primary statistical analyses, and contributed in manuscript writing, while AM performed more advanced statistical analyses.
CONFLICT OF INTEREST
The authors have no competing interests to declare.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGMENTS
Christina Egnell is supported by funding from the Swedish Childhood Cancer Foundation (Barncancerfonden, grant numbers TJ2018‐0093; PR2019‐0075; TJ2019‐0048). This work is part of the Danish nation‐wide research program Childhood Oncology Network Targeting Research, Organisation & Life expectancy (CONTROL) and supported by the Danish Cancer Society (R‐257‐A14720) and the Danish Childhood Cancer Foundation (2019‐5934 and 2020‐5769).
Egnell C, Närhinen H, Merker A, et al. Changes in body mass index during treatment of childhood acute lymphoblastic leukemia with the Nordic ALL2008 protocol. Eur J Haematol. 2022;109(6):656‐663. doi: 10.1111/ejh.13848
Funding information Danish Childhood Cancer Foundation, Grant/Award Number: 2019‐5934; the Danish Cancer Society, Grant/Award Number: R‐257‐A14720; Swedish Childhood Cancer Foundation, Grant/Award Number: TJ2018‐0093
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Wang Y, Lim H. The global childhood obesity epidemic and the association between socio‐economic status and childhood obesity. Int Rev Psychiatry. 2012;24(3):176‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hohwu L, Gissler M, Sjoberg A, Biehl AM, Kristjansson AL, Obel C. Prevalence of overweight in 2 to 17 year‐old children and adolescents whose parents live separately: a Nordic cross‐sectional study. BMC Public Health. 2014;14:1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amankwah EK, Saenz AM, Hale GA, Brown PA. Association between body mass index at diagnosis and pediatric leukemia mortality and relapse: a systematic review and meta‐analysis. Leuk Lymphoma. 2016;57(5):1140‐1148. [DOI] [PubMed] [Google Scholar]
- 4. Orgel E, Genkinger JM, Aggarwal D, Sung L, Nieder M, Ladas EJ. Association of body mass index and survival in pediatric leukemia: a meta‐analysis. Am J Clin Nutr. 2016;103(3):808‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gelelete CB, Pereira SH, Azevedo AM, et al. Overweight as a prognostic factor in children with acute lymphoblastic leukemia. Obesity (Silver Spring). 2011;19(9):1908‐1911. [DOI] [PubMed] [Google Scholar]
- 6. Egnell C, Ranta S, Banerjee J, et al. Impact of body mass index on relapse in children with acute lymphoblastic leukemia treated according to Nordic treatment protocols. Eur J Haematol. 2020;105(6):797‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butturini AM, Dorey FJ, Lange BJ, et al. Obesity and outcome in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2007;25(15):2063‐2069. [DOI] [PubMed] [Google Scholar]
- 8. Orgel E, Sposto R, Malvar J, et al. Impact on survival and toxicity by duration of weight extremes during treatment for pediatric acute lymphoblastic leukemia: a report from the Children's oncology group. J Clin Oncol. 2014;32(13):1331‐1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Browne EK, Zhou Y, Chemaitilly W, et al. Changes in body mass index, height, and weight in children during and after therapy for acute lymphoblastic leukemia. Cancer. 2018;124(21):4248‐4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Withycombe JS, Smith LM, Meza JL, et al. Weight change during childhood acute lymphoblastic leukemia induction therapy predicts obesity: a report from the Children's oncology group. Pediatr Blood Cancer. 2015;62(3):434‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hunter MP, Ismail N, Zhang X, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3(11):e3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foster KL, Kern KD, Chambers TM, et al. Weight trends in a multiethnic cohort of pediatric acute lymphoblastic leukemia survivors: a longitudinal analysis. PLoS One. 2019;14(5):e0217932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Belle FN, Wenke‐Zobler J, Cignacco E, et al. Overweight in childhood cancer patients at diagnosis and throughout therapy: a multicentre cohort study. Clin Nutr. 2019;38(2):835‐841. [DOI] [PubMed] [Google Scholar]
- 14. Reilly JJ, Ventham JC, Newell J, Aitchison T, Wallace WH, Gibson BE. Risk factors for excess weight gain in children treated for acute lymphoblastic leukaemia. Int J Obes Relat Metab Disord. 2000;24(11):1537‐1541. [DOI] [PubMed] [Google Scholar]
- 15. Zhang FF, Liu S, Chung M, Kelly MJ. Growth patterns during and after treatment in patients with pediatric ALL: a meta‐analysis. Pediatr Blood Cancer. 2015;62(8):1452‐1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arpe ML, Rorvig S, Kok K, Molgaard C, Frandsen TL. The association between glucocorticoid therapy and BMI z‐score changes in children with acute lymphoblastic leukemia. Support Care Cancer. 2015;23(12):3573‐3580. [DOI] [PubMed] [Google Scholar]
- 17. Orgel E, Framson C, Buxton R, et al. Caloric and nutrient restriction to augment chemotherapy efficacy for acute lymphoblastic leukemia: the IDEAL trial. Blood Adv. 2021;5(7):1853‐1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. den Hoed MA, Pluijm SM, de Groot‐Kruseman HA, et al. The negative impact of being underweight and weight loss on survival of children with acute lymphoblastic leukemia. Haematologica. 2015;100(1):62‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toft N, Birgens H, Abrahamsson J, et al. Risk group assignment differs for children and adults 1‐45 yr with acute lymphoblastic leukemia treated by the NOPHO ALL‐2008 protocol. Eur J Haematol. 2013;90(5):404‐412. [DOI] [PubMed] [Google Scholar]
- 20. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ. 2007;335(7612):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatr Obes. 2012;7(4):284‐294. [DOI] [PubMed] [Google Scholar]
- 23. Dangour AD, Schilg S, Hulse JA, Cole TJ. Sitting height and subischial leg length centile curves for boys and girls from Southeast England. Ann Hum Biol. 2002;29(3):290‐305. [DOI] [PubMed] [Google Scholar]
- 24. Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child. 1995;73(1):17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73(1):25‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17(4):407‐429. [PubMed] [Google Scholar]
- 27. Ahmed SF, Tucker P, Mushtaq T, Wallace AM, Williams DM, Hughes IA. Short‐term effects on linear growth and bone turnover in children randomized to receive prednisolone or dexamethasone. Clin Endocrinol (Oxf). 2002;57(2):185‐191. [DOI] [PubMed] [Google Scholar]
- 28. Wallace AM, Tucker P, Williams DM, Hughes IA, Ahmed SF. Short‐term effects of prednisolone and dexamethasone on circulating concentrations of leptin and sex hormone‐binding globulin in children being treated for acute lymphoblastic leukaemia. Clin Endocrinol (Oxf). 2003;58(6):770‐776. [DOI] [PubMed] [Google Scholar]
- 29. Sharma V, Coleman S, Nixon J, et al. A systematic review and meta‐analysis estimating the population prevalence of comorbidities in children and adolescents aged 5 to 18 years. Obes Rev. 2019;20(10):1341‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rao WW, Zong QQ, Zhang JW, et al. Obesity increases the risk of depression in children and adolescents: results from a systematic review and meta‐analysis. J Affect Disord. 2020;267:78‐85. [DOI] [PubMed] [Google Scholar]
- 31. Zhang FF, Rodday AM, Kelly MJ, et al. Predictors of being overweight or obese in survivors of pediatric acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer. 2014;61(7):1263‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang JS, Dillon L, Terrones L, et al. Fit4Life: a weight loss intervention for children who have survived childhood leukemia. Pediatr Blood Cancer. 2014;61(5):894‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang FF, Kelly M, Du M, et al. Early lifestyle intervention for obesity prevention in pediatric survivors of acute lymphoblastic leukemia. Nutrients. 2019;11(11):2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bygdell M, Celind J, Lilja L, et al. Prevalence of overweight and obesity from 5 to 19 years of age in Gothenburg. Sweden Acta Paediatr. 2021;110(12):3349‐3355. [DOI] [PubMed] [Google Scholar]
- 35. Group WHOMGRS . WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76‐85. [DOI] [PubMed] [Google Scholar]
- 36. Bruzzi P, Predieri B, Corrias A, et al. Final height and body mass index in adult survivors of childhood acute lymphoblastic leukemia treated without cranial radiotherapy: a retrospective longitudinal multicenter Italian study. BMC Pediatr. 2014;14:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vanderwall C, Eickhoff J, Randall Clark R, Carrel AL. BMI z‐score in obese children is a poor predictor of adiposity changes over time. BMC Pediatr. 2018;18(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Orgel E, Mueske NM, Sposto R, Gilsanz V, Freyer DR, Mittelman SD. Limitations of body mass index to assess body composition due to sarcopenic obesity during leukemia therapy. Leuk Lymphoma. 2018;59(1):138‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rayar M, Webber CE, Nayiager T, Sala A, Barr RD. Sarcopenia in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2013;35(2):98‐102. [DOI] [PubMed] [Google Scholar]
- 40. Marriott CJC, Beaumont LF, Farncombe TH, et al. Body composition in long‐term survivors of acute lymphoblastic leukemia diagnosed in childhood and adolescence: a focus on sarcopenic obesity. Cancer. 2018;124(6):1225‐1231. [DOI] [PubMed] [Google Scholar]
- 41. Seo YG, Kim JH, Kim Y, et al. Validation of body composition using bioelectrical impedance analysis in children according to the degree of obesity. Scand J Med Sci Sports. 2018;28(10):2207‐2215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


