Abstract
The therapeutic landscape for chronic lymphocytic leukemia (CLL) has changed dramatically over the past decade as our understanding of the biology of CLL has advanced, allowing the development of oral therapies targeting key drivers of CLL. Currently, inhibitors of Bruton's Tyrosine Kinase and the BH3 mimetic venetoclax are standards of care for both frontline and relapsed/refractory CLL. Sequencing of available therapies, therefore, has become a major challenge of therapy. In this review, we will focus on the current landscape for the treatment of relapsed/refractory CLL. We will also discuss important considerations when sequencing these available treatments. The recent advances in this disease are significant steps forward, and raise new questions of how these available drugs should be given as well as how we can continue to improve the treatment of CLL.
1. INTRODUCTION
Therapy for relapsed/refractory Chronic Lymphocytic Leukemia (CLL) has changed dramatically over the past 10 years with the approval of novel agents targeting the B‐cell receptor signaling pathway and anti‐apoptotic protein BCL2. These agents, including the covalent BTK inhibitors (BTKis) ibrutinib, acalabrutinib, and zanubrutinib, and the BCL2 inhibitor venetoclax dramatically changed therapy for relapsed and now treatment‐naïve disease. According to the National Comprehensive Cancer Network (NCCN) guidelines, the recommended therapies for relapsed/refractory CLL are generally the same as those recommended for treatment‐naïve disease. As a result, the choice of treatment for relapsed CLL is heavily driven by which drug was given in the frontline setting.
In this review, we will discuss the agents currently available for the treatment of relapsed/refractory CLL and then discuss considerations for sequencing of these agents.
2. INHIBITORS OF BTK IN RELAPSED/REFRACTORY CLL
2.1. Ibrutinib
The first in class covalent BTK inhibitor ibrutinib was approved by the US Food and Drug Administration in 2014 following completion of the RESONATE study. This trial compared ibrutinib to the anti‐CD20 antibody ofatumumab, which would have been a reasonable standard of care at that time. Median PFS for ibrutinib was 44.1 months compared with 8.1 with ofatumumab (HR 0.148; 95% CI 0.113–0.196) and OS favored ibrutinib as well (HR 0.639; 95% CI 0.418–0.975). 1 Use of ibrutinib in earlier lines of therapy was associated with longer PFS (not reached for those with 1 prior therapy vs. 27.3 months for those with 5 or more prior therapies). Del (17p)/TP53 mutation was associated with shorter remission duration (40.6 months for those with del(17p) compared with not reached for patients with neither del(11q) nor del(17p)). IGHV mutational status was not associated with outcomes.
Retrospective analyses of large single center experiences have shown that complex karyotype, defined as 3 or more cytogenetic abnormalities on stimulated karyotype, is associated with shorter PFS with ibrutinib. 2 , 3 In addition, when evaluating karyotype complexity as a continuous variable, each additional abnormality increases the risk of progression. 4
Safety with ibrutinib has been a major focus of recent study given the long duration of therapy most patients can expect. As a class, BTKi are associated with specific toxicities including hypertension, atrial fibrillation, ventricular arrhythmias, and bleeding. (Table 1) On the RESONATE study, only 16% of patients discontinued therapy due to toxicity. Toxicities tended to decrease with time with the exception of hypertension and bruising. With a follow‐up of 74 months, 12% of patients experienced atrial fibrillation, 21% experienced hypertension of any grade, and major bleeding was seen in 10%. Grade 3 or higher infections were seen in 45% of patients. Real‐world data have shown more variable but in general similar or higher rates of toxicity. A large multicenter retrospective study of 231 patients treated in the relapsed setting showed a discontinuation rate of 20.9% due to toxicity, with atrial fibrillation, infection, pneumonitis, bleeding, and diarrhea being the most common toxicities leading to discontinuation. 5 Another US based study and data from the Swedish CLL group also showed a discontinuation rate of around 20% due to toxicity. 6 , 7
TABLE 1.
Specific adverse events of clinical interest with BTK inhibitors
| Rates with Ibrutinib (RESONATE/phase 3 ELEVATE RR/phase 3 ALPINE) | Rates with acalabrutinib (phase 3 ASCEND/phase 3 ELEVATE RR) | Rates with zanubrutinib (phase 1/phase 3 ALPINE) | |
|---|---|---|---|
| Atrial fibrillation | 12%/16%/10.1% | 5%/9.4% | 1%/2.5% |
| Hypertension | 21%/22.8%/13% | 3%/8.6% | 5.3%/15.7% |
| High‐grade bleeding | 10%/5.3%/3.9% | 1%/4.5% | 1%/2.9% |
| Neutropenia (grade 3+) | 25%/22.8%/15% | 15%/19.5% | 6.4%/18.6% |
| Infections (grade 3+) | 45%/30.0%/17.9% | 15%/30.8% | NR/12.7% |
Note: Median follow‐up differs on these trials: RESONATE‐median 65 months; ELEVATE RR‐ median 40.9 months; ALPINE‐median 15 months; ASCEND—16.1 months; phase 1 zanubrutinib—13.7 months.
Abbreviation: NR, not reported.
2.2. Acalabrutinib
Acalabrutinib is a covalent BTK inhibitor designed to have improved specificity over ibrutinib, with the goal of diminishing toxicity. It is suspected that alternative targets of ibrutinib, including ITK, EGFR, and TEC may be responsible for some of the adverse events such as diarrhea, rash, and bruising, 8 and cardiac side effects may be the result of inhibition of PI3K‐Akt signaling 9 or C‐terminal Src kinase. 10 The kinase profile of acalabrutinib suggests that many of these targets are spared with acalabrutinib versus ibrutinib. 11
FDA approval for acalabrutinib in relapsed CLL came as result of the ASCEND study, which compared acalabrutinib versus dealers choice of idelalisib/rituximab versus bendamustine/rituximab. 12 , 13 In this trial, 36 month PFS rate was 63% for acalabrutinib versus 21% for standard of care, with bendamustine/rituximab and idelalisib/rituximab performing similarly. At this time, neither del17p/TP53 mutation nor IGVH mutation status altered PFS with acalabrutinib. Side effect profile is favorable, with atrial fibrillation observed in 7% of patients and major bleeding in 5%. Eleven percent of patients discontinued treatment for toxicity.
The first head‐to‐head trial of BTK inhibitor monotherapy was the ELEVATE RR study which compared ibrutinib and acalabrutinib in high risk (defined as del(17p) or del(11q)) relapsed/refractory CLL. At a median follow‐up of 40.9 months, acalabrutinib was found to be non‐inferior to ibrutinib, with a median PFS of 38.4 months in both arms. 14 Though efficacy was similar, toxicity with acalabrutinib was significantly lower, with grade 3 toxicity seen in 75% of ibrutinib‐treated patients compared with 69% of acalabrutinib treated patients. Atrial fibrillation of any grade was seen in 9.4% of acalabrutinib‐treated patients and 16% of ibrutinib‐treated patients, and hypertension seen in 9.4% of acalabrutinib‐treated patients and 23.2% of ibrutinib‐treated patients. Toxicity led to treatment discontinuation in 15% of acalabrutinib and 21% of ibrutinib treated patients. Infection rate and major bleeding were not different between the two drugs. Overall, these data favor the use of acalabrutinib for most patients.
2.3. Zanubrutinib
Another newer selective BTK inhibitor is zanubrutinb, which is currently FDA approved for mantle cell lymphoma, Waldenstrom's macroglobulinemia, and marginal zone lymphoma. Among other differentiating properties, zanubrutinib has been shown to have less effects on platelet aggregation than ibrutinib. 15 In phase 1 study, zanubrutinib was shown to have overall response rates of 94.6% in patients with relapsed/refractory CLL and showed higher BTK occupancy in the blood and lymph node than previously observed with ibrutinib. 16 Long term follow‐up of this trial has shown median PFS of 61.4 months. 17
The ALPINE study is a head‐to‐head comparison of ibrutinib and zanubrutinib in relapsed/refractory CLL. Important distinctions of this trial design that limit direct comparisons with ELEVATE RR are the use of overall response rate as the primary endpoint and inclusion of all‐comers with relapsed/refractory CLL, not limited to high‐risk patients. 18 At an early median follow‐up of 15 months, zanubrutinib had a superior overall response rate to ibrutinib (78.3% vs. 62.5%, respectively). Twelve‐month PFS was also higher with zanubrutinib than ibrutinib (94.9% vs. 84%, respectively). Although these efficacy results should be interpreted with caution given the early stage of follow‐up, they are nonetheless outstanding and suggest that zanubrutinib will be at least as effective as ibrutinib with longer follow‐up.
Safety data with zanubrutinib has been as expected for a selective BTKi. A large pooled analysis over multiple B cell malignancies showed that the most common side effects were consistent with other BTKi. 19 In the ALPINE study, rates of atrial fibrillation were significantly lower with zanubrutinib (2.5% vs. 10.1%, respectively). Lower rates of atrial fibrillation versus ibrutinib were also seen in the phase 3 ASPEN study in Waldenstrom macroglobulinemia. 20 Major bleeding was similar but trended toward lower with zanubrutinib (2.9 vs. 3.9%, respectively). Neutropenia was higher with zanubrutinib, however, grade 3 infections trended toward being lower with zanubrutinib. 18 Overall, the ALPINE data favor the use of zanubrutinib rather than ibrutinib for most patients.
2.4. Venetoclax in relapsed/refractory CLL
BCL2 is an anti‐apoptotic protein which is upregulated in a variety of cancers including CLL. 21 , 22 , 23 Venetoclax is an orally bioavailable, selective inhibitor of BCL2 that has shown excellent activity in relapsed CLL. In the phase 1 study as continuous monotherapy, overall response rate was 82% for the patients treated at the recommended dose, with a median PFS of 25 months (95% CI: 17–30 months), and a lower duration of remission observed in patients with del(17p) of 16 months (95% CI: 11–25 months). 24 A contemporary phase 2 study of patients with del(17p) similarly showed an overall response rate of 77% and a 12‐month PFS of 72% (95% CI: 61.8–79.8). 25 Following these initial studies and realization of high rates of undetectable minimal residual disease (uMRD) allowing successful therapy discontinuation, further development of venetoclax has been in time‐limited approaches. In addition, continuous venetoclax has been associated with resistance and clonal hematopoiesis. 26 , 27
The definitive study of venetoclax in relapsed/refractory CLL is the MURANO trial, which compared 2‐year fixed duration venetoclax plus rituximab with bendamustine plus rituximab in relapsed/refractory CLL. 28 Overall response rate was 92.3% with a complete response rate of 26.8% and uMRD rate of 83.5%. Long‐term follow‐up has shown a median PFS of 53.6 months, with a median time to MRD conversion from end of treatment of 19.4 months and a median time from MRD conversion to progressive disease of 25.2 months (95% CI: 19.4–30.4 months). 29 Among patients that were uMRD at end of treatment, presence of del(17p), genomic complexity, and unmutated IGHV were all associated with increased risk of MRD conversion and subsequent disease progression. A pooled analysis of 4 early‐stage clinical trials of venetoclax also identified bulky lymph nodes, refractory to B cell receptor inhibitor, TP53 abnormalities, and NOTCH1 mutation as factors independently associated with shorter remissions with venetoclax therapy. Conversely, uMRD by 24 months and CR by 9 months were associated with longer remissions. 30
Among venetoclax trials, a common finding has been uMRD as a predictor of subsequent PFS. For this reason, multiple studies are focusing on using MRD to direct treatment, either incorporating shorter or longer treatment regimens based upon MRD. Although this is currently an area of active investigation, current standard is to administer venetoclax for 2 years (in combination with an anti‐CD20 monoclonal antibody) regardless of MRD status at end of treatment. The one potential exception to this is in those patients previously treated with a kinase inhibitor, where the only trial in this setting administered venetoclax on a continuous basis. 31
Venetoclax has generally been well tolerated. In the MURANO study, the most common high‐grade toxicity was neutropenia at 57.7% in the venetoclax plus rituximab group, however, incidence of febrile neutropenia was low (3.6%). 28 Real‐world data where venetoclax could be given alone or in combination has shown similar rates of neutropenia (47.4%) but higher rates of neutropenic fever (11.6%). Opportunistic infections were also relatively common at 7.8% including primarily pneumocystis jirovecii, invasive fungal infections, and toxoplasmosis. 32 Tumor lysis syndrome has been relatively uncommon in more modern data, with a 3.1% incidence of high‐grade tumor lysis syndrome seen in the MURANO study.
2.5. Other therapies for relapsed/refractory CLL
BTKi and venetoclax regimens are the most effective regimens for relapsed/refractory CLL, so most patients should be treated with these unless a specific contraindication exists. For patients with dual‐refractory disease (refractory to both BTKi and venetoclax), limited data exist that any other currently approved therapies will be of benefit, 33 and most of these patients should be considered for therapy on a clinical trial. However, data do exist in the relapsed setting for other agents that may be considered for patients not appropriate for BTKi or venetoclax, as a bridge to a trial, or for patients who are not candidates for clinical trials. Select alternative agents are reviewed here.
2.6. PI3k inhibitors
There are two PI3 kinase delta inhibitors currently FDA approved for use in relapsed/refractory CLL: idelalisib and duvelisib. In patients with relapsed/refractory CLL, idelalisib in combination with rituximab has an overall response rate of 85.5% 34 and median PFS of 20.3 months. 35 Toxicity to idelalisib, and this class in general, is immune‐mediated. Short term toxicities include diarrhea, pneumonitis, and transaminitis. Prolonged exposure to idelalisib increases risk of diarrhea/colitis (grade 3 or higher diarrhea 16.4%, colitis 8.2%) and pneumonitis (grade 3 or higher 6.4%). 35 Duvelisib was investigated in the phase 3 DUO study, which compared duvelisib to ofatumumab in relapsed CLL. Overall response rate was 74% with a median PFS of 13.3 months. 36 Grade 3 or higher colitis was seen in 12% of patients, with grade 3 or higher diarrhea in 15%. Grade 3 or higher pneumonitis was seen in 3% of patients. However, despite the PFS advantage seen in these studies, recent data from a US FDA Oncologic Drugs Advisory Committee (ODAC) meeting on PI3 kinase inhibitors revealed that for a number of randomized clinical trials with idelalisib and duvelisib, long‐term overall survival trended toward favoring the control arms, raising the question of whether these drugs should be used in CLL. 37
2.7. Lenalidomide
Lenalidomide is a cereblon‐targeting agents that has immunomodulatory effects in CLL that may allow for tumor recognition and immune activation. reviewed in Reference 38. In relapsed/refractory CLL it has an overall response rate of 47%, with a median PFS of 19.4 months. 39 At doses tolerated in other malignancies, severe toxicities including tumor lysis syndrome, cytopenias, and tumor flare have been seen in CLL, so most patients are treated with doses of 2.5–5 mg. Combination therapy with rituximab has also had some encouraging results, with an overall response rate of 66% and median time to treatment failure of 17.4 months. 40
2.8. Transplant
The role of transplant in the treatment of relapsed/refractory CLL has diminished over the past decade due to availability of effective and more tolerable therapies. reviewed in Reference 41. However, it remains one of the few curative options for CLL and for select patients remains a viable therapeutic option. There are no randomized clinical trials comparing transplant to other therapies in CLL, and results of transplant studies must also be interpreted with caution as generally only very high‐risk patients are treated in this manner. Long term results from a number of trials demonstrate that reduced intensity allogeneic stem cell transplants will induce durable remission and cure for about 40% of patients. Long term results of the German CLL3X study which included 100 patients demonstrated a 10 year PFS of 34% and overall survival of 51%. 42 TP53 status did not play a role in outcomes, but active disease at transplant significantly shortened PFS. For young, fit patients with very high‐risk disease who have progressed despite multiple treatments stem cell transplant still plays a role, but with the availability of targeted agents both approved and in clinical trials as well as chimeric antigen receptor T (CAR‐T) cells in clinical trials, very few patients and physicians will likely continue to choose this treatment modality.
2.9. Sequencing of therapy: BTKi and venetoclax
For most patients with CLL, frontline therapy can consist either of a BTK inhibitor or venetoclax. In addition, many patients are, or have been, treated with chemoimmunotherapy in the past. All of the studies that led to FDA approval of therapies for relapsed/refractory CLL were performed prior to the approval of other novel therapies, so patients on the RESONATE study, for example, were not previously treated with venetoclax, and patients on the MURANO study had not previously received a BTKi. Therefore, these data can be easily cited when choosing therapy for a patient previously treated with chemotherapy, but must be interpreted with caution for patients previously treated with a novel agent. When choosing how best to sequence therapies for patients with relapsed/refractory CLL, the most important consideration is what their prior therapy was. A suggested algorithm is found in Figure 1.
FIGURE 1.
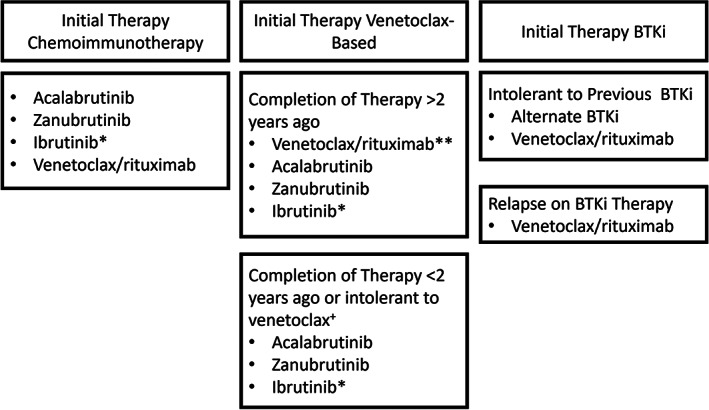
Therapeutic options for relapsed/refractory CLL. *In randomized clinical trials of ibrutinib versus acalabrutinib and ibrutinib versus zanubrutinib, ibrutinib has shown equivalent efficacy to both second‐generation inhibitors, and both acalabrutinib and zanubrutinib had a more favorable safety profile compared with ibrutinib. Therefore, acalabrutinib or zanubrutinib are the preferred agents for most patients. **For patients who tolerated venetoclax‐based regimens well and had a long remission duration, venetoclax/rituximab (or alternatively venetoclax/obinutuzumab) is likely to be the preferred regimen, although long‐term data for this strategy is not yet available. +For patients who relapse quickly after discontinuation of venetoclax, it may be reasonable in some circumstances to restart venetoclax rather than switch to alternative therapy.
2.10. Sequencing when initial treatment was chemotherapy
As mentioned, the clinical trials of BTKi and venetoclax in the relapsed setting primarily included patients previously treated with chemoimmunotherapy. The choice in the relapsed setting for these patients is very similar to that in patients with treatment‐naïve disease, where efficacy, safety, and intangible factors must all be considered and discussed. For standard‐risk patients, BTKi and venetoclax have similar PFS in the relapsed setting. It is more difficult to compare outcomes for patients with higher risk disease due to differences in analysis methods between studies, however, it does appear that patients with TP53 abnormalities may have superior PFS with BTKi based regimens. 25 , 43
Safety considerations and differential toxicity may help inform choices among BTKi and venetoclax. Patients with cardiac disorders, arrhythmias, or hypertension may wish to avoid BTKi or at least ibrutinib. Those with high tumor lysis risk, renal disease, or volume overload may wish to avoid venetoclax due to need for hydration with dose ramp‐up.
Intangible considerations are very important when choosing between BTKi and venetoclax‐based regimens, similar to the front‐line setting. Most patients prefer time‐limited therapy, although some prefer the convenience of BTKi initiation and consider indefinite therapy a reasonable trade‐off for the ease of starting treatment. As well, in times of COVID 19 surges, patients and physicians might prefer BTKi to limit visits to the hospital or infusion center. It is important to discuss the risks and benefits of each treatment approach so that patients can make an informed decision.
2.11. Sequencing when initial treatment was BTKi therapy
For patients initially treated with a BTKi who present with relapsed CLL in need of therapy, the first consideration is whether the patient relapsed while taking a BTKi or after discontinuation due to intolerance or other factors.
For patients who discontinued BTKi due to toxicity and present with relapsed disease, the reason for discontinuation will determine whether an alternative BTKi could be considered. Data exist for acalabrutinib in patients intolerant to ibrutinib, 44 and zanubrutinib in patients intolerant to ibrutinib or acalabrutinib. 45 Many irritating side effects from ibrutinib such as arthralgias, skin/hair changes, or rash, can be alleviated with a switch to either acalabrutinib or zanubrutinib. As well, for patients with atrial fibrillation or uncontrolled hypertension on ibrutinib, acalabrutinib or zanubrutinib could be considered. The available data suggest that lowest rates of atrial fibrillation are seen with zanubrutinib and lowest rates of hypertension with acalabrutinib. 14 , 18 For patients with ventricular arrhythmias or major hemorrhage, risk/benefit ratio probably favors switching drug classes entirely.
If a patient discontinues BTKi due to relapse on therapy, the patient should be switched to venetoclax or another class of therapy. All covalent BTKi, including ibrutinib, acalabrutinib, and zanubrutinib, share the same drug binding site and share common resistance mechanisms, so resistance to one BTKi indicates resistance to all covalent inhibitors. Venetoclax has been studied in patients relapsed after ibrutinib, where the majority of patients progressed on therapy. 31 In this trial of continuous venetoclax monotherapy, overall response rate was 65% (95% CI: 53%–74%), and median PFS 24.7 months. uMRD was uncommon in this setting with this regimen. Most patients currently are treated with venetoclax in combination with an anti‐CD20 antibody, but it is not known whether post‐BTKi patients should be treated with fixed duration or continuous venetoclax. This is a scenario where knowledge of MRD status at 2 years may be helpful to guide discontinuation decisions.
2.12. Sequencing when initial treatment was venetoclax
For patients who relapse after being treated with a venetoclax‐based therapy, considerations when thinking about next line of therapy include whether venetoclax was stopped early due to intolerance, what was the MRD status at end of treatment, and how long was the remission. Unfortunately, robust prospective data do not exist in this setting, but smaller prospective cohorts and retrospective data provide some evidence.
If patients did not tolerate venetoclax as their previous therapy, re‐treatment is most likely not an option, but could be considered in certain circumstances. For example, if therapy was stopped due to cytopenias that occurred early in therapy (when bone marrow involvement was significant), or stopped prior to trial of growth factor, re‐treatment could be considered, potentially without an anti‐CD20 antibody.
For patients with a long duration of remission after previous venetoclax therapy, re‐treatment can be considered. Long‐term data from the MURANO study showed that re‐treatment is successful for many patients, with an overall response rate of 72.2%. 29 Duration of remission is not known at this time. In a retrospective study of 25 patients, overall response rate was the same as reported in MURANO, at 72.2% with an estimated 12 month PFS of 69.1%, 46 suggesting that patients likely have a shorter remission to re‐treatment with venetoclax. Clinical trials are ongoing to evaluate the benefit of venetoclax re‐treatment.
BTKi can also be considered following treatment with venetoclax. Little data exist, but the data that are present demonstrates efficacy of this sequence. In the MURANO study follow‐up, BTKi as subsequent therapy had a response rate of 100%, however, remission durations are unknown. 29 A large retrospective study of 74 patients treated with BTKi following venetoclax. In these patients, estimated median PFS was 32 months for those patients who had not previously been treated with a BTKi. 47 Other small retrospective studies have also shown benefit of this approach. 48 , 49
2.13. Future therapies for relapsed/refractory CLL
There are multiple agents currently in clinical trials for CLL that have the potential to change standards of care once again for relapsed/refractory disease. Reversible inhibitors of BTK including pirtobrutinib 50 and nemtabrutinib 51 have shown outstanding efficacy in early clinical trials, and the safety particularly of pirtobrutinib make this an attractive agent. Although resistance mechanisms associated with pirtobrutinib would suggest that this drug should be sequenced after covalent inhibitors, 52 if the efficacy of this agent in the BTKi‐naïve setting is long enough, pirtobrutinib may develop a niche prior to other BTKi. Other agents which inhibit the B‐cell receptor signaling pathway are also promising in early clinical trials.
CAR‐T cells are also showing exciting efficacy for patients with relapsed/refractory CLL. In general, responses and durability of response for CAR‐T in CLL has been lower than other diseases where this modality is FDA approved, due to disease‐related immunosuppression suppressing T cell proliferation in vitro and in vivo. Improved antibody engineering and more potent co‐stimulatory molecules have simproved responses over time, 53 , 54 and incorporation of small molecules like BTKi which potentiate T cell effects 55 , 56 are of interest as well.
Currently, most CAR‐T cells are directed against CD19, however, other targets are of interest and under active investigation in clinical trials. As this modality improves, it has the potential to overtake transplant as a cellular therapy for CLL and may be of interest in earlier lines of therapy for young patients and those with high genetic risk disease.
3. CONCLUSIONS
At this time, we are fortunate that patients with relapsed/refractory CLL have a number of therapeutic options that are both safe and effective. However, for patients who relapse after multiple therapies, those with high‐risk disease, and those who are very young, more options are needed. A challenge in CLL over the next decade will be to continue to perform high quality large‐scale clinical trials to bring new drugs forward in CLL and determine which drugs and combinations are best for individual patients. Only through continued research will we achieve the goal of curing CLL or at least providing effective disease suppression to allow patients to live the duration of their natural lives.
FUNDING INFORMATION
Jennifer A Woyach is a clinical scholar of the Leukemia and Lymphoma Society.
CONFLICT OF INTEREST
Jennifer A Woyach has consulted for Abbvie, AstraZeneca, Beigene, Genentech, Janssen, Loxo, Newave, Pharmacyclics, and Schrodinger and receives research funding from Abbvie, Janssen, Morphosys, and Schrodinger.
Woyach JA. Management of relapsed/refractory Chronic Lymphocytic Leukemia. Am J Hematol. 2022;97(S2):S11‐S18. doi: 10.1002/ajh.26683
Continuing Medical Education: Please Click Here to complete an accredited learning activity for this article and receive 1.5 AMA PRA Category 1 Credits™.
Funding information Leukemia and Lymphoma Society
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article.
REFERENCES
- 1. Munir T, Brown JR, O'Brien S, et al. Final analysis from RESONATE: up to six years of follow‐up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353‐1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of Ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson PA, O'Brien SM, Wierda WG, et al. Complex karyotype is a stronger predictor than del(17p) for an inferior outcome in relapsed or refractory chronic lymphocytic leukemia patients treated with ibrutinib‐based regimens. Cancer. 2015;121(20):3612‐3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kittai AS, Miller C, Goldstein D, et al. The impact of increasing karyotypic complexity and evolution on survival in patients with CLL treated with ibrutinib. Blood. 2021;138(23):2372‐2382. [DOI] [PubMed] [Google Scholar]
- 5. Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib‐treated patients in the United States: a real‐world analysis. Haematologica. 2018;103(5):874‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hou JZ, Ryan K, Du S, et al. Real‐world ibrutinib dose reductions, holds and discontinuations in chronic lymphocytic leukemia. Future Oncol. 2021;17(35):4959‐4969. [DOI] [PubMed] [Google Scholar]
- 7. Winqvist M, Andersson PO, Asklid A, et al. Long‐term real‐world results of ibrutinib therapy in patients with relapsed or refractory chronic lymphocytic leukemia: 30‐month follow up of the Swedish compassionate use cohort. Haematologica. 2019;104(5):e208‐e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI‐32765). Leuk Lymphoma. 2013;54(11):2385‐2391. [DOI] [PubMed] [Google Scholar]
- 9. McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K‐Akt signaling. Blood. 2014;124(25):3829‐3830. [DOI] [PubMed] [Google Scholar]
- 10. Xiao L, Salem JE, Clauss S, et al. Ibrutinib‐mediated atrial fibrillation attributable to inhibition of C‐terminal Src kinase. Circulation. 2020;142(25):2443‐2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herman SEM, Montraveta A, Niemann CU, et al. The Bruton tyrosine kinase (BTK) inhibitor Acalabrutinib demonstrates potent on‐target effects and efficacy in two mouse models of chronic lymphocytic leukemia. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2017;23(11):2831‐2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of Acalabrutinib versus Idelalisib plus rituximab or Bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849‐2861. [DOI] [PubMed] [Google Scholar]
- 13. Jurczak W, Pluta A, Wach M, et al. Three‐year follow‐up of the Ascend trial: Acalabrutinib versus rituximab plus Idelalisib or Bendamustine in relapsed/refractory chronic lymphocytic leukemia. Blood (ASH Annual Meeting Abstracts). 2021:Abstract 393;138:393. [Google Scholar]
- 14. Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus Ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441‐3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dobie G, Kuriri FA, Omar MMA, et al. Ibrutinib, but not zanubrutinib, induces platelet receptor shedding of GPIb‐IX‐V complex and integrin alphaIIbbeta3 in mice and humans. Blood Adv. 2019;3(24):4298‐4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B‐cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cull G, Burger JA, Opat S, et al. Zanubrutinib for treatment‐naive and relapsed/refractory chronic lymphocytic leukaemia: long‐term follow‐up of the phase I/II AU‐003 study. Br J Haematol. 2022;196(5):1209‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hillmen P, Eichhorst B, Brown JR, et al. First interim analysis of alpine study: Results of a phase 3 randomized study of zanubrutinib versus ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Haematologica (EHA Abstracts). 2021; LBA 4. [Google Scholar]
- 19. Tam C, Opat S, Zhu J, et al. Pooled analysis of safety data from monotherapy studies of the BRUTON tyrosine kinase (BTK) inhibitor, ZANUBRUTINIB (BGB‐3111), in b‐cell malignancies. Haematologica (EHA Abstracts). 2019;3 (S1):526. [Google Scholar]
- 20. Tam CS, Opat S, D'Sa S, et al. A randomized phase 3 trial of zanubrutinib versus ibrutinib in symptomatic Waldenstrom macroglobulinemia: the ASPEN study. Blood. 2020;136(18):2038‐2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Papakonstantinou G, Verbeke C, Hastka J, Bohrer M, Hehlmann R. Bcl‐2 expression in non‐Hodgkin's lymphomas is not associated with bcl‐2 gene rearrangements. Br J Haematol. 2001;113(2):383‐390. [DOI] [PubMed] [Google Scholar]
- 22. Cimmino A, Calin GA, Fabbri M, et al. miR‐15 and miR‐16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102(39):13944‐13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buggins AG, Pepper CJ. The role of Bcl‐2 family proteins in chronic lymphocytic leukaemia. Leuk Res. 2010;34(7):837‐842. [DOI] [PubMed] [Google Scholar]
- 24. Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with Venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open‐label, phase 2 study. Lancet Oncol. 2016;17(6):768‐778. [DOI] [PubMed] [Google Scholar]
- 26. Blombery P, Anderson MA, Gong JN, et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to Venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 2019;9(3):342‐353. [DOI] [PubMed] [Google Scholar]
- 27. Blombery P, Lew TE, Dengler MA, et al. Clonal hematopoiesis, myeloid disorders and BAX‐mutated myelopoiesis in patients receiving venetoclax for CLL. Blood. 2022;139(8):1198‐1207. [DOI] [PubMed] [Google Scholar]
- 28. Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax–rituximab in relapsed or refractory chronic lymphocytic leukemia. New England Journal of Medicine. 2018;378(12):1107‐1120. [DOI] [PubMed] [Google Scholar]
- 29. Kater A, Kipps T, Eichhorst B, et al. Five‐year analysis of Murano study demonstrates enduring undetectable minimal residual disease (uMRD) in a subset of relapsed/refractory chronic lymphocytic leukemia (R/R CLL) patients (pts) following fixed‐duration Venetoclax‐rituximab (VenR) therapy (Tx). Blood (ASH Annual Meeting Abstracts). 2021:Abstract 125;136:19‐21. [Google Scholar]
- 30. Roberts AW, Ma S, Kipps TJ, et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood. 2019;134(2):111‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jones JA, Mato AR, Wierda WG, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open‐label, phase 2 trial. Lancet Oncol. 2018;19(1):65‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mato AR, Thompson M, Allan JN, et al. Real‐world outcomes and management strategies for venetoclax‐treated chronic lymphocytic leukemia patients in the United States. Haematologica. 2018;103(9):1511‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mato AR, Davids MS, Sharman J, et al. Recognizing unmet need in the era of targeted therapy for CLL/SLL: "What's past is prologue" (Shakespeare). Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2021;28:603‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharman JP, Coutre SE, Furman RR, et al. Final results of a randomized, phase III study of rituximab with or without Idelalisib followed by open‐label Idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol. 2019;37(16):1391‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446‐2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. https://urldefense.com/v3/__https://www.fda.gov/media/157762/download__;!!AU3bcTlGKuA!A_ODakipEd_Pa‐5Nr6RLcHTZC25o6vLN‐2xFDUxJRz7Af2fP6TA0fEKS7gBnpTZGLWWhYUDHUavzDd_IRd0BOQWMYGX8hdrtJF8$.
- 38. Itchaki G, Brown JR. Lenalidomide in the treatment of chronic lymphocytic leukemia. Expert Opin Investig Drugs. 2017;26(5):633‐650. [DOI] [PubMed] [Google Scholar]
- 39. Chanan‐Khan A, Miller KC, Musial L, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24(34):5343‐5349. [DOI] [PubMed] [Google Scholar]
- 40. Badoux XC, Keating MJ, Wen S, et al. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2013;31(5):584‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gribben JG. How and when I do allogeneic transplant in CLL. Blood. 2018;132(1):31‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kramer I, Stilgenbauer S, Dietrich S, et al. Allogeneic hematopoietic cell transplantation for high‐risk CLL: 10‐year follow‐up of the GCLLSG CLL3X trial. Blood. 2017;130(12):1477‐1480. [DOI] [PubMed] [Google Scholar]
- 43. Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rogers KA, Thompson PA, Allan JN, et al. Phase II study of acalabrutinib in ibrutinib‐intolerant patients with relapsed/refractory chronic lymphocytic leukemia. Haematologica. 2021;106(9):2364‐2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shadman M, Sharman J, Levy M, et al. Preliminary results of the phase 2 study of zanubrutinib in patients with previously treated B‐cell malignancies intolerant to ibrutinib and/or acalabrutinib. J Clin Oncol. 2021;39(15s):Abstract e19506. [Google Scholar]
- 46. Thompson M, Allan JN, Sail K, et al. Venetoclax re‐treatment of chronic lymphocytic leukemia (CLL) patients after a previous Venetoclax‐based regimen. Blood. 2020;136(Supplement 1):39‐41. [Google Scholar]
- 47. Mato AR, Roeker LE, Jacobs R, et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res. 2020;26(14):3589‐3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brown JR, Davids MS, Chang J, et al. Outcomes of ibrutinib (Ibr) therapy in Ibr‐naïve patients (pts) with chronic lymphocytic leukemia (CLL) progressing after venetoclax (Ven). Blood. 2019;134(Supplement 1): 4320. [Google Scholar]
- 49. Lin VS, Lew TE, Handunnetti SM, et al. BTK inhibitor therapy is effective in patients with CLL resistant to venetoclax. Blood. 2020;135(25):2266‐2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mato A, Pagel JM, Coombs CC, et al. LOXO‐305, a next generation, highly selective, non‐covalent BTK inhibitor in previously treated CLL/SLL: results from the phase 1/2 BRUIN study. Blood. 2020;136(Supplement 1):35‐37. [Google Scholar]
- 51. Woyach J, Flinn I, Awan F, et al. Preliminary efficacy and safety of MK‐1026, a non‐covalent inhibitor of wild‐type and C481S mutated Bruton tyrosine kinase, in B‐cell malignancies: a phase 2 dose expansion study. Blood (ASH Annual Meeting Abstracts). 2021:Abstract 392;138:392. [Google Scholar]
- 52. Wang E, Mi X, Thompson MC, et al. Mechanisms of resistance to noncovalent Bruton's tyrosine kinase inhibitors. N Engl J Med. 2022;386(8):735‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor‐modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Siddiqi T, Soumerai JD, Dorritie KA, et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood. 2022;139(12):1794‐1806. [DOI] [PubMed] [Google Scholar]
- 55. Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T‐cell engraftment and efficacy in leukemia. Blood. 2016;127(9):1117‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gill MSI, Vides BV, Frey NV, et al. Prospective clinical trial of anti‐CD19 CAR T cells in combination with Ibrutinib for the treatment of chronic lymphocytic leukemia shows a high response rate. Blood (ASH Annual Meeting Abstracts). 2018:Abstract 298;132:298. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article.


