Abstract
Aims
Pressurised metered‐dose inhalers (MDIs) have a much higher carbon footprint than dry powder inhalers (DPIs). We aimed to describe variations of inhaler options in local adult asthma prescribing guidance.
Methods
We reviewed local clinical commissioning group (CCG) adult asthma prescribing guidance for primary care in England in 2019 and recorded DPI and MDI inclusion. The relationship to prescribing data from OpenPrescribing.net was examined.
Results
In total, 58 unique guidance documents were analysed covering 144 out of 191 CCGs in England. Only 3% of CCG guidelines expressed an overall preference for DPIs, while 12% explicitly preferred MDIs. The inclusion of DPIs first‐line was 77% for short‐acting β‐agonists, 78% for low‐dose inhaled corticosteroid (ICS) inhalers and 90–96% for combination long‐acting β‐agonist/ICS inhalers. MDIs were included first‐line in 98–100% of these classes. In 26% of CCGs, there was no first‐line DPI option for at least 1 asthma management step. Ten percent of CCGs had no DPI included first‐line for any of the 5 classes examined. Many CCGs recommended higher carbon footprint options; Ventolin MDI (25.6%), inhalers containing HFA227ea (57.9%) and ICS regimes recommending 2 puffs of a lower dose over 1 puff of higher dose (94.2%). MDIs were prescribed more in CCGs that recommended them.
Conclusion
Before the COVID pandemic, there was substantial variation between CCGs in adult asthma prescribing guidance regarding higher and lower carbon footprint options. There may still be scope to amend local guidance to improve clinical and environmental outcomes. This study provides a method and baseline for further investigation of this.
Keywords: asthma, climate change, prescribing, respiratory medicine
What is already known about this subject
Metered‐dose inhalers (MDIs) have a substantially larger carbon footprint than dry powder inhalers due to the propellant gases used.
There is variation among clinical commissioning groups in the relative proportion of dry powder inhalers and MDIs prescribed in primary care in England.
The impact of variation in local prescribing guidelines is not known.
What this study adds
Local prescribing guidelines before the COVID pandemic differed hugely in their recommendations.
This variation may have influenced the proportion of inhalers prescribed as MDIs.
Local guidelines still may not be optimised for carbon footprint or patient outcomes and this work provides a method and baseline for further investigation of this.
1. INTRODUCTION
Inhalers, which contribute 13% of the NHS's carbon footprint related to delivery of care and 3% of the carbon footprint plus (which the health service can influence) of the NHS in England, are an important area for action as healthcare systems seek to de‐carbonise in response to the climate emergency. 1 , 2 Inhalers can be divided into high carbon footprint inhalers that contain hydrofluoroalkane propellant such as pressurised metered‐dose inhalers (MDIs); and low carbon footprint inhalers that do not, such as dry powder inhalers (DPIs) and soft mist inhalers. In response to the Montreal Protocol, chlorofluorocarbon propellants were replaced with hydrofluoroalkanes (HFAs). However, HFAs have a high global warming potential, so MDIs have an estimated carbon footprint 9–200 times greater than DPIs. 3 , 4
There is significant variation between countries in the use of MDIs. In the UK 70% of inhaler prescriptions are for MDIs, compared to the European mean of 47.5%. Scandinavian countries prescribe fewer than 30% MDIs. 5 There is also significant variation within England. 6 In 2000 around 2/3 of inhaled corticosteroid (ICS) and 40% of short‐acting β‐agonist (SABA) prescriptions for asthma in the UK were DPIs. In the following 20 years, there has been a switch to MDIs, and DPIs now represent <10% of ICS and SABA prescriptions. 7 SABA overuse is associated with poor asthma outcomes and in the UK SABA use and resultant emissions are approximately treble those of other European countries. 8 , 9
National guidance and strategy, notably the NHS England Net Zero National Health Service report, has shifted in recent years to support greater use of low carbon inhalers. 1 The NHS National Institute for Health and Care Excellence (NICE) include carbon footprint information in their Inhaler Device Decision Aid. 10 The 2019 British Thoracic Society and Scottish Intercollegiate Guidelines Network (BTS/SIGN) asthma guideline states “Choice of reliever inhaler for stable asthma should be based on patient preference and assessment of correct use. Many patients will not be prepared to carry a spacer.” BTS/SIGN also recommend that “… inhalers with low global‐warming potential should be used when they are likely to be equally effective. Where there is no alternative to MDIs, lower volume HFA134a inhalers should be used in preference to large volume or HFA227ea inhalers”. 11 The most common examples in England of higher carbon footprint treatment options are Ventolin MDI, a large volume HFA134a inhaler with a carbon footprint of 28 kg per inhaler, and Flutiform, which uses HFA227ea and has a carbon footprint of over 36 kg per inhaler. 3 , 12 , 13
Understanding the drivers of variation in prescribing patterns between areas may help policymakers identify the best interventions to encourage a shift towards greater use of low carbon footprint inhalers, where clinically appropriate. This paper seeks to describe variation in prescribing guidance as a potential driver for prescribing, by first describing the variation and then relating this to prescribing data. Our focus was on variations that might affect the carbon footprint of inhalers prescribed in primary care. Our data collection and analysis were carried out prior to the COVID pandemic and local guidance may have been updated since this time. Therefore, this work provides a baseline and method for further investigation in the future.
At the time this work was undertaken, specific guidance on which inhalers to prescribe in primary care were produced locally in England by Clinical Commissioning Groups (CCGs) or Area Prescribing Committees. This guidance may have been unique to 1 CCG or shared among many. In contrast to nationally produced guidance, these local documents commonly named specific brands. National guidelines recommend that generic prescribing should be avoided, as patients may inadvertently get unfamiliar devices. 14
2. METHODS
2.1. Identification of prescribing guidance
A systematic approach was used to identify a prescribing guidance for adult asthma for each of the 191 CCGs in England. An initial web search (described in Appendix 1) was undertaken and then followed by email contact with CCGs if guidance had not been found online. This was undertaken between 09 January 2020 and 31 January 2020.
2.2. Description of variation in guidance
Once identified, preset questions were asked of each guidance by 2 different reviewers independently. These scores were then discussed and a consensus reached. We examined each guidance for the presence of a statement of overall preference for DPI or MDI. We split inhalers by type and dose of medicine, according to the BTS/SIGN asthma treatment pathway, resulting in 5 distinct classes. These were: SABA, low‐dose ICS, and low‐, medium‐ and high‐dose inhalers containing ICS and a long‐acting β‐agonist (ICS + LABA). For each document, we recorded whether DPIs and/or MDIs were recommended as a first‐line option for each class. A device was considered a first‐line option if a prescriber following the guidance could reasonably prescribe that device before trialling any others in a situation where there was clinical equipoise, by which we mean no clear patient individual factors making MDIs or DPIs clinically inappropriate. To illustrate this, guidance which recommended a first choice ICS which was an MDI and presented a DPI as a second or even third choice to be used only if MDI was not suitable was considered to not have first‐line inclusion of ICS DPI. This did not mean a DPI could never be prescribed but that DPI use was not presented as a first‐line option. This contrasted with guidance which presented ICS MDI and DPI as equally valid first‐line options. We considered 4 further specific questions that considered the presence of Ventolin Evohaler and HFA227ea MDIs, whether 2 puffs bi‐daily was the recommended ICS regimen, and device disposal.
For each question, we calculated the proportion of analysed CCGs that fulfilled the criteria. Because we were not able to answer every question for every CCG analysed, the denominator differed between questions. When guidance was shared by multiple CCGs, each 1 received identical results.
2.3. Relationship to prescribing data
Prescribing data from primary care are England are collected and made available by the NHS Business Services Authority. The EBM DataLab at Oxford University, which exists to “help make complex medical and scientific data more accessible and more impactful in the real world”, then analyse and present the data on their OpenPrescribing platform. This platform includes an inhalers metric; the proportion of MDIs prescribed relative to all inhaler types (excluding salbutamol) by CCG. 6 This aggregate prescribing metric could not be disaggregated by age or underlying condition.
To discover how the variation we found in CCG guidance documents was associated with real‐world prescribing, we related 3 of our parameters to the OpenPrescribing metric. The 3 parameters selected were explicit overall device preference, inclusion of low‐dose ICS DPI first‐line and inclusion of low‐dose ICS + LABA DPI first‐line. We considered these to be the most likely to influence overall proportion of nonsalbutamol MDIs used. Overall device preference will affect the prescribing of DPIs in every class of device, and given the exclusion of salbutamol from the dataset, low‐dose ICS and low‐dose ICS + LABA inhalers are likely to be the 2 largest device classes in the data. CCGs were grouped using these parameters.
For overall device preference we grouped CCGs into 3 groups; DPIs recommended, MDIs recommended and no recommendation. For DPI inclusion first‐line in both low‐dose ICS and low‐dose ICS + LABA we grouped CCGs into 2 groups: Yes and No. For each group within each parameter, we then examined the proportion of nonsalbutamol inhalers prescribed as MDIs for each CCG in 2019 (from OpenPrescribing) and presented these as boxplots. For overall device preference a Kruskal–Wallis H test with Bonferroni correction was used. For the other parameters a Mann–Whitney U test was used. Significance was set at P < .05.
2.4. Data statement
Only publicly available data were used for this work. Individual guidance documents can be found on CCG or prescribing group websites. OpenPrescribing data are available at https://openprescribing.net. The data that support the findings of this study are available from the corresponding author upon reasonable request.
3. RESULTS
3.1. Prescribing guidance identified
Prescribing guidance was found for 158 of 191 CCGs in England (83%), as summarised in Appendices 2 and 3. This excludes those that did not provide us with their guidance, and, in 2 cases, a guidance document, which was replaced with an updated version over the analysis period. Of these 158 CCGs, 144 used a locally produced guideline and 14 used national guidance (NICE, BTS/SIGN). As national guidelines do not recommend specific devices, these CCGs were not included in our analysis. Twenty‐two of the 144 CCGs, with local guidance, used a document unique to themselves, while the remainder were part of a local area prescribing committee or similar which produced guidance shared across more than 1 CCG. This meant that there were 58 unique local guidance documents to review with each document being used by between 1 and 9 CCGs. The majority of guidance which recorded date of creation or amendment were from within 2 years of our study (113/130, 87%).
3.2. Description of variation in guidance
The findings of our analysis of 144 CCGs are summarised in Tables 1 and 2. Of 144 CCG guidance documents, 3.5% (5) stated an explicit overall preference for DPIs in contrast to 11.8% (17/144) which explicitly preferred MDIs. The inclusion of DPIs as a first‐line recommended option was lower than for MDIs at all treatment steps, with MDIs nearly universally included as first‐line recommended option. MDIs were included as first‐line in 100% of guidelines for SABA and ICS inhalers (133/133 and 144/144 respectively), whereas DPIs were only first‐line for SABAs in 77% of guidelines (103/133) and for low‐dose ICS in 78% (113/144); 74% of CCG guidelines had a DPI included as first‐line in every class in their guidance (106/144), compared to 98% for MDIs (141/144), and 10% of CCGs had a DPI included as first‐line for none of the classes their guidance covered (14/144), compared to 0% for MDIs (0/144). When examining the guidance for inclusion of high carbon footprint options we found that 26.2% (34/130) included Ventolin MDI as a named option, 58.5% (83/142) included inhalers containing HFA227ea and 94.0% (126/134) included the use of inhaled corticosteroid regimes recommending 2 puffs of a lower dose rather than 1 puff of higher dose. We found no mention in any document of device disposal (0/144).
TABLE 1.
First‐line inclusion of metered‐dose inhalers (MDIs) and dry powder inhalers (DPIs)
| Criteria | Proportion of CCGs in accordance with criteria for each device type (%) | |
|---|---|---|
| DPIs | MDIs | |
| SABA: Inclusion first‐line, n (%) | 103/133 (77) | 133/133 (100) |
| Low‐dose ICS: Inclusion first‐line, n (%) | 113/144 (78) | 144/144 (100) |
| Low‐dose ICS + LABA: Inclusion first‐line, n (%) | 130/144 (90) | 143/144 (99) |
| Med dose ICS + LABA: Inclusion first‐line, n (%) | 130/144 (90) | 143/144 (99) |
| High‐dose ICS + LABA: Inclusion first‐line, n (%) | 128/133 (96) | 130/133 (98) |
| Device included first‐line for ALL included steps in guidance, n (%) | 106/144 (74) | 141/144 (98) |
| Device included first‐line in ZERO included steps in guidance, n (%) | 14/144 (10) | 0/144 (0) |
Column 1 (Criteria) lists the questions applied to each guidance document. Columns 2 and 3 show the number of CCGs in accordance with each criterion. Each question is applied to the guidance for both DPIs and MDIs. For some of these questions, the denominator in Column 2 and 3 is lower than 144. This is because some guidance documents did not include advice for certain classes of device, in which case the response to the question was left as null. Rows 6 and 7 relate to included steps in each guidance document; this relates to the BTS/SIGN asthma treatment guidance. Not every document contained device recommendations for each step, so this value was calculated based only on the presence of DPIs or MDIs on all the steps included in any particular document, NOT necessarily all 5 steps.
CCG, clinical commissioning group; ICS, inhaled corticosteroid; LABA, long‐acting β‐agonist; SABA, short‐acting β‐agonist.
TABLE 2.
Environmental consideration of guidance
| Environmental policy | CCGs using policy—No. (%) | ||
|---|---|---|---|
| Explicit statement of preference | For MDIs | None stated | For DPIs |
| 17/144 (12) | 122/144 (85) | 5/144 (3) | |
| Presence of Ventolin MDI | 34/130 (26) | ||
| Presence of HFA 227ea MDIs | 83/142 (58) | ||
| Low‐dose ICS: Option of 2 puffs bi‐daily | 126/134 (94) | ||
| Recommendation about disposal (either returning to pharmacy, incineration, or recycling) | 0/144 (0) | ||
Column 1 lists 5 potential features on a guidance document relating to greenhouse emissions. For each feature column 2 states the number and percentage of CCGs that used a guidance document containing that feature in 2019. Denominators vary as not every document contained adequate information to answer this question. For example, some documents did not contain any mention of short‐acting β‐agonist inhalers, so information on Ventolin could not be collected. The difference in denominators between this table and Table 2 is explained in full detail in Appendix 4.
CCG, clinical commissioning group; ICS, inhaled corticosteroid; MDI, metered‐dose inhaler; DPI, dry powder inhaler.
3.3. Relationship to prescribing data
Amongst CCGs where guidance made a specific overall recommendation for MDIs there was a higher median proportion of nonsalbutamol inhaler prescriptions that were MDIs; 56% in those recommending MDIs, 52% in CCGs with no device recommendation and 48% in those recommending DPIs (Figure 1). We found no significant association between having an overall DPI recommendation and proportion of nonsalbutamol inhalers prescriptions that were MDIs, but as only 5/144 CCGs made a general recommendation for DPIs, a very large effect size would have been required.
FIGURE 1.
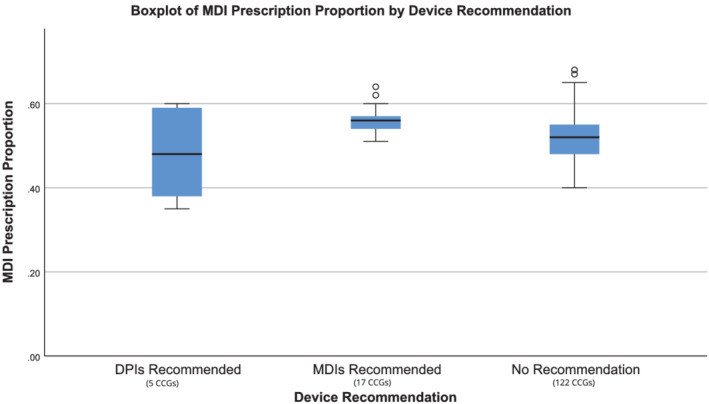
Metered‐dose inhaler (MDI) prescription proportion by statement of explicit preference for inhaler type in guidance. Point estimate and distribution of MDI prescriptions (as a proportion of total inhaler prescribing) is shown for 3 groups of clinical commissioning groups (CCGs); those with no recommendation in their guidance, those that explicitly recommended dry powder inhalers (DPIs) overall and those that explicitly recommended MDIs overall. Prescription data include all indications for inhalers (including asthma and chronic obstructive pulmonary disease) but excludes salbutamol relievers. For each group, the blue box represents the interquartile range, the black bar shows the median value and the whiskers show 1.5× interquartile range outside of the first and third quartiles. Data points outside this range are shown as unfilled circles, each representing 1 CCG. On pairwise comparisons with the Bonferroni correction the only significant difference is between MDIs recommended and no recommendation (Kruskal–Wallis H = 9.02, 2), adjusted significance: P = .011). No significant difference was found between DPIs recommended and MDIs recommended or DPIs recommended and no recommendation (adjusted significance: P = .146, P > .999)
The inclusion of DPIs first‐line in the low‐dose ICS class had a significant association with the proportion of nonsalbutamol inhalers prescriptions which were MDIs (Figure 2). Where DPIs were included first‐line, the proportion of all nonsalbutamol inhalers prescribed that were MDIs was 52%, compared to 55% when they were not. For low‐dose combination inhalers we found that even when DPIs are not recommended first‐line, the proportion of all nonsalbutamol inhalers prescribed that were MDIs was similar to that of CCGs where they are recommended (Figure 3).
FIGURE 2.
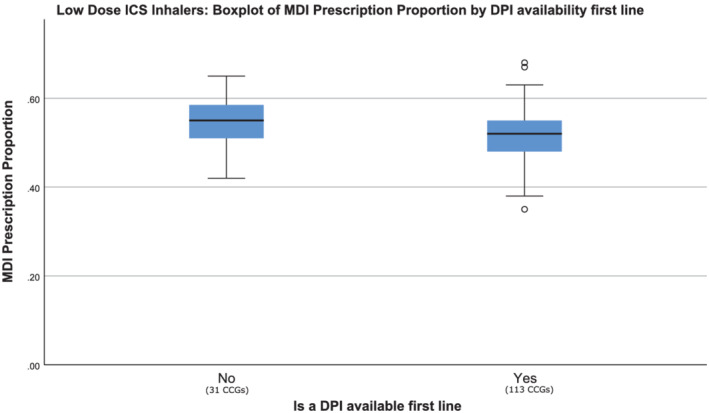
Metered‐dose inhalers (MDI) proportion by low‐dose inhaled corticosteroid (ICS) device recommendation in guidance. Point estimate and distribution MDI prescription proportion for 2 groups; clinical commissioning groups (CCGs) that did have dry powder inhalers (DPIs) containing a low‐dose ICS included first‐line, and those that did not. Prescription data include all indications for inhalers (including asthma and chronic obstructive pulmonary disease) but excludes salbutamol relievers. For each group, the blue box represents the interquartile range, the black bar shows the median value and the whiskers show 1.5× interquartile range outside of the first and third quartiles. Data points outside this range are shown as unfilled circles, each representing 1 CCG. The difference between the 2 groups was statistically significant (Mann–Whitney U = 1255, P = .016).
FIGURE 3.
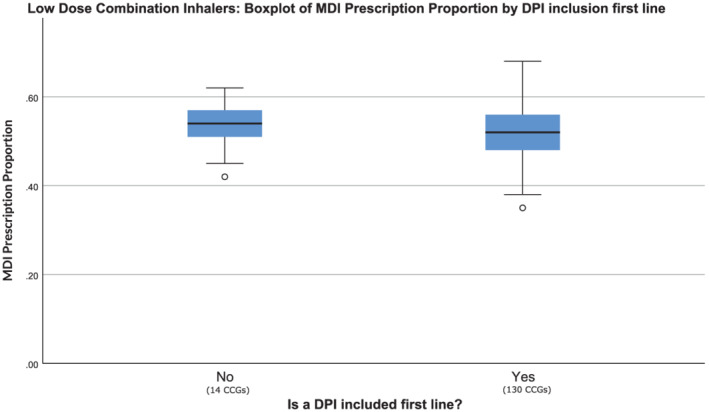
Metered‐dose inhaler (MDI) proportion by low‐dose combination device recommendation in guidance. Point estimate and distribution in MDI prescription proportion for 2 groups; clinical commissioning group (CCGs) that did have low‐dose combination dry powder inhalers (DPIs) included first‐line, and those that did not. Prescription data include all indications for inhalers (including asthma and chronic obstructive pulmonary disease) but excludes salbutamol relievers. For each group, the blue box represents the interquartile range, the black bar shows the median value and the whiskers show 1.5× interquartile range outside of the first and third quartiles. Data points outside this range are shown as unfilled circles, each representing 1 CCG. There was no significant difference between the 2 groups (Mann–Whitney U = 810, P = .42).
4. DISCUSSION
The inclusion of DPIs as first‐line prescribing options was substantially lower than that of MDIs in local adult asthma guidance in England. This was true in all treatment steps but was most pronounced for initial therapy with SABA and low‐dose ICS inhalers. This may help explain why such a high proportion of these were MDIs. Clinicians may, as a result of the variation described in local guidance, have been driven towards greater MDI use and so not be optimising care to the device that is best suited for individual patients. Increasing DPI inclusion in guidance would expand the first‐line device options for practitioners and could improve clinical outcomes. Our findings are time‐specific with guidance collected in early 2020 prior to the COVID pandemic in the UK. We are aware that some CCGs have updated their guidance to address some of the issues raised above. We hope that the findings provide a useful baseline for future further investigation and a valuable method for undertaking this.
The majority of guidance documents did not express a general preference over device type. Among the 15% that did, 3 times more recommended MDIs than DPIs. In many CCGs, recommended first‐line options were limited as only 74% had DPIs included first‐line for every step, and 10% had no DPIs included first‐line at all. This suggests prescribers are often steered towards MDI use even in the absence of an explicit overarching device preference.
Two aspects of the guidance documents were associated with higher MDI use in local data. Firstly, the presence of an explicit statement of overall preference recommending the use of MDIs had a statistically significant association with a higher proportion of MDI nonsalbutamol inhalers. Whilst only a minority of CCGs had guidance with such a statement (12%), this suggests that these documents may have influenced local prescribing practices. Secondly, the first‐line inclusion of DPIs in the low‐dose ICS class also had a statistically significant association with the proportion of MDIs prescribed. This may imply that the guidance relating to this class alone has a disproportionate impact on overall MDI use in England.
We were able to obtain a local guidance document for 144/191 CCGs in England (75%). The missing CCG guidance could have introduced bias. However, review of the characteristics of the CCGs (Appendix 3) suggests that we did not disproportionately omit CCGs of any particular size or region.
The use of the OpenPrescribing dataset has several limitations. The data are not limited by age or diagnosis, so include inhalers prescribed for childhood asthma, chronic obstructive pulmonary disease and other conditions. Prescribing in these cases will be influenced by different guidelines. An additional limitation is that the OpenPrescribing analysis excludes salbutamol inhalers, so we could not assess the impact of first‐line inclusion of SABA DPIs. These limitations are likely to have substantially diluted the visibility of the relationship we were looking for between local prescribing guidance and prescribing patterns. The interpretation of the analysis related to low dose ICS‐LABA inhalers may have been further complicated and weakened by the use of this type of inhalers as maintenance and reliever therapy prescribing is likely to be influenced by guidance over many years and our analysis only includes a cross sectional snapshot. We expect prescribing habits and responses to changes in guidance to vary between practitioners. 15 Our analysis of CCG prescribing guidance is based on the hypothesis that local guidance is an important driver of prescribing behaviour in primary care. While we cannot establish causation from the comparison of our findings to prescribing data, it is consistent with our hypothesis. Further research is required to determine the impact of local guidance on prescribing.
Variation in guidance is 1 of many factors that may drive prescribing, including cultural beliefs of prescribers and patients, inhaler pricing and wider healthcare system characteristics. More research is needed to understand these. This work should not be considered in isolation by those creating local prescribing guidance. More work is needed to support local guidance authors to optimally combine factors such as clinical efficacy, financial costs, environmental factors and patient preferences.
It is fundamental in any discussion of prescribing to consider patient outcomes. Effective inhaled therapy in asthma depends critically on finding a device that patients are willing and able to use, and each patient’s device should be matched to their abilities and preferences as part of a shared decision‐making process. 14 Despite this, some CCGs did not recommend the DPIs as a first‐line option. This may have resulted in lower rates of DPI prescriptions, and in effect resulted in some patients being prescribed a device which might not have been the most clinically effective for them. SABA use is a main driver of greenhouse gas release and high SABA use has been associated with poor asthma control. 16 Getting the optimal controller device is therefore vital and the lack of DPI option for ICS is potentially detrimental for asthma care, as well as for its carbon footprint. Better asthma control with reductions in SABA use and reduced greenhouse gas release could potentially be achieved by addressing the lack of DPI options in prescribing guidance where this remains.
In considering the clinical effectiveness of MDIs and DPIs, it is important to recognise that the majority of MDIs are not used with spacers even though the importance of spacers for clinical effectiveness has long been recognised. 17 BTS guidelines advise that adolescents have access to DPIs because spacers are unpopular. 14 Adults may show similar preferences.
One reason often cited for avoiding DPIs is patients lacking the inspiratory flow rate to effectively use an MDI, particularly in an exacerbation. This is an important concern for some patients, particularly young children. Other factors must be considered, including patient preference, the strength to actuate the device, the ability to coordinate actuation and inhalation and to breathe in at the correct rate for the device, ability to track number of doses remaining and willingness to use a spacer. Where there are concerns about inspiratory flow rates during acute severe exacerbations, rescue packs of MDI and spacer have been suggested. 18 However, a review of 23 clinical studies of SABAs delivered by DPIs concluded that they were as effective as MDI + spacer or nebulisers. 19 Randomised trials do not necessarily predict outcomes in clinical practice, but real world studies of inhaler switching have generally favoured DPIs over MDIs. 20 , 21 In a recent study where patients were offered a wide array of placebo inhalers, most patients selected a multi‐dose DPI as their preferred device. 22 Not including DPIs as a first‐line recommended option is therefore likely to be detrimental.
A more fundamental question is whether we should be using ICS and as required SABA as separate inhalers. Once‐daily long‐acting ICS + LABA therapy, with a DPI, has been shown in a pragmatic randomised controlled trial to improve asthma control and for patients who prioritise once‐daily treatment this is likely to be a more effective option. 23 Alternatively, the Global Initiative for Asthma (GINA) recommends an as required ICS + LABA inhaler containing formoterol as first‐line therapy, to ensure underlying airway inflammation is controlled as well as symptoms. This approach has the potential to simplify therapy, reduce SABA overuse, reduce overall steroid dose and reduce the risk of severe exacerbations. 24 It also inevitably involves a switch to lower carbon footprint inhalers, as most of the licensed devices and the evidence of effectiveness is for DPIs. Moreover, there is likely to be reduced waste using ICS + LABA inhalers, which all include dose counters, compared to SABAs, which lack dose counters in the UK, and are commonly disposed of when half‐full. 25
As DPIs are not suitable for all patients, reducing the environmental impact of asthma prescribing necessitates measures to lower the impact of the MDIs used. New MDIs with lower carbon footprint propellant gas are expected in coming years but will not have as low a footprint as DPIs and soft mist inhalers. 13 , 26 CCGs may want to consider excluding higher footprint MDIs such as Ventolin Evohaler, Flutiform and Symbicort MDI except in circumstances where no acceptable similarly effective lower footprint device could be found. At the individual level, changing the prescribing of ICS to single rather than double puff regimes could simplify the instructions, halve the greenhouse gas emissions, and reduce the number of prescriptions, trips to the pharmacy and number of inhalers needing disposal. With each prescription there is a risk of delay and subsequent treatment gap so this may improve patient outcomes.
Our results show that there has been a disparity between DPIs and MDIs in local adult asthma guidance in England. There has also been variation in the inclusion or exclusion of high carbon footprint options. There has been in effect a postcode lottery that influences the carbon footprint and potentially clinical quality of treatment. The absence of DPIs as recommended first‐line options is an issue that, where it remains, must be addressed if local commissioners are to comply with the aims of the NHS Long Term Plan (2019) to “switch to dry powder inhalers where clinically appropriate”. 27 It is unlikely that the desired and necessary changes in adult asthma prescribing can be achieved without local guidance, which supports these changes.
5. CONCLUSION
Clinicians in the 21st century have the dual challenges of trying to optimise the clinical care for patients, while also minimising the environmental impact, and consequent harm to health, of that care. Prior to the COVID pandemic prescribing guidance for inhalers in England has not been optimised for either clinical outcomes or environmental impact, with an overdependence on MDIs. Altering local guidance, notably to increase the first‐line inclusion of DPIs where this has not already been done, could be a highly significant and impactful way to improve clinical outcomes and support efforts to address the climate emergency.
COMPETING INTEREST
A.T. has no competing interests. A.W. is a member of the UN Medical and Chemical Technical Options Committee, the NHSE/I inhaler experts working group, and has made unpaid contributions to research on the carbon footprint of inhalers which was funded by AstraZeneca and GlaxoSmithKline. J.S. is married to a general practitioner who is a partner in a dispensing practice in Cambridgeshire.
CONTRIBUTORS
There were no contributing authors other than the named authors.
ACKNOWLEDGEMENTS
We would like to acknowledge Dr Mary Fortune, Department of Public Health and Primary Care, who advised on statistical methods. There was no funding received for this work.
APPENDIX A. METHODS—Logical web searching method:
-
1Google search ‘NHS _______ CCG’ and click on the CCG [clinical commissioning group] website
- Perform an internal search for ‘asthma guidance’
- If there are no suitable results on the first page, then search ‘asthma’ (often these come with 10s of pages of results so it was not feasible to look through the entirety for each CCG)
- If no pathway has been found so far look on CCG website for ‘Guidelines’ heading
Click if present and look for ‘Asthma’ in alphabetical list
If not present, go to ‘Respiratory’ section and look for attached documents
-
2If guidelines heading does not exist, then Google search ‘NHS _______ CCG Formulary’
-
IClick on relevant result (Results may link to a joint formulary website with other CCGs)
-
IIOn formulary website search ‘Asthma’
-
I
- Look in results for any guidelines or pathways
-
IIIIf no suitable results then go to the Respiratory Chapter of the formulary, looking for linked documents on Asthma guidelines
-
III
-
3
If nothing has been found so far, or only national guidelines found, then email CCG (Using general contact email if available, failing this a website contact form and, if that does not exist either, then the FOI dedicated email address)
APPENDIX B.
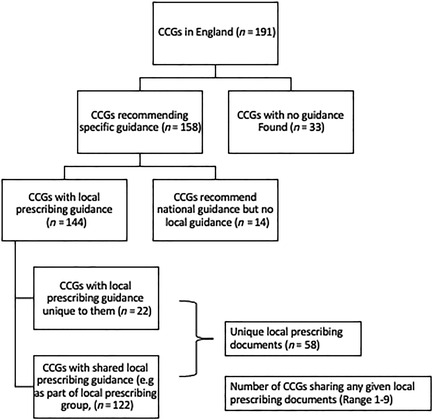
FIGURE 4 Flowchart of output of search for guidance documents as per method in Appendix 1. CCG, clinical commissioning group; MDI, metered‐dose inhaler; DPI, dry powder inhaler
APPENDIX C. Characteristics of the clinical commissioning groups (CCGs) included
The 158 CCGs included in our analysis are compared to the 33 we were unable to include. Our analysis does not appear to disproportionately include or exclude any geographical region or CCG size
| Characteristic 28 | Included CCGs (n = 158) | Absent CCGs (n = 33) |
|---|---|---|
| Geographical region: n (% of CCGs in that region) | ||
| North west | 22 (73) | 8 (27) |
| North east and Yorkshire | 27 (84) | 5 (16) |
| Midlands | 29 (81) | 7 (19) |
| East of England | 17 (85) | 3 (15) |
| London | 27 (84) | 5 (16) |
| South east | 28 (88*) | 4 (13*) |
| South west | 8 (89) | 1 (11) |
| Population size: n (%) | ||
| Small (<250 000) | 80 (83) | 16 (17) |
| Intermediate (250 000–500 000) | 59 (80) | 15 (20) |
| Large (>500 000) | 19 (90) | 2 (10) |
*The percentage values shown for the South east region are an artefact of rounding (87.5 and 12.5%).
APPENDIX D. RESULTS: Denominator variation between Tables 2 and 3
Presence of Ventolin metered‐dose inhaler (MDI) is listed as 34/130, whereas short‐acting β‐agonist inclusion first‐line is 103/133. This difference relates entirely to 2 documents, 1 providing guidance for NHS Somerset clinical commissioning group (CCG) and 1 joint document for NHS Rotherham CCG and NHS Sheffield CCG. NHS Somerset CCG informed us that they allowed GPs to prescribe either a dry powder inhaler (DPI) or MDI at every BTS step, but did not provide information on specific MDI brands offered. The document used by Rotherham and Sheffield states that “all patients should have a salbutamol MDI + Volumatic” but does not give detail on brands.
Presence of HFA 227ea MDIs is written as 83/142, but both low‐ and medium‐dose combination inhalers are given out of 144. This is due to the shared guidance of NHS Hull and NHS East Riding of Yorkshire CCGs, which names Symbicort as an option, but does not clarify if it is the DPI or MDI version.
Low‐dose inhaled corticosteroid (ICS): Option of 2 puffs bi‐daily is given as 126/134 but low‐dose ICS inclusion is given as 113/144. The guidance for 10 CCGs contained no mention of puffs in the low‐dose ICS class so this question could not be answered.
Twigg AJ, Wilkinson A, Smith JN. Local variation in low carbon footprint inhalers in pre‐COVID pandemic primary care prescribing guidelines for adult asthma in England and its potential impact. Br J Clin Pharmacol. 2022;88(12):5083‐5092. doi: 10.1111/bcp.15511
Guarantor Dr James Nicholas Smith.
DATA AVAILABILITY STATEMENT
Only publicly available data was used for this work. Individual guidance documents can be found on CCG or prescribing group websites. OpenPrescribing data is available at https://openprescribing.net. The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. The NHS Net Zero Expert Panel. Delivering a ‘Net Zero’ National Health Service . NHS. Published online 2020:12. https://www.england.nhs.uk/greenernhs/wp-content/uploads/sites/51/2020/10/delivering-a-net-zero-national-health-service.pdf
- 2. Tennison I, Roschnik S, Ashby B, et al. Health care's response to climate change: a carbon footprint assessment of the NHS in England. Lancet Planet Heal. 2021;5(2):e84‐e92. doi: 10.1016/S2542-5196(20)30271-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilkinson AJK, Braggins R, Steinbach I, Smith J. Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England. BMJ Open. 2019;9(10):e028763. doi: 10.1136/bmjopen-2018-028763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fulford B, Mezzi K, Whiting A, Aumônier S. Life‐Cycle Assessment of the Breezhaler® Breath‐Actuated Dry Powder Inhaler. Sustainability. 2021;13(12):6657. doi: 10.3390/SU13126657 [DOI] [Google Scholar]
- 5. Lavorini F, Corrigan CJ, Barnes PJ, et al. Retail sales of inhalation devices in European countries: So much for a global policy. Respir Med. 2011;105(7):1099‐1103. doi: 10.1016/j.rmed.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 6. OpenPrescribing.net. EBM DataLab, University of Oxford. Published 2020. https://openprescribing.net/
- 7. Bloom CI, Douglas I, Olney J, D'ancona G, Smeeth L, Quint JK. Cost saving of switching to equivalent inhalers and its effect on health outcomes. Thorax. 2019;74(11):1078‐1086. doi: 10.1136/thoraxjnl-2018-212957 [DOI] [PubMed] [Google Scholar]
- 8. Wilkinson A, Menzies‐Gow A, Sawyer M, et al. S26 An assessment of short‐acting β2‐agonist (SABA) use and subsequent greenhouse gas (GHG) emissions in five European countries and the consequence of their potential overuse for asthma in the UK. Thorax. 2021;76:A19.1‐A19. doi: 10.1136/thorax-2020-btsabstracts.32 [DOI] [Google Scholar]
- 9. Janson C, Menzies‐Gow A, Nan C, et al. SABINA: An Overview of Short‐Acting β2‐Agonist Use in Asthma in European Countries. Adv Ther. 2020;37(3):1124‐1135. doi: 10.1007/s12325-020-01233-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institute For Health and Care Excellence. Patient Decision Aid Inhalers for Asthma . 2019.
- 11. British Thoracic Society . Position Statement Environment and Lung Health 2019. Published online 2019. https://www.brit-thoracic.org.uk/document-library/governance-and-policy-documents/position-statements/environment-and-lung-health-position-statement-2019/
- 12. Janson C, Henderson R, Löfdahl M, Hedberg M, Sharma R, Wilkinson AJK. Carbon footprint impact of the choice of inhalers for asthma and COPD. Thorax. 2020;75(1):82‐84. doi: 10.1136/thoraxjnl-2019-213744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jeswani HK, Azapagic A. Life cycle environmental impacts of inhalers. J Clean Prod. 2019;237:117733. doi: 10.1016/j.jclepro.2019.117733 [DOI] [Google Scholar]
- 14. British Thoracic Society/SIGN . British Guideline on the Management of Asthma; 2019. [Google Scholar]
- 15. Walker AJ, Pretis F, Powell‐Smith A, Goldacre B. Variation in responsiveness to warranted behaviour change among NHS clinicians: Novel implementation of change detection methods in longitudinal prescribing data. BMJ. 2019;367:l5205. doi: 10.1136/bmj.l5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bloom CI, Cabrera C, Arnetorp S, et al. Asthma‐Related Health Outcomes Associated with Short‐Acting β2‐Agonist Inhaler Use: An Observational UK Study as Part of the SABINA Global Program. Adv Ther. 2020;37(10):4190‐4208. doi: 10.1007/s12325-020-01444-5 [DOI] [PubMed] [Google Scholar]
- 17. Vincken W, Levy ML, Scullion J, Usmani OS, Dekhuijzen PNR, Corrigan CJ. Spacer devices for inhaled therapy: why use them, and how? ERJ Open Res. 2018;4(2):00065‐2018. doi: 10.1183/23120541.00065-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keeley D, Partridge MR. Emergency MDI and spacer packs for asthma and COPD. Lancet Respir Med. 2019;7(5):380‐382. doi: 10.1016/S2213-2600(19)30046-3 [DOI] [PubMed] [Google Scholar]
- 19. Selroos O. Dry‐powder inhalers in acute asthma. Ther Deliv. 2014;5(1):69‐81. doi: 10.4155/tde.13.132 [DOI] [PubMed] [Google Scholar]
- 20. Price D, Thomas V, von Ziegenweidt J, Gould S, Hutton C, King C. Switching patients from other inhaled corticosteroid devices to the Easyhaler®: Historical, matched‐cohort study of real‐life asthma patients. J Asthma Allergy. 2014;7(7):31‐51. doi: 10.2147/JAA.S59386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Price D, Haughney J, Sims E, et al. Effectiveness of inhaler types for real‐world asthma management: Retrospective observational study using the GPRD. J Asthma Allergy. 2011;4:37‐47. doi: 10.2147/JAA.S17709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schreiber J, Sonnenburg T, Luecke E. Inhaler devices in asthma and COPD patients – a prospective cross‐sectional study on inhaler preferences and error rates. BMC Pulm Med. 2020;20(1):1‐12. doi: 10.1186/s12890-020-01246-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woodcock A, Vestbo J, Bakerly ND, et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open‐label, parallel group, randomised controlled trial. Lancet. 2017;390(10109):2247‐2255. doi: 10.1016/S0140-6736(17)32397-8 [DOI] [PubMed] [Google Scholar]
- 24. Cusack RP, Satia I, O’Byrne PM. Asthma maintenance and reliever therapy: Should this be the standard of care? Ann Allergy Asthma Immunol. 2020;125(2):150‐155. doi: 10.1016/j.anai.2020.04.009 [DOI] [PubMed] [Google Scholar]
- 25. Wilkinson AJK, Anderson G. Sustainability in Inhaled Drug Delivery. Pharmaceut Med. 2020;34(3):191‐199. doi: 10.1007/s40290-020-00339-8 [DOI] [PubMed] [Google Scholar]
- 26. Panigone S, Sandri F, Ferri R, Volpato A, Nudo E, Nicolini G. Environmental impact of inhalers for respiratory diseases: decreasing the carbon footprint while preserving patient‐tailored treatment. BMJ Open Respir Res. 2020;7(1):e000571 doi: 10.1136/bmjresp-2020-000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. NHS . The NHS Long Term Plan. 2019. Accessed March 17, 2020. www.longtermplan.nhs.uk
- 28. Office for National Statistics licensed under the Open Government Licence . Mid‐2018 Population Estimates for CCGs in England. 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Only publicly available data was used for this work. Individual guidance documents can be found on CCG or prescribing group websites. OpenPrescribing data is available at https://openprescribing.net. The data that support the findings of this study are available from the corresponding author upon reasonable request.


