Abstract
Introduction
Overactive bladder (OAB) and frailty are independently associated with patient burden. However, economic burden and treatment‐taking behavior have not been well characterized among frail patients with OAB, which, given the varying safety and tolerability profiles of available treatments, is crucial.
Objectives
To assess costs, health care resource utilization, treatment‐taking behavior (persistence and adherence) to OAB medication in older, frail OAB patients.
Methods
This was a retrospective cohort study using international business machines MarketScan Medicare Supplemental claims data. Eligible frail patients (per Claims‐based Frailty Index score) initiating mirabegron were 1:2 propensity score matched (based on age, sex, and other characteristics) with those initiating antimuscarinics and were followed up to 1 year. All‐cause, per‐person, per‐month costs, health care encounters, persistence (median days to discontinuation assessed using Kaplan−Meier methods) and adherence (≥80% of proportion of days covered at Day 365) were compared.
Results
From 2527 patients with incident mirabegron (21%) or antimuscarinic (79%) dispensations, 516 incident mirabegron users (median age: 82 years, 64% female) were matched to 1032 incident antimuscarinic users (median age: 81 years, 62% female). Median cost was higher in mirabegron group ($1581 vs. $1197 per month); this was primarily driven by medication cost. There was no difference in medical encounters. Adherence (39.1% vs. 33.8%) and persistence (103 vs. 90 days) were higher in mirabegron users.
Conclusions
Among frail older adults with OAB, mirabegron use was associated with higher costs and potential improvements in treatment‐taking behaviors, particularly with respect to treatment adherence, versus those initiating antimuscarinics.
Keywords: adherence, costs, frail, health care resource utilization, persistence
1. INTRODUCTION
Overactive bladder (OAB) is a urologic condition characterized by urgency, with or without urge urinary incontinence, and accompanied by frequency and nocturia. 1 , 2 Estimates of OAB prevalence among the general US population range between 16.5% and 23.3%. 3 Research using the Timed Up and Go Test, as a parsimonious measure of frailty, has demonstrated a highly significant association between OAB and frailty, even after adjusting for age and other risk factors. 4
Frailty is associated with older age and is a risk factor for adverse outcomes including increased health services use, institutionalization, and death. 5 Given the association between frailty and excess health care costs, the Center for Medicare and Medicaid Services applies a survey‐based frailty adjustment to payments for enrollees in Program of All‐Inclusive Care for the Elderly organizations. 6 , 7 To characterize frailty among larger populations, an index was developed and was adapted for use with administrative claims data (the claims‐based frailty index [CFI]). 8 The CFI is the most widely used measure to assess frailty in administrative claims data; its use improves the prediction of adverse outcomes when added to demographics and comorbidities, and there is evidence that higher scores are associated with increased mobility impairment, falls, disability, and mortality. 8 , 9 , 10
Anticholinergics are prescribed for a variety of conditions (e.g., depression, chronic obstructive pulmonary disease, Parkinson's disease, and OAB) and are associated with numerous adverse effects on physical and mental function, 11 , 12 , 13 particularly among older frail individuals. 14 First‐line pharmacotherapy for OAB typically comprises es oral antimuscarinics, which are part of the class of anticholinergic medications indicated for and used for the treatment of OAB, and beta‐3 adrenergic agonists (mirabegron and vibegron). 15 , 16 Recent American Urological Association Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU) guidelines for OAB management state that clinicians should use caution in prescribing antimuscarinics in patients who are using other medications with anticholinergic properties, and antimuscarinics and mirabegron (the only beta‐3 adrenergic agonist available at the time of guideline drafting) for frail OAB patients. These cautions are due to concerns about cumulative anticholinergic burden and that OAB medications may have a lower therapeutic index among frail individuals and a poorer safety profile. 15 However, there is an overall lack of evidence regarding symptom management among frail older adults with OAB. 17
OAB and frailty are each associated with economic burden in the United States, although the impact of frailty on OAB‐related costs has not been described. The direct costs of OAB in the United State was estimated to be $49.1 billion in 2007 (most recent data) and was projected to increase by 168% by 2020. 18 Studies have consistently demonstrated an association between frailty and increased health care costs. 19 , 20 , 21 In the United State, application of the CFI to Medicare claims data showed that the incremental annual costs associated with frailty were $2712, $7915, and $16 449 for prefrail, mildly frail, and moderately to severely frail patients, respectively. 22
It is important to understand the impact of frailty on OAB‐related costs, particularly among users of the most commonly prescribed OAB medications (mirabegron and antimuscarinics). Additionally, persistence and adherence among frail individuals using different OAB therapies should be evaluated to determine whether any treatment‐related differences in safety or therapeutic index manifest as higher costs or poorer treatment‐taking behaviors. Therefore, among a cohort of frail older adults with OAB, the objectives of this study were to compare all‐cause costs and encounters and persistence and adherence between incident antimuscarinic and incident mirabegron users.
2. MATERIALS AND METHODS
2.1. Study design and data source
This was a retrospective cohort study using international business machines MarketScan Medicare Supplemental claims data, comprising approximately 11 million individuals covered by Medicare Supplemental plan. Elements collected include patient demographics, geographic data, inpatient and outpatient medical encounters, payment both by Medicare and commercial, and drug information. 23
2.2. Claims‐Based assessment of frailty
Frailty was assessed using the CFI. 8 Categories of frailty derived from the calculated CFI include robust (CFI < 0.15), prefrail (0.15−0.24) mildly frail (0.25−0.34) and moderate‐to‐severely frail (≥0.35). Patient diagnoses used in the CFI were captured by International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes. 10 , 24
2.3. Study population
Patients were identified based on the presence of ≥1 dispensation for mirabegron (vibegron was not available at the time of the study) or an antimuscarinic between October 1, 2016, and September 30, 2018. Patients were required to be 65 years of age on the date of the first observed claim for mirabegron or an antimuscarinic (i.e., the index date). Exclusion criteria were simultaneous prescriptions of both drugs at index and a record of neurogenic bladder during any point of the study. Patients were required to have ≥1 year of continuous enrollment data before index date (pre‐index period) and ≥1 month of follow‐up post‐index. A ≤ 45‐day gap in enrollment was permitted during the pre‐index period.
CFI score was estimated during the pre‐index period. Patients who had a CFI score of 0.25 were included in the study cohort.
2.4. Study outcomes
2.4.1. Costs and encounters
All‐cause costs were calculated as the sum of all encounters and associated costs from inpatient, outpatient, emergency room, and outpatient pharmacy settings. Encounters and costs that were specific to OAB were ascertained according to the following: Medical claims with one or more ICD‐9/10 codes for OAB diagnosis (regardless of whether OAB is a primary or secondary diagnosis), medical claims with one or more Current Procedural Terminology codes for onabotulinumtoxinA, sacral nerve stimulation, or percutaneous tibial nerve stimulation, and/or pharmacy claims for mirabegron or antimuscarinics.
2.4.2. Adherence and persistence
Adherence was determined by the proportion of days covered (PDC, %), where the number of days a patient was known to have medication coverage over the period is divided by the number of days in the period. Patients were considered adherent if the PDC was 0.80. 25 Treatment persistence was assessed by Kaplan−Meier methods as the days to discontinuation, which was defined as (1) the absence of a prescription fill within the defined 30‐day allowable gap following the end of available therapy (calculated from fill dates and days of supply) or (2) the presence of a claim for the other study treatment indicating a potential switch or add‐on, whichever occurred first. Antimuscarinic drugs were treated as therapeutic equivalents; switches between antimuscarinic treatments were not considered as discontinuation events. The end of the data period and Day 365 were considered censoring events.
2.5. Statistical analysis
2.5.1. Propensity score (PS) matching
PS matching was used to balance the distribution of baseline characteristics that might affect the outcomes. 26 Variables included in the PS were age, sex, CFI category, Charlson Comorbidity Index (CCI) comorbidities, plan type, geographic region, pre‐index health care costs, and other OAB medication class use. Nearest neighbor matching of mirabegron to antimuscarinic users was done on a 1:2 ratio, using a recommended caliper width of 0.2. 27 Incident antimuscarinic users who were within a caliper of 0.2 of the standard deviation (SD) of the logit of the PS were considered as potential matches for the incident mirabegron users. The first and second closest matches based on the smallest absolute difference in PS were selected as matched patients for the mirabegron user. If there was a tie, the match was selected randomly. Matching was conducted without replacement. Mirabegron users who were not matched to two antimuscarinic users were dropped from the matched analysis. Absolute standardized mean differences (SMD) were calculated to assess the balance between the groups before and after the matching: SMD values less than 0.1 were considered negligible. 28
2.5.2. Statistical comparisons
For costs and encounters, differences were estimated between matched pairs, and the medians of these differences were calculated. Distribution‐free confidence limits (95% CL) for the median difference were derived using previously published methods. 29 An estimate with a CL that did not include 0 was considered statistically significant. Mean/median costs were calculated for the duration of patient follow‐up and per‐patient per‐month (PPPM). Regarding the latter, the post‐index period for each patient was partitioned into 30‐day intervals, each of which was considered an analytic month. Partial months (< 30 days) were dropped from the analysis. Costs and encounters were calculated for each month for each patient. Mean and median PPPM were summarized across patients and analytic months.
Kaplan−Meier (KM) analyses were used to estimate the time to treatment discontinuation for each treatment group. The proportion of individuals still on treatment at 90, 180, and 365 days was reported with 95% confidence intervals (CIs). Mean PDC and proportion of individuals adherent to treatment were tabulated at 90, 180, and 365 days. Tests for between‐group differences were conducted using the Fisher Exact test.
2.5.3. Sensitivity analyses
Two sensitivity analyses were conducted to assess the impact of different assumptions on the study findings. First, health care costs, number of encounters and treatment adherence were calculated from index date to time of index treatment discontinuation (vs. the duration of patient follow‐up). Second, the permissible gap of 30 days in the assessment of persistence was extended to 60 days.
3. RESULTS
Among 2527 patients identified as meeting the study criteria (Table 1) before matching, 537 initiated mirabegron and 1990 initiated an antimuscarinic. Oxybutynin was the most frequently observed antimuscarinic (65.2%). The mean (SD) age was 81.3 (7.3) years for mirabegron users and 80.2 (7.96) years for antimuscarinic users; the majority of both groups were female. Groups differed somewhat in terms of geographic distribution, insurance plan type and median enrollment.
Table 1.
Baseline characteristics of the incident and matched‐incident mirabegron and antimuscarinic groups
| Overall cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|
| Category | Mirabegron users (n = 537) | Antimuscarinic users (n = 1990) | Standardized mean differencea | Mirabegron users (n = 516) | Antimuscarinic users (n = 1032) | Standardized mean differencea |
| Year of index date [ b ], n (%) | ||||||
| 2016 | 166 (30.9) | 477 (24.0) | 0.1561 | 150 (29.1) | 270 (26.2) | 0.0651 |
| 2017 | 323 (60.1) | 1394 (70.1) | 0.2089 | 319 (61.8) | 661 (64.1) | 0.0462 |
| 2018 | 48 (8.9) | 119 (6.0) | 0.1128 | 47 (9.1) | 101 (9.8) | 0.0232 |
| Sex, n (%) | ||||||
| Female | 344 (64.1) | 1372 (68.9) | 0.1036 | 332 (64.3) | 638 (61.8) | 0.0522 |
| Male | 193 (35.9) | 618 (31.1) | 184 (35.7) | 394 (38.2) | ||
| Age at index date (years old) | ||||||
| Mean (SD) | 81.3 (7.26) | 80.2 (7.85) | 0.1453 | 81.3 (7.29) | 80.5 (7.92) | 0.1124 |
| Median (Q1–Q3) | 82.0 (76.0–87.0) | 80.0 (74.0–86.0) | 82.0 (76.0–87.0) | 81.0 (74.0–87.0) | ||
| Min–max | 65–98 | 65–104 | 65–98 | 65–104 | ||
| Age category at index date, n (%) | ||||||
| 65c | 4 (0.7) | 10 (0.5) | 0.0308 | 3 (0.6) | 3 (0.3) | 0.0441 |
| 65–69 | 30 (5.6) | 191 (9.6) | 0.1519 | 29 (5.6) | 111 (10.8) | 0.1881 |
| 70–74 | 75 (14.0) | 362 (18.2) | 0.1152 | 72 (14.0) | 159 (15.4) | 0.0411 |
| 75–79 | 102 (19.0) | 373 (18.7) | 0.0064 | 100 (19.4) | 187 (18.1) | 0.0323 |
| 80–84 | 141 (26.3) | 442 (22.2) | 0.0945 | 133 (25.8) | 232 (22.5) | 0.0771 |
| 85+ | 189 (35.2) | 622 (31.3) | 0.0837 | 182 (35.3) | 343 (33.2) | 0.0429 |
| Region of residence at index date, n (%) | ||||||
| Northeast | 151 (28.1) | 393 (19.7) | 0.1971 | 150 (29.1) | 298 (28.9) | 0.0043 |
| North central | 118 (22.0) | 836 (42.0) | 0.4398 | 107 (20.7) | 231 (22.4) | 0.0401 |
| South | 228 (42.5) | 633 (31.8) | 0.2218 | 220 (42.6) | 434 (42.1) | 0.0118 |
| West | 40 (7.4) | 125 (6.3) | 0.0462 | 39 (7.6) | 68 (6.6) | 0.0378 |
| Unknown | 0 (0) | 3 (0.2) | 0.0550 | 0 (0) | 1 (0.1) | 0.0440 |
| Claims‐based frailty index during the pre‐index periodd | ||||||
| Mean (SD) | 0.318 (0.076) | 0.319 (0.074) | 0.0139 | 0.318 (0.075) | 0.318 (0.071) | 0.0092 |
| Median (Q1–Q3) | 0.289 (0.266–0.338) | 0.293 (0.268–0.345) | 0.289 (0.266–0.337) | 0.294 (0.268–0.344) | ||
| Min–max | 0.25–0.63 | 0.25–0.68 | 0.25–0.63 | 0.25–0.65 | ||
| Claims‐based frailty index during the pre‐index period, n (%) | ||||||
| 0.25 ≤ CFI < 0.35 | 418 (77.8) | 1519 (76.3) | 0.0359 | 404 (78.3) | 796 (77.1) | 0.0279 |
| CFI > = 0.35 | 119 (22.2) | 471 (23.7) | 112 (21.7) | 236 (22.9) | ||
| Charlson comorbidity index score | ||||||
| Mean (SD) | 6.6 (2.98) | 6.5 (2.90) | 0.0359 | 6.6 (2.95) | 6.6 (2.88) | 0.0080 |
| Median (Q1–Q3) | 6.0 (4.0–9.0) | 6.0 (4.0–8.0) | 6.0 (4.0–9.0) | 6.0 (5.0–8.0) | ||
| Min–max | 0–21 | 0–18 | 0–16 | 0–18 | ||
| Charlson comorbidity index, n (%) | ||||||
| Myocardial infarction | 128 (23.8) | 524 (26.3) | 0.0576 | 127 (24.6) | 255 (24.7) | 0.0022 |
| Congestive Heart Failure | 300 (55.9) | 1138 (57.2) | 0.0266 | 285 (55.2) | 582 (56.4) | 0.0234 |
| Peripheral vascular disease | 342 (63.7) | 1237 (62.2) | 0.0316 | 330 (64.0) | 643 (62.3) | 0.0341 |
| Cerebrovascular disease | 356 (66.3) | 1256 (63.1) | 0.0666 | 339 (65.7) | 688 (66.7) | 0.0205 |
| Dementia | 238 (44.3) | 770 (38.7) | 0.1144 | 227 (44.0) | 410 (39.7) | 0.0865 |
| Chronic pulmonary disease | 340 (63.3) | 1296 (65.1) | 0.0378 | 324 (62.8) | 662 (64.1) | 0.0282 |
| Rheumatic disease | 78 (14.5) | 256 (12.9) | 0.0483 | 77 (14.9) | 151 (14.6) | 0.0082 |
| Peptic ulcer disease | 40 (7.4) | 150 (7.5) | 0.0034 | 40 (7.8) | 81 (7.8) | 0.0036 |
| Mild liver disease | 71 (13.2) | 272 (13.7) | 0.0131 | 66 (12.8) | 176 (17.1) | 0.1199 |
| Moderate or severe liver disease | 6 (1.1) | 23 (1.2) | 0.0036 | 6 (1.2) | 10 (1.0) | 0.0189 |
| Diabetes without chronic complication | 78 (14.5) | 323 (16.2) | 0.0473 | 76 (14.7) | 156 (15.1) | 0.0109 |
| Diabetes with chronic complication | 228 (42.5) | 848 (42.6) | 0.0031 | 223 (43.2) | 419 (40.6) | 0.0530 |
| Hemiplegia or paraplegia | 61 (11.4) | 260 (13.1) | 0.0521 | 57 (11.0) | 114 (11.0) | 0.0000 |
| Renal disease | 254 (47.3) | 934 (46.9) | 0.0073 | 244 (47.3) | 486 (47.1) | 0.0039 |
| Any malignancye | 128 (23.8) | 417 (21.0) | 0.0692 | 120 (23.3) | 252 (24.4) | 0.0273 |
| Metastatic solid tumor | 37 (6.9) | 131 (6.6) | 0.0123 | 35 (6.8) | 78 (7.6) | 0.0300 |
| AIDS/HIV | 2 (0.4) | 1 (0.1) | 0.0702 | 1 (0.2) | 1 (0.1) | 0.0254 |
| Plan type at index date, n (%) | ||||||
| Comprehensive | 208 (38.7) | 1180 (59.3) | 0.4203 | 206 (39.9) | 442 (42.8) | 0.0590 |
| Preferred Provider Organization | 301 (56.1) | 696 (35.0) | 0.4331 | 282 (54.7) | 518 (50.2) | 0.0893 |
| All other fee service plan | 28 (5.2) | 114 (5.7) | 0.0226 | 28 (5.4) | 72 (7.0) | 0.0643 |
| Type of antimuscarinic at index date (among antimuscarinic users),f n (%) | ||||||
| Darifenacin | 0 (0) | 26 (1.3) | 0 (0) | 15 (1.5) | ||
| Fesoterodine | 0 (0) | 25 (1.3) | 0 (0) | 12 (1.2) | ||
| Oxybutynin | 0 (0) | 1297 (65.2) | 0 (0) | 677 (65.6) | ||
| Solifenacin | 0 (0) | 209 (10.5) | 0 (0) | 135 (13.1) | ||
| Tolterodine | 0 (0) | 360 (18.1) | 0 (0) | 162 (15.7) | ||
| Trospium | 0 (0) | 75 (3.8) | 0 (0) | 32 (3.1) | ||
| All‐cause health care costs during pre‐index period ($) | ||||||
| Mean (SD) | $76 059 ($111 311) | $65 112 ($89 867) | 0.1082 | $76 687 ($112 355) | $80 220 ($110 839) | 0.0317 |
| Median (Q1–Q3) | $46 415 ($23 407–$92 813) | $39 025 ($20 048–$73 621) | $47 177 ($23 457–$94 247) | $45 227 ($23 004–$92 709) | ||
| Min–max | $1021–$1 505 117 | $604–$1 054 954 | $1021–$1 505 117 | $1235–$1 054 954 | ||
| Previous use of any OAB medication during pre‐index period, n (%) | ||||||
| Yes | 75 (14.0) | 52 (2.6) | 0.4208 | 64 (12.4) | 20 (1.9) | 0.4142 |
| Mirabegron | 0 (0) | 52 (2.6) | 0 (0) | 20 (1.9) | ||
| Antimuscarinic | 75 (14.0) | 0 (0) | 64 (12.4) | 0 (0) | ||
| Days of follow‐upg | ||||||
| Mean (SD) | 285.1 (115.2) | 256.3 (117.8) | 290.8 (111.1) | 270.3 (118.7) | ||
| Median (Q1–Q3) | 365.0 (207.0–365.0) | 304.0 (145.0–365.0) | 365.0 (220.5–365.0) | 365.0 (152.0–365.0) | ||
| Min–max | 30–365 | 30–365 | 30–365 | 31–365 | ||
| 12‐month enrollment; n (%) | 316 (59.0) | 814 (41.0) | 312 (60.0) | 526 (51.0) | ||
Abbreviations: OAB, Overactive Bladder; SD, standard deviation.
Absolute value of standardized mean difference is shown. A standardized mean difference < 0.1 is considered well‐balanced with negligible difference between the groups.
Index date was defined as the first claim date of Mirabegron or antimuscarinics during the ascertainment period (January 10, 2016–September 09, 2019).
A small percentage of patients were anticipated in the 65 year old age. Age 65 is presented separately for cohort understanding. Overlap of 65 and 65–70 age groups was intentional.
Pre‐index period was defined as the 12‐month period before index date.
Any malignancy, including lymphoma and leukemia, except malignant neoplasm of skin.
Sum of proportions may be greater than 100%.
The period of follow‐up began on the index date and ended at the end of enrollment or 1 year‐post index date, whichever occurred first.
In terms of clinical characteristics, the median CFI score was similar among mirabegron and antimuscarinic users; 23.7% of antimuscarinic users had moderate/severe frailty versus 22.2% of mirabegron users. The median CCI score for both groups was 6.0. A weak positive association between CCI score and CFI score was found (Pearson correlation coefficient = 0.2), however, there was no interaction between CFI score and individual CCI conditions in the logistic regression model which used for PS match. The presence of dementia was higher among mirabegron users (44.3%) than antimuscarinic users (38.7%). 14% of mirabegron users had received an antimuscarinic in the pre‐index period, while 2.6% of antimuscarinic users had received mirabegron during the pre‐index period.
The median enrollment time for mirabegron users was longer than that of antimuscarinic users, as was the percentage of those who contributed a full year of follow‐up (Table 1). Pre‐index median health care costs were higher for mirabegron users versus antimuscarinic users (Table 1).
After PS‐matching, the study sample consisted of 516 mirabegron users and 1032 antimuscarinic users. Baseline characteristics were generally well balanced (Table 1), although the SMD for age remained above 0.1 (SMD = 0.1124), as well as the SMD for previous use of an OAB medication during the pre‐index period (SMD = 0.4142). Patient attrition is presented in Figure 1.
Figure 1.
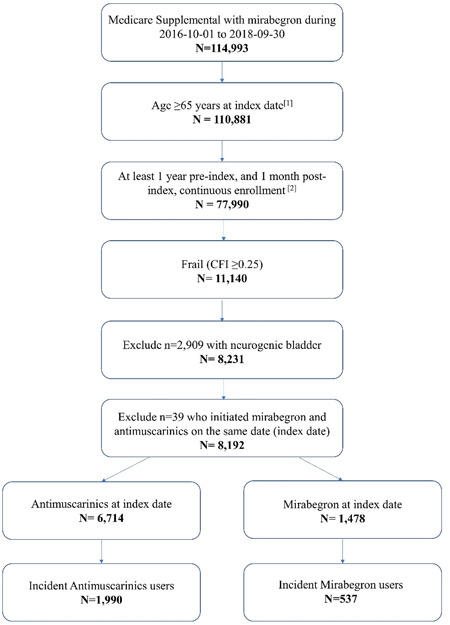
Patient Attrition. [1] Index date was defined as the first claim date of MIRA or AMs during the ascertainment period (January 10, 2016−September 30, 2019). [2] A gap in enrollment up to 45 days was allowed in the pre‐index, while no gap in enrollment was allowed in the post‐index period. [3] First claim observed in the identification period is for MIRA (or AM) medication, and there is absence of MIRA (or AM) claims in the pre‐index period. AM, antimuscarinic; CFI, Claims‐based Frailty Index; MIRA, mirabegron.
3.1. Costs
Among matched cohorts, the largest contributor to all‐cause health care costs PPPM was outpatient costs, followed by inpatient costs, and then pharmacy costs (Table 2). The mean and median total health care costs PPPM were higher among mirabegron users (mean: $6010; median: $1581) than antimuscarinic users (mean: $5515; median: $1197). The median difference in total health care costs PPPM was $308 (95% CL: $178−$470) more among mirabegron users, a difference which was statistically significant. The largest median difference was observed for pharmacy costs, which was statistically significantly higher ($218 [95% CL: $169−$263]) among mirabegron users versus antimuscarinic users. There were no differences in inpatient and outpatient costs between groups. The trends observed for OAB‐related costs mirrored those for total health care costs (Table 2).
Table 2.
All‐cause and OAB‐related costs and encounters for matched cohorts
| Category | Statistics | Mirabegron users (n = 516) | Antimuscarinic users (n = 1032) | Median difference (Mira‐AM), (95% CL) |
|---|---|---|---|---|
| All‐cause health care (inpatient, outpatient, and outpatient pharmacy) costsa | ||||
| Total health care costs | Mean (SD) | $56 590 ($89 982) | $48 084 ($88 142) | $4881 ($2560−$7267) |
| Median (Q1–Q3) | $25 370 ($10 866–$67 180) | $19 651 ($6472–$50 198) | ||
| Min–max | $213–$1239 664 | $31–$1 023 475 | ||
| Total health care costs (PPPM) | Mean (SD) | $6010 ($17 429) | $5515 ($18 015) | $308 ($178–$470) |
| Median (Q1–Q3) | $1581 ($676–$3929) | $1,197 ($434–$3283) | ||
| Min–max | $0–$426 313 | $0–$471 260 | ||
| All‐cause inpatient costs | ||||
| Hospitalization costs | Mean (SD) | $20 098 ($49 234) | $17 866 ($47 660) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$17 020) | $0 ($0–$15 673) | ||
| Min–max | $0–$494 527 | $0–$474 883 | ||
| Hospitalization costs (PPPM) | Mean (SD) | $2134 ($14 491) | $2049 ($15 037) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$0) | $0 ($0–$0) | ||
| Min–max | $0–$422 878 | $0–$468 397 | ||
| All‐cause outpatient costs | ||||
| Outpatient services costs | Mean (SD) | $27 019 ($64 510) | $23 849 ($58 295) | $1046 ($228–$2100) |
| Median (Q1–Q3) | $10 365 ($3482–$30 857) | $8417 ($2436–$24,283) | ||
| Min–max | $0–$1 180 053 | $0–$896 078 | ||
| Outpatient services costs (PPPM) | Mean (SD) | $2869 ($8638) | $2735 ($8475) | $25 ($24–$68) |
| Median (Q1–Q3) | $549 ($144–$1975) | $498 ($131–$1769) | ||
| Min–max | $0–$130 878 | $0–$219 452 | ||
| Total outpatient office costs | Mean (SD) | $5293 ($10 009) | $4559 ($10254) | $324 ($125–$548) |
| Median (Q1–Q3) | $2412 ($522–$6115) | $1495 ($411–$4649) | ||
| Min–max | $0–$147 020 | $0–$199 132 | ||
| Total outpatient office costs (PPPM) | Mean (SD) | $562 ($1302) | $523 ($1538) | $11 ($0–$33) |
| Median (Q1–Q3) | $181 ($0–$569) | $145 ($0–$464) | ||
| Min–max | $0–$27 539 | $0–$38 485 | ||
| Median (Q1–Q3) | $44 ($0–$367) | $0 ($0–$254) | ||
| Min–max | $0–$27 265 | $0–$38 485 | ||
| All‐cause outpatient pharmacy costs | ||||
| Prescription claim costs | Mean (SD) | $9473 ($13 174) | $6369 ($13021) | $2021 ($1665–$2583) |
| Median (Q1–Q3) | $5974 ($2883–$11 004) | $3091 ($1089–$7220) | ||
| Min–max | $117–$118 706 | $6–$205 579 | ||
| Prescription claim costs (PPPM) | Mean (SD) | $1006 ($1750) | $731 ($2004) | $218 ($169–$263) |
| Median (Q1–Q3) | $536 ($120–$1182) | $255 ($52–$805) | ||
| Min–max | $0–$33 063 | $0–$77 703 | ||
| All‐cause inpatient encounters | ||||
| Hospitalization encounters | N (%) | 213 (41.3%) | 398 (38.6%) | 0 (0–0) |
| Mean (SD) | 0.547 (0.755) | 0.525 (0.800) | ||
| Median (Q1–Q3) | 0 (0–1) | 0 (0–1) | ||
| Min–max | 0–5 | 0–7 | ||
| Hospitalization encounters (PPPM) | Mean (SD) | 0.058 (0.237) | 0.060 (0.247) | 0 (0–0) |
| Median (Q1–Q3) | 0 (0–0) | 0 (0–0) | ||
| Min–max | 0–2 | 0–3 | ||
| All‐cause outpatient services | ||||
| Outpatient services encounters | N (%) | 511 (99.0%) | 1024 (99.2%) | 4 (2–7) |
| Mean (SD) | 37.727 (29.137) | 32.842 (28.569) | ||
| Median (Q1–Q3) | 31.5 (16–53) | 27 (12–47) | ||
| Min–max | 0–139 | 0–349 | ||
| Outpatient services encounters (PPPM) | Mean (SD) | 4.006 (3.810) | 3.767 (3.755) | 0 (0–0.5) |
| Median (Q1–Q3) | 3 (1–6) | 3 (1–5) | ||
| Min–max | 0–27 | 0–30 | ||
| Total outpatient office encounters | N (%) | 492 (95.3%) | 951 (92.2%) | 2 (1–4) |
| Mean (SD) | 21.339 (21.468) | 17.093 (18.482) | ||
| Median (Q1–Q3) | 15 (5–31.5) | 11 (3–24.5) | ||
| Min–max | 0–124 | 0–123 | ||
| Total outpatient office encounters (PPPM) | Mean (SD) | 2.266 (2.546) | 1.960 (2.271) | 0 (0–0) |
| Median (Q1–Q3) | 2 (0–3) | 1 (0–3) | ||
| Min–max | 0–18 | 0–23 | ||
| All‐cause outpatient pharmacy | ||||
| Prescription claim encounters | N (%) | 516 (100%) | 1032 (100%) | 5 (0–9) |
| Mean (SD) | 59.285 (41.798) | 53.592 (44.029) | ||
| Median (Q1–Q3) | 52 (27–80) | 43 (21–72) | ||
| Min–max | 1–246 | 1–297 | ||
| Prescription claim encounters (PPPM) | Mean (SD) | 6.296 (4.746) | 6.147 (5.181) | 0.25 (0–0.5) |
| Median (Q1–Q3) | 5 (3–9) | 5 (3–9) | ||
| Min–max | 0–43 | 0–116 | ||
| OAB‐related health care (inpatient, outpatient, and outpatient pharmacy) costsa | ||||
| Total health care costs | Mean (SD) | $2919 ($3421) | $1205 ($2988) | $1238 ($1047–$1546) |
| Median (Q1–Q3) | $2147 ($825–$4199) | $335 ($103–$1138) | ||
| Min–max | $142–$35393 | $2–$39044 | ||
| Total health care costs (PPPM) | Mean (SD) | $310 ($886) | $138 ($924) | $37 ($0–$64) |
| Median (Q1–Q3) | $43 ($0–$363) | $0 ($0–$74) | ||
| Min–max | $0–$28 913 | $0–$38 719 | ||
| OAB‐related inpatient costs | ||||
| Hospitalization costs | Mean (SD) | $125 ($1694) | $246 ($2469) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$0) | $0 ($0–$0) | ||
| Min–max | $0–$28 913 | $0–$38 719 | ||
| Hospitalization costs (PPPM) | Mean (SD) | $13 ($553) | $28 ($839) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$0) | $0 ($0–$0) | ||
| Min–max | $0–$28 913 | $0–$38 719 | ||
| OAB‐related outpatient services costs | ||||
| Outpatient services costs | Mean (SD) | $618 ($2165) | $352 ($1241) | $0 ($0–$2) |
| Median (Q1–Q3) | $109 ($0–$382) | $70 ($0–$253) | ||
| Min–max | $0–$32,079 | $0–$17,351 | ||
| Outpatient services costs (PPPM) | Mean (SD) | $66 ($600) | $40 ($339) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$0) | $0 ($0–$0) | ||
| Min–max | $0–$22 782 | $0–$17 351 | ||
| Total outpatient office costs | Mean (SD) | $299 ($714) | $230 ($878) | $0 ($0–$0) |
| Median (Q1–Q3) | $83 ($0–$284) | $0 ($0–$180) | ||
| Min–max | $0–$8498 | $0–$15 277 | ||
| Total outpatient office costs (PPPM) | Mean (SD) | $32 ($172) | $26 ($187) | $0 ($0 ‐ $0) |
| Median (Q1–Q3) | $0 ($0–$0) | $0 ($0–$0) | ||
| Min–max | $0–$5,042 | $0–$5,957 | ||
| Primary care provider outpatient office costsb | Mean (SD) | $60 ($210) | $58 ($211) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$0) | $0 ($0–$0) | ||
| Min–max | $0–$2813 | $0–$3625 | ||
| Primary care provider outpatient office costs (PPPM) | Mean (SD) | $6 ($52) | $7 ($60) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$0) | $0 ($0–$0) | ||
| Min–max | $0–$1911 | $0 ‐ $3519 | ||
| Obstetrics/gynecology outpatient office costsc | Mean (SD) | $25 ($269) | $4 ($43) | $0 ($0 ‐ $0) |
| Median (Q1–Q3) | $0 ($0–$0) | $0 ($0–$0) | ||
| Min–max | $0–$5483 | $0–$810 | ||
| Obstetrics/gynecology outpatient office costs (PPPM) | Mean (SD) | $3 ($72) | $0 ($13) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$0) | $0 ($0–$0) | ||
| Min–max | $0–$4516 | $0–$810 | ||
| Urologist outpatient office costsd | Mean (SD) | $188 ($604) | $141 ($816) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$143) | $0 ($0–$0) | ||
| Min–max | $0–$8498 | $0 ‐ $15 277 | ||
| Urologist outpatient office costs (PPPM) | Mean (SD) | $20 ($142) | $16 ($171) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$0) | $0 ($0–$0) | ||
| Min–max | $0–$3883 | $0–$5957 | ||
| Other outpatient office costs | Mean (SD) | $26 ($128) | $27 ($214) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$0) | $0 ($0–$0) | ||
| Min–max | $0–$1742 | $0–$5638 | ||
| Other outpatient office costs (PPPM) | Mean (SD) | $3 ($29) | $3 ($45) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$0) | $0 ($0–$0) | ||
| Min–max | $0–$752 | $0–$2581 | ||
| OAB‐related outpatient pharmacy costs | ||||
| Prescription claim costs | Mean (SD) | $2176 ($1873) | $607 ($1096) | $1095 ($927–$1341) |
| Median (Q1–Q3) | $1787 ($657–$3545) | $158 ($38–$599) | ||
| Min–max | $78–$19 159 | $2–$13 150 | ||
| Prescription claim costs (PPPM) | Mean (SD) | $231 ($344) | $70 ($189) | $0 ($0–$0) |
| Median (Q1–Q3) | $0 ($0–$348) | $0 ($0–$32) | ||
| Min–max | $0–$4044 | $0–$3985 | ||
| OAB‐related inpatient encounters | ||||
| Hospitalization encounters | N (%) | 3 (0.6%) | 15 (1.5%) | 0 (0–0) |
| Mean (SD) | 0.006 (0.076) | 0.015 (0.120) | ||
| Median (Q1–Q3) | 0 (0–0) | 0 (0–0) | ||
| Min–max | 0–1 | 0–1 | ||
| Hospitalization encounters (PPPM) | Mean (SD) | 0.001 (0.025) | 0.002 (0.041) | 0 (0–0) |
| Median (Q1–Q3) | 0 (0–0) | 0 (0–0) | ||
| Min–max | 0–1 | 0–1 | ||
| OAB‐related outpatient services | ||||
| Outpatient services encounters | N (%) | 319 (61.8%) | 556 (53.9%) | 0 (0–0) |
| Mean (SD) | 1.787 (2.459) | 1.359 (2.273) | ||
| Median (Q1–Q3) | 1 (0–3) | 1 (0–2) | ||
| Min–max | 0–17 | 0–31 | ||
| Outpatient services encounters (PPPM) | Mean (SD) | 0.190 (0.515) | 0.156 (0.482) | 0 (0–0) |
| Median (Q1–Q3) | 0 (0–0) | 0 (0–0) | ||
| Min–max | 0–7 | 0–15 | ||
| OAB‐related outpatient services | ||||
| Total outpatient office encounters | N (%) | 291 (56.4%) | 463 (44.9%) | 0 (0–0) |
| Mean (SD) | 1.463 (2.140) | 1.080 (2.080) | ||
| Median (Q1–Q3) | 1 (0–2) | 0 (0–1) | ||
| Min–max | 0–17 | 0–31 | ||
| Total outpatient office encounters (PPPM) | Mean (SD) | 0.155 (0.452) | 0.124 (0.434) | 0 (0–0) |
| Median (Q1–Q3) | 0 (0–0) | 0 (0–0) | ||
| Min–max | 0–7 | 0–15 | ||
| Primary care provider outpatient office encountersb | N (%) | 112 (21.7%) | 253 (24.5%) | 0 (0–0) |
| Mean (SD) | 0.395 (1.064) | 0.420 (0.937) | ||
| Median (Q1–Q3) | 0 (0–0) | 0 (0–0) | ||
| Min–max | 0–11 | 0–8 | ||
| Primary care provider outpatient office encounters (PPPM) | Mean (SD) | 0.042 (0.243) | 0.048 (0.230) | 0 (0–0) |
| Median (Q1–Q3) | 0 (0–0) | 0 (0–0) | ||
| Min–max | 0–6 | 0–3 | ||
| OAB‐related outpatient services | ||||
| Obstetrics/gynecology outpatient office encountersc | N (%) | 19 (3.7%) | 16 (1.6%) | 0 (0–0) |
| Mean (SD) | 0.089 (0.665) | 0.026 (0.299) | ||
| Median (Q1–Q3) | 0 (0–0) | 0 (0–0) | ||
| Min–max | 0–11 | 0–8 | ||
| Obstetrics/gynecology outpatient office encounters (PPPM) | Mean (SD) | 0.009 (0.131) | 0.003 (0.062) | 0 (0–0) |
| Median (Q1–Q3) | 0 (0–0) | 0 (0–0) | ||
| Min–max | 0–5 | 0–2 | ||
| Urologist outpatient office encountersd | N (%) | 175 (33.9%) | 195 (18.9%) | 0 (0–0) |
| Mean (SD) | 0.808 (1.526) | 0.449 (1.255) | ||
| Median (Q1–Q3) | 0 (0–1) | 0 (0–0) | ||
| Min–max | 0–9 | 0–13 | ||
| Urologist outpatient office encounters (PPPM) | Mean (SD) | 0.086 (0.317) | 0.051 (0.251) | 0 (0–0) |
| Median (Q1–Q3) | 0 (0–0) | 0 (0–0) | ||
| Min–max | 0–4 | 0–4 | ||
| OAB‐related outpatient services | ||||
| Other outpatient office encounters | N (%) | 49 (9.5%) | 79 (7.7%) | 0 (0–0) |
| Mean (SD) | 0.171 (0.701) | 0.186 (1.272) | ||
| Median (Q1–Q3) | 0 (0–0) | 0 (0–0) | ||
| Min–max | 0–10 | 0–30 | ||
| Other outpatient office encounters (PPPM) | Mean (SD) | 0.018 (0.156) | 0.021 (0.263) | 0 (0–0) |
| Median (Q1–Q3) | 0 (0–0) | 0 (0–0) | ||
| Min–max | 0–4 | 0–15 | ||
| OAB‐related outpatient pharmacy | ||||
| Prescription claim encounters | N (%) | 516 (100%) | 1032 (100%) | 1 (0–1) |
| Mean (SD) | 5.355 (4.703) | 4.176 (3.938) | ||
| Median (Q1–Q3) | 4 (2–8) | 3 (1–6) | ||
| Min–max | 1–28 | 1–28 | ||
| Prescription claim encounters (PPPM) | Mean (SD) | 0.569 (0.709) | 0.479 (0.682) | 0 (0–0) |
| Median (Q1–Q3) | 0 (0–1) | 0 (0–1) | ||
| Min–max | 0–5 | 0–9 | ||
Note: 95% CL for the median are distribution‐free confidence limits of median; Median differences were derived using the CIPCTLDF option on PROC UNIVARIATE statement in SAS.
Abbreviations: CL, confidence limit; SD, standard deviation; Q1, 25th percentile; Q3, 75th percentile; min, minimum; max, maximum; PPPM, per patient per month.
Total inpatient and outpatient costs include inpatient admission, outpatient service and outpatient pharmacy costs.
Primary care provider includes the following physician specialties: Family Practice, Internal Medicine, MultiSpecialty Physician Group, and Medical Doctor.
Obstetrics/gynecology includes the following specialties: OBGY.
Urologist includes the following specialties: Urology.
Notes: 95% CI for the median are distribution‐free confidence intervals for the median.
3.2. Encounters
Regarding all‐cause medical encounters, the largest median difference observed was for pharmacy claims, estimated at 0.25 more claims PPPM among mirabegron users (95% CL: 0.0−0.5; Table 2) but not statistically significant. Trends in number of OAB‐related medical encounters were similar to those observed for all‐cause medical encounters (Table 2). Median differences in OAB‐related medical encounters PPPM were not statistically significant between groups. When health care costs and encounters were calculated from index date to time of index treatment discontinuation (instead of through end of follow‐up), the observed trends were unchanged (Supporting Information: Table 1).
3.3. Treatment persistence
The median time to treatment discontinuation (Figure 2) was nonstatistically significantly higher among mirabegron users (103 days; 95% CI: 30−360) versus antimuscarinic users (90 days; 95% CI: 30−326). A higher percentage of mirabegron users were persistent to therapy versus antimuscarinic users at all time points evaluated (90, 180, and 365 days; Table 3). Extending the allowable gap in treatment to 60 days did not change the findings (Supporting Information: Table 2).
Figure 2.
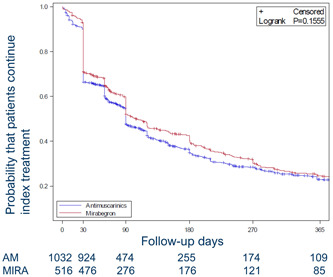
Kaplan−Meier plots of persistence by index medication for matched cohorts (with number of patients with index medication). Abbreviations: AM, antimuscarinic; MIRA, mirabegron.
Table 3.
Persistence and adherence (PDC) for matched cohorts
| Persistence | ||
|---|---|---|
| Kaplan−Meier estimates (95% CLs) | Mirabegron users (n = 516) | Antimuscarinic users (n = 1032) |
| 90 days | 52.0% (47.5−56.3) | 47.7% (44.5−50.9) |
| 180 days | 40.2% (35.7−44.6) | 34.8% (31.7−38.0) |
| 365 days | 24.6% (20.6−28.8) | 23.0% (20.0−26.1) |
| Adherence (PDC) | ||
| PDC through 90 days | ||
| Mean (SD) | 0.763 (0.279) | 0.701 (0.312) |
| Median (Q1−Q3) | 0.925 (0.515−1.000) | 0.789 (0.333−1.000) |
| Min−max | 0.022−1.000 | 0.011−1.000 |
| Median difference (Mira‐AM), (95%CI) | 0.000 (0.000−0.000) | |
| PDC through 180 days | ||
| Mean (SD) | 0.664 (0.322) | 0.601 (0.340) |
| Median (Q1−Q3) | 0.753 (0.333−0.994) | 0.656 (0.246−0.967) |
| Min−max | 0.022−1.000 | 0.006−1.000 |
| Median difference (Mira‐AM), (95% CI) | 0.011 (0.000−0.046) | |
| PDC through 365 days | ||
| Mean (SD) | 0.589 (0.343) | 0.535 (0.355) |
| Median (Q1−Q3) | 0.652 (0.247−0.935) | 0.507 (0.173−0.921) |
| Min−max | 0.019−1.000 | 0.003−1.000 |
| Median difference (Mira‐AM), (95%CI) | 0.009 (0.000−0.049) | |
| PDC ≥ 0.80 | ||
| Through 90 days | 298 (57.8%) | 515 (49.9%) |
| Difference (95% CI) | 7.85% (2.61%−13.09%) | |
| p Value* | 0.0036 | |
| Through 180 days | 247 (47.9%) | 415 (40.2%) |
| Difference (95% CI) | 7.66% (2.41%−12.90%) | |
| p Value* | 0.0046 | |
| Through 365 days | 202 (39.1%) | 349 (33.8%) |
| Difference (95% CI) | 5.33% (0.22%−10.43%) | |
| p Value* | 0.0426 | |
Note: 95% CI for the median are distribution‐free confidence intervals for the median. p Value was derived from Fisher's Exact Test.
Abbreviations: KM, Kaplan−Meier estimate; NE, not estimated; PDC (proportion of days covered); Q1, 25th percentile; Q3, 75th percentile; 95% CL, 95% confidence limits.
3.4. Treatment adherence
Treatment adherence was higher among mirabegron users compared to matched antimuscarinic users (Table 3), although mirabegron users had a longer average follow‐up time. The proportion of patients adherent to treatment (i.e., proportion with PDC ≥ 0.80) was 57.8% among mirabegron users and 49.9% among antimuscarinic users at 90 days (p < 0.01), and 39.1% among mirabegron users and 33.8% among antimuscarinic users at 365 days (p = 0.04). However, no differences were noted in PDC between the matched groups across all time points considered (90, 180, and 365 days) when adherence was assessed over time on treatment (Supporting Information: Table 2).
4. DISCUSSION
In this retrospective study, a large, US claims data set was used to assess differences in health care spending and to characterize treatment‐taking behaviors among frail older patients with OAB who were newly treated with either mirabegron or antimuscarinics. To our knowledge, this is the first study to assess these outcomes among frail older adults with OAB.
New users of mirabegron had higher health care costs than new users of antimuscarinics. This difference was driven by medication costs rather than the number of claims, and no unexpected differences in terms of medical encounters were noted among frail patients taking mirabegron compared to those on antimuscarinics. The difference in pharmacy costs could indicate that those who received mirabegron had broader and more generous prescription coverage than those who received antimuscarinics. Mirabegron users were more (nonsignificantly) persistent than antimuscarinic users and had higher adherence across all time points considered. However, mirabegron users had longer follow‐up times than antimuscarinic users, which allowed for more observation of medication coverage. Sensitivity analyses that considered time on treatment versus the full follow‐up period were conducted to examine whether PDC was a function of longer treatment time rather than gaps in therapy. No differences in adherence were observed, which indicates that the differences observed when PDC was considered over time on treatment are likely not due to gaps in therapy. It should be noted that safety‐related discontinuations could also result in decreased costs; however, reason for discontinuation were not available in the current analysis and it was not possible to determine whether they contributed to observed differences in costs.
Findings from the present study regarding treatment adherence and persistence are in line with those derived from a general OAB population. In a retrospective claims analysis of OAB patients receiving either mirabegron or anticholinergic therapies (including antimuscarinics), Sussman et al. found that 44% of patients treated with mirabegron and 31% of patients treated with anticholinergics adhered to their index medication during the 12‐month study period (defined as PDC ≥0.80). 25 In the present study, adherence was also higher among mirabegron users versus antimuscarinic users, although this could have been impacted by a longer persistence rate among mirabegron users. Furthermore, using a definition similar to that employed by the present study, Sussman et al. reported that 19% of mirabegron users and 12% of anticholinergic users were persistent to therapy at 12 months. The present study found higher treatment persistence among mirabegron users (25%) versus antimuscarinic users (23%) at 12 months. This suggests that relative to a general OAB population, older, frail OAB patients who receive mirabegron do not exhibit poorer treatment‐taking behaviors that arise as a result of unique safety issues or decreased treatment efficacy. Overall, these findings are also consistent with observations that mirabegron is associated with better adherence and persistence than antimuscarinics, given its association with significantly fewer side effects. 30 , 31
Findings from the present study suggest that compared to a general OAB population, frail OAB patients have higher health care costs. A study by Durden et al. using Truven MarketScan data reported that subsequent to PS‐matching, the mean all‐cause health care costs PPPM among a general population of patients with OAB were estimated at $1625 (SD: $4293; estimates in 2013 United Stated Dollars). 32 In the present study, costs were estimated to be $6010 ($17 429) in the matched mirabegron group, and $5515 ($18 015) in the matched antimuscarinic group; higher costs were driven by pharmacy costs. Both studies overlap in terms of study population; whereas the present study includes only patients from the MarketScan Medicare Supplemental database, Durden et al. includes patients from both the MarketScan commercial and Medicare Supplemental databases. Considering Medicare reimbursement rates for health care are generally lower than those seen with Commercial coverage, the magnitude of the difference between the findings observed in the present study versus those observed in Durden et al. is possibly in part attributable to differences in the study populations (general OAB vs. older frail OAB).
This study used Medicare Supplemental data, which is limited to adults who have commercial insurance. Therefore, the results of this study may limit the generalizability to that population. Other limitations include those that are inherent to administrative claims data: dispensation of medication does not indicate whether the medication was actually taken, ascertainment of conditions and treatments rely on administrative codes, which are subject to coding error, and data on subjects before enrollment is unavailable. Additionally, it is not possible to adjust for the generosity of drug benefit, as the specifics related to plan benefits are not contained in the database. Therefore, it is possible that patients who received mirabegron had a more generous drug benefit that extended to other prescription drugs.
While administrative claims data provides robust information regarding health care costs, encounters, and treatment patterns, it does not fully capture OAB‐related costs not covered by insurance (e.g., containment products) or clinical characteristics beyond diagnoses and treatment. In the current study, specific disease characteristics that may guide physicians to choose mirabegron over antimuscarinics cannot be ascertained. Other clinically relevant outcomes, including the specific reasons for OAB treatment discontinuation and treatment efficacy, as well as the use of non‐pharmacologic OAB treatments, are also not captured in these data. Instead, this study provides a broad overview of treatment‐taking behavior and health care costs among frail, older adults with OAB.
It should be noted that we considered all antimuscarinics in one treatment category; however, there is evidence that differences exist in persistence amongst oxybutynin and the newer generation antimuscarinics (e.g., tolterodine, solifenacin). 33 , 34 Finally, the 1‐year follow‐up time does not allow for the assessment of long‐term costs and outcomes, including the advancement to more aggressive treatments, the occurrence of a prescribing cascade, and the implications of anticholinergic burden. 35 , 36
5. CONCLUSIONS
This study provides evidence regarding treatment‐taking behaviors and all‐cause medical costs and encounters among a population of frail older adults with OAB. There were no differences in number of encounters between treatment groups. Costs were higher among those who started mirabegron, which was driven by differences in costs specific to pharmacy claims. Incident mirabegron use was associated with potential improvements in treatment‐taking behaviors, particularly with respect to treatment adherence, versus those initiating antimuscarinics.
CONFLICTS OF INTEREST
All authors all fulfill the ICMJE guidelines for authorship. D. W. and A. L. are employees of Astellas Pharma Global Development and T. K., D. N., and B. J., of Astellas Pharma US. T. M. J. received consulting fees from Astellas for the conduct of this study, as well as research support from Medtronic and Dexcom, consultation fees from Eisai, and authorship royalties from UpToDate. G. L. O. is an employee of Broadstreet HEOR which received a contract for consultant services for this study.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This study was funded by Astellas Pharma Global Development. Medical writing/editorial support was provided by Alexis Mickle from Broadstreet HEOR and funded by the study sponsor. This study was funded by Astellas Pharma Global Development.
Johnson TM, Walker D, Lockefeer A, et al. Mirabegron and antimuscarinic use in frail overactive bladder patients in the United States Medicare population. Neurourol Urodyn. 2022;41:1872‐1889. 10.1002/nau.25040
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx
REFERENCES
- 1. Abrams P, Andersson KE, Apostolidis A, et al. International consultation on incontinence. recommendations of the international scientific committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse and faecal incontinence. Neurourol Urodyn. 2018;37(7):2271‐2272. 10.1002/nau.23551 [DOI] [PubMed] [Google Scholar]
- 2. Leron E, Weintraub AY, Mastrolia SA, Schwarzman P. Overactive bladder syndrome: evaluation and management. Curr Urol. 2018;11(3):117‐125. 10.1159/000447205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reynolds WS, Fowke J, Dmochowski R. The burden of overactive bladder on US public health. Curr Bladder Dysfunct Rep. 2016;11(1):8‐13. 10.1007/s11884-016-0344-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suskind AM, Quanstrom K, Zhao S, et al. Overactive bladder is strongly associated with frailty in older individuals. Urology. 2017;106:26‐31. 10.1016/j.urology.2017.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722‐727. 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 6. Johnston KJ, Bynum JPW, Joynt Maddox KE. The need to incorporate additional patient information into risk adjustment for Medicare beneficiaries. JAMA. 2020;323(10):925‐926. 10.1001/jama.2019.22370 [DOI] [PubMed] [Google Scholar]
- 7. Kautter J, Ingber M, Pope GC. Medicare risk adjustment for the frail elderly. Health Care Financ Rev. Winter 2008;30(2):83‐93. [PMC free article] [PubMed] [Google Scholar]
- 8. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a Claims‐Based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980‐987. 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shashikumar SA, Luke AA, Johnston KJ, Joynt Maddox KE. Assessment of HF outcomes using a Claims‐Based frailty index. JACC Heart Fail. 2020;8(6):481‐488. 10.1016/j.jchf.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 10. Kim DH, Patorno E, Pawar A, Lee H, Schneeweiss S, Glynn RJ. Measuring frailty in administrative claims data: comparative performance of four claims‐based frailty measures in the U.S. Medicare data. J Gerontol A Biol Sci Med Sci. 2020;75(6):1120‐1125. 10.1093/gerona/glz224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley‐Cox J. Anticholinergic drug exposure and the risk of dementia: a nested Case‐Control study. JAMA Intern Med. 2019;179(8):1084‐1093. 10.1001/jamainternmed.2019.0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richardson K, Fox C, Maidment I, et al. Anticholinergic drugs and risk of dementia: case‐control study. BMJ. 2018;361:k1315. 10.1136/bmj.k1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mur J, Russ TC, Cox SR, Marioni RE, Muniz‐Terrera G. Association between anticholinergic burden and dementia in UK Biobank. Alzheimers Dement (N Y). 2022;8(1):e12290. 10.1002/trc2.12290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landi F, Russo A, Liperoti R, et al. Anticholinergic drugs and physical function among frail elderly population. Clin Pharmacol Ther. 2007;81(2):235‐241. 10.1038/sj.clpt.6100035 [DOI] [PubMed] [Google Scholar]
- 15. Lightner DJ, Gomelsky A, Souter L, Vasavada SP. Diagnosis and treatment of overactive bladder (non‐neurogenic) in adults: AUA/SUFU guideline amendment 2019. J Urol. 2019;202(3):558‐563. 10.1097/JU.0000000000000309 [DOI] [PubMed] [Google Scholar]
- 16. Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. Sep 2011;10(5):751‐765. 10.1517/14740338.2011.579899 [DOI] [PubMed] [Google Scholar]
- 17. Gibson W, Johnson T, Kirschner‐Hermanns R, et al. Incontinence in frail elderly persons: report of the 6th international consultation on incontinence. Neurourol Urodyn. 2021;40(1):38‐54. 10.1002/nau.24549 [DOI] [PubMed] [Google Scholar]
- 18. Ganz ML, Smalarz AM, Krupski TL, et al. Economic costs of overactive bladder in the United States. Urology. 2010;75(3):526‐532. 10.1016/j.urology.2009.06.096 [DOI] [PubMed] [Google Scholar]
- 19. García‐Nogueras I, Aranda‐Reneo I, Peña‐Longobardo LM, Oliva‐Moreno J, Abizanda P. Use of health resources and healthcare costs associated with frailty: the FRADEA study. J Nutr Health Aging. 2017;21(2):207‐214. 10.1007/s12603-016-0727-9 [DOI] [PubMed] [Google Scholar]
- 20. Kojima G. Increased healthcare costs associated with frailty among community‐dwelling older people: A systematic review and meta‐analysis. Arch Gerontol Geriatr. 2019;84:103898. 10.1016/j.archger.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 21. Bock J‐O, König H‐H, Brenner H, et al. Associations of frailty with health care costs – results of the ESTHER cohort study. BMC Health Serv Res. 2016;16(1):128. 10.1186/s12913-016-1360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnston KJ, Wen H, Joynt Maddox KE. Relationship of a claims‐based frailty index to annualized Medicare costs: a cohort study. Ann Intern Med. 2020;172(8):533‐540. 10.7326/m19-3261 [DOI] [PubMed] [Google Scholar]
- 23. Health IW 2019. IBM MarketScan Research Databases for Health Services Researchers. https://www.ibm.com/downloads/cas/6KNYVVQ2
- 24. Gautam N, Bessette L, Pawar A, Levin R, Kim DH. Updating international classification of diseases 9th revision to 10th revision of a claims‐based frailty index. J Gerontol A Biol Sci Med Sci. 2021;76(7):1316‐1317. 10.1093/gerona/glaa150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sussman D, Yehoshua A, Kowalski J, et al. Adherence and persistence of mirabegron and anticholinergic therapies in patients with overactive bladder: a real‐world claims data analysis. Int J Clin Pract. 2017;71(3−4):e12824. 10.1111/ijcp.12824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399‐424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150‐161. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen T‐L, Collins GS, Spence J, et al. Double‐adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol. 2017;17(1):78. 10.1186/s12874-017-0338-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hogg RVTE. Probability and Statistical Inference. 8th ed. Macmillan; 2008. [Google Scholar]
- 30. Kelleher C, Hakimi Z, Zur R, et al. Efficacy and tolerability of mirabegron compared with antimuscarinic monotherapy or combination therapies for overactive bladder: a systematic review and network meta‐analysis. Eur Urol. 2018;74(3):324‐333. 10.1016/j.eururo.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 31. Chapple CR, Nazir J, Hakimi Z, et al. Persistence and adherence with mirabegron versus antimuscarinic agents in patients with overactive bladder: A retrospective observational study in UK clinical practice. Eur Urol. 2017;72(3):389‐399. 10.1016/j.eururo.2017.01.037 [DOI] [PubMed] [Google Scholar]
- 32. Durden E, Walker D, Gray S, Fowler R, Juneau P, Gooch K. The direct and indirect costs associated with overactive bladder within a commercially‐insured population in the United States. J Occup Environ Med. 2018;60(9):847‐852. 10.1097/jom.0000000000001367 [DOI] [PubMed] [Google Scholar]
- 33. Gomes T, Juurlink DN, Mamdani MM. Comparative adherence to oxybutynin or tolterodine among older patients. Eur J Clin Pharmacol. 2012;68(1):97‐99. 10.1007/s00228-011-1090-8 [DOI] [PubMed] [Google Scholar]
- 34. Wagg A, Compion G, Fahey A, Siddiqui E. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int. 2012;110(11):1767‐1774. 10.1111/j.1464-410X.2012.11023.x [DOI] [PubMed] [Google Scholar]
- 35. Teramura‐Grönblad M, Muurinen S, Soini H, Suominen M, Pitkälä KH. Use of anticholinergic drugs and cholinesterase inhibitors and their association with psychological well‐being among frail older adults in residential care facilities. Ann Pharmacother. 2011;45(5):596‐602. 10.1345/aph.1P650 [DOI] [PubMed] [Google Scholar]
- 36. Chatterjee S, Talwar A, Aparasu RR. Anticholinergic medications and risk of dementia in older adults: where are we now. Expert Opin Drug Saf. 2020;19(10):1251‐1267. 10.1080/14740338.2020.1811227 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx


