Abstract
Textile fibers alone are highly prevalent in our environment, and not only are there a wide variety of fibers, but generally, consumer textiles are colored. Given the variety of crime locations where dyes are encountered and the potential circumstances, a rapid, preparation free analysis of samples is highly beneficial. This study has characterized a collection of commercially available textiles dyes by verifying the chemical structure, collecting reference spectra, and developing a method to analyze dyed fibers via Direct Analysis in Real‐Time (DART) mass spectrometry. A methodology for direct analysis of pieces of fabric and single thread samples of polyester fibers dyed with disperse dyes was developed. The presence of 31 target dyes on fibers whose structures were previously established via high‐resolution mass spectrometry was confirmed. Dyed fabrics containing mixtures of dyes in varying concentrations were also evaluated to determine whether each dye in the composition could be detected. The DART‐MS methodology was sensitive and positively characterized disperse dyes in polyester fibers, allowing for blind identification of mixtures with the assistance of a high‐resolution mass spectrometry database.
Keywords: ambient ionization, DART (direct analysis in real‐time), disperse dyes, fibers, high‐resolution mass spectrometry, polyester
Highlights.
In situ analysis of evidence limits the effects that sample preparation may have on the accuracy of analyses.
Rapid evaluation limits degradation, allows for fast throughput and could relieve back‐logs in processing.
Fabrics with known dye concentrations were analyzed in order to validate the method.
Multiple dyes in a mixture can be detected, making the combination of dyes a unique characteristic.
1. INTRODUCTION
Textile fibers are a highly prevalent source of trace evidence, with polyester as the most widely used synthetic fiber at approximately 52% of global fiber production in 2018–2020 [1, 2]. In a forensic investigation, fibers from clothing, home furnishing, automobiles, and a variety of other sources may need to be evaluated either as a whole article or as trace evidence in both original and degraded forms. The identity of the fiber itself usually does not provide enough unique identifying information to characterize a sample in a forensic setting. Hence, a sample's color, finish, and texture provide greater detail. Forensic laboratories generally evaluate textiles using microscopy and micro‐visible spectrophotometry, however, the same color and absorbance may be generated by different dyes. Colorants are extremely common not only in textiles, but also in plastics, foods, paints, and inks and come in a variety of structures dictated by their application and appearance. Due to these reasons, identifying dyes themselves as opposed to simply using perceived color can be more valuable.
Synthetic dyes depend on extended conjugation to facilitate the mobilization of electrons following photoexcitation, allowing light to be absorbed and re‐emitted at a particular wavelength to produce color. There are a variety of chromophores (chemical groups central to color generation), including azo (most common), anthraquinone, methine, quinoline, and others that are less common. There are several classes of synthetic dye molecules characterized by their preferred fiber and performance regarding textile applications. Fiber reactive and direct dyes used for cellulosic materials bond to fibers covalently and ionically using triazines/sulfato ethyl sulfones and solubilizing groups respectively. Acid (anionic) dyes bond ionically to wool and other fibers with cationic groups such as amides (nylons), and basic (cationic) dyes have an affinity for fibers like acrylic. Finally, disperse dyes are used for synthetic fibers that do not possess the functionality that other dye classes use to adhere to, such as polyester. Disperse dyes are generally small, non‐ionic molecules that can be characterized as high, medium, or low energy based upon their molecular weight.
Mass spectrometry alone meets the specificity and sensitivity required for forensic analysis, and Direct Analysis in Real‐Time Mass Spectrometry (DART‐MS) is no different [3, 4, 5]. DART‐MS can be used to analyze solid, liquid, and gas samples rapidly and with little to no sample preparation. This reduces the amount of time and expertise required to process and prepare materials for MS analysis compared with other ionization methods like electrospray ionization (ESI). DART is an ionization method that directs a heated stream of metastable gas (generally He, H2, N2, or Ar) to the sample, desorbing and ionizing it into a gaseous phase before sending the analyte on to the mass analyzer. As the name suggests, spectra can be observed in real time and collection can be both continuous and pulsed [4, 6, 7]. The lack of sample preparation required allows for rapid, easy testing that has great potential for use in forensic sciences [5, 8, 9, 10, 11, 12, 13]. Case and evidence backlogs are a significant problem in forensic laboratories, so high turnover during testing and the potential to test on‐site are both desirable attributes for an analytical method. The ability to test materials directly helps to prevent degradation, destruction, or alteration of the original sample, allowing evidence to survive for additional or separate testing and presentation at trial [14, 15, 16, 17]. In related studies, DART has been used in the analysis of historical colorants, such as pigments from organic sources used in prints and paints from artifacts due to its speed, sensitivity, and lack of sample preparation needed, allowing for conservation of archeological and otherwise significant objects [5, 8, 9, 10, 11, 12].
2. DESIGN OF EXPERIMENT
This study aimed to observe and characterize the ionization of disperse dyes from polyester fibers in situ via DART‐MS. Several commercial disperse dyes were collected and characterized using electrospray ionization (ESI) coupled with quadrupole time‐of‐flight (Q‐TOF) mass spectrometry. Each dye's ion identity and isotopic distribution were confirmed, and spectra were collected for reference from dyes in solution using ESI‐MS before the dyes were applied to polyester (PET) fabrics. Dye molecules were extracted from fibers and introduced in solution to the DART source along with the same solubilized dyes used in ESI‐MS evaluations. The fabric surface and individual fibers mounted on IonSense QuickStrips were then directly measured using DART ionization and the resulting spectra compared with the ESI references to confirm and compare ionization behavior. Finally, mixtures of varying concentrations as well as ternary mixtures of dyes were evaluated to determine whether each dye in the composition would be detectable and if correlation in varying concentrations in a set could be accurately assessed using abundance.
3. INSTRUMENTATION
An Agilent 1200 series HPLC system was used for sample introduction via direct injection, bypassing the column compartment as reference and extraction solutions were already in simple solution matrices. An Agilent 6520 Accurate Mass Q‐TOF mass spectrometer (MS) with an Agilent ESI source was used during structural confirmation. DART analysis was completed using an IonSense DART‐SVP ionization source and VAPOR® interface set‐up, including an 87 mm ceramic transfer tube and pump at the MS interface to direct ions from the sample to the atmospheric pressure inlet of the MS and remove excess He gas. The DART‐MS analysis was undertaken using the same Agilent 6520 Q‐TOF instrument.
4. MATERIALS
HPLC grade acetonitrile (ACN), methanol (MeOH), water and acetone were purchased from Sigma‐Aldrich and used without further purification. IonSense DART QuickStrips (IonSense), and glass melting point tubes were purchased from Sigma‐Aldrich. The following materials were used for producing samples: polyester (PET) woven fabric, scoured to remove spin finish/oil, glacial acetic acid (Fisher Scientific), commercial textile dyes (DyStar; Standard Colors; Archroma; Huntsman).
5. METHODOLOGY
5.1. LCMS method/ESI parameters
Dye solutions composed of 80:20 ACN/water and approximately 20 ppm of dye were introduced via the LCMS system by direct injection. The mobile phase was a mixture of acetonitrile and water at a ratio of 9:1, the flow rate was set to 0.5 ml/min, and the total collection time for each sample was 3 min. Electrospray ionization was carried out in positive mode with the following parameters: gas temperature 325°C, drying gas 8 L/min, nebulizer 50 psi, Vcap voltage 4000 V, and fragmentor voltage at 170 V. Additionally, a solution of mass reference mix obtained from Agilent Technologies was introduced via a secondary ESI needle to improve mass accuracy. The exact mass of suspected structures was calculated, and resultant spectra were searched for the expected mass‐to‐charge ratio (m/z) of the protonated molecule; if the m/z value of the dye of interest was not present on the spectrum, common losses or fragments were considered before setting aside a dye as unconfirmed. Tandem mass spectrometry for ESI and DART analyses were completed using the targeted MS/MS mode, in which the system automatically isolates a series of precursor ions and fragments them using Collision‐Induced Dissociation (CID). In CID, nitrogen gas was used as collision gas, isolation width was set to a 1.3 mass‐to‐charge ratio, and collision energy was set to 5, 10, 20, 30, 40, and 50 eV.
5.2. Fabric dyeing
Commercial textile disperse dyes were applied to polyester fabrics according to manufacturer guidelines to replicate the fixation found in potential real‐world samples. Standards of a single color were generated for all confirmed disperse dyes, as well as monochromatic (2 dyes, one color), dichromatic, and trichromatic mixtures. A standard of 2% dye powder on weight of the fabric was dissolved in water generating a 20:1 liquor ratio in a 300 ml sealing beaker for an IR heated beaker dyeing machine (Ahiba IR, Datacolor). Acetic acid was added to acidify the bath, though additional leveling agents were omitted to provide a cleaner sample for initial analytical analysis. The dyebaths were heated to 130°C at a 2°C/min gradient and held for 30, 45, or 60 min depending on the dye or mixture (structure and depth of shade considerations). Dyebaths were then cooled and the fabric rinsed before a post‐scour using 1 g/L ApolloScour SDRS and 2 g/L soda ash at 100°C for 15 min, additional rinsing, and drying. Acid dye references on nylon and reactive dyes on cotton samples were also prepared using the manufacturer's recommended time, temperature, and auxiliaries, along with an identical post‐scour.
5.3. DART parameters and method development
Helium (He) gas was used (at 2.5 L/min) to promote the most efficient ionization mechanism employed by DART; the ionization of water in the air and subsequent ionization of the sample. Temperature trials for this polyester fiber (single thread, approximately 30 filament fibers) were run between 200–500°C to determine the optimum ionization temperature while limiting sample degradation/destruction. The trials were run in triplicate with an extended, 30 s exposure. However, the final analysis requires significantly less time for ionization and detection of dye molecules. It was found that an ionization temperature of 200°C or below had a negligible effect on the thread, while 300–350°C showed some signs of distortion with extended exposure. 400°C and above instantaneously melted the fibers placed in the stream. Balancing ion abundance and physical disruption of the substrate, 300°C was chosen for this method. Narrowing of DART‐to‐interface spacing was also required, with the highest clear response occurring at a spacing of approximately 1–1.5 cm; a larger spacing decreased the resolution and abundance of ionized dye molecules, so ultimately, a 1 cm spacing was settled on for analysis.
Multiple introduction methods were observed to verify the versatility of DART‐MS for disperse dye analysis in multiple matrices. Solutions were analyzed first to confirm the dye molecules themselves would ionize and be detectable in the DART gas stream before introducing them into a more solid medium. Solutions (dye dissolved in acetone as well as in 80:20 ACN/water) were introduced via a glass probe (the closed end of a glass melting point tube) from both fiber extractions and spiked solutions. Single threads and thin solid and perforated strips of fabric were introduced both by using tweezers and by mounting each on IonSense QuickStrip wire mesh cards, with care taken not to obstruct the flow of gas. All methods were comparable where ionization is concerned, though introduction via mesh card on a sliding rail provides the most reproducible measurement, standardization, and removes error or differences in introduction angle, the distance between source and MS inlet, etc. The mesh cards also benefit the method by preventing loose fibers from dislodging and entering the mass spectrometer.
6. RESULTS
Thirty‐one commercial disperse dyes have been characterized using this methodology, and their spectra used to generate a collection for later reference. High‐resolution ESI‐MS analysis for disperse dyes showed positive, singly charged protonated molecules, [M + H]+, in each case, generally with a ppm error (parts‐per‐million) of approximately 3.0 or less (calculated by Qualitative Analysis B.06.00 software) when compared with the ion exact mass for suspected chemical formulas based on provided chemical structures. The results of this analysis are reported in Table 1.
TABLE 1.
ESI‐MS ionized disperse dye structural verification, including each dye's exact mass, molecular weight, chemical formula, and observed ESI and DART exact masses
| CI | Exact mass | MW | Chemical formula | Observed mass | Ppm error | DART mass |
|---|---|---|---|---|---|---|
| DB 27 | 420.0958 | 420.377 | C22H16N2O7 | 421.0885 | −0.33 | 421.1055* |
| DB 287 | 448.1859 | 448.483 | C23H24N6O4 | 449.1945 | −2.4 | 449.1951 |
| DB 291 | 508.0706 | 509.317 | C19H21BrN6O6 | 509.0784 | 0.94 | 509.0725 |
| DB 56 | 347.9746 | 349.140 | C14H9BrN2O4 | 348.9813 | 2.36 | 348.9776 |
| DB 60 | 379.1168 | 379.372 | C20H17N3O5 | 380.1243 | 0.31 | 380.1087 |
| DB 77 | 376.0695 | 376.070 | C20H12N2O6 | 377.0764 | 2.2 | 377.0628 |
| DB 79: 1 | 624.0816 | 625.381 | C23H25BrN6O10 | 625.0889 | −0.77 | 625.0900* |
| DO 73 | 443.1594 | 443.463 | C24H21N5O4 | 444.1665 | 0.23 | 444.1540 |
| DR 15 | 239.0582 | 239.230 | C14H9NO3 | 240.0653 | 0.83 | 240.0587 |
| DR 153 | 403.0425 | 404.313 | C18H15Cl2N5S | 404.0497 | 0.19 | 404.0345 |
| DR 167:1 | 505.1364 | 505.907 | C22H24ClN5O7 | 506.1438 | −0.99 | 506.145* |
| DR 367 | 486.1315 | 486.476 | C28H22O8 | 487.1392 | −1.03 | 487.1313 |
| DR 4 | 269.0688 | 269.256 | C15H11NO4 | 270.076 | 0.17 | 270.0823 |
| DR 60 | 331.0845 | 331.327 | C20H13NO4 | 332.092 | 0.72 | 332.0916 |
| DR 73 | 348.1335 | 348.366 | C18H16N6O2 | 349.1415 | −2 | 349.1377 |
| DR 82 | 439.1492 | 439.428 | C21H21N5O6 | 440.156 | 0.07 | 440.1432 |
| DR 86 | 422.455 | 422.455 | C22H18N2O5S | 423.1005 | 2.36 | 423.0990 |
| DV 28 | 305.9963 | 307.130 | C14H8Cl2N2O2 | 306.9956 | 1.82 | 306.9995 |
| DY 114 | 424.0841 | 424.431 | C20H16N4O5S | 425.0902 | 2.84 | 425.0865 |
| DY 211 | 361.0578 | 361.742 | C15H12ClN5O4 | 362.0652 | −0.28 | 362.0638 |
| DY 64 | 366.9844 | 368.186 | C18H10BrNO3 | 290.0813 | −0.34 | 290.0814 |
| DY 82 | 333.1477 | 333.391 | C20H19N3O2 | 334.1548 | 0.6 | 334.1530 |
| DB 3 | 296.1161 | 296.319 | C17H16N2O3 | 297.1239 | −0.98 | 297.1245* |
| DO 30 | 449.0658 | 450.274 | C19H17Cl2N5O4 | 450.0725 | 1.11 | 450.0785* |
| DY 54 | 289.0739 | 289.284 | C18H11NO3 | 290.0813 | −0.34 | 290.0802* |
| DO 29 | 377.1124 | 377.352 | C19H15N5O4 | 378.1191 | 1.59 | 378.1207* |
| DR 311 | 516.1605 | 516.459 | C22H24N6O9 | 517.161 | 13.15 | 517.1709* |
| DB 183 | 474.1015 | 475.347 | C20H23BrN6O3 | 475.0911 | 0.82 | 475.1016* |
| DB 73 | 376.1059 | 376.368 | C21H16N2O5 | 377.1155 | −4.77 | 377.1101* |
| DB 87–1 | 379.1168 | 379.372 | C20H17N3O5 | 380.1235 | 1.58 | 380.1300* |
| DB 87–2 | 378.1328 | 378.388 | C20H18N4O4 | 379.1421 | −5.28 | 379.1391* |
Note: Masses marked with an asterisk (*) were not dyed to the fabric and only measured from solutions.
Abbreviations: B, blue; D, disperse; O, Orange; R, red; Y, yellow.
Disperse dyes are particularly applicable to this form of ionization compared with other classes of textile dyes due to the nature of their fixation in the fiber. Acid and fiber reactive dyes affix to their respective fibers via ionic and covalent bonds, and acid and reactive dyes did not readily ionize from the fibers using DART. On the other hand, disperse dyes are physically entrapped in a synthetic fiber following heat induced swelling and dispersion into the structure. The heated gas stream used in DART can open the fiber structure and blow the dye molecules out of the fibers and into the mass spectrometer (Figure 1). This can be clearly exemplified by bleaching that occurs following extended or repeated exposure to the gas stream. Along with dislodging dye molecules from the fiber, the metastable helium gas used in this method protonates the water in the air, generating reagent ions that in turn ionized the sample according to Penning ionization, as shown in Equation 1.
FIGURE 1.
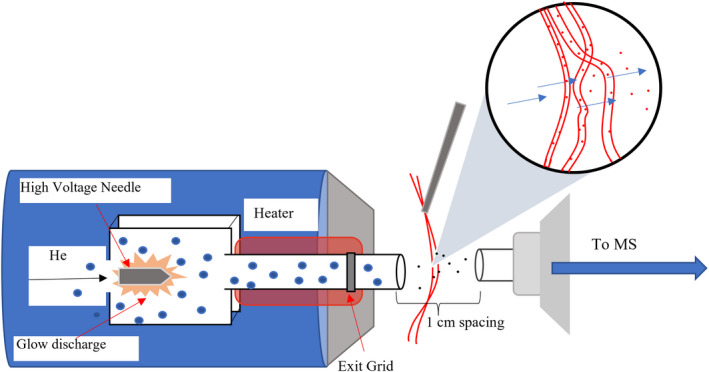
IonSense DART‐SVP ionization source and model of interaction with dyed polyester fibers.
Equation 1: Formation of reagent ions from air via metastable gas interaction to generate molecular ions (5)
| (1) |
Analysis of solutions and dyed fibers via DART‐MS show consistent results with ESI‐MS; for example, three thread samples obtained from PET fabrics dyed with Disperse Red 153, Disperse Yellow 211, and Disperse Blue 56. Direct analysis of the Disperse Red 153 thread provided a signal with an ion of m/z 404.0499 detected by QTOF, and the m/z difference between isotopic distribution peaks suggested that it was a singly charged ion. Using the formula generation function in Agilent MassHunter Qualitative analysis software, a formula of C18H15Cl2N5S was obtained with a relative mass measurement error of 0.38 ppm, which is consistent with the provided chemical formula of Disperse Red 153. The identification of C.I. Disperse Red 153 was reinforced by fragmentation patterns obtained from Collision‐Induced‐Dissociation (CID) in tandem mass spectrometry. Using the same methodology, Disperse Yellow 211 and Disperse Blue 56 were also identified, suggesting that the combination of DART with QTOF mass spectrometry can detect and identify the disperse dyes directly from fabric threads with minimal sample preparation (attachment to sampling card). A trichromatic combination of these three dyes to generate a black fabric (0.99% red, 0.52% yellow, and 2.19% blue on weight of fabric) were similarly analyzed, and each dye was confirmed present. This result suggests that the combination of DART with QTOF mass spectrometry could rapidly (<30 s) and simultaneously identify the different dye components in a black thread sample. Another set of three disperse dyes (Disperse Red 15, Disperse Yellow 82, and Disperse Blue 287) individually and in a black mixture were similarly evaluated and had the same success (see Figure 2).
FIGURE 2.
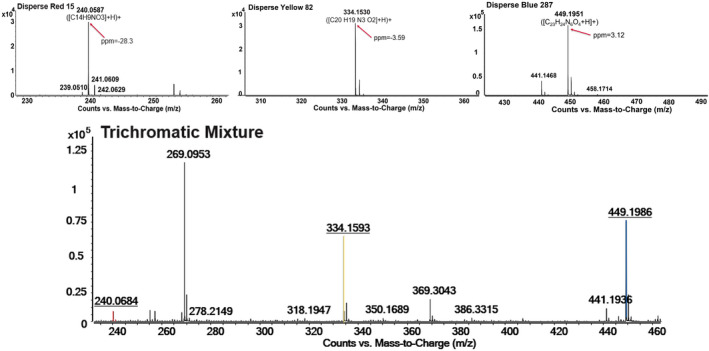
Example individually dyed and trichromatic DART mass spectra of Disperse Red 15, Yellow 82, and Blue 287 dyed fibers measured at 300° for 10–20 s.
Monochromatic and dichromatic mixtures (two dyes of similar color and two dyes of different colors) were generated at ratios of 1:99, 20:80, 50:50, 80:20, and 99:1 of a total of 2% dye on weight of fiber and analyzed. The purpose of the ratios was to attempt to estimate the relative amount of each dye in comparison to the other by their intensity. As an example, a mixture of Disperse Blue 60 ([380.1243] and 77 [377.0764]) was run. While DART analysis was able to detect each dye readily and accurately at each ratio, the relative abundance of each component was not entirely consistent with the applied ratio. While the first two ratios (1:99 and 20:80) follow an expected pattern, the 50:50 sample does not and shows Blue 60 at a significantly higher intensity (see Figure 3). This could be the result of one dye's signal interfering with the other and overshadowing it, or varying rates of fixation between dyes or manufacturers, which may alter the actual percentage present in fibers compared with what was applied. Analysis of other similar ratios of different dyes will determine whether this ratio estimation is possible, as this test was inconclusive.
FIGURE 3.
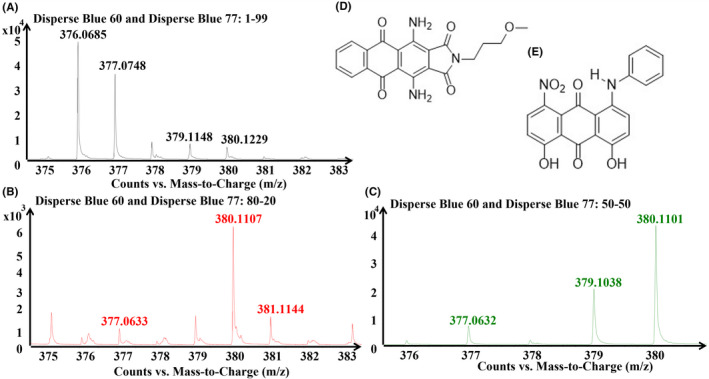
Comparison of the abundance of different ratios of dyes in a binary mixture of Disperse Blue 60 (exact mass 380.1243) and Blue 77 (377.0764) in varying ratios dyed on polyester and analyzed using the developed DART methodology. Concentrations are (A) 1:99, (B) 20:80, and (C) 50:50 of a total 3% on weight of fabric dyeing. Also provided are the chemical structures for the two dyes, (D) Disperse Blue 60 and (E) Disperse Blue 77.
7. BLIND STUDY
Fabric samples generated using structurally confirmed fiber reactive, acid, and disperse dyes in an unknown mixture were provided to a student that was not intimately familiar with dye chemistry for analysis (see Table 2). Multiple dye classes were included to provide internal information regarding the collection of spectra and ionization behavior for fiber reactive and acid dyes, as well as test the viability of this method of identification in a more varied situation. The trichromatic mixture's composition was chosen to generate a black color for the sample, and the 1:99 compositions were chosen to determine whether a minimal amount of those dyes were distinguishable within the mixture in the manual blind study. Chemical treatments were completed for each of the unknown dye/fiber mixtures in duplicate (x3) using 0.15% sodium hydroxide at 80° C for 1 h with constant mixing. The samples were cooled, neutralized using 1 M HCl solution, and the solvent removed and filtered using a PTFE filter. The resultant solutions were observed using the ESI methodology established previously in this study.
TABLE 2.
Samples analyzed in blind study, including fiber and dye identity, proportion of dyes applied to the fabric samples, and dyes that were manually identified
| Sample | Fabric | Dyes | Dye proportion | Dyes identified |
|---|---|---|---|---|
| A | Cotton | R. Red 153, R. Blue 4, R. Yellow 84 | 1:13:70 | R. Red 153 |
| B | Polyester | D. Yellow 211, D. Blue 60 | 1:99 | D. Blue 60 |
| C | Nylon | A. Blue 62, A. Red 337 | 1:99 | A. Red 337 |
| D | Poly‐Cotton | D. Red 153, R. Red 123 | 1:99 | D. Red 153 |
Note: The proportions are listed in order of dye appearance in the “dyes” column. Abbreviations used are: R. for fiber reactive, D. for disperse, and A. for acid dyes
Blank fibers were evaluated for each base fabric (nylon, cotton, polyester, and polyester/cotton blend) using DART as well as blank fiber extractions evaluated via ESI in both positive and negative mode. The lists (one for each class) of all confirmed dye exact masses, high‐resolution observed masses and ion distributions, chemical formulas and structures were then provided to the student, who compared the lists to the spectra collected. Some assumptions could be made about the identity of dyes used based on the fiber, and the student was told to look for either 2 or 3 dyes per sample. Acid dyes and fiber reactive dyes are negatively charged and are only observable in ESI extraction solutions; these classes of dyes have not been observable from the fibers using DART, whereas the disperse dyes are visible via ESI from extractions as well as directly from fibers by DART‐MS. The student was able to positively identify Reactive Red 153, Disperse Blue 60, Acid Red 337, and Disperse Red 153. It is expected in the near future to apply a computer‐driven search to aid the identification of dyes more automatically in comparison to manually.
8. CONCLUSIONS
Many dye structures accessible in the literature ultimately match with mass spectra obtained from this research, with a few exceptions for fragmentation, lack of signal, or an incorrectly provided formula. Comparison between LC/MS spectra for a confirmed dye and the corresponding dye's DART spectra also confirms that DART‐QTOF mass spectrometry is an applicable method for forensic analysis of dyed fibers, including mixtures, in the samples of this work. Using the narrowed methodology, each dye was detectable from the fiber surface with increasing abundance with increasing temperature, though the settled upon 300 °C analysis minimizes sample degradation. Each of the individual components of mixtures can be elucidated. Not enough data has been collected yet to determine whether there are consistent trends in response to factors like the ratio of one dye to another. The approximate proportion was not immediately evident, likely due to ion suppression and varying degree of fixation between dyes that may not correlate with intended/attempted concentration at dyeing. Acid dyed nylon and fiber reactive dyed cotton samples' dye molecules were not readily observed via DART‐MS using this methodology and no sample preparation. Ultimately, the simplicity, speed, and sensitivity of DART‐MS give this method good potential for application in the field of forensic analyses. A database for spectral reference would allow identifying characteristics to be determined for rapid comparison, preliminarily exemplified by positive identification of dyes in a blind study. A collection for this purpose has been initiated with the Max Weaver Dye Library, a collection of more than 98,000 dyes [18].
9. CONTINUING WORK
Treatment for acid dyes in solution and on fibers to allow for DART‐MS evaluation is currently being investigated. The viability of other fibers and method considerations concerning the non‐destructive application of DART to their analysis should also be investigated, as well as the further determination of sample size limitations for this method. Further research should be done to determine the effect on the fiber surface of this method microscopically (by both light microscopy and Scanning Electron Microscopy), which could be valuable for determining the amount and type of sample alteration that may occur, which would have an impact on forensic applications. Additionally, the mass spectra information generated in this project could be compiled into a reference library for future unknown dye identification; the availability of reference libraries is crucial for forensic analysis as manipulated samples are generally unknown to begin with. Further sampling and confirmation to both verify, improve, and expand the project and resultant libraries and procedures are required. A set of base parameters has been confirmed as successful for LC/MS analysis of dye solutions, however, most of the work done so far is preliminary for the goal of a larger project (continued collection and incorporation into searchable database).
Millbern Z, Vinueza NR. The characterization of disperse dyes in polyester fibers using DART mass spectrometry. J Forensic Sci. 2022;67:2291–2298. 10.1111/1556-4029.15129
Presented at the 74th Annual Scientific Conference of the American Academy of Forensic Sciences, February 21‐26, 2022, in Seattle, WA.
Funding information
This work was supported by the Department of Justice, National Institute of Justice Grant No. 2018‐DU‐BX‐0178.
Disclaimer
The following is not intended to advertise or require the use of IonSense technology.
REFERENCES
- 1. Opperskalski S, Siew S, Tan E, Truscott L. Preferred fiber & materials market report. 2019. https://textileexchange.org/preferred‐fiber‐and‐materials‐market‐report/. Accessed 15 Dec 2021.
- 2. Fernandez L. Distribution of textile fibers production worldwide in 2020, by type. https://www.statista.com/statistics/1250812/global‐fiber‐production‐share‐type/. Accessed 15 Dec 2021.
- 3. Goodpaster JV, Liszewski EA. Forensic analysis of dyed fibers. Anal Bioanal Chem. 2009;394:2009–18. 10.1007/s00216-009-2885-7 [DOI] [PubMed] [Google Scholar]
- 4. Li X, Wang X, Li L, Bai Y, Liu H. Direct analysis in real time mass spectrometry: a powerful tool for fast analysis. Mass Spectrom Lett. 2015;6(1):1–6. 10.5478/MSL.2015.6.1.1 [DOI] [Google Scholar]
- 5. Gross JH. Direct analysis in real time – a critical review on DART‐MS. Anal Bioanal Chem. 2014;406:63–80. 10.1007/s00216-013-7316-0 [DOI] [PubMed] [Google Scholar]
- 6. Habe TT, Morlock GE. Office chromatography: precise printing of sample solutions on miniaturized thin‐layer phases and utilization for scanning direct analysis in real time mass spectrometry. J Chromatogr A. 2015;1413:127–34. 10.1016/j.chroma.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 7. Li F, Musselman B. High‐throughput extraction and detection of drugs in urine: parallel sampling with solid‐phase microextraction (SPME) fibers coupled with direct analysis in real time‐mass spectrometry (DART‐MS) detection. Methods Mol Biol. 2018;1810:97–106. 10.1007/978-1-4939-8579-1_9 [DOI] [PubMed] [Google Scholar]
- 8. Armitage RA, Fraser D, Degano I, Colombini MP. The analysis of the Saltzman collection of Peruvian dyes by high performance liquid chromatography and ambient ionisation mass spectrometry. Herit Sci. 2019;7:81. 10.1186/s40494-019-0319-1 [DOI] [Google Scholar]
- 9. DeRoo CS, Armitage RA. Direct identification of dyes in textiles by direct analysis in real time‐time of flight mass spectrometry. Anal Chem. 2011;83(18):6924–8. 10.1021/ac201747s [DOI] [PubMed] [Google Scholar]
- 10. Liang J, Frazier J, Benefield V, Chong NS, Zhang M. Forensic fiber analysis by thermal desorption/pyrolysis‐direct analysis in real time‐mass spectrometry. Anal Chem. 2019;92:1925–33. 10.1021/acs.analchem.9b04167 [DOI] [PubMed] [Google Scholar]
- 11. Drury N, Ramotowski R, Moini M. A comparison between DART‐MS and DSA‐MS in the forensic analysis of writing inks. Forensic Sci Int. 2018;289:27–32. 10.1016/j.forsciint.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zughaibi TA, Steiner RR. Forensic analysis of polymeric carpet fibers using direct analysis in real time coupled to an AccuTOF mass spectrometer. Polymers (Basel). 2021;13:2687. 10.3390/polym13162687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sultana N, Gunning S, Furst SJ, Garrard KP, Dow TA, Vinueza NR. Direct analysis of textile dyes from trace fibers by automated microfluidics extraction system coupled with Q‐TOF mass spectrometer for forensic applications. Forensic Sci Int. 2018;289:67–74. 10.1016/j.forsciint.2018.05.020 [DOI] [PubMed] [Google Scholar]
- 14. Strom KJ, Hickman MJ. Forensic science and the administration of justice: critical issues and directions. Thousand Oaks, CA: SAGE Publications Inc; 2016. p. 43–54. [Google Scholar]
- 15. Lovrich NP, Pratt TC, Gaffney MJ, Johnson CL, Asplen CH, Hurst LH, et al. National forensic DNA study report, final report. Pullman, WA: National Institute of Justice, U.S. Department of Justice; 2004 Feb. Document No: 203970.
- 16. Pratt TC, Gaffney MJ, Lovrich NP, Johnson CL. This Isn't CSI: estimating the national backlog of forensic DNA cases and the barriers associated with case processing. Crim Justice Policy Rev. 2006;17:32–47. 10.1177/0887403405278815 [DOI] [Google Scholar]
- 17. Strom KJ, Hickman MJ. Unanalyzed evidence in law‐enforcement agencies. Criminol Public Policy. 2010;9:381–404. 10.1111/j.1745-9133.2010.00635.x [DOI] [Google Scholar]
- 18. Kuenemann MA, Szymczyk M, Chen Y, Sultana N, Hinks D, Freeman HS, et al. Weaver's historic accessible collection of synthetic dyes: a cheminformatics analysis. Chem Sci. 2017;8:4334–9. 10.1039/C7SC00567A [DOI] [PMC free article] [PubMed] [Google Scholar]


