Abstract
Background
Neoadjuvant chemotherapy (NAC) is standard for many females with breast cancer (FBC). The efficacy of NAC in male breast cancer (MaBC) is unclear. The aim of this study was to compare proportions of pathologic complete response (pCR) between MaBC and FBC by tumor subtype (TS).
Methods
MaBC and FBC treated with NAC between 2010 and 2016, with known TS, were evaluated from the National Cancer Database. Proportions of pCR (ypT0/Tis ypN0) were compared between sexes within TS by Fisher test. Multivariable logistic regression assessed the independent association of sex with pCR. Overall survival (OS) was estimated by Kaplan–Meier.
Results
A total of 385 MaBC and 68,065 FBC were included. Median time from initiation of NAC to surgery was 143 days in MaBC and 148 days in FBC. Proportions of pCR in MaBC and FBC by TS were: hormone receptor–positive/human epidermal growth factor receptor 2–negative (HR+/HER2–): 4.9% vs 9.7%, p = .01; HR+/HER2+: 16.1% vs 33.6%, p < .001; HR–/HER2+: 44.0% vs 53.2%, p = .42; and HR–/HER2–: 21.4% vs 32.1%, p = .18, respectively. FBC had twice the odds of pCR than MaBC (adjusted odds ratio, 2.0; 95% CI, 1.5–2.8; p < .001). Five‐year OS for MaBC with pCR vs not was 90% vs 64.7%; p = .02. Five‐year OS for FBC with pCR vs not was 91.9% vs 75.3%; p < .01.
Conclusions
Proportions and odds of pCR to NAC were numerically lower in MaBC compared with FBC for each TS and statistically significant for HR+/HER2– and HR+/HER2+. The independent association of sex with pCR was confirmed in multivariable analysis. pCR is prognostic in both MaBC and FBC.
Keywords: breast cancer in men, neoadjuvant therapy, pCR, prognosis, tumor subtypes
Short abstract
Using the National Cancer Database, the authors show that the proportions of pathologic complete response (pCR) following neoadjuvant chemotherapy by tumor subtype in breast cancer were uniformly lower in men compared with women. pCR may serve as an endpoint for clinical trials in men with certain breast cancer subtypes and facilitate the identification of more effective therapies using smaller sample sizes.
INTRODUCTION
Outcomes in male breast cancer (MaBC) have historically lagged behind those of female breast cancer (FBC), in part because of its rarity (~1% of all breast cancers) and the lack of prospective trials in men. Current treatment guidelines recommend that men with breast cancer follow the same neoadjuvant chemotherapy (NAC) and anti‐HER2 treatment strategies as women with breast cancer. 1 , 2
NAC is a standard of care for many women with breast cancer because it allows for higher rates of breast‐conserving surgery, axillary downstaging, in vivo assessment of tumor sensitivity to systemic therapy, and tailoring adjuvant therapy based on the response achieved. 3 , 4 One efficacy measure for NAC is pathologic complete response (pCR), which is particularly relevant because it reflects and can be analyzed as a surrogate for overall survival (OS), disease‐free survival, and event‐free survival. 5 NAC use is lower in MaBC than in FBC 6 ; however, the efficacy of NAC in MaBC and its outcomes are largely unknown.
With the goal of understanding the comparative efficacy of NAC between MaBC and FBC, we analyzed the National Cancer Database (NCDB) for patients with breast cancer on NAC and compared the proportion of pCR by tumor subtype between sexes. We also evaluated clinical response and OS.
MATERIALS AND METHODS
Data source and study design
Data for this study were obtained from the NCDB. 7 NCDB currently collects and publishes cancer data nationwide, covering more than 70% of newly diagnosed invasive cancer cases at a variety of medical center types (eg, academic, community, Veterans Affairs) that are accredited by the Commission on Cancer Care. 8 The data undergo quality checks and include tumor characteristics (including size, metastases, node, and grade), clinical and pathological staging, surgical and pharmacological treatments, outcomes, and demographics and socioeconomic information. 7
Using NCDB data, we evaluated men and women who were diagnosed with invasive, nonmetastatic breast cancer treated with NAC followed by surgery to the breast and axillary nodes between January 1, 2010, and December 31, 2016. Although the NCDB does not provide information on the specific type of chemotherapy administered, the NCDB does report the dates of chemotherapy initiation and surgical excision. We defined NAC as patients who received their chemotherapy before surgical excision and had 90 to 210 days between starting NAC and undergoing surgery. These limits were selected based on the optimal time required to complete most NAC regimens, regardless of the specific agents included. Patients were excluded if the information about hormone receptor (HR), human epidermal growth factor receptor 2 (HER2), clinical T, clinical N, pathologic T, or pathologic N were unknown. Clinical and pathological cancer stage were recorded per the American Joint Committee on Cancer staging system, seventh edition. 9 Figure 1 shows the flow diagram of the patient population included in the study.
FIGURE 1.
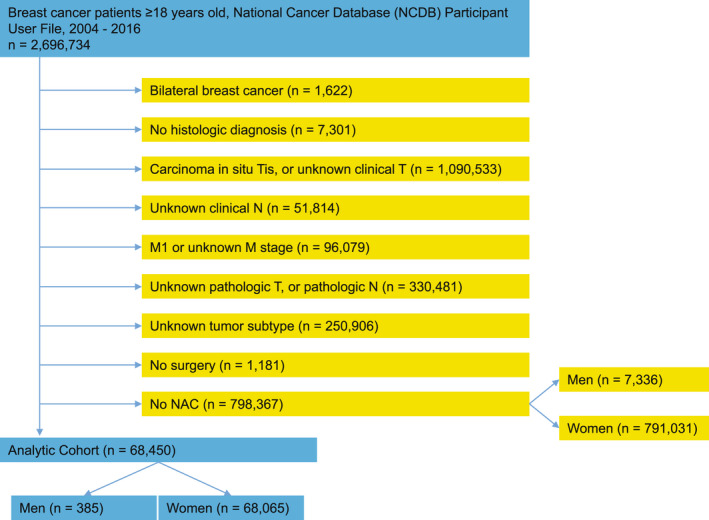
Flow diagram of patient population. NAC indicates neoadjuvant chemotherapy.
Patients were categorized based on the information on their receptor status: HR+/HER2– (estrogen receptor [ER] and/or progesterone receptor [PR] positive and HER2 negative); HR+/HER2+ (ER and/or PR positive and HER2 positive); HR–/HER2+ (ER and PR negative and HER2 positive); and triple‐negative (ER, PR, and HER2 negative).
The study was exempted from institutional review board approval because we used deidentified, publicly available data.
Statistical analysis
Comparisons of patient characteristics between men and women were made by χ2 test and Fisher exact test, as appropriate.
The primary end point of the study was pCR, defined as the absence of invasive cancer in the breast and axillary nodes, regardless of ductal carcinoma in situ (ypT0/Tis, ypN0). We proceeded to compare proportion of patients with pCR for MaBC and FBC for each tumor subtype by Fisher exact test. Odds ratios (ORs) of pCR were evaluated by logistic regression. We conducted a multivariable logistic regression model to evaluate the impact of sex on the odds of pCR, adjusted for tumor subtype, tumor grade, tumor size, nodal status, age at diagnosis, race, Charlson‐Deyo comorbidity score, and median income. Given that specific type of chemotherapy is unavailable in the NCDB, we included those variables in the adjusted model because these are factors associated with the type of chemotherapy used and with social determinants of health.
Secondary end points included clinical response and OS. Clinical response data were collected from the NCDB variable “CS_SITESPECIFIC_FACTOR_21,” which categorizes response to neoadjuvant therapy as complete clinical response, partial response, response (unknown if complete or partial), and no response. Proportions of clinical responses were compared between MaBC and FBC using the Fisher exact test. OS was defined as the time from diagnosis of breast cancer until death from any cause or last follow‐up for patients who were censored. We selected date of breast cancer diagnosis (rather than date of surgery) for the starting point of all OS analyses because, in this retrospective study, the date of surgery is not uniform and using date of surgery would introduce potential lead‐time bias given that patients could have undergone surgery as early as 90 days vs up to 210 days after diagnosis. We estimated OS by Kaplan–Meier method and compared OS by sex and by pCR using the log‐rank test. In addition, we performed a multivariable Cox regression model to evaluate the impact of sex on OS, adjusted for tumor subtype, tumor grade, tumor size, nodal status, pathologic response, age at diagnosis, race, Charlson‐Deyo comorbidity score, and median income. All p values reported were two‐sided, and p values < .05 were considered statistically significant. All statistical analyses were performed using STATA 12.0 (Stata Corporation, College Station, TX) and SPSS 20.0 (IBM Corporation, Armonk, NY).
RESULTS
Patient characteristics
Among 7721 MaBC and 859,096 FBC patients, 385 MaBC (5%) and 68,065 FBC (7.9%) underwent NAC and were included in the study (Fig. 1). Table 1 describes the demographics of subjects included from the NCDB. Median age for MaBC was 58 years (range, 23–88 years) and for FBC was 53 years (range, 18–90 years). Charlson‐Deyo comorbidity score was significantly higher for men than women (p = .038). Compared with FBC, MaBC had lower tumor grade and higher clinical stage. Statistically significant differences in tumor subtype distribution were seen when comparing MaBC with FBC (p < .0001). Among the male population, 206 patients (53.5%) had HR+/HER2– tumors, 112 patients (29.1%) had HR+/HER2+ tumors, 25 patients (6.5%) had HR–/HER2+ tumors, and 42 patients (10.9%) had triple‐negative disease. The distribution of tumor subtypes in FBC were as follows: 37.2% HR+/HER2–, 22.8% HR+/HER2+, 12.0% HR–/HER2+, and 27.9% triple‐negative. Median time from initial breast cancer diagnosis until initiation of NAC was 31 days in MaBC and 30 days in FBC. Median time from initiation of NAC to surgery was 143 days in MaBC and 148 days in FBC. Mastectomy was the most common surgery in both sexes, and it was more often performed in men than in women (81.0% vs. 63.8%, respectively; p < .0001).
TABLE 1.
Patient Characteristics
| Characteristics | Male | Female | |||
|---|---|---|---|---|---|
| No. | % | No. | % | p | |
| All patients | 385 | 0.6 | 68,065 | 99.4 | |
| Age at diagnosis, y | |||||
| <50 | 124 | 32.2 | 27,099 | 39.8 | <.0001 |
| 50–64 | 142 | 36.9 | 29,158 | 42.8 | |
| >64 | 119 | 30.9 | 11,808 | 17.3 | |
| Race | |||||
| White | 299 | 77.7 | 52,110 | 76.6 | .235 |
| Black | 69 | 17.9 | 11,421 | 16.8 | |
| Other | 15 | 3.9 | 3995 | 5.9 | |
| Unknowna | 2 | 0.5 | 539 | .8 | |
| Median income | |||||
| <$40,227 | 72 | 18.7 | 11,434 | 16.8 | .409 |
| $40,227–$50,353 | 84 | 21.8 | 13,635 | 20.0 | |
| $50,354–$63,332 | 90 | 23.4 | 15,707 | 23.1 | |
| ≥$63,333 | 135 | 35.1 | 26,475 | 38.9 | |
| Unknowna | 4 | 1.0 | 814 | 1.2 | |
| Charlson‐Deyo score | |||||
| 0 | 325 | 84.4 | 59,463 | 87.4 | .038 |
| 1 | 44 | 11.4 | 7050 | 10.4 | |
| 2 | 10 | 2.6 | 1155 | 1.7 | |
| ≥3 | 6 | 1.6 | 397 | .6 | |
| Histology | |||||
| Ductal | 330 | 85.7 | 56,835 | 83.5 | .281 |
| Lobular | 13 | 3.4 | 3619 | 5.3 | |
| Mixed ductal and lobular | 11 | 2.9 | 2307 | 3.4 | |
| Mixed ductal and other | 12 | 3.1 | 1490 | 2.2 | |
| Carcinoma | 19 | 4.9 | 3814 | 5.6 | |
| Grade | |||||
| I | 29 | 7.5 | 3410 | 5.0 | <.0001 |
| II | 159 | 41.3 | 21,728 | 31.9 | |
| III/IV | 167 | 43.4 | 38,075 | 55.9 | |
| Unknowna | 30 | 7.8 | 4852 | 7.1 | |
| Clinical stage | |||||
| I | 33 | 8.6 | 7450 | 10.9 | .004 |
| II | 207 | 53.8 | 40,145 | 59.0 | |
| III | 145 | 37.7 | 20,470 | 30.1 | |
| Tumor subtype | |||||
| HR+/HER2– | 206 | 53.5 | 25,326 | 37.2 | <.0001 |
| HR+/HER2+ | 112 | 29.1 | 15,539 | 22.8 | |
| HR–/HER2+ | 25 | 6.5 | 8198 | 12.0 | |
| Triple negative | 42 | 10.9 | 19,002 | 27.9 | |
| Surgery | |||||
| Mastectomy | 312 | 81.0 | 43,451 | 63.8 | <0.0001 |
| Partial mastectomy | 73 | 19.0 | 24,593 | 36.1 | |
| Unknown | 0 | 0.0 | 21 | .0% | |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor.
Unknown patients are excluded from the comparative analysis.
Pathologic response to neoadjuvant chemotherapy
Proportions and odds of pCR to NAC were numerically lower in MaBC compared with FBC for each tumor subtype and statistically significant for HR+/HER2– and HR+/HER2+ (Table 2). Rates of pCR for HR+/HER2– breast cancer in women and men were 9.7% and 4.9%, respectively (p = .01; OR, 2.1; 95% CI, 1.1–4.0). In HR+/HER2+ breast cancer, rates of pCR in women and men were 33.6% and 16.1%, respectively (p < .001; OR, 2.6; 95% CI, 1.6–4.4).
TABLE 2.
pCR by Tumor Subtype for Men and Women Receiving NAC
| Tumor subtype | Male | Female | p | Odds ratio (95% CI) pCR female v male | ||
|---|---|---|---|---|---|---|
| No. | pCR (ypT0/Tis ypN0) (%) | No. | pCR (ypT0/Tis ypN0) (%) | |||
| HR+/HER2– | 206 | 4.9 | 25,326 | 9.7 | .01 | 2.1 (1.1–4.0) |
| HR+/HER2+ | 112 | 16.1 | 15,539 | 33.6 | <.001 | 2.6 (1.6–4.4) |
| HR–/HER2+ | 25 | 44 | 8198 | 53.2 | .42 | 1.4 (0.7–3.2) |
| Triple negative | 42 | 21.4 | 19,002 | 32.1 | .18 | 1.7 (0.8–3.6) |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor; NAC, neoadjuvant chemotherapy; pCR, pathologic complete response.
To further evaluate the difference in pCR between MaBC and FBC, we conducted a multivariable model (Table 3). After adjusting for patient and tumor characteristics, including tumor subtype, women had twice the odds of pCR than men (p < .001; OR, 2.0; 95% CI, 1.5–2.8).
TABLE 3.
Multivariable Logistic Regression for Pathologic Complete Response
| Variable | Odds ratio | p | 95% CI for odds ratio | |
|---|---|---|---|---|
| Lower | Upper | |||
| Sex (female vs male) | 1.998 | <.001 | 1.449 | 2.755 |
Adjusted for: tumor subtype (HR+/HER2–, HR+/HER2+, HR‐/HER2+, triple negative); tumor grade (I, II, III/IV, unknown); tumor size (T1, T2, T3, T4); nodal status (N0, N1, N2, N3); age at diagnosis (<50 years, 50–64 years, >64 years); race (White, Black, other, unknown); Charlson‐Deyo score (0, 1, 2, ≥3); median income (<$40,227; $40,227–$50,353; $50,354–$63,332; ≥$63,333; unknown).
Abbreviations: CI, confidence interval; HER2, human epidermal growth factor receptor 2; HR, hormone receptor.
Clinical response to neoadjuvant chemotherapy
The degree of clinical response to NAC across all tumor subtypes is shown in Table S1. Among patients with known clinical response information, MaBC had a lower proportion of complete clinical response than FBC (13.5% vs 23.3%, respectively) and MaBC had a higher proportion of no clinical response than FBC (10.6% vs 5.0%, respectively) (p < .001).
Survival analysis
As a secondary end point, we evaluated the associations of OS with pCR and sex. A total of 297 men (77.1% of the MaBC cohort) and 53,826 women (79.1% of the FBC cohort) had available information on both survival time and vital status and were included in the analyses of OS. Median follow‐up for MaBC was 33 months (interquartile range, 20 months–47 months). Median follow‐up for FBC was 35 months (interquartile range, 22 months–54 months).
Figures 2A and 2B show that pCR was significantly associated with OS in both men and women. Specifically, among MaBC who achieved pCR vs residual disease, the 5‐year OS rate was 90.0% vs. 64.7% (p = .02) (Fig. 2A). In FBC who achieved pCR vs residual disease, the 5‐year OS rate was 91.9% vs 75.3% (p < .001) (Fig. 2B).
FIGURE 2.
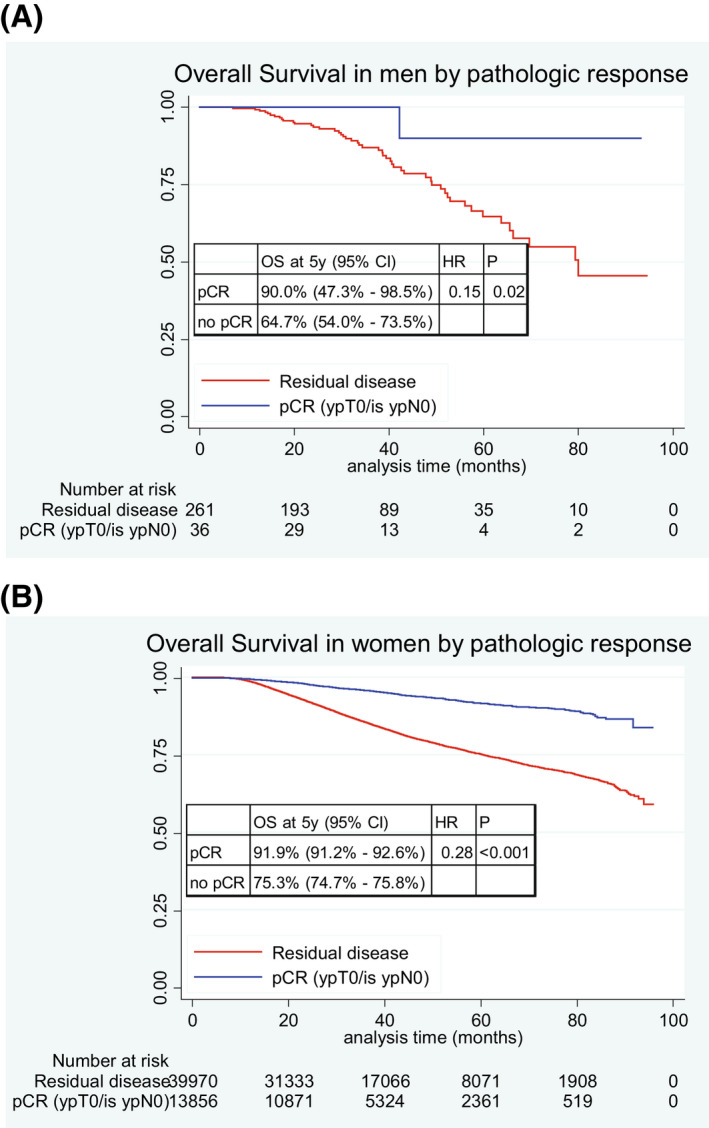
Kaplan–Meier curves for overall survival by pathologic response in (A) men, and (B) women. HR indicates hazard ratio; OS, overall survival; pCR, pathologic complete response.
Univariate comparisons of OS between MaBC and FBC are shown in Figure 3A‐C. Overall, FBC had better OS than MaBC with 5‐year OS rate of 79.0% and 67.1%, respectively (p = .02) (Fig. 3A). There was no statistically significant difference in OS between FBC and MaBC when both achieved pCR (5‐year OS rate 91.9% and 90.0%, respectively; p = .66) (Fig. 3B). We observed a numerical difference in OS between FBC and MaBC when both had residual disease (5‐year OS rate 75.3% and 64.7%, respectively; p = .11) (Fig. 3C).
FIGURE 3.
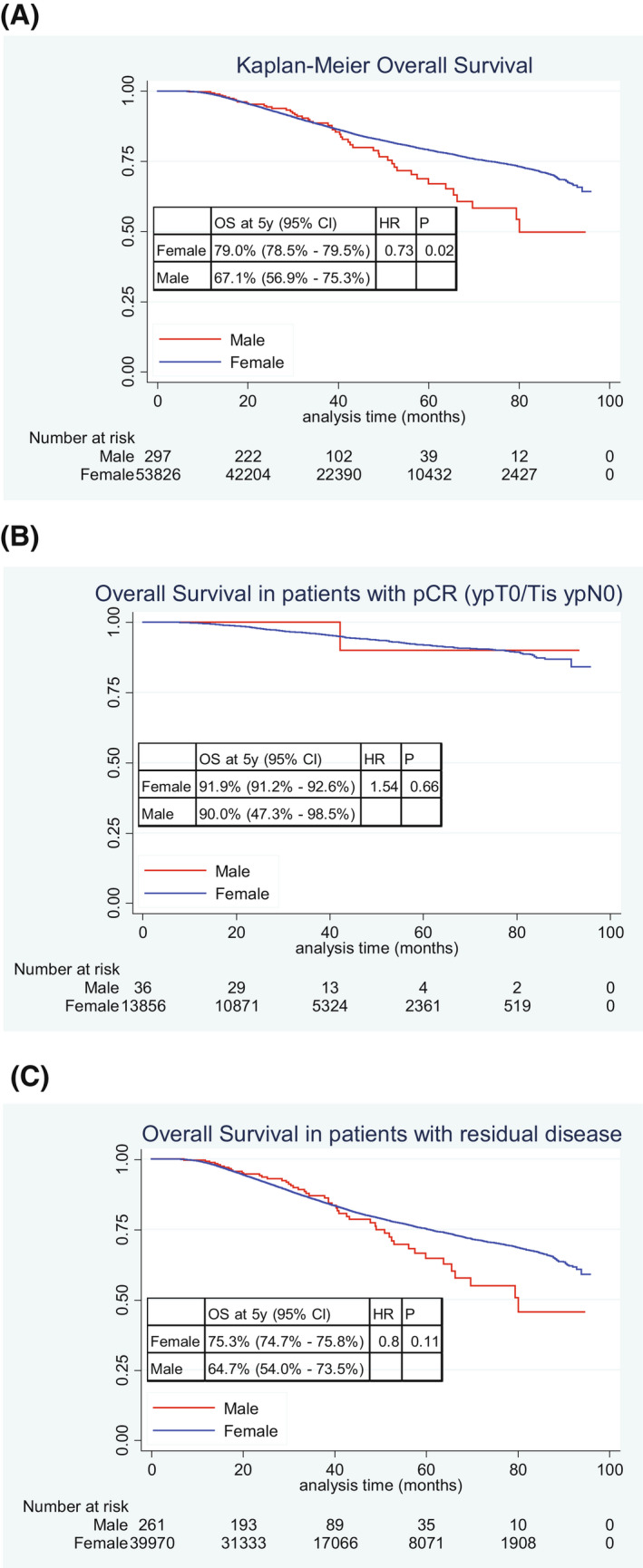
Kaplan–Meier curves for overall survival: (A) all men compared with all women; (B) men with pCR compared with women with pCR; and (C) men with residual disease compared with women with residual disease. Abbreviations: HR indicates hazard ratio; OS, overall survival; pCR, pathologic complete response.
In multivariable Cox regression analysis adjusted for patient and tumor characteristics and pathologic response, we observed no statistically significant difference in OS between FBC and MaBC (hazard ratio, 0.9; 95% CI, 0.7–1.2) (Table S2).
DISCUSSION
The outcomes of NAC for MaBC are not well described in the literature. We designed this study to compare the efficacy of NAC in MaBC with FBC according to tumor subtype. Our study showed that the rates of pCR in MaBC were numerically lower than in FBC within each tumor subtype with statistically significant differences in HR+/HER2– and HR+/HER2+ subtypes. Multivariable analysis confirmed that the odds of pCR after NAC in men were half those in women. Similarly, clinical responses to NAC in men were lower than those in women. A possible explanation for the lower rate of pCR in men compared with women may be that breast cancer in men is more luminal driven. A recent study evaluated 67 MaBC samples by PAM50 and showed that 90% were luminal A or B. 10 Similarly, Christensen et al. conducted PAM50 analysis in 37 MaBC samples and reported 95% of cases having luminal A or B subtype. 11 The high prevalence of luminal disease in men may be associated with lower sensitivity to NAC and in turn, lower rates of pCR compared with women. Another possible explanation may be related to differences in the tumor microenvironment between MaBC and FBC. In fact, MaBC appears to have lower rates of immune cell infiltration and higher proportion of exhausted T cells. 12 Lower tumor‐infiltrating lymphocytes are a known predictor of lower odds of pCR to NAC. 13
Two large meta‐analyses have reported pCR rates to NAC in women with breast cancer and their association with recurrence‐free survival. 5 , 14 The rates of pCR in those meta‐analyses are remarkably similar to the rates of pCR in women in our study. Specifically, in HR+/HER2– breast cancer, the rate of pCR in our study was 9.7%, compared with 9.3% in Spring et al. In HR+/HER2+ breast cancer, our observed rate of pCR was 33.6%, vs 30.9% in Cortazar et al. In HR–/HER2+ disease, we observed a pCR rate of 53.2%, compared with 50.3% in Cortazar et al. Last, in triple‐negative breast cancer, the rate of pCR in our study was 32.1% vs 32.6% in Spring et al. and 33.6% in Cortazar et al. 5 , 14
Prior studies evaluating differences in prognosis between MaBC and FBC have shown worse survival in men. 15 , 16 However, there are several factors that can affect OS, such as age at diagnosis, stage of breast cancer, tumor subtype, treatment received, and compliance, among others. Our study evaluated both men and women undergoing NAC. When comparing OS between MaBC and FBC in our study overall (Fig. 3A), we observed that men had significantly worse survival than women, in line with those prior studies. This finding, however, may be due in part to the difference in pCR rates between sexes. In fact, when we compared the survival of men and women when both achieved pCR and when both had residual disease (non‐pCR), differences in OS were not statistically significant, suggesting that pCR is prognostic for both men and women in a similar manner. Moreover, when we adjusted the analysis of OS for multiple relevant covariates, including pathologic response, OS was not significantly different between men and women. Nonetheless, the disparity in pCR rates warrants further investigation to improve outcomes in men with this disease.
Two prior studies from our group demonstrated that men with HER2+ breast cancer have worse OS and breast cancer–specific survival than men with HR+/HER2– disease. 17 , 18 In addition, men with HER2+ breast cancer have worse OS and breast cancer–specific survival than women with HER2+ disease. 15 , 16 The present study showed that, when compared with women with the same subtypes, men had a significantly lower rate of pCR in HR+/HER2+ and a numerically lower rate of pCR in HR–/HER2+ breast cancer. Two prior studies evaluated the distribution of intrinsic subtypes by PAM50 in male breast cancer samples and identified that ≥90% were genomically luminal B or luminal A. 10 , 11 There was only one HER2+ case among both studies, and therefore the distribution of intrinsic subtypes within HER2+ male breast cancer remains unknown. Collectively, the data from the previously referenced studies 10 , 11 , 15 , 16 , 17 , 18 and our present analysis suggest that HER2+ breast cancer in men may be more luminal driven and may derive less benefit from anti‐HER2 therapies. Further studies on the mechanism of resistance of anti‐HER2 therapies in men, as well as whether certain anti‐HER2 drugs may be more effective in MaBC, are warranted.
We recognize that our study has several limitations. Detailed systemic therapy regimen information is unavailable in NCDB, which represents a major limitation when comparing pCR between sexes without being able to confirm that treatments administered were similar. In addition, the duration of NAC is unknown. NCDB provides the start date of NAC and the date of surgery, but the completion date of NAC is not provided. To address this limitation, we required that the timeframe from start of NAC to surgery was between 90 and 210 days, which is the standard duration for most of the NAC regimens and excludes any patient who may have received short course therapy as a bridge to surgery, or patients who may have been inoperable. In this regard, the similarity in pCR rates between our study and the meta‐analyses are reassuring. 5 , 14 Given that specific chemotherapy regimen information was not available, we were not able to include type of chemotherapy as a variable in the multivariable analyses. To mitigate this limitation, we included age at diagnosis, tumor size, nodal status, and comorbidities in both adjusted models because these are variables that are correlated with the type of chemotherapy administered in routine clinical practice. This multivariable model also allowed to adjust the pCR results for existing imbalances in characteristics between the sexes, such as higher stage and higher comorbidities in MaBC, among others. Some tumor subtypes in MaBC had small sample sizes, which unfortunately may have affected the possibility to observe significance in the HR–/HER2+ and triple‐negative subtypes. This represents a significant limitation in our study, given that there are men with those subtypes of breast cancer for whom we need additional data. Despite the use of a very large database, the small sample size of men with HR–/HER2+ and triple‐negative subtypes is expected because these tumor subtypes are very uncommon in men. 18 This precluded us from being able to conduct multivariable analyses within specific tumor subtypes. Because of small sample size, some survival estimates have wide CIs and therefore should be interpreted with caution. The data on clinical response in our study are derived from the NCDB variable “CS_SITESPECIFIC_FACTOR_21,” which collects responses as documented by the individual medical centers and does not necessarily follow a response criteria used in clinical trials. Unfortunately, approximately 22% of patients had missing survival data for the secondary end point of OS. In addition, the NCDB does not report data on disease‐free survival or breast cancer–specific survival. Last, given that baseline characteristics between men and women who received NAC were different, there may be a risk of potential confounding by indication to receive NAC between MaBC and FBC, particularly by tumor stage. However, in the multivariable analyses, we included several important covariates (including tumor size and nodal status) to address this limitation. Moreover, existing data suggest that the likelihood of pCR is regardless of tumor size and nodal status. 5 , 19
Despite the limitations, our study has several strengths. First, our study compared pCR rates between MaBC and FBC by specific tumor subtypes. This is important, because almost 90% of men have HR+/HER2– breast cancer, 18 and if compared only by sex, men would have lower pCR rates than women. In addition, we conducted multivariable analyses that included key clinical and pathologic covariates and confirmed the independent association of sex with the likelihood of pCR. Another important strength is that our study evaluated a very clinically relevant question as we now know that pathologic responses to NAC are not only prognostic, but also dictate the type of therapy that should be administered in the adjuvant setting. 4 In this regard, the lower likelihood of pCR in MaBC seen in our study is a provocative finding and warrants further research in men with residual disease to improve outcomes. The results from our study are valuable to inform about the comparative efficacy of NAC between men and women with breast cancer, given that there has never been a prospective trial evaluating the efficacy of NAC in MaBC, and there are no prospective comparisons of the efficacy of NAC between sexes.
In summary, our study showed that men receiving NAC for breast cancer achieved lower proportions of pCR than women. These results suggest that, compared with FBC of the same subtypes, HR+/HER2– and HR+/HER2+ MaBC may be more resistant to NAC. We identified that pCR is prognostic both in men and women with breast cancer. Therefore, pCR may serve as an end point for clinical trials in MaBC, which is a rare disease, and facilitate the identification of effective therapies using smaller sample sizes.
AUTHOR CONTRIBUTIONS
José Pablo Leone: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, writing – original draft, and writing – review & editing. Michael J. Hassett: Methodology and writing – review & editing. Julieta Leone: Investigation, methodology, writing – original draft, and writing – review & editing. Sara M. Tolaney: Methodology and writing – review & editing. Carlos T. Vallejo: Methodology and writing – review & editing. Bernardo A. Leone: Investigation, conceptualization, methodology, software, and supervision. Eric P. Winer: Methodology, supervision, and writing – review & editing. Nancy U. Lin: Investigation, methodology, supervision, and writing – review & editing.
CONFLICTS OF INTEREST
J.P.L. received research funding from Kazia Therapeutics and consulting honoraria from Minerva Biotechnologies. S.M.T. receives institutional research funding from AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics/Gilead, Exelixis, Bristol‐Myers Squibb, Eisai, Nanostring, Cyclacel, Odonate, Sanofi, and Seattle Genetics; has served as an advisor/consultant to, and/or received honoraria from, AstraZeneca, Eli Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics/Gilead, Bristol‐Myers Squibb, Eisai, Nanostring, Puma, Sanofi, Silverback Therapeutics, G1 Therapeutics, Athenex, OncoPep, Kyowa Kirin Pharmaceuticals, Daiichi‐Sankyo, Ellipsis, Infinity, 4D Pharma, Samsung Bioepsis Inc, Chugai Pharmaceuticals, BeyondSpring Pharmaceuticals, OncXerna, OncoSec Medical Incorporated, Odonate, Certara, Mersana Therapeutics, CytomX, Zymeworks, Zentalis, Blueprint Pharmaceuticals, and Seattle Genetics. E.P.W. reports institutional research funding from Genentech/Roche; serving as a consultant for Athenex, Carrick Therapeutics, G1 Therapeutics, Genentech/Roche, Genomic Health, Gilead, GlaxoSmithKline, GSK, Jounce, Lilly, St. Lucia, Syros, and Zymeworks; a nonpaid scientific advisory board membership at Leap Therapeutics; and serving as President‐Elect of the American Society of Clinical Oncology (ASCO). N.U.L. reports institutional research funding from Genentech, Merck, Pfizer, Seattle Genetics, AstraZeneca, Zion Pharmaceuticals, and Olema Pharmaceuticals; consultant/advisory board work for Pfizer, Puma, Seattle Genetics, Daiichi Sankyo, AstraZeneca, Prelude Therapeutics, Denali Therapeutics, Olema Pharmaceuticals, Aleta BioPharma, Affinia Therapeutics, Voyager Therapeutics, and Artera, Inc; royalties from UpToDate; and stock or other ownership interests in Artera Inc (a startup with no current value, but options only valued at <5% and < $50,000 will be provided at a later date). The remaining authors made no disclosures.
Supporting information
Appendix S1
Present Address Eric P. Winer, Yale Cancer Center, New Haven, CT 06510, USA.
This study was presented on June 5, 2021, in part at the 2020 Annual Meeting of the American Society of Clinical Oncology as Abstract 587.
Bernardo A. Leone died on May 25, 2021. This manuscript is dedicated to his memory.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the National Cancer Database, at: https://www.facs.org/quality‐programs/cancer/ncdb
REFERENCES
- 1. Cardoso F, Kyriakides S, Ohno S, Penault‐Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E, ESMO Guidelines Committee . Electronic address: clinicalguidelines@esmo.org. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐updagger. Ann Oncol 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173 [DOI] [PubMed] [Google Scholar]
- 2. Hassett MJ, Somerfield MR, Baker ER, et al. Management of male breast cancer: ASCO Guideline. J Clin Oncol. 2020;38(16):1849‐1863. doi: 10.1200/JClinOncol.19.03120 [DOI] [PubMed] [Google Scholar]
- 3. National Comprehensive Cancer Network . Breast Cancer (version 5.2021), http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed March 24, 2022.
- 4. Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO Guideline. J Clin Oncol. 2021;39(13):1485‐1505. doi: 10.1200/JCO.20.03399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long‐term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164‐172. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 6. Cao L, Hue JJ, Freyvogel M, et al. Despite equivalent outcomes, men receive neoadjuvant chemotherapy less often than women for lymph node‐positive breast cancer. Ann Surg Oncol. 2021;28:438‐439. doi: 10.1245/s10434-021-09857-4 [DOI] [PubMed] [Google Scholar]
- 7. Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683‐690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. About the National Cancer Database . Accessed March 24, 2022. https://www.facs.org/quality‐programs/cancer/ncdb/about
- 9. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471‐1474. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 10. Sanchez‐Munoz A, Vicioso L, Santonja A, et al. Male breast cancer: correlation between immunohistochemical subtyping and PAM50 intrinsic subtypes, and the subsequent clinical outcomes. Mod Pathol. 2018;31(2):299‐306. doi: 10.1038/modpathol.2017.129 [DOI] [PubMed] [Google Scholar]
- 11. Christensen LG, Lautrup MD, Lyng MB, Moller S, Jylling AMB. Subtyping of male breast cancer by PAM50 and immunohistochemistry: a pilot study of a consecutive Danish cohort. APMIS. 2020;128(9):523‐530. doi: 10.1111/apm.13068 [DOI] [PubMed] [Google Scholar]
- 12. Sun H, Wang Z, Zhu Y, Yang C, Wang Q, Wei J, Ding Q Abstract PS18‐50: Single‐cell transcriptomic analysis reveals tumor microenvironment in male breast cancer. Cancer Res 2021;81(4_suppl):PS18–PS50, PS18‐50. [Google Scholar]
- 13. Gao ZH, Li CX, Liu M, Jiang JY. Predictive and prognostic role of tumour‐infiltrating lymphocytes in breast cancer patients with different molecular subtypes: a meta‐analysis. BMC Cancer. 2020;20(1):1150. doi: 10.1186/s12885-020-07654-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spring LM, Fell G, Arfe A, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta‐analysis. Clin Cancer Res. 2020;26(12):2838‐2848. doi: 10.1158/1078-0432.CCR-19-3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang F, Shu X, Meszoely I, et al. Overall mortality after diagnosis of breast cancer in men vs women. JAMA Oncol. 2019;5(11):1589‐1596. doi: 10.1001/jamaoncol.2019.2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leone J, Zwenger AO, Leone BA, Vallejo CT, Leone JP. Overall survival of men and women with breast cancer according to tumor subtype: a population‐based study. Am J Clin Oncol. 2019;42(2):215‐220. doi: 10.1097/COC.0000000000000497 [DOI] [PubMed] [Google Scholar]
- 17. Leone JP, Leone J, Zwenger AO, Iturbe J, Vallejo CT, Leone BA. Prognostic significance of tumor subtypes in male breast cancer: a population‐based study. Breast Cancer Res Treat. 2015;152(3):601‐609. doi: 10.1007/s10549-015-3488-y [DOI] [PubMed] [Google Scholar]
- 18. Leone J, Freedman RA, Lin NU, et al. Tumor subtypes and survival in male breast cancer. Breast Cancer Res Treat. 2021;188(3):695‐702. doi: 10.1007/s10549-021-06182-y [DOI] [PubMed] [Google Scholar]
- 19. Baron P, Beitsch P, Boselli D, et al. Impact of tumor size on probability of pathologic complete response after neoadjuvant chemotherapy. Ann Surg Oncol. 2016;23(5):1522‐1529. doi: 10.1245/s10434-015-5030-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data underlying this article are available in the National Cancer Database, at: https://www.facs.org/quality‐programs/cancer/ncdb


