Abstract
Lobar atelectasis may be a complication of pulmonary exacerbations in cystic fibrosis (CF). There are no established guidelines on the management of this condition in patients with CF. Therapeutic bronchoscopy with recombinant human deoxyribonuclease (rhDNase) instillation has been described to be successful in patients not responding to conservative measures. We describe a case of a young man with CF, with previously mild impaired lung function, presenting with cough, desaturation, and worsening dyspnea, persisting for over 6 weeks, despite conservative therapy. Thoracic imaging showed right lower lobe atelectasis, which was successfully treated with bronchoscopy and instillation of rhDNase. Long‐term resolution of the atelectasis was confirmed with chest magnetic resonance imaging follow‐up.
Keywords: atelectasis, chest MRI, cystic fibrosis, lung pathology, rhDNase, therapeutic bronchoscopy
Pulmonary exacerbations in cystic fibrosis (CF) may be complicated by lobar atelectasis, mainly occurring as a result of mucus plugging. There are no established guidelines on the management of atelectasis in people with CF (pwCF). The most common approach consists of the first‐line treatment with intravenous (iv) antibiotics, associated with enhanced chest physiotherapy; if no improvement is obtained, a second‐line treatment consists of a therapeutic bronchoscopy with intrabronchial recombinant human deoxyribonuclease (rhDNase) instillation. 1 , 2 These procedures might be uneffective if the persistence of atelectasis has already led to a state of parenchymal fibrosis.
1. CASE REPORT
A 21‐year‐old male patient with CF (R347P/4382delA), with pancreatic sufficiency, was admitted to our regional CF reference center for progressive dyspnea and worsening of pulmonary function tests. He had a good nutritional status (BMI 26) and FEV1 values in the previous 5 years varied from 75% to 96% of predicted. During the same period he was chronically infected with methicillin‐sensitive Staphylococcus aureus and intermittently by Pseudomonas aeruginosa, but his last sputum culture tested positive for methicillin‐resistant S. aureus (MRSA). The patient was regularly treated with nebulized rhDNase and inhaled budesonide–formoterol for a history of seasonal allergies and asthma. Before hospitalization he was evaluated as an outpatient for frequent cough, SpO2 was 94% in room air, and FEV1 was markedly reduced (55% of predicted, as compared to 75% at the previous visit). The patient refused hospitalization and was therefore given oral cotrimoxazole. After about 6 weeks he presented to the emergency department due to the persistence of symptoms, associated with low SpO2 (91%) and dyspnea. Blood tests revealed a negative C‐reactive protein and a normal white blood cell count. Total immunoglobulin E (IgE) was 452 kUA/L, while Aspergillus fumigatus‐specific IgE and IgG levels as well as the Galactomannan index were negative. Chest x‐ray showed a right paracardiac hypodiaphany of uncertain significance, described as a possible area of dysventilation. The triangular wedge‐shaped opacity correspondent to the collapsed lower lobe was poorly visible and accessory signs of atelectasis (downward shift of the hilum, mediastinal shift, and diaphragm obscuration) were not present.
On admission, he was started on iv vancomycin and meropenem. On Day 5, a pulmonary magnetic resonance imaging (MRI) was performed (Figure 1A,B), which showed right lower lobe atelectasis. The diagnosis was confirmed by a chest CT scan (Figure 2A).
Figure 1.
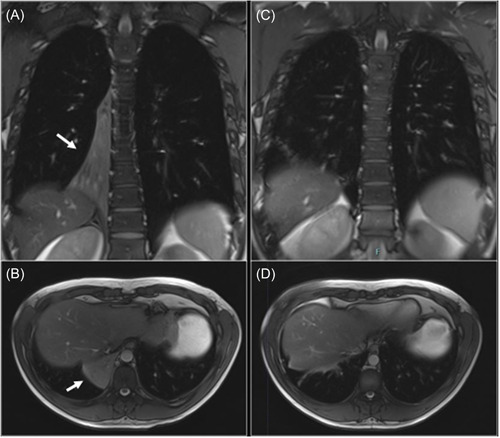
Chest magnetic resonance imaging T2‐weighted images. (A and B) The right lower lobe atelectasis (indicated by the white arrows) in the coronal and transverse sections, respectively. (C and D) The resolution of the atelectasis after bronchoscopic instillation of rhDNase, in coronal and transverse sections, respectively.
Figure 2.
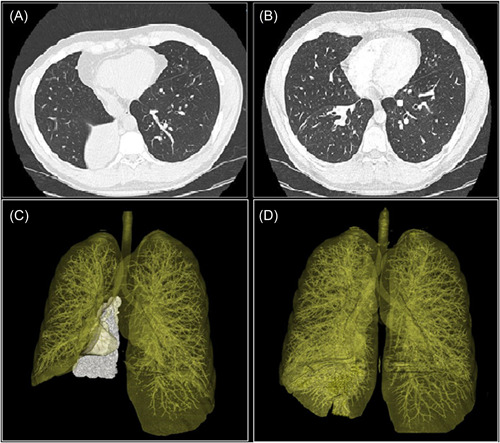
Axial and three‐dimensional volume rendering reconstruction of the chest CT scan. (A and C) The right lower lobe atelectasis and (B and D) the resolution of the atelectasis after treatment. [Color figure can be viewed at wileyonlinelibrary.com]
Hence, chest physiotherapy was enhanced by the application of high‐frequency percussive ventilation and continuous positive airway pressure. On Day 10, considering persistent desaturation and dyspnea, the patient underwent bronchoscopy with bronchoalveolar lavage (BAL) and instillation of N‐acetyl cysteine in the right inferior lobar bronchus, that appeared obstructed by a mucus plug. A postprocedural chest x‐ray did not show relevant changes, thus after 48 h, a second bronchoscopy was performed, with instillation of rhDNase. BAL fluid analysis tested positive for MRSA and A. fumigatus, so iv voriconazole was added to the treatment. In the next few days, the patient showed a progressive clinical improvement, confirmed also by the normalization of SpO2 values.
On Day 16 a CT scan (Figure 2B) documented an almost complete resolution of the atelectasis; iv antibiotic treatment and voriconazole were continued for 14 days and then switched to oral therapy. MRI performed on Day 26 (Figure 1C,D) confirmed the clinical improvement.
At the time of discharge (Day 27), SpO2 was 96% in room air and FEV1 reached 66% of predicted. Oral antibiotics and antifungal therapy were continued at home. A follow‐up with MRI every 4–6 weeks confirmed a persistent resolution of the atelectasis.
2. DISCUSSION
In CF, atelectasis occurs as a consequence of poor clearance of inflammatory debris, smooth muscle constriction, and edema of the bronchial walls, leading to complete intrabronchial obstruction.
The atelectatic lung region is perfused, generating a physiologic shunt, which may explain the hypoxemia observed in our patient. Hypoxic vasoconstriction may act as an adaptive mechanism, diverting blood flow from the nonventilated lung region, and it may be speculated that, once fibrosis has developed, blood flow is fully diverted from the atelectatic lung regions, with the restoration of normal SpO2. 3 In our patient SpO2 did not normalize, suggesting that the atelectatic lung was still viable.
Patients with atelectasis generally respond to iv antibiotics and to intensive chest physiotherapy. Even if there are no controlled trials to support the role of bronchoscopy with rhDNase instillation for the treatment of lobar atelectasis, this approach has been successfully applied in pwCF not responding to standard medical management and shown to be resolutive 7 weeks after the onset of symptoms. 1 , 2 , 4 , 5
Recent advances in MRI have made this tool comparable to CT in detecting morphological changes in CF lung. MRI has the advantage of being nonionizing and therefore repeatable without any radiation risk, making it suitable for frequent longitudinal monitoring and to assess personalized patient care. Furthermore, lung MRI also offers functional evaluation and provides ventilation and perfusion assessment in the same setting. Finally, it is possible to improve image quality with proper MRI protocols and to reduce MRI scan time with judicious planning and adopting tailored MR protocols. 6 , 7
In conclusion, lobar atelectasis may be a complication of pulmonary exacerbations in CF. Bronchoscopy with rhDNase instillation represents a valid therapeutic option in pwCF failing to respond to conservative treatment, particularly in those with persistently suboptimal SpO2 values, since this nonadaptive condition is present until atelectasis becomes irreversible. In these cases, chest MRI has proved to be a valid diagnostic and follow‐up tool, that avoids repeated irradiation.
AUTHOR CONTRIBUTIONS
Valeria Daccò: Writing – original draft. Calogero Sathya Sciarrabba: Resources. Fabiola Corti: Writing – review and editing. Chiara Rosazza: Resources. Anna Malfitano: Resources. Irene Borzani: Data curation; writing – review and editing. Carla Colombo: Writing – original draft; writing – review and editing.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Milano within the CRUI‐CARE Agreement.
Daccò V, Sciarrabba CS, Corti F, et al. A successful treatment of a lobar atelectasis in a patient with cystic fibrosis. Pediatric Pulmonology. 2022;57:2868‐2871. 10.1002/ppul.26094
REFERENCES
- 1. Whitaker P, Brownlee K, Lee T, Conway S, Etherington C, Peckham D. Sequential bronchoscopy in the management of lobar atelectasis secondary to allergic bronchopulmonary aspergillosis. J Bronchol Intervent Pulmonol. 2011;18:57‐60. [DOI] [PubMed] [Google Scholar]
- 2. Slattery DM, Waltz DA, Denham B, O'Mahony M, Greally P. Bronchoscopically administered recombinant human DNase for lobar atelectasis in cystic fibrosis. Pediat Pulmonol. 2001;31:383‐388. [DOI] [PubMed] [Google Scholar]
- 3. Gregory J, Redding MD. Atelectasis in childhood. Pediatr Clin North Am . 1984:891‐905. [DOI] [PubMed]
- 4. Nagakumar P, Hilliard T. Recurrent lobar atelectasis in a child with cystic fibrosis. J R Soc Med. 2012;105(S2):S50‐S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McLaughlin AM, McGrath E, Barry R, Egan JJ, Gallagher CG. Treatment of lobar atelectasis with bronchoscopically administered recombinant human deoxyribonuclease in cystic fibrosis? Clin Respir J. 2008;2:123‐126. [DOI] [PubMed] [Google Scholar]
- 6. Pennati F, Borzani I, Moroni L, et al. Longitudinal assessment of patients with cystic fibrosis lung disease with multivolume noncontrast MRI and spirometry. J Magn Reson Imaging. 2021;53(5):1570‐1580. [DOI] [PubMed] [Google Scholar]
- 7. Sodhi KS, Ciet P, Vasanawala S, Biederer J. Practical protocol for lung magnetic resonance imaging and common clinical indications. Pediatr Radiol. 2022;52(2):295‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]


