Abstract
Background
Psoriatic arthritis (PsA) is a chronic inflammatory arthritis characterised by musculoskeletal and extra‐articular manifestations, most notably psoriasis. While the underlying pathogenetic mechanisms are not yet fully understood, a central role has been identified for the IL‐23/IL‐17 pathway.
Objectives
We briefly describe the role of IL‐23 in the pathogenesis of PsA and go on to describe the available anti‐IL‐23 agents and their place in the management of PsA.
Methods
This is a narrative review of the current literature, focussing on the results of the phase 3 studies in PsA for the IL‐12/23 p40 inhibitor ustekinumab and the more recent IL‐23 p19 inhibitors guselkumab, risankizumab and tildrakizumab.
Results
IL‐23 triggers expression of IL‐17 and other effector cytokines in a variety of cells, leading to tissue inflammation and injury. Targeting IL‐23, particularly with p19 inhibitors, appears to be an effective and safe strategy for multiple clinical domains in PsA, most notably the skin, with some differences in efficacy emerging between these agents.
Conclusion
The development of IL‐23 inhibitors represents a significant advance in the management of psoriatic disease. In the absence of head‐to‐head studies, future data emerging from real‐world experiences of individual IL‐23 p19 inhibitors will help inform the use of these agents in relation to other biologics in PsA.
Keywords: guselkumab, interleukin‐23, psoriatic arthritis, risankizumab, tildrakizumab, ustekinumab
1. INTRODUCTION
Psoriatic arthritis (PsA) is a chronic inflammatory arthritis, belonging to the spectrum of spondyloarthritis (SpA) (Lopez‐Medina et al., 2021). It is characterised by inflammatory musculoskeletal manifestations, including peripheral arthritis, enthesitis, dactylitis and axial disease and an association with cutaneous psoriasis, resulting in a heterogeneous clinical picture. In addition, PsA can be associated with eye (uveitis) and bowel (inflammatory bowel disease) inflammation (Ritchlin et al., 2017), as well as comorbidities like cardiometabolic and mental health disorders (Fragoulis et al., 2020), leading some investigators to adopt the term ‘psoriatic disease’ to collectively describe the whole clinical spectrum. The clinical heterogeneity of PsA is reflected in the numerous indices used to monitor clinical activity and assess response to treatment in PsA trials and clinical practice (Gialouri & Fragoulis, 2021; Kerschbaumer et al., 2018).
The pathogenesis of PsA is complex and distinct from that of rheumatoid arthritis (Merola et al., 2018). In addition to dysfunction of innate and adaptive immunity, there are, as yet incompletely understood, contributory roles for barrier, microbiome and metabolic dysfunction, as well as mechanical stress (Schett, Rahman, et al., 2022). Recognition and understanding of the central role of the IL‐23/IL‐17 pathway in psoriatic disease (Fragoulis et al., 2016) has led to the development of multiple effective agents targeting this pathway, which are now established in clinical practice (Coates, Soriano, et al., 2022; Gossec et al., 2020). Herein, after briefly describing the role of IL‐23 in the pathogenesis of PsA, we provide a narrative review of the current knowledge about anti‐IL‐23 agents and their place in the management of PsA.
2. THE IL‐23/IL‐17 AXIS IN PSORIATIC DISEASE
The central pathogenic role of the IL‐23/IL‐17 pathway, and efficacy of therapies targeting this pathway, in psoriatic disease was first demonstrated in the skin in psoriasis (Liu et al., 2020). This was subsequently also confirmed in PsA, with compelling evidence ranging from genome‐wide association studies (Vecellio et al., 2020), circulating and tissue cytokine studies and, most importantly, efficacy in randomized controlled trials (RCTs).
IL‐23, originating from dendritic cells, monocytes and macrophages, acts on a plethora of other cells, including Th17, Tγδ, type 3 innate lymphoid cells (ILC3s) and natural killer cells, leading to production of IL‐17 and other pro‐inflammatory cytokines and chemokines, such as tumour necrosis factor (TNF), IL‐21 and IL‐22, and ultimately to the observed clinical phenotype (Fragoulis et al., 2016; Siebert et al., 2020). IL‐23 is a heterodimer, comprising a unique p19 subunit and a p40 subunit that is shared with IL‐12 (Figure 1). Engagement of IL‐23 with the IL‐23 receptor (IL‐23R), expressed on a range of cells, as indicated above, results in signalling, mainly through janus kinase 2 and TYK2, leading to phosphorylation of signal transducer and activator of transcription 3 and subsequent expression of the transcription factor RORγt and production of IL‐17 (Sherlock & Cua, 2021). In this model, IL‐23 is considered to be the regulator and IL‐17, and other downstream cytokines, the effector cytokines, although other signalling pathways and models have been identified in a number of tissues (Pastor‐Fernandez et al., 2020). The main homoeostatic role of the IL‐23/IL‐17 pathway appears to be host defence against fungal and bacterial pathogens at mucosal barriers, as well as regulating barrier function (Gaffen et al., 2014).
FIGURE 1.
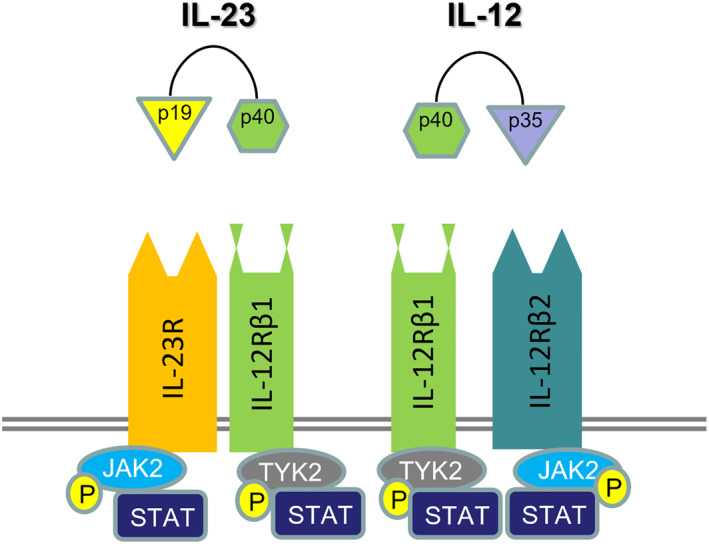
IL‐23 and IL‐12 signal transduction. IL‐23 is a heterodimer consisting of p19 and p40 subunits. The latter is shared with IL‐12. IL‐23 mediates its signal mainly through the (Janus kinase‐signal transducer and activator of transcription) JAK/STAT pathway. P (in circle): phosphorylation R: receptor
Consistent with this model, IL‐23/IL‐23R appear to be orchestrators of pathogenesis in PsA, and SpA more generally, being present in many anatomical sites affected in these conditions (Schett, Rahman, et al., 2022). In a landmark paper, Sherlock et al. described, in a mouse model that T‐cells at the sites of tendon insertion into bone (entheses) express IL‐23R and respond to systemically administered IL‐23 to produce IL‐17, IL‐22 and IL‐6, and inflammatory manifestations in these sites (Sherlock et al., 2012). In humans, myeloid CD14+ cells expressing IL‐23 have recently been identified in spinal entheses obtained from healthy individuals (Bridgewood et al., 2019) and IL‐23 has been shown to be present in facet joints of patients with ankylosing spondylitis (Appel et al., 2013), suggesting there may be similar process in the entheses in humans.
The initial triggers for excessive IL‐23 production (e.g. mechanical stress causing entheseal microtrauma [Van Mechelen & Lories, 2016]) and how this mediates inflammation and tissue injury at remote sites in PsA remain to be elucidated. While the dominant role of the IL‐23/IL‐17 axis is clear‐cut in the skin in psoriasis, the pathogenesis in the musculoskeletal compartment in PsA is more mixed and complex (Belasco et al., 2015). Emerging evidence suggests IL‐23‐activated cells may migrate to sites such as the joints and entheses in PsA and produce cytokines, like IL‐17 and TNF, that further facilitate the local inflammatory process (Gracey et al., 2020; Schett, Rahman, et al., 2022). Along these lines, in a mouse model overexpressing IL‐23 in the skin, the animals initially developed psoriatic‐like skin lesions and subsequently also suggestive musculoskeletal features, such as enthesitis, synovitis and dactylitis. Interestingly, these latter manifestations were not due to higher circulating IL‐23 levels (Chen et al., 2020).
It is increasingly recognised that IL‐17 can be also produced independently of IL‐23 by some cell sub‐populations, like mucosal‐associated invariant T cells, spine‐entheseal Tγδ cells (Cuthbert et al., 2019) and ILC3s (Gracey et al., 2016), or in certain sites, such as the IL‐23‐independent role of IL‐17A in the regulation of epithelial permeability in the gut (Lee et al., 2015). Therefore, the effects of the IL‐23/IL‐17 pathway appear highly cell type and tissue specific. Furthermore, the impact may also be context specific and vary over time. In an animal model of HLA‐B27/Huβ2m transgenic rats, IL‐23 inhibition (with IL‐23R blockade) suppressed arthritis when given before the onset of disease but not in established disease (van Tok et al., 2018); in contrast, in the same model, inhibition of IL‐17A was effective both prophylactically and in established disease (van Tok et al., 2019). Similar results were reported in a collagen induced arthritis mouse model in which treatment with anti‐IL‐23 p19 inhibition led to arthritis amelioration only when this was given before the onset of clinical disease (Cornelissen et al., 2013). This suggests that IL‐23 may play a key role in the development of these diseases, although this is difficult to study in humans and remains poorly understood.
Most importantly, the key role of IL‐23/IL‐17 pathway in the pathogenesis of psoriatic disease is confirmed by the efficacy of multiple agents blocking IL‐23 and IL‐17 in psoriasis and subsequently also PsA (Ghoreschi et al., 2021).
3. IL‐23 INHIBITORS FOR THE TREATMENT OF PSA
There are currently four anti‐IL‐23 agents approved or in the approval stages for psoriasis and/or PsA. The first agent to reach the market was ustekinumab, which blocks both IL‐23 and IL‐12 as it targets their common p40 subunit, while guselkumab, risankizumab and tildrakizumab are monoclonal antibodies against the p19 subunit of IL‐23 (summarised in Table 1). Despite positive results in phase 3 studies in psoriasis, the manufacturers (Eli Lilly) of a fourth IL‐23 p19 inhibitor, mirikizumab, discontinued the psoriasis approval programme for this agent to focus on inflammatory bowel disease (Blauvelt et al., 2022; Fierce Biotech, 2021, ‘www.fiercebiotech.com/biotech/lilly‐scraps‐il‐23‐psoriasis‐program‐despite‐phase‐3‐success‐focuses‐ibd‐race‐against’). Mirikizumab is not discussed further here.
TABLE 1.
IL‐23 targeting drugs approved (or registered for approval) for psoriatic arthritis (PsA)
| Generic name (Trade name) | Target | Approved or in phase 3 studies | Licenced dose for PsA |
|---|---|---|---|
| Ustekinumab (Stelara) | p40 subunit of IL‐23 and IL‐12 | Psoriasis, PsA, Crohn's disease, ulcerative colitis | SC: 45 mg at weeks 0 and 4, then every 12 weeks. |
| Phase 3 stage: Dermatomyositis polymyositis, Vasculitis | If co‐existent severe psoriasis or weight >100 Kg: recommended dose is 90 mg instead of 45 mg | ||
| Guselkumab (Tremfya) | p19 subunit of IL‐23 | Psoriasis, PsA | SC: 100 mg at weeks 0 and 4, then every 8 weeks |
| Phase 3 stage: Crohn's disease, ulcerative colitis | |||
| Risankizumab (Skyrizi) | p19 subunit of IL‐23 | Psoriasis, PsA, Crohn's disease | SC: 150 mg at weeks 0 and 4, then every 12 weeks |
| Phase 3 stage: ulcerative colitis | |||
| Tildrakizumab (Illumetri) | p19 subunit of IL‐23 | Psoriasis, PsA a | Not yet approved (psoriasis: SC 100–200 mg at weeks 0 and 4, then every 12 weeks) |
Abbreviations: mg, milligrammes; PsA, psoriatic arthritis; SC, subcutaneous.
Pre‐registration phase.
3.1. Ustekinumab (IL‐12/23 p40i)
Ustekinumab is a human IgG1 kappa monoclonal antibody and is the only IL‐12/23 p40 inhibitor approved for psoriasis or PsA. The efficacy of ustekinumab in patients with active PsA was evaluated in the PSUMMIT phase 3 RCTs. The primary outcome (ACR20 response at week 24) was achieved more frequently in those who received one of two ustekinumab doses compared to placebo (PSUMMIT‐1: 42.4% for 45 mg dose; 49.5% for 90 mg dose; placebo: 22.8%. PSUMMIT‐2: 43.8% combined for both doses; placebo: 20.2%) (Figure 2) (McInnes et al., 2013; Ritchlin et al., 2014). In PSUMMIT‐2, ustekinumab also demonstrated efficacy in TNF‐experienced patients compared to placebo (36.6% vs. 14.5%). The results were maintained through 2 years (Kavanaugh et al., 2015), with integrated data analysis demonstrating significant reduction of radiographic progression with ustekinumab in patients with active PsA (Kavanaugh et al., 2014). In addition to high hurdle responses in cutaneous psoriasis (Griffiths et al., 2010; Leonardi et al., 2008), ustekinumab, also achieved secondary endpoints in PsA, suggesting efficacy for the other musculoskeletal features, such as enthesitis and dactylitis, in PsA. A small (n = 47) open‐label RCT (ECLIPSA) of PsA patients with enthesitis reported better clearance of enthesitis outcomes at week 24 with ustekinumab compared to those who received TNF‐inhibitors (Araujo et al., 2019). However, the best therapeutic approach for treating enthesitis in PsA remains unclear. In the PsABio real‐world multicentre observational study, outcomes and persistence at 6‐month and 1 year were similar for ustekinumab and TNF‐inhibitors in PsA (Gossec et al., 2022; Smolen et al., 2021).
FIGURE 2.
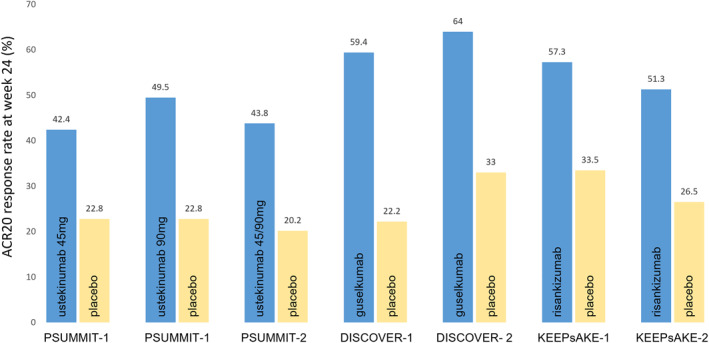
American College of Rheumatology (ACR) 20 response rates in the major phase 3 randomised controlled trials of licenced IL‐23 blocking reagents in psoriatic arthritis (PsA). Percentages of patients achieving ACR20 at week 24, compared to placebo. The ACR20 response rates cannot be directly compared as they are derived from different studies. mg: milligrammes
In psoriasis, ustekinumab demonstrated superior efficacy compared to etanercept (soluble receptor TNF‐inhibitor) (Griffiths et al., 2010), but subsequent studies indicated that targeting IL‐23 with p19 inhibition was superior to p40 inhibition in psoriasis (Gordon et al., 2018), suggesting IL‐12 may have protective effects in psoriasis. As a result of this and the excellent outcomes with p19 inhibition in psoriasis, p19 inhibition has largely become the preferred mode of IL‐23 inhibition in dermatology practice.
3.2. Guselkumab (IL‐23 p19i)
Guselkumab is a fully human IgG1 lambda monoclonal antibody. The DISCOVER phase 3 studies evaluated the efficacy and safety of guselkumab in PsA.
In DISCOVER‐2, 741 biologic‐naïve patients with active PsA (defined by higher entry criteria of ≥5 swollen and ≥5 tender joints, CRP ≥0.6 mg/dl) were randomized (1:1:1) to receive guselkumab 100 mg every 4 weeks, guselkumab 100 mg every 8 weeks or placebo (Mease et al., 2020). Significantly more patients treated with guselkumab achieved the primary ACR20 endpoint at week 24 (64% every 4 weeks, 64% every 8 weeks) than those who received placebo (33%) (Figure 2). Other secondary endpoints were also significantly better at week 24 in both guselkumab groups compared to placebo (Mease et al., 2020). Sustained improvements and safety were maintained through 2 years (McInnes et al., 2022). DISCOVER‐1 evaluated a combination of TNF‐experienced and TNF‐naïve patients. In this study, 381 patients with active PsA (defined as ≥3 swollen and ≥3 tender joints, C reactive protein (CRP) level ≥0.3 mg/dL) were randomized (1:1:1) to receive guselkumab every 4 weeks, guselkumab every 8 weeks or placebo (Deodhar et al., 2020). The study included 118 (30.9%) participants who had previously been exposed to one or two TNF‐inhibitors. A greater proportion of DISCOVER‐1 participants treated with guselkumab (59.4% for every 4 weeks, 52.0% for every 8 weeks) achieved the primary ACR20 endpoint at week 24 compared to those who received placebo (22.2%) (Figure 2) (Deodhar et al., 2020). Response rates were comparable between TNF‐experienced and TNF‐naïve patients. The observed ACR20 response rates were already greater in both guselkumab arms, compared to placebo, by week 8 of the study. Major secondary endpoints were also significantly greater in the guselkumab groups compared to placebo.
Additionally, combined data from DISCOVER‐1 and DISCOVER‐2 showed that dactylitis at week 24 was resolved in more patients treated with guselkumab (59%–64%) compared to placebo (42%). Similar results were observed for resolution of enthesitis (guselkumab 45%–50%; placebo 29%) (Mease et al., 2020). Post hoc analysis of DISCOVER‐1 and DISCOVER‐2 data indicated that baseline characteristics, including sex, body mass index, PsA duration, swollen/tender joint counts, CRP level, extent of psoriasis and conventional synthetic disease‐modifying antirheumatic drug (DMARD) use did not affect the primary or most secondary responses to guselkumab in these trials (Ritchlin et al., 2022).
The safety profile was comparable between guselkumab‐treated and placebo‐treated patients in both DISCOVER trials (Deodhar et al., 2020; Mease et al., 2020).
The subsequent phase 3b COSMOS study specifically evaluated the efficacy of guselkumab in patients with active PsA (≥3 swollen and ≥3 tender joints) who were inadequate responders to TNF inhibitors (Coates, Gossec, et al., 2022). A total of 285 participants were randomised 2:1 to guselkumab 100 mg 8 weekly (after a 4‐week loading regimen) or placebo. Significantly more participants receiving guselkumab, compared to placebo, achieved the primary ACR20 outcome at week 24 (44.4% vs. 19.8%, respectively) and key secondary endpoints, with a favourable efficacy‐safety profile maintained through 56 weeks.
Biomarkers collected in a representative subset of participants in the DISCOVER studies indicated that circulating IL‐17A, IL‐17F and IL‐22 levels correlated with baseline disease activity in the skin but not the joints (Sweet et al., 2021). Treatment with guselkumab led to rapid (week 4) reduction of these cytokine levels, which after 24 weeks of guselkumab treatment achieved levels similar to those seen in matched healthy controls without PsA, suggesting normalisation of IL‐23/IL‐17 effector cytokines with guselkumab treatment. Interestingly, the reductions in IL‐17A and IL‐17F levels were greater in patients treated with guselkumab than ustekinumab, despite similar reductions in CRP levels (Sweet et al., 2021). Furthermore, guselkumab treatment also led to reductions in serum levels of collagen degradation biomarkers that are elevated in PsA (Schett, Loza, et al., 2022)
3.3. Risankizumab (IL‐23 p19i)
Risankizumab is a humanised IgG1 kappa monoclonal antibody. The efficacy of risankizumab was evaluated in PsA in the KEEPsAKE phase 3 RCTs. The KEEPsAKE‐1 study randomised (1:1) 964 biologic‐naïve patients with active PsA (defined as ≥5 swollen and ≥5 tender joints, ≥1 erosion or CRP ≥3 mg/L) despite at least one conventional synthetic DMARD to risankizumab 150 mg or placebo (Kristensen et al., 2022). At week 24, a greater proportion of participants treated with risankizumab achieved ACR20 response (57.3%) compared to those who received placebo (33.5%) (Figure 2). Significant improvements, compared to placebo, were also observed for key secondary endpoints, including enthesitis, dactylitis, cutaneous psoriasis and nail disease.
Risankizumab was well tolerated with a similar safety profile to placebo. The companion KEEPsAKE‐2 trial recruited 444 participants, including participants (46.5%) who had previous inadequate response or intolerance to ≤2 biologic therapies (Ostor et al., 2022). At week 24, significantly more patients treated with risankizumab (51.3%) achieved the primary ACR20 endpoint compared to those who received placebo (26.5%). ACR20 rates were similar when only biologic DMARD‐inadequate responders were compared (risankizumab 45.7% vs. placebo 14.9%).
3.4. Tildrakizumab (IL‐23 p19i)
Tildrakizumab is a humanised IgG1 kappa monoclonal antibody. A phase 2b RCT evaluated four doses of tildrakizumab in patients with active PsA (defined as ≥3 swollen and ≥3 tender joints) (Mease, Chohan, et al., 2021). At week 24, a higher proportion of participants treated with any dose of tildrakizumab (71.4%–79.5%) achieved the primary ACR20 endpoint compared to placebo (50.6%). Response rates were numerically lower for TNF‐experienced compared to TNF‐naïve participants, although response patterns were generally similar. While response rates in the skin were higher in the tildrakizumab groups, results were mixed for other outcomes, with no improvement observed in enthesitis or dactylitis scores following any dose of tildrakizumab. The authors note that relatively low numbers of patients with these features were included in the study, so the study may not have been sufficiently powered to detect differences (Mease, Chohan, et al., 2021).
3.5. Implications and considerations for PsA management relating to IL‐23 inhibitors
The development of IL‐23 inhibitors represents a significant advance in the management of psoriatic disease. While these agents have been used for longer for the treatment of psoriasis, the p19 inhibitors have only more recently become available in clinical practice for PsA, so there is still limited experience and data outside RCTs for PsA. In the absence of head‐to‐head studies to inform treatment choice, the exact positioning in PsA of the IL‐23 inhibitors relative to the other biologic DMARDs remains to be fully established. The updated Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2021 treatment recommendations for PsA include strong recommendations for IL‐23 inhibitors for all domains in PsA, with the exception of dominant axial disease (Coates, Soriano, et al., 2022); the earlier European Alliance of Associations for Rheumatology 2019 recommendations predated the approval of the IL‐23 p19 inhibitors but included the IL‐12/23 p40 inhibitor ustekinumab together with the other licenced biologic DMARDs for patients with peripheral arthritis who had an inadequate response to at least one conventional synthetic DMARD (Gossec et al., 2020).
In clinical practice, patients have heterogeneous and often complex clinical presentations, with variable involvement of multiple tissue domains and associated comorbidities, making the application of treatment guidelines and choice of appropriate biologic therapy difficult. It should also be noted that the phase 3 RCTs used for licencing purposes in PsA specifically recruited patients with active peripheral arthritis, so these participants do not fully represent the disease spectrum of real‐world patient populations (Vandendorpe et al., 2019). In clinical practice, the efficacy of IL‐23 inhibitors across multiple domains in PsA and good safety profile need to be considered when selecting biologic DMARD class. In addition to the efficacy of IL‐23 inhibition for peripheral arthritis, enthesitis and dactylitis described previously, there are other domains that warrant specific consideration when making treatment decisions (Gialouri et al., 2022).
Patients with PsA may also have inflammatory axial involvement, although the prevalence of this is unclear, reflecting the lack of agreed definition for what has been termed ‘axial PsA’. Following the failure of both IL‐23 p19 and p40 inhibition in axial spondyloarthritis (Baeten et al., 2018; Deodhar et al., 2019; Siebert et al., 2019), despite compelling pathophysiological data to support this strategy, there has been much interest and discussion about axial PsA. While axial PsA resembles classical axial spondyloarthritis in many ways, there appear to be demographic, clinical and imaging differences (Feld et al., 2020; Fragoulis et al., 2022; Jadon et al., 2017), leading to uncertainty about whether the results from the ustekinumab and risankizumab studies in axial spondyloarthritis apply to axial PsA. Post hoc analyses of IL‐23 inhibitor phase 3 RCTs in PsA have been reported as suggesting that IL‐23 inhibitors may work for axial involvement in PsA (Helliwell et al., 2020; Mease, Helliwell, et al., 2021). However, there are issues about how axial involvement was retrospectively defined and assessed in these participants who were recruited on the basis of active peripheral joint disease, with the potential for significant bystander effect rather than a true direct benefit in the spine (Braun & Landewe, 2022; Siebert & Marzo‐Ortega, 2021). The issue of axial PsA is currently unresolved and international initiatives are ongoing to better define the concept of axial PsA (Poddubnyy et al., 2021) to facilitate robust RCTs to evaluate the efficacy of IL‐23 inhibitors and other therapies for this. A phase 4 study (STAR) is already underway, evaluating the efficacy of guselkumab, compared to placebo, in biologic‐naïve patients with active axial PsA, defined using imaging (MRI) and clinical (axial and peripheral) criteria (Gladman et al., 2022).
In terms of extra‐articular manifestations of PsA, the IL‐23 p19 inhibitors have demonstrated impressive high hurdle responses, in excess of those reported for TNF inhibitors, in psoriasis (Armstrong et al., 2020; Blauvelt et al., 2017; Sawyer et al., 2019), while there are also interesting data emerging to suggest potential longer term differences between IL‐23 and IL‐17 inhibition in psoriasis, which are beyond the scope of this review but worth noting (Reich et al., 2019). In addition to differences between therapeutic classes, including between the IL‐23 p19 and p40 inhibitors, there also appear to be emerging differences between the various IL‐23 p19 antibodies with observed differences in clinical efficacy and treatment persistence in psoriasis (Yiu et al., 2022). These within class differences have been postulated to relate to the different molecular attributes of the antibodies. For example, guselkumab (which contains a native Fc region) has recently been shown to bind CD64+ myeloid cells (Krueger et al., 2022), which are enriched in psoriatic skin and are the dominant source of IL‐23 (Mehta et al., 2021); in contrast, risankizumab, which has a mutated Fc region, did not bind CD64 (Krueger et al., 2022). Similarly, meta‐analysis suggests that tildrakizumab may be less effective in psoriasis than other IL‐23 p19 inhibitors, which may relate to its lower binding affinity for p19 (Armstrong et al., 2020; Ghoreschi et al., 2021; Sawyer et al., 2019). Understanding the effects of these molecular differences on efficacy in the musculoskeletal domains in PsA will be important. Beyond the skin, the efficacy of ustekinumab (Sands et al., 2019) and the promising results of p19 inhibitors for the treatment of inflammatory bowel disease (Parigi et al., 2022; Sewell & Kaser, 2022) are reassuring in the context of their use in PsA patients with these associated comorbidities. This contrasts with the negative trial results of IL‐17 inhibitors for active Crohn's disease (Hueber et al., 2012; Targan et al., 2016).
3.6. Future directions and conclusions
IL‐23 inhibitors are another important weapon in rheumatologists' arsenal for the treatment PsA, where there remains much unmet clinical need. Targeting IL‐23, particularly with p19 inhibitors, appears to be an effective and safe strategy for multiple clinical domains in PsA, with efficacy on par to that observed for other biologic DMARDs in the joints and superior to TNF inhibitors for cutaneous psoriasis. In the absence of robust head‐to‐head RCTs, data emerging from real‐world experiences of individual IL‐23 p19 inhibitors will further inform the use of these agents in relation to other biologics in PsA, while RCTs in axial PsA will be required to address the uncertainty in this domain. Until these data and/or predictive theranostic biomarkers are available, the choice of biologic therapy for an individual with PsA continues to require careful evaluation and consideration of all affected domains, including extra‐articular manifestations and comorbidities, combined with detailed knowledge of the efficacy of specific biologic therapies in those domains and an appreciation of the likely underlying disease pathogenesis.
AUTHOR CONTRIBUTIONS
All authors made contributed to the concept, review of literature, and writing of the manuscript.
CONFLICT OF INTEREST
George E Fragoulis declares: honoraria from: Abbvie, UCB,Janssen, Novartis, PFizer, Genesis, Lilly, Aenorasis. Stefan Siebert declares research funding from Amgen (previously Celgene), Boehringer‐Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Janssen, and UCB and speaker/consultancy fees from AbbVie, Eli Lilly, GSK, Janssen, and UCB.
ETHICS STATEMENT
This review of the literature does not require ethical approval or informed consent as it does not involve human subjects.
Fragoulis, G. E. , & Siebert, S. (2022). The role of IL‐23 and the use of IL‐23 inhibitors in psoriatic arthritis. Musculoskeletal Care, 20(S1), S12–S21. 10.1002/msc.1694
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Appel, H. , Maier, R. , Bleil, J. , Hempfing, A. , Loddenkemper, C. , Schlichting, U. , Syrbe, U. , & Sieper, J. (2013). In situ analysis of interleukin‐23‐ and interleukin‐12‐positive cells in the spine of patients with ankylosing spondylitis. Arthritis & Rheumatism, 65(6), 1522–1529. 10.1002/art.37937 [DOI] [PubMed] [Google Scholar]
- Araujo, E. G. , Englbrecht, M. , Hoepken, S. , Finzel, S. , Kampylafka, E. , Kleyer, A. , Bayat, S. , Schoenau, V. , Hueber, A. , Rech, J. , & Schett, G. (2019). Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: Results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Seminars in Arthritis and Rheumatism, 48(4), 632–637. 10.1016/j.semarthrit.2018.05.011 [DOI] [PubMed] [Google Scholar]
- Armstrong, A. W. , Puig, L. , Joshi, A. , Skup, M. , Williams, D. , Li, J. , Betts, K. A. , & Augustin, M. (2020). Comparison of biologics and oral treatments for plaque psoriasis: A meta‐analysis. JAMA Dermatol, 156(3), 258–269. 10.1001/jamadermatol.2019.4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten, D. , Ostergaard, M. , Wei, J. C. , Sieper, J. , Jarvinen, P. , Tam, L. S. , Salvarani, C. , Kim, T. H. , Solinger, A. , Datsenko, Y. , Pamulapati, C. , Visvanathan, S. , Hall, D. B. , Aslanyan, S. , Scholl, P. , & Padula, S. J. (2018). Risankizumab, an IL‐23 inhibitor, for ankylosing spondylitis: Results of a randomised, double‐blind, placebo‐controlled, proof‐of‐concept, dose‐finding phase 2 study. Annals of the Rheumatic Diseases, 77(9), 1295–1302. 10.1136/annrheumdis-2018-213328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco, J. , Louie, J. S. , Gulati, N. , Wei, N. , Nograles, K. , Fuentes‐Duculan, J. , Mitsui, H. , Suarez‐Farinas, M. , & Krueger, J. G. (2015). Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis & Rheumatology, 67(4), 934–944. 10.1002/art.38995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt, A. , Kimball, A. B. , Augustin, M. , Okubo, Y. , Witte, M. M. , Rodriguez Capriles, C. , Sontag, A. , Arora, V. , Osuntokun, O. , & Strober, B. (2022). Efficacy and safety of mirikizumab in psoriasis: Results from a 52‐week, double‐blinded, placebo‐controlled, randomised withdrawal, phase III trial (OASIS‐1). British Journal of Dermatology. 10.1111/bjd.21743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt, A. , Papp, K. A. , Griffiths, C. E. , Randazzo, B. , Wasfi, Y. , Shen, Y. K. , Li, S. , & Kimball, A. B. (2017). Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. Journal of the American Academy of Dermatology, 76(3), 405–417. 10.1016/j.jaad.2016.11.041 [DOI] [PubMed] [Google Scholar]
- Braun, J. , & Landewe, R. B. (2022). No efficacy of anti‐IL‐23 therapy for axial spondyloarthritis in randomised controlled trials but in post‐hoc analyses of psoriatic arthritis‐related 'physician‐reported spondylitis'. Annals of the Rheumatic Diseases, 81(4), 466–468. 10.1136/annrheumdis-2021-221422 [DOI] [PubMed] [Google Scholar]
- Bridgewood, C. , Watad, A. , Russell, T. , Palmer, T. M. , Marzo‐Ortega, H. , Khan, A. , Millner, P. A. , Dunsmuir, R. , Rao, A. , Loughenbury, P. , Wittmann, M. , Cuthbert, R. J. , & McGonagle, D. G. (2019). Identification of myeloid cells in the human enthesis as the main source of local IL‐23 production. Annals of the Rheumatic Diseases, 78(7), 929–933. 10.1136/annrheumdis-2018-214944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Deshpande, M. , Grisotto, M. , Smaldini, P. , Garcia, R. , He, Z. , Gulko, P. S. , Lira, S. A. , & Furtado, G. C. (2020). Skin expression of IL‐23 drives the development of psoriasis and psoriatic arthritis in mice. Scientific Reports, 10(1), 8259. 10.1038/s41598-020-65269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates, L. C. , Gossec, L. , Theander, E. , Bergmans, P. , Neuhold, M. , Karyekar, C. S. , Shawi, M. , Noel, W. , Schett, G. , & McInnes, I. B. (2022). Efficacy and safety of guselkumab in patients with active psoriatic arthritis who are inadequate responders to tumour necrosis factor inhibitors: Results through one year of a phase IIIb, randomised, controlled study (COSMOS). Annals of the Rheumatic Diseases, 81(3), 359–369. 10.1136/annrheumdis-2021-220991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates, L. C. , Soriano, E. R. , Corp, N. , Bertheussen, H. , Callis Duffin, K. , Campanholo, C. B. , Chau, J. , Eder, L. , Fernandez‐Avila, D. G. , FitzGerald, O. , Garg, A. , Gladman, D. D. , Goel, N. , Helliwell, P. S. , Husni, M. E. , Jadon, D. R. , Katz, A. , Laheru, D. , Latella, J. , & subcommittees, G. T. R. d. (2022). Group for research and assessment of psoriasis and psoriatic arthritis (GRAPPA): Updated treatment recommendations for psoriatic arthritis 2021. Nature Reviews Rheumatology, 18(8), 465–479. 10.1038/s41584-022-00798-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen, F. , Asmawidjaja, P. S. , Mus, A. M. , Corneth, O. , Kikly, K. , & Lubberts, E. (2013). IL‐23 dependent and independent stages of experimental arthritis: No clinical effect of therapeutic IL‐23 p19 inhibition in collagen‐induced arthritis. PLoS One, 8(2), e57553. 10.1371/journal.pone.0057553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert, R. J. , Watad, A. , Fragkakis, E. M. , Dunsmuir, R. , Loughenbury, P. , Khan, A. , Millner, P. A. , Davison, A. , Marzo‐Ortega, H. , Newton, D. , Bridgewood, C. , & McGonagle, D. G. (2019). Evidence that tissue resident human enthesis gammadeltaT‐cells can produce IL‐17A independently of IL‐23R transcript expression. Annals of the Rheumatic Diseases, 78(11), 1559–1565. 10.1136/annrheumdis-2019-215210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deodhar, A. , Gensler, L. S. , Sieper, J. , Clark, M. , Calderon, C. , Wang, Y. , Zhou, Y. , Leu, J. H. , Campbell, K. , Sweet, K. , Harrison, D. D. , Hsia, E. C. , Ariel, F. , Asnal, C. A. , Berman, A. , Citera, G. , Rodriguez, G. , Savio, V. G. , & van der Heijde, D. (2019). Three multicenter, randomized, double‐blind, placebo‐controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis & Rheumatology, 71(2), 258–270. 10.1002/art.40728 [DOI] [PubMed] [Google Scholar]
- Deodhar, A. , Helliwell, P. S. , Boehncke, W. H. , Kollmeier, A. P. , Hsia, E. C. , Subramanian, R. A. , Xu, X. L. , Sheng, S. , Agarwal, P. , Zhou, B. , Zhuang, Y. , Ritchlin, C. T. , & Group, D.‐S. (2020). Guselkumab in patients with active psoriatic arthritis who were biologic‐naive or had previously received TNFalpha inhibitor treatment (DISCOVER‐1): A double‐blind, randomised, placebo‐controlled phase 3 trial. Lancet, 395(10230), 1115–1125. 10.1016/S0140-6736(20)30265-8 [DOI] [PubMed] [Google Scholar]
- Feld, J. , Ye, J. Y. , Chandran, V. , Inman, R. D. , Haroon, N. , Cook, R. , & Gladman, D. D. (2020). Is axial psoriatic arthritis distinct from ankylosing spondylitis with and without concomitant psoriasis? Rheumatology, 59(6), 1340–1346. 10.1093/rheumatology/kez457 [DOI] [PubMed] [Google Scholar]
- Fierce Biotech . (2021). Lilly scraps IL‐23 psoriasis program despite phase 3 success, focuses on IBD race against AbbVie, J&J. Retrieved 27 July 2022, from www.fiercebiotech.com/biotech/lilly‐scraps‐il‐23‐psoriasis‐program‐despite‐phase‐3‐success‐focuses‐ibd‐race‐against
- Fragoulis, G. E. , Evangelatos, G. , Tentolouris, N. , Fragkiadaki, K. , Panopoulos, S. , Konstantonis, G. , Iliopoulos, A. , Chatzidionysiou, K. , Sfikakis, P. P. , & Tektonidou, M. G. (2020). Higher depression rates and similar cardiovascular comorbidity in psoriatic arthritis compared with rheumatoid arthritis and diabetes mellitus. Therapeutic Advances in Musculoskeletal Disease, 12. 10.1177/1759720X20976975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoulis, G. E. , Pappa, M. , Evangelatos, G. , Iliopoulos, A. , Sfikakis, P. P. , & Tektonidou, M. G. (2022). Axial psoriatic arthritis and ankylosing spondylitis: Same or different? A real‐world study with emphasis on comorbidities. Clinical & Experimental Rheumatology, 40(7), 1267–1272. 10.55563/clinexprheumatol/8zn9z8 [DOI] [PubMed] [Google Scholar]
- Fragoulis, G. E. , Siebert, S. , & McInnes, I. B. (2016). Therapeutic targeting of IL‐17 and IL‐23 cytokines in immune‐mediated diseases. Annual Review of Medicine, 67(1), 337–353. 10.1146/annurev-med-051914-021944 [DOI] [PubMed] [Google Scholar]
- Gaffen, S. L. , Jain, R. , Garg, A. V. , & Cua, D. J. (2014). The IL‐23‐IL‐17 immune axis: From mechanisms to therapeutic testing. Nature Reviews Immunology, 14(9), 585–600. 10.1038/nri3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi, K. , Balato, A. , Enerback, C. , & Sabat, R. (2021). Therapeutics targeting the IL‐23 and IL‐17 pathway in psoriasis. Lancet, 397(10275), 754–766. 10.1016/S0140-6736(21)00184-7 [DOI] [PubMed] [Google Scholar]
- Gialouri, C. G. , Evangelatos, G. , & Fragoulis, G. E. (2022). Choosing the appropriate target for the treatment of psoriatic arthritis: TNFα, IL‐17, IL‐23 or JAK inhibitors? Mediterranean Journal of Rheumatology, 33(Suppl 1), 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialouri, C. G. , & Fragoulis, G. E. (2021). Disease activity indices in psoriatic arthritis: Current and evolving concepts. Clinical Rheumatology, 40(11), 4427–4435. 10.1007/s10067-021-05774-9 [DOI] [PubMed] [Google Scholar]
- Gladman, D. D. , Mease, P. J. , Bird, P. , Soriano, E. R. , Chakravarty, S. D. , Shawi, M. , Xu, S. , Quinn, S. T. , Gong, C. , Leibowitz, E. , Poddubnyy, D. , Tam, L. S. , Helliwell, P. S. , Kavanaugh, A. , Deodhar, A. , Østergaard, M. , & Baraliakos, X. (2022). Efficacy and safety of guselkumab in biologic‐naïve patients with active axial psoriatic arthritis: Study protocol for STAR, a phase 4, randomized, double‐blinded, placebo‐controlled trial. Trials, 23(1), 743. 10.1186/s13063-022-06589-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, K. B. , Strober, B. , Lebwohl, M. , Augustin, M. , Blauvelt, A. , Poulin, Y. , Papp, K. A. , Sofen, H. , Puig, L. , Foley, P. , Ohtsuki, M. , Flack, M. , Geng, Z. , Gu, Y. , Valdes, J. M. , Thompson, E. H. Z. , & Bachelez, H. (2018). Efficacy and safety of risankizumab in moderate‐to‐severe plaque psoriasis (UltIMMa‐1 and UltIMMa‐2): Results from two double‐blind, randomised, placebo‐controlled and ustekinumab‐controlled phase 3 trials. Lancet, 392(10148), 650–661. 10.1016/S0140-6736(18)31713-6 [DOI] [PubMed] [Google Scholar]
- Gossec, L. , Baraliakos, X. , Kerschbaumer, A. , de Wit, M. , McInnes, I. , Dougados, M. , Primdahl, J. , McGonagle, D. G. , Aletaha, D. , Balanescu, A. , Balint, P. V. , Bertheussen, H. , Boehncke, W. H. , Burmester, G. R. , Canete, J. D. , Damjanov, N. S. , Kragstrup, T. W. , Kvien, T. K. , Landewe, R. B. M. , … Smolen, J. S. (2020). EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Annals of the Rheumatic Diseases, 79(6), 700–712. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossec, L. , Siebert, S. , Bergmans, P. , de Vlam, K. , Gremese, E. , Joven‐Ibanez, B. , Korotaeva, T. V. , Lavie, F. , Noel, W. , Nurmohamed, M. T. , Sfikakis, P. P. , Theander, E. , & Smolen, J. S. (2022). Persistence and effectiveness of the IL‐12/23 pathway inhibitor ustekinumab or tumour necrosis factor inhibitor treatment in patients with psoriatic arthritis: 1‐year results from the real‐world PsABio study. Annals of the Rheumatic Diseases, 81(6), 823–830. 10.1136/annrheumdis-2021-221640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracey, E. , Qaiyum, Z. , Almaghlouth, I. , Lawson, D. , Karki, S. , Avvaru, N. , Zhang, Z. , Yao, Y. , Ranganathan, V. , Baglaenko, Y. , & Inman, R. D. (2016). IL‐7 primes IL‐17 in mucosal‐associated invariant T (MAIT) cells, which contribute to the Th17‐axis in ankylosing spondylitis. Annals of the Rheumatic Diseases, 75(12), 2124–2132. 10.1136/annrheumdis-2015-208902 [DOI] [PubMed] [Google Scholar]
- Gracey, E. , Vereecke, L. , McGovern, D. , Frohling, M. , Schett, G. , Danese, S. , De Vos, M. , Van den Bosch, F. , & Elewaut, D. (2020). Publisher correction: Revisiting the gut‐joint axis: Links between gut inflammation and spondyloarthritis. Nature Reviews Rheumatology, 16(9), 536. 10.1038/s41584-020-0486-1 [DOI] [PubMed] [Google Scholar]
- Griffiths, C. E. , Strober, B. E. , van de Kerkhof, P. , Ho, V. , Fidelus‐Gort, R. , Yeilding, N. , Guzzo, C. , Xia, Y. , Zhou, B. , Li, S. , Dooley, L. T. , Goldstein, N. H. , Menter, A. , & Group, A. S. (2010). Comparison of ustekinumab and etanercept for moderate‐to‐severe psoriasis. New England Journal of Medicine, 362(2), 118–128. 10.1056/NEJMoa0810652 [DOI] [PubMed] [Google Scholar]
- Helliwell, P. S. , Gladman, D. D. , Chakravarty, S. D. , Kafka, S. , Karyekar, C. S. , You, Y. , Campbell, K. , Sweet, K. , Kavanaugh, A. , & Gensler, L. S. (2020). Effects of ustekinumab on spondylitis‐associated endpoints in TNFi‐naive active psoriatic arthritis patients with physician‐reported spondylitis: Pooled results from two phase 3, randomised, controlled trials. RMD Open, 6(1), e001149. 10.1136/rmdopen-2019-001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber, W. , Sands, B. E. , Lewitzky, S. , Vandemeulebroecke, M. , Reinisch, W. , Higgins, P. D. , Wehkamp, J. , Feagan, B. G. , Yao, M. D. , Karczewski, M. , Karczewski, J. , Pezous, N. , Bek, S. , Bruin, G. , Mellgard, B. , Berger, C. , Londei, M. , Bertolino, A. P. , Tougas, G. , Travis, S. P. L. , … Secukinumab in Crohn's Disease Study, G . (2012). Secukinumab, a human anti‐IL‐17A monoclonal antibody, for moderate to severe Crohn's disease: Unexpected results of a randomised, double‐blind placebo‐controlled trial. Gut, 61(12), 1693–1700. 10.1136/gutjnl-2011-301668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadon, D. R. , Sengupta, R. , Nightingale, A. , Lindsay, M. , Korendowych, E. , Robinson, G. , Jobling, A. , Shaddick, G. , Bi, J. , Winchester, R. , Giles, J. T. , & McHugh, N. J. (2017). Axial disease in psoriatic arthritis study: Defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Annals of the Rheumatic Diseases, 76(4), 701–707. 10.1136/annrheumdis-2016-209853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh, A. , Puig, L. , Gottlieb, A. B. , Ritchlin, C. , Li, S. , Wang, Y. , Mendelsohn, A. M. , Song, M. , Zhu, Y. , Rahman, P. , McInnes, I. B. , & Group, P. S. (2015). Maintenance of clinical efficacy and radiographic benefit through two years of ustekinumab therapy in patients with active psoriatic arthritis: Results from a randomized, placebo‐controlled phase III trial. Arthritis Care & Research, 67(12), 1739–1749. 10.1002/acr.22645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh, A. , Ritchlin, C. , Rahman, P. , Puig, L. , Gottlieb, A. B. , Li, S. , Wang, Y. , Noonan, L. , Brodmerkel, C. , Song, M. , Mendelsohn, A. M. , McInnes, I. B. , & Study, G. (2014). Ustekinumab, an anti‐IL‐12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: Results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double‐blind, placebo‐controlled PSUMMIT‐1 and PSUMMIT‐2 trials. Annals of the Rheumatic Diseases, 73(6), 1000–1006. 10.1136/annrheumdis-2013-204741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschbaumer, A. , Smolen, J. S. , & Aletaha, D. (2018). Disease activity assessment in patients with psoriatic arthritis. Best Practice & Research Clinical Rheumatology, 32(3), 401–414. 10.1016/j.berh.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Kristensen, L. E. , Keiserman, M. , Papp, K. , McCasland, L. , White, D. , Lu, W. , Wang, Z. , Soliman, A. M. , Eldred, A. , Barcomb, L. , & Behrens, F. (2022). Efficacy and safety of risankizumab for active psoriatic arthritis: 24‐week results from the randomised, double‐blind, phase 3 KEEPsAKE 1 trial. Annals of the Rheumatic Diseases, 81(2), 225–231. 10.1136/annrheumdis-2021-221019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger, J. G. , Eyerich, K. , Geving, C. , Sachen, K. , Hammaker, D. , Bao, P. , & Fourie, A. (2022). Differentiation of therapeutic antibodies targeting IL‐23. In Paper presented at the society for investigative dermatology (SID) 2022 annual meeting. [Google Scholar]
- Lee, J. S. , Tato, C. M. , Joyce‐Shaikh, B. , Gulen, M. F. , Cayatte, C. , Chen, Y. , Blumenschein, W. , Judo, M. , Ayanoglu, G. , McClanahan, T. , Li, X. , & Cua, D. J. (2015). Interleukin‐23‐Independent IL‐17 production regulates intestinal epithelial permeability. Immunity, 43(4), 727–738. 10.1016/j.immuni.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi, C. L. , Kimball, A. B. , Papp, K. A. , Yeilding, N. , Guzzo, C. , Wang, Y. , Li, S. , Dooley, L. T. , Gordon, K. B. , & investigators, P. s. (2008). Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 76‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 1). Lancet, 371(9625), 1665–1674. 10.1016/S0140-6736(08)60725-4 [DOI] [PubMed] [Google Scholar]
- Liu, T. , Li, S. , Ying, S. , Tang, S. , Ding, Y. , Li, Y. , Qiao, J. , & Fang, H. (2020). The IL‐23/IL‐17 pathway in inflammatory skin diseases: From bench to bedside. Frontiers in Immunology, 11, 594735. 10.3389/fimmu.2020.594735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Medina, C. , Molto, A. , Sieper, J. , Duruoz, T. , Kiltz, U. , Elzorkany, B. , Hajjaj‐Hassouni, N. , Burgos‐Vargas, R. , Maldonado‐Cocco, J. , Ziade, N. , Gavali, M. , Navarro‐Compan, V. , Luo, S. F. , Monti, S. , Tae‐Jong, K. , Kishimoto, M. , Pimentel‐Santos, F. M. , Gu, J. , Schiotis, R. , … Dougados, M. (2021). Prevalence and distribution of peripheral musculoskeletal manifestations in spondyloarthritis including psoriatic arthritis: Results of the worldwide, cross‐sectional ASAS‐PerSpA study. RMD Open, 7(1), e001450. 10.1136/rmdopen-2020-001450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes, I. B. , Kavanaugh, A. , Gottlieb, A. B. , Puig, L. , Rahman, P. , Ritchlin, C. , Brodmerkel, C. , Li, S. , Wang, Y. , Mendelsohn, A. M. , Doyle, M. K. , & Group, P. S. (2013). Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double‐blind, placebo‐controlled PSUMMIT 1 trial. Lancet, 382(9894), 780–789. 10.1016/S0140-6736(13)60594-2 [DOI] [PubMed] [Google Scholar]
- McInnes, I. B. , Rahman, P. , Gottlieb, A. B. , Hsia, E. C. , Kollmeier, A. P. , Xu, X. L. , Jiang, Y. , Sheng, S. , Shawi, M. , Chakravarty, S. D. , van der Heijde, D. , & Mease, P. J. (2022). Long‐term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin‐23, through two years: Results from a phase III, randomized, double‐blind, placebo‐controlled study conducted in biologic‐naive patients with active psoriatic arthritis. Arthritis & Rheumatology, 74(3), 475–485. 10.1002/art.42010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease, P. J. , Chohan, S. , Fructuoso, F. J. G. , Luggen, M. E. , Rahman, P. , Raychaudhuri, S. P. , Chou, R. C. , Mendelsohn, A. M. , Rozzo, S. J. , & Gottlieb, A. (2021). Efficacy and safety of tildrakizumab in patients with active psoriatic arthritis: Results of a randomised, double‐blind, placebo‐controlled, multiple‐dose, 52‐week phase IIb study. Annals of the Rheumatic Diseases, 80(9), 1147–1157. 10.1136/annrheumdis-2020-219014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease, P. J. , Helliwell, P. S. , Gladman, D. D. , Poddubnyy, D. , Baraliakos, X. , Chakravarty, S. D. , Kollmeier, A. P. , Hsia, E. C. , Xu, X. L. , Sheng, S. , Agarwal, P. , Zhou, B. , Sweet, K. , Shawi, M. , Karyekar, C. S. , Deodhar, A. , & van der Heijde, D. (2021). Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: A post‐hoc analysis of the phase 3 DISCOVER‐1 and DISCOVER‐2 studies. Lancet Rheumatology, 3(10), e715–e723. 10.1016/s2665-9913(21)00105-3 [DOI] [PubMed] [Google Scholar]
- Mease, P. J. , Rahman, P. , Gottlieb, A. B. , Kollmeier, A. P. , Hsia, E. C. , Xu, X. L. , Sheng, S. , Agarwal, P. , Zhou, B. , Zhuang, Y. , van der Heijde, D. , McInnes, I. B. , & Group, D.‐S. (2020). Guselkumab in biologic‐naive patients with active psoriatic arthritis (DISCOVER‐2): A double‐blind, randomised, placebo‐controlled phase 3 trial. Lancet, 395(10230), 1126–1136. 10.1016/S0140-6736(20)30263-4 [DOI] [PubMed] [Google Scholar]
- Mehta, H. , Mashiko, S. , Angsana, J. , Rubio, M. , Hsieh, Y. M. , Maari, C. , Reich, K. , Blauvelt, A. , Bissonnette, R. , Munoz‐Elias, E. J. , & Sarfati, M. (2021). Differential changes in inflammatory mononuclear phagocyte and T‐cell profiles within psoriatic skin during treatment with guselkumab vs. Secukinumab. Journal of Investigative Dermatology, 141(7), 1707–1718. e1709. 10.1016/j.jid.2021.01.005 [DOI] [PubMed] [Google Scholar]
- Merola, J. F. , Espinoza, L. R. , & Fleischmann, R. (2018). Distinguishing rheumatoid arthritis from psoriatic arthritis. RMD Open, 4(2), e000656. 10.1136/rmdopen-2018-000656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostor, A. , Van den Bosch, F. , Papp, K. , Asnal, C. , Blanco, R. , Aelion, J. , Alperovich, G. , Lu, W. , Wang, Z. , Soliman, A. M. , Eldred, A. , Barcomb, L. , & Kivitz, A. (2022). Efficacy and safety of risankizumab for active psoriatic arthritis: 24‐week results from the randomised, double‐blind, phase 3 KEEPsAKE 2 trial. Annals of the Rheumatic Diseases, 81(3), 351–358. 10.1136/annrheumdis-2021-221048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parigi, T. L. , Iacucci, M. , & Ghosh, S. (2022). Blockade of IL‐23: What is in the pipeline? Journal of Crohn's and Colitis, 16(Supplement_2), ii64–ii72. 10.1093/ecco-jcc/jjab185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor‐Fernandez, G. , Mariblanca, I. R. , & Navarro, M. N. (2020). Decoding IL‐23 signaling cascade for new therapeutic opportunities. Cells, 9(9), 2044. 10.3390/cells9092044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddubnyy, D. , Baraliakos, X. , Van den Bosch, F. , Braun, J. , Coates, L. C. , Chandran, V. , Diekhoff, T. , van Gaalen, F. A. , Gensler, L. S. , Goel, N. , Gottlieb, A. B. , van der Heijde, D. , Helliwell, P. S. , Hermann, K. G. A. , Jadon, D. , Lambert, R. G. , Maksymowych, W. P. , Mease, P. , Nash, P. , … Gladman, D. D. (2021). Axial involvement in psoriatic arthritis cohort (AXIS): The protocol of a joint project of the assessment of SpondyloArthritis international society (ASAS) and the group for research and assessment of psoriasis and psoriatic arthritis (GRAPPA). Therapeutic Advances in Musculoskeletal Disease, 13. 10.1177/1759720X211057975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich, K. , Armstrong, A. W. , Langley, R. G. , Flavin, S. , Randazzo, B. , Li, S. , Hsu, M. C. , Branigan, P. , & Blauvelt, A. (2019). Guselkumab versus secukinumab for the treatment of moderate‐to‐severe psoriasis (ECLIPSE): Results from a phase 3, randomised controlled trial. Lancet, 394(10201), 831–839. 10.1016/S0140-6736(19)31773-8 [DOI] [PubMed] [Google Scholar]
- Ritchlin, C. T. , Colbert, R. A. , & Gladman, D. D. (2017). Psoriatic arthritis. New England Journal of Medicine, 376(10), 957–970. 10.1056/NEJMra1505557 [DOI] [PubMed] [Google Scholar]
- Ritchlin, C. T. , Mease, P. J. , Boehncke, W. H. , Tesser, J. , Schiopu, E. , Chakravarty, S. D. , Kollmeier, A. P. , Xu, X. L. , Shawi, M. , Jiang, Y. , Sheng, S. , Wang, Y. , Xu, S. , Merola, J. F. , McInnes, I. B. , & Deodhar, A. (2022). Sustained and improved guselkumab response in patients with active psoriatic arthritis regardless of baseline demographic and disease characteristics: Pooled results through week 52 of two phase III, randomised, placebo‐controlled studies. RMD Open, 8(1), e002195. 10.1136/rmdopen-2022-002195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchlin, C. T. , Rahman, P. , Kavanaugh, A. , McInnes, I. B. , Puig, L. , Li, S. , Wang, Y. , Shen, Y. K. , Doyle, M. K. , Mendelsohn, A. M. , Gottlieb, A. B. , & Group, P. S. (2014). Efficacy and safety of the anti‐IL‐12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non‐biological and biological anti‐tumour necrosis factor therapy: 6‐month and 1‐year results of the phase 3, multicentre, double‐blind, placebo‐controlled, randomised PSUMMIT 2 trial. Annals of the Rheumatic Diseases, 73(6), 990–999. 10.1136/annrheumdis-2013-204655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands, B. E. , Sandborn, W. J. , Panaccione, R. , O'Brien, C. D. , Zhang, H. , Johanns, J. , Adedokun, O. J. , Li, K. , Peyrin‐Biroulet, L. , Van Assche, G. , Danese, S. , Targan, S. , Abreu, M. T. , Hisamatsu, T. , Szapary, P. , & Marano, C. , & Group, U. S. (2019). Ustekinumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine, 381(13), 1201–1214. 10.1056/NEJMoa1900750 [DOI] [PubMed] [Google Scholar]
- Sawyer, L. M. , Malottki, K. , Sabry‐Grant, C. , Yasmeen, N. , Wright, E. , Sohrt, A. , Borg, E. , & Warren, R. B. (2019). Assessing the relative efficacy of interleukin‐17 and interleukin‐23 targeted treatments for moderate‐to‐severe plaque psoriasis: A systematic review and network meta‐analysis of PASI response. PLoS One, 14(8), e0220868. 10.1371/journal.pone.0220868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett, G. , Loza, M. J. , Palanichamy, A. , FitzGerald, O. , Ritchlin, C. , Bay‐Jensen, A. C. , Nielsen, S. H. , Gao, S. , Hsia, E. C. , Kollmeier, A. P. , Xu, X. L. , Baribaud, F. , & Sweet, K. (2022). Collagen turnover biomarkers associate with active psoriatic arthritis and decrease with guselkumab Treatment in a phase 3 clinical trial (DISCOVER-2). Rheumatology and Therapy, 9(4), 1017–1030. 10.1007/s40744-022-00444-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett, G. , Rahman, P. , Ritchlin, C. , McInnes, I. B. , Elewaut, D. , & Scher, J. U. (2022). Psoriatic arthritis from a mechanistic perspective. Nature Reviews Rheumatology, 18(6), 311–325. 10.1038/s41584-022-00776-6 [DOI] [PubMed] [Google Scholar]
- Sewell, G. W. , & Kaser, A. (2022). Interleukin‐23 in the pathogenesis of inflammatory bowel disease and implications for therapeutic intervention. Journal of Crohn's and Colitis, 16(Supplement_2), ii3–ii19. 10.1093/ecco-jcc/jjac034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock, J. P. , & Cua, D. J. (2021). Interleukin‐23 in perspective. Rheumatology, 60(Suppl 4), iv1–iv3. 10.1093/rheumatology/keab461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock, J. P. , Joyce‐Shaikh, B. , Turner, S. P. , Chao, C. C. , Sathe, M. , Grein, J. , Gorman, D. M. , Bowman, E. P. , McClanahan, T. K. , Yearley, J. H. , Eberl, G. , Buckley, C. D. , Kastelein, R. A. , Pierce, R. H. , LaFace, D. M. , & Cua, D. J. (2012). IL‐23 induces spondyloarthropathy by acting on ROR‐gammat+ CD3+CD4‐CD8‐entheseal resident T cells. Nature Medicine, 18(7), 1069–1076. 10.1038/nm.2817 [DOI] [PubMed] [Google Scholar]
- Siebert, S. , & Marzo‐Ortega, H. (2021). Correspondence on 'No efficacy of anti‐IL‐23 therapy for axial spondyloarthritis in randomised controlled trials but in post‐hoc analyses of psoriatic arthritis‐related 'physician‐reported spondylitis'?' by Braun and Landewe. Annals of the Rheumatic Diseases. 10.1136/annrheumdis-2021-221799 [DOI] [PubMed] [Google Scholar]
- Siebert, S. , McGucken, A. , & McInnes, I. B. (2020). The IL‐23/IL‐17A axis in spondyloarthritis: Therapeutics informing pathogenesis? Current Opinion in Rheumatology, 32(4), 349–356. 10.1097/BOR.0000000000000719 [DOI] [PubMed] [Google Scholar]
- Siebert, S. , Millar, N. L. , & McInnes, I. B. (2019). Why did IL‐23p19 inhibition fail in AS: A tale of tissues, trials or translation? Annals of the Rheumatic Diseases, 78(8), 1015–1018. 10.1136/annrheumdis-2018-213654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen, J. S. , Siebert, S. , Korotaeva, T. V. , Selmi, C. , Bergmans, P. , Gremese, E. , Joven‐Ibanez, B. , Katsifis, G. , Noel, W. , Nurmohamed, M. T. , Richette, P. , Sfikakis, P. P. , de Vlam, K. , Theander, E. , & Gossec, L. (2021). Effectiveness of IL‐12/23 inhibition (ustekinumab) versus tumour necrosis factor inhibition in psoriatic arthritis: Observational PsABio study results. Annals of the Rheumatic Diseases, 80(11), 1419–1428. 10.1136/annrheumdis-2021-220263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet, K. , Song, Q. , Loza, M. J. , McInnes, I. B. , Ma, K. , Leander, K. , Lakshminarayanan, V. , Franks, C. , Cooper, P. , & Siebert, S. (2021). Guselkumab induces robust reduction in acute phase proteins and type 17 effector cytokines in active psoriatic arthritis: Results from phase 3 trials. RMD Open, 7(2), e001679. 10.1136/rmdopen-2021-001679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan, S. R. , Feagan, B. , Vermeire, S. , Panaccione, R. , Melmed, G. Y. , Landers, C. , Li, D. , Russell, C. , Newmark, R. , Zhang, N. , Chon, Y. , Hsu, Y. H. , Lin, S. L. , & Klekotka, P. (2016). A randomized, double‐blind, placebo‐controlled phase 2 study of brodalumab in patients with moderate‐to‐severe Crohn's disease. American Journal of Gastroenterology, 111(11), 1599–1607. 10.1038/ajg.2016.298 [DOI] [PubMed] [Google Scholar]
- Vandendorpe, A. S. , de Vlam, K. , & Lories, R. (2019). Evolution of psoriatic arthritis study patient population characteristics in the era of biological treatments. RMD Open, 5(1), e000779. 10.1136/rmdopen-2018-000779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mechelen, M. , & Lories, R. J. (2016). Microtrauma: No longer to be ignored in spondyloarthritis? Current Opinion in Rheumatology, 28(2), 176–180. 10.1097/BOR.0000000000000254 [DOI] [PubMed] [Google Scholar]
- van Tok, M. N. , Na, S. , Lao, C. R. , Alvi, M. , Pots, D. , van de Sande, M. G. H. , Taurog, J. D. , Sedgwick, J. D. , Baeten, D. L. , van Duivenvoorde, L. M. , & van Duivenvoorde, L. M. (2018). The initiation, but not the persistence, of experimental spondyloarthritis is dependent on interleukin‐23 signaling. Frontiers in Immunology, 9, 1550. 10.3389/fimmu.2018.01550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tok, M. N. , van Duivenvoorde, L. M. , Kramer, I. , Ingold, P. , Pfister, S. , Roth, L. , Blijdorp, I. C. , Sande, M. G. H. , Taurog, J. D. , Kolbinger, F. , & Baeten, D. L. (2019). Interleukin‐17A inhibition diminishes inflammation and new bone formation in experimental spondyloarthritis. Arthritis & Rheumatology, 71(4), 612–625. 10.1002/art.40770 [DOI] [PubMed] [Google Scholar]
- Vecellio, M. , Hake, V. X. , Davidson, C. , Carena, M. C. , Wordsworth, B. P. , & Selmi, C. (2020). The IL‐17/IL‐23 Axis and its genetic contribution to psoriatic arthritis. Frontiers in Immunology, 11, 596086. 10.3389/fimmu.2020.596086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu, Z. , Becher, G. , Kirby, B. , Laws, P. , Reynolds, N. J. , Smith, C. H. , Warren, R. B. , Griffiths, C. , & BADBIR Study Group (2022). Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA Dermatology, e222909. Advance online publication. 10.1001/jamadermatol.2022.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


