Abstract
Objectives
To analyze the effects of a fast‐velocity concentric resistance training (FVCRT) program on maximum strength of upper and lower limb, gait speed, walking endurance, fatigue, physical self‐perception, and catastrophizing pain in people with multiple sclerosis (MS).
Materials and Methods
Participants were randomized to either an experimental [EG] (n = 18) or a control [CG] (n = 12) group. The EG carried out 10‐weeks of lower limb FVCRT. The CG did not perform any intervention. The maximum isometric voluntary contraction (MVIC) during knee extension, hand‐grip strength, gait speed, walking endurance, fatigue, physical self‐perception, and catastrophizing pain were measured.
Results
Inter‐group differences after intervention were found on the right and left sides in MVIC (p = .032; ES = ‐0.7 and p = .009; ES = ‐0.9), and hand grip strength (p = .003; ES = ‐1.0 and p = .029; ES = ‐0.7). After FVCRT, there was in increase in MVIC (p < .001; ES = ‐1.7 and p < .001; ES = ‐1.3) and hand grip strength (p < .001; ES = ‐1.3 and p < .001; ES = ‐1.3) on both right and left sides, respectively. In addition, gait speed (p = .023; ES = 1.3), walking endurance (p < .001; ES = ‐1.0), symptomatic fatigue (p = .004; ES = 0.6), and catastrophizing pain (p < .001; ES = 1.0) improved in EG.
Conclusion
Lower limb FVCRT improved the upper and lower limb strength, walking, symptomatic fatigue, and catastrophizing pain in MS participants.
Keywords: functional capacity, mobility, neurological disorders, psychological state, strength training
1. INTRODUCTION
Multiple sclerosis (MS) is a chronic, demyelinating disease of the central nervous system, which is commonly diagnosed in young adults. The worldwide prevalence of MS is estimated around 2.2 million. 1 During the course of the disease, there is a degeneration of myelin around the axons, resulting in impaired neural drive. Consequently, people with MS develop both motor and sensory deficiencies. The severity of these problems depends on the disease progress, which can range from minor issues to serious problems that significantly increase the disability status of people with MS. Among the many symptoms of the disease, muscle weakness is commonly observed in persons with MS compared with matched groups without pathologies. 2 One explanation for this is that people with MS have a lower capacity to activate motor units of the lower limb muscles. 2 Similarly, maximum voluntary strength is impaired in this population, 2 , 3 which may imply important clinical problems, such as poor balance or impaired functional capacity. 4 Thus, the neuromuscular disability can gravely affect gait and mobility in people with MS. 5 Negative effects on gait kinematics, gait speed, and walking endurance can lead to a significant decrease in the quality of life. 6 For this reason, improving walking ability in people with MS is a major goal. In addition to neural and motor problems, people with MS present different psycho‐physiological problems such as a high perception of symptomatic fatigue, 7 pain catastrophizing, and a low physical self‐perception. 8
In an effort to reverse the symptomatology of MS, traditional resistance training is an effective tool to improve the activation of lower limb muscles. 9 Different meta‐analyses have shown improvement in maximum voluntary contraction (MVIC) following traditional resistance training. 10 There are also neuromuscular benefits (e.g., neural drive) following 3‐weeks of maximal strength training 9 as well as improvements in walking endurance and gait speed in people with MS 11 compared with control group. Specifically, a meta‐analysis carried out by Pearson et al. 12 demonstrated that resistance training improved walking endurance and gait speed in MS participants. Furthermore, previous studies have concluded that there is a close relationship between lower limb strength and walking, which suggests the need to improve neuromuscular performance to achieve benefits in overall mobility and quality of life. 4
Recently, some studies have examined the benefits of resistance training using maximum‐velocity contractions during the concentric phase of the movement, called fast‐velocity concentric resistance training (FVCRT), on strength and functional capacity in healthy adults 13 and in the elderly. 14 , 15 Together, these results suggest that FVCRT elicits greater neuromuscular adaptations compared with other types of physical training programs. 13 , 14 , 15 The intention to produce force at maximal velocity during the concentric phase leads to greater neural demands. 16 , 17 In addition, these neuromuscular adaptations lead to further gains in functional capacity and balance, among others. 14 However, FVCRT has yet to be investigated in people with MS.
Along with the benefits of resistance training on motor control, physical exercise also provides improvements in psychological state and fatigue perception in populations with and without pathologies. 18 Resistance training has a positive impact on physical self‐perception in people with MS. 19 In addition, catastrophizing pain and symptomatic fatigue can also be diminished after a resistance training program in the MS population. 19
Although recent studies have analyzed the benefits of resistance training on maximal strength of trained limbs, there are no studies that have investigated improvements in upper limb strength following lower limb focused training. In addition, the neural improvements derived from FVCRT has been studied in other populations, 14 but not in MS. FVCRT may be an appropriate type of training for the MS population as it may generate greater increases in rate of force development (RFD), 20 which in turn can enhance gait and balance.
Therefore, the main objectives of this randomized clinical trial (RCT) were as follows: (1) to analyze the benefits of a 10‐week lower‐limb FVCRT on upper and lower limb maximum strength; (2) to know the impact of this training on gait speed and walking endurance; and (3) to analyze the benefit of FVCRT on fatigue perception, catastrophizing pain, and physical self‐perception measured through questionnaires in people with MS. Our hypothesis was that FVCRT will have a large effect on lower limb maximum strength and upper‐limb strength (due to neural gains). In addition, we hypothesized that FVCRT will lead to an increase in walking performance and physical self‐perception, as well as a decrease in symptomatic fatigue and catastrophizing pain.
2. MATERIALS AND METHODS
2.1. Study design and testing procedure
A single‐blinded, RCT was conducted with two arms (experimental [EG] and control [CG] groups) and consisted of a 10‐week intervention. All training and testing sessions were carried out in the UCAM Sports Center (Murcia, Spain). All participants were evaluated during the same time of the day to minimize differing responses due to circadian rhythm changes. The temperature (21–22°C) and humidity (55%–60%) of the room were controlled during the testing sessions. To analyze the benefits of FVCRT on maximum strength, gait speed, walking endurance, fatigue perception, physical self‐perception, and catastrophizing pain, measurements were carried out at pre‐ and post‐10 weeks of FVCRT in both EG and CG. The trial was approved by the Catholic University of Murcia Ethics Committee and was in accordance with the Declaration of Helsinki. The trial design followed Consort guidelines for RCT. This study was registered in ClinicalTrials.gov (identifier: NCT04452760).
2.2. Participants
Thirty individuals with MS were recruited through the local MS association. A board‐certified neurologist diagnosed the participants with either Relapsing–Remitting MS or Secondary Progressive MS, based on the McDonald criteria. Following Kim & Shin, 21 a randomization sequence was created using Excel 2016 (Microsoft, Redmond, WA, USA) with a 3:2 (e.g., 3 in EG, 2 in CG) allocation using a random number table by one of the research staff member that specialized in statistical analysis. People with MS had to be in the stable phase of the disease and were ambulatory for more than 100 meters. The exclusion criteria were as follows: 1) Expanded Disability Status Scale <1 or >6, 2) experienced a relapse in the prior 12 months, 3) on corticosteroid treatment 2 months before study inclusion, and 4) partook in resistance training in the prior 4 months. The informed consent document was read and signed by the participants before participating in the study. Participants were excluded if they experienced an exacerbation that affected pyramidal functions or if they incomplete >10% of the planned training sessions.
2.3. Procedures
The 10‐week lower‐limb FVCRT was performed 3 times per week with at least 48 h of rest between sessions, in the UCAM Sports Center (Murcia, Spain). Prior to FVCRT, a standardized warm‐up protocol (5‐min on a stationary bicycle, mobility of lower‐limbs, and 5 repetitions at 40% 1‐RM on each machine) was conducted. Afterward, the EG performed bilateral leg press, unilateral leg extension, unilateral hip extension, and bilateral seated calf raise on conventional weight machines (Technogym, Cesena, Italy). Intensity, sets, repetitions, and rest between sets are presented in Table 1. Participants were told to prevent muscle failure and leave two repetitions in reserve. Supervisors instructed participants to lower the weight in a controlled manner and have a short pause at the end of movement, then to maximally contract the muscle as quickly as possible (concentric phase) to maximize the neural component. The individualized training load was determined from the 1‐repetition maximum (1‐RM) of each exercise before the start of the study. The 1‐RM load was estimated using the following protocol: 1 set of 10 repetitions at 50% of perceived 1‐RM, 1 set of 5 repetitions at 75% of the perceived 1‐RM, and 1 set of 1 repetition at 100% of 1‐RM. Five min of rest were given between sets. If a participant could complete >1 repetition in the latter set, the 1‐RM was estimated. The load was increased by 2%–5% when the participants were able to achieve 2 more repetitions than the predetermined ones, always with 2 repetitions in reserve. At the end of each session, participants filled‐in a log with the details of each exercise: weight lifted, repetitions, and sets completed. A group of up to 4 participants trained together, and all sessions had the same supervisor, specialized in strength and conditioning training, and certified by NSCA‐CPT. The CG did not partake in any intervention.
TABLE 1.
FVCRT training program
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Intensity (% 1‐RM) | 60 | 65 | 70 | 75 | 60 | 65 | 70 | 75 | 75 | 60 |
| Sets for exercise | 2 | 3 | 3 | 4 | 2 | 3 | 3 | 3 | 4 | 2 |
| Repetitions | 15 | 13 | 9 | 8 | 15 | 13 | 9 | 8 | 8 | 15 |
| Rest between set (s) | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 |
Note: FVCRT, fast‐velocity concentric resistance training. 1‐RM, one‐repetition maximum.
2.4. Outcomes measures
The same researcher conducted each assessment. Participants were not blinded to the intervention due to the peculiarity of the intervention (resistance training program vs. no exercise). Participants who were allocated to the CG were invited to complete the 10‐weeks of lower‐limb FVCRT after completion of the control post‐measurements. A blinded researcher who was not directly involved in the training program and group allocation analyzed all outcome measures.
2.5. Maximum strength of upper and lower limb
2.5.1. Lower limb
Participants sat on the isokinetic dynamometer chair (Biodex Medical System, NY) with both legs flexed at 90° and the testing leg's ankle strapped directly to a customized apparatus with a load cell (Model SML500, Interface Scottsdale, AZ, USA). To assess maximal torque (MVIC) in each leg, participants were verbally encouraged to apply “as much force as possible” throughout the 2 consecutive maximal contractions. Participants performed two 5‐s MVICs with 3 min of rest between contractions. The right leg was always evaluated first, and the trial with the highest MVIC was used for analysis.
2.5.2. Upper limb
Participants stood with their elbows fully extended and separated from the trunk. In this position, isometric handgrip strength was measured for 5 s using the electronic hand dynamometer (TL‐LSC100, Trailite, Ahaus, Germany). Participants performed three trials in each hand (right and left) and a 60‐s rest interval was given between attempts. The highest value was recorded.
2.6. Walking variables
2.6.1. Gait speed
Gait speed was measured with the 10‐meter walk test (10‐MWT), where 2 photocells (Witty, Microgate, Italy) were placed at 6‐ and 10‐m to record the time. Participants performed the test as fast as possible without running, twice with 2 min of rest in between. Participants were encouraged throughout the 10‐MWT. The lowest walking time (s) was used for analysis.
2.6.2. Walking endurance
After the 10‐MWT, participants performed the 6‐min walk test (6‐MWT) using a self‐selected preferred speed to measure walking endurance. The testing track was rectangular, and cones defined the corners. Rest during the test was allowed if the participant needed it, but the time did not stop during the resting period. The total distance (m) covered was recorded. The investigator accompanied the participants during the test but did not make conversation.
2.7. Physical self‐perception
The physical self‐perception consisted of six subscales that assessed self‐perception in sports competence, physical condition, attractive body, physical strength, general physical self‐perception, and general self‐perception. The answers were structured on a 5‐point Likert scale, where each subscale score could range from 6 to 36 points. A higher score indicated good physical self‐perception.
2.8. Catastrophizing Pain Scale
The Catastrophizing Pain Scale was utilized to assess catastrophic feelings related to pain (e.g., painful experiences). Three subscales scores examined rumination and helplessness. Each of the 13 questions had a scale of 5 scores with the end points were <0 > not at all and <4 > all the time. A lower score indicated low or no catastrophizing pain.
2.9. Fatigue Severity Scale
Fatigue perception was measured with the one‐dimensional Fatigue Severity Scale (FSS), a validated questionnaire for the MS population. The test–retest reproducibility of FSS is high. A higher score in FSS indicated high level of fatigue.
2.10. Statistical analyses
Statistical analysis was performed using the software SPSS (v.24.0). Descriptive analyses (mean and standard deviation) were calculated. The Shapiro–Wilk test verified the assumption of normality before using parametric tests, and the Levene's test determined the homogeneity of variance. A two‐way, repeated measures analysis of variance (ANOVA) analyzed the effects of lower‐limb resistance training program (general linear model; 2 time points (pre‐ and post‐intervention) × 2 groups (EG and CG). Post‐hoc tests (Bonferroni) were performed when significant interaction (group x time) effects were observed. Eta squared partial (η 2 p ) for variance analysis calculated the effect size, and Cohen's d (ES) evaluated the standardized difference between two means. An η2p of 0.1–0.24 indicated a small effect, 0.25–0.36 a medium effect and ≥0.37 a large effect. The Cohen scale was used to demarcate effect sizes, where 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. A level of p < .05 established statistical significance.
3. RESULTS
The inclusion flowchart and the participant's characteristics presented in Table 2 and Figure 1, respectively. The period of recruitment was between June and July of 2020, and the study trial started in July 2020 and ended in November 2020. All participants completed the intervention and were included in the data analysis. No participant showed adverse effects related to resistance training. In addition, there were no group differences observed at baseline.
TABLE 2.
Participant characteristic
| Characteristics | All (n = 30) | Experimental group (n = 18) | Control group (n = 12) | P |
|---|---|---|---|---|
| Sex (men: women) | 15:15 | 10:8 | 5:7 | |
| MS phenotype (RR:SP) | 27:3 | 16:2 | 11:1 | |
| Age (years) | 46.21 ± 10.43 | 44.89 ± 10.62 | 48.36 ± 10.23 | .394 |
| EDSS | 3.21 ± 1.51 | 3.17 ± 1.65 | 3.27 ± 1.33 | .858 |
| Weight (kg) | 68.51 ± 11.55 | 67.19 ± 10.63 | 70.67 ± 13.17 | .442 |
| Height (cm) | 166.86 ± 6.95 | 166.44 ± 7.32 | 167.54 ± 6.58 | .687 |
| BMI (kg⋅m−2) | 24.56 ± 3.29 | 24.26 ± 3.12 | 25.06 ± 3.64 | .534 |
| Fat mass (%) | 26.47 ± 8.72 | 25.92 ± 8.28 | 27.34 ± 9.69 | .680 |
Abbreviations: Data are presented as mean ± standard deviation. Significance was set at p = .05. BMI, body mass index. EDSS, expanded disability status scale. MS, multiple sclerosis. RR, relapsing–remitting. SP, secondary‐progressive.
FIGURE 1.
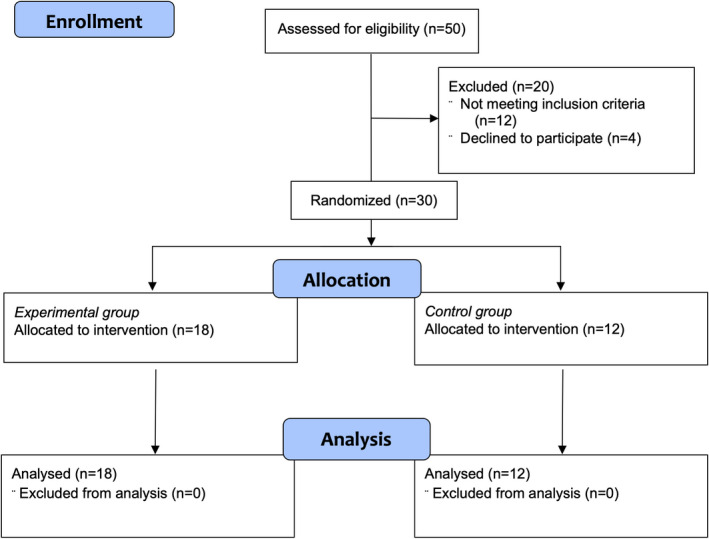
Participant enrollment, allocation, and analysis flowchart
Table 3 shows the strength results. There was a group x time interaction effect in MVIC of both limbs (Right: p < .001; Left: p < .001), showing a significant improvement from pre‐ to post‐training in EG (Right: p < .001; Left: p < .001) and significant differences between EG and CG at the end of the program (Right: p = .032; Left: p = .009). Additionally, there was a group x time interaction effect in hand‐grip strength of both hands (Right: p = .004; Left: p < .004) with a significant improvement in EG due to training program (Right: p < .001; Left: p < .001) and significant differences between EG and CG at the end of the program (Right: p = .003; Left: p = .029).
TABLE 3.
Right and left maximum upper and lower limb strength
| ANOVA (F, p, η 2 p ) | Post Hoc (Bonferroni) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE‐training | POST‐training | Time effect | Group effect | Time x Group effect | Intra | Inter | |||||||||||||
| Outcome | Group | Mean | SD | Mean | SD | F | p | η2p | F | p | η2p | F | p | η2p | p | ES | M | p | ES |
| Right Side | |||||||||||||||||||
| MVIC (N) | CG | 366.9 | 125.0 | 373.6 | 131.5 | 29.7 | <.001* | 0.54 | 2.4 | .137 | 0.09 | 23.9 | <.001* | 0.49 | .711 | −0.2 | Pre | .580 | −0.067 |
| EG | 395.6 | 134.3 | 519.3 | 182.1 | <.001* | −1.7 | Post | .032 | −0.724 | ||||||||||
| Hand‐grip (kg) | CG | 26.3 | 8.6 | 26.9 | 8.3 | 18.8 | <.001* | 0.43 | 7.4 | .012* | 0.23 | 9.9 | .004* | 0.28 | .436 | −0.3 | Pre | .043 | −0.694 |
| EG | 33.5 | 8.7 | 37.7 | 8.6 | <.001* | −1.3 | Post | .003 | −1.075 | ||||||||||
| Left Side | |||||||||||||||||||
| MVIC (N) | CG | 283.5 | 143.6 | 284.1 | 143.6 | 20.5 | <.001* | 0.45 | 5.5 | .027* | 0.18 | 20.0 | <.001* | 0.44 | .973 | 0.0 | Pre | .106 | −0.535 |
| EG | 376.6 | 140.5 | 480.1 | 195.5 | <.001* | −1.3 | Post | .009 | −0.941 | ||||||||||
| Hand‐grip (kg) | CG | 25.2 | 7.8 | 30.6 | 9.3 | 12.3 | .002* | 0.33 | 3.9 | .059 | 0.14 | 10.0 | .004* | 0.29 | .823 | −0.1 | Pre | .123 | −0.470 |
| EG | 25.3 | 7.7 | 33.5 | 9.7 | <.001* | −1.3 | Post | .029 | −0.740 | ||||||||||
Abbreviations: CG, control group. EG, experimental group. MVIC, maximal voluntary isometric contraction. SD, standard deviation. 6‐MWT, 6‐min walk test. 10‐MWT, 10‐meters walk test.
p < .05.
Concerning gait speed and walking endurance (Table 4), no interaction group × time effect was observed in any of the variables. There was a main effect of time in 10‐MWT (p = .010) and 6‐MWT (p < .001) walk tests, showing a significant improvement of EG from pre‐ to post‐training program (10‐MWT: p = .023; 6‐MWT: p < .001). In addition, significant differences were observed at the end of the program in 10‐MWT in EG (p = .041).
TABLE 4.
Gait speed and walking endurance
| ANOVA (F, p, η 2 p ) | Post Hoc (Bonferroni) | ||||||||||||||||||
| PRE‐training | POST‐training | Time effect | Group effect | Time x Group effect | Intra | Inter | |||||||||||||
| Outcome | Group | Mean | SD | Mean | SD | F | p | η 2 p | F | p | η 2 p | F | p | η 2 p | p | ES | M | p | ES |
| 10‐MWT (s) | CG | 8.7 | 7.7 | 7.96 | 7.2 | 8.9 | .010* | 0.26 | 4.36 | .05 | 0.14 | 0 | .97 | 0 | .071 | 0.4 | Pre | .056 | 0.8 |
| EG | 4.5 | 3.0 | 3.8 | 2.8 | .023* | 1.3 | Post | .041* | 0.8 | ||||||||||
| 6‐MWT (m) | CG | 356.2 | 246.7 | 393.9 | 310.6 | 13.7 | .010* | 0.35 | 1.82 | .19 | 0.07 | 3.45 | .08 | 0.12 | .248 | −0.4 | Pre | .324 | −0.4 |
| EG | 445.7 | 218.8 | 559.3 | 237.5 | <.001* | −1.0 | Post | .123 | −0.6 | ||||||||||
Abbreviations: CG, control group; EG, experimental group; SD, standard deviation; 6‐MWT, 6‐min walk test; 10‐MWT, 10‐meters walk test.
p < .05.
Finally, Table 5 shows the physical self‐perception, fatigue, and catastrophizing pain results. The items in the physical self‐perception questionnaire that showed a group x time interaction effect were physical condition (p = .010) and general physical self‐perception (p = .030). The items in the catastrophizing pain questionnaire that revealed a group x time interaction were helplessness (p = .003), magnification (p = .020), and total catastrophizing pain scale (p = .010). A significant impairment in physical condition was observed in CG (p = .043), while an improvement in helplessness (p = .001), magnification (p < .001), and total pain catastrophizing scale (p < .001) were shown in EG at the end of the program. No other group x time interaction effect was observed in any of the analyzed variables.
TABLE 5.
Physical self‐perception, fatigue severity, and catastrophizing pain questionnaires
| ANOVA (F, p, η 2 p ) | Post Hoc (Bonferroni) | ||||||||||||||||||
| PRE‐training | POST‐training | Time effect | Group effect | Time x Group effect | Intra | Inter | |||||||||||||
| Outcome | Group | Mean | SD | Mean | SD | F | p | η 2 p | F | p | η 2 p | F | p | η 2 p | p | ES | M | p | ES |
| Physical Self‐Perception | |||||||||||||||||||
| Sports competence | CG | 16.9 | 1.7 | 15.8 | 2.3 | 0.3 | .610 | 0.01 | 0.7 | .420 | 0.02 | 0.7 | .400 | 0.03 | .391 | 0.449 | Pre | .671 | ‐0.164 |
| EG | 17.9 | 7.8 | 18.2 | 6.7 | .778 | ‐0.057 | Post | .264 | ‐0.437 | ||||||||||
| Physical condition | CG | 16.3 | 3.3 | 14.4 | 2.5 | 0.4 | .520 | 0.02 | 0.3 | .590 | 0.01 | 7.3 | .010* | 0.21 | .043* | 1.052 | Pre | .209 | 0.492 |
| EG | 13.6 | 6.5 | 14.7 | 7.4 | .108 | ‐0.335 | Post | .878 | ‐0.059 | ||||||||||
| Attractive body | CG | 18.9 | 3.1 | 18.0 | 3.4 | 0.4 | .540 | 0.01 | 0.9 | .370 | 0.03 | 0.5 | .490 | 0.02 | .404 | 0.292 | Pre | .516 | ‐0.252 |
| EG | 20.4 | 7.0 | 20.4 | 6.8 | .948 | ‐0.015 | Post | .281 | ‐0.421 | ||||||||||
| Physical strength | CG | 16.2 | 3.3 | 15.6 | 3.4 | 0.2 | .630 | 0.01 | <0.1 | .870 | <0.01 | 0.2 | .630 | 0.01 | .538 | 0.399 | Pre | .993 | 0.004 |
| EG | 16.2 | 4.7 | 16.2 | 4.6 | 1.000 | 0.000 | Post | .746 | ‐0.125 | ||||||||||
| General Physical‐Self Perception | CG | 16.5 | 2.8 | 15.3 | 2.9 | 0.1 | .850 | 0.01 | 3.5 | .070 | 0.12 | 5.2 | .030* | 0.16 | .756 | 0.609 | Pre | .207 | ‐0.495 |
| EG | 19.1 | 6.3 | 20.1 | 6.3 | .591 | ‐0.359 | Post | .026* | ‐0.898 | ||||||||||
| General Self‐Perception | CG | 16.1* | 2.2 | 16.4 | 2.2 | 0.4 | .540 | 0.01 | 6.0 | .020* | 0.18 | <0.1 | .910 | 0.01 | .745 | ‐0.140 | Pre | .024* | ‐0.915 |
| EG | 19.6 | 4.5 | 19.9 | 5.1 | .554 | ‐0.124 | Post | .035* | ‐0.848 | ||||||||||
| Fatigue Severity Scale | |||||||||||||||||||
| Fatigue Perception | CG | 42.9 | 10.9 | 42.1 | 10.0 | 0.8 | .040* | 0.14 | 1.9 | .180 | 0.06 | 3.0 | .090 | 0.10 | .811 | 0.162 | Pre | .568 | 0.221 |
| EG | 39.4 | 17.9 | 31.1 | 16.1 | .004 | 0.610 | Post | .053 | 0.774 | ||||||||||
| Catastrophizing Pain Scale | |||||||||||||||||||
| Rumination | CG | 5.6 | 4.4 | 5.4 | 4.8 | 6.8 | .020* | 0.2 | 0.8 | .380 | 0.03 | 3.4 | .080 | 0.11 | .552 | 0.183 | Pre | .659 | 0.171 |
| EG | 4.9 | 4.4 | 3.3 | 3.5 | .001 | 0.775 | Post | .198 | 0.505 | ||||||||||
| Helplessness | CG | 6.0 | 5.3 | 6.8 | 5.5 | 1.9 | .180 | 0.07 | 1.0 | .330 | 0.04 | 10.4 | .010* | 0.28 | .254 | ‐0.302 | Pre | .949 | 0.024 |
| EG | 5.9 | 4.0 | 3.8 | 2.6 | .001* | 0.992 | Post | .057 | 0.760 | ||||||||||
| Magnification | CG | 3.8 | 3.0 | 3.8 | 3.3 | 6.6 | .020* | 0.20 | 1.5 | .230 | 0.05 | 6.6 | .020* | 0.20 | 1.000 | 0.000 | Pre | .600 | 0.203 |
| EG | 3.2 | 2.9 | 1.9 | 2.0 | <.001* | 0.972 | Post | .057 | 0.761 | ||||||||||
| Total | CG | 15.5 | 12.4 | 16.0 | 13.2 | 5.9 | .020* | 0.18 | 1.2 | .290 | 0.04 | 9.1 | .010* | 0.25 | .706 | ‐1.200 | Pre | .738 | 0.129 |
| EG | 14.0 | 10.5 | 9.1 | 6.9 | <.001* | 1.018 | Post | .073 | 0.715 | ||||||||||
Abbreviations: CG, control group; EG, experimental group; SD, standard deviation; STAI, state–trait anxiety inventory.
p < .05 compared to experimental group post‐training.
4. DISCUSSION
The present RCT demonstrates that lower‐limb FVCRT can improve not only lower‐limb strength, but also upper‐limb strength. In addition, gait speed, walking endurance, and to some extent symptomatic fatigue and catastrophizing pain, improved after the FVCRT.
4.1. Maximum strength
Our training program focused on lower limb exercises mainly for two reasons. The first is based on the greater neuromuscular deficit that people with MS present in the lower limbs compared with the upper limbs. 3 The second reason is the relationship between lower limb strength and other impaired variables in this population, such as walking and balance. 4 Our study showed that 10 weeks of lower‐limb FVCRT improves knee extensor maximum strength by 31.7% in the right leg (differences between groups at post of 69%) and by 26.7% in the left leg (differences between groups at post of 69%). Interestingly, hand‐grip strength also increased by 12.53% for the right hand (differences between groups at post of 40%) and by 32.41% for the left hand (differences between groups at post of 9.5%). In line with our results, previous studies have shown increases in maximum strength, mainly in the lower limb muscles, after 8–12 weeks of traditional resistance training programs in people with MS. 22 , 23 A deficit of maximum lower limb strength is one of the main problems in people with MS, so improving it is a crucial aspect of rehabilitation in this population. 3 In addition, enhanced maximum strength has been associated with improved gait, increased mobility, and reduced risk of falls 24 in this population.
Interestingly, maximum hand‐grip strength increased significantly in both hands after 10 weeks of FVCRT in the EG, even though the training program only targeted lower limb muscles. Improvements in upper body strength (untrained limb) show adaptations at the neural level. Improvements in the untrained limbs in our suggest that there were improvements in recruitment and synchronization of motor units, higher firing rates, greater spinal motor‐neuronal excitability, and an increase in efferent motor drive. 16 , 25 There was a large ES in hand‐grip strength observed, suggesting possible neuromuscular adaptation in the untrained upper limbs. Hand‐grip strength is an indicator of overall strength and is associated with numerous clinically relevant health outcomes. 26 Previous research has shown a close relationship of hand grip values with mortality, risk of suffering cardiovascular diseases, quality of life, and functional capacity. 27 Even a relationship between upper limb strength and walking capacity has been established in people with MS. 28
It is suggested that FVCRT elicits greater neural adaptations than resistance training at low‐moderate concentric velocities. 29 The intention to produce force at maximal velocity during the concentric phase leads to greater synchronization and recruitment of motor units, 16 higher firing rates, greater spinal motor‐neuronal excitability and higher efferent motor drive. 17 These factors may be the underlying mechanisms that explain the greater neural adaptations of this type of training. Thus, in our study, even though we only trained the lower limb muscles, it appears that the neural adaptations achieved during the 10‐week of FVCRT improved strength in the untrained upper limb as well. This improvement in strength in the untrained limbs may be a promising alternative for people with MS who present with high levels of lower limb disability, as they could perform upper limb FVCRT and could increase lower limb muscle strength through neural adaptation.
4.2. Gait speed and walking endurance
Both gait speed (10‐MWT; Δ −15.6% and large ES) and walking endurance (6‐MWT; Δ 25.5% and large ES) significantly improved in EG after 10 weeks of lower‐limb resistance training. Gait improvements are one of the targeted goals in MS rehabilitation, mainly due to the high prevalence of gait deficits present in people with MS. 30 Physical therapy, 31 aerobic training, 32 and robot‐assisted gait training 33 have been extensively examined by others and have found moderate improvements in gait performance. Also, in accordance with our results, resistance training has also been shown to be an effective tool in improving both gait speed and walking endurance in people with moderate MS disability. 22 A meta‐analysis carried out by Pearson et al. 12 established that resistance training would have a greater impact on walking endurance than aerobic training or other types of training. These authors affirm that combined training (endurance and resistance training) was the type of training with the greatest effect on gait speed. The underlying mechanism for improvements in walking may be due to the increases in lower body strength (MVIC), as well as neural adaptations evidenced by improved upper limb strength found in EG.
According to the previous studies, a 12% 34 to 20% 35 change in walking tests is generally considered to be clinically significant in people with moderate MS disability. In our study, EG improved gait speed by 16.9% (differences between groups at post of 47%) and walking endurance by 25.5% (differences between groups at post of 42%) after FVCRT, which is within the aforementioned range. A previous meta‐analysis establishes that training programs of less than 3 months are effective in improving walking performance. 36 Therefore, our results suggest that lower‐limb FVCRT may lead to greater effects, in a shorter period of time, on gait speed and walking endurance than other exercise protocols proposed for this population, because of greater neural adaptations compared with other types or resistance training. However, more studies are needed to examine the effect of different durations and types of resistance training programs on gait variables in the MS population.
4.3. Pain catastrophizing, physical self‐perception, and fatigue perception
The EG significantly decreased the levels of fatigue perception and all subscales of the Catastrophizing Pain Scale (rumination, helplessness, magnification, and total) after the intervention with moderate and large effects. The CG experienced no pre‐post changes. However, the levels of physical self‐perception did not change in either group. A decrease in fatigue perception is a crucial objective in MS rehabilitation, since there is a high prevalence of symptomatic fatigue in 64%–81% of people with this disease and, thus, being one of the most disabling factors in this population. 37 Fatigue perception is correlated with other important factors, such as quality of life, sleep quality, anxiety, depression, and cognition. 37 A previous study associates physical inactivity and sedentary behavior to fatigue perception in people with MS. 38 Therefore, implementing a training program in this population should be a fundamental pillar in their rehabilitation process. In agreement with our results, several studies have found improvements in symptomatic fatigue perception after resistance training programs with intensities ranging from 40% to 80% 1‐RM and durations ranging from 4 to 16 weeks. 19 , 39 , 40 However, to the best of our knowledge, only one study has reported a trend towards increasing fatigue perception after an 8‐week resistance training intervention using 85%–90% 1‐RM in a sample of 7 people with moderate MS disability. 41 The discrepancy may be due to the low sample size and the high intensity used in the program. Therefore, it is important to consider that resistance‐training programs, in addition to the neuromuscular improvement, have a large impact on psycho‐physiological variables, such as fatigue perception, and participation adherence to training programs.
All subscales of Catastrophizing Pain Scale also decreased after FVCRT in EG. The catastrophizing pain, together with the aforementioned variables, strongly affects quality of life. Reducing catastrophizing pain in the MS population would lead to improvements in the psychological component, a fundamental aspect in the management of a progressive disease. 42 Finally, no changes in either EG or CG after FVCRT were found in our study, which contrasts findings from Dalgas et al. 19 that have found improvements in physical self‐perception after progressive resistance training. Nevertheless, other authors found no improvement in the self‐concept of physical health after a 6‐week resistance training program. 43
5. LIMITATIONS OF THE STUDY
The present study has some limitations. The first is the heterogeneity of the sample, composed of different disease phenotypes (relapsing–remitting and secondary progressive) and of both sexes (men and women). In addition, variables such as motor unit firing rate, which could have provided us with more direct information regarding neuromuscular activation, were not measured. Future lines of research should compare the effects of traditional resistance training vs FVCRT on structural and neural adaptations in people with MS.
6. CONCLUSIONS
The FVCRT is an effective tool in improving strength, gait, perception of fatigue and catastrophizing pain. In addition, improvements in upper limb strength, which was not trained, provide evidence of substantial neural gains caused by this new type of training.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ane.13704.
ACKNOWLEDGMENTS
This work has been supported partially by the Spanish Ministry of Education, Culture, and Science through the National Program FPU.
Andreu‐Caravaca, L. , Ramos‐Campo, D. J. , Chung, L. H. , Manonelles, P. , Abellán‐Aynés, O. & Rubio‐Arias, J. Á. (2022). Effects of fast‐velocity concentric resistance training in people with multiple sclerosis: A randomized controlled trial. Acta Neurologica Scandinavica, 146, 652–661. 10.1111/ane.13704
Registration number: This study was registered in ClinicalTrials.gov (identifier: NCT04452760).
Contributor Information
Luis Andreu‐Caravaca, Email: landreu@ucam.edu.
Jacobo Á. Rubio‐Arias, Email: jararias@ual.es.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ng AV, Miller RG, Gelinas D, Kent‐Braun JA. Functional relationships of central and peripheral muscle alterations in multiple sclerosis. Muscle & Nerve. 2004;29(6):843‐852. [DOI] [PubMed] [Google Scholar]
- 3. Jørgensen MLK, Dalgas U, Wens I, Hvid LG. Muscle strength and power in persons with multiple sclerosis – A systematic review and meta‐analysis. J Neurol Sci. 2017;376:225‐241. [DOI] [PubMed] [Google Scholar]
- 4. Ramari C, Hvid LG, David AC, Dalgas U. The importance of lower‐extremity muscle strength for lower‐limb functional capacity in multiple sclerosis: Systematic review. Ann Phys Rehabil Med. 2020;63(2):123‐137. [DOI] [PubMed] [Google Scholar]
- 5. Bethoux F. Gait disorders in multiple sclerosis. Continuum (Minneap Minn). 2013;19(4):1007‐1022. [DOI] [PubMed] [Google Scholar]
- 6. Straudi S, Fanciullacci C, Martinuzzi C, et al. The effects of robot‐assisted gait training in progressive multiple sclerosis: A randomized controlled trial. Mult Scler. 2016;22:373‐384. [DOI] [PubMed] [Google Scholar]
- 7. Manjaly ZM, Harrison NA, Critchley HD, et al. Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2019;90(6):642‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korwin‐Piotrowska K, Korwin‐Piotrowska T, Samochowiec J. Self perception among patients with multiple sclerosis. Arch Psychiatry Psychother. 2010;12(3):63‐68. [Google Scholar]
- 9. Fimland MS, Helgerud J, Gruber M, Leivseth G, Hoff J. Enhanced neural drive after maximal strength training in multiple sclerosis patients. Eur J Appl Physiol. 2010;110(2):435‐443. [DOI] [PubMed] [Google Scholar]
- 10. Platta ME, Ensari I, Motl RW, Pilutti LA. Effect of Exercise Training on Fitness in Multiple Sclerosis: A Meta‐Analysis. Arch Phys Med Rehabil. 2016;97(9):1564‐1572. [DOI] [PubMed] [Google Scholar]
- 11. Taul‐Madsen L, Connolly L, Dennett R, Freeman J, Dalgas U, Hvid LG. Is aerobic or resistance training the most effective exercise modality for improving lower extremity physical function and perceived fatigue in people with multiple sclerosis? A systematic review and meta‐analysis. Arch Phys Med Rehabil. 2021;102(10):2032‐2048. [DOI] [PubMed] [Google Scholar]
- 12. Pearson M, Dieberg G, Smart N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: a meta‐analysis. Arch Phys Med Rehabil. 2015;96:1339‐1348. [DOI] [PubMed] [Google Scholar]
- 13. Davies TB, Kuang K, Orr R, Halaki M, Hackett D. Effect of Movement Velocity During Resistance Training on Dynamic Muscular Strength: A Systematic Review and Meta‐Analysis. Sports Med. 2017;47(8):1603‐1617. [DOI] [PubMed] [Google Scholar]
- 14. da Rosa Orssatto LB, Cadore EL, Andersen LL, Diefenthaeler F. Why fast velocity resistance training should be prioritized for elderly people. Strength Cond J. 2019;41(1):105‐114. [Google Scholar]
- 15. da Rosa Orssatto LB, de la Rocha FC, Shield AJ, Silveira Pinto R, Trajano GS. Effects of resistance training concentric velocity on older adults' functional capacity: A systematic review and meta‐analysis of randomised trials. Exp Gerontol. 2019;127:110731. [DOI] [PubMed] [Google Scholar]
- 16. Jenkins NDM, Miramonti AA, Hill EC, et al. Greater neural adaptations following high‐ vs. low‐load resistance training. Front Physiol. 2017;8:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramírez‐Campillo R, Martínez C, De La Fuente CI, et al. High‐speed resistance training in older women: The role of supervision. J Aging Phys Act. 2017;25(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 18. Hart PD, Buck DJ. The effect of resistance training on health‐related quality of life in older adults: Systematic review and meta‐analysis. Heal Promot Perspect. 2019;9(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dalgas U, Stenager E, Jakobsen J, et al. Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult Scler. 2010;16(4):480‐490. [DOI] [PubMed] [Google Scholar]
- 20. Andreu‐Caravaca L, Ramos‐Campo DJ, Chung LH, Manonelles P, Boas JPV, Rubio‐Arias JÁ. Fast‐velocity Resistance Training Improves Force Development and Mobility in Multiple Sclerosis. Int J Sports Med. 2021;43(7):593‐599. [DOI] [PubMed] [Google Scholar]
- 21. Kim J, Shin W. How to Do Random Allocation (Randomization). Clin Orthop Surg. 2014;6:103‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. White LJ, McCoy SC, Castellano V, et al. Resistance training improves strength and functional capacity in persons with multiple sclerosis. Mult Scler. 2004;10:668‐674. [DOI] [PubMed] [Google Scholar]
- 23. Medina‐Perez C, De Souza‐Teixeira F, Fernandez‐Gonzalo R, De Paz‐Fernandez JA. Effects of a resistance training program and subsequent detraining on muscle strength and muscle power in multiple sclerosis patients. NeuroRehabilitation. 2014;34(3):523‐530. [DOI] [PubMed] [Google Scholar]
- 24. Kasser SL, Jacobs JV, Foley JT, Cardinal BJ, Maddalozzo GF. A prospective evaluation of balance, gait, and strength to predict falling in women with multiple sclerosis. Arch Phys Med Rehabil. 2011;92(11):1840‐1846. [DOI] [PubMed] [Google Scholar]
- 25. Del Vecchio A, Negro F, Holobar A, et al. You are as fast as your motor neurons: speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans. J Physiol. 2019;597:2445‐2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGrath RP, Kraemer WJ, Al SS, Peterson MD. Handgrip Strength and Health in Aging Adults. Sports Med. 2018;48:1993‐2000. [DOI] [PubMed] [Google Scholar]
- 27. Tietjen‐Smith T, Smith SW, Martin M, Henry R, Weeks S, Bryant A. Grip strength in relation to overall strength and functional capacity in very old and oldest old females. Phys Occup Ther Geriatr. 2006;24(6):63‐78. [Google Scholar]
- 28. Broekmans T, Gijbels D, Eijnde BO, et al. The relationship between upper leg muscle strength and walking capacity in persons with multiple sclerosis. Mult Scler J. 2013;19(1):112‐119. [DOI] [PubMed] [Google Scholar]
- 29. Behm DG, Sale DG. Velocity Specificity of Resistance Training. Sport Med. 1993;15(6):374‐388. [DOI] [PubMed] [Google Scholar]
- 30. Comber L, Galvin R, Coote S. Gait deficits in people with multiple sclerosis: A systematic review and meta‐analysis. Gait Posture. 2017;51:25‐35. [DOI] [PubMed] [Google Scholar]
- 31. Kalron A, Rosenblum U, Frid L, Achiron A. Pilates exercise training vs. physical therapy for improving walking and balance in people with multiple sclerosis: A randomized controlled trial. Clin Rehabil. 2017;31(3):319‐328. [DOI] [PubMed] [Google Scholar]
- 32. Rampello A, Franceschini M, Piepoli M, et al. Effect of aerobic training on walking capacity and maximal exercise tolerance in patients with multiple sclerosis: a randomized crossover controlled study. Phys Ther. 2007;87:545‐555. [DOI] [PubMed] [Google Scholar]
- 33. Straudi S, Manfredini F, Lamberti N, Martinuzzi C, Maietti E, Basaglia N. Robot‐assisted gait training is not superior to intensive overground walking in multiple sclerosis with severe disability (the RAGTIME study): A randomized controlled trial. Mult Scler. 2019;26(6):716‐724. [DOI] [PubMed] [Google Scholar]
- 34. Learmonth YC, Dlugonski DD, Pilutti LA, Sandroff BM, Motl RW. The reliability, precision and clinically meaningful change of walking assessments in multiple sclerosis. Mult Scler J. 2013;19(13):1784‐1791. [DOI] [PubMed] [Google Scholar]
- 35. Kieser M, Friede T, Gondan M. Assessment of statistical significance and clinical relevance. Stat Med. 2013;32(10):1707‐1719. [DOI] [PubMed] [Google Scholar]
- 36. Snook EM, Motl RW. Effect of exercise training on walking mobility in multiple sclerosis: A meta‐analysis. Neurorehabil Neural Repair. 2009;23(2):108‐116. [DOI] [PubMed] [Google Scholar]
- 37. Rooney S, Wood L, Moffat F, Paul L. Prevalence of fatigue and its association with clinical features in progressive and non‐progressive forms of Multiple Sclerosis. Mult Scler Relat Disord. 2019;28:276‐282. [DOI] [PubMed] [Google Scholar]
- 38. Neal WN, Cederberg KL, Jeng B, Sasaki JE, Motl RW. Is Symptomatic Fatigue Associated With Physical Activity and Sedentary Behaviors Among Persons With Multiple Sclerosis? Neurorehabil Neural Repair. 2020;34(6):505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dodd KJ, Taylor NF, Shields N, Prasad D, McDonald E, Gillon A. Progressive resistance training did not improve walking but can improve muscle performance, quality of life and fatigue in adults with multiple sclerosis: A randomized controlled trial. Mult Scler J. 2011;17(11):1362‐1374. [DOI] [PubMed] [Google Scholar]
- 40. Callesen J, Cattaneo D, Brincks J, Kjeldgaard Jørgensen ML, Dalgas U. How do resistance training and balance and motor control training affect gait performance and fatigue impact in people with multiple sclerosis? A randomized controlled multi‐center study. Mult Scler J. 2020;26(11):1420‐1432. [DOI] [PubMed] [Google Scholar]
- 41. Karpatkin HI, Cohen ET, Klein S, Park D, Wright C, Zervas M. The Effect of Maximal Strength Training on Strength, Walking, and Balance in People with Multiple Sclerosis: A Pilot Study. Mult Scler Int. 2016;2016:5235971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kerns R, Otis JD. Pain in multiple sclerosis: A biopsychosocial perspective. J Rehabil Res Dev. 2002;39(2):225‐232. [PubMed] [Google Scholar]
- 43. Keser I, Meric A, Kirdi N, Kurne A, Karabudak R. Comparing routine neurorehabilitation programme with callisthenic exercises in multiple sclerosis. NeuroRehabilitation. 2011;29(1):91‐98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


