Abstract
Context
Studies from high‐income countries indicates that infants born preterm are at increased risk of respiratory infections; however in the low and middle‐income countries (LMICs) data are limited. Our aim was to systematically review the studies evaluating the risk of respiratory infections in preterm children born in LMICs.
Methods
We searched Medline, PubMed, Cumulative Index of Nursing and Allied Health Literature, Embase, and Psych‐INFO databases for studies reporting respiratory outcomes in children born preterm in LMICs. Two authors extracted the data and evaluated the risk of bias with appropriate assessment methods independently.
Results
Twelve observational studies evaluating 5969 children were included in the review. The risk of lower respiratory tract infection varied from 5% to 73.9%. Similarly, respiratory syncytial virus (RSV) infection risk ranged from 4.4% to 22.7%. The unadjusted relative risk for any respiratory tract infection or lower respiratory tract infection was significantly higher in the children born preterm than in children born at term (1.52 [95% confidence interval 1.25–1.85]). We also noted wide‐ranging risk of respiratory infections requiring in‐hospital or emergency care (range: 0.5%–27.7%) and hospital stay in children born preterm (range: 6–14.3 days).
Conclusions
Preterm‐born children in LMICs are at risk of increased respiratory infections compared to term‐born children; however, the baseline risk is variable, although substantial; This highlights the need for preventive strategies, including RSV immunoprophylaxis.
Keywords: bronchiolitis, low birth weight, LRTI, pneumonia, premature, respiratory syncytial virus, ventilation
1. INTRODUCTION
Each year 15 million infants are born preterm, and the preterm birth rates vary from as low as 5%–7% in high‐income countries (HICs) to as high as 15%–18% in low‐ and middle‐income countries (LMICs). 1 Most of these infants are born in African and Southeast Asian countries, contributing to more than 60% of the burden. 2 Preterm infants are at a potential immunological disadvantage, 3 have immature lungs, 4 and some have a developmental disability, all of which make them susceptible to infections, resulting in hospitalization, seeking emergency care, and increased utilization of healthcare costs. 3 , 4 , 5 Respiratory infections are of primary concern out of all the infections and are the leading cause of death during infancy and childhood. 5
Many observational studies and their systematic reviews from HICs have shown that infants born preterm are at higher risk of developing respiratory infections, including respiratory syncytial virus (RSV) infection when followed up during the first few years of life. 6 Hence, clinicians tend to follow preterm infants more closely and offer strategies such as improving nutrition, vaccination, and reduced exposure to smoke to curtail the risk of such respiratory infections in these high‐risk children. One particularly strategy includes palivizumab for preventing severe respiratory RSV infection in children, 7 as data from HICs show that RSV infection is common and palivizumab immunoprophylaxis costs exceed the economic benefit of preventing RSV‐associated hospitalization in high‐risk children. 8 However, whether such immunoprophylaxis is helpful in children in LMICs is unknown as the burden of RSV infection in LMICs is undetermined.
Studies from LMICs assessing the risk of developing respiratory infections, including RSV infections, in children born preterm are sparse. It is vital to understand the burden of respiratory infections, the type of respiratory infections, causative organisms, and the impact on healthcare, including hospitalization and the need for respiratory support, in LMICs, as the risks are amplified due to overcrowding, poor vaccination status, suboptimal follow‐up, and poor nutritional status after discharge. 9 , 10 Hence, a systematic review is required to assess the burden of respiratory infections in children born preterm in LMICs. The objective of the systematic review is to evaluate the risk of respiratory infection (outcome) in children born preterm (population and exposure) compared to term children (control) in LMICs (setting).
2. METHODS
This systematic review was conducted as per the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) reporting guideline. 11 The protocol was registered with PROSPERO (registration number, CRD42021272375), the prospective international register for systematic reviews. The study is described according to the PRISMA checklist.
2.1. Search strategy
A systematic search of the literature was conducted using an appropriate prespecified search strategy across Medline, PubMed, Cumulative Index of Nursing and Allied Health Literature, Embase, and Psych‐INFO databases from January 1, 2000, through July 31, 2021, without language restriction. The details of the search strategy across each database are provided in the online Supporting Information. In addition, the reference lists from studies that were included in the systematic review were also searched.
2.2. Eligibility criteria
2.2.1. Population
Children less than 2‐year‐old born in LMICs were included. Children with known congenital heart disease, immunodeficiency disorders, chromosomal disorders, and life‐threatening congenital anomalies were excluded. We defined LMIC as per World Bank classification criteria based on Gross National Income (GNI) per capita (current US$). (a) Low‐income countries: those with GNI per capita, calculated using the World Bank Atlas method, of $1045 or less. (b) Middle‐income countries: those with GNI per capita of >$1045 but <$12736. 12
2.2.2. Exposure
Preterm infants less than 37 weeks of gestational age (GA) born in LMICs were included.
2.2.3. Comparator
In observational studies, term infants more than 37 weeks of GA born in LMICs will serve as a control. Whereas in descriptive studies, there will be no comparator.
2.2.4. Study design
We included observational studies (cohort and case–control) comparing the outcomes between preterm and term infants born in LMICs. Descriptive studies providing data on preterm infants without control were also included. Studies were only included if they followed up with infants (minimum two follow‐ups, any frequency) during the first 2 years of life to identify the relevant outcomes. Case series, narrative reviews, systematic reviews, studies from HICs, and studies with no follow‐up were excluded.
2.2.5. Outcomes
Studies must report one or more following outcomes identified in our systematic review.
2.3. Primary outcome
Respiratory infections (as defined by authors) during the first 2 years of life
-
1.
Any respiratory infection (upper or lower respiratory tract infection), as defined by the authors.
2.4. Secondary outcomes
-
1.
Any lower respiratory tract infection, including pneumonia, bronchopneumonia, and bronchiolitis, as defined by the authors.
-
2.
RSV infection (any) during the first 2 years of life.
-
3.
Respiratory infections (any) requiring hospitalization or emergency treatment including ventilatory care during the first 2 years of life.
2.5. Study selection and data extraction
The authors (S. D./A. R.) searched the literature in the databases mentioned above and assimilated a final list of literature using a web‐based reference management platform, Rayyan software (https://www.rayyan.ai/). Then, S. D. and A. P. screened the titles and abstracts to determine relevant reports. The shortlisted literature after the title and abstract screening was assessed by the authors (S. D., A. P., A. R., and R. S.) independently to determine the eligibility for inclusion based on the eligibility criteria. Included reports were examined for all the relevant information, including study design, population, inclusion and exclusion criteria, outcomes, and risk of bias, independently by the authors (S. D., A. P., A. R., and R. S.). No blinding strategies were employed. Any discrepancies were resolved by discussion and consensus.
2.6. Assessment of risk of bias
The risk of bias for included studies was evaluated using a modified Newcastle–Ottawa scale (NOS) 13 and modified Leboeuf‐Yde and Lauritsen tool by Hoy et al. 14 for observational studies and descriptive studies, respectively. The following domains were evaluated in the modified NOS: selection, comparability, and outcome. A priori, a score of ≤3/9 was deemed high risk, a score of 4–6/9 was deemed a moderate risk, and a score of ≥7/9 was deemed a low risk of bias. 13 The authors (S. D., A. P., A. R., and R. S.) independently evaluated the risk of bias and resolved the conflicts through discussion and consensus. In the modified tool by Hoy et al. 14 a priori, a score of 0–3/9 is deemed low risk, 4–6/9 is deemed a moderate risk, and 7–9/9 is deemed a high risk of bias.
2.7. Data synthesis and statistical analysis
The odds ratio risk (OR) with its 95% confidence intervals (CI) and p value were calculated for each study if the study (comparative studies only) provided the raw data. There was insufficient data to pool for meta‐analysis for any of primary or the secondary outcomes.
3. RESULTS
The study selection log is shown in Figure 1. There were 15552 articles identified through all databases, and 4081 records were included for the title and abstract screening. One hundred and one studies were included for full‐text screening. Eighty‐nine were excluded and 12 studies were included in the systematic review. The details of the risk of bias assessment of included studies are provided in Table 1, and all the studies were adjudged to be at low risk of bias. (Table 2)
Figure 1.
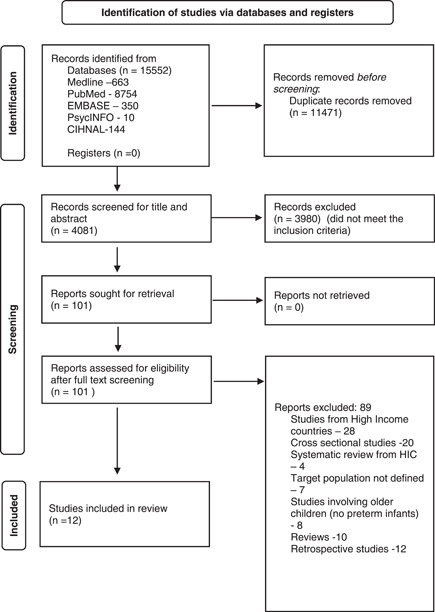
Flowchart of search results (adapted from PRISMA 2021). PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐analyses.
Table 1.
Summary table
| Study ID | Study setting | Study design and follow‐up plan | Sample size (exposure and control) | Inclusion criteria | Exclusion criteria | Outcomes assessed |
|---|---|---|---|---|---|---|
| Arruda (2014) | Brazil, Peru, three hospitals (Curitiba, Porto Alegre, and Ribeirão Preto) | Prospective cohort study; infants followed up for 1 year (monthly for the first 6 months and bimonthly for the next 6 months) telephonically | N = 303, ≤35 weeks (no controls) | ≤35 weeks and <6 months at enrollment b/w January 2008–December 2010 | Prior RSV infection or if they had received palivizumab or other RSV‐specific immunoglobulin products before the start of the study |
Primary outcome: 174/303 (58.1%) experienced LRTI. 27.8% experienced severe LRTI. RSV (30/45, 66.7%) than with other viruses. RSV was present in 33.1% (143/432) of LRTI events tested, and 57.3% (82/143) were coinfections. RSV was the most frequently associated with severe LRTIs (34/56 events, 60.7%), 50% (17/34 events), single and 50% coinfections. Longer hospital stays—RSV coinfections >RSV single infections (p = 0.012). |
| Armanian (2015) | Shahid Beheshti Hospital, Isfahan, Iran, Single center | Retrospective study: followed up for 1 year of life | N = 495, low birth weight preterm infants | All preterm low birth weight infants | significant congenital anomalies and delayed discharge due to multiple parities | 15.3% (76) were readmitted during the first year of life. 32.9% (25) were admitted with pneumonia |
| Etiler (2002) | Antalya, Turkey, two primary healthcare centers | Prospective cohort study: infants followed up every 2 months until 1 year of age or moved out from the area | N = 216, term and preterm infants | All infants born in two primary healthcare centers during the study period | Not specified |
Acute respiratory infection: preterm versus term infants (RR [95% CI] 1.52 [1.25–1.85]). Death resulting from respiratory infection (1/216) but unclear whether the infant was a term or preterm |
| Maksic (2018) | Bosnia and Herzegovina, Europe, multiple centers | Prospective cohort study; one‐time follow‐up (end of RSV season) | N = 160, late preterm infants (no controls) | Preterm infants 33–35 weeks GA were born during the study period | Chronic lung disease, congenital heart disease, RSV prophylaxis, and unable to follow‐up |
Respiratory infection requiring hospitalization (11.2%). RSV infection (requiring hospitalization) (4.37%). Hospitalization days median (IQR) 6 (3–14) |
| Martins (2015) | Ararangua, Brazil, maternity hospital | Prospective cohort study: infants followed up every 2 months until 1 year of age | N = 187, term and preterm (or low birth weight) infants | All infants born in a maternity hospital during the study period | Not specified | Pneumonia: adjusted RR 5.96 (1.75–20.4). Bronchiolitis: Adjusted RR 0.78 (0.31–1.94) |
| Miller (2012) | Bueno Aires, Argentina, Children's Hospital | Prospective cohort study; infants followed up every month until 1 year of age | N = 119 VLBW infants (no controls) | VLBW infants | Short life expectancy (6 months), known bleeding disorders, immune deficiencies, or orofacial malformations |
Acute lower respiratory infection (73.9%). Respiratory infection requiring hospitalization (27.7%) |
| Muller (2021) | Cape Town, South Africa, two governmental hospitals | Prospective cohort study; infants followed up until 1 year of age (1 week, 1, 3, 5, and 12 months) | N = 53 preterm infants (no controls) | Preterm infants 29–34 weeks GA were born during the study period | Unable to follow‐up, maternal HIV infection, any acute illness 2 weeks before enrollment or any known chronic illness |
Death resulting from respiratory infection (1.8%). Authors captured respiratory infections and hospitalization, but absolute rates are not provided |
| Ochoa (2014) | Lima, Peru, four hospitals (details not available) | Prospective cohort study; infants followed up every 2 weeks until 1 year of age to assess for respiratory infection | N = 222 VLBW infants (no controls) | <37 weeks and <1500 gm hospitalized infants b/w March 2009 and March 2010 | Unable to follow‐up for 1 year, infants who received immunoglobulin prophylaxis (such as RSV Ig, CMV Ig, or hyperimmunoglobulin) before discharge, congenital malformations, and infants older than 6 months at the time of discharge |
RSV infection rate (17%) Respiratory infection requiring hospitalization (22.7%). Death resulting from respiratory infection (0.47%) |
| Guerra (2019) | San Luis Potosi, Mexico, two hospitals | Prospective cohort study; infants followed up every month by telephonic calls and outpatient visits until 1 year of age | N = 294 infants (no controls) | <37 weeks hospitalized infants | Infants who died before discharge from the neonatal unit or those who we were unable to locate after discharge from the hospital were excluded from analysis |
18% (53) have one episode of ARI during the first 12 months of life. 4.8% (14) had confirmed RSV infection. The overall ARI hospitalization incidence was 278 episodes/1000 child‐years of follow‐up. Up to 22% of children require admission due to an ARI by 1 year of life |
| Chu (2016) | Nepal | Prospective cohort (no controls) Weekly visits for 180 days. Part of maternal randomized control trial on influenza vaccine | No details | All infants born in the time period to women who were enrolled in the influenza study | No details | 311 (9%) of 3509 RSV 0.220 infants (71%) were evaluated in the health system, and 41 (19%) visited hospital or physician. 203/287 (71%) had LRTI |
| Oncel (2012) | Turkey | Retrospective study. Main aim‐evaluate the cost‐effectiveness of palivizumab | 272 infants, 201 (Group 1), and 71 (Group 2) | <32 weeks, Gp1 with Pavlivizumab and Gp2 without pavlizumab | No details |
LRTI requiring hospitalization 18/272 (6.6%) LRTIs were identified using the ICD10 coding system (J12, J16, J17, and J18 for pneumonia; J21 for acute bronchiolitis) |
| Linder‐Jackson (2014) | Argentina | Prospective cohort study | 139 ARI samples, 66% were positive for HRV, and 53% of |
Premature infants weighing <1500 gm were enrolled in the NICU from June 2011 through October 2012. Infants were followed for the first year of life |
No details | 92 (66%) HRV positive. Seventy‐four of which have bronchiolitis, 32% hospitalization (50% for HRV, 15% RSV, and 15% MPV) |
Abbreviations: ARI, acute respiratory infection; CI, confidence interval; CMV, cytomegalovirus; GA, gestational age; HRV, human rhinovirus; HIV, human immunodeficiency virus; ICD, international classification of diseases; IQR, interquartile range; LRTI, lower respiratory tract infection; MPV, metapneumovirus; NICU, neonatal intensive care unit; RR, relative risk; RSV, respiratory syncytial virus; VLBW, very lower birth weight.
Table 2.
Risk of bias for studies analyzing outcome only in the exposed cohort (descriptive, prevalence studies)
| Risk of bias items | Arruda (2014) | Armanian (2015) | Ochoa (2014) | Etiler (2002) | Muller (2021) | Miller (2012) | Martins (2015) | Maksic (2018) | Guerra (2019) | Chu (2016) | Oncel (2012) | Linder Jackson (2014) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Was the study's target population a close representation of the national population about relevant variables, for example, age, sex, occupation? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No (from two hospitals) | Yes—only for age and sex. Not occupation | Unclear. Only two hospitals | No |
| Was the sampling frame an accurate or close representation of the target population? | No (some patients, such as those who received immunoglobulin prophylaxis and infants who were older than 6 months, were excluded) | Yes | No (some patients, such as those who received immunoglobulin prophylaxis and infants who were older than 6 months, were excluded) | Yes | No (some exclusions were not relevant) | Yes | Yes | No (some exclusions were not relevant) | No iInfants who died before discharge from the neonatal unit or those who we were unable to locate after discharge from the hospital were excluded) | No—rural population only. Not urban | Yes | Yes |
| Was some form of random selection used to select the sample, OR, was a census undertaken? | Unclear (study nurses enrolled preterm neonates admitted in the first 72 h of life) | No (preterm neonates, low birth weight who were admitted were followed up) | Yes (continuous sampling of 1 year) | Yes (continuous sampling for a prespecified period) | Yes (nested study) | Yes (continuous sampling for a prespecified period) | Yes (continuous sampling for a prespecified period) | Yes (continuous sampling for a prespecified period) | Yes (continuous sampling of 1 year) | Yes(continuous sampling of 6 months) | Yes (only children admitted to the hospital were selected) | No |
| Was the likelihood of nonresponse bias minimal? | No, (LTFU = 18.8%) | Yes | Yes | No (only 58.3% completed 1 year follow‐up) | Yes | Yes | Yes | Yes | Yes (LTFU [2.8%]) | Yes | Yes | Yes |
| Were data collected directly from the subjects (as opposed to a proxy)? | Yes | No (data was retrospectively collected from hospital records) | Yes | No (Researchers asked parents about the illness) | Yes | Yes | Yes | Yes | Yes | Yes | No (records) | Yes |
| Was an acceptable case definition used in the study? | Yes (LRTIs were diagnosed based on symptoms noted during the physical examination and included respiratory rate, retractions, wheezing and crackles on auscultation, and, whenever present, radiologic signs were compatible with viral bronchiolitis or pneumonia. Severe LRTI was defined as an event that required hospitalization because of these signs and symptoms) | No (no case definition for pneumonia, LRTI was used) | Unclear (mostly no, respiratory infection defined by attending at the hospital and the details are not provided) | Yes | Unclear (likely yes) | Yes | Yes | Yes | Yes (the age at first ARI‐associated hospitalization was used for time survival analysis. The incidence per 1000 infant‐years of follow‐up was determined) | Yes (Infant ARI was defined as having any of the following: fever, cough, wheeze, difficult or rapid breathing, or a draining ear) | Yes | Yes |
| Was the study instrument that measured the parameter of interest (e.g., X‐ray or ultrasound, or microbiological assay for diagnosis of pneumonia) shown to have reliability and validity (if necessary)? | Yes (RSV PCR using commercial assay technique | Unclear | Yes (RSV detected using commercial assay) | Yes (they used clinical criteria but did not apply directly; instead used as a proxy) | Unclear (likely yes) | Yes (appropriate respiratory viruses testing) | Yes (chest X‐ray) | Yes | Yes (RSV detected using RT‐PCR) | Yes (An RSV ARI was defined as the presence of any symptoms occurring for a minimum of 1 day with a mid‐nasal swab obtained and RSV detected on qPCR assay) | Yes | Yes |
| Was the same mode of data collection used for all subjects? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the numerator(s) and denominator(s) for the parameter of interest appropriate | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Risk of bias score | 2 | 3 | 2 | 2 | 1 | 0 | 0 | 1 | 2 | 1 | 3 | 3 |
| Summary of risk of bias | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Abbreviations: ARI, acute respiratory infections; LRTI, lower respiratory tract infection; PCR, polymerase chain reaction; RSV, respiratory syncytial virus.
The details of the 12 studies included in the systematic review are presented in Table 1. Three studies were comparative, and nine were descriptive studies. In addition, 10 studies were carried out prospectively, while 2 were carried out retrospectively. Of the 12 studies, 2 each were from Brazil, 15 , 16 Turkey, 17 , 18 Argentina, 19 , 20 and one each from Nepal, 21 Peru, 22 South Africa, 23 Bosnia and Herzegovina, 24 Iran, 25 and Mexico. 26 The majority of studies were conducted in the last decade except for one study conducted in 2002. 17 The sample size across studies ranged from 53 to 495, included infants from 29 weeks and the follow‐up period varied across studies ranging from 1 week of life to 12 months.
3.1. Primary outcome
3.1.1. Any respiratory tract infection
One study provided comparative data, and the unadjusted risk for any respiratory tract infection was significantly higher in the children born preterm as compared to children born at term (relative risk [RR] [95% CI] 1.52 [1.25–1.85]). 17 Another study provided that only risk of respiratory tract infection in preterm infants was 66% (92/139). 19 No meta‐analysis was performed as one was comparative and another was a descriptive study.
3.2. Secondary outcomes
3.2.1. Any lower respiratory tract infection
No meta‐analysis was performed as only one study provided adjusted risk for any lower respiratory tract infection, which was significantly higher in children born preterm as compared to children born at term (R. R. [95% CI] 1.52 [1.25–1.85]). 17 Additionally, five studies provided data only for preterm infants and the risk of any lower respiratory tract infection varied from 5% to 73.9% 20 , 25 across the studies.
3.2.2. RSV infection
Only four studies 16 , 17 , 24 , 26 provided the risk data for children born preterm, and the risk varied from 4.4% to 22.7% across the studies.
3.2.3. Respiratory infections (any) requiring hospitalization or emergency treatment
Seven studies 16 , 17 , 19 , 20 , 23 , 25 , 26 provided descriptive data for children born preterm on respiratory infections requiring in‐hospital or emergency care. The risk varied widely from 0.5% to 27.7%. The hospital stay in children born preterm ranged from 6 to 14.3 days. No studies provided data on ventilation days.
4. DISCUSSION
Our systematic review summarizes the evidence from 12 observational studies, including 5969 children on the risk of respiratory infection in children born preterm followed up until the first 2 years of life in LMICs. The review finds, compared to children born at term, children born preterm are at significantly higher risk of developing any respiratory infection (unadjusted comparison, one study) and any acute lower respiratory infection (adjusted comparison, one study) during the first 2 years of life. However, it also highlights a substantial variation in the risk of acute lower respiratory tract infection (5%–73.9%), RSV infection (4.4%–22.7%), and respiratory infections requiring hospitalization or emergency care treatment (0.5%–22.7%) in children born preterm during the first 2 years of life. Lastly, the review also finds a considerable increase in length of hospital stay (6 to 14.3 days) but a small risk of death (0.3%–0.68%) among children born preterm who require in‐hospital care due to respiratory infections.
This review is the first systematic review summarizing the risk of respiratory infections and other vital outcomes in children born preterm in LMICs. In this systematic review, we sought to find the risk of respiratory infections in children born preterm in LMICs and explore differences in the risk compared to HICs. However, we identified limited studies, mainly descriptive, and hence unable to quantify the actual burden of respiratory infection in this population in LMICs. Nonetheless, the review summarizes the available literature, shows that children born preterm are at increased risk of infection because they have reduced antimicrobial peptides and proteins along with abundance of goblet cells and few ciliated cells in the respiratory tract, and further highlights the considerable variation in the risk of respiratory infections in the LMIC cohort. Furthermore, the review also highlights the need for comparative studies to explore the baseline risk of respiratory infections in children born preterm in LMICs.
Studies from HICs show preterm infants, compared to term infants, are at risk of respiratory tract infections, including RSV infections, and respiratory‐related morbidities, such as prolonged hospitalization and increased risk of death. 27 , 28 A recent systematic review and meta‐analysis evaluating 55 studies showed that a substantial proportion of RSV‐associated morbidity occurred in the first year of life, with global RSV‐acute respiratory infections (ARI) hospitalization estimates of 63.85 (95% CI 37.52, 109.70) per 1000 children per year among preterm children <1 year compared to 19.19 (95% CI 15.04, 24.48) per 1000 children per year among overall children. 6 Similarly, other studies show an increased risk of hospitalizations in preterm infants with varying estimates in proportion to the GA.
In comparison to term infants, the risk of hospitalization from bronchiolitis was much higher in very preterm infants (<32 weeks GA; RR 2.6 [95% CI 2.32–2.89]) than in moderate preterm infants (32–34 weeks GA; RR 1.9 [95% CI 1.32–1.67]) and late preterm infants (34 to 36 weeks GA; RR 1.25 [95% CI 11.8–1.33]). 27 In LMICs, the burden of respiratory infection in preterm infants could be just the tip of the iceberg as only infants who get hospitalized or need intensive care admission to get reported. Besides, studies evaluating or reporting such risk or burden in LMICs are limited. Most of the mild to moderate cases remain untested or underreported, which could mask the actual burden of all‐cause LRTI in these infants. Lack of regional/national registry, no standardized definition, and lack of viral testing facilities add to the under‐reporting issue. We believe these reasons contribute to the heterogeneity between studies estimating the risk of respiratory infections in preterm children in LMICs, as noted in our systematic review.
There is an urgent need to evaluate the burden of respiratory infections in preterm children in LMICs as they contribute to a substantial economic burden. A study evaluating acute respiratory illness‐related hospitalization costs has shown the cost can vary from $54 to $120 in public to $135–$355 in private healthcare settings, which is relatively high per median per capita income. 29 Therefore, it is crucial to evaluate cost‐effective ARI prevention strategies, such as vaccination, to reduce the economic burden.
Immunoprophylaxis for preventing RSV has been a center of the debate over the last few decades, and palivizumab, a humanized monoclonal antibody against the RSV F glycoprotein, is effective in reducing RSV hospitalizations from 101 to 50 per 1000 among high‐risk infants, as compared to placebo. 30 Hence, in many countries, such as the United States, Canada, and the United Kingdom, it has been licensed for use in preterm infants to reduce the risk of RSV morbidity and hospitalizations although debate arise about its cost‐effectiveness. 31 , 32 Nonetheless, the cost‐effectiveness of RSV prophylaxis still tends to be more favorable in populations with greater risk, such as preterm infants. 33
Preterm infants in their first 6 months, children with underlying cardiac or pulmonary disease in the first 2 years, immunocompromised children are at highest risk of severe RSV infection. These children are most likely to require RSV prophylaxis because of longer hospital stays and admission to intensive care unit. 10 , 34 It is important to note that the cost‐to‐benefit ratio is likely favorable if the baseline risk of RSV disease or hospitalization is high, and hence it may likely be helpful in LMIC countries like India, where the RSV disease burden in preterm infants could be substantial. Other ways to prevent respiratory infection in children in LMIC are adequate nutrition and hygiene, improving literacy status, antiviral agents, and vaccines. However, these speculations must be evaluated prospectively in LMICs on a large scale.
5. CONCLUSION
The current systematic review of 12 observational studies from LMICs shows that children born preterm are at significantly higher risk of developing any respiratory infection and also highlights a substantial variation in the risk of acute lower respiratory tract infections, RSV infections, and respiratory infections requiring hospitalization or emergency care treatment and a considerable increase in length of hospital stay, which therefore underlines the need for preventive strategies, including RSV immunoprophylaxis.
5.1. Strengths and weaknesses
This is the first systematic review summarizing the literature on the risk of respiratory infection in preterm children until 2 years of age from LMICs. We searched the literature comprehensively, appraised the evidence using prespecified eligibility criteria, performed the methodological assessment using standardized tools, registered the review prospectively, and reported it using PRISMA guidelines. However, the review was limited to fewer and heterogeneous studies, including descriptive studies with no comparative data. Nonetheless, it summarizes the literature comprehensively from all LMICs to date and provides an impression of the burden of respiratory infections in LMICs.
5.2. Implications for future research
As our review highlights the lack of factual data to evaluate the risk of respiratory infections in LMICs, it is necessary to study them in the future. Studies should assess the risk of respiratory infections prospectively and longitudinally by following children adequately for at least 2 years. In addition, researchers should attempt to include the term children parallelly and quantify the risk to provide comparative data. Furthermore, one should use robust and standard methods to define and detect respiratory infections, including various organisms causing respiratory diseases. Finally, immunization programs and preventive steps must be scrutinized to curtail the risk of respiratory infections, and studies evaluating the cost‐effectiveness of RSV immunoprophylaxis in preterm infants in LMICs must be explored.
AUTHOR CONTRIBUTIONS
Shivashankar Diggikar: Writing – original draft; methodology; Writing – review & editing; formal analysis; data curation; conceptualization. Abhishek Paul: Conceptualization; writing – original draft; data curation; writing – review & editing. Abdul Razak: Conceptualization; writing – original draft; methodology; validation; writing – review & editing; data curation. Manigandan Chandrasekaran: Supervision; writing – review & editing. Ravi Shankar Swamy: Conceptualization; investigation; supervision; writing – original draft; writing – review & editing; methodology; formal analysis.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
None.
Diggikar S, Paul A, Razak A, Chandrasekaran M, Swamy RS. Respiratory infections in children born preterm in low and middle‐income countries: a systematic review. Pediatric Pulmonology. 2022;57:2903‐2914. 10.1002/ppul.26128
Contributor Information
Abdul Razak, Email: abdul.razak@monash.edu.
Ravi Shankar Swamy, Email: docraviswamy@gmail.com.
DATA AVAILABILITY STATEMENT
All data relevant to the study are included in the article or uploaded as Supporting Information.
REFERENCES
- 1. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162‐2172. [DOI] [PubMed] [Google Scholar]
- 2. Lawn JE, Davidge R, Paul VK, et al. Born too soon: care for the preterm baby. Reprod Health. 2013;10:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collins A, Weitkamp J‐H, Wynn JL. Why are preterm newborns at increased risk of infection? Arch Dis Child Fetal Neonatal Ed. 2018;103(4):F391‐F394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants—2010 update. Neonatology. 2010;97:402‐417. [DOI] [PubMed] [Google Scholar]
- 5. Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. 2016;21:68‐73. [DOI] [PubMed] [Google Scholar]
- 6. Stein RT, Bont LJ, Zar H, et al. Respiratory syncytial virus hospitalization and mortality: systematic review and meta‐analysis. Pediatr Pulmonol. 2017;52:556‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luna MS, Manzoni P, Paes B, et al. Expert consensus on palivizumab use for respiratory syncytial virus in developed countries. Paediatr Respir Rev. 2020;33:33‐44. [DOI] [PubMed] [Google Scholar]
- 8. Wang D, Cummins C, Bayliss S, Sandercock J, Burls A. Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation. Health Technol Assess. 2008;12:(36). 10.3310/hta12360 [DOI] [PubMed] [Google Scholar]
- 9. Kumar Sg, Roy G, Suguna E. Prevalence and risk factors of acute respiratory infection among school children in coastal South India. J Glob Infect Dis. 2014;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi T, Balsells E, Wastnedge E, et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: systematic review and meta‐analysis. J Glob Health. 2015;5:020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fantom N, Serajuddin U. The world bank's classification of countries by income [Internet]. 2016. Accessed June 22, 2022. http://econ.worldbank.org
- 13. Wells G, Shea B, O′Connell D. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses; 2012.
- 14. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934‐939. [DOI] [PubMed] [Google Scholar]
- 15. Martins ALO, da Silva Fernandes Nascimento D, Schneider IJC, Schuelter‐Trevisol F. Incidence of community‐acquired infections of lower airways among infants. Rev Paul Pediatr. 2016;34:204‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arruda E, Jones MH, Escremim de Paula F, et al. The burden of single virus and viral coinfections on severe lower respiratory tract infections among preterm infants a prospective birth cohort study in Brazil. Pediatr Infect Dis J. 2014;33:997‐1003. [DOI] [PubMed] [Google Scholar]
- 17. Etiler N, Velipasaoglu S, Aktekin M. Incidence of acute respiratory infections and the relationship with some factors in infancy in Antalya, Turkey. Pediatr Int. 2002;44:64‐69. [DOI] [PubMed] [Google Scholar]
- 18. Oncel MY, Mutlu B, Kavurt S, et al. Respiratory syncytial virus prophylaxis in preterm infants: a cost‐effectiveness study from Turkey. Turk J Pediatr. 2012;54:344‐351. [PubMed] [Google Scholar]
- 19. Jung JA, Kim JH, Lee JS, et al. Association of vitamin D status with recurrent wheezers. Journal of Allergy and Clinical Immunology . 2014; 133(2) (suppl AB192). [Google Scholar]
- 20. Kathryn Miller E, Bugna J, Libster R, et al. Human rhinoviruses in severe respiratory disease in very low birth weight infants. Pediatrics. 2012;129:e60‐e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chu HY, Katz J, Tielsch J, et al. Respiratory syncytial virus infection in infants in rural Nepal. J Infect. 2016;73:145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bautista R, Ochoa TJ, Salazar JA, et al. Respiratory syncytial virus‐associated hospitalizations in pre‐mature infants in Lima. Am J Trop Med Hyg. 2014;91:1029‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muller SJ, Zar HJ, Tooke L. Illness episodes in a cohort of preterm infants in their first year of life. S Afr J Child Health. 2021;15:44‐49. [Google Scholar]
- 24. Maksić H, Heljić S, Skokić F, et al. Predictors and incidence of hospitalization due to respiratory syncytial virus (RSV)‐associated lower respiratory tract infection (LRTI) in non‐prophylaxed moderate‐to‐late preterm infants in Bosnia and Herzegovina. Bosn J Basic Med Sci. 2018;18:279‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohammad Armanian A, Armanian A‐M, Mohammadzadeh M, Soleimani R, Salehimehr N, Hasanzadeh A. The duration of hospitalization and readmission rate of low birth weight infants in a tertiary referral hospital in isfahan, Iran. Iran J Neonatol. 2015;6:17‐21. [Google Scholar]
- 26. Benítez‐Guerra D, Piña‐Flores C, Zamora‐López M, et al. Respiratory syncytial virus acute respiratory infection‐associated hospitalizations in preterm Mexican infants: a cohort study. Influenza Other Respir Viruses. 2020;14:182‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haataja P, Korhonen P, Ojala R, et al. Hospital admissions for lower respiratory tract infections in children born moderately/late preterm. Pediatr Pulmonol. 2018;53:209‐217. [DOI] [PubMed] [Google Scholar]
- 28. Anderson EJ, Carbonell‐Estrany X, Blanken M, et al. Burden of severe respiratory syncytial virus disease among 33‐35 weeks' gestational age infants born during multiple respiratory syncytial virus seasons. Pediatr Infect Dis J. 2017;36:160‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peasah SK, Purakayastha DR, Koul PA, et al. The cost of acute respiratory infections in Northern India: a multi‐site study. BMC Public Health. 2015;15:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andabaka T, Nickerson JW, Rojas‐Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev. 2013;30(4):CD0006602. [DOI] [PubMed] [Google Scholar]
- 31. Mammas IN, Drysdale SB, Rath B, et al. Update on current views and advances on RSV infection (review). Int J Mol Med. 2020;46:509‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Willem L, Antillon M, Bilcke J, Jit M, Beutels P. Health and economic burden of respiratory syncytial virus (RSV) disease and the cost‐effectiveness of potential interventions against RSV among children under 5 years in 72 Gavi‐Eligible countries. BMC Med. 2020;18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prescott WA, Doloresco F, Brown J, Paladino JA. Cost effectiveness of respiratory syncytial virus prophylaxis. Pharmacoeconomics. 2010;28:279‐293. [DOI] [PubMed] [Google Scholar]
- 34. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. Lancet. 2010;375:1545‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as Supporting Information.


