Abstract
Background
Alcohol consumption produces feelings of well‐being and stimulation, but also impairs psychomotor performance, disturbs cardiovascular function and sleep, and can disrupt next‐day mood and behavior. A deeper understanding of how the acute effects of alcohol relate to its sleep and morning‐after effects is needed to minimize harm resulting from its use. This study examined relationships between the effects of a high dose of alcohol on subjective and psychomotor measures, nocturnal heart rate, sleep quality, and morning‐after mood and behavior. We hypothesized that alcohol would produce disturbances in cardiovascular and sleep regulation during the night, which would predict morning‐after mood and behavioral performance.
Methods
Thirty‐one men and women participated in two overnight laboratory visits during which they consumed either alcohol (1.0 g/kg for men, 0.85 g/kg for women) or placebo (randomized, crossover design). They consumed the beverage from 8 to 9 pm, and remained in the laboratory overnight for polysomnographic sleep recording. Subjective and behavioral measures were obtained during consumption and at 7–8 am the morning after.
Results
Alcohol increased both negative and positive arousal, urge to drink and sedation, and it impaired performance on behavioral tasks. During sleep, alcohol produced expected tachycardia and detriments in sleep quality including decreased total sleep time, sleep efficiency, and altered sleep architecture. Only modest effects on mood or performance were detected the following morning. The acute sedative‐like effects of alcohol were related to increases in N2 sleep, but not to other disruptions in sleep or nocturnal heart rate, and neither sleep impairments nor nocturnal heart rate were related to mood or task performance the morning after.
Conclusions
The effects of alcohol on sleep and nocturnal heart rate were not strongly related to either its acute or morning‐after effects. These findings do not provide strong support for the idea that alcohol‐induced sleep disruptions underlie morning‐after effects.
Keywords: alcohol, hyperarousal, morning‐after, REM, sleep
Short abstract
Alcohol produces arousal and stimulation, but also impairs psychomotor performance, disturbs cardiovascular function and sleep, and disrupts next‐day mood and behavior. In a sample of healthy adult alcohol drinkers, we examined relationships between the acute effects of alcohol, nocturnal heart rate, sleep quality and morning‐after mood and behavior. During sleep, alcohol increased heart rate and decreased sleep quality, yet modest morning‐after effects were detected. Neither sleep quality nor nocturnal heart rate were related to either acute or morning‐after effects of alcohol.
INTRODUCTION
In 2020, over half of American adults aged 18 years and older reported drinking alcohol in the past month, and approximately one in four adults reported at least one binge drinking episode (4+ drinks in 2 h for females, 5+ for males; NSDUH, 2020). These levels of consumption are associated with long‐term health risks (Stahre et al., 2014) as well as shorter‐term risks related to acute intoxication including impairments in cognitive and psychomotor performance, and cardiovascular function (Buckman et al., 2015; Hunt & Witt, 1994; Oscar‐Berman & Marinković, 2007). Immediately following the period of acute intoxication, alcohol has further adverse effects including disruptions in sleep and cardiovagal tone during sleep, as well as residual next‐day effects including impairments in mood and cognitive function (Arnedt et al., 2011; Earleywine & Martin, 1993; Greenlund et al., 2021a, 2021b; Gunn et al., 2018; Howland et al., 2008a, 2010a, 2010b; Roehrs et al., 1991; Rohsenow et al., 2010; Verster et al., 2020). To understand the mechanisms that mediate these complex effects of alcohol, it is useful to examine the interrelationships among the mood, behavioral and physiological responses to the drug. For example, are acute behavioral responses to alcohol related to its effects on nocturnal cardiovascular function or sleep, and are either of these related to adverse effects the morning‐after alcohol consumption?
The effects of alcohol on sleep have been well documented using polysomnography (PSG; Arnedt et al., 2011; Rohsenow et al., 2010; Roehrs et al., 1999; Stone, 1980; Kleitman, 1939). Alcohol produces variable effects on sleep latency and efficiency, and alters sleep architecture (Arnedt et al., 2011; Greenlund et al., 2021b; Roehrs et al., 1999; Stone, 1980). During the early part of the night, alcohol suppresses REM sleep, but later it increases wakefulness and there is a rebound in REM sleep (Feige et al., 2006; Roehrs & Roth, 2001a, 2001b; Williams et al., 1983). Paradoxically, people sometimes report that alcohol improves their sleep, perhaps because of the reduced objective or subjective latency to sleep (Roehrs & Roth, 2018), and this misperception may contribute to continued use often seen in clinical poor sleep populations like insomnia to cope with presleep cognitive hyperarousal. The hyperarousal arousal theory of insomnia states that heightened cognitive arousal before sleep increases sleep latency and impairs overall sleep quality (Riemann et al., 2010). Heightened cognitive arousal may present with physiological phenomenon like elevated wake and nocturnal heart rate (HR; Freeman et al., 2012). Indeed, a single dose of alcohol (i.e., 0.6 g/kg dose) in patients diagnosed with insomnia objectively improved total sleep time (TST) and increased slow wave sleep (SWS). However, repeated consumption of alcohol decreases TST and SWS (Roehrs & Roth, 2018), leading to what has been proposed as a feed‐forward allostatic framework (Koob & Colrain, 2020) perhaps perpetuating sleep hyperarousal. In this framework, binge intoxication leads to disturbed sleep, which, in turn, leads to further drinking in an attempt to self‐medicate, but instead leads to compromised sleep quality (Koob & Colrain, 2020). The degree to which alcohol‐induced hyperarousal (i.e., indexed via HR) is related to sleep quality and next‐day performance impairments remains unknown. The fact that sleep disruptions lead to poorer cognitive performance (Roehrs et al., 1989a, 1989b), suggest that the effects of alcohol on sleep may contribute to next‐day impairments.
One previous study examined whether the acute mood and cognitive effects of alcohol predict its effects on sleep, and whether the disruptions in sleep account for impairments the following day (Rohsenow et al., 2010). Rohsenow et al. (2010) examined effects of a high dose of alcohol or placebo on sleep and next‐day mood and performance in 95 heavy drinkers. Alcohol decreased sleep efficiency and percent of time in rapid eye movement sleep, and increased the number of awakenings. The morning following alcohol consumption, the subjects showed impaired performance on the Continuous Performance Test and Psychomotor Vigilance Task, and reported dysphoric mood, a component of “hangover.” The dysphoric mood was related to sleep disruptions, but the impairments in performance were not. This suggests that next day negative mood states are somewhat independent of next‐day impairments in performance, and that sleep disruption may mainly affect negative mood states. The next‐day results reported by Rohsenow et al. (2010) are consistent with other studies indicating that disruptions of sleep reduce alertness and increase sleepiness the morning after (Carskadon & Dement, 1982; Roehrs et al., 1989a). However, these studies did not specifically assess the effects of alcohol on HR, which is relevant to the hyperarousal hypothesis.
Other studies have examined whether impairments after acute administration of alcohol are related to morning‐after impairments. For example, McKinney et al. (2012) examined performance during consumption of alcohol or placebo and the day after consumption, and observed similar impairments in performance after acute administration and the next day. Indeed, the impairments on reaction time, divided attention, selective attention, and Stroop interference were of comparable magnitude during acute administration and the next morning, and performance on delayed recognition and irregular interstimulus reaction time was worse during hangover when compared with intoxication.
This study extended these studies in several ways. First, we included measures of nocturnal HR, during the post‐beverage sleep period. Alcohol is known to increase HR, and reduce both the high frequency and time‐domain components of heart rate variability during sleep (Greenlund et al., 2021a), effects that could mediate the disruptions in sleep especially later in the night, and thus the morning‐after effects. Second, we focused on the relationships among different measures of response to alcohol, including its acute effects, its effects on sleep and nocturnal HR, and the morning‐after effects on mood and performance. We hypothesized that the magnitude of responses during consumption (mood or psychomotor) would be related to sleep and cardiovascular disruptions, and that sleep and HR disruptions would be related to morning‐after mood and psychomotor performance impairments.
MATERIALS AND METHODS
Design
This study was part of a larger study on the effects of a high dose of alcohol on sleep, autonomic indices, and cardiovascular function (Greenlund et al., 2021a, 2021b). This study examined the acute subjective and performance effects of evening alcohol consumption in relation to subsequent sleep and cardiovascular function, and the effects of alcohol on sleep in relation to morning‐after mood and performance. Subjects participated in two overnight sessions, in which they consumed alcohol (1.0 g/kg for men, 0.85 g/kg dose for women) or a fluid control beverage.
Participants
Healthy men and women aged 21–45 years were recruited from Michigan Technological University, Montana State University and surrounding communities. Inclusion criteria were body mass index (BMI) 18.5–35 kg/m2 and at least one self‐reported binge drinking episode (i.e., 4–5 drink equivalents within 2 h) within the past 6 months. Participants were excluded if they reported a history of daily tobacco use, a diagnosis of diabetes, current blood pressure or related medications, a diagnosis or current treatment for obstructive sleep apnea, or moderate to severe alcohol use disorder (AUD; DSM‐V American Psychiatric Association, 2013). We only included women who reported a normal menstrual cycle (i.e., 25–32 day average), were not pregnant, breastfeeding, or using hormonal contraceptives.
Procedure
After completing an initial phone screen, eligible participants were invited to the laboratory for an orientation session, where they were familiarized with the study protocol and informed consent process. Subjects underwent an aldehyde dehydrogenase 2 (ALDH‐2) function questionnaire and completed an at‐home sleep apnea screen before participating (excluded if positive). They provided actigraphy data of their sleep (total sleep time, sleep onset latency, sleep efficiency, wake after sleep onset, and awakenings) at home for four nights before the sessions (See Table S1). Procedures were approved by the Michigan Technological University and Montana State University Institutional Review Boards, and are in accordance with the Declaration of Helsinki. This study is part of an ongoing randomized control trial (NCT 03567434; Greenlund et al., 2021a, 2021b).
Participants reported to the sleep research laboratory for three overnight visits. The first visit was a familiarization session to provide subjects with experience with the procedures and confirm the absence of any sleep disorders. The familiarization session included a routine polysomnography (PSG) study assessing obstructive sleep apnea and periodic limb movements. The next two visits used a randomized crossover design for testing with alcohol and placebo, separated by 1 month. These visits were conducted from 4 pm until 8 am the following morning. Upon arrival in the laboratory, participants provided breath and blood samples to confirm abstinence from alcohol. A standardized dinner was provided approximately 2 h before the session began. At 7:00 pm, subjects completed baseline preconsumption questionnaires (mood and subjective state, see below) and behavioral psychomotor tasks. At 8:00 pm and 9:00 pm, subjects consumed a beverage containing alcohol (0.5 g/kg at 8 pm and another 0.5 g/kg at 9 pm; total dose 1 g/kg) or placebo. The dose was adjusted to 0.85 g/kg for women to account for differences in body composition. Alcohol was diluted in a 1:3 ratio of 95% EtOH to desired volume using fruit juice (i.e., orange or cranberry juice). Participants drank the beverage in three equal portions, every 5 minutes over a 15 min period. The fluid control condition (placebo) consisted of the same volume of fruit juice with 1 ml of EtOH to mask the smell. Each drink was served in opaque lidded cups and was sprayed with a mist of 95% EtOH. Breath alcohol content was measured using an Intoximeter Alco‐Sensor breathalyzer (Intoximeters Inc.) every 15 minutes from 8:00 pm to 11:00 pm, except directly after beverage consumption (8:15 and 9:15 pm), and once the following morning at 7 am (Figure 1).
FIGURE 1.
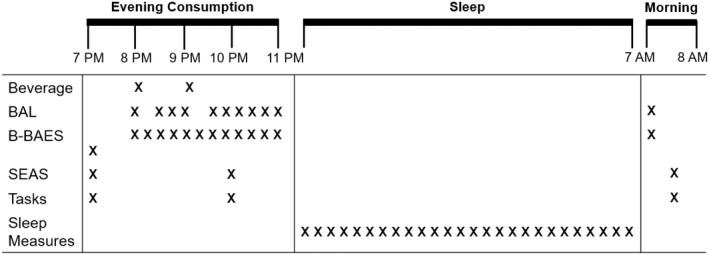
Session timeline, including (i) evening beverage consumption (7–11 pm), (ii) sleep (11 pm – 7 am), and (iii) morning after (7–8 am); BAL: Breath alcohol level, B‐BAES: Brief‐Biphasic Alcohol Effects Scale, SEAS: Subjective Effects of Alcohol Scale; Tasks included Flanker, Pursuit Rotor, Digit Span, Two Column Addition, Sleep measures included HR, total sleep time, sleep latency, sleep efficiency, awakenings, arousals, and percentage of time spent in N1, N2, N3, rapid eye movement (REM) sleep.
To assess subjective responses to the beverage, participants completed the Brief‐Biphasic Alcohol Effects scale (B‐BAES) before the beverage, every 15 minutes from 8:00 pm to 11:00 pm and once the following morning at 7 am. They also completed the Subjective Effects of Alcohol Scale (SEAS) before the beverage, at 10 pm, and the morning after. Participants completed the same series of behavioral psychomotor tasks at 10 pm at the expected time of peak alcohol effects, and again the following morning at 7:30 am. Participants were asked to sleep at 11 pm (i.e., “lights out”), and they were awakened at 7:00 am (Figure 1). They were observed through the night by a registered polysomnography technician who obtained regular measures of heart rate and sleep quality and duration.
Polysomnography
Participants underwent a standard polysomnographic sleep study (Natus Medical; Middleton, WI) on each session. Sleep electroencephalography (EEG) was recorded and scored via 10–20 electrode placement with two frontal, central, and occipital leads referenced to contralateral electrodes placed at the mastoid process on the opposite side of the head. Electrooculography (EOG) and electromyography (EMG) were recorded via two electrodes near the eyes and three electrodes placed on the chin, respectively. HR and rhythm was monitored via pulse oximetry and two‐lead electrocardiogram (ECG), respectively. All physiologic signals were sampled at 250–500 Hz. Sleep staging and arousals were defined and scored according to the AASM by a board‐certified sleep physician. For the present analysis, we report the following measures: Nocturnal HR, total sleep time, sleep latency, sleep efficiency, awakenings, arousals, and proportion of sleep time spent in N1, N2, N3, and Rapid‐Eye‐Movement (REM) sleep.
Measures of subjective effects of alcohol
Brief‐Biphasic Alcohol Effects Scale (B‐BAES; Rueger & King, 2013). The B‐BAES is a 6‐item questionnaire on which subjects rate the degree to which they feel stimulant and sedative effects on 11‐point likert‐type scales.
Subjective Effects of Alcohol Scale (SEAS; Morean et al., 2013). The SEAS is a 14‐item questionnaire on which subjects rate the degree to which they feel effects of alcohol on 11‐point likert‐type scales. It consists of four subscales: Low Arousal Negative, High Arousal Negative, Low Arousal Positive, and High Arousal Positive.
Behavioral psychomotor tasks
Psychomotor tasks were used to assess specific cognitive processes during each session and the morning after. These tasks included a Flanker task to assess attention and distractibility, a pursuit rotor task to assess motor control, the digit span task to assess working memory, and a two column addition task (Psychology Experiment Building Language; PEBL; Mueller & Piper, 2014).
Flanker task
The flanker task in PEBL is a modified version of the Eriksen flanker task (Eriksen & Eriksen, 1974), which assesses conditional accuracy and reaction time with distractors. Participants respond by indicating the direction toward which a central arrow is pointing. On some trials the central arrow is flanked by congruent arrows (pointing in the same direction), on other trials by incongruent arrows (pointing in the opposite direction), dashes, or nothing. The primary outcome measures are congruent flanker response time, noncongruent flanker response time, and total number of errors.
Pursuit rotor
The pursuit rotor task assesses motor dexterity by requiring the participant to use a computer mouse to follow a target moving at a fixed rate along a circular track (Piper et al., 2015). The target moves at a fixed rate, and participants perform four 15‐s trials. The total time on the target in milliseconds is the primary outcome measurement.
Digit span
The digit span task is a working memory task that consists of visual presentation of a series of numbers, one at a time. After the series of numbers is displayed, the participant must respond by typing the numbers in the same in order in which they appeared. The number of digits displayed is increased by one digit every two trials beginning at 3 digits, up to a maximum of 10. The task ends when the participant incorrectly responds on 2 consecutive trials or after 10 digits. The primary outcome measures are memory span (the maximum number of digits correctly recalled).
Two column addition task
The Two Column Addition Task is a measure of cognitive processing speed in which subjects perform mathematical calculations as rapidly as possible. Three two‐digit numbers are shown on the screen and the individual has 15 seconds to type in the sum of these numbers, starting with the left‐most digit. They begin with three practice trials followed by 30 included trials. The primary outcome was accuracy, at 7 pm, at 10 pm following beverage ingestion, and at 7:30 am the following morning.
Statistical analysis
Analyses were conducted using IBM SPSS statistical software (IBM Corp. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
Direct effects of alcohol
We assessed the effects of beverage condition (i.e., alcohol or placebo) on mood (SEAS and B‐BAES) and behavioral task performance (Digit Span, Flanker, Pursuit Rotor, Two Column Addition) the evening of and the morning after consumption, using 2x2 repeated measures ANOVAs (drug [alcohol, placebo] by time prebeverage, postbeverage peak or prebeverage, morning after). For sleep parameters (total sleep time, sleep efficiency, sleep onset latency, arousals, awakenings) and nocturnal HR, we used paired t‐tests to compare alcohol and placebo beverage conditions.
Relationships between direct effects of alcohol and sleep
To determine whether acute responses to alcohol during the evening were related to sleep disruptions or HR during the ensuing night, we correlated peak mood and behavioral effects during consumption with sleep parameters and nocturnal HR. First, for placebo and alcohol sessions separately, we calculated peak change from prebeverage baseline for all mood and behavioral measures. We then subtracted placebo session peak change scores from alcohol peak changes scores to isolate the alcohol‐induced effect. Then we calculated the difference between sleep parameters and nocturnal HR between placebo and alcohol (alcohol minus placebo). We then correlated the peak change difference scores with the sleep parameter and nocturnal change scores to examine potential associations.
Relationships between nocturnal effects of alcohol and morning‐after mood and behavior
To determine whether alcohol‐induced changes in sleep or nocturnal HR were related to morning‐after mood and behavior, we correlated the same sleep parameter and nocturnal HR difference scores (alcohol minus placebo) used in the earlier analyses with any significant morning‐after mood and behavior effects. First, we calculated morning‐after effects for placebo and alcohol sessions separately by subtracting morning‐after responses from prebeverage responses. Then we subtracted placebo session morning‐after change from prebeverage from alcohol morning‐after change from prebeverage to isolate the morning‐after alcohol effect. We then correlated the sleep parameter and nocturnal change scores with morning‐after mood and behavioral alcohol effects to examine potential associations.
RESULTS
Participants
Participants were male and female social drinkers in their mid‐20s (Table 1). Most participants were Caucasian, and reported fewer than 5 episodes of binge alcohol use in the past 6 months. Participants' sleep patterns (total sleep time, sleep onset latency, sleep efficiency, wake after sleep onset, and awakenings) were normal during the 4 prestudy nights and did not differ significantly before placebo and alcohol sessions (Supplementary Table S1).
TABLE 1.
Demographic characteristics [N = 31].
| Sex (M:F) | 15:16 |
|---|---|
| Age | 25.3 (1.1) |
| Height | 170.5 (1.9) |
| Weight | 78.9 (2.5) |
| Race | |
| White | 90.4% |
| Asian | 9.7% |
| Ethnicity | |
| Hispanic | 12.9% |
| Not Hispanic | 87.1% |
| Binge Episodes* (last 6 months) | 4.7 (0.6) |
Data represented as mean (SEM) or percentage. * A binge was defined as 5 or more drinks per occasion in men, and 4 or more drinks per occasion in women.
Breath alcohol content
Mean BAC values during the sessions showed that on average subjects attained expected alcohol levels (Figure 2, mean peak BAC = 0.1%, SD = 0.02%), and men and women did not differ.
FIGURE 2.
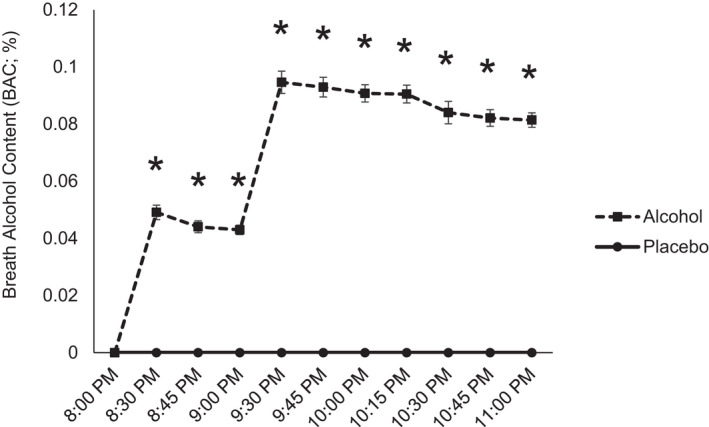
Mean (±SEM) Breath Alcohol Content (mg/L) after consumption of placebo (solid line, circle) and alcohol (dashed line, diamond) during the session and the following morning. * paired t‐test p > 0.05.
Data distribution transformations
Data from the SEAS—High Arousal Negative, Low Arousal Negative, Urge to Drink, B‐BAES—Sedation and Flanker Total Errors was heavily right skewed (>1.5) and contained zero values. Box‐Cox transformations were performed to normalize skewed data (Box & Cox, 1964) for subsequent analyses. Following Box‐Cox transformation, SEAS—High Arousal Negative was still heavily skewed (>1.5), thus we excluded it from subsequent analyses due to an apparent floor effect.
Acute evening effects of alcohol on mood and behavior
Alcohol acutely increased SEAS—Low Arousal Negative (drug*time, p < 0.001), High Arousal Positive (drug*time, p < 0.005), and Urge to Drink ratings (drug*time, p < 0.05), and B‐BAES – Sedation (drug*time, p < 0.005), from prebeverage to postbeverage (Figure 3). Alcohol also acutely impaired psychomotor performance (Figure 4) on the Flanker (Congruent Flanker RT drug*time, p < 0.05, Total Errors drug*time, p < 0.05), Pursuit Rotor (drug*time, p < 0.05), and Two Column Addition tasks (drug*time, p < 0.05).
FIGURE 3.
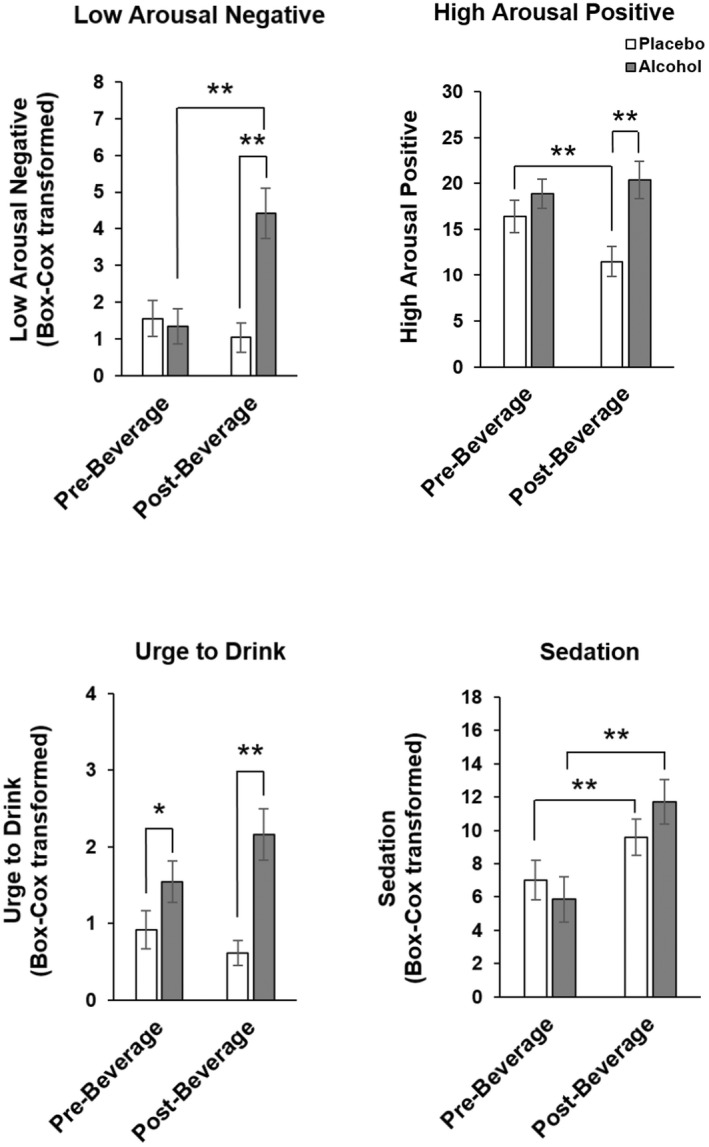
Acute evening effects of alcohol on mood. Data represented as mean (± SEM) SEAS Low Arousal Negative, High Arousal Positive and Urge to Drin and BBAES Sedation ratings pre‐ and postbeverage. Pairwise t‐test *p < 0.05, **p < 0.005.
FIGURE 4.
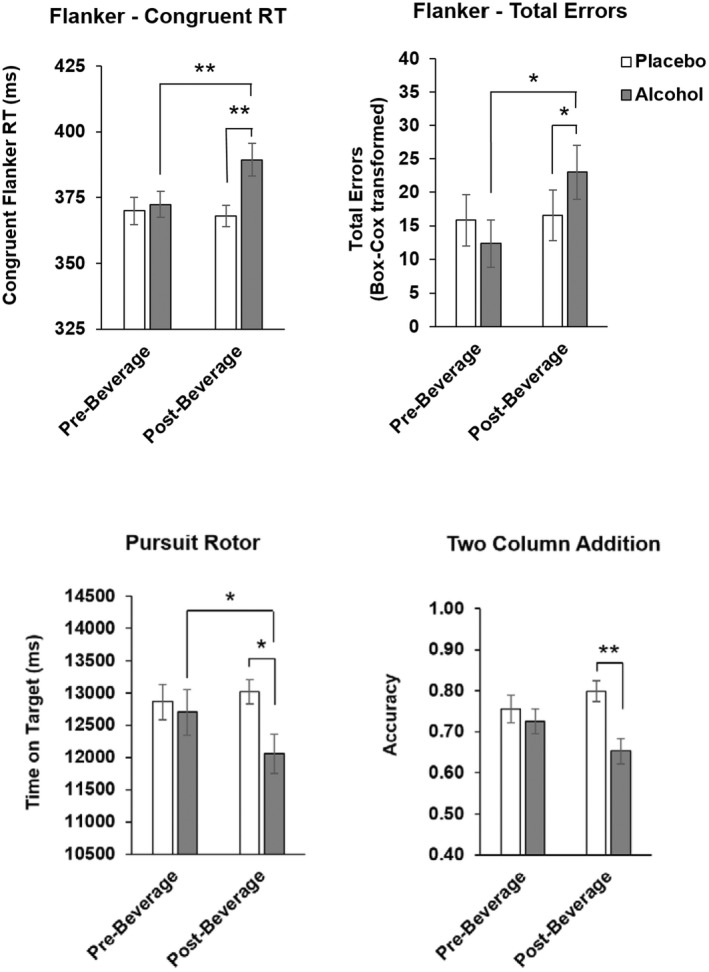
Acute evening effects of alcohol on psychomotor task performance. Data represented as mean (± SEM) Flanker Task Congruent Flanker RT (ms) and Total Errors, Pursuit rotor Time on Target (ms), and Two Column Addition Accuracy pre‐ and postbeverage. Pairwise t‐test *p < 0.05, **p < 0.005.
Effects of alcohol on sleep and nocturnal HR
Alcohol significantly decreased total sleep time, sleep efficiency, and percentage of time spent in REM sleep, while significantly increasing percentage of time spent in N2 stage sleep (Table 2; previously reported in Greenlund et al., 2021a, 2021b). Alcohol also significantly increased nocturnal HR when compared to placebo (Table 2).
TABLE 2.
Effects of alcohol on sleep and nocturnal heart rate (HR).
| Measure | Placebo | Alcohol | T‐test p‐value |
|---|---|---|---|
| Total Sleep Time (min) | 436.6 (4.5) | 421.3 (7.1) | p < 0.05 |
| Sleep Efficiency (%) | 91.2 (1.0) | 88.0 (1.5) | p < 0.05 |
| Sleep Latency (min) | 6.9 (1.2) | 5.3 (0.9) | ns |
| Arousals (#) | 55.5 (22.1) | 54.7 (3.4) | ns |
| Awakenings Index | 3.0 (0.2) | 3.3 (0.2) | ns |
| Arousal Index | 7.9 (0.5) | 8.2 (0.5) | ns |
| N1 (%) | 5.3 (0.5) | 5.3 (0.4) | ns |
| N2 (%) | 51.4 (1.4) | 54.2 (1.1) | p < 0.05 |
| N3 (%) | 23.6 (1.5) | 23.9 (1.0) | ns |
| REM (%) | 20.0 (0.9) | 16.5 (0.9) | p < 0.005 |
| Nocturnal HR | 56.4 (1.6) | 65.0 (2.0) | p < 0.001 |
All data represented as mean (SEM), all significant differences are bolded.
Morning‐after effects of alcohol on mood and behavior
The morning after, alcohol had modest mood effects compared to placebo, increasing SEAS—Low Arousal Negative (drug*time, p < 0.005) and B‐BAES sedation (drug*time, p < 0.05) and decreasing Urge to Drink (drug*time, p < 0.05; Figure 5). Digit Span performance improved the morning after alcohol (drug*time, p = 0.044; Figure 5).
FIGURE 5.
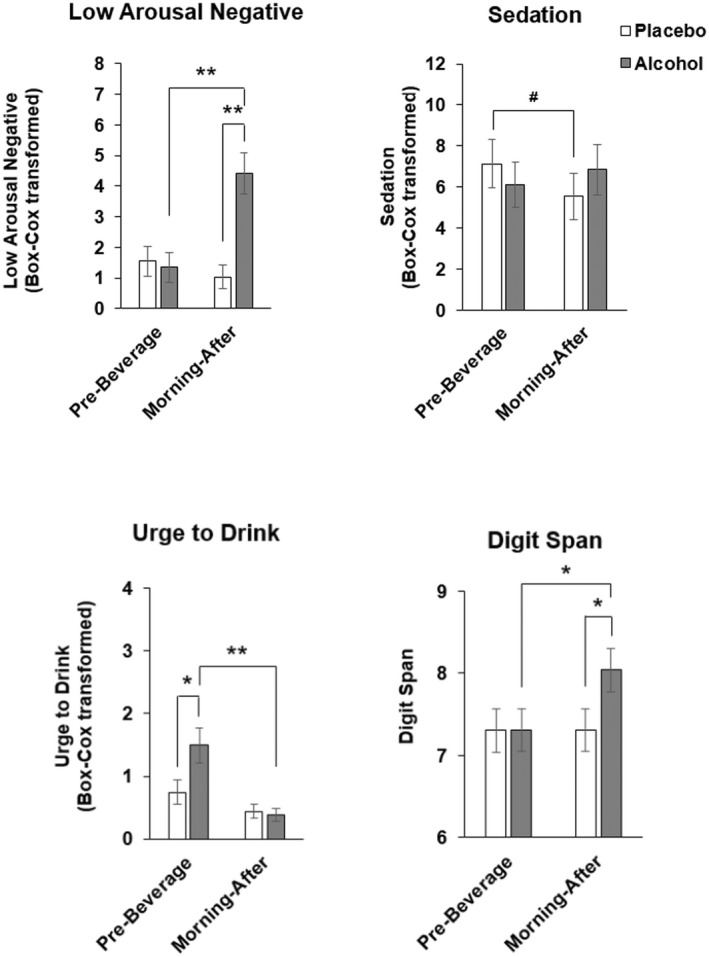
Morning‐after effects of alcohol on mood and psychomotor task performance. Data represented as mean (± SEM) SEAS Low Arousal Negative and Urge to Drink, BBAES Sedation and Digit Span prebeverage and morning after. Pairwise t‐test # p = 0.07, *p < 0.05, **p < 0.005.
Relationships between acute evening effects of alcohol, sleep disruptions, and nocturnal HR
Most of the acute subjective and behavioral effects of alcohol were not directly related to changes in sleep parameters or nocturnal HR. Only alcohol‐induced increase in subjective sedation (B‐BAES—Sedation scale) was significantly correlated (r = 0.5, p < 0.05) with more time spent in N2 stage sleep (Figure 6).
FIGURE 6.
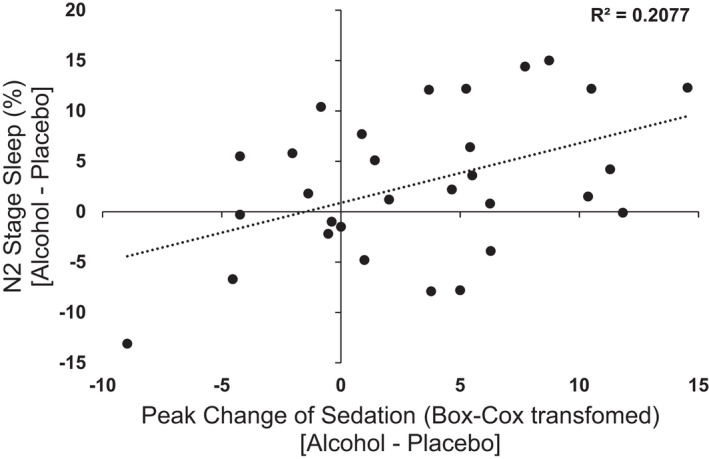
Scatterplot of peak change in evening BBAES Sedation ratings [Alcohol – Placebo] and percentage of time spent in N2 stage sleep [Alcohol – Placebo]. Data represented as individual participant data points. Increased sedation during evening consumption was positively associated with increase in percentage of time spent in N2 stage sleep.
Relationships between nocturnal effects of alcohol and morning‐after mood and behavior
None of the nocturnal effects of alcohol on sleep and HR were significantly associated with any morning‐after mood and behavior changes.
In secondary analyses, we also examined responses to alcohol (subjective, behavioral and sleep) during the sessions in relation to habitual alcohol consumption, but found no significant relationships.
DISCUSSION
This study examined the effects of a high dose of alcohol on subjective and psychomotor measures, cardiovascular function, sleep quality, and morning‐after mood and behavior in healthy adults. We hypothesized alcohol‐induced disturbances in cardiovascular and sleep function during the night would predict morning‐after mood states and behavioral performance. Alcohol produced its expected acute effects on mood, behavior, and sleep. Immediately after consumption, it increased ratings of low arousal negative, high arousal positive, urge to drink and sedation, and it impaired psychomotor performance. During the night, alcohol decreased total sleep time, sleep efficiency, and percentage of time spent in REM stage sleep and increased nocturnal HR. The increase in nocturnal HR was consistent with an alcohol‐induced state of hyperarousal during the night, although other measures did not support this idea, such as sleep latency or arousal index. The acute subjective sedative effects of alcohol were related to increases in N2 stage sleep, but not to other disruptions in sleep or nocturnal cardiovascular function. The morning after alcohol administration, subjects reported increases in negative, low arousal states and feeling sedated, decreased urge to drink and, unexpectedly, improved digit span performance. Impairments in sleep and nocturnal cardiovascular function were not related to mood or task performance the morning after. Thus, although alcohol produced its expected acute effects, including elevated nocturnal cardiovascular activity, these were not related to residual morning‐after effects.
These findings did not support the idea that hyperarousal accounted for the morning‐after effects. The hyperarousal theory of insomnia suggests increased cognitive and autonomic activity preceding and during sleep leads to increased sleep onset latency, decreased total sleep time and sleep efficiency, and overall poorer sleep quality (Kalmbach et al., 2020; Riemann et al., 2010). These sleep disruptions are related to daytime cognitive impairment in individuals with insomnia, and may also play a role in cognitive impairments the morning after alcohol consumption (Fortier‐Brochu et al., 2012; Fortier‐Brochu & Morin, 2014). In this study, alcohol increased nocturnal HR and impaired sleep, but did not significantly increase sleep latency or number of awakenings. Further, sleep disruptions were not related to morning‐after mood and behavior. It is possible this dose of alcohol was not high enough to produce appreciable levels of hyperarousal in these participants.
A novel relationship identified within this study was the association between subjective sedating effects of alcohol and percentage of time spent in N2 stage sleep. Although alcohol has previously been shown to increase both subjective sedation and N2 stage sleep (Greenlund et al., 2021b; Hendler et al., 2013; King et al., 2011; Martin et al., 1993), their interrelationship has not been reported. N2 sleep occurs repeatedly throughout sleep, preceding N3 stage SWS, and can account of 50% of sleep time (Shrivastava et al., 2014). In this study, the increase in N2 stage sleep was accompanied by decreased REM sleep, consistent with overall lighter sleep after alcohol. Rohsenow et al. (2006) reported that subjective sedation after alcohol was associated with decreased sleep onset latency and improved perceived sleep quality. In contrast, we did not detect changes in sleep onset, but subjects' self‐reported sedation was associated with overall lighter sleep quality. These findings provide some evidence that individuals who experience more sedating effects of alcohol may exhibit larger alcohol‐related sleep disruptions, which is not intuitively consistent with the hyperarousal hypothesis.
Neither the effects of alcohol on nocturnal cardiovascular function nor sleep were related to morning‐after alcohol effects. These results parallel those of Rohsenow et al. (2010), in which vodka or bourbon (mean BAC of 0.11%) decreased sleep efficiency and REM stage sleep in heavy drinkers. However, as in this study, the sleep disruptions did not mediate next‐day effects. The finding that alcohol‐induced sleep disruptions are not directly related to next‐day psychomotor or cognitive function is somewhat surprising in view of the associations between sleep quality and performance in clinical populations with insomnia (Blackwell et al., 2006; Fortier‐Brochu et al., 2012; Wardle‐Pinkston et al., 2019). It is possible that insomnia entails more severe sleep disruption than a moderate dose of alcohol. Alternatively, the disruptions seen in clinical populations may be the result of cumulative impairments after repeated sleep disruptions across multiple nights. Similarly, alcohol may produce greater impairments in individuals who habitually consume large amounts of alcohol over repeated days. These questions remain to be addressed in future studies.
Surprisingly, subjects' performance on the digit span task was better the morning after alcohol compared to placebo. This is inconsistent with reports that both acute alcohol and sleep deprivation impair working memory function (Alhola & Polo‐Kantola, 2007; Frenda & Fenn, 2016; Martinez‐Cancino et al., 2016; Mintzer, 2007; Scott Saults et al., 2007; Stevens et al., 2022). Yet, other studies either did not detect the effects of intoxication on next‐day cognitive and behavioral performance (Rohsenow et al., 2006, 2010) or found nonsignificant improvements in performance (Howland et al., 2010b). Performance tasks vary in their sensitivity to the effects of alcohol (Scott Saults et al., 2007), and indeed, in this study the acute dose of alcohol failed to impair digit span performance. Yet, there is a possibility that the increase in N2 stage sleep after alcohol contributed to the improved performance the morning after. Memory consolidation is strongly associated with N2 stage sleep (Okuda et al., 2021; Rauchs et al., 2005; Stickgold, 2005). Sleep spindles, distinct waxing, and waning electrical neural oscillations (11–15 Hz), occur most frequently and prominently during N2 stage sleep (De Gennaro & Ferrara, 2003; Fogel & Smith, 2011). These spindles contribute to brain plasticity and memory consolidation (Boutin & Doyon, 2020; Fogel et al., 2017; Fogel & Smith, 2011; Rasch & Born, 2013). Although in this study the improved performance was seen with new stimuli viewed during the morning test, these associations between sleep and working memory function might have contributed to the improved memory performance the morning after. Whether there is a truly paradoxical improvement in task performance the morning after alcohol consumption, or whether this effect reflected simple variation in performance, remains to be determined.
The study had several limitations. First, the analysis combined men and women in spite of known sex and hormone‐related differences in responses to alcohol (Flores‐Bonilla & Richardson, 2020; Warren et al., 2021). Possible sex differences will be addressed in a future study with a larger N. Second, in this subject sample, and under these conditions, alcohol produced relatively modest stimulating effects during the consumption period, and little impairment the morning after. The controlled laboratory environment may have dampened some of the expected mood effects of alcohol. It is likely that greater impairments would be seen with a higher dose of alcohol, or a less resilient sample of social drinker who do not report significant history of binge drinking. In this study, the alcohol was administered across two fifteen‐minute intervals separated by an hour. The subjects were free of other drugs at the time of testing, and had good sleep the nights before the session. In contrast, in many naturalistic drinking settings, individuals consume alcohol over many hours, they consume alcohol on consecutive days, and often combine alcohol with other drugs and sleep deprivation. Further, in natural settings there may be disruptions to sleep that interact with the alcohol effects, whereas in the study the laboratory environment was quiet and uninterrupted. Further, it has been reported that some individuals are resistant to hangover (review Howland et al., 2008b), which may be related to personality traits (Earleywine, 1993; MacAndrew, 1965) or genetic predisposition (Newlin & Pretorius, 1990; Wall et al., 2005). It is possible that our study sampled individuals who were resistant to hangover effects.
This study adds to our understanding of the effects of alcohol on sleep, nocturnal cardiovascular function, and morning‐after mood and behavior. Consistent with previous findings, alcohol disrupted sleep and impacted morning‐after mood, yet alcohol‐induced sleep disturbances were not related to morning‐after effects. We found little support for the idea that alcohol‐induced increase in nocturnal HR accounted for morning‐after impairments. It is likely that higher doses of alcohol, or repeated doses, would produce greater behavioral and sleep disruptions, and that more pronounced effects would be observed in more vulnerable subject samples. Further research is needed to identify the behavioral and physiological mechanisms underlying the adverse effects of alcohol consumption.
FUNDING INFORMATION
This research was supported by National Institute on Alcohol Abuse and Alcoholism (R01 AA024892; JRC). EP was supported by National Institute on Drug Abuse (T32 DA043469 and F31 DA049391).
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.
Supporting information
Table S1
Pabon, E. , Greenlund, I.M. , Carter, J.R. & de Wit, H. (2022) Effects of alcohol on sleep and nocturnal heart rate: Relationships to intoxication and morning‐after effects. Alcoholism: Clinical and Experimental Research, 46, 1875–1887. Available from: 10.1111/acer.14921
REFERENCES
- Alhola, P. & Polo‐Kantola, P. (2007) Sleep deprivation: impact on cognitive performance. Neuropsychiatric Disease and Treatment, 3(5), 553–567. [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013) Diagnostic and statistical manual of mental disorders, 5th edition. Washington, DC: American Psychiatric Association Publishing. [Google Scholar]
- Arnedt, J.T. , Rohsenow, D.J. , Almeida, A.B. , Hunt, S.K. , Gokhale, M. , Gottlieb, D.J. et al. (2011) Sleep following alcohol intoxication in healthy, young adults: effects of sex and family history of alcoholism. Alcoholism, Clinical and Experimental Research, 35, 870–878. 10.1111/j.1530-0277.2010.01417.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell, T. , Yaffe, K. , Ancoli‐Israel, S. , Schneider, J.L. , Cauley, J.A. , Hillier, T.A. et al. (2006) Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61, 405–410. [DOI] [PubMed] [Google Scholar]
- Boutin, A. & Doyon, J. (2020) A sleep spindle framework for motor memory consolidation. Philosophical Transactions of the Royal Society, B: Biological Sciences, 375(1799), 20190232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box, G.E.P. & Cox, D.R. (1964) An analysis of transformations. Journal of the Royal Statistical Society: Series B: Methodological, 26(2), 211–243. [Google Scholar]
- Buckman, J.F. , Eddie, D. , Vaschillo, E.G. , Vaschillo, B. , Garcia, A. & Bates, M.E. (2015) Immediate and complex cardiovascular adaptation to an acute alcohol dose. Alcoholism, Clinical and Experimental Research, 39, 2334–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon, M.A. & Dement, W.C. (1982) Nocturnal determinants of daytime sleepiness. Sleep, 5(suppl_2), S73–S81. [DOI] [PubMed] [Google Scholar]
- De Gennaro, L. & Ferrara, M. (2003) Sleep spindles: an overview. Sleep Medicine Reviews, 7(5), 423–440. [DOI] [PubMed] [Google Scholar]
- Earleywine, M. (1993) Personality risk for alcoholism covaries with hangover symptoms. Addictive Behaviors, 18(4), 415–420. [DOI] [PubMed] [Google Scholar]
- Earleywine, M. & Martin, C.S. (1993) Anticipated stimulant and sedative effects of alcohol vary with dosage and limb of the blood alcohol curve. Alcoholism: Clinical and Experimental Research, 17(1), 135–139. [DOI] [PubMed] [Google Scholar]
- Eriksen, B.A. & Eriksen, C.W. (1974) Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. [Google Scholar]
- Feige, B. , Gann, H. , Brueck, R. , Hornyak, M. , Litsch, S. , Hohagen, F. et al. (2006) Effects of alcohol on polysomnographically recorded sleep in healthy subjects. Alcoholism, Clinical and Experimental Research, 30, 1527–1537. [DOI] [PubMed] [Google Scholar]
- Flores‐Bonilla, A. & Richardson, H.N. (2020) Sex differences in the neurobiology of alcohol use disorder. Alcohol Research: Current Reviews, 40(2), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel, S.M. & Smith, C.T. (2011) The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep‐dependent memory consolidation. Neuroscience & Biobehavioral Reviews, 35(5), 1154–1165. [DOI] [PubMed] [Google Scholar]
- Fogel, S. , Vien, C. , Karni, A. , Benali, H. , Carrier, J. & Doyon, J. (2017) Sleep spindles: a physiological marker of age‐related changes in gray matter in brain regions supporting motor skill memory consolidation. Neurobiology of Aging, 49, 154–164. [DOI] [PubMed] [Google Scholar]
- Fortier‐Brochu, E. & Morin, C.M. (2014) Cognitive impairment in individuals with insomnia: clinical significance and correlates. Sleep, 37(11), 1787–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier‐Brochu, É. , Beaulieu‐Bonneau, S. , Ivers, H. & Morin, C.M. (2012) Insomnia and daytime cognitive performance: a meta‐analysis. Sleep Medicine Reviews, 16, 83–94. [DOI] [PubMed] [Google Scholar]
- Freeman, D. , Stahl, D. , McManus, S. , Meltzer, H. , Brugha, T. , Wiles, N. et al. (2012) Insomnia, worry, anxiety and depression as predictors of the occurrence and persistence of paranoid thinking. Social Psychiatry and Psychiatric Epidemiology, 47, 1195–1203. [DOI] [PubMed] [Google Scholar]
- Frenda, S.J. & Fenn, K.M. (2016) Sleep less, think worse: the effect of sleep deprivation on working memory. Journal of Applied Research in Memory and Cognition, 5(4), 463–469. [Google Scholar]
- Greenlund, I.M. , Bigalke, J.A. , Tikkanen, A.L. , Durocher, J.J. , Smoot, C.A. & Carter, J.R. (2021a) Evening binge alcohol disrupts cardiovagal tone and baroreflex function during polysomnographic sleep. Sleep, 44, zsab130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlund, I.M. , Cunningham, H.A. , Tikkanen, A.L. , Bigalke, J.A. , Smoot, C.A. , Durocher, J.J. et al. (2021b) Morning sympathetic activity after evening binge alcohol consumption. American Journal of Physiology. Heart and Circulatory Physiology, 320, H305–H315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn, C. , Mackus, M. , Griffin, C. , Munafò, M.R. & Adams, S. (2018) A systematic review of the next‐day effects of heavy alcohol consumption on cognitive performance. Addiction, 113(12), 2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler, R.A. , Ramchandani, V.A. , Gilman, J. & Hommer, D.W. (2013) Stimulant and sedative effects of alcohol. Current Topics in Behavioral Neurosciences, 13, 489–509. [DOI] [PubMed] [Google Scholar]
- Howland, J. , Rohsenow, D. & Edwards, E. (2008a) Are some drinkers resistant to hangover? A literature review. CDAR, 1, 42–46. [DOI] [PubMed] [Google Scholar]
- Howland, J. , Rohsenow, D.J. , Allensworth‐Davies, D. , Greece, J. , Almeida, A. , Minsky, S.J. et al. (2008b) The incidence and severity of hangover the morning after moderate alcohol intoxication. Addiction, 103, 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland, J. , Rohsenow, D.J. , Bliss, C.A. , Almeida, A.B. , Calise, T.V. , Heeren, T. et al. (2010a) Hangover predicts residual alcohol effects on psychomotor vigilance the morning after intoxication. Journal of Addiction Research and Therapy, 1, 1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland, J. , Rohsenow, D.J. , Greece, J.A. , Littlefield, C.A. , Almeida, A. , Heeren, T. et al. (2010b) The effects of binge drinking on college students' next‐day academic test‐taking performance and mood state. Addiction, 105, 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt, W.A. & Witt, E.D. (1994) Behavioral effects of alcohol ingestion: implications for drug testing. Toxic Substances Journal, 13, 41–49. [Google Scholar]
- Kalmbach, D.A. , Buysse, D.J. , Cheng, P. , Roth, T. , Yang, A. & Drake, C.L. (2020) Nocturnal cognitive arousal is associated with objective sleep disturbance and indicators of physiologic hyperarousal in good sleepers and individuals with insomnia disorder. Sleep Medicine, 71, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, A.C. , de Wit, H. , McNamara, P.J. & Cao, D. (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of General Psychiatry, 68, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitman, N. (1939) Sleep and wakefulness as alternating phases in the cycle of existence. JAMA, 113, 2086. [Google Scholar]
- Koob, G.F. & Colrain, I.M. (2020) Alcohol use disorder and sleep disturbances: a feed‐forward allostatic framework. Neuropsychopharmacology, 45, 141–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAndrew, C. (1965) The differentiation of male alcoholic outpatients from nonalcoholic psychiatric outpatients by means of the MMPI. Quarterly Journal of Studies on Alcohol, 26, 238–246. [PubMed] [Google Scholar]
- Martin, C.S. , Earleywine, M. , Musty, R.E. , Perrine, M.W. & Swift, R.M. (1993) Development and validation of the biphasic alcohol effects scale. Alcoholism, Clinical and Experimental Research, 17, 140–146. [DOI] [PubMed] [Google Scholar]
- Martinez‐Cancino, D.P. , Azpiroz‐Leehan, J. , Jimenez‐Angeles, L. , Garcia‐Quintanar, A. & Santana‐Miranda, R. (2016) Effects of high frequency rTMS on sleep deprivation: a pilot study. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). Presented at the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). Orlando, FL: IEEE, pp. 5937–5940. [DOI] [PubMed] [Google Scholar]
- McKinney, A. , Coyle, K. & Verster, J. (2012) Direct comparison of the cognitive effects of acute alcohol with the morning after a normal night's drinking: acute and hangover alcohol performance. Human Psychopharmacology: Clinical and Experimental, 27, 295–304. [DOI] [PubMed] [Google Scholar]
- Mintzer, M.Ζ. (2007) The acute effects of alcohol on memory: a review of laboratory studies in healthy adults. International Journal on Disability and Human Development, 6, 397–403. [Google Scholar]
- Morean, M.E. , Corbin, W.R. & Treat, T.A. (2013) The subjective effects of alcohol scale: development and psychometric evaluation of a novel assessment tool for measuring subjective response to alcohol. Psychological Assessment, 25, 780–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, S.T. & Piper, B.J. (2014) The psychology experiment building language (PEBL) and PEBL test battery. Journal of Neuroscience Methods, 222, 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Survey on Drug Use and Health . (2020) The NSDUH report. Rockville, MD: U.S. Department of Health & Human Services, Substance Abuse & Mental Health Services Administration, Office of Applied Studies. Available from: https://datafiles.samhsa.gov/ [Google Scholar]
- Newlin, D.B. & Pretorius, M.B. (1990) Sons of alcoholics report greater hangover symptoms than sons of nonalcoholics: a pilot study. Alcoholism, Clinical and Experimental Research, 14, 713–716. [DOI] [PubMed] [Google Scholar]
- Okuda, M. , Noda, A. , Mabuchi, S. , Iwamoto, K. , Banno, M. , Miyata, S. et al. (2021) Sleep fragmentation and working memory in healthy adults. Sleep Science, 14, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar‐Berman, M. & Marinković, K. (2007) Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology Review, 17, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper, B.J. , Mueller, S.T. , Geerken, A.R. , Dixon, K.L. , Kroliczak, G. , Olsen, R.H.J. et al. (2015) Reliability and validity of neurobehavioral function on the psychology experimental building language test battery in young adults. PeerJ, 3, e1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch, B. & Born, J. (2013) About sleep's role in memory. Physiological Reviews, 93, 681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchs, G. , Desgranges, B. , Foret, J. & Eustache, F. (2005) The relationships between memory systems and sleep stages. Journal of Sleep Research, 14, 123–140. [DOI] [PubMed] [Google Scholar]
- Riemann, D. , Spiegelhalder, K. , Feige, B. , Voderholzer, U. , Berger, M. , Perlis, M. et al. (2010) The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Medicine Reviews, 14, 19–31. [DOI] [PubMed] [Google Scholar]
- Roehrs, T. & Roth, T. (2001a) Sleep, sleepiness, and alcohol use. Alcohol Research & Health, 25, 101–109. [PMC free article] [PubMed] [Google Scholar]
- Roehrs, T. & Roth, T. (2001b) Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Medicine Reviews, 5, 287–297. [DOI] [PubMed] [Google Scholar]
- Roehrs, T. & Roth, T. (2018) Insomnia as a path to alcoholism: tolerance development and dose escalation. Sleep, 41, zsy091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrs, T. , Zorick, F. , Wittig, R. , Conway, W. & Roth, T. (1989a) Predictors of objective level of daytime sleepiness in patients with sleep‐related breathing disorders. Chest, 95, 1202–1206. [DOI] [PubMed] [Google Scholar]
- Roehrs, T. , Zwyghuizen‐Doorenbos, A. , Timms, V. , Zorick, F. & Roth, T. (1989b) Sleep extension, enhanced alertness and the sedating effects of ethanol. Pharmacology, Biochemistry, and Behavior, 34, 321–324. [DOI] [PubMed] [Google Scholar]
- Roehrs, T. , Yoon, J. & Roth, T. (1991) Nocturnal and next‐day effects of ethanol and basal level of sleepiness. Human Psychopharmacology: Clinical and Experimental, 6, 307–311. [Google Scholar]
- Roehrs, T. , Papineau, K. , Rosenthal, L. & Roth, T. (1999) Ethanol as a hypnotic in insomniacs: self administration and effects on sleep and mood. Neuropsychopharmacology, 20, 279–286. [DOI] [PubMed] [Google Scholar]
- Rohsenow, D.J. , Howland, J. , Minsky, S.J. & Arnedt, J.T. (2006) Effects of heavy drinking by maritime academy cadets on hangover, perceived sleep, and next‐day ship power plant operation. Journal of Studies on Alcohol, 67, 406–415. [DOI] [PubMed] [Google Scholar]
- Rohsenow, D.J. , Howland, J. , Arnedt, J.T. , Almeida, A.B. , Greece, J. , Minsky, S. et al. (2010) Intoxication with Bourbon versus vodka: effects on hangover, sleep, and next‐day neurocognitive performance in young adults. Alcoholism: Clinical and Experimental Research, 34, 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueger, S.Y. & King, A.C. (2013) Validation of the brief biphasic alcohol effects scale (B‐BAES). Alcoholism, Clinical and Experimental Research, 37, 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott Saults, J. , Cowan, N. , Sher, K.J. & Moreno, M.V. (2007) Differential effects of alcohol on working memory: distinguishing multiple processes. Experimental and Clinical Psychopharmacology, 15, 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava, D. , Jung, S. , Saadat, M. , Sirohi, R. & Crewson, K. (2014) How to interpret the results of a sleep study. Journal of Community Hospital Internal Medicine Perspectives, 4, 24983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahre, M. , Roeber, J. , Kanny, D. , Brewer, R.D. & Zhang, X. (2014) Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Preventing Chronic Disease, 11, 130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, D. , D'Rozario, A. , Openshaw, H. , Bartlett, D. , Rae, C.D. , Catcheside, P. et al. (2022) Clinical predictors of working memory performance in obstructive sleep apnea patients before and during extended wakefulness. Sleep, 45, zsab289. [DOI] [PubMed] [Google Scholar]
- Stickgold, R. (2005) Sleep‐dependent memory consolidation. Nature, 437, 1272–1278. [DOI] [PubMed] [Google Scholar]
- Stone, B.M. (1980) Sleep and low doses of alcohol. Electroencephalography and Clinical Neurophysiology, 48, 706–709. [DOI] [PubMed] [Google Scholar]
- Verster, J.C. , Scholey, A. , van de Loo, A.J.A.E. , Benson, S. & Stock, A.‐K. (2020) Updating the definition of the alcohol hangover. Journal of Clinical Medicine, 9, E823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall, T.L. , Shea, S.H. , Luczak, S.E. , Cook, T.A.R. & Carr, L.G. (2005) Genetic associations of alcohol dehydrogenase with alcohol use disorders and endophenotypes in white college students. Journal of Abnormal Psychology, 114, 456–465. [DOI] [PubMed] [Google Scholar]
- Wardle‐Pinkston, S. , Slavish, D.C. & Taylor, D.J. (2019) Insomnia and cognitive performance: a systematic review and meta‐analysis. Sleep Medicine Reviews, 48, 101205. [DOI] [PubMed] [Google Scholar]
- Warren, J.G. , Fallon, V.M. , Goodwin, L. , Gage, S.H. & Rose, A.K. (2021) Menstrual cycle phase, hormonal contraception, and alcohol consumption in premenopausal females: a systematic review. Frontiers in Global Women's Health, 2, 745263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, D.L. , MacLean, A.W. & Cairns, J. (1983) Dose‐response effects of ethanol on the sleep of young women. Journal of Studies on Alcohol, 44, 515–523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


