Abstract
Human naïve B cells are notoriously difficult to differentiate into antibody‐secreting cells (ASCs) in vitro while maintaining sufficient cell numbers to evaluate the differentiation process. B cells require T follicular helper (TFH) cell‐derived signals like CD40L and IL‐21 during germinal center (GC) responses to undergo differentiation into ASCs. Cognate interactions between B and TFH cells are transient; after TFH contact, B cells cycle between GC light and dark zones where TFH contact is present and absent, respectively. Here, we elucidated that the efficacy of naïve B cells in ACS differentiation is dramatically enhanced by the release of CD40L stimulation. Multiparameter phospho‐flow and transcription factor (TF)‐flow cytometry revealed that termination of CD40L stimulation downmodulates NF‐κB and STAT3 signaling. Furthermore, the termination of CD40 signaling downmodulates C‐MYC, while promoting ASC TFs BLIMP1 and XBP‐1s. Reduced levels of C‐MYC in the differentiating B cells are later associated with crucial downmodulation of the B cell signature TF PAX5 specifically upon the termination of CD40 signaling, resulting in the differentiation of BLIMP1 high expressing cells into ASCs. The data presented here are the first steps to provide further insights how the transient nature of CD40 signaling is in fact needed for efficient human naïve B cell differentiation to ASCs.
Keywords: CD40L, human B cells, IL‐21, phospho‐signaling, transcriptional regulation
B cells require transient T cell help during germinal center responses in order to undergo antibody‐secreting cell differentiation. We show through in‐depth multiparameter phospho‐flow and transcription factor‐flow cytometry that in fact, termination of CD40L stimulation is required for downmodulation of PAX5, upregulation of BLIMP1 and subsequent differentiation into antibody‐secreting cells

Introduction
One key aspect of humoral immunity is the ability of B cells to respond to pathogens and differentiate into antibody‐secreting cells (ASCs). Essential to the generation of high‐affinity antibody production are follicular T helper cells (TFH) that regulate GC formation and B cell differentiation by providing cognate B cells with co‐stimulatory signals and specific cytokines [1, 2, 3, 4]. Naïve B cells are activated when an antigen is recognized by the BCR. The antigen is subsequently phagocytosed, degraded, loaded onto MHC class II molecules, and presented to TFH cells to allow cognate recognition [5]. Positive recognition results in strong synapse formation between B and TFH cells and allows TFH cells to stimulate B cells through membrane‐bound CD40L that activates CD40 on the B cell surface [6, 7]. Additionally, TFH cells secrete cytokines that promote B cell differentiation. Of these, the TFH hallmark cytokine IL‐21, which triggers the IL‐21 receptor, is indispensable for B cell differentiation [8, 9].
The timing and regulation of CD40L and IL‐21 stimulation within GC responses are highly complex and dynamic [10, 11]. Interactions between TFH cells and B cells are spatio‐dynamically regulated and therefore temporary [12]. B cells actively move away from initial cognate T cell interactions at the B/T cell border in secondary lymphoid organs to form GC dark zones. Here, B cells expand rapidly and can undergo class‐switch recombination (CSR) and somatic hyper‐mutation (SHM). Subsequently, B cells migrate out of the GC dark zones to establish GC light zones where the B cells re‐acquire antigen, followed by reacquisition of TFH help, consisting of additional CD40L and (among others) IL‐21‐mediated TFH cell stimulation [13]. After TFH cell interactions, differentiating B cells may migrate back to the GC dark zone to undergo more proliferation, SHM, and/or CSR or undergo early B memory formation. After several rounds of GC cycling and memory formation, B cells differentiate into ASCs. Still, questions remain how exactly the latter process is regulated. CD40L stimulation induces activation of NF‐κB pathways while IL‐21R stimulation mainly induces phosphorylation of STAT3 (p‐STAT3) [14, 15, 16]. These signaling pathways subsequently regulate an intricate network of transcription factors (TFs) governing B cell identity, GC cell regulation, and ASC differentiation [11, 17]. Expression of PAX5 maintains B cell identity and is induced by CD40L stimulation [18]. Additionally, CD40L stimulation and NF‐κB signaling induce expression of C‐MYC [19], which regulates proliferation and cycling of GC B cells [20, 21], and IRF4 [22], which at low levels promotes GC B cell development [23]. Differentiation of B cells into ASCs requires the downregulation of PAX5 and C‐MYC and the concomitant upregulation of BLIMP1, master regulator of ASC differentiation [17, 24, 25]. BLIMP1 expression is mainly induced by IL‐21 mediated p‐STAT3 signaling [1, 9, 26, 27] while CD40L co‐stimulation has been shown to inhibit BLIMP1 expression [6] but both CD40L and IL‐21 however, are essential for ASC differentiation [1]. Additionally, alternative splicing of XBP1 into XBP‐1s is required for the maintenance of the unfolded‐protein response (UPR), allowing the massive production of antibodies [28]. Within this complicated network, TFs can also positively and negatively feedback on the expression of other TFs in the network. Most well‐known is the fact that PAX5 and BLIMP1 inhibit each other [17]. However, PAX5 has also been shown to inhibit C‐MYC [29, 30] and XBP1 expression, possibly through the downregulation of BLIMP1 [31]. Additionally, high levels of IRF4 promote the expression of BLIMP1 and thus downregulation of PAX5 [23, 32].
As the T‐dependent B cell response is complex and still not fully elucidated, insights into factors determining B cell to ASC differentiation are highly desired. Over the years in vitro cultures have been applied to study human ASC generation with varying success [33, 34, 35, 36]. Most cultures rely on continued CD40L and IL‐21 stimulation over the course of several days. Based on the in vivo situation of spatiotemporal regulation of B cell to ASC differentiation and intermittent TFH cell help, we hypothesized that temporal instead of continued TFH stimulation contributes to the T‐dependent B cells response. Recently, a similar mechanism was found for the IL‐4 / STAT6 pathway for which it was demonstrated that ASC differentiation requires the downregulation of this pathway [37]. Additionally, we have shown that phospho‐ STAT1, ‐STAT3, ‐STAT6, and activated NF‐κB p65 signaling in in vitro generated ASC is also downregulated compared to other B cell subsets [38]. Here we demonstrate that targeted termination of CD40L stimulation with continuous IL‐21 stimulation, promotes human B cell to ASC differentiation. Interestingly, abrogation of CD40L stimulation both extinguished NF‐κB and STAT3 signaling during B cell differentiation. Our data show that even though BLIMP‐1 expression may be induced independently of termination of CD40L stimulation, both downregulation of the B cell identity TF PAX5 and upregulation of the ASC‐associated TF XBP‐1s are dependent on termination of CD40L stimulation and extinguishment the NF‐κB signaling pathway. Termination of CD40L stimulation during B cell differentiation is therefore a direct factor involved in B cell differentiation into ASCs.
Results
Termination of CD40L co‐stimulation inhibits B cell expansion while promoting differentiation
The effect of termination of CD40L co‐stimulation on human naive B cell proliferation and differentiation was investigated by activating naive human B cells via CD40L‐expressing 3T3 cells and IL‐21 and the addition of CD40L blocking antibodies at several points in time. When CD40L blocking antibodies were present before activation almost no proliferation occurred, showing that, in accordance with the literature [36], B cell proliferation is highly reliant on CD40L and IL‐21 stimulation (Fig. 1A; Fig. S1B). Proliferation was also significantly reduced when CD40L blocking antibodies were added at later time points during stimulation (Fig. 1A), showing that CD40L blocking antibodies efficiently inhibit CD40L‐induced proliferation even after initial stimulation. Termination of CD40L co‐stimulation at all timepoints also significantly reduced actual expansion of CD19+ cells in culture (Fig. 1B). These data demonstrate that termination of CD40L co‐stimulation inhibits proliferation and expansion of CD19+ naïve B cells even when CD40L stimulation is blocked at a later stage of the culture.
Figure 1.
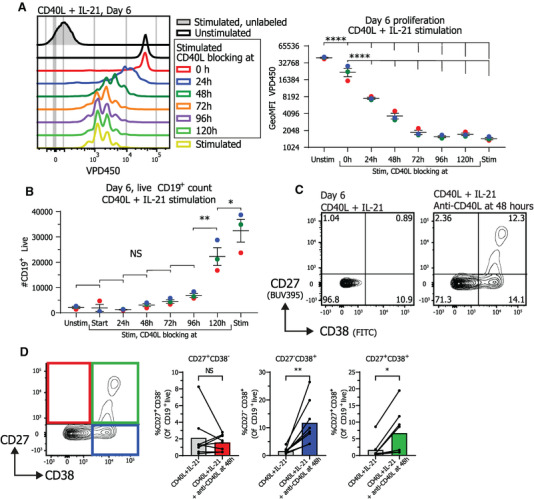
Termination of CD40L stimulation after CD40L and IL‐21 stimulation inhibits proliferation and promotes differentiation into ASCs Naïve B cells were sorted and 25.000 cells were stimulated with a human‐CD40L expressing 3T3 feeder layer and recombinant IL‐21 (50 ng/ml). After the indicated culture duration CD40L blocking antibodies (13 μg/ml) were added and cells were analyzed at day 6 by flow cytometry. (A) Naïve B cells were labeled with proliferation dye VPD450 prior to culture. Representative histogram overlay (left) and quantification of VPD450 GeoMFI after 6 days of culture under indicated conditions. Data from a single independent experiment with the mean of three individual donors replicates marked as red, green, or blue dots with triplicate measurements (n = 3). (B) Number of live CD19+ cells was analyzed at day 6 after adding CD40L blocking antibodies at indicated time points (n = 3). Data from a single independent experiment with the mean of three individual donors marked as red, green or blue dots with triplicate measurements (n = 3), scatter plots show mean ± SD, p‐values were calculated using one‐way ANOVA with Tukey's multiple comparison test. (C) Representative contour plot of CD27 and CD38 expression profiles after 6 days of culture with indicated conditions. (D) Quantification of the relative percentages of CD27 and CD38 subpopulations after 6 days of culture with the indicated conditions (n = 8). Combined data from 7 independent experiments, each performed with 1–2 donors. Each data point represents the mean of an individual donor with duplicate or triplicate measurements. Mean values are represented as bars. P‐values were calculated using paired t‐test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Further investigation of the effects of termination of CD40L stimulation on naive B cell differentiation showed that naive B cell differentiation into CD27+CD38+ ASC was prominently induced upon the termination of CD40L co‐stimulation after 2 to 4 days of culture, while ASC differentiation was nearly absent when stimulated continuously with CD40L and IL‐21 (Fig. 1C; Fig. S1C). In order to mimic the in vivo situation as close as possible, by aiming to terminate CD40L stimulation as early as possible after the initial stimulus, while maintaining sufficient numbers of CD19+ cells for differentiation analysis, CD40L stimulation was blocked after 48 h in the rest of the experiments. Termination of CD40L co‐stimulation after 48 h significantly increased differentiation into both CD38+ B cells and CD27+CD38+ ASCs at day 6 (Fig. 1D).
To further elucidate the effect of CD40L co‐stimulation and termination thereof in more detail, cultures were analyzed for proliferation, expansion, and differentiation every 24 h during continued or terminated CD40L stimulation. Unstimulated naïve B cells were analyzed as controls and did not proliferate or expand and were therefore not analyzed after the 72‐h time point in any further analyses (Fig. 2A). Termination of CD40L co‐stimulation after 48 h resulted in significantly reduced B cell expansion, compared to continued stimulation, after 120 h of culture (Fig. 2A). This observation is in line with the fact that proliferation stagnated at 96 h and later time points compared to continued stimulation (Fig. 2B). These data interestingly also demonstrate that B cells continue to proliferate in the first days after the termination of the CD40L stimulation.
Figure 2.
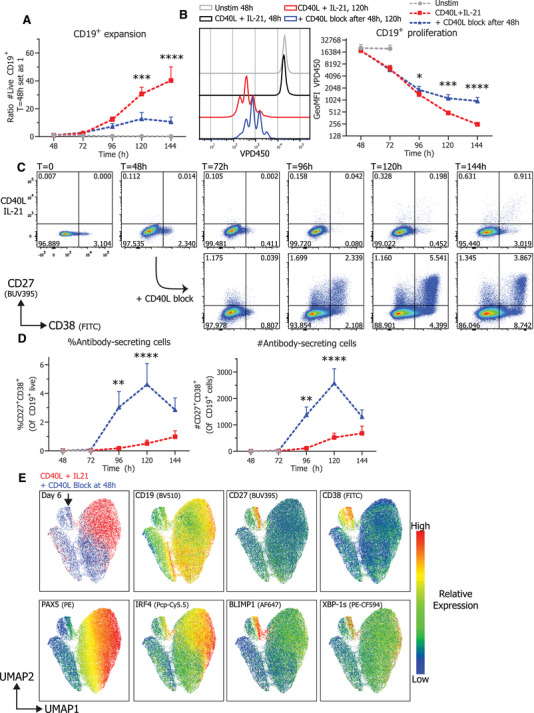
Termination of CD40L stimulation inhibits CD19+ expansion and proliferation and results in efficient ASC differentiation Naïve B cells were sorted, labeled with VPD450, and 25.000 cells were stimulated with a human‐CD40L expressing 3T3 feeder layer and recombinant IL‐21 (50 ng/ml). After 48 h, CD40L blocking antibodies (13 μg/ml) were added or not. At indicated timepoints, cultures were harvested, stained for membrane markers, fixed, and stored until day 6 when all samples were analyzed by flow cytometry. (A) Quantification of live CD19+ cells per day (n = 8) (B) Representative histogram overlay (left) and quantification (right) of VPD450 GeoMFI per day (n = 8). Combined data from 4 independent experiments, each performed with two donors. Symbols represent the mean and error bars show ± SEM. (C) Representative pseudocolor dot plots of CD27 and CD38 expression profiles per day under indicated conditions. (D) Quantification of relative percentages (left) and absolute counts (right) of CD27+CD38+ ASC per day (n = 6). Combined data from three independent experiments, each performed with two donors. Symbols represent the mean and error bars show ± SEM. p‐Values were calculated by comparing the red CD40L + IL‐21 condition and the blue CD40L stimulation terminated after 48 h condition, using two‐way ANOVA with Sidak's multiple comparison test. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. (E) UMAP clustering analysis on day 6 cultured B cells stained for CD19, CD27, CD38, PAX5, IRF4, BLIMP1, and XBP‐1s. The different conditions (red and blue) are shown overlaid at the top left, the ASC population is marked by a black arrow and relative protein expressions are represented as a heatmap. UMAP settings were as follows: distance function, Euclidean; number of neighbors, 30; minimal distance, 0.5; and number of components, 2. UMAP generated with data from a single representative donor measured in duplicate.
Additionally, the effect of CD40L blockade on the dynamics of CD27+CD38+ ASC differentiation was investigated. Remarkably, termination of CD40L co‐stimulation after 48 h significantly increased differentiation into CD27+CD38+ ASCs already within 48 h after CD40L stimulation was blocked (Fig. 2C and D). These data demonstrate that the B cell differentiation in CD40L terminated cultures is not only elevated but also that differentiation is quicker and already detectable at 96 h of culture and reaches a peak after 120 h. After this time point however, CD27+CD38+ ASCs numbers declined indicating that these differentiated cells do not survive in longer cultures. Utilizing Uniform Manifold Approximation and Projection (UMAP) analyses of expression of TFs regulating B cell identity and ASC differentiation revealed that termination of CD40L co‐stimulation strongly induced relatively high expression of ASC TFs IRF4, BLIMP1, and XBP‐1s and relatively low expression of B cell identity TF PAX5 in the CD27+CD38+ compartment (Fig. 2E, below the black arrow). Additionally, measuring secreted antibodies in culture supernatant demonstrated that termination of CD40L stimulation also promotes secretion of both IgM and IgG (Supporting Information 2A). Finally, isotype control antibodies were added to the culture next to a media control, demonstrating that the increased ASC differentiation is a result of the CD40L blocking antibodies (Supporting Information 2B). Altogether, these data clearly show that termination of CD40L co‐stimulation with continuous IL‐21 inhibits B cell proliferation and expansion while promoting rapid differentiation of stimulated B cells into CD27+CD38+ ASCs.
Termination of CD40L stimulation results in downregulation of active NF‐κB p65 and p‐STAT3 pathways
To elucidate in more detail how the termination of CD40L co‐stimulation leads to ASC differentiation intracellular (phospho) signaling pathways were investigated. Stimulation by CD40L is known to activate NF‐κB signaling and this subsequently leads to the survival and proliferation of B cells [15]. Additionally, IL‐21 is known to mainly induce phosphorylation of STAT3 that in turn is crucial for the induction of BLIMP1 [27]. Therefore, activated NF‐κB p65 and (phosphorylated) STAT3 by CD40L and IL‐21 stimulation (Fig. 3A) were analyzed over time during continued stimulation or in cultures with terminated CD40L co‐stimulation after 48 hours. Continued CD40L and IL‐21 stimulation induced high levels of activated NF‐κB p65 after 72 h, after which it decreased again (Fig. 3B). Termination of CD40L stimulation with continued IL‐21 stimulation however resulted in an immediate and clear inhibition of activated NF‐κB p65 induction (Fig. 3B). The activated NF‐κB p65 levels after CD40L termination declined to a level that is higher than the unstimulated condition, indicating that residual active NF‐κB p65 signaling remains after the termination of CD40L co‐stimulation. Partly this residual staining could be attributed to an increased cell size after stimulation (Fig. S3). Remarkably, termination of CD40L stimulation with continued IL‐21 stimulation also resulted in significant inhibition of both STAT3 and p‐STAT3 levels (Fig. 3C and D), indicating crosstalk between the CD40/NF‐κB and IL‐21R pathways. After termination of CD40L stimulation p‐STAT3/STAT3 signaling was not reduced to baseline levels, again indicating that a low level of signaling remains even without CD40L stimulation present. This could however not be attributed to the increased cell size as with the residual activated NF‐κB p65 and STAT3 expression (Fig. S3). Together these data show that termination of CD40L stimulation rapidly inhibits not only NF‐κB but also downmodulates STAT3 signaling.
Figure 3.
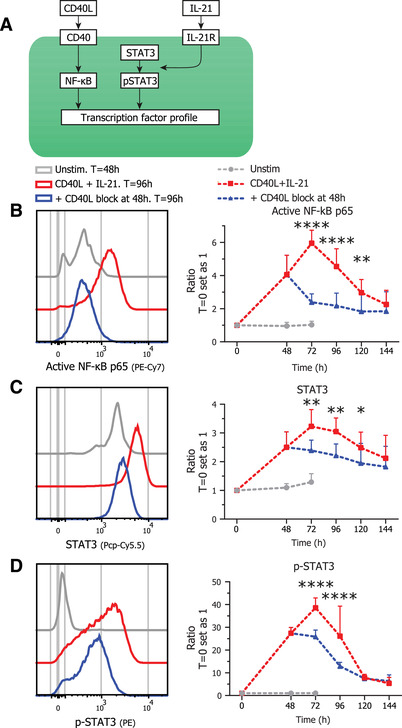
Termination of CD40L stimulation results in downregulation of NF‐kB and pSTAT3/STAT3 signaling Naïve B cells were sorted and 25.000 cells were stimulated with a human‐CD40L expressing 3T3 feeder layer and recombinant IL‐21 (50 ng/ml). After 48 hours CD40L blocking antibodies (13 ug/ml) were added or not. At indicated timepoints, cultures were harvested, stained for membrane markers, fixed, and stored until day 6 when all samples were stained intracellularly and analyzed by flow cytometry. (A) Schematic representation of CD40L and IL‐21 signaling pathways. (B) Representative histogram overlay (left) and quantification (right) of activated NF‐κB p65 GeoMFI per day under indicated conditions (n = 4). (C) Representative histogram overlay (left) and quantification (right) of STAT3 GeoMFI per day under indicated conditions (n = 4). (D) Representative histogram overlay (left) and quantification (right) of p‐STAT3 GeoMFI per day under indicated conditions (n = 4). p‐Values were calculated by comparing the red CD40L + IL‐21 condition and the blue CD40L stimulation terminated after 48 h condition, using two‐way ANOVA with Sidak's multiple comparison test Combined data from two independent experiments, each performed with two donors. Symbols represent the mean and error bars show ± SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Termination of CD40L stimulation downregulates C‐MYC and promotes the ASC transcriptional program
B cell to ASC differentiation is tightly regulated by a complex network of multiple inducible transcriptional regulators, as summarized in Fig. 4A [11]. Analysis of these transcriptional regulators during CD40L stimulation and termination shows that CD40L and IL‐21 stimulation induced increased IRF4 expression (Fig. 4B). Interestingly, while termination of CD40L co‐stimulation does reduce expression of IRF4 significantly, a high IRF4 expressing population remained at 96 hours of culture (Fig. 4B, left) coinciding with the generation of CD27+CD38+ ASCs (Fig. 2D). Second, the regulator of GC maintenance and B cell proliferation C‐MYC was rapidly induced upon CD40L and IL‐21 stimulation in CD19+ cells. Similarly to activated NF‐κB p65, C‐MYC expression was significantly inhibited after termination of CD40L stimulation (Fig. 3B and 4C). CD40L and IL‐21 stimulation steadily induced PAX5 expression over the course of the culture (Fig. 4D). Termination of CD40L co‐stimulation first stabilized expression of PAX5 until 96 h of culture, after which PAX5 expression was significantly decreased. Strikingly, the downregulation of PAX5 occurred after the downregulation of IRF4, indicating that these two TFs are regulated differently upon the termination of CD40L stimulation. In addition, termination of CD40L stimulation with continued IL‐21 stimulation resulted in a prominent increase in the percentage of BLIMP1High expressing cells (Fig. 4E, middle). This increase in BLIMPHigh expressing cells already occurred after 72 hours of culture, preceding the downregulation of PAX5 and the generation of CD27+CD38+ ASCs (Fig. 2D). However, even though differentiation into CD27+CD38+ ASCs in both percentages and absolute counts is significantly higher in the CD40L blocked condition (Fig. 2D) this is not the case for the absolute counts of BLIMP1High expressing cells (Fig. 4E, right) indicating that high BLIMP1 expression alone is not sufficient for ASC differentiation. Altogether these data demonstrate that termination of CD40L co‐stimulation reduces proliferation and expression of C‐MYC while subsequently PAX5 expression is downregulated and B cell differentiation into ASCs is enhanced. However, as high levels of IRF4 are found in both CD40L stimulated and terminated conditions and that absolute numbers of BLIMP1High expressing cells are the same for both conditions, we next investigated these high BLIMP1 expressing cells in more detail as these are expected to differentiate into ASCs.
Figure 4.
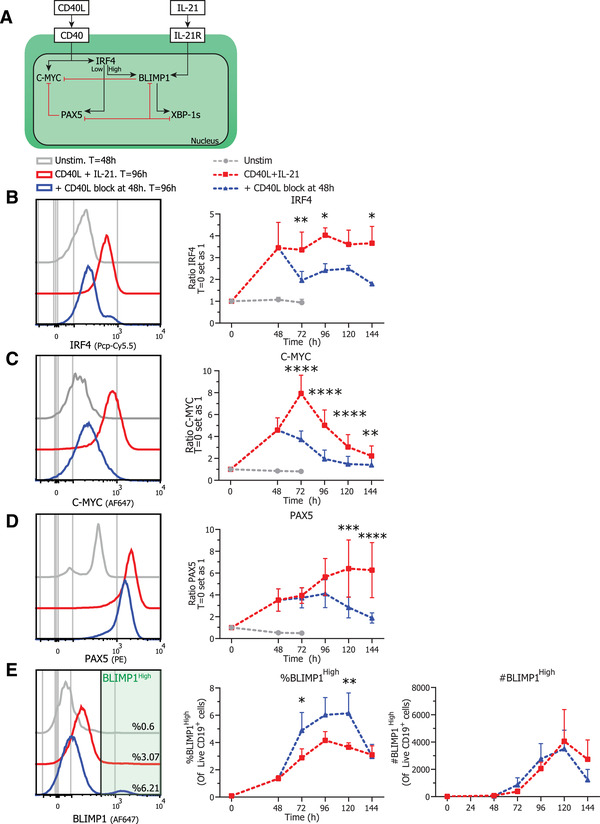
Termination of CD40L stimulation inhibits expression of C‐MYC, IRF4 and PAX5 Naïve B cells were sorted and 25.000 cells were stimulated with a human‐CD40L expressing 3T3 feeder layer and recombinant IL‐21 (50 ng/ml). After 48 h, CD40L blocking antibodies (13 μg/ml) were added or not. At indicated timepoints, cultures were harvested, stained for membrane markers, fixed, and stored until day 6 when all samples were stained for TFs and analyzed by flow cytometry. (A) Schematic representation of CD40L and IL‐21 induced TF network. CD40L stimulation induces expression of IRF4, PAX5, and proliferation regulator C‐MYC while IL‐21 induces expression of BLIMP1 that is the crucial regulator of ASC differentiation. BLIMP1 expression inhibits and is inhibited by PAX5. Depending on the level of IRF4 expression either PAX5 or BLIMP1 expression is promoted. Additionally, TF XBP‐1s is repressed by PAX5 and promoted by BLIMP1. While B cells are known to express high levels of PAX5, ASCs express high levels of IRF4, BLIMP1, and XBP‐1s. (B) Representative histogram overlay (left) and quantification (right) of C‐MYC GeoMFI per day under indicated conditions (n = 4). (C) Representative histogram overlay (left) and quantification (right) of IRF4 GeoMFI per day under indicated conditions (n = 4). Combined data from 2 independent experiments, both performed with two donors. Symbols represent the mean and error bars show ±SEM. (D) Representative histogram overlay (left) and quantification (right) of PAX5 GeoMFI per day under indicated conditions (n = 6). (E) Representative histogram overlay (left), relative percentages (middle) and absolute counts (right) of BLIMP1High expressing cells under indicated conditions (n = 6). Combined data from three independent experiments, each performed with two donors. Symbols represent the mean and error bars show ±SEM. p‐Values were calculated by comparing the red CD40L + IL‐21 condition and the blue CD40L stimulation terminated after 48 h condition, using two‐way ANOVA with Sidak's multiple comparison test. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
The phenotype of the BLIMP1High cells was analyzed to determine the CD27+CD38+ ASCs within this population (Fig. 5A, left). This clearly demonstrates that termination of CD40L stimulation results in significantly more differentiation of BLIMP1High cells into CD27+CD38+ ASCs (Fig. 5A, right). In addition, differentiation into CD27+CD38+ cells was preceded by significantly increased differentiation into CD27+CD38− cells after 72 h of culture (Fig. S4). Next to increased differentiation, significantly more XBP‐1sHigh expressing cells were found in BLIMP1High cells when CD40L stimulation was terminated. Increased secretion of antibodies (Supporting Information 2) in CD40L terminated cultures was observed, in line with the function of XBP‐1s as a crucial regulator of the UPR that is required for the efficient production of antibodies. Next, analysis of expression of IRF4 and PAX5 within CD19+ and BLIMP1High expressing cells (Fig. 5C) revealed that induction of high levels of IRF4 is similar in continuous CD40L and IL‐21 stimulated and CD40L terminated conditions within BLIMP1High expressing cells (Fig. 5D). However, a negative regulator of ASC differentiation PAX5 is prominently downregulated in BLIMP1High expressing cells when CD40L co‐stimulation is terminated (Fig. 5C and E). These data demonstrate that termination of CD40L stimulation with continued IL‐21 stimulation reduced PAX5 expression in BLIMP1High expressing cells that maintained high expression of IRF4 and increased XBP‐1s expression, ultimately leading to the inhibition of the B cell program and promotion of a true ASC differentiation program resulting in the efficient generation of CD27+CD38+ ASCs.
Figure 5.
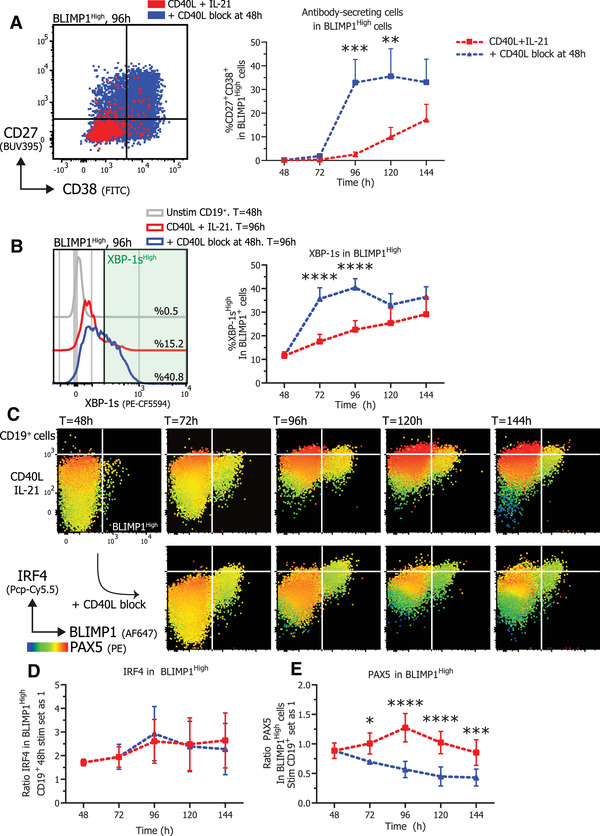
Termination of CD40L stimulation promotes differentiation of BLIMP1High B cells that express XBP‐1s while inhibiting PAX5 expression. Naïve B cells were sorted and 25.000 cells were stimulated with a human‐CD40L expressing 3T3 feeder layer and recombinant IL‐21 (50 ng/ml). After 48 h, CD40L blocking antibodies (13 ug/ml) were added or not. At indicated timepoints, cultures were harvested, stained for membrane markers, fixed, and stored until day 6 when all samples were stained for TFs and analyzed by flow cytometry. (A) Representative overlay of CD27 and CD38 expression profiles (left) of BLIMPHigh gated cells under indicated conditions after 96 h of culture and quantification (right) of the relative percentages of CD27+CD38+ ASCs within BLIMP1High gated cells per day (n = 6). (B) Representative histogram overlay (left) and quantification (right) of the relative percentages of XBP‐1sHigh expressing cells within BLIMP1High gated cells per day (n = 6). (C) Representative heatmap dot plots of IRF4, BLIMP1, and PAX5 expression profiles per day under indicated conditions gated on CD19+ cells. (D) Quantification of IRF4 GeoMFI per day under indicated conditions in BLIMP1High expressing cells (n = 4). (E) Quantification of PAX5 GeoMFI per day under indicated conditions in BLIMP1High expressing cells (n = 6). Combined data from two or three independent experiments, each performed with two donors. Symbols represent the mean and error bars show ±SEM. p‐Values were calculated by comparing the red CD40L + IL‐21 condition and the blue CD40L stimulation terminated after 48 h condition, using two‐way ANOVA with Sidak's multiple comparison test. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001
Discussion
During T‐cell dependent B cell responses, naïve B cells require stimulation from TFH cells in the form of CD40L and IL‐21 and this stimulation has often been mimicked in vitro [33, 34, 35, 36]. However, a key limitation in in vitro studies is that the duration of stimulation has never been investigated. It has been shown that the T‐B cell interactions are short‐lived and B cells actively move away from T cell stimulation [12, 39, 40] and thus we hypothesized that the termination of T cell stimulation contributes to B cell differentiation. In this study, we show that after CD40L and IL‐21 stimulation, termination of CD40L stimulation promotes efficient and rapid differentiation of human naïve B cells into ASCs in vitro. These data are of importance as in mice, CD40L termination did not result in more ASC formation [41]. This discrepancy could also be explained by the differences in the experimental setup, rather than being a difference in human‐versus‐mouse B cell responses, and requires additional investigation. Here, by using in‐depth analysis of intracellular signaling pathways and TF expression profiles [38] during culture we assessed the mechanism underlying the increased ASC differentiation after CD40L stimulation termination of human differentiating naïve B cells. These findings are summarized in Figure 6 where our observed expression profiles are depicted in a model in which direct connections between signaling pathways and transcription factors are depicted based on extensive literature [11, 17, 42].
Figure 6.
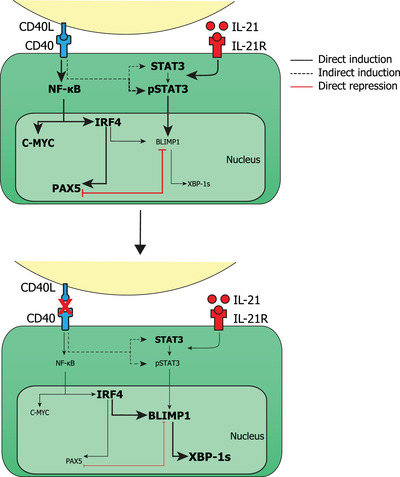
Schematic overview of CD40L stimulated and CD40L stimulation terminated B cells with phospho‐signaling and transcription factor profiles. Stimulation of CD40 by CD40L activates the NF‐κB pathway which in turn upregulates the expression of C‐MYC and IRF4. IL‐21 stimulation results in the phosphorylation of STAT3 which in turn can upregulate the expression of BLIMP1. Continuous CD40L stimulation results in an increased expression of NF‐κB, C‐MYC, IRF4, and PAX5 while preventing differentiation into ASCs. Upon termination of CD40L stimulation, NF‐κB, C‐MYC, and PAX5 expression is downregulated while expression of IRF4, BLIMP1, and XBP‐1s is increased, resulting in efficient ASC differentiation.
As shown before [14, 15, 16], CD40L and IL‐21 induced high levels of NF‐κB and p‐STAT3 signaling. Analysis of NF‐kB p65 and pSTAT3/STAT3 pathways demonstrated that release of CD40L co‐stimulation not only downregulates NF‐κB but also pSTAT3/STAT3 signaling, indicating a link between CD40 and STAT3 pathways as there was continued IL‐21 stimulation present in the culture (Fig. 6, dashed line). One possible explanation is that CD40L co‐stimulation promotes the expression of IL‐21R [1], the release of CD40L stimulation could subsequently lead to downregulation of IL‐21R and thus downregulation STAT3 phosphorylation. As CD40L stimulation induces expression of PAX5 [31] and, through NF‐kB induction, C‐MYC [19], it was to be expected that both PAX5 and C‐MYC are downregulated upon release of CD40L stimulation. As C‐MYC drives a proliferative state instead of a differentiative state, downregulation of C‐MYC is required for terminal differentiation to occur [43]. This alone is not sufficient however as PAX5 must also be downregulated in order to reach a true ASC transcriptional profile [42, 44–46]. Interestingly it has also been shown that during B cell development, C‐MYC expression is able to regulate PAX5 expression indirectly [47]. Although these separate findings are in agreement with the data presented here, we are the first to show that termination of CD40L co‐stimulation instigates first the required downregulation of C‐MYC and subsequently PAX5. Furthermore, a higher proportion of BLIMP1High‐expressing cells was found in CD40L stimulation terminated cultures. However, as there was no increase in the absolute count of BLIMP1High expressing cells this warranted further investigation. Analysis into BLIMP1High cells showed that upon the termination of CD40L co‐stimulation IRF4 expression was maintained, while PAX5 was downmodulated. In line with these findings, termination of CD40 signaling efficiently increased the differentiation of these cells into CD27+CD38+ ASCs. Actual antibody secretion was observed, in line with increased expression of XBP‐1s (Fig. 6, below). Together these data demonstrate that termination of CD40L stimulation with continued IL‐21 stimulation induces a true ASC transcriptional profile, explaining the increased generation of both ASCs and Igs in these cultures. Furthermore, BLIMP1 and PAX5 are known to be mutually inhibitory and as a consequence, it is often assumed that upregulation of BLIMP1 is always accompanied by downregulation of PAX5 and that upregulation of BLIMP1 is leading the induction of ASC differentiation. Our data show that cells that do not exhibit an CD27+CD38+ ASC phenotype, and are still in the B cell state, can show upregulation of BLIMP1. In this case, however, they still express PAX5. Only upregulation of BLIMP1 with concomitant downregulation of PAX5 leads to the CD27+CD38+ ASC phenotype with accompanying elevated expression of XBP‐1s. Although cyclic migration of B cells to and away from TFH cell stimulation is a hallmark of in vivo GC reactions [10, 12, 39, 40], the importance of termination of T cell stimulation has always been overlooked in in vitro B cell cultures. We show efficient ASC differentiation in this culture where naïve B cells only receive and are released from CD40L stimulation once. Potentially, ASC differentiation can be further increased by introducing multiple rounds of stimulation, and termination thereof, as in vivo this cyclic stimulation also occurs multiple times [10]. Indeed, our previous work demonstrated that secondary CD40L and IL‐21 stimulation induces downregulation of B cell identity gene PAX5 and thus promotes ASC differentiation [36]. Additionally, in vivo T‐B cell interactions only last for a few minutes and can occur multiple times over several hours [12, 39]. Thus, future in vitro cultures should allow for dynamic control of B cell stimulation in order to investigate how specific timing and duration of stimulation impact the formation of ASCs. Our data already demonstrates that prolonged stimulation promotes proliferation of B cells while inhibiting ASC differentiation and others have shown that in mice, constitutive CD40L stimulation in fact promotes ASC differentiation [41] while the transient release of T‐cell help promotes GC B cell transition [48]. In addition, prolonged (IL‐4) stimulation in human B cells impairs differentiation [37]. These findings clearly show that dynamic B cell stimulation and termination of stimulation are key in controlling B cell fate decision. Finally, several groups have sought to quantitatively model signal integration in order to interrogate what transcriptional thresholds are in place that control B cell differentiation [49, 50, 51] and several descriptive models have been proposed that explain B cell fate decision‐making [52]. Each model still requires a deeper investigation into underlying mechanisms of how signaling integration results in crossing a transcriptional threshold in order to undergo ASC differentiation. In the future, dynamically controlled in vitro systems can contribute to these models and will improve our understanding of the temporal requirements for B cell to ASC differentiation. Altogether, the literature and data presented here demonstrate that the next step for in vitro human B cell differentiation studies should be the generation of an in vitro system that allows for transient and controlled B cell stimulation, in combination with secondary or even cyclic stimulation in order to mimic in vivo TFH stimulation dynamics as closely as possible. This system could lead to even more B cell to ASC differentiation and that system could then be interrogated to determine what temporal factors drive this differentiation. This will ultimately result in a better understanding of how desired B cell responses can be promoted and how undesired responses, such as auto‐ and allo‐immunity [53, 54, 55, 56], can be inhibited.
The generation of ASC from naïve B cells by TFH cell stimulation remains incompletely understood and insights in the mechanisms underlying this response are highly required as secreted antibodies form a crucial layer of immune protection. The data presented here show, for the first time, that termination of the initially essential CD40L stimulation is required in order to downregulate signaling and proliferation and subsequently reach a true ASC transcription factor profile where termination specifically lowers PAX5 and C‐MYC, in a background where IRF4, BLIMP1, and XBP‐1s are highly induced and maintained by initial CD40 signaling. These insights will aid future research on T‐dependent B cell responses both for improving vaccination strategies and treating B cell mediated diseases.
Materials and methods
Cell lines
NIH3T3 fibroblasts expressing human CD40L (3T3‐CD40L+) [57] were cultured in IMDM (Lonza) containing 10% FCS (Serana), 100 U/ml penicillin (Invitrogen), 100 μg/ml streptomycin (Invitrogen), 2mM L‐glutamine (Invitrogen), 50 μM β‐mercaptoethanol (Sigma Aldrich) and 500 μg/ml G418 (Life Technologies).
Isolation of B Cells from Human Healthy Donors
Buffy coats were collected from voluntary, non‐remunerated, adult healthy blood donors (Sanquin Blood Supply, Amsterdam, the Netherlands), who provided written informed consent for the use of remainders of their donation for research as part of routine donor selection and blood collection procedures. Peripheral blood mononucleated cells (PBMCs) were isolated from buffy coats using a Lymphoprep (Axis‐Shield PoC AS, Dundee, Scotland) density gradient. Afterward, CD19+ B‐cells were separated using magnetic anti‐CD19 Dynabeads and DETACHaBEAD (Invitrogen, Carlsbad, CA, USA) according to manufacturer's instructions with purity > 99%.
In vitro B cell stimulation cultures
B cell cultures were carried out as described before [36] with a few adjustments. In short, 3T3 ‐CD40L+ were harvested, irradiated with 30 Gy and seeded in B cell medium (RPMI 1640 (Gibco) without phenol red containing 5% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l‐glutamine, 50 μM β‐mercaptoethanol, and 20 μg/ml human apo‐transferrin (Sigma–Aldrich; depleted for human IgG with protein G sepharose (GE Healthcare)) on 96‐well flat‐bottom plates (Nunc) to allow adherence overnight. 3T3 ‐CD40L+ were seeded at 10.000 cells per 96‐well. The next day, CD19+ B cells were thawed after cryopreservation and CD19+CD27−IgG− naive B cells were sorted on a FACSAria II. 2.5 × 104 naive B cells were cultured on the irradiated CD40L‐expressing 3T3 fibroblasts in the presence of IL‐21 (50 ng/mL; Peprotech, London W6 8LL, UK) for 6 days. After 48 h or at indicated timepoints either medium or anti‐CD40L clone 5C8 antibodies (13 μg/ml) were added to the cultures.
Violet Proliferation Dye 450 labeling
B cells were labeled according to manufacturer's instructions. In short, sorted B cells were washed with 10 ml PBS twice and resuspended to a concentration of 2 × 107 cells/ml in PBS. Cells and 4 μM Violet Proliferation Dye 450 (VPD450, BD Biosciences) in PBS were mixed at a 1:1 ratio and incubated 15 min in a 37°C water bath in the dark, vortexing the tube every 5 min to ensure uniform staining. Cells were washed twice using a 10 times volume of cold culture medium to end labeling. Thereafter, B cells were cultured according to the protocol described above.
Phosphoflow analysis
Flow cytometric samples were prepared as described previously [38] with a few adjustments. In short, wells were harvested at indicated time points and multiple culture wells were pooled, up to a full 96‐well plate per replicate, in 96‐well V‐bottom plates. After harvest cells were kept on ice or at 4°C at all times. Cells were washed with ice‐cold PBS/0.1% bovine serum albumin (BSA; Sigma–Aldrich), centrifuged, and pooled. Samples were stained in with LIVE/DEAD Fixable Near‐IR Dead cell stain kit (Invitrogen) and anti‐CD19 BV510 (BD) and anti‐CD38 FITC (Beckman Coulter) diluted in ice‐cold PBS/0.1% BSA for 15 min on ice. Samples were washed once with ice‐cold PBS/0.1% BSA, centrifuged, and fixed with 37°C 4% paraformaldehyde (PFA; Sigma) for 10 min at 37°C. After fixation, samples were centrifuged, washed once with PBS/0.1% BSA, and permeabilized with 90% methanol from a −20°C freezer. Samples were incubated for at least 30 min or stored at −20°C till the day of FACS analysis. After permeabilization, samples were centrifuged followed by two consecutive washes with PBS/0.1% BSA. Samples were then stained with anti‐CD27 BUV395 (BD), anti‐NF‐κB p65 (pS529) PE‐Cy7 (BD), anti‐STAT3 Percp‐Cy5.5 (BD), anti‐p‐STAT3 PE (BD), and anti‐C‐MYC AF647 (CST) diluted in PBS/0.1% BSA. Samples were incubated for 30 minutes on a plate shaker at room temperature. Samples were washed and resuspended in PBS/0.1%BSA before measuring on a BD FACSymphony A5 machine and analyzed using FlowJo Software v10.8. (Treestar). Cells were pre‐gated on live cells, lymphocytes, two single cells gates, and live CD19+ gate unless stated otherwise (Fig. S1A). We adhered to the ‘Guidelines for the use of flow cytometry and cell sorting in immunological studies’ [58].
Transcription factor flow analysis
Flow cytometric samples were prepared as described previously[38] with a few adjustments. In short, wells were harvested at indicated time points and multiple culture wells were pooled, up to 20 wells per replicate, in 96‐well V‐bottom plates. After harvest cells were kept on ice or at 4°C at all times. Samples were centrifuged and stained with 25 μl staining mix LIVE/DEAD Fixable Near‐IR Dead cell stain kit, anti‐CD19, anti‐CD27, and anti‐CD38 antibodies, and incubated for 15 min at 4°C. Samples were washed with ice‐cold PBS/0.1% and centrifuged. Samples were then fixated with 100 μl Foxp3 fixation buffer (eBioscience) for 30 minutes at 4°C. Next, Foxp3 permeabilization buffer (eBioscience) was added, samples were centrifuged and stored in PBS/0.1%BSA at 4°C till the day of FACS analysis. After permeabilization or storage, samples were centrifuged and washed with Foxp3 permeabilization buffer. After removing the supernatant, samples were stained with anti‐PAX5 PE, anti‐IRF4 PercpCy5.5, anti‐BLIMP1 AF647, and anti‐XBP‐1s PE‐CF594 diluted in Foxp3 permeabilization buffer and incubated for 30 min in the fridge. Samples were washed with Foxp3 permeabilization buffer and resuspended in PBS/0.1%BSA before measuring on a BD FACSymphony A5 machine and analyzed using FlowJo Software v10.8. (Treestar). Cells were pre‐gated on live cells, lymphocytes, two single cells gates, and live CD19+ gate unless stated otherwise (Fig. S1A). We adhered to the ‘Guidelines for the use of flow cytometry and cell sorting in immunological studies’ [58].
Multimarker analysis using UMAP
After gating for live CD19+ B cells, samples were randomly down‐sampled to 25.000 events and subsequently stimulated and CD40L stimulation terminated samples were concatenated into a single 50.000 event FCS file using the DownSample plugin in FlowJo v10.8.0. Next, the concatenated sample was analyzed using the Uniform Manifold Approximation and Projection (UMAP) plugin v3.1 in FlowJo v10.8.0. UMAP is a machine learning algorithm used for dimensionality reduction to visualize high parameter datasets in a two‐dimensional space. UMAP plugin settings were as followed: Distance function Euclidean; number of neighbors 30; minimal distance 0.5 and number of components 2. The UMAP dot plot generated can be manipulated as a standard dot plot and allows for multiple parameter and heatmap overlays in the FlowJo layout‐editor. The DownSample and UMAP FlowJo plugins can be found on the FlowJo Exchange website.
IgM and IgG ELISA of Culture Supernatants
IgM and IgG levels in supernatants were measured as previously described [59]. In short, plates were coated with monoclonal anti‐IgM or anti‐IgG (2 μg/mL; clone MH15‐1 and MH16‐1, respectively; Sanquin Reagents, Amsterdam, the Netherlands) and for detection, HRP‐conjugated mouse‐anti‐human‐IgM or mouse‐anti‐human‐ IgG (1 μg/mL in HPE; clone MH15‐1 and MH16‐1, respectively; Sanquin Reagents, Amsterdam, the Netherlands) were used. The ELISA was developed with 100 μg/mL tetramethylbenzidine in 0.11 M/L sodium acetate (pH 5.5) containing 0.003% (v/v) H2O2. The reaction was stopped with 2 M H2SO4. Absorption at 450 and 540 nm was measured with a Synergy 2 microplate reader (Biotek, Winooski, VT, USA). Results were related to a titration curve of a serum pool in each plate. The lower‐limit detection levels of IgM and IgG ELISA were 3 ng/mL and 2.8 ng/mL, respectively.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 8; GraphPad Software). Data were analyzed using t‐tests, Repeated Measures one‐way ANOVA with Tukey's multiple comparison test, Repeated Measures two‐way ANOVA with Sidak's multiple comparison test, or mixed‐effects analysis with Dunnett's multiple comparison test where appropriate. Results were considered significant at p < 0.05. Significance was depicted as *(p < 0.05) or **(p < 0.01), ***(p < 0.001) or ****(p < 0.0001).
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Author contributions
C.M., NJM.V., A.t.B., and S.M.v.H. designed the research. C.M., M.S., and T.J. performed the research. C.M. analyzed the data. L.B. provided reagents. All authors critically reviewed the manuscript, gave final approval of the version to be published and agreed to be accountable for all aspects of the work ensuring that questions related to the accuracy or intergrity of any part of the work are appropriately investigated and resolved.
Ethics approval statement
Buffy coats were obtained from anonymized healthy adult donors with written informed consent in accordance to the guidelines established by the Sanquin Medical Ethical Committee and in line with the Declaration of Helsinki.
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.202249972.
Abbreviations
- ASC
antibody‐secreting cell
- CSR
class‐switch recombination
- SHM
somatic hyper‐mutation
- TFH
follicular T helper cells
Supporting information
Supporting Information
Acknowledgments
The authors thank Simon Tol, Erik Mul, Mark Hoogenboezem, and Tom Ebbes of the Sanquin central facility for cell sorting on the FACS AriaIII and maintenance and calibration of FACS machines. We thank Gijs van Schijndel and Ninotska Derksen for their help with ELISA assays. This project was funded by the Landsteiner Foundation for Blood Transfusion Research, project grant number: LSBR 1609 and Sanquin Product and Process Development Call 2020.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Ding, B. B. , Bi, E. , Chen, H. , Yu, J. J. and Ye, B. H. , IL‐21 and CD40L Synergistically Promote Plasma Cell Differentiation through Upregulation of Blimp‐1 in Human B Cells. The Journal of Immunology. 2013. 190: 1827–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moens, L. and Tangye, S. G. , Cytokine‐mediated regulation of plasma cell generation: IL‐21 takes center stage. Frontiers in Immunology. 2014. 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinstein, J. S. , Herman, E. I. , Lainez, B. , Licona‐Limón, P., Esplugues, E. , Flavell, R. and Craft, J. , TFH cells progressively differentiate to regulate the germinal center response. Nature Immunology. 2016. 17: 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weisel, F. J. , Zuccarino‐Catania, G.V. , Chikina, M. and Shlomchik, M. J. , A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity. 2016. 44: 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McHeyzer‐Williams, L. J. , Pelletier, N. , Mark, L. , Fazilleau, N. and McHeyzer‐Williams, M. G. , Follicular helper T cells as cognate regulators of B cell immunity. Current Opinion in Immunology. 2009. 21: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Upadhyay, M. , Priya, G. K. , Ramesh, P. , Madhavi, M. B. , Rath, S. , Bal, V. , George, A. et al., CD40 signaling drives B lymphocytes into an intermediate memory‐like state, poised between naïve and plasma cells. J Cell Physiol. 2014. 229: 1387–1396. [DOI] [PubMed] [Google Scholar]
- 7. Luo, W. , Weisel, F. and Shlomchik, M. J. , B Cell Receptor and CD40 Signaling Are Rewired for synergistic induction of the c‐Myc transcription factor in germinal center B cells. Immunity. 2018. 48: 313–326.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avery, D. T. , Ma, C. S. , Bryant, V. L. , Santner‐Nanan, B. , Nanan, R. , Wong, M. , Fulcher, D. A. et al., STAT3 is required for IL‐21 induced secretion of IgE from human naive B cells. Blood. 2008. 112: 1784–1793. [DOI] [PubMed] [Google Scholar]
- 9. Avery, D. T. , Deenick, E. K. , Ma, C. S. , Suryani, S. , Simpson, N. , Chew, G. Y. , Chan, T. D. et al., B cell–intrinsic signaling through IL‐21 receptor and STAT3 is required for establishing long‐lived antibody responses in humans. The Journal of Experimental Medicine. 2010. 207: 155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mesin, L. , Ersching, J. and Victora, G. D. , Germinal Center B Cell Dynamics. Immunity. 2016. 45: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verstegen, N. J. M. , Ubels, V. , Westerhoff, H V. , van Ham, S. M. and Barberis, M. , System‐Level Scenarios for the Elucidation of T Cell‐Mediated Germinal Center B Cell Differentiation. Frontiers in Immunology. 2021. 12: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shulman, Z. , Gitlin, A. D. , Weinstein, J. S. , Lainez, B. A. , Esplugues, E. , Flavell, R. A. , Craft, J. E. , et al., Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science (1979). 2014. 345: 1058–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Victora, G. D. and Nussenzweig, M. C. , Germinal Centers. 10.1146/annurev-immunol-020711-075032 [DOI]
- 14. Craxton, A. , Shu, G. , Graves, J. D. , Saklatvala, J. , Krebs, E. G. and Clark, E. A. , p38 MAPK is required for CD40‐induced gene expression and proliferation in B lymphocytes. J Immunol. 1998. 161: 3225–3236. [PubMed] [Google Scholar]
- 15. Chen, D. , Ireland, S. J. , Remington, G. , Alvarez, E. , Racke, M. K. , Greenberg, B. , Frohman, E. M. et al., CD40‐Mediated NF‐κB activation in B Cells Is increased in multiple sclerosis and modulated by therapeutics. The Journal of Immunology. 2016. 197: 4257–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deenick, E. K. , Avery, D. T. , Chan, A. , Berglund, L. J. , Ives, M. L. , Moens, L. , Stoddard, J. L. et al., Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody‐secreting plasma cells. J Exp Med. 2013. 210: 2739–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nutt, S. L. , Hodgkin, P. D. , Tarlinton, D. M. and Corcoran, L. M. , The generation of antibody‐secreting plasma cells. Nat Rev Immunol. 2015. 15: 160–171. [DOI] [PubMed] [Google Scholar]
- 18. Merluzzi, S. , Moretti, M. , Altamura, S. , Zwollo, P. , Sigvardsson, M. , Vitale, G. and Pucillo, C. , CD40 stimulation induces Pax5/BSAP and EBF activation through a APE/Ref‐1‐dependent redox mechanism. J Biol Chem. 2004. 279: 1777–1786. [DOI] [PubMed] [Google Scholar]
- 19. Grumont, R. J. , Strasser, A. and Gerondakis, S. , B cell growth is controlled by phosphatidylinosotol 3‐kinase‐dependent induction of Rel/NF‐kappaB regulated c‐myc transcription. Mol Cell. 2002. 10: 1283–1294. [DOI] [PubMed] [Google Scholar]
- 20. Dominguez‐Sola, D. , Victora, G. D. , Ying, C. Y. , Phan, R. T. , Saito, M. , Nussenzweig, M. C. and Dalla‐Favera, R. , c‐MYC is required for germinal center selection and cyclic re‐entry HHS public access. Nat Immunol. 2012. 13: 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Finkin, S. , Hartweger, H. , Oliveira, T. Y. , Kara, E. E. and Nussenzweig, M. C. , Protein amounts of the MYC Transcription Factor Determine Germinal Center B Cell Division Capacity. Immunity. 2019. 51: 324–336.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grumont, R. J. and Gerondakis, S. , Rel induces interferon regulatory factor 4 (IRF‐4) expression in lymphocytes: modulation of interferon‐regulated gene expression by rel/nuclear factor kappaB. J Exp Med. 2000. 191: 1281–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sciammas, R. , Shaffer, A. L. , Schatz, J. H. , Zhao, H. , Staudt, L. M. and Singh, H. , Graded Expression of Interferon Regulatory Factor‐4 Coordinates Isotype Switching with Plasma Cell Differentiation. Immunity. 2006. 25: 225–236. [DOI] [PubMed] [Google Scholar]
- 24. Fairfax, K. A. , Kallies, A. , Nutt, S. L. and Tarlinton, D. M. , Plasma cell development: From B‐cell subsets to long‐term survival niches. Seminars in Immunology. 2008. 20: 49–58. [DOI] [PubMed] [Google Scholar]
- 25. Méndez, A. and Mendoza, L. , A Network Model to Describe the Terminal Differentiation of B Cells. PLoS Computational Biology. 2016. 12: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scheeren, F. A. , Naspetti, M. , Diehl, S. , Schotte, R. , Nagasawa, M. , Wijnands, E. , Gimeno, R. et al., STAT5 regulates the self‐renewal capacity and differentiation of human memory B cells and controls Bcl‐6 expression. Nature Immunology. 2005. 6: 303–313. [DOI] [PubMed] [Google Scholar]
- 27. Kwon, H. , Thierry‐Mieg, D. , Thierry‐Mieg, J. , Kim, H. ‐. P. , Oh, J. , Tunyaplin, C. , Carotta, S. et al., Analysis of Interleukin‐21‐Induced Prdm1 Gene Regulation Reveals Functional Cooperation of STAT3 and IRF4 Transcription Factors. Immunity. 2009. 31: 941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tellier, J. , Shi, W. , Minnich, M. , Liao, Y. , Crawford, S. , Smyth, G. K. , Kallies, A. et al., Blimp‐1 controls plasma cell function through the regulation of immunoglobulin secretion and the unfolded protein response. Nat Immunol. 2016. 17: 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chung, E. Y. , Psathas, J. N. , Yu, D. , Li, Y. , Weiss, M. J. and Thomas‐Tikhonenko, A. , CD19 is a major B cell receptor‐independent activator of MYC‐driven B‐lymphomagenesis. J Clin Invest. 2012. 122: 2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Somasundaram, R. , Jensen, C. T. , Tingvall‐Gustafsson, J. , Ã…Hsberg, J. , Okuyama, K. , Prasad, M. , Hagman, J. R. , Wang, X. , Soneji, S. et al., EBF1 and PAX5 control pro‐B cell expansion via opposing regulation of the Myc gene. Blood. 2021. 137: 3037–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reimold, A. M. , Ponath, P. D. , Li, Y. S. , Hardy, R. R. , David, C. S. , Strominger, J. L. , Glimcher, L. H. et al., Transcription factor B cell lineage‐specific activator protein regulates the gene for human X‐box binding protein 1. Journal of Experimental Medicine. 1996. 183: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ochiai, K. , Maienschein‐Cline, M. , Simonetti, G. , Chen, J. , Rosenthal, R. , Brink, R. , Chong, A. S. et al., Transcriptional Regulation of Germinal Center B and Plasma Cell Fates by Dynamical Control of IRF4. Immunity. 2013. 38: 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ettinger, R. , Sims, G. P. , Fairhurst, A. M. , Robbins, R. , Da Silva, Y. S. , Spolski, R. , Leonard, W. J. et al., IL‐21 Induces Differentiation of Human Naive and Memory B Cells into Antibody‐Secreting Plasma Cells. The Journal of Immunology. 2005. 175: 7867–7879. [DOI] [PubMed] [Google Scholar]
- 34. Marasco, E. , Farroni, C. , Cascioli, S. , Marcellini, V. , Scarsella, M. , Giorda, E. , Piano Mortari, E. et al., B‐cell activation with CD40L or CpG measures the function of B‐cell subsets and identifies specific defects in immunodeficient patients. European Journal of Immunology. 2017. 47: 131–143. [DOI] [PubMed] [Google Scholar]
- 35. Tuijnenburg, P. , aan de Kerk, D. J. , Jansen, M. H. , Morris, B. , Lieftink, C. , Beijersbergen, R. L. , van Leeuwen, E. M. M. et al., High‐throughput compound screen reveals mTOR inhibitors as potential therapeutics to reduce (auto)antibody production by human plasma cells. European Journal of Immunology. 2020. 50: 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Unger, P. P. A. , Verstegen, N. J. M. , Marsman, C. , Jorritsma, T. , Rispens, T. , Ten Brinke, A. and Van Ham, S. M. , Minimalistic in vitro culture to drive human Naive B cell differentiation into antibody‐secreting Cells. Cells. 2021. 10: 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pignarre, A. , Chatonnet, F. , Caron, G. , Haas, M. , Desmots, F. and Fest, T. , Plasmablasts derive from CD23– activated B cells after the extinction of IL‐4/STAT6 signaling and IRF4 induction. Blood. 2021. 137: 1166–1180. [DOI] [PubMed] [Google Scholar]
- 38. Marsman, C. , Jorritsma, T. , Ten Brinke, A. and van Ham, S. M. , Flow Cytometric Methods for the Detection of Intracellular Signaling Proteins and Transcription Factors Reveal Heterogeneity in Differentiating Human B Cell Subsets. Cells. 2020. 9: 2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Victora, G. D. , Schwickert, T. A. , Fooksman, D. R. , Kamphorst, A. O. , Meyer‐Hermann, M. , Dustin, M. L. , Nussenzweig, M. C. , Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010. 143: 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shulman, Z. , Gitlin, A. D. , Targ, S. , Jankovic, M. , Pasqual, G. , Nussenzweig, M. C. , Victora, G. D. , T follicular helper cell dynamics in germinal centers. Science (1979). 2013. 341: 673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bolduc, A. , Long, E. , Stapler, D. , Cascalho, M. , Tsubata, T. , Koni, P A. and Shimoda, M. , Constitutive CD40L Expression on B Cells Prematurely Terminates Germinal Center Response and Leads to Augmented Plasma Cell Production in T Cell Areas. The Journal of Immunology. 2010. 185: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song, S. and Matthias, P. D. , The transcriptional regulation of germinal center formation. Frontiers in Immunology. 2018. 9: 2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin, K. I. , Lin, Y. and Calame, K. , Repression of c‐ myc Is Necessary but Not Sufficient for Terminal Differentiation of B Lymphocytes In Vitro. Molecular and Cellular Biology. 2000. 20: 8684–8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reimold, A. M. , Iwakoshi, N. N. , Manis, J. , Vallabhajosyula, P. , Szomolanyi‐Tsuda, E. , Gravallese, E. M. , Friend, D. et al., Plasma cell differentiation requires the transcription factor XBP‐1. Nature 2001 412:6844. 2001. 412: 300–307. [DOI] [PubMed] [Google Scholar]
- 45. Lin, K. I. , Angelin‐Duclos, C. , Kuo, T. C. and Calame, K. , Blimp‐1‐Dependent Repression of Pax‐5 Is Required for Differentiation of B Cells to Immunoglobulin M‐Secreting Plasma Cells. Molecular and Cellular Biology. 2002. 22: 4771–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nutt, S. L. and Tarlinton, D. M. , Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat Immunol. 2011. 12: 472–477. [DOI] [PubMed] [Google Scholar]
- 47. Vallespinós, M. , Fernández, D. , Rodríguez, L. , Alvaro‐Blanco, J. , Baena, E. , Ortiz, M. , Dukovska, D. et al., B Lymphocyte Commitment Program Is Driven by the Proto‐Oncogene c‐myc. The Journal of Immunology. 2011. 186: 6726–6736. [DOI] [PubMed] [Google Scholar]
- 48. Zhang, T. ‐ T. , Gonzalez, D. G. , Cote, C. M. , Kerfoot, S. M. , Deng, S. , Cheng, Y. , Magari, M. et al., Germinal center B cell development has distinctly regulated stages completed by disengagement from T cell help. Elife. 2017. 6: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martínez, M. R. , Corradin, A. , Klein, U. , Álvarez, M. J. , Toffolo, G. M. , di Camillo, B. , Califano, A. et al., Quantitative modeling of the terminal differentiation of B cells and mechanisms of lymphomagenesis. Proc Natl Acad Sci U S A. 2012. 109: 2672–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tejero, E. M. , Lashgari, D. , García‐Valiente, R. , Gao, X. , Crauste, F. , Robert, P. A. , Meyer‐Hermann, M. et al., Multiscale Modeling of Germinal Center Recapitulates the Temporal Transition From Memory B Cells to Plasma Cells Differentiation as Regulated by Antigen Affinity‐Based Tfh Cell Help. Frontiers in Immunology. 2021. 11: 620716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tejero, E. M. , Lashgari, D. , García‐Valiente, R. , He, J. , Robert, P. A. , Meyer‐Hermann, M. , Guikema, J. E. J. et al., Coupled Antigen and BLIMP1 Asymmetric Division With a Large Segregation Between Daughter Cells Recapitulates the Temporal Transition From Memory B Cells to Plasma Cells and a DZ‐to‐LZ Ratio in the Germinal Center. Frontiers in Immunology. 2021. 12: 3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Laidlaw, B. J. and Cyster, J. G. , Transcriptional regulation of memory B cell differentiation. Nature Reviews Immunology 2020 21:4. 2020. 21: 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zimring, J. C. and Hudson, K. E. , Cellular immune responses in red blood cell alloimmunization. Hematology Am Soc Hematol Educ Program. 2016. 2016: 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bugatti, S. , Vitolo, B. , Caporali, R. , Montecucco, C. and Manzo, A. , B cells in rheumatoid arthritis: From pathogenic players to disease biomarkers. BioMed Research International. 2014. 2014: 681678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yap, D. Y. H. and Chan, T. M. , B cell abnormalities in systemic lupus erythematosus and lupus nephritis—role in pathogenesis and effect of immunosuppressive treatments. International Journal of Molecular Sciences. 2019. 20: 6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McClure, M. , Gopaluni, S. , Jayne, D. and Jones, R. , B cell therapy in ANCA‐associated vasculitis: current and emerging treatment options. Nature Reviews Rheumatology. 2018. 14: 580–591. [DOI] [PubMed] [Google Scholar]
- 57. Urashima, M. , Chauhan, D. , Uchiyama, H. , Freeman, G. J. and Anderson, K. C. , CD40 ligand triggered interleukin‐6 secretion in multiple myeloma. Blood. 1995. 85: 1903–1912. [PubMed] [Google Scholar]
- 58. Cossarizza, A. , Chang, H. D. , Radbruch, A. , Abrignani, S. , Addo, R. , Akdis, M. , Andrä, I. et al., Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition). European Journal of Immunology. 2021. 51: 2708–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Souwer, Y. , Griekspoor, A. , Jorritsma, T. , De Wit, J. , Janssen, H. , Neefjes, J. and Van Ham, S. M. , B Cell Receptor‐Mediated Internalization of Salmonella : A Novel Pathway for Autonomous B Cell Activation and Antibody Production. The Journal of Immunology. 2009. 182: 7473–7481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


