Summary
Background
After 1 year of ursodeoxycholic acid (UDCA), patients with primary biliary cholangitis (PBC) may have a normal GLOBE score despite high alkaline phosphatase (ALP) levels.
Aim
To assess the association between ALP and liver transplantation (LT)‐free survival according to the GLOBE score
Methods
Among patients with a normal or elevated GLOBE score in the Global PBC cohort, the association between ALP after 1 year of UDCA and the risk of LT/death was assessed. The LT‐free survival was compared with that of a matched general population.
Results
After 1 year of UDCA, ALP was associated with the risk of LT/death (aHR 1.31, 95% CI 1.003–1.72, p = 0.048) among 2729 patients with a normal GLOBE score. The 10‐year LT‐free survival among these patients with an ALP >2.0 × ULN was 94.0% (95% CI 90.1–97.9) for those <50 years, and 82.6% (95% CI 76.5–88.7) for those ≥50 years, which was significantly lower (p = 0.040) and similar (p = 0.736) to that of the matched population, respectively. The 10‐year LT‐free survival in patients ≥50 years with normal GLOBE score and normal ALP (90.8%, 95% CI 87.7–93.9) was significantly higher (p = 0.022) than the matched population. Among 1045 patients with an elevated GLOBE score, ALP was associated with LT/death only in those <50 years (aHR 1.38, 95% CI 1.06–1.81, p = 0.016).
Conclusion
The LT‐free survival of patients with PBC with a normal GLOBE score is optimal in case of normal ALP levels, also in relation to the general population. Despite their generally favourable prognosis, an elevated ALP level may still indicate a need for add‐on therapy.
Keywords: GLOBE score, primary biliary cholangitis, second‐line treatment, treatment response
Abstract
xxx

Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AMA
anti‐mitochondrial antibody
- AST
aspartate aminotransferase
- CI
confidence interval
- HR
hazard ratio
- IQR
interquartile range
- LLN
lower limit of normal
- LT
liver transplantation
- OCA
obeticholic acid
- PBC
primary biliary cholangitis
- SD
standard deviation
- UDCA
ursodeoxycholic acid
- ULN
upper limit of normal
1. INTRODUCTION
Primary biliary cholangitis (PBC) is a chronic cholestatic liver disease with auto‐immune features, histologically characterised by the destruction of the small intrahepatic bile ducts. Patients with PBC may silently progress towards cirrhosis with the risk of liver failure and hepatocellular carcinoma, resulting in an impaired survival in this population. 1 , 2 Ursodeoxycholic acid (UDCA) is currently the standard treatment for PBC. 3 , 4 Patients with lower alkaline phosphatase (ALP) and bilirubin levels during UDCA therapy showed an improved liver transplantation (LT)‐free survival. Yet, up to 40% of the patients are considered to respond incompletely to UDCA. 5 , 6 , 7 For these patients additional therapy with obeticholic acid (OCA) and/or fibrates may optimise their prognosis, as randomised controlled clinical trials showed a decrease in ALP and bilirubin with these drugs. 8 , 9 , 10
To identify patients who could benefit from second‐line therapy, the degree of response to UDCA treatment is traditionally determined by dichotomous UDCA response criteria, such as Paris I/II, Barcelona and Toronto. 6 , 11 , 12 , 13 Since then, continuous models including additional parameters (such as the GLOBE score) were developed to more accurately predict the prognosis of UDCA‐treated PBC patients. 14 , 15 Over the last years, the GLOBE score is increasingly recognised as a tool to assess the patient's prognosis and aid in treatment decisions. For optimal use in daily clinical practice, however, accurate interpretation of the patient's GLOBE score is important.
At present, patients with GLOBE scores below their age‐specific threshold are considered to have a normal survival in relation to their age‐ and sex‐matched peers. Second‐line treatment may not be fully considered in these patients, while patients with low GLOBE scores may still present with high ALP levels in case the other model parameters are favourable. It is ALP normalisation, however, which was recently shown to be associated with the most optimal survival. 16 Therefore, to optimise the interpretation of the GLOBE score, the aim of this study was to assess the association between ALP or bilirubin and the LT‐free survival in PBC patients according to their GLOBE score response status, also in relation to a matched general population.
2. PATIENTS AND METHODS
2.1. Study population and design
Patients were derived from the Global PBC Study Group database. This database contains individual patient data from long‐term follow‐up cohorts from 15 liver units across Europe and Northern America. All patients had an established diagnosis of PBC according to the internationally accepted guidelines. 3 , 4 In this study, only UDCA‐treated patients with sufficient follow‐up (≥12 months and ≥2 recorded visits) and known dates of clinical events were eligible for inclusion. Patients with overt overlapping features of auto‐immune hepatitis according to the Paris criteria were excluded. 17
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the international research board of the corresponding center and at each participating center, in accordance with their local regulations.
2.2. Data collection
Clinical and laboratory data were collected at baseline, which was the start of UDCA treatment, and during follow‐up. Clinical data included the following: age, gender, details about PBC diagnosis, baseline anti‐mitochondrial antibody (AMA) serological status, liver histology obtained within 1 year from baseline (classified according to Ludwig and Scheuer's criteria 18 , 19 ), treatment and clinical outcome (LT or death). Laboratory data included ALP, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, albumin and platelet count and was collected at baseline and after 1 year. Biochemical disease stage was classified according to the Rotterdam criteria. 5
Data of the original cohorts were collected through the end of December 2012. For three centers, which entered the study group more recently, data collection occurred through the end of December 2015.
2.3. Statistical analyses
Data are presented as mean with standard deviation (SD), median with interquartile range (IQR) or as proportions. In the current study, patients were categorised according to the age‐specific threshold of the GLOBE score. The GLOBE score was considered ‘normal’ if below the threshold and ‘elevated’ if above the threshold. At present, patients with a GLOBE score below their age‐specific thresholds are considered to have a survival comparable with that of the age‐ and sex‐matched general population, in contrast with those with GLOBE scores above the threshold. 14 , 20 For visualisation purposes, patients were stratified according to their age at baseline (<50 years and ≥50 years based on a round cut‐off close to the average age in the cohort). For comparisons of categorical and continuous variables the chi‐squared test, students' t‐test, one‐way ANOVA or Kruskal–Wallis test were used, where appropriate. The GLOBE score was calculated with the formula: 0.044378 × age + 0.93982 × LN(bilirubin) + 0.335648 × LN(ALP) + 2.266708 × albumin + 0.002581 × platelets (per 109/L) + 1.216865 (bilirubin and ALP in ‘times upper limit of normal’ and albumin in ‘times lower limit of normal’).
The primary outcome of this study was the combined endpoint of LT and all‐cause mortality. Patients were followed until LT or death or censored at their last visit in case no event occurred. Univariate and multivariate Cox proportional hazards' regression analyses (hazard ratio [HR] with 95% confidence interval [CI]) were performed to assess the association between ALP, bilirubin and the risk of LT or death. Biochemical variables were log transformed in case of non‐normality. The survival rates were based on the Kaplan Meier method and compared using the log‐rank test. For these analyses, start of follow‐up was set at 1 year after start of UDCA treatment. To plot the survival, patients were categorised according to their level of ALP (≤1.0, 1.0–2.0 and >2.0 × ULN) or level of bilirubin (≤0.6, 0.6–1.0 and >1.0 × ULN) after 1 year of UDCA therapy. The lower cut‐offs were based on a prior report indicating that the risk of LT or death starts to increase from these levels onwards. 16 As the relation between ALP or bilirubin and the LT‐free survival follows a log‐linear pattern hereafter, it is difficult to define optimal upper cut‐off. 21 The upper cut‐off for ALP (2.0 × ULN) was arbitrary and partly driven by the number of patients and events in our cohort, whereas the upper cut‐off for bilirubin (1.0 × ULN) has been repeatedly used in the traditional response criteria. The LT‐free survival of each group was compared with the survival of an age‐, sex‐ and calendar time‐matched general population using the life table method and the Wilcoxon (Gehan) test as previously described. 14 Data of this matched population were retrieved from a Dutch registry (Statistics Netherlands, www.cbs.nl). According to the life table data of the World Health Organisation (http://www.who.int/) the median life expectancy of the Dutch population at age 60 was similar to the average median life expectancy of the other represented countries in our global cohort.
Missing data at baseline and at year 1 of UDCA therapy was handled by means of multiple imputation using SAS version 9.4 (SAS Institute Inc.), as in previous reports from our study group. 16 , 20 , 22 Hereto, 10 databases were generated and Rubin's rules were used to obtain pooled parameters and corresponding standard errors. Only continuous biochemical values were imputed: ALP, AST, ALT, bilirubin, albumin and platelet count. For the cumulative LT‐free survival estimates, patients were categorised in specific subgroups based on their individual mean GLOBE score and ALP or bilirubin level over the 10 imputed databases.
All statistical tests were two‐sided, and a p‐value <0.05 was considered to be statistically significant. Statistical analyses were performed in SPSS Statistics V.25.0 (IBM Corp.).
3. RESULTS
3.1. Cohort characteristics
In total, 3774 UDCA‐treated patients with PBC were included. The mean (SD) age was 52.2 (11.8) years, and the majority of patients was female (n = 3431; 90.7%). According to the GLOBE score, 2729 (72.3%) patients had a normal GLOBE score and 1045 (27.7%) patients had an elevated GLOBE score after 1 year of UDCA treatment. In both groups, patients <50 years showed statistically significantly higher ALP, aminotransaminases, albumin and platelets at baseline (Table 1). Patients ≥50 years had more advanced biochemical disease stage at baseline, especially those with an elevated GLOBE score. Patients were followed for a median of 7.2 (IQR 3.7–11.5) years, during which a total of 253 patients underwent LT and 477 patients died. The primary endpoint of LT or death occurred in 252 patients with a normal GLOBE score and in 475 patients with an elevated GLOBE score. The cumulative 10‐year LT‐free survival rates were 91.1% (95% CI 88.4–93.8) versus 53.2% (95% CI 49.7–56.7) in patients with a normal GLOBE score and elevated GLOBE score, respectively (p < 0.001).
TABLE 1.
Baseline characteristics
| Overall | Normal GLOBE score | Elevated GLOBE score | |||||
|---|---|---|---|---|---|---|---|
| <50 years | ≥50 years | p‐value | <50 years | ≥50 years | p‐value | ||
| (n = 3774) | (n = 981) | (n = 1748) | (n = 557) | (n = 488) | |||
| Age at diagnosis, years a | 52.3 (11.7) | 41.0 (6.4) | 60.3 (7.7) | <0.001 | 41.2 (5.7) | 59.0 (8.2) | <0.001 |
| Female, n (%) | 3423/3774 (90.9) | 925/981 (94.3) | 1583/1748 (90.6) | 0.001 | 503/557 (90.3) | 412/488 (84.4) | 0.004 |
| AMA positive, n (%) | 3394/3732 (90.9) | 881/971 (90.7) | 1585/1732 (91.5) | 0.391 | 492/548 (89.8) | 435/480 (90.6) | 0.085 |
| Year of diagnosis b | 1997 (1991–2004) | 1997 (1991–2003) | 1999 (1993–2005) | <0.001 | 1992 (1987–2000) | 1996 (1989–2003) | <0.001 |
| Histological disease stage, n (%) c | 0.796 | <0.001 | |||||
| Stage I | 827/2191 (37.7) | 279/636 (43.9) | 423/981 (43.1) | 80/326 (24.5) | 45/248 (18.1) | ||
| Stage II | 676/2191 (30.9) | 202/636 (31.8) | 324/981 (33.0) | 104/326 (31.9) | 46/248 (18.5) | ||
| Stage III | 355/2191 (16.2) | 81/636 (12.7) | 133/981 (13.6) | 79/326 (24.2) | 62/248 (25.0) | ||
| Stage IV | 333/2191 (15.2) | 74/636 (11.6) | 101/981 (12.3) | 63/326 (19.3) | 95/240 (38.2) | ||
| Serum bilirubin (ULN) b | 0.66 (0.47–0.99) | 0.55 (0.41–0.73) | 0.57 (0.43–0.75) | 0.167 | 1.19 (0.83–1.91) | 1.25 (0.91–1.85) | 0.279 |
| Serum ALP (ULN) b | 2.34 (1.49–3.80) | 2.17 (1.40–3.26) | 1.90 (1.31–2.87) | <0.001 | 4.11 (2.65–5.89) | 3.20 (2.00–4.92) | <0.001 |
| Serum AST (ULN) b | 1.60 (1.12–2.29) | 1.53 (1.11–2.10) | 1.33 (0.98–1.83) | <0.001 | 2.40 (1.85–3.33) | 2.07 (1.55–2.77) | <0.001 |
| Serum ALT (ULN) b | 1.82 (1.20–2.69) | 2.02 (1.37–2.88) | 1.48 (1.00–2.10) | <0.001 | 2.88 (2.05–4.00) | 1.91 (1.36–2.75) | <0.001 |
| Serum albumin (LLN) b | 1.16 (1.08–1.23) | 1.20 (1.14–1.27) | 1.17 (1.10–1.24) | <0.001 | 1.11 (1.03–1.19) | 1.06 (0.97–1.14) | <0.001 |
| Platelet count (×103/mm3) b | 250 (202–298) | 280 (238–325) | 252 (210–298) | <0.001 | 227 (174–281) | 192 (131–239) | <0.001 |
| Biochemical disease stage, n (%) d | 0.020 | <0.001 | |||||
| Early | 2731/3774 (72.4) | 896/981 (91.3) | 1536/1748 (87.9) | 171/557 (30.7) | 128/488 (26.2) | ||
| Moderately advanced | 853/3774 (22.6) | 82/981 (8.4) | 203/1748 (11.6) | 320 /557 (57.5) | 248/488 (50.8) | ||
| Advanced | 190/3774 (5.0) | 3/981 (0.3) | 9/1748 (0.5) | 66/557 (11.8) | 112/488 (23.0) | ||
Note: Serum bilirubin was missing for 1047 (28%) patients, serum ALP for 1036 (27%), serum AST for 1099 (29%), serum ALT for 1169 (31%), serum albumin for 1518 (40%) and platelet count for 1573 (42%). AMA status was missing for 43 (1.1%).
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA, anti‐mitochondrial antibody; AST, aspartate aminotransferase; LLN, lower limit of normal; ULN, upper limit of normal.
Data are expressed as mean and standard deviation.
Data are expressed as median and interquartile range.
Biochemical disease stage according to Rotterdam criteria. 5
3.2. Patients with a normal GLOBE score
Of the 2729 patients with a normal GLOBE score, 981 (35.9%) patients were <50 years and 1748 (64.1%) were ≥50 years (Table 1) among which 57 and 195 underwent LT or died, respectively. Overall, high ALP levels (×ULN) after 1 year of UDCA were associated with an increased risk of LT or death (HR 1.31, 95% CI 1.04–1.66, p = 0.025) in patients with a normal GLOBE score. After adjusting for age, gender, year of diagnosis and baseline biochemical liver tests (albumin, bilirubin, platelet count, ALT and AST), the ALP level remained statistically significantly associated with LT or death (HR 1.31, 95% CI 1.003–1.72, p = 0.048). There was no statistically significant interaction between ALP and the age category with respect to the occurrence of LT or death (p = 0.475).
In patients <50 years with a normal GLOBE score, the cumulative 10‐year LT‐free survival was 97.1% (95% CI 94.7–99.5) in those with normal ALP after 1 year of UDCA treatment (n = 352; 35.9%), 96.2% (95% CI 93.8–98.6) in those with an ALP between 1.0 and 2.0 × ULN (n = 428; 43.6%) and 94.0% (95% CI 90.1–97.9) in those with an ALP >2.0 × ULN (n = 201; 20.5%) (log rank over three categories: p = 0.090). Compared with an age‐ and sex‐matched general population, the LT‐free survival among patients <50 years with a normal GLOBE score did not differ statistically significantly from the matched population in case of ALP <1.0 × ULN (p = 0.352) or ALP of 1.0–2.0 × ULN (p = 0.508). However, those who remained with ALP levels >2.0 × ULN after 1 year of UDCA had a statistically significantly impaired LT‐free survival compared with the matched population (p = 0.040) (Figure 1).
FIGURE 1.
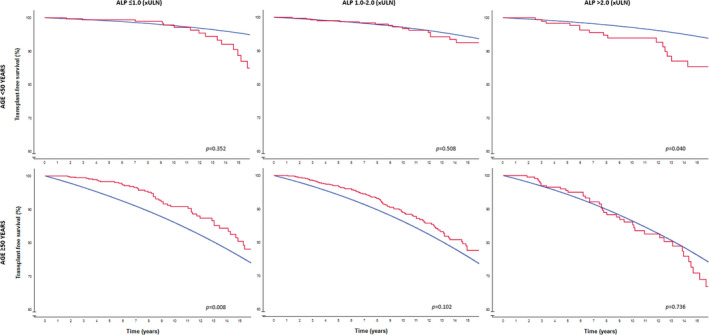
LT‐free survival in patients with a normal GLOBE score according to age and ALP categories compared with the age‐ and sex‐matched general population. The red line represents the observed LT‐free survival among patients with PBC. The blue line represents the LT‐free survival of the matched general population per ALP risk category. ALP, alkaline phosphatase; ULN; upper limit of normal; LT, liver transplantation; PBC, primary biliary cholangitis.
Of the 201 patients with a normal GLOBE score and an ALP >2.0 × ULN after 1 year of UDCA therapy, 193 patients were in follow‐up at year 2. At this time, the ALP level remained >2.0 × ULN in 129 (66.8%) patients, of which 105 (81.4%) were still classified as patients with a normal GLOBE score. The LT‐free survival among these patients was statistically significantly impaired compared with the matched population (p = 0.009), also when adding the 37 patients with an increase in ALP from ≤2.0 × ULN at year 1 to >2.0 × ULN at year 2 (p = 0.004). Those 64 patients with a normal GLOBE score and a decrease in ALP from >2.0 × ULN at year 1 to ≤2.0 × ULN at year 2 showed an LT‐free survival comparable with their matched population (p = 0.950).
In patients ≥50 years with a normal GLOBE score, the cumulative 10‐year LT‐free survival rates were 90.8% (95% CI 87.7–93.9), 87.8% (95% CI 84.9–90.7) and 82.6% (95% CI 76.5–88.7) for those with an ALP level ≤1.0 × ULN (n = 650; 37.2%), 1.0–2.0 × ULN (n = 847; 48.5%), or >2.0 × ULN (n = 251; 14.4%), respectively (log‐rank over three categories: p = 0.036). While the LT‐free survival did not differ statistically significantly from the matched population in case of an ALP between 1.0 and 2.0 × ULN (p = 0.102) or an ALP >2.0 × ULN in patients ≥50 years (p = 0.736), these older patients had a significantly better outcome in case of a normal ALP after 1 year of UDCA therapy compared with the matched general population (p = 0.022) (Figure 1).
Results were comparable when patients with a normal GLOBE score were stratified according to their bilirubin levels after 1 year of UDCA (Figure 2). Only three events occurred among the 18 patients <50 years with a bilirubin >1.0x ULN. Patients ≥50 years with a bilirubin level <0.6 × ULN (n = 1223) showed a cumulative 10‐year LT‐free survival of 90.4% (95% CI 88.2–92.6), which was statistically significantly better compared with the matched general population (p < 0.008).
FIGURE 2.
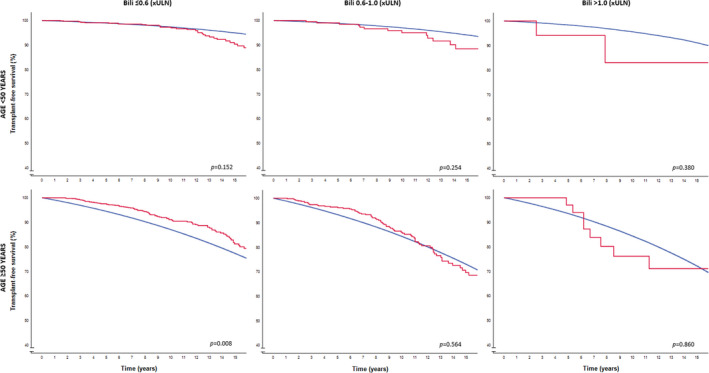
LT‐free survival in patients with a normal GLOBE score according to age and bilirubin categories compared with the age‐ and sex‐matched general population. The red line represents the observed LT‐free survival among patients with PBC. The blue line represents the LT‐free survival of the matched general population per bilirubin risk category. ULN; upper limit of normal; LT, liver transplantation; PBC, primary biliary cholangitis.
3.3. Patients with an elevated GLOBE score
Among the 1045 patients with an elevated GLOBE score, 557 (53.3%) patients were <50 years and 488 (46.7%) were ≥50 years. Patients ≥50 years had a lower median platelet count and more advanced disease (both biochemically and histologically) compared with younger patients (Table 1). The primary endpoint of LT or death was reached by 225 patients <50 years and 250 patients ≥50 years. There was a statistically significant interaction between ALP and the age category with respect to the occurrence of LT or death (p = 0.020) in those with an elevated GLOBE score. Among patients <50 years, the ALP level after 1 year of UDCA therapy was associated with LT/death (aHR 1.38, 95% CI 1.06–1.81, p = 0.016). In contrast, among those ≥50 years, there was no statistically significant association between ALP and the risk of LT or death (aHR 0.98, 95% CI 0.75–1.28, p = 0.889).
The cumulative 10‐year LT‐free survival rates were 68.4% (95% CI 46.6–90.2), 72.6% (95% CI 63.8–81.4) and 58.9% (95% CI 53.4–64.4) for patients <50 years with an elevated GLOBE score with an ALP level ≤1.0 × ULN (n = 27; 4.8%), 1.0–2.0 × ULN (n = 135; 24.2%), or >2.0 × ULN (n = 395; 70.9%), respectively (Log rank over three categories: p = 0.010). For patients ≥50 years with an elevated GLOBE score, the cumulative 10‐year LT‐free survival was 32.7% (95% CI 12.9–52.2) in those with normal ALP (n = 43; 9.6%), 49.4% (95% CI 40.0–58.8) in those with an ALP between 1.0 and 2.0 × ULN (n = 173; 38.6%) and 38.0% (95% CI 30.9–45.1) in those with an ALP >2.0 × ULN (n = 272; 60.7%), respectively (Log rank over three categories: p = 0.443). Compared with the matched general population, the LT‐free survival was impaired in all patients with an elevated GLOBE score, independent of the age or ALP category (range p‐value: <0.001–0.012) (Figure 3). Similar results were observed when patients with an elevated GLOBE score were stratified according to their bilirubin levels after 1 year of UDCA (Figure 4).
FIGURE 3.
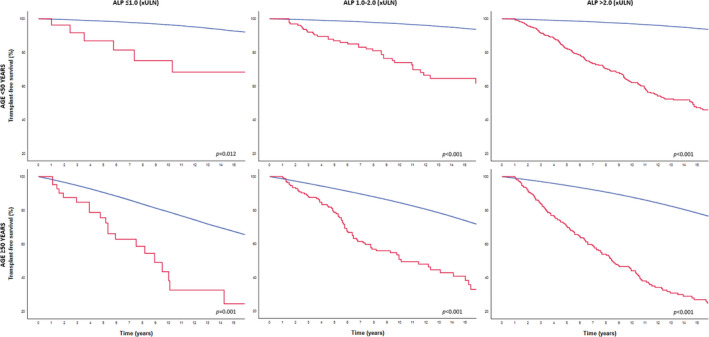
LT‐free survival in patients with an elevated GLOBE score according to age and ALP categories compared with the age‐ and sex‐matched general population. The red line represents the observed LT‐free survival among patients with PBC. The blue line represents the LT‐free survival of the matched general population per ALP risk category. ALP, alkaline phosphatase; ULN; upper limit of normal; LT, liver transplantation; PBC, primary biliary cholangitis.
FIGURE 4.
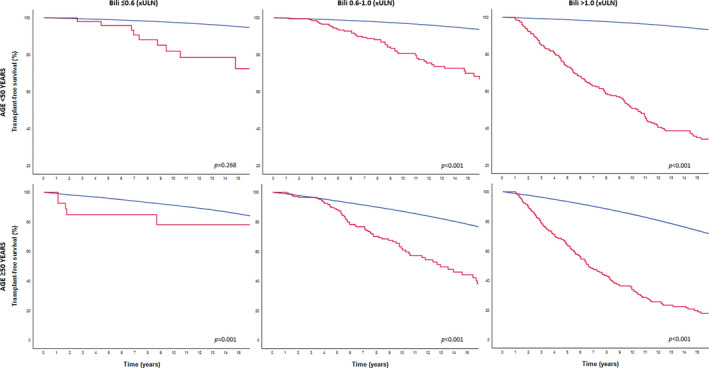
LT‐free survival in patients with an elevated GLOBE score according to age and bilirubin categories compared with the age‐ and sex‐matched general population. The red line represents the observed LT‐free survival among patients with PBC. The blue line represents the LT‐free survival of the matched general population per bilirubin risk category. ULN; upper limit of normal; LT, liver transplantation; PBC, primary biliary cholangitis.
4. DISCUSSION
In this large international cohort of patients with PBC, the level of ALP after 1 year of UDCA therapy was associated with the risk of LT or death among those with a GLOBE scores below their age‐specific threshold. Despite a GLOBE score below the threshold, patients <50 years with an ALP level >2.0 × ULN had a statistically significantly impaired LT‐free survival compared with a matched general population. Although the LT‐free survival did not deviate from normal in case of an elevated ALP in patients ≥50 years with a GLOBE score below the threshold, those with a normal ALP showed a significantly better survival compared with the matched population. These results suggest that the previously suggested dichotomous interpretation of the GLOBE score may require refinement.
The GLOBE score is an accurate model to predict the LT‐free survival of individual PBC patients by incorporating five independent continuous predictors of LT or death: age, ALP, total bilirubin, albumin and platelet count. 14 As suggested in the original GLOBE score development study, the score can be interpreted dichotomously using age‐specific thresholds (based on the average prognosis in relation to the survival of the matched general population). 14 When used as such, the GLOBE score parallels the concept of traditional response criteria. 20 Here, we show that about two‐thirds of patients with a ‘normal GLOBE score’ remain with an elevated ALP level after 1 year of UDCA therapy, which is likely explained by favourable parameters reflecting their stage of liver disease (albumin, bilirubin and platelets) (Table 1). These parameters have an important impact on the models estimates and, if the GLOBE score is taken into consideration for that purpose, on decisions related to second‐line therapy. 4 , 23 As we present here, it is, thus, important to recognise that patients with elevated ALP levels had a suboptimal survival despite GLOBE scores below their age‐specific threshold. New treatment options with proposed clinical efficacy based on their ability to lower ALP levels should, thus, be fully considered in these patients.
Our findings re‐enforce prior analyses from our group, which indicated that a normal ALP level may be the preferred goal of PBC therapy, as the risk of LT or death started to increase from the ULN of ALP upwards. 16 We extend this observation with comparisons with the survival of the general population, as well as the subgroup of patients with the best predicted outcome (those with low GLOBE scores). Still, the prognosis of patients with a low GLOBE score can be considered good irrespective of the ALP level, so that the absolute survival gain of add‐on therapy may be modest. 22 Especially for young patients, however, optimised outcome may be preferred. 24 The expected survival gain with additional therapy should thus be balanced against potential side‐effects and costs of different second‐line treatment options for the individual patient.
Among the 650 patients ≥50 years with a GLOBE score below their age‐specific threshold and a normal ALP in our study, the LT‐free survival was significantly better than that of an age and sex‐matched general population. We suggest that this may have to do with extrahepatic comorbidities being an important factor for survival in these patients. Their PBC‐related prognosis is generally good with a strong response to UDCA. Early detection and treatment of comorbidities may be eased by frequent medical monitoring. Second, it could be hypothesized that patients diagnosed with a chronic disease such as PBC, are more aware of their health behaviour. Favourable lifestyle changes after the diagnosis of PBC may contribute to a better outcome than that of non‐affected peers.
The LT‐free survival of patients with an elevated GLOBE score after 1 year of UDCA, was impaired compared with the matched general population irrespective of the ALP level or age category. This confirms their general need for additional therapy. While improving the prognosis as measured by a decrease in ALP is the main argument to administer OCA and/or fibrates, ALP was not a statistically significant predictor of LT or death among patients ≥50 years with an elevated GLOBE score in our study. This may be explained by the higher proportion of advanced disease in this subgroup. 21 While bilirubin remains a relevant cholestatic surrogate parameter in these patients, which was suggested to stabilise with OCA or fibrate therapy, the proposed anti‐fibrotic properties of new drugs may represent an important target mechanism. Both the UK‐PBC Group and the Global PBC Group showed that the degree of fibrosis was indeed associated with long‐term outcome in PBC, independent of the biochemical UDCA response. 25 , 26 Further clinical studies on the efficacy of farnesoid X receptor agonists and peroxisome proliferator‐activated receptor agonists in halting or reversing hepatic fibrosis are thus awaited.
Some limitations to our study should be noted. First, the current analyses were performed in a largely retrospectively constructed dataset. Long‐term prospective follow‐up studies are, however, unlikely to follow considering the course of UDCA‐treated PBC, which is also a relatively rare disease. As a result, adequate data on the cause of death are lacking. Second, the follow‐up of the patients in our cohort does not extend beyond 2015. While this is not considered to have had a major influence on the validity of our results, future studies should evaluate our findings in the setting of combination therapy. Third, despite the respective size of the Global cohort, we were unable to perform all desired subgroup analyses. Although we consider that the current data should be interpreted from a continuous perspective, we had to categorise the patients according to their age and biochemistry to visualise the results. The chosen cut‐offs are largely in line with prior PBC research, but can be considered arbitrary to some extent. We lack power to adequately assess optimal age cut‐off(s) with respect to the differences in LT‐free survival compared with the normal population. While further extension of worldwide initiatives could perhaps enable such analyses, the clinical consequences of such efforts may be questioned. Physicians may, for instance, be reluctant to risk PBC progression while awaiting patients to age above an ‘optimal’ cut‐off related to their outcome versus the normal population, based on the well‐described relation between ALP and prognosis. Fourth, we were unable to assess the UK‐PBC score due to lacking data on the cause of death. Since the UK‐PBC score also comprises objective disease stage parameters, we would expect that the concept of our results applies to this prognostic score as well. Last, the majority of the patients included in this study were treated in tertiary liver centers, which may have led to a selection bias.
In conclusion, the results of this study indicate that the clinical outcome of UDCA‐treated PBC patients with a GLOBE score below their age‐specific threshold is most optimal in case of normal ALP levels, also in relation to the matched general population. Thus, despite a relatively favourable prognosis according to the GLOBE score, the ALP level may still indicate that add‐on therapy to UDCA should be considered.
AUTHORSHIP
Guarantor of the article: Rozanne C. de Veer and Adriaan J. van der Meer had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of data analyses.
AUTHOR CONTRIBUTIONS
Rozanne C. de Veer: Conceptualization (lead); formal analysis (lead); writing – original draft (lead); writing – review and editing (lead). Maren H. Harms: Conceptualization (equal); data curation (equal); formal analysis (supporting); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Christophe Corpechot: Data curation (equal); investigation (equal); writing – review and editing (equal). Douglas Thorburn: Data curation (equal); investigation (equal); writing – review and editing (equal). Pietro Invernizzi: Data curation (equal); investigation (equal); writing – review and editing (equal). Harry L. Janssen: Data curation (equal); investigation (equal); writing – review and editing (equal). Pier Maria Battezzati: Data curation (equal); investigation (equal); writing – review and editing (equal). Frederik Nevens: Data curation (equal); investigation (equal); writing – review and editing (equal). Keith D Lindor: Data curation (equal); investigation (equal); writing – review and editing (equal). Annarosa Floreani: Data curation (equal); investigation (equal); writing – review and editing (equal). Cyriel Ponsioen: Data curation (equal); investigation (equal); writing – review and editing (equal). Marlyn Mayo: Data curation (equal); investigation (equal); writing – review and editing (equal). Albert Pares: Data curation (equal); investigation (equal); writing – review and editing (equal). Andrew Mason: Data curation (equal); investigation (equal); writing – review and editing (equal). Kris Kowdley: Data curation (equal); investigation (equal); writing – review and editing (equal). Palak J Trivedi: Data curation (supporting); investigation (equal); writing – review and editing (equal). Gideon Hirschfield: Data curation (equal); investigation (equal); writing – review and editing (equal). Tony Bruns: Data curation (equal); investigation (equal); writing – review and editing (equal). George Dalekos: Data curation (equal); investigation (equal); writing – review and editing (equal). Nikolaos K Gatselis: Data curation (equal); investigation (equal); writing – review and editing (equal). Xavier Patrick Verhelst: Data curation (equal); investigation (equal); writing – review and editing (equal). Willem Lammers: Data curation (equal); investigation (equal); writing – review and editing (equal). Bettina Hansen: Data curation (equal); funding acquisition (lead); investigation (equal); supervision (supporting); writing – original draft (supporting); writing – review and editing (equal). Henk R van Buuren: Conceptualization (equal); data curation (equal); funding acquisition (lead); investigation (equal); supervision (supporting); writing – original draft (supporting); writing – review and editing (equal). Ad van der Meer: Conceptualization (lead); data curation (equal); formal analysis (equal); methodology (lead); supervision (lead); writing – original draft (lead); writing – review and editing (lead).
FUNDING INFORMATION
This investigator‐initiated study was supported by an unrestricted grant from Intercept Pharmaceuticals and was funded by the Foundation for Liver and Gastrointestinal Research (a not‐for‐profit foundation) in Rotterdam, the Netherlands. The supporting parties had no influence on the study design, data collection and analyses, writing of the manuscript, or on the decision to submit the manuscript for publication.
CONFLICT OF INTEREST
The following authors declared that they have no conflicts of interest: Rozanne C. de Veer and Pier M. Battezzati. Maren H. Harms received a speakers fee from Zambon Nederland B.V. and thesis printing reimbursements from Intercept Pharmaceuticals, Pentax, Norgine, Tramedico, Dr. Falk, Sysmex, Astellas and Zambon Nederland B.V. Christophe Corpechot reports receiving consulting fees from Intercept Pharmaceuticals, Cymabay, Genkyotex and Inventiva, grant support from Arrow and Intercept Pharmaceuticals, and fees for teaching from GlaxoSmithKline. Douglas Thorburn reports consulting activities for Intercept Pharmaceuticals. Pietro Invernizzi reports personal fees from Intercept and non‐financial support from Bruschettini and Menarini Diagnostics. Harry L.A. Janssen reports grants from and consulting work for AbbVie Pharmaceuticals, Bristol‐Myers Squibb, Gilead Sciences, Innogenetics, Merck, Novartis, Roche, Intercept Pharmaceuticals and Janssen. Frederik Nevens reports Advisory boards for Astellas, Janssen‐Cilag, AbbVie, Gilead, CAF, Intercept, Gore, BMS, Novartis, MSD, Janssen‐Cilag, Promethera Biosciences, Ono Pharma, Durect, Roche, Ferring. Research grants from Roche, Ferring and Novartis. Keith D. Lindor reports that he is an unpaid advisor for Intercept Pharmaceuticals and Shire. Annerosa Floreani reports consulting activities for Intercept Pharmaceuticals. Cyriel Y. Ponsioen has received grant support from Takeda, speaker's fees from Tillotts and Takeda, consultancy fee from Pliant and served as consultant for Takeda. Marlyn J. Mayo reports being on advisory committees or review panels for GSK, Target, Regeneron; grant/research support from Cymabay, Intercept, Mallinckrodt, Target, GSK and Salix. Albert Parés reports consulting services for Intercept Pharmaceuticals and Novartis Pharma. Andrew L. Mason reports advisory services for Intercept Pharmaceuticals, AbbVie and Novartis; and research funding resources from the Canadian Institutes of Health Research, Canadian Liver Foundation, American Kennel Club, Intercept Pharmaceuticals Inc., AbbVie and Gilead Sciences. Kris V. Kowdley reports personal fees from Gilead Sciences, Intercept Pharmaceuticals and Novartis; and grants from Gilead Sciences and Intercept Pharmaceuticals. Palak J. Trivedi receives institutional salary support from the NIHR Birmingham Liver Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. PJT has received research grant funding from the Wellcome trust, the Core Digestive Diseases Charity, Intercept Pharmaceuticals, Bristol Myers' Squibb, Innovate UK, the Medical Research Foundation, EASL and PSC Support. He has also received speaker fees from Dr. Falk Pharma, Intercept Pharmaceuticals and Perspectum Diagnostics. He has received consulting and advisory board fees from Dr. Falk Pharma, Intercept Pharmaceuticals and GSK. Gideon M. Hirschfield reports advisory services for Intercept Pharmaceuticals, Novartis and GlaxoSmithKline Pharmaceuticals. Tony Bruns has received honoraria from Intercept Pharmaceuticals, Falk Foundation, Abbvie and Norgine, and travel expenses from Gilead. George N Dalekos is an advisor or lecturer for Ipsen, Pfizer, Genkyotex, Novartis, Sobi, received research grants from Abbvie, Gilead and has served as PI in studies for Abbvie, Novartis, Gilead, Novo Nordisk, Genkyotex, Regulus Therapeutics Inc, Tiziana Life Sciences, Bayer, Astellas, Pfizer, Amyndas Pharmaceuticals, CymaBay Therapeutics Inc. and Intercept Pharmaceuticals. Nikolaos K. Gatselis is an advisor or lecturer for Gilead and has acted as co‐investigator in studies for Abbvie, Novartis, Gilead, Novo Nordisk, Genkyotex, Regulus Therapeutics Inc, Tiziana Life Sciences, Bayer, Astellas, Pfizer, Amyndas Pharmaceuticals, CymaBay Therapeutics Inc. and Intercept Pharmaceuticals. Xavier Verhelst received grants from Gilead, Abbvie, Dr Phalk Pharma and MSD and acted as a consultant for Gilead, Abbvie and MSD. Willem J. Lammers reports consulting services for Intercept Pharmaceuticals. Bettina E. Hansen reports grants from Intercept Pharmaceuticals, Cymabay Therapeutics and Zambon Nederland B.V. and consulting work for Intercept Pharmaceuticals, Cymabay Therapeutics, Albireo AB and Novartis. Henk R. van Buuren is a consultant for Intercept Pharma Benelux and received unrestricted research grants from Intercept Pharmaceuticals and from Zambon Nederland B.V. A.J. van der Meer reports speakers fees from Zambon Nederland B.V., received an unrestricted grant from Gilead Sciences, AbbVie, MSD and Zambon Nederland B.V. and consulting work for AOP Pharma.
de Veer RC, Harms MH, Corpechot C, Thorburn D, Invernizzi P, Janssen HLA, et al. Liver transplant‐free survival according to alkaline phosphatase and GLOBE score in patients with primary biliary cholangitis treated with ursodeoxycholic acid. Aliment Pharmacol Ther. 2022;56:1408–1418. 10.1111/apt.17226
The Handling Editor for this article was Dr Colin Howden, and it was accepted for publication after full peer‐review.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386(10003):1565–75. [DOI] [PubMed] [Google Scholar]
- 2. Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353(12):1261–73. [DOI] [PubMed] [Google Scholar]
- 3. Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69(1):394–419. [DOI] [PubMed] [Google Scholar]
- 4. European Association for the Study of the Liver . Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67(1):145–72. [DOI] [PubMed] [Google Scholar]
- 5. Kuiper EM, Hansen BE, de Vries RA, den Ouden‐Muller JW, van Ditzhuijsen TJ, Haagsma EB, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136(4):1281–7. [DOI] [PubMed] [Google Scholar]
- 6. Corpechot C, Abenavoli L, Rabahi N, Chretien Y, Andreani T, Johanet C, et al. Biochemical response to ursodeoxycholic acid and long‐term prognosis in primary biliary cirrhosis. Hepatology. 2008;48(3):871–7. [DOI] [PubMed] [Google Scholar]
- 7. Gatselis NK, Zachou K, Lygoura V, Azariadis K, Arvaniti P, Spyrou E, et al. Geoepidemiology, clinical manifestations and outcome of primary biliary cholangitis in Greece. Eur J Intern Med. 2017;42:81–8. [DOI] [PubMed] [Google Scholar]
- 8. Corpechot C, Chazouilleres O, Rousseau A, Le Gruyer A, Habersetzer F, Mathurin P, et al. A placebo‐controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med. 2018;378(23):2171–81. [DOI] [PubMed] [Google Scholar]
- 9. Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, et al. A placebo‐controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375(7):631–43. [DOI] [PubMed] [Google Scholar]
- 10. Jones D, Boudes PF, Swain MG, Bowlus CL, Galambos MR, Bacon BR, et al. Seladelpar (MBX‐8025), a selective PPAR‐delta agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double‐blind, randomised, placebo‐controlled, phase 2, proof‐of‐concept study. Lancet Gastroenterol Hepatol. 2017;2(10):716–26. [DOI] [PubMed] [Google Scholar]
- 11. Corpechot C, Chazouilleres O, Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long‐term outcome. J Hepatol. 2011;55(6):1361–7. [DOI] [PubMed] [Google Scholar]
- 12. Pares A, Caballeria L, Rodes J. Excellent long‐term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130(3):715–20. [DOI] [PubMed] [Google Scholar]
- 13. Kumagi T, Guindi M, Fischer SE, Arenovich T, Abdalian R, Coltescu C, et al. Baseline ductopenia and treatment response predict long‐term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105(10):2186–94. [DOI] [PubMed] [Google Scholar]
- 14. Lammers WJ, Hirschfield GM, Corpechot C, Nevens F, Lindor KD, Janssen HL, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149(7):1804–12 e4. [DOI] [PubMed] [Google Scholar]
- 15. Carbone M, Sharp SJ, Flack S, Paximadas D, Spiess K, Adgey C, et al. The UK‐PBC risk scores: derivation and validation of a scoring system for long‐term prediction of end‐stage liver disease in primary biliary cholangitis. Hepatology. 2016;63(3):930–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murillo Perez CF, Harms MH, Lindor KD, van Buuren HR, Hirschfield GM, Corpechot C, et al. Goals of treatment for improved survival in primary biliary cholangitis: treatment target should be bilirubin within the normal range and normalization of alkaline phosphatase. Am J Gastroenterol. 2020;115(7):1066–74. [DOI] [PubMed] [Google Scholar]
- 17. Chazouilleres O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis‐autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28(2):296–301. [DOI] [PubMed] [Google Scholar]
- 18. Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol. 1978;379(2):103–12. [DOI] [PubMed] [Google Scholar]
- 19. Scheuer P. Primary biliary cirrhosis. Proc R Soc Med. 1967;60(12):1257–60. [PMC free article] [PubMed] [Google Scholar]
- 20. Harms MH, van Buuren HR, Corpechot C, Thorburn D, Janssen HLA, Lindor KD, et al. Ursodeoxycholic acid therapy and liver transplant‐free survival in patients with primary biliary cholangitis. J Hepatol. 2019;71(2):357–65. [DOI] [PubMed] [Google Scholar]
- 21. Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HL, Invernizzi P, Mason AL, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow‐up study. Gastroenterology. 2014;147(6):1338–49 e5. quiz e15. [DOI] [PubMed] [Google Scholar]
- 22. Harms MH, de Veer RC, Lammers WJ, Corpechot C, Thorburn D, Janssen HLA, et al. Number needed to treat with ursodeoxycholic acid therapy to prevent liver transplantation or death in primary biliary cholangitis. Gut. 2020;69(8):1502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montano‐Loza AJ, Corpechot C. Definition and management of patients with primary biliary cholangitis and an incomplete response to therapy. Clin Gastroenterol Hepatol. 2021;19(11):2241–51 e1. [DOI] [PubMed] [Google Scholar]
- 24. Cheung AC, Lammers WJ, Murillo Perez CF, van Buuren HR, Gulamhusein A, Trivedi PJ, et al. Effects of age and sex of response to ursodeoxycholic acid and transplant‐free survival in patients with primary biliary cholangitis. Clin Gastroenterol Hepatol. 2019;17(10):2076–84 e2. [DOI] [PubMed] [Google Scholar]
- 25. Murillo Perez CF, Hirschfield GM, Corpechot C, Floreani A, Mayo MJ, van der Meer A, et al. Fibrosis stage is an independent predictor of outcome in primary biliary cholangitis despite biochemical treatment response. Aliment Pharmacol Ther. 2019;50(10):1127–36. [DOI] [PubMed] [Google Scholar]
- 26. Carbone M, Sharp SJ, Heneghan MA, Neuberger JM, Hirschfield G, Burroughs AK, et al. P1198: histological stage is relevant for risk‐stratification in primary biliary cirrhosis. J Hepatol. 2015;62(suppl 2):S805. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


