Abstract
Microglia are tissue‐resident macrophages responsible for the surveillance, neuronal support, and immune defense of the brain parenchyma. Recently, the role played by microglia in the formation and function of neuronal circuits has garnered substantial attention. During development, microglia have been shown to engulf neuronal precursors and participate in pruning mechanisms while, in the mature brain, they influence synaptic signaling, provide trophic support and shape synaptic plasticity. Recently, studies have unveiled different microglial characteristics associated with specific brain regions. This emerging view suggests that the maturation and function of distinct neuronal circuits may be potentially associated with the molecular identity microglia adopts across the brain. Here, we review and summarize the known role of these cells in the thalamus, hippocampus, cortex, and cerebellum. We focus on in vivo studies to highlight the characteristics of microglia that may be important in the remodeling of these neuronal circuits and in relation to neurodevelopmental and neuropsychiatric disorders.
Keywords: brain wiring, cerebellum, cortex, hippocampus, microglia, neurodevelopment, thalamus
Here we review and synthesize current knowledge on the role of microglia in the development and maintenance of brain homeostasis. We highlight the characteristics and roles played by microglia across different neural circuits and their potential impact in neuropsychiatric disorders.
Abbreviations
- ASD
autism spectrum disorder
- BDNF
brain‐derived neurotrophic factor
- DAM
disease‐associated microglia
- DG
dentate gyrus
- EPSC
excitatory postsynaptic currents
- IBA‐1
ionized calcium binding adaptor molecule 1
- IL
interleukin
- LGN
lateral geniculate nucleus
- LPS
lipopolysaccharide
- LTD
long‐term depression
- LTP
long‐term potentiation
- M‐CSF
macrophage‐stimulating factor 1
- MIA
maternal immune activation
- PCs
Purkinje cells
- PGRN
progranulin
- Poly I:C
polyinosinic:polycytidylic acid
- PS
phosphatidylserine
- RGC
retinal ganglion cells
- SVZ
subventricular zone
- TGF‐β
transforming growth factor β
- TNF‐α
tumor necrosis factor α
- TTX
tetrodotoxin
- WM
white matter
1. INTRODUCTION
Proper brain wiring requires the assembly of microcircuits and long‐range connections formed during development. Within each circuit, glial cells, such as astrocytes and oligodendrocytes, assist neuronal activity by providing trophic signaling, clearing neurotransmitters, and regulating synaptic strength and plasticity. Microglia are specialized macrophages mainly recognized for providing immune surveillance within the brain parenchyma, that also participate in the regulation of synapses and neuronal circuit efficiency.
Microglia derive from primitive hematopoietic progenitors of the extra‐embryonic yolk sac that migrate to the early brain during embryonic development (Figure 1) (Ginhoux et al., 2010). In mice, at embryonic day (E)8, yolk sac CD45− c‐kit+ erythromyeloid progenitors have been identified as the earliest microglial precursors (Kierdorf et al., 2013). Between E9.5 and 14, these progenitors mature into CD45+ c‐kit− which initiate the colonization of the neural tube and start to express CX3CR1, the fractalkine receptor, and CSFR1, the receptor of the macrophage stimulating factor 1 (M‐CSF) (Kierdorf et al., 2013). While the mechanisms regulating microglia migration to the developing brain are largely unknown, some observations suggest this process occurs via the bloodstream, since mice with abnormal cardiovascular circulation show a lower number of microglia (Ginhoux et al., 2010).
FIGURE 1.
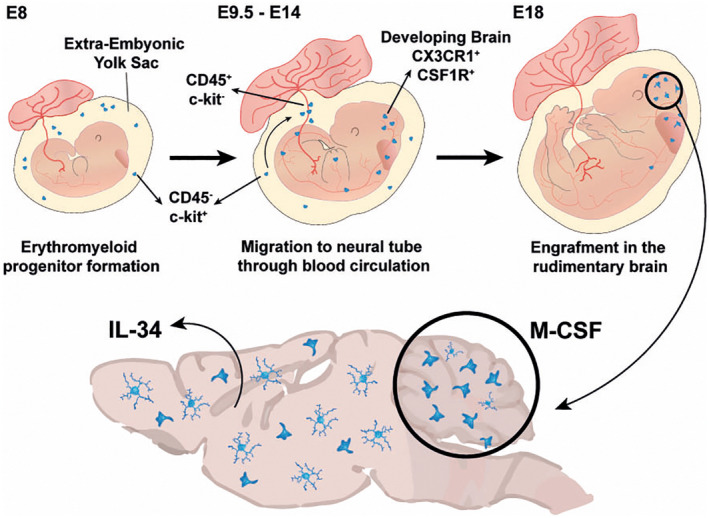
Microglia ontogeny and development. Microglia derive from primitive CD45− c‐kit+ hematopoietic progenitors of the extra‐embryonic yolk sac (YS), that mature to CD45+ c‐kit− progenitors between E9.5 and 14. During this time, microglia begin the colonization of the neural tube and start to express CX3CR1, the fractalkine receptor, and CSFR1. In the rudimentary brain, microglia develop at different paces, with microglia from the cortex, for example, developing faster than those of the cerebellum, becoming more ramified and less amoeboid at an earlier age. CSFR1 signaling, required for proper microglia development, is mediated by M‐CSF in the cerebellum, while in the rest of the brain this is promoted by IL‐34.
Engraftment of microglia in the developing brain is accompanied by the expression of another microglia marker, the ionized calcium‐binding adaptor molecule 1 (IBA‐1) and is dependent on the transcription factors PU.1 and IRF8 (Figure 1) (Kierdorf et al., 2013). Additionally, contrary to what is observed for other tissue‐resident macrophages, whose development depends on the expression of both CSFR1 and its classical ligand M‐CSF (Dai et al., 2002; Wiktor‐Jedrzejczak et al., 1982), microglia only require the expression of the receptor (Ginhoux et al., 2010). Reports have suggested this may be because of the presence of interleukin (IL)‐34 (Wang et al., 2012), a CSFR1 alternative ligand expressed by neurons in almost every region of the brain, with the exception of the cerebellum, where M‐CSF drives microglia identity (Kana et al., 2019) (Figure 1). Furthermore, the presence of transforming growth factor β (TGF‐β) in the brain milieu has been shown as necessary for the establishment of a proper microglial signature (Butovsky et al., 2014; Utz et al., 2020).
During development, microglia follow a stepwise maturation program characterized by discrete transcriptional phases (Matcovitch‐Natan et al., 2016; Thion, Low, et al., 2018). Early postnatal microglia are very heterogeneous compared to adult cells (Hammond et al., 2019; Li et al., 2019). However, the level to which this diversity is maintained in adulthood and the importance of this heterogeneity in different periods of life is a topic of debate. Even so, in the adult brain, microglia from different regions can vary in density, morphology, lysosomal content, and membrane properties, suggesting that microenvironmental cues may be crucial for the determination of region‐specific phenotypes.
Despite their myeloid origin, the pool of microglia in the adult brain is capable of self‐renewal and is independent from bone‐marrow‐derived progenitors (Bruttger et al., 2015; Huang et al., 2018). Interestingly, microglia from different brain regions also present distinct turnover rates. For example, microglia from the olfactory bulb and cerebellum display higher renewal rates compared to those from the cortex (Tay et al., 2017). Despite the slow turnover rate of cortical microglia, they can repopulate the entire visual cortex in only 3 days after depletion, in a process independent on P2RY12 (Mendes et al., 2021). This self‐renewal property allows microglia to tightly regulate their proliferation in response to environmental challenges without reinforcements from the peripheral immune system.
Environmental cues influence a variety of tissue‐specific enhancers that hierarchically collaborate with PU.1 to determine microglial phenotypes (Gosselin et al., 2014). This ability to sense the extracellular milieu is regulated by “sensome” genes, such as P2ry12, Tmem119, Gpr34, Siglech, Cx3cr1, Hexb, Trem2, P2ry6, and P2ry13 (Hickman et al., 2013) that are highly expressed in microglia when compared other myeloid and brain cells mostly found in the brain barriers (Butovsky et al., 2014; Hickman et al., 2013).
Because of the importance of deficits in micro‐ and long‐range circuits (Peça & Feng, 2012) and challenges to microglia (Suzuki et al., 2013) in neurodevelopmental and psychiatric disorders, it is critical to synthetize the current knowledge on microglial‐dependent remodeling of neuronal circuits. Here, we will review the function of microglia during development and in the adult brain, highlighting specificities of discrete microcircuits and how these may bidirectionally interact with microglia.
2. MICROGLIA IN NEURODEVELOPMENT
2.1. Microglia maturation and heterogeneity
The migration of microglia precursors to the embryonic brain, their proliferation, distribution, differentiation, and formation of the microglial network occurs concomitantly with the maturation of neuronal circuits (Figure 2) (reviewed in [Thion, Ginhoux, & Garel, 2018]). Following engraftment and proliferation, microglia drastically change their position and morphology from an amoeboid‐like phenotype to a ramified morphology (Cunningham et al., 2013). This conspicuous change may be driven by two non‐mutually exclusive scenarios: (1) as a response to cues released to the microenvironment ‐ by neurons and glial cells ‐, or (2) as a consequence of an intrinsic and cell‐autonomous developmental program. Recent ontogeny studies have presented strong evidence supporting both hypotheses. On the one hand, Cronk et al. showed that exposure to the adult brain environment is not sufficient to promote the differentiation of bone‐marrow‐derived macrophages into microglia (Cronk et al., 2018). On the other hand, yolk sac‐derived cells can still attain microglial identity following engraftment even after pre‐exposure to a cell culture environment (Bennett et al., 2018). These results suggest that both yolk sac origin and exposure to specific cues during neurodevelopment are critical factors for microglial differentiation.
FIGURE 2.
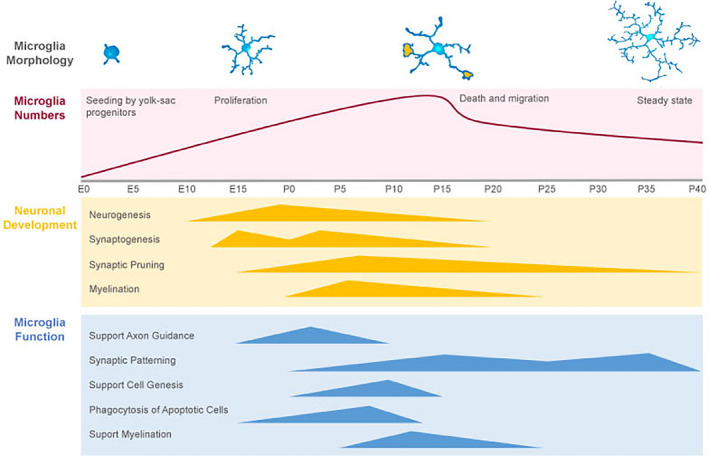
Microglia matures alongside neuronal circuits. As brain circuits develop, microglia colonize and mature alongside in a bidirectionally regulated process. During this time, these cells participate in several of developmental steps, from neurogenesis to synaptogenesis and circuit refinement through synaptic pruning, being essential for the proper establishment of these circuits.
Neurons and their progenitors can regulate some aspects of microglia maturation. For example, neuronal progenitors can directly regulate microglia distribution, since their ablation impairs microglia recruitment to the subventricular zone (SVZ) (Arnò et al., 2014). Additionally, programmed cell death is thought to determine the number of microglia in the brain of zebrafish, as reducing neuronal cell death decreases microglia numbers, whereas increasing apoptosis enhances brain colonization by these cells (Casano et al., 2016).
Recent single‐cell RNA sequencing studies have shed light on the heterogeneity of microglia during development. In addition to the identification of expression clusters unique to developing microglia, Hammond and colleagues found specific microglia populations that appear in narrow time‐windows and niches, such as axon tract‐associated microglia (Hammond et al., 2019). Li and colleagues also observed the presence of microglia heterogeneity during development, identifying expression patterns associated with homeostatic, embryonic‐like, proliferative, or immature microglia (Li et al., 2019). These authors further described proliferative‐region‐associated microglia, as these cells appeared to be closely related to disease‐associated microglia (DAM), with an upregulation in genes like Spp1, Gpnmb, Igf1, and Clec7a (Li et al., 2019). Masuda and colleagues found similar heterogeneity in embryonic and juvenile microglia, but disclosed a region‐specific difference in the proportion of the observed expression clusters, with some being more prevalent in the hippocampus and cortex and others in the cerebellum and corpus callosum (Masuda et al., 2019). However, in contrast with earlier studies (Ayata et al., 2018; Grabert et al., 2016), these three independent studies identified limited heterogeneity and regional differences in adult homeostatic microglia, suggesting a transcriptional continuum rather than specific microglia populations (Masuda et al., 2019). Furthermore, there was also limited sex differences in transcriptional clusters (Hammond et al., 2019), which does not eliminate the possibility of marked sex‐ and region‐specific differences in microglial phenotypes in response to a wider spectrum of environmental cues (Caetano et al., 2017).
2.2. The role of microglia in the formation of neuronal circuits
Microglia maturation occurs in tandem with several processes linked to neuronal circuit development, such as proliferation and migration of neuronal precursors, neuronal differentiation, axonal growth, myelination, synaptogenesis, and synaptic pruning (Figure 2).
Microglia have also been shown to play a crucial role in several of these processes (Figure 3 and Table 1). Their privileged location in neurogenic niches (Arnò et al., 2014; Cunningham et al., 2013) and the distinct phenotypes they adopt (Ribeiro Xavier et al., 2015) suggest that microglia contribute to the regulation of the number and position of proliferative neuronal precursors. On the one hand, it is thought that microglia may directly impact neurogenesis, since mice lacking CSFR1 show reduced numbers of basal neuronal progenitors (Arnò et al., 2014) and CX3CR1‐deleted microglia fail to support the survival of cortical neurons (Ueno et al., 2013). On the other hand, microglia have been shown to actively phagocyte SVZ progenitors in non‐human primates in the prenatal period (Cunningham et al., 2013) and viable newborn cells in the rat amygdala during postnatal stages (VanRyzin et al., 2019). These studies shed light on the controversial debate on whether microglia function only as clearers of apoptotic cells, or if they play an active role in eliminating live cells. Evidence suggests microglia may indeed induce neuronal apoptosis (Marín‐Teva et al., 2004) in a process dependent on CD11b and the DAP12/TREM2 axis (Wakselman et al., 2008), while blocking microglia phagocytosis with an antibody against CD11b, increased the number of viable newborn cells in the target region (VanRyzin et al., 2019).
FIGURE 3.
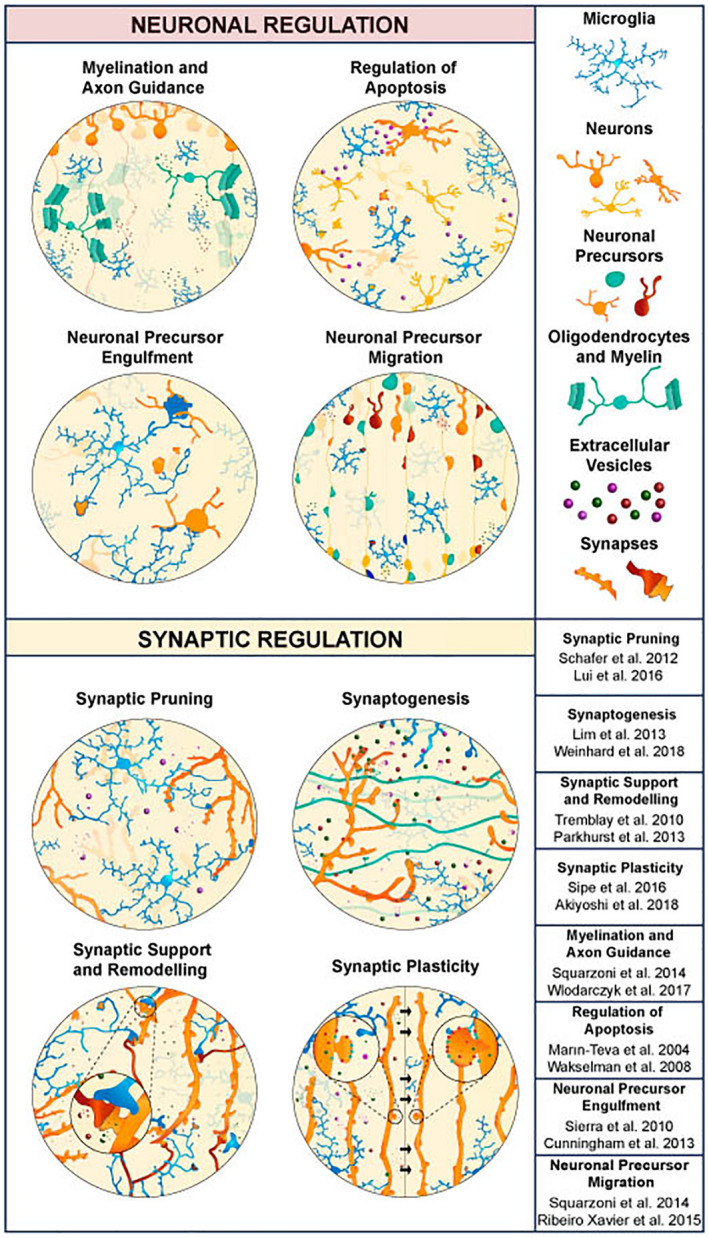
The many roles of microglia throughout development. Microglia participate in key steps of brain development and are essential for the proper shaping of neuronal circuits. These cells play an important role in ensuring proper neuronal migration and axonal connectivity, trimming excess neurons, and clearing apoptotic cells and debris. Microglia also extensively engage in the regulation of synapses, performing either synaptic pruning or promoting synaptogenesis, providing support, and modulating synaptic plasticity to ensure proper neuronal connectivity.
TABLE 1.
Role of microglia in the formation and maturation of neuronal circuits during development
| Function | Signaling | Output | References |
|---|---|---|---|
| Neurogenesis | CSF1R | Lack of Csfr1 reduced the numbers of basal neuronal progenitors | Arnò et al. (2014) |
| CX3CR1 and IGF‐1 | Cx3cr1‐deleted microglia fail to support the survival of cortical neurons | Ueno et al. (2013) | |
| Engulfment of neuronal precursors | ND | Phagocytosis of SVZ progenitors in non‐human primates is dependent on microglia activation | Cunningham et al. (2013) |
|
Androgen‐induced endocannabinoids Complement (CR3) |
Phagocytosis of viable newborn cells is dependent on CD11b | VanRyzin et al. (2019) | |
| Induction of apoptosis |
CD11b DAP12/TREM2 Superoxide ions |
Blocking apoptosis with anti‐CD11b antibodies decreases neuronal death | Wakselman et al. (2008) |
| Superoxide ions | Elimination of microglia increased Purkinje cell number | Marín‐Teva et al. (2004) | |
| Neuronal migration |
CX3CR1 CR3 DAP12 |
Perturbing microglia activity affects the outgrowth of dopaminergic axons in the forebrain and the laminar positioning of subsets of neocortical interneurons | Squarzoni et al. (2014) |
| Axonal outgrowth | PU.1 | While depleting microglia increases the extension of dopaminergic axons, MIA decreases it | Ribeiro Xavier et al., 2015 |
| Myelination |
IGF‐1 transglutaminase‐2/GPR56 |
Lack of Igf1 disrupted primary myelination Loss of Tgm2 and Adgrg decreases myelination |
Wlodarczyk et al. (2017) Giera et al. (2018) |
| Axonal pruning |
Complement Dependent on neuronal activity |
Expression of C3 reduced the size of RGC axons Decreased neuronal activity increased myelin inclusions per microglia |
Lim and Ruthazer (2021) Hughes and Appel (2020) |
| Synaptogenesis | ND | Microglia ablation reduces cortical synapses |
Miyamoto et al. (2016) Weinhard et al. (2018) |
| Synaptic pruning |
CX3CR1 Complement (CR3, C3, C1Q) Neuronal activity TREM2 IL‐33 |
Deletion of Cx3cr1, Cr3, C3, C1q and Trem2 increases the number of synapses Deletion of Il33 from astrocytes increases the number of synapses |
Paolicelli et al. (2011) Gunner et al. (2019) Schafer et al. (2012) Filipello et al. (2018) Vainchtein et al. (2018) |
Abbreviations: ND, not determined; MIA, maternal immune activation; RGC, retinal ganglion cell.
During embryonic development, microglia also play a role in regulating the migration of cortical interneurons to their correct laminar position (Squarzoni et al., 2014), thereby influencing the distribution of neuronal types within specific circuits. In this study, the authors showed that microglia regulates the outgrowth of dopaminergic axons (Squarzoni et al., 2014). Furthermore, microglia are enriched in white matter (WM) areas and have been shown to also play a role in axon pruning in the cerebellum (Nakayama et al., 2018; Ueno et al., 2013). This localization has also been observed in brain human samples (Mildner et al., 2017), suggesting that proximity to axonal fibers may be a general characteristic of microglia in the mammalian brain. Recently, Lim et al. used a Xenopus laevis model to demonstrate that microglia engage in the trogocytosis of retinal ganglion cell axons in a complement‐dependent manner (Lim & Ruthazer, 2021). However, microglia are also involved in myelination, since a CD11c+ subset of microglia was identified as a major source of myelinogenic IGF‐1 (Wlodarczyk et al., 2017). Microglia‐derived transglutaminase‐2 was shown to signal to G‐Protein‐Coupled Receptor 56 (GPR56) in oligodendrocyte precursor cells to promote their proliferation (Giera et al., 2018). Myelin sheaths can also be regulated by microglia, as these cells survey and phagocyte these structures, in a process regulated by neuronal activity (Hughes & Appel, 2020). Together, these studies suggest that microglia influence both microcircuitry and long‐range connections by playing diverse roles in the formation, elimination, and migration of neurons, but also in shaping axonal projections.
Microglia also regulate synaptogenesis and synaptic pruning during the early stages of circuit maturation. In addition to secreting growth factors, such as nerve growth factor and brain‐derived neurotrophic factor (BDNF) (Liao et al., 2008), IL‐10 released by microglia was shown to induce the formation of both excitatory and inhibitory synapses in hippocampal neurons (Lim et al., 2013). Additionally, microglia contacts induce spine filopodia in both cortical and hippocampal neurons (Miyamoto et al., 2016; Weinhard et al., 2018).
Synaptic pruning is the process by which synapses are eliminated in a latter phase of neuronal circuit refinement. This process is thought to be essential for the maintenance of stronger connections and elimination of weaker synapses. Microglia have been shown to participate in synaptic pruning processes in several brain circuits and, because of its broad distribution in the adult brain, it is likely that this is a widespread mechanism (Kettenmann et al., 2013). However, studies on microglia engulfment of synapses are based on observations showing labeled synaptic terminals (Schafer et al., 2012), or synaptic proteins, such as GluR1 AMPA subunits (Sipe et al., 2016), PSD‐95 (Paolicelli et al., 2011; Vainchtein et al., 2018) or synaptophysin (Lui et al., 2016) inside microglia. Because of this, some authors argue that these observations do not necessarily demonstrate specific phagocytosis of entire synapses or spines, but rather that these contents could derive from engulfment of larger structures (dendrites or neurons). Additionally, other mechanisms, such as trogocytosis or “nibbling” of synaptic structures, may also be taking place (Weinhard et al., 2018). Thus, it is still unclear whether phagocytosis and/or trogocytosis occurs for whole synapses or for pre and/or postsynaptic terminals.
Regardless, it is generally accepted that microglia participate, in some extent, in synaptic pruning during the maturation of neuronal circuits. Several signaling pathways have been suggested to mediate the interaction and engulfment of synapses by microglia in different brain regions, such as the CX3CR1/CX3CL1 axis (Paolicelli et al., 2011), the complement cascade (Lui et al., 2016; Schafer et al., 2012), TREM2‐dependent pathways (Filipello et al., 2018), and IL‐33 released from astrocytes (Vainchtein et al., 2018). Neuron‐to‐microglia signaling can also strongly modulate this activity. Dampening cortical activity by trimming mouse whiskers postnatally, Gunner et al. demonstrated an enhancement in microglia‐mediated synapse elimination in cortical layer IV neurons, in a process dependent on the CX3CR1/CX3CL1 axis (Gunner et al., 2019). Two proteins of the complement system, C1q and C3, whose main function in the periphery is to recognize foreign pathogens for elimination, have also been shown to tag synapses for elimination (Stevens et al., 2007). In particular, C1q‐labeled synaptosomes enriched for synaptophysin display high levels of caspase‐3 and annexin V, suggesting that selective C1q tagging depends on the occurrence of local apoptotic‐like processes, both in mouse and human tissue (Györffy et al., 2018). Additionally, phosphatidylserine (PS), whose expression peaks during brain development, has been shown to function as an “eat‐me signal” in a process that requires the presence of C1q. In this regard, C1q KO animals present an accumulation of PS in presynaptic terminals and reduced PS engulfment by microglia (Scott‐Hewitt et al., 2020). Together these studies suggest that microglia‐mediated elimination of synapses, similarly to engulfment of whole cells, might also be dependent on apoptosis‐related signals.
3. MICROGLIA IN THE ADULT BRAIN
3.1. Microglia in homeostasis
In the adult brain, microglia numbers are regulated through a balance of high rates of local proliferation and apoptosis (Askew et al., 2017). At a molecular level, CSF1R signaling is crucial for microglial survival, since inhibiting this receptor eliminates more than 90% of these cells (Elmore et al., 2014). In contrast, loss of CX3CR1 only transiently alters microglia numbers (Paolicelli et al., 2011). Adult microglia normally display a ramified phenotype, with each soma being evenly distributed in a non‐overlapping way (Cunningham et al., 2013; Kettenmann et al., 2013; Nakayama et al., 2018; Squarzoni et al., 2014). Pioneer in vivo microscopy studies have shown that microglia processes dynamically penetrate the parenchyma in a constant surveillance of their territory (Davalos et al., 2005; Nimmerjahn et al., 2005). This phenotype is mediated by purinergic receptors, such as P2RY receptors, which play a key role in the rapid response to focal damage (Davalos et al., 2005). P2RY12, for instance, is essential to regulate microglial motility in the homeostatic brain (Sipe et al., 2016).
3.2. The role of microglia in modulating the function of neuronal circuits
Although in adulthood the remodeling of neuronal circuits is less marked, it is important to acknowledge that microglia still regulate the number of adult newborn neurons and actively participate in synaptic remodeling (Figure 3 and Table 2). The adult SVZ is enriched in cytokines that promote neurogenesis, and depletion of SVZ microglia disrupts neuroblast survival and migration (Ribeiro Xavier et al. 2015). On the other hand, microglia phagocyte SVZ progenitors that undergo apoptosis (Sierra et al., 2010). Moreover, mice deficient in microglial Mer and Axl display a significant accumulation of apoptotic cells in neurogenic regions (Fourgeaud et al., 2016). Interestingly, Damisah and colleagues employed two photon‐mediated photochemically induced apoptosis to trigger cell death in individual neurons and precisely image their clearance by microglia (Damisah et al., 2020). Within 2 to 3 hours of the stimulus, nearby microglia competed among themselves to engage in the clearance of the apoptotic cell (Damisah et al., 2020). They also observed that microglia specialized in the engulfment of the soma and nearby dendritic branches, worked in concert with astrocytes, which engulfed the remaining neuritic fragments (Damisah et al., 2020).
TABLE 2.
Influence of microglia on neuronal circuit function in the adult brain
| Function | Signaling | Output | References |
|---|---|---|---|
| Neuronal migration | ND | Depletion of SVZ‐microglia disrupts neuroblast migration | Ribeiro Xavier et al. (2015) |
| Microglial phagocytosis of apoptotic newborn neurons | MERTK/AXL2 |
Clearance of apoptotic cells in SVZ Deletion of TAM receptor tyrosine kinases increased the accumulation of apoptotic neurons in SVZ |
Sierra et al. (2010) Fourgeaud et al. (2016) |
| Elimination of dead neurons externally induced | MERTK | Deletion of MERTK delays microglial detection and clearance of apoptotic cells | Damisah et al. (2020) |
| Experience‐dependent synaptic plasticity |
P2RY12 Fn14‐TWEAK axis |
Light deprivation affected microglial morphology, motility, increased their phagocytic structures and their localization in synaptic clefts GluR1 puncta found inside microglia following monocular deprivation In the absence of TWEAK, relay neurons display a significant increase in the number of bulbous spines |
Tremblay et al. (2010) Sipe et al. (2016) Cheadle et al. (2020) |
| Regulation of synaptic activity |
ATP/A1R CD39 THIK‐1 |
Microglia contact active synapses Lack of CD39 was associated with an increase in striatal neuron PKA activity, a decrease in striatal adenosine levels and increased susceptibility to D1 agonist‐induced seizures Inhibition of microglia induces hyperexcitability Deletion or inhibition of THIK‐1 decreases microglia surveillance and impairs remyelination Anesthesia may impact microglia surveillance |
Li et al. (2012) Wake et al. (2009) Akiyoshi et al. (2018) Badimon et al. (2020) Merlini et al. (2021) Madry et al. (2018) Madry et al. (2018); Ronzano et al. (2021) Liu et al. (2019) |
Abbreviations: ND, not determined; SVZ, subventricular zone.
The close association of microglia with synaptic structures also suggests they actively participate in their remodeling in the adult brain and that neuronal activity may influence this function. Experience‐dependent plasticity in the visual cortex, induced by light deprivation, affects microglial morphology, motility, and increases their phagocytic structures and localization in synaptic clefts (Tremblay et al., 2010). These results suggest that microglia in the visual cortex play a role in remodeling synapses that become weakened because of light deprivation. Indeed, a separate study showed that monocular deprivation altered microglia morphology and motility in the contralateral hemisphere of the deprived eye, thus affecting synaptic pruning (Sipe et al., 2016). Recently, Cheadle et al. described the neuron–microglia Fn14‐TWEAK axis as important in the regulation of retinogeniculate circuit synapses (Cheadle et al., 2020). These authors demonstrated that sensory experience induces expression of Fn14 in neurons and TWEAK (Fn14 ligand) in microglia and that TWEAK binding to Fn14 antagonizes the ability of Fn14 to enhance bulbous spine numbers (Cheadle et al., 2020). In a motor learning paradigm, microglia have also been observed to play a critical role in synaptic remodeling, since changes in the formation and elimination rate of synapses in the motor cortex, as a consequence of rotarod training, are critically affected following microglia deletion (Parkhurst et al., 2013). These studies suggest that microglia display the ability to engulf synaptic material or reinforce synaptic activity in a sensory‐ and experience‐dependent manner.
Although at least two early studies propose that neuronal activity does not influence microglia (Chen et al., 2010; Wu & Zhuo, 2008), other compelling evidence registered an effect of neuronal activity in the function of these cells (Akiyoshi et al., 2018; Li et al., 2012; Wake et al., 2009). In the cortex, microglia processes contact synapses for approximately 5 min, at a frequency of once per hour. The frequency of these contacts was reduced with decreasing neuronal activity (Akiyoshi et al., 2018; Wake et al., 2009). These studies suggest that activation of synapses steers microglia processes to favor contact with most active synapses. Additionally, neuronal activity‐induced synaptic release of ATP may act as a chemoattractant to microglial processes. ATP is then processed to adenosine by microglial CD39 and CD73, leading to suppressed neuronal activity via adenosine receptor A1R (Badimon et al., 2020). Suppression of synaptic activity by microglia was also shown to be important for the regulation of network hyperexcitability. Inhibition of microglial Gi‐dependent pathways reduces microglial surveillance and induced spontaneous seizures upon physiological neuronal activity stimulation, suggesting that microglia dynamics is essential to prevent hyperexcitability (Merlini et al., 2021).
One possibility is that microglia directly respond to neurotransmitters released to the synaptic cleft or to changes in local ion concentrations because of continued membrane depolarization. Direct application of glutamate is able to induce microglia chemotaxis (Liu et al., 2009), while glutamate uncaging was shown to attract microglial processes (Li et al., 2012). Interestingly, using agonists and antagonists of AMPA and GABA receptors, Fontainhas and colleagues showed that microglial process motility increased with glutamatergic neurotransmission and decreased with GABAergic neurotransmission (Fontainhas et al., 2011). Together these studies demonstrated that microglia dynamics is affected by the binding of neurotransmitters to neuronal ionotropic receptors, suggesting that the signaling is indirect and activity‐driven, while microglia per se can directly respond to certain neuromodulators, such as purines. Additionally, the activity of microglial THIK‐1 has been shown to function as a potassium sensor when in close proximity to both axons and synapses and may function as a readout for neuronal membrane activity (Madry et al., 2018; Ronzano et al., 2021). Indeed, abrogation or inhibition of THIK‐1 leads to an increase in synaptic density because of defective microglia pruning (Izquierdo et al., 2021).
Still in this context, it is important to note two recently published studies demonstrating a strong connection between neuronal activity in different wake states and microglial motility and surveillance. While Madry and colleagues suggest that anesthesia evokes retraction of microglia processes and inhibits surveillance without affecting motility (in hippocampal slices [Madry et al., 2018]), Liu and coworkers claim that, in vivo, microglial process surveillance increases after general anesthesia (Liu et al., 2019). Although with apparent contradictory results, these studies highlight the possible impact of awake states for microglia dynamics and reinforce the importance of careful consideration of anesthesia methodology in microglia research.
4. CHALLENGES TO MICROGLIA INFLUENCE NEURODEVELOPMENT
“Microglia activation” is a broad and heterogeneous terminology utilized to refer to any microglial response occurring following an insult, such as a viral or bacterial infection, acute brain damage or long‐term accumulation of toxic products derived from neurodegenerative processes. Microglia responses vary from clonal expansion (Tay et al., 2017), recruitment to the site of injury (Davalos et al., 2005), secretion of inflammatory cytokines (Sousa et al., 2018) or activation of clearing mechanisms, such as phagocytosis (Cunningham et al., 2013), although it is now acknowledged that phenotypes change widely depending on macro and microenvironment variables. Microglial responses have been extensively studied in vitro and in rodent models of disease, however, it is still unclear how different phenotypes may be induced, if the responses are region‐ and age‐dependent, and what is the contribution of each microglia phenotype across different scenarios in health and disease. Although newer nomenclature and recommendation for describing microglia have been recently proposed (Paolicelli et al., 2022), on occasion we will preserve the terminology used by the original authors.
Considering the importance of microglia in the above‐mentioned processes of formation and function of neuronal circuits, several studies have tried to discern how microglia status influences neurodevelopment and behavior. In rodents and non‐human primates, lipopolysaccharide (LPS) and other stimuli, such as polyinosinic:polycytidylic acid (poly I:C) ‐ a drug that simulates viral infections ‐ have been used to mimic infections during pregnancy, a challenge which is associated with greater risk for neuropsychiatric diseases in the offspring (Knuesel et al., 2014). In fact, LPS‐maternal immune activation (MIA) has been shown to significantly increase the number of synapses in granule cells of the hippocampus of the offspring and reduce the expression of CX3CR1 (Fernández de Cossío et al., 2017), as well as promote brain overgrowth and autism spectrum disorder (ASD)‐related behavioral abnormalities (Le Belle et al., 2014). Poly I:C MIA has also been shown to induce expression of both pro‐ and anti‐inflammatory cytokines in maternal blood and fetal brain in both rats (Missault et al., 2014) and non‐human primates (Rose et al., 2017). The offspring of poly I:C‐injected mothers presents behavioral deficits, such as alterations in sensorimotor gating, social behavior and memory, which can be reverted with minocycline (Mattei et al., 2017). Importantly, Choi and colleagues showed that poly I:C‐dependent MIA increases maternal IL‐17a which induces disorganized cortical cytoarchitecture and ASD‐like behaviors in the offspring (Choi et al., 2016). This phenotype was also achieved by optogenetic activation of pyramidal neurons of the dysgranular zone of the primary somatosensory cortex, a region where MIA‐dependent abnormalities are more salient, while reduction of neuronal activity rescued ASD‐related behaviors (Shin et al., 2017). Importantly, it has been shown that poly I:C results in an accelerated developmental program of microglia, since the transcriptome of early stage microglia in pups from dams injected with poly I:C is more similar to adult microglia (Matcovitch‐Natan et al., 2016). Again, this suggests that at least some characteristics of microglia activation phenotypes may be essential during development and that a transient perturbation in the kinetics of the microglial developmental program might result in implications to the function of neuronal circuits in adult animals.
5. MICROGLIA IN DIFFERENT BRAIN REGIONS
Although in the past two decades our knowledge on microglia has grown exponential, it is hard to interpret all data available on the physiology of these cells, when considering that most studies are performed in different brain regions and at different ages. In this context, some important questions remain: are there different microglia populations defined by specific transcriptomes that translate into discrete functional phenotypes? Or is there a continuum of phenotypes in the same cell, according to environmental factors? Are there regional specificities that determine microglia fate? Do microglia perform different functions along their lifetime, determined by the requirements of each circuit? Or are microglia performing similar roles in all regions?
Although these questions remain largely unanswered, in the next sections we aggregate information on microglia physiology in four specific brain regions, which may hint at regional specificity (thalamus, hippocampus, cortex, and cerebellum; Table 3). These regions were chosen based on their importance for neurodevelopmental conditions and existing evidence for these circuits being influenced by microglia.
TABLE 3.
Reference table of microglia studies across different brain regions
| Brain region | Function | References |
|---|---|---|
| Thalamus | Myelination | Kalish et al. (2018) |
| Synaptic pruning |
Schafer et al. (2012) Vainchtein et al. (2018) Lehrman et al. (2018) Lui et al. (2016) |
|
| Neuronal apoptosis | Zhang et al. (2020) | |
| Hippocampus | Phagocytosis of adult newborn neurons | Diaz‐Aparicio et al. (2020) |
| Neurogenesis |
Stefani et al. (2018) Zhang et al. (2021) |
|
| Synaptic pruning |
Paolicelli et al. (2011) Zhan et al. (2014) Basilico et al. (2019) Kim et al. (2017) Filipello et al. (2018) Wang et al. (2020) |
|
| Synaptogenesis |
Nguyen et al. (2020) Roumier et al. (2004) |
|
| Modulation of neuronal activity |
Eyo et al. (2014) Pagani et al. (2015) Basilico et al. (2019) Pfeiffer et al. (2016) Riazi et al. (2015) Wang et al. (2020) |
|
| Cortex | Neurogenesis |
Cunningham et al. (2013) Arnò et al. (2014) Shigemoto‐Mogami et al. (2014) |
| Phagocytosis of neuronal precursors | Cunningham et al. (2013) | |
| Neuronal survival | Ueno et al. (2013) | |
| Migration and position of neurons |
Squarzoni et al. (2014) Yu et al. (2022) |
|
| Synaptogenesis |
Miyamoto et al., 2016 Parkhurst et al. (2013) |
|
| Synaptic pruning |
Chu et al. (2010) Hoshiko et al. (2012) Yilmaz et al. (2021) |
|
| Cerebellum | Purkinje cell apoptosis | Marín‐Teva et al. (2004) |
| Surveillance of Purkinje cells | Stowell et al. (2018) | |
| Pruning of climbing fibers | Nakayama et al. (2018) | |
| Synaptic pruning | Kana et al. (2019) |
5.1. Thalamus
Although most of the developmental processes in which microglia participate are not yet described for the different nuclei of the thalamus, a specific subregion of the isothalamus, the lateral geniculate nucleus (LGN), was the first in which microglia‐mediated synaptic pruning was molecularly dissected and where microglia regulation of myelination was observed. Recently, a single‐cell transcriptomic study revealed that one of the highly upregulated genes in LGN microglia at P16 is the secreted enzyme autotaxin, which is involved in lipid metabolism and myelination (Kalish et al., 2018). The LGN is one of the best models to study synaptic pruning, since axons from the retinal ganglion cells (RGC) innervate non‐overlapping eye‐specific regions in the LGN. In rodents, the process of segregation occurs between P8 and P30 and is characterized by the loss of innervation of LGN neurons by RGC axons that are progressively eliminated until each LGN neuron receives inputs from only one or two RGCs. This pruning is activity‐dependent as tetrodotoxin (TTX) infusion in the eye drastically changes the elimination of connections in favor of stronger ones (Schafer et al., 2012). The process of synaptic pruning in the LGN was shown to be dependent on microglia (Schafer et al., 2012), astrocytes (Chung et al., 2013) and several proteins of the complement cascade, such as C1q (Stevens et al., 2007). C1q was found to co‐localize with synapses in vivo, while its expression was downregulated throughout development (Stevens et al., 2007). In C1q KO mice, the innervation of LGN neurons by RGCs was no longer region‐specific (Stevens et al., 2007). This impairment led to an increase in the number of multiple innervated cells as observed by patch‐clamp experiments in LGN neurons, where each cell presented numerous small indistinct inputs following injection of incremental current steps, instead of one or two large distinct inputs that are characteristic of a pruned circuit (Stevens et al., 2007). Interestingly, similar results were obtained for C3 KO mice, another mouse model in which the complement cascade is compromised (Stevens et al., 2007). Using the same LGN system and intraocular TTX injections, Schafer and co‐workers (Schafer et al., 2012) showed that RGC inputs are regulated by activity such that microglia preferentially engulf inputs from the “weakest” eye. Moreover, they also observed that engulfment was decreased in mice in which the CR3/C3 signaling was compromised (Schafer et al., 2012), again implicating the complement system and a phagocytosis signaling pathway in LGN synaptic pruning by microglia.
Other genes, such as Il33, have been shown to regulate neuronal development in the intrathalamic circuit between the ventrobasal nucleus and the reticular nucleus, where IL‐33 is highly expressed (Vainchtein et al., 2018). In young mice, stimulating the cortical afferent fibers passing in the internal capsule evoked enhanced excitatory activity in the absence of IL‐33, as well as elevated spontaneous activity, which derived, at least in part, from an increased number of excitatory synapses of ventrobasal nucleus neurons (Vainchtein et al., 2018). Interestingly, deleting Il33 specifically from astrocytes led to increased number of excitatory and inhibitory inputs in spinal cord motor neurons, which are the primary inputs of the sensorimotor circuit, because of a failure of microglia‐derived synaptic engulfment (Vainchtein et al., 2018). This study highlighted the role of the interplay between astrocytic IL‐33 and the IL1RL1 microglial receptor in fine‐tuning thalamocortical circuits, showing the importance and interconnection of glial cells for the maturation of thalamic circuits.
While the complement proteins C1q and C3 have been shown to tag synapses for elimination in physiological (Györffy et al., 2018) and pathological conditions, such as Alzheimer's Disease (Hong et al., 2016) or viral infections (Vasek et al., 2016), the opposite has been observed for CD47‐labeled synapses, which seem to be protected from pruning (Lehrman et al., 2018). CD47 and its receptor SIRPα have been classified in the immune system as a “don't eat me” signal, since their binding inhibits phagocytosis by macrophages (Okazawa et al., 2005). These two molecules have been shown to be enriched in the LGN during the peak of pruning and SIRPα is highly expressed in microglia (Lehrman et al., 2018). KO mice for either Cd47 or Sirpα presented increased engulfment of RGC presynaptic terminals by microglia (Lehrman et al., 2018), since microglia of Cd47 KO mice lost the ability to perform activity‐dependent engulfment of inactive synapses after TTX injection. Importantly, following Cd47 deletion, LGN adult neurons received fewer retinal inputs, as observed by decreased excitatory postsynaptic currents (EPSCs) recorded in response to increasing stimulation of the optic tract (Lehrman et al., 2018).
Lastly, progranulin (PGRN), has also been implicated in the pruning of synapses in the ventral thalamus. Deficiency in the Grn gene, which encodes PGRN, leads to an age‐dependent increase in the expression of microglial lysosomal and innate immune genes such as C1q which, in turn, was associated with frontotemporal dementia development (Lui et al., 2016). In fact, Grn KO mice showed an accumulation of C1q in both excitatory and inhibitory synapses of the ventral thalamus, although only inhibitory VGAT+ synapses from parvalbumin inhibitory neurons were preferentially pruned (Lui et al., 2016). Since these neurons are the source of inhibition between the ventral thalamus and the layer IV of the somatosensory cortex, it is not surprising that Grn KO mice display hyperexcitability in this circuit (Lui et al., 2016). Interestingly, several of the behavioral abnormalities these mice present, such as OCD‐like behaviors, could be reverted by eliminating C1q expression (Lui et al., 2016). In a more recent paper, the same authors showed death of neurons induced by the deletion of PGRN from microglia (Zhang et al., 2020). Both studies highlight the role of complement and lysosomal proteins in the mechanisms of microglia‐mediated toxicity in frontotemporal dementia and suggest that microglia may also be involved in age‐dependent regulation of synaptic connections and number of neurons.
5.2. Hippocampus
Several microglia‐dependent mechanisms of circuit remodeling were first described in the hippocampus. Microglia precursors arrive at the primitive hippocampal formation at E14 in rats, further arranging themselves along subregions and layers according to an ontogenic program that mimics the neuronal differentiation patterns (Dalmau et al., 1998). Microglia follows an outside‐to‐inside distribution, starting from the hippocampal fissure, first reaching the main layers of the Ammon's horn and then the dentate gyrus (DG). Microglia differentiation proceeds from birth to ⁓P18 (Dalmau et al., 1998), progressing from a round or pleomorphic shape with few filopodia, to an oval or slightly elongated shape, characteristic of primitive ramified microglia. At P12, mature homeostatic microglia are present with fully developed processes.
Primitive microglia often display round structures in the underdeveloped processes, which present a beaded shape, consistent with the presence of engulfed material in their interior (Kim et al., 2015). This morphology is more frequent in the hippocampus between P0 and P12, which overlaps ‐ temporarily and spatially ‐ with the elimination of excess cells and exuberant axonal projections. Eyo and co‐workers showed that the number of apoptotic cells in the hippocampus peaks around P4 concomitantly with an increase in microglia density and a peak in microglia motility (Eyo et al., 2016). These results suggest that microglia may mediate the engulfment of apoptotic neurons in this region. Wakselman and colleagues reported that neuronal death in the hippocampus can be triggered by the activation of CD11b and DAP12, which directly contribute to the production and release of superoxide ions, in a process that closely resembles the mechanism by which macrophages trigger the death of invading pathogens (Wakselman et al., 2008). Nevertheless, in some regions of the developing hippocampus, such as the fimbria, no apoptotic cells are found near DAP12+ and CD11b+ microglia, indicating that the expression of these proteins may not be sufficient to trigger neuronal death and suggesting that neurons committed to die need to present specific signals that trigger microglia‐mediated reactive oxygen species production and engulfment.
Another important contribution of microglia to the regulation of neuronal numbers in the hippocampus is its participation in neurogenesis. Microglial phagocytosis influences the adult neurogenic cascade through a mechanism dependent on the MERTK/AXL axis and P2RY12 (Diaz‐Aparicio et al., 2020). In this study, the authors described that microglia rapidly clear newborn cells that undergo apoptosis, and that impairments in this pathway directly disrupt neurogenesis (Diaz‐Aparicio et al., 2020). Stefani and colleagues reported that disrupting the microglial P2RY13 receptor led to a decrease in microglia complexity in the subgranular zone, coupled with an increase in progenitor cell proliferation and formation of new neurons (Stefani et al., 2018). Since P2RY13 activation is associated with the release of pro‐inflammatory cytokines, including TNF‐α, IL‐1β, and IL‐6, it is plausible to hypothesize that these cytokines may be crucial for the regulation of neurogenesis under basal conditions. In line with this, it was recently shown that IL‐4‐driven ARG1+ microglia are also essential for hippocampal adult neurogenesis, in a process reliant on microglial BDNF production (Zhang et al., 2021). These observations suggest that some microglial activation features, or at least inflammatory mediators that drive microglia function, might be important for hippocampal circuits.
An important aspect to consider when addressing brain development is that hormone‐induced sexual differentiation takes place during the perinatal period, leading to important sex differences in cell proliferation, dendritic spines, cell death, and microglia numbers and morphology. Nelson and colleagues showed that male mice have higher levels of microglia proliferation compared to females, while females presented an overall higher phagocytic activity (Nelson et al., 2017). This was illustrated through an increase in the number of phagocytic cups around P3, a higher expression of phagocytic genes Tyrobp, Cd68, and Trem2, and an increase in the engulfment of SOX2+ progenitor cells. Interestingly, the authors did not observe sex differences in the number of newly born or apoptotic cells targeted for elimination by microglia, only an increase in the number of progenitor cells that were eliminated. These results illustrate that microglia in the hippocampus are subjected to sex‐driven effects during the early neonatal period, and that fundamental biological differences may have important consequences for circuit wiring and hippocampal function.
In addition to microglial role in neurogenesis and neuronal apoptosis and clearance, the hippocampus was also one of the first regions where microglia were associated with developmental synaptic pruning. These cells were shown to contribute to the maturation of excitatory Schaffer collateral inputs into CA1 neurons, through a process that is dependent on the CX3CL1/CX3CR1 axis. In a landmark study, Paolicelli and colleagues found both postsynaptic and presynaptic contents inside microglia using super‐resolution and electron microscopy, suggesting that microglia actively engulf synaptic material during the second and third postnatal weeks (Paolicelli et al., 2011). In Cx3cr1 KO mice, the authors found that there was a transient reduction in microglia numbers in the hippocampus in this period, which correlated with an increased spine density which, together with defective hippocampal long‐term depression (LTD), incriminate microglia in hippocampal wiring (Paolicelli et al., 2011). In a follow‐up study, LTD defects and increase in synaptic inputs were shown to be transient in this animal model, although deficiency in synaptic multiplicity was maintained until adulthood, resulting in a decreased functional connectivity across different brain regions (Zhan et al., 2014). Importantly, Cx3cr1 KO mice displayed behavioral changes, including defects in social interaction and increased repetitive behaviors (Zhan et al., 2014). This same animal model displayed profound deficits in glutamatergic neurotransmission in the hippocampus, including alterations in the AMPA/NMDA ratio across the developmental period, resulting from defects in the AMPA component of EPSC, as well as alterations at the presynaptic level, including a decrease in the probability of glutamate release (Basilico et al., 2019). Consistently, these animals showed a higher number of failures following minimal stimulation, which suggests a higher number of silenced synapses (Basilico et al., 2019) that may help explain the persistent deficits in synaptic multiplicity previously observed by Zhan and co‐workers in adult animals (Zhan et al., 2014).
The involvement of microglia in synapse elimination in the hippocampus has also been associated with microglial autophagy. Kim and co‐workers reported that deletion of Atg7, a gene vital for autophagy in myeloid cells, resulted in an increase in dendritic spines and synaptic markers in the hippocampus, as well as altered connectivity between brain regions (Kim et al., 2017). These alterations led to behavioral changes including impaired sociability and increased repetitive behaviors. The authors suggested that this phenotype was because of impaired degradation of synapses through a process that is autophagy‐dependent. TREM2 was also shown to be necessary for microglia‐mediated synaptic pruning in the hippocampus, since Trem2 KO mice presented lower levels of microglia activation during the early stages of development. This resulted in impaired synapse elimination and enhanced excitatory neurotransmission, as observed by the increase in mEPSC frequency in CA1 hippocampal neurons (Filipello et al., 2018). These early‐life changes translated into reduced long‐range connectivity coupled with behavioral alterations similar to those of Cx3cr1 KO animals, including repetitive behaviors and altered sociability (Filipello et al., 2018). The authors also found that TREM2 protein levels were decreased in autism patients. Taken together, these studies support the hypothesis that disruptions in microglia activity, specifically related to synaptic pruning, could contribute to neurodevelopmental defects and behaviors associated with neuropsychiatric disorders.
Considering the important role of microglia in eliminating unnecessary synapses, it is plausible that they might also regulate synaptic formation and maturation. Recently, Nguyen and colleagues demonstrated that experience‐dependent release of IL‐33 by neurons was sufficient to drive spine formation and newborn neuron integration in a microglia‐dependent manner (Nguyen et al., 2020). Importantly, DAP12 was also found to be a crucial regulator of synapse maturation, since Dap12 mutant mice displayed enhanced long‐term potentiation (LTP), which was partly NMDA receptor (NMDAR)‐independent, and changes in synaptic glutamate receptor content (Roumier et al., 2004).
In addition to microglial role on synaptic maturation, these cells have also been associated with the modulation of neuronal activity, synaptic efficiency, and plasticity in the adult hippocampus. In a model of kainate‐induced seizures, global increase in glutamate levels triggered microglial process extension through activation of NMDAR and calcium influx in neurons, followed by ATP release and subsequent microglial response through P2RY12 (Eyo et al., 2014). More recent studies confirmed that hippocampal microglia recruitment and translocation is dependent on the activation of this receptor (Eyo et al., 2018; Madry et al., 2018). Functionally, deletion of P2RY12 had strong deleterious effects, decreasing the number of microglia processes in the vicinity of neurons and exacerbating seizure activity (Eyo et al., 2014). Microglial ability to extend processes in response to local levels of ATP is also dependent on CX3CR1, since Cx3cr1 KO mice display a reduction in K+ outward currents (Pagani et al., 2015), which are crucial to maintain microglial membrane potential and ramification trough THIK‐1 (Madry et al., 2018). Importantly, these mice show a decrease in microglial ATP‐induced mobility and branching, together with a variety of neuronal defects such as an immature AMPA/NMDA synaptic ratio and reduced presynaptic functionality (Basilico et al., 2019), thought to result from the transient microglial dysfunction observed during the critical postnatal period (Paolicelli et al., 2011).
Neuronal activity of hippocampal neurons also influences microglial phenotypes. Pfeiffer and co‐workers employed time‐lapse two‐photon imaging and electrophysiological recordings in acute brain slices to characterize the morphological dynamics of microglia before, and after, the induction of LTP. Their results clearly show that, during hippocampal LTP, microglia increase their process numbers while prolonging contact with dendritic spines, through a process that is dependent on the activation of NMDAR (Pfeiffer et al., 2016). In another study, Riazi and colleagues observed, using a model of peripheral inflammation, the presence of a microglia‐mediated inflammatory response in the hippocampus that resulted in significant synaptic changes (Riazi et al., 2015). These authors reported significant postsynaptic effects, including enhanced excitatory field potentials in the CA1 stratum radiatum, enhanced AMPA‐ and NMDA‐mediated synaptic currents and reduced LTP and LTD. These changes in synaptic plasticity were reverted upon chronic administration of minocycline (Riazi et al., 2015), demonstrating the possible impact of pushing microglia activation status on synapse dynamics and local connectivity. To reinforce the importance of microglia on hippocampal synapses, a recent paper demonstrated that these cells are an essential component for the forgetting of memories (Wang et al., 2020). First, these authors showed that microglia engulf synapses in the healthy adult hippocampus, and that reducing the levels of this process by administering minocycline after contextual fear conditioning, led to preserved freezing behavior, indicating that mice were not forgetting, in a process shown to be linked to the complement system. Second, using a combination of transgenic mouse models and viral injections, they were able to induce expression of CD55 on memory engram cells, which was sufficient to lead to the previously observed forgetting of memories, this effect also being mimicked by inhibiting engram cell activity through the usage of DREADs. Overall, these results demonstrate that the process of forgetting requires a delicate interplay between neuronal activity, the complement system, and microglia engulfment of synapses (Wang et al., 2020).
5.3. Cortex
In mice, microglia colonization of the developing cortex starts at E11.5, near the pial surface and lateral ventricles, from which they slowly distribute along the cortex. Around E15.5, both amoeboid and ramified microglia cells can be found in the parenchyma (Swinnen et al., 2013). Microglia colonization is strongly correlated with the positioning and migration of neuronal progenitors in the cortex of several different animal species. Cunningham and colleagues demonstrated that, in the developing human, macaque, and rodent brains, microglia selectively colonize neuroproliferative zones, with relatively few microglia cells present in the cortical plate during neurogenic periods (Cunningham et al., 2013). Cortical neurogenesis dynamically modulates microglia proliferation, as Emx2 KO mice, which display significant deficits in neurogenesis because of impairments of radial glial self‐renewal, present reduced microglia numbers (Arnò et al., 2014). In contrast, an increase in basal progenitor cells, triggered by Notch inhibition, increased microglia numbers (Arnò et al., 2014). At the same time, microglia are also essential for supporting neurogenesis in this brain region, as Csf1r KO mice display reduced numbers of basal progenitors into the cerebral cortex (Arnò et al., 2014). Moreover, microglia were shown to phagocyte viable TBR2+ and PAX6+ neuronal precursors in the SVZ of non‐human primates, in a process that regulates neuron production in the prenatal brain by regulating the size of the precursors’ cell pool (Cunningham et al., 2013). This process was also dependent on characteristics of microglia activation, since LPS and minocycline treatments led to a significant decrease and increase in the number of neuronal progenitors, respectively (Cunningham et al., 2013).
In addition to controlling neurogenesis in the cortex, microglia also play a role in the correct formation of cortical circuitry by promoting both the survival, migration, and positioning of several subtypes of excitatory and inhibitory neurons at different developmental time points. While layer V cortical neurons require microglial support for survival during postnatal development, in a process dependent of the fractalkine CX3CL1/CX3CR1 interaction and the microglia‐derived IGF‐1 (Ueno et al., 2013), microglia also regulate the migration and the laminar position of cortical interneurons during the prenatal stage (Squarzoni et al., 2014). In embryos that lack microglia or that were exposed to MIA, a specific subtype of interneurons expressing LHX‐6 were found to prematurely enter the cortical plate and to present an abnormal distribution (Squarzoni et al., 2014). These abnormalities were maintained until the end of the migration period and the alteration in distribution affected fast‐spiking interneurons that were increased in layers III/IV. This ultimately led to severe alterations in excitatory/inhibitory balance (Squarzoni et al., 2014). Importantly, a recent study by Yu and colleagues showed that deleting microglial Gpr56 mimics MIA‐induced parvalbumin neuron reduction in the neocortex, but not in the hippocampus or striatum (Yu et al., 2022). The decrease in this type of inhibitory interneurons again suggests that microglia dysfunction, resultant from inflammation during development, may greatly impact the inhibitory tonus essential for the functional rhythms and activity of neural circuits in the cortex. Considering that both MIA (Choi et al., 2016) and disrupting interneuron number/function can cause ASD (Lauber et al., 2018), it may be speculated that microglia could play a central role in the pathogenesis of this condition through similar mechanisms.
The contribution of microglia to the formation of new synapses has been demonstrated in the somatosensory cortex, using in vivo two‐photon microscopy to observe microglia‐dendrite contacts. This interaction results in the formation of new stable dendritic filopodia in pyramidal neurons between P8 and P10 ‐ a period of intense synaptogenesis ‐ (Miyamoto et al., 2016). Interestingly, this process was shown to be dependent on characteristics of microglia activation since treatment with minocycline significantly reduced the filopodia formation rate. Furthermore, genetic ablation of microglia led to a decrease in spine density and in the frequency of mEPSC in L2/L3 cortical neurons, ultimately resulting in altered excitatory synaptic connectivity from L4 to L2/3 (Miyamoto et al., 2016). The timing of microglia ablation may justify these defects in synaptogenesis and not synaptic pruning (which is thought to occur after). Later in development, microglia were also shown to be essential for learning‐dependent synapse formation as the same model shows significant decrease in motor‐learning‐induced formation of dendritic spines. Interestingly, similar results were achieved by inhibiting microglia‐mediated secretion of BDNF, which indicates that microglial BDNF may play an important role regulating the formation of new synapses associated with learning (Parkhurst et al., 2013).
Conversely, microglia are also important for synapse elimination. Several studies have reported that alterations in microglia activity during the critical period of synaptic pruning led to several types of abnormalities in synapse numbers and synaptic connectivity both within the cortex microcircuitry and in the cortical connections with other brain regions. Chu and colleagues showed that mice deficient in C1q displayed defects in neocortical synaptic elimination, ultimately resulting in enhanced excitatory synaptic connectivity on layer V pyramidal neurons, as seen by an increase in both spontaneous EPSCs and mEPSCs (Chu et al., 2010). Interestingly, these mice did not present alterations in inhibitory connectivity, indicating that C1q is important for microglia‐mediated elimination of excitatory but not inhibitory synapses in this region. The CX3CL1/CX3CR1 signaling pathway also regulates the refinement of cortical circuits, particularly in the thalamocortical pathway. A study from Hoshiko and colleagues demonstrated that deletion of CX3CR1 during development of the barrel field in the mouse somatosensory cortex impairs microglia entry in this region, leading to an impaired functional maturation of thalamocortical synapses (Hoshiko et al., 2012). CX3CR1 deletion did not affect the probability of glutamate release, nor AMPA‐mediated evoked postsynaptic currents. However, there was a change in AMPA/NMDA ratio because of an impairment in the normal developmental switch of NMDAR subunits from GluN2B to GluN2A (Hoshiko et al., 2012).
Alterations in microglia‐mediated synaptic remodeling in the cortex have been linked to schizophrenia. Yilmaz and colleagues used a mouse model overexpressing human C4A to show that this complement protein can bind more efficiently to cortical synapses, when compared with its C4B counterpart. This binding led to increased microglial engulfment and decreased cortical synapse density, as well as deficits in social behavior, spatial working memory, and anxiety‐like behaviors (Yilmaz et al., 2021). Although the authors observed no alterations in cortical synapse density in mice lacking C4, this study elucidates possible disease mechanisms through alterations in microglia‐mediated synaptic remodeling.
5.4. Cerebellum
The cerebellar circuit undergoes critical development in the early postnatal period and recent studies have shown that microglia are essential in this process. Namely, within the cerebellar cortex, Purkinje cells (PCs) experience axon collateral sprouting followed by pruning, maturation (Gianola et al., 2003), and exuberant dendritic growth until P30 (Riazi et al., 2015). Still, during the second postnatal week, the external granular layer disappears as the precursors of granule cells migrate inwards to the granular layer, while leaving behind their T‐shaped axons, the parallel fibers. Importantly, the pruning of climbing fibers also occurs in this same time‐window until each PC is enervated by a single climbing fiber.
Concomitantly with the development of the cerebellar circuit, microglia also undergo maturation while populating the cerebellum. An early study showed that cerebellar microglia surround the developing cerebellum at E11, starting to infiltrate the WM around birth (Ashwell, 1990). Recently, it was shown that microglia start entering the WM at P0, probably through intracerebellar blood vessels and meninges (Groteklaes et al., 2020). At P4 they are mostly restricted to the WM, with very few cells in the GL (Groteklaes et al., 2020), followed by progressive outward migration between P5 and P21, concentrating around the PC layer at P8‐P9, and only adopting their prototypical evenly spread network by P60 (Nakayama et al., 2018). Microglia were also suggested to be actively involved in phagocytosis during cerebellar development and to undergo progressive morphological changes, transitioning from amoeboid and stout morphologies in the first postnatal week to a mature form with thin processes after the third week onwards (Perez‐Pouchoulen et al., 2015).
Following the developmental period, cerebellar microglia establish themselves as a unique population with respect to microglia from other brain circuits. Compared to cortical microglia, cerebellar microglia display lower arborization complexity, in some cases looking almost bipolar (Stowell et al., 2018). Additionally, Tay and colleagues observed a lower density of microglia in the cerebellum compared to the cortex and hippocampus (Tay et al., 2017). This study also showed the cerebellum to have a higher rate of microglial turnover than several other brain regions under basal conditions (Tay et al., 2017). This turnover rate of microglia is comparable to that of neurons in the cerebellum (Ayata et al., 2018), suggesting a functional connection between the different cell types in this specific brain region, such that eliminating microglia from the brain results in a significant increase in apoptotic cells in the cerebellum, but not the striatum (Ayata et al., 2018). In fact, cerebellar microglia appear to express a large number of genes associated with energy production when compared with other brain regions (Grabert et al., 2016), indicative of a high metabolic rate. Despite their reduced complexity and lower numbers in the cerebellum, their cell somas are uncommonly mobile, being able to achieve fast displacements that compensate for a lower coverage area (Stowell et al., 2018).
Morphology and density are only part of the distinctive factors between cerebellar microglia and that of other brain regions. Transcriptomic data revealed that microglia in cerebellum are significantly distinct when compared with those in the cortex, hippocampus, and striatum (Grabert et al., 2016). Among several clusters of genes, “immune response” and “energy metabolism” sets were found upregulated in cerebellar microglia (Grabert et al., 2016). A later study employed translating ribosome affinity purification coupled to bulk RNA‐seq to identify several cerebellar microglia‐enriched genes in comparison with striatal microglia, including targets related to apoptotic cell detection, engulfment and catabolism (Ayata et al., 2018). Importantly, in basal conditions, cerebellar microglia were shown to display a higher expression of genes commonly associated with microglia challenge via LPS or IL‐4, as well as of genes related to receptors with tyrosine‐based activation motifs, known to regulate the strength of immune responses (Grabert et al., 2016). Moreover, an increase in cerebellar microglia CD68+ lysosome content and a magnified efficiency in the engulfment of apoptotic cells have also been observed (Ayata et al., 2018). Together these studies suggest that cerebellar microglia are characterized by: (1) diminished ramification and reduced number, but increase motility during surveillance; (2) increased basal expression of activation‐related genes and decreased expression of immunomodulatory molecules; (3) enhanced engulfment of neuronal material and clearance of debris.
Although recent transcriptomic studies found more limited microglia regional‐specific differences in adulthood, the cerebellum was still highlighted as an outlier. Li and colleagues observed a cluster of Spp1 + Gpnmb + cells at P7 present almost exclusively in the corpus callosum and cerebellar WM that participate in oligodendrocyte and possibly astrocyte phagocytosis, presenting a gene expression profile similar to DAM (Li et al., 2019). This specific population of microglia was also identified in a separate study, where it was shown that the transcriptomic profile of P7‐P10 microglia from the cerebellar WM is significantly different from cortical microglia, being enriched for genes related to phagocytosis and migration, while P10 to P42 microglia present upregulation of apoptosis‐ and necrosis‐related genes (Staszewski & Hagemeyer, 2019). These authors also found enhanced expression of priming genes related to aging and disease such as Axl, Mrc1, Clec7a, and Spp1 (Staszewski & Hagemeyer, 2019). Hammond and colleagues found a cerebellum and corpus callosum‐specific microglia population only present around P4/P5 in axonal tracks that were Spp1 + and rich in CD68, and a phenotype suggestive of phagocytic activity (Hammond et al., 2019).
The above‐mentioned phenotypes of cerebellar microglia may be influenced by both intrinsic factors and their environmental milieu. On the one hand, cerebellar microglia epigenetic profile is important for the maintenance of their phenotype (Ayata et al., 2018), as evidenced by the interplay between the suppressive chromatin modification H3K27me3 and lysine‐specific demethylase A and B (KDM6A/B). On the other hand, one environmental factor influencing cerebellar microglia phenotype may be the unique high levels of expression of M‐CSF and low levels of IL‐34 in the cerebellum, both during development and in adulthood (Wei et al., 2010). A more recent study further demonstrated that M‐CSF stimulation of forebrain microglia induced a gene expression profile similar to microglia of the cerebellum, with IL‐34 having opposite effects (Kana et al., 2019). These observations point to a regional epigenetic regulation of the microglial phenotype, which may be modulated by both the exposure to high amounts of apoptotic cells and the signaling provided by factors almost exclusive to the cerebellum, such as M‐CSF.
Although it is not clear if these differences in cerebellar microglia are required for specifications in the maturation process of the cerebellar cortex, like in other brain regions, cerebellar microglia participate in induction of apoptosis and pruning. Microglia are closely linked to PC development, as they are able to promote the apoptosis of supernumerary PCs (Marín‐Teva et al., 2004), then continue to thoroughly survey them in adulthood (Stowell et al., 2018). Additionally, the complement system was also shown to influence the pruning of climbing fibers. This pruning mechanism is dependent on C1ql1 and the G‐protein‐coupled receptor B3 (BAI3) (Hashimoto et al., 2009). Besides PCs, BAI3 expression is also present in adult cerebellar microglia (Ayata et al., 2018), suggesting that C1ql1 may be signaling its effect through both cell types. Interestingly, Nakayama and colleagues have shown that microglia are indeed necessary for correct pruning of climbing fibers (Nakayama et al., 2018). By depleting microglia through liposomal clodronate infusion in the cerebellum and using a KO of Csf1r, these authors showed that climbing fiber pruning was impaired (Nakayama et al., 2018), confirming microglial involvement in the process of climbing fiber maturation. Recently, Kana and colleagues also demonstrated that CSF‐1 depletion originated morphological and functional alterations in PCs, as well as motor learning and sociability defects (Kana et al., 2019). Because these authors found an increase in mEPSC in Csf1r KO mice, these results may suggest that cerebellar microglia prune synapses of PCs.
Microglia have also been shown to regulate neuronal activity in the adult cerebellum. LPS injection can induce changes in PC plasticity and activity in a microglia‐dependent manner, originating in different behavioral deficits such as depressive‐like or autistic‐like behaviors (Yamamoto et al., 2019). Interestingly, a post‐mortem study of brains of autism patients showed marked activation of microglia and astrocytes, with the cerebellum being the most affected region (Vargas et al., 2005), while positron emission tomography of autism patients has revealed high levels of microglia activation especially in the cerebellum (Suzuki et al., 2013). As the cerebellum is recognized to be highly involved in ASD‐related behaviors (Fatemi et al., 2012), it is becoming increasingly important to understand the role of microglia and their different phenotypes in cerebellar development and homeostasis in connection to neuropsychiatric disorders. Interestingly, poly I:C MIA has also been shown to induce dynamic changes in the cerebellar cytokine milieu, coupled with a reduction in the levels of synaptic proteins in PCs, such as cerebellin 1 and GluRδ2, as well as reduction in the density of glutamatergic synapses (Pendyala et al., 2017). These alterations were accompanied by unusual behavioral phenotypes in terms of social interaction, repetitive behaviors, and ultrasonic vocalizations (Pendyala et al., 2017). In a separate study, poly I:C MIA led to increases in PC numbers and deficits in motor and social behaviors in adolescent mice (Aavani et al., 2015).
6. CONCLUSION
Microglia are cells with immune ontogeny and function that, in the last decade, have been consistently linked with the formation, maturation and maintenance of neuronal circuits. Our knowledge on the importance of these cells first derived from their role as the macrophages of the brain, regulated by a multitude of signaling pathways originally described under the framework of fighting infection. However, it is now widely accepted that their nature goes beyond this function and extends to regulation of circuit wiring. In this review, we described microglia‐mediated processes essential for the correct wiring of several brain regions and proposed that different neuronal circuits might create unique microenvironments that influence microglia function. Although we are beginning to uncover the differential needs for each circuit‐specific architecture, microglia are emerging as the sculptors of those needs. This suggests that the microenvironment of neuronal circuits and brain regions may influence microglia activity which, in turn, act to support the proper function of those neuronal connections. We believe that dissecting these mechanisms is of crucial importance to understand not only how microglia supports and shapes healthy brain development, but also to understand the role they may play in neurodevelopmental and neuropsychiatric disorders. Considering microglia as interpreters of environmental cues, defective microglia may present a huge impact in the function of neuronal circuits and, consequently, in abnormal behaviors and cognitive processes associated with brain diseases, from neurodevelopmental to neurodegenerative conditions. Therefore, defining the time‐ and region‐specific rules regarding microglia interactions within specific neuronal circuits may be critical for a fundamental understanding of the brain.
AUTHOR CONTRIBUTION
All authors contributed to the writing and editing of the manuscript.
CONFLICT OF INTERESTS
The authors have no conflicts of interest to declare that are relevant to the content of this article.
ACKNOWLEDGMENTS
This work was financed by the European Regional Development Fund (ERDF), through the Centro 2020 Regional Operational Programme, under project CENTRO‐01‐0145‐FEDER‐000008 (BrainHealth 2020), the COMPETE 2020—Operational Programme for Competitiveness and Internationalization and Portuguese national funds via FCT—Fundação para a Ciência e Tecnologia, under projects POCI‐01‐0145‐FEDER‐007440, UIBD/04539/2020, PTDC/NEU‐SCC/3247/2014 and PTDC/MED‐NEU/5993/2020. This research was supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (#20733), Bial Foundation Grant (266/2016 and 264/2016), 2019 Pfizer Prize in Basic Sciences and a 2020 IBRO Early Career Award. The authors of this work were also funded by “Programa Operacional Potencial Humano” (POPH) through the fellowships SFRH/BPD/120611/2016 (to JRG), SFRH/BD/144224/2019 (to PAF), SFRH/BD/144875/2019 (to JMC).
Guedes, J. R. , Ferreira, P. A. , Costa, J. M. , Cardoso, A. L. , & Peça, J. (2022). Microglia‐dependent remodeling of neuronal circuits. Journal of Neurochemistry, 163, 74–93. 10.1111/jnc.15689
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Aavani, T. , Rana, S. A. , Hawkes, R. , & Pittman, Q. J. (2015). Maternal immune activation produces cerebellar hyperplasia and alterations in motor and social behaviors in male and female mice. Cerebellum, 14, 491–505. [DOI] [PubMed] [Google Scholar]
- Akiyoshi R., Wake H., Kato D., Horiuchi H., Ono R., Ikegami A., Haruwaka K., Omori T., Tachibana Y., Moorhouse A. J., Nabekura J. (2018) Microglia enhance synapse activity to promote local network synchronization. eNeuro 5, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnò, B. , Grassivaro, F. , Rossi, C. , Bergamaschi, A. , Castiglioni, V. , Furlan, R. , Greter, M. , Favaro, R. , Comi, G. , Becher, B. , Martino, G. , & Muzio, L. (2014). Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nature Communications, 5, 5611. [DOI] [PubMed] [Google Scholar]
- Ashwell, K. (1990). Microglia and cell death in the developing mouse cerebellum. Brain Research Developmental Brain Research, 55, 219–230. [DOI] [PubMed] [Google Scholar]
- Askew, K. , Li, K. , Olmos‐Alonso, A. , Garcia‐Moreno, F. , Liang, Y. , Richardson, P. , Tipton, T. , Chapman, M. A. , Riecken, K. , Beccari, S. , Sierra, A. , Molnár, Z. , Cragg, M. S. , Garaschuk, O. , Perry, V. H. , & Gomez‐Nicola, D. (2017). Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Reports, 18, 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayata, P. , Badimon, A. , Strasburger, H. J. , Duff, M. K. , Montgomery, S. E. , Loh, Y.‐H. E. , Ebert, A. , Pimenova, A. A. , Ramirez, B. R. , Chan, A. T. , Sullivan, J. M. , Purushothaman, I. , Scarpa, J. R. , Goate, A. M. , Busslinger, M. , Shen, L. , Losic, B. , & Schaefer, A. (2018). Epigenetic regulation of brain region‐specific microglia clearance activity. Nature Neuroscience, 21, 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badimon, A. , Strasburger, H. J. , Ayata, P. , Chen, X. , Nair, A. , Ikegami, A. , Hwang, P. , Chan, A. T. , Graves, S. M. , Uweru, J. O. , Ledderose, C. , Kutlu, M. G. , Wheeler, M. A. , Kahan, A. , Ishikawa, M. , Wang, Y. C. , Loh, Y. H. E. , Jiang, J. X. , Surmeier, D. J. , … Schaefer, A. (2020). Negative feedback control of neuronal activity by microglia. Nature, 586, 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico, B. , Pagani, F. , Grimaldi, A. , Cortese, B. , Di Angelantonio, S. , Weinhard, L. , Gross, C. , Limatola, C. , Maggi, L. , & Ragozzino, D. (2019). Microglia shape presynaptic properties at developing glutamatergic synapses. Glia, 67, 53–67. [DOI] [PubMed] [Google Scholar]
- Bennett, F. C. , Bennett, M. L. , Yaqoob, F. , Mulinyawe, S. B. , Grant, G. A. , Hayden, G. M. , Plowey, E. D. , & Barres, B. A. (2018). A combination of ontogeny and CNS environment establishes microglial identity. Neuron, 98, 1170–1183.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruttger, J. , Karram, K. , Wörtge, S. , Regen, T. , Marini, F. , Hoppmann, N. , Klein, M. , Blank, T. , Yona, S. , Wolf, Y. , Mack, M. , Pinteaux, E. , Müller, W. , Zipp, F. , Binder, H. , Bopp, T. , Prinz, M. , Jung, S. , & Waisman, A. (2015). Genetic cell ablation reveals clusters of local self‐renewing microglia in the mammalian central nervous system. Immunity, 43, 92–106. [DOI] [PubMed] [Google Scholar]
- Butovsky, O. , Jedrychowski, M. P. , Moore, C. S. , Cialic, R. , Lanser, A. J. , Gabriely, G. , Koeglsperger, T. , Dake, B. , Wu, P. M. , Doykan, C. E. , Fanek, Z. , Liu, L. P. , Chen, Z. , Rothstein, J. D. , Ransohoff, R. M. , Gygi, S. P. , Antel, J. P. , & Weiner, H. L. (2014). Identification of a unique TGF‐β‐dependent molecular and functional signature in microglia. Nature Neuroscience, 17, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano, L. , Pinheiro, H. , Patrício, P. , Mateus‐Pinheiro, A. , Alves, N. D. , Coimbra, B. , Baptista, F. I. , Henriques, S. N. , Cunha, C. , Santos, A. R. , Ferreira, S. G. , Sardinha, V. M. , Oliveira, J. F. , Ambrósio, A. F. , Sousa, N. , Cunha, R. A. , Rodrigues, A. J. , Pinto, L. , & Gomes, C. A. (2017). Adenosine A2A receptor regulation of microglia morphological remodeling‐gender bias in physiology and in a model of chronic anxiety. Molecular Psychiatry, 22, 1035–1043. [DOI] [PubMed] [Google Scholar]
- Casano, A. M. , Albert, M. , & Peri, F. (2016). Developmental apoptosis mediates entry and positioning of microglia in the zebrafish brain. Cell Reports, 16, 897–906. [DOI] [PubMed] [Google Scholar]
- Cheadle, L. , Rivera, S. A. , Phelps, J. S. , Ennis, K. A. , Stevens, B. , Burkly, L. C. , Lee, W.‐C. A. , & Greenberg, M. E. (2020). Sensory experience engages microglia to shape neural connectivity through a non‐phagocytic mechanism. Neuron, 108, 451–468.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. , Koga, K. , Li, X.‐Y. , & Zhuo, M. (2010). Spinal microglial motility is independent of neuronal activity and plasticity in adult mice. Molecular Pain, 6, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, G. B. , Yim, Y. S. , Wong, H. , Kim, S. , Kim, H. , Kim, S. V. , Hoeffer, C. A. , Littman, D. R. , & Huh, J. R. (2016). The maternal interleukin‐17a pathway in mice promotes autism‐like phenotypes in offspring. Science, 351, 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, Y. , Jin, X. , Parada, I. , Pesic, A. , Stevens, B. , Barres, B. , & Prince, D. A. (2010). Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proceedings of the National Academy of Sciences of the United States of America, 107, 7975–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, W.‐S. , Clarke, L. E. , Wang, G. X. , Stafford, B. K. , Sher, A. , Chakraborty, C. , Joung, J. , Foo, L. C. , Thompson, A. , Chen, C. , Smith, S. J. , & Barres, B. A. (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature, 504, 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk, J. C. , Filiano, A. J. , Louveau, A. , Marin, I. , Marsh, R. , Ji, E. , Goldman, D. H. , Smirnov, I. , Geraci, N. , Acton, S. , Overall, C. C. , & Kipnis, J. (2018). Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. The Journal of Experimental Medicine, 215, 1627–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, C. L. , Martínez‐Cerdeño, V. , & Noctor, S. C. (2013). Microglia regulate the number of neural precursor cells in the developing cerebral cortex. The Journal of Neuroscience, 33, 4216–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, X.‐M. , Ryan, G. R. , Hapel, A. J. , Dominguez, M. G. , Russell, R. G. , Kapp, S. , Sylvestre, V. , & Stanley, E. R. (2002). Targeted disruption of the mouse colony‐stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood, 99, 111–120. [DOI] [PubMed] [Google Scholar]
- Dalmau, I. , Finsen, B. , Zimmer, J. , González, B. , & Castellano, B. (1998). Development of microglia in the postnatal rat hippocampus. Hippocampus, 8, 458–474. [DOI] [PubMed] [Google Scholar]
- Damisah, E. C. , Hill, R. A. , Rai, A. , Chen, F. , Rothlin, C. V. , Ghosh, S. , & Grutzendler, J. (2020). Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Science Advances, 6, eaba3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos, D. , Grutzendler, J. , Yang, G. , Kim, J. V. , Zuo, Y. , Jung, S. , Littman, D. R. , Dustin, M. L. , & Gan, W. B. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nature Neuroscience, 8, 752–758. [DOI] [PubMed] [Google Scholar]
- Diaz‐Aparicio, I. , Paris, I. , Sierra‐Torre, V. , Plaza‐Zabala, A. , Rodríguez‐Iglesias, N. , Márquez‐Ropero, M. , Beccari, S. , Huguet, P. , Abiega, O. , Alberdi, E. , Matute, C. , Bernales, I. , Schulz, A. , Otrokocsi, L. , Sperlagh, B. , Happonen, K. E. , Lemke, G. , Maletic‐Savatic, M. , Valero, J. , & Sierra, A. (2020). Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. The Journal of Neuroscience, 40, 1453–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore, M. R. P. , Najafi, A. R. , Koike, M. A. , Dagher, N. N. , Spangenberg, E. E. , Rice, R. A. , Kitazawa, M. , Matusow, B. , Nguyen, H. , West, B. L. , & Green, K. N. (2014). Colony‐stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron, 82, 380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo, U. B. , Miner, S. A. , Weiner, J. A. , & Dailey, M. E. (2016). Developmental Changes in Microglial Mobilization are Independent of Apoptosis in the Neonatal Mouse Hippocampus. Brain, Behavior, and Immunity, 55, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo, U. B. , Mo, M. , Yi, M.‐H. , Murugan, M. , Liu, J. , Yarlagadda, R. , Margolis, D. J. , Xu, P. , & Wu, L. J. (2018). P2Y12R‐Dependent translocation mechanisms gate the changing microglial landscape. Cell Reports, 23, 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo, U. B. , Peng, J. , Swiatkowski, P. , Mukherjee, A. , Bispo, A. , & Wu, L. J. (2014). Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. The Journal of Neuroscience, 34, 10528–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi, S. H. , Aldinger, K. A. , Ashwood, P. , Bauman, M. L. , Blaha, C. D. , Blatt, G. J. , Chauhan, A. , Chauhan, V. , Dager, S. R. , Dickson, P. E. , Estes, A. M. , Goldowitz, D. , Heck, D. H. , Kemper, T. L. , King, B. H. , Martin, L. A. , Millen, K. J. , Mittleman, G. , Mosconi, M. W. , … Welsh, J. P. (2012). Consensus paper: pathological role of the cerebellum in autism. Cerebellum, 11, 777–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández de Cossío, L. , Guzmán, A. , van der Veldt, S. , & Luheshi, G. N. (2017). Prenatal infection leads to ASD‐like behavior and altered synaptic pruning in the mouse offspring. Brain, Behavior, and Immunity, 63, 88–98. [DOI] [PubMed] [Google Scholar]
- Filipello, F. , Morini, R. , Corradini, I. , Zerbi, V. , Canzi, A. , Michalski, B. , Erreni, M. , Markicevic, M. , Starvaggi‐Cucuzza, C. , Otero, K. , Piccio, L. , Cignarella, F. , Perrucci, F. , Tamborini, M. , Genua, M. , Rajendran, L. , Menna, E. , Vetrano, S. , Fahnestock, M. , … Matteoli, M. (2018). The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity, 48, 979–991.e8. [DOI] [PubMed] [Google Scholar]
- Fontainhas, A. M. , Wang, M. , Liang, K. J. , Chen, S. , Mettu, P. , Damani, M. , Fariss, R. N. , Li, W. , & Wong, W. T. (2011). Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One, 6, e15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud, L. , Través, P. G. , Tufail, Y. , Leal‐Bailey, H. , Lew, E. D. , Burrola, P. G. , Callaway, P. , Zagórska, A. , Rothlin, C. V. , Nimmerjahn, A. , & Lemke, G. (2016). TAM receptors regulate multiple features of microglial physiology. Nature, 532, 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianola, S. , Savio, T. , Schwab, M. E. , & Rossi, F. (2003). Cell‐autonomous mechanisms and myelin‐associated factors contribute to the development of Purkinje axon intracortical plexus in the rat cerebellum. The Journal of Neuroscience, 23, 4613–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giera, S. , Luo, R. , Ying, Y. , Ackerman, S. D. , Jeong, S.‐J. , Stoveken, H. M. , Folts, C. J. , Welsh, C. A. , Tall, G. G. , Stevens, B. , Monk, K. R. , & Piao, X. (2018). Microglial transglutaminase‐2 drives myelination and myelin repair via GPR56/ADGRG1 in oligodendrocyte precursor cells. eLife, 7, e33385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux, F. , Greter, M. , Leboeuf, M. , Nandi, S. , See, P. , Gokhan, S. , Mehler, M. F. , Conway, S. J. , Ng, L. G. , Stanley, E. R. , Samokhvalov, I. M. , & Merad, M. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science, 330, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin, D. , Link, V. M. , Romanoski, C. E. , Fonseca, G. J. , Eichenfield, D. Z. , Spann, N. J. , Stender, J. D. , Chun, H. B. , Garner, H. , Geissmann, F. , & Glass, C. K. (2014). Environment drives selection and function of enhancers controlling tissue‐specific macrophage identities. Cell, 159, 1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabert, K. , Michoel, T. , Karavolos, M. H. , Clohisey, S. , Baillie, J. K. , Stevens, M. P. , Freeman, T. C. , Summers, K. M. , & McColl, B. W. (2016). Microglial brain region‐dependent diversity and selective regional sensitivities to aging. Nature Neuroscience, 19, 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groteklaes, A. , Bönisch, C. , Eiberger, B. , Christ, A. , & Schilling, K. (2020). Developmental maturation of the cerebellar white matter‐an instructive environment for cerebellar inhibitory interneurons. Cerebellum, 19, 286–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunner, G. , Cheadle, L. , Johnson, K. M. , Ayata, P. , Badimon, A. , Mondo, E. , Nagy, M. A. , Liu, L. , Bemiller, S. M. , Kim, K. W. , Lira, S. A. , Lamb, B. T. , Tapper, A. R. , Ransohoff, R. M. , Greenberg, M. E. , Schaefer, A. , & Schafer, D. P. (2019). Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nature Neuroscience, 22, 1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györffy, B. A. , Kun, J. , Török, G. , Bulyáki, É. , Borhegyi, Z. , Gulyássy, P. , Kis, V. , Szocsics, P. , Micsonai, A. , Matkó, J. , Drahos, L. , Juhász, G. , Kékesi, K. A. , & Kardos, J. (2018). Local apoptotic‐like mechanisms underlie complement‐mediated synaptic pruning. Proceedings of the National Academy of Sciences of the United States of America, 115, 6303–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, T. R. , Dufort, C. , Dissing‐Olesen, L. , Giera, S. , Young, A. , Wysoker, A. , Walker, A. J. , Gergits, F. , Segel, M. , Nemesh, J. , Marsh, S. E. , Saunders, A. , Macosko, E. , Ginhoux, F. , Chen, J. , Franklin, R. J. M. , Piao, X. , McCarroll, S. A. , & Stevens, B. (2019). Single‐cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell‐state changes. Immunity, 50, 253–271.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, K. , Ichikawa, R. , Kitamura, K. , Watanabe, M. , & Kano, M. (2009). Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron, 63, 106–118. [DOI] [PubMed] [Google Scholar]
- Hickman, S. E. , Kingery, N. D. , Ohsumi, T. K. , Borowsky, M. L. , Wang, L. , Means, T. K. , & El Khoury, J. (2013). The microglial sensome revealed by direct RNA sequencing. Nature Neuroscience, 16, 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, S. , Beja‐Glasser, V. F. , Nfonoyim, B. M. , Frouin, A. , Li, S. , Ramakrishnan, S. , Merry, K. M. , Shi, Q. , Rosenthal, A. , Barres, B. A. , Lemere, C. A. , Selkoe, D. J. , & Stevens, B. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science, 352, 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiko, M. , Arnoux, I. , Avignone, E. , Yamamoto, N. , & Audinat, E. (2012). Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. The Journal of Neuroscience, 32, 15106–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Xu, Z. , Xiong, S. , Sun, F. , Qin, G. , Hu, G. , Wang, J. , Zhao, L. , Liang, Y. X. , Wu, T. , Lu, Z. , Humayun, M. S. , So, K. F. , Pan, Y. , Li, N. , Yuan, T. F. , Rao, Y. , & Peng, B. (2018). Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nature Neuroscience, 21, 530–540. [DOI] [PubMed] [Google Scholar]
- Hughes, A. N. , & Appel, B. (2020). Microglia phagocytose myelin sheaths to modify developmental myelination. Nature Neuroscience, 23, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo, P. , Shiina, H. , Hirunpattarasilp, C. , Gillis, G. , & Attwell, D. (2021). Synapse development is regulated by microglial THIK‐1 K+ channels. Proceedings of the National Academy of Sciences, 118, e2106294118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish, B. T. , Cheadle, L. , Hrvatin, S. , Nagy, M. A. , Rivera, S. , Crow, M. , Gillis, J. , Kirchner, R. , & Greenberg, M. E. (2018). Single‐cell transcriptomics of the developing lateral geniculate nucleus reveals insights into circuit assembly and refinement. Proceedings of the National Academy of Sciences of the United States of America, 115, E1051–E1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana, V. , Desland, F. A. , Casanova‐Acebes, M. , Ayata, P. , Badimon, A. , Nabel, E. , Yamamuro, K. , Sneeboer, M. , Tan, I. L. , Flanigan, M. E. , Rose, S. A. , Chang, C. , Leader, A. , le Bourhis, H. , Sweet, E. S. , Tung, N. , Wroblewska, A. , Lavin, Y. , See, P. , … Merad, M. (2019). CSF‐1 controls cerebellar microglia and is required for motor function and social interaction. The Journal of Experimental Medicine, 216, 2265–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann, H. , Kirchhoff, F. , & Verkhratsky, A. (2013). Microglia: New roles for the synaptic stripper. Neuron, 77, 10–18. [DOI] [PubMed] [Google Scholar]
- Kierdorf, K. , Erny, D. , Goldmann, T. , Sander, V. , Schulz, C. , Perdiguero, E. G. , Wieghofer, P. , Heinrich, A. , Riemke, P. , Hölscher, C. , Müller, D. N. , Luckow, B. , Brocker, T. , Debowski, K. , Fritz, G. , Opdenakker, G. , Diefenbach, A. , Biber, K. , Heikenwalder, M. , … Prinz, M. (2013). Microglia emerge from erythromyeloid precursors via Pu.1‐ and Irf8‐dependent pathways. Nature Neuroscience, 16, 273–280. [DOI] [PubMed] [Google Scholar]
- Kim, H.‐J. , Cho, M.‐H. , Shim, W. H. , Kim, J. K. , Jeon, E.‐Y. , Kim, D.‐H. , & Yoon, S.‐Y. (2017). Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Molecular Psychiatry, 22, 1576–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, I. , Mlsna, L. M. , Yoon, S. , Le, B. , Yu, S. , Xu, D. , & Koh, S. (2015). A postnatal peak in microglial development in the mouse hippocampus is correlated with heightened sensitivity to seizure triggers. Brain and Behavior: A Cognitive Neuroscience Perspective, 5, e00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel, I. , Chicha, L. , Britschgi, M. , Schobel, S. A. , Bodmer, M. , Hellings, J. A. , Toovey, S. , & Prinssen, E. P. (2014). Maternal immune activation and abnormal brain development across CNS disorders. Nature Reviews. Neurology, 10, 643–660. [DOI] [PubMed] [Google Scholar]
- Lauber, E. , Filice, F. , & Schwaller, B. (2018). Parvalbumin neurons as a hub in autism spectrum disorders. Journal of Neuroscience Research, 96, 360–361. [DOI] [PubMed] [Google Scholar]
- Le Belle, J. E. , Sperry, J. , Ngo, A. , Ghochani, Y. , Laks, D. R. , López‐Aranda, M. , Silva, A. J. , & Kornblum, H. I. (2014). Maternal inflammation contributes to brain overgrowth and autism‐associated behaviors through altered redox signaling in stem and progenitor cells. Stem Cell Reports, 3, 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman, E. K. , Wilton, D. K. , Litvina, E. Y. , Welsh, C. A. , Chang, S. T. , Frouin, A. , Walker, A. J. , Heller, M. D. , Umemori, H. , Chen, C. , & Stevens, B. (2018). CD47 protects synapses from excess microglia‐mediated pruning during development. Neuron, 100, 120–134.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Cheng, Z. , Zhou, L. , Darmanis, S. , Neff, N. F. , Okamoto, J. , Gulati, G. , Bennett, M. L. , Sun, L. O. , Clarke, L. E. , Marschallinger, J. , Yu, G. , Quake, S. R. , Wyss‐Coray, T. , & Barres, B. A. (2019). Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single‐cell RNA sequencing. Neuron, 101, 207–223.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Du, X.‐F. , Liu, C.‐S. , Wen, Z.‐L. , & Du, J.‐L. (2012). Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Developmental Cell, 23, 1189–1202. [DOI] [PubMed] [Google Scholar]
- Liao, H. , Huang, W. , Niu, R. , Sun, L. , & Zhang, L. (2008). Cross‐talk between the epidermal growth factor‐like repeats/fibronectin 6‐8 repeats domains of Tenascin‐R and microglia modulates neural stem/progenitor cell proliferation and differentiation. Journal of Neuroscience Research, 86, 27–34. [DOI] [PubMed] [Google Scholar]
- Lim, S.‐H. , Park, E. , You, B. , Jung, Y. , Park, A.‐R. , Park, S. G. , & Lee, J. R. (2013). Neuronal synapse formation induced by microglia and interleukin 10. PLoS One, 8, e81218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, T. K. , & Ruthazer, E. S. (2021). Microglial trogocytosis and the complement system regulate axonal pruning in vivo. eLife, 0, e62167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G. J. , Nagarajah, R. , Banati, R. B. , & Bennett, M. R. (2009). Glutamate induces directed chemotaxis of microglia. The European Journal of Neuroscience, 29, 1108–1118. [DOI] [PubMed] [Google Scholar]
- Liu, Y. U. , Ying, Y. , Li, Y. , Eyo, U. B. , Chen, T. , Zheng, J. , Umpierre, A. D. , Zhu, J. , Bosco, D. B. , Dong, H. , & Wu, L. J. (2019). Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nature Neuroscience, 22, 1771–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui, H. , Zhang, J. , Makinson, S. R. , Cahill, M. K. , Kelley, K. W. , Huang, H.‐Y. , Shang, Y. , Oldham, M. C. , Martens, L. H. , Gao, F. , Coppola, G. , Sloan, S. A. , Hsieh, C. L. , Kim, C. C. , Bigio, E. H. , Weintraub, S. , Mesulam, M. M. , Rademakers, R. , Mackenzie, I. R. , … Huang, E. J. (2016). Progranulin deficiency promotes circuit‐specific synaptic pruning by microglia via complement activation. Cell, 165, 921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madry, C. , Kyrargyri, V. , Arancibia‐Cárcamo, I. L. , Jolivet, R. , Kohsaka, S. , Bryan, R. M. , & Attwell, D. (2018). Microglial ramification, surveillance, and interleukin‐1β release are regulated by the two‐pore domain K+ channel THIK‐1. Neuron, 97, 299–312.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín‐Teva, J. L. , Dusart, I. , Colin, C. , Gervais, A. , Rooijen, N. , & van Mallat, M. (2004). Microglia promote the death of developing Purkinje cells. Neuron, 41, 535–547. [DOI] [PubMed] [Google Scholar]
- Masuda, T. , Sankowski, R. , Staszewski, O. , Böttcher, C. , Amann, L. , Scheiwe, C. , Nessler, S. , Kunz, P. , van Loo, G. , Coenen, V. A. , Reinacher, P. C. , Michel, A. , Sure, U. , Gold, R. , Grün, D. , Priller, J. , Stadelmann, C. , & Prinz, M. (2019). Spatial and temporal heterogeneity of mouse and human microglia at single‐cell resolution. Nature, 566, 388–392. [DOI] [PubMed] [Google Scholar]
- Matcovitch‐Natan, O. , Winter, D. R. , Giladi, A. , Vargas, A. S. , Spinrad, A. , Sarrazin, S. , Ben‐Yehuda, H. , David, E. , González, F. Z. , Perrin, P. , Keren‐Shaul, H. , Gury, M. , Lara‐Astaiso, D. , Thaiss, C. A. , Cohen, M. , Halpern, K. B. , Baruch, K. , Deczkowska, A. , Lorenzo‐Vivas, E. , … Amit, I. (2016). Microglia development follows a stepwise program to regulate brain homeostasis. Science, 353, aad8670. [DOI] [PubMed] [Google Scholar]
- Mattei, D. , Ivanov, A. , Ferrai, C. , Jordan, P. , Guneykaya, D. , Buonfiglioli, A. , Schaafsma, W. , Przanowski, P. , Deuther‐Conrad, W. , Brust, P. , Hesse, S. , Patt, M. , Sabri, O. , Ross, T. L. , Eggen, B. J. L. , Boddeke, E. W. G. M. , Kaminska, B. , Beule, D. , Pombo, A. , … Wolf, S. A. (2017). Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Translational Psychiatry, 7, e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes, M. S. , Le, L. , Atlas, J. , Brehm, Z. , Ladron‐de‐Guevara, A. , Matei, E. , Lamantia, C. , McCall, M. N. , & Majewska, A. K. (2021). The role of P2Y12 in the kinetics of microglial self‐renewal and maturation in the adult visual cortex in vivo. eLife, 10, e61173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini, M. , Rafalski, V. A. , Ma, K. , Kim, K.‐Y. , Bushong, E. A. , Rios Coronado, P. E. , Yan, Z. , Mendiola, A. S. , Sozmen, E. G. , Ryu, J. K. , Haberl, M. G. , Madany, M. , Sampson, D. N. , Petersen, M. A. , Bardehle, S. , Tognatta, R. , Dean Jr, T. , Acevedo, R. M. , Cabriga, B. , … Akassoglou, K. (2021). Microglial Gi‐dependent dynamics regulate brain network hyperexcitability. Nature Neuroscience, 24, 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner, A. , Huang, H. , Radke, J. , Stenzel, W. , & Priller, J. (2017). P2Y12 receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia, 65, 375–387. [DOI] [PubMed] [Google Scholar]
- Missault, S. , Van den Eynde, K. , Vanden, B. W. , Fransen, E. , Weeren, A. , Timmermans, J. P. , Kumar‐Singh, S. , & Dedeurwaerdere, S. (2014). The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain, Behavior, and Immunity, 42, 138–146. [DOI] [PubMed] [Google Scholar]
- Miyamoto, A. , Wake, H. , Ishikawa, A. W. , Eto, K. , Shibata, K. , Murakoshi, H. , Koizumi, S. , Moorhouse, A. J. , Yoshimura, Y. , & Nabekura, J. (2016). Microglia contact induces synapse formation in developing somatosensory cortex. Nature Communications, 7, 12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama, H. , Abe, M. , Morimoto, C. , Iida, T. , Okabe, S. , Sakimura, K. , & Hashimoto, K. (2018). Microglia permit climbing fiber elimination by promoting GABAergic inhibition in the developing cerebellum. Nature Communications, 9, 2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, L. H. , Warden, S. , & Lenz, K. M. (2017). Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain, Behavior, and Immunity, 64, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, P. T. , Dorman, L. C. , Pan, S. , Vainchtein, I. D. , Han, R. T. , Nakao‐Inoue, H. , Taloma, S. E. , Barron, J. J. , Molofsky, A. B. , Kheirbek, M. A. , & Molofsky, A. V. (2020). Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell, 182, 388–403.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn, A. , Kirchhoff, F. , & Helmchen, F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science, 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Okazawa, H. , Motegi, S. , Ohyama, N. , Ohnishi, H. , Tomizawa, T. , Kaneko, Y. , Oldenborg, P.‐A. , Ishikawa, O. , & Matozaki, T. (2005). Negative regulation of phagocytosis in macrophages by the CD47‐SHPS‐1 system. Journal of Immunology, 174, 2004–2011. [DOI] [PubMed] [Google Scholar]
- Pagani, F. , Paolicelli, R. C. , Murana, E. , Cortese, B. , Di Angelantonio, S. , Zurolo, E. , Guiducci, E. , Ferreira, T. A. , Garofalo, S. , Catalano, M. , D'Alessandro, G. , Porzia, A. , Peruzzi, G. , Mainiero, F. , Limatola, C. , Gross, C. T. , & Ragozzino, D. (2015). Defective microglial development in the hippocampus of Cx3cr1 deficient mice. Frontiers in Cellular Neuroscience, 9, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli, R. , Sierra, A. , Stevens, B. , Tremblay, M. E. , Aguzzi, A. , Ajami, B. , Amit, I. , Audinat, E. , Bechmann, I. , Bennett, M. , Bennett, F. , Bessis, A. , Biber, K. , Bilbo, S. , Blurton‐Jones, M. , Boddeke, E. , Brites, D. , Brône, B. , Brown, G. C. , … Wyss‐Coray, T. (2022). Defining microglial states and nomenclature: A roadmap to 2030. SSRN Electronic Journal. [Google Scholar]
- Paolicelli, R. C. , Bolasco, G. , Pagani, F. , Maggi, L. , Scianni, M. , Panzanelli, P. , Giustetto, M. , Ferreira, T. A. , Guiducci, E. , Dumas, L. , Ragozzino, D. , & Gross, C. T. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science, 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- Parkhurst, C. N. , Yang, G. , Ninan, I. , Savas, J. N. , Yates, J. R. , Lafaille, J. J. , Hempstead, B. L. , Littman, D. R. , & Gan, W. B. (2013). Microglia promote learning‐dependent synapse formation through brain‐derived neurotrophic factor. Cell, 155, 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peça, J. , & Feng, G. (2012). Cellular and synaptic network defects in autism. Current Opinion in Neurobiology, 22, 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala, G. , Chou, S. , Jung, Y. , Coiro, P. , Spartz, E. , Padmashri, R. , Li, M. , & Dunaevsky, A. (2017). Maternal immune activation causes behavioral impairments and altered cerebellar cytokine and synaptic protein expression. Neuropsychopharmacology, 42, 1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Pouchoulen M., VanRyzin J. W., & McCarthy M. M. (2015) Morphological and phagocytic profile of microglia in the developing rat cerebellum eNeuro 2, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer, T. , Avignone, E. , & Nägerl, U. V. (2016). Induction of hippocampal long‐term potentiation increases the morphological dynamics of microglial processes and prolongs their contacts with dendritic spines. Scientific Reports, 6, 32422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazi, K. , Galic, M. A. , Kentner, A. C. , Reid, A. Y. , Sharkey, K. A. , & Pittman, Q. J. (2015). Microglia‐dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. The Journal of Neuroscience, 35, 4942–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Xavier, A. L. , Kress, B. T. , Goldman, S. A. , Lacerda de Menezes, J. R. , & Nedergaard, M. (2015). A distinct population of microglia supports adult neurogenesis in the subventricular zone. The Journal of Neuroscience, 35, 11848–11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzano, R. , Roux, T. , Thetiot, M. , Aigrot, M. S. , Richard, L. , Lejeune, F. X. , Mazuir, E. , Vallat, J. M. , Lubetzki, C. , & Desmazières, A. (2021). Microglia‐neuron interaction at nodes of Ranvier depends on neuronal activity through potassium release and contributes to remyelination. Nature Communications, 12, 5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, D. R. , Careaga, M. , Van de Water, J. , McAllister, K. , Bauman, M. D. , & Ashwood, P. (2017). Long‐term altered immune responses following fetal priming in a non‐human primate model of maternal immune activation. Brain, Behavior, and Immunity, 63, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumier, A. , Béchade, C. , Poncer, J.‐C. , Smalla, K.‐H. , Tomasello, E. , Vivier, E. , Gundelfinger, E. D. , Triller, A. , & Bessis, A. (2004). Impaired synaptic function in the microglial KARAP/DAP12‐deficient mouse. The Journal of Neuroscience, 24, 11421–11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, D. P. , Lehrman, E. K. , Kautzman, A. G. , Koyama, R. , Mardinly, A. R. , Yamasaki, R. , Ransohoff, R. M. , Greenberg, M. E. , Barres, B. A. , & Stevens, B. (2012). Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron, 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott‐Hewitt, N. , Perrucci, F. , Morini, R. , Erreni, M. , Mahoney, M. , Witkowska, A. , Carey, A. , Faggiani, E. , Schuetz, L. T. , Mason, S. , Tamborini, M. , Bizzotto, M. , Passoni, L. , Filipello, F. , Jahn, R. , Stevens, B. , & Matteoli, M. (2020). Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. The EMBO Journal, 39, e105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, Y. Y. , Park, A. , Berrios, J. , Lafourcade, M. , Pascual, L. M. , Soares, N. , Yeon, K. J. , Kim, S. , Kim, H. , Waisman, A. , Littman, D. R. , Wickersham, I. R. , Harnett, M. T. , Huh, J. R. , & Choi, G. B. (2017). Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature, 549, 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra, A. , Encinas, J. M. , Deudero, J. J. P. , Chancey, J. H. , Enikolopov, G. , Overstreet‐Wadiche, L. S. , Tsirka, S. E. , & Maletic‐Savatic, M. (2010). Microglia shape adult hippocampal neurogenesis through apoptosis‐coupled phagocytosis. Cell Stem Cell, 7, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe, G. O. , Lowery, R. L. , Tremblay, M. È. , Kelly, E. A. , Lamantia, C. E. , & Majewska, A. K. (2016). Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nature Communications, 7, 10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa, C. , Golebiewska, A. , Poovathingal, S. K. , Kaoma, T. , Pires‐Afonso, Y. , Martina, S. , Coowar, D. , Azuaje, F. , Skupin, A. , Balling, R. , Biber, K. , Niclou, S. P. , & Michelucci, A. (2018). Single‐cell transcriptomics reveals distinct inflammation‐induced microglia signatures. EMBO Reports, 19, e46171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni, P. , Oller, G. , Hoeffel, G. , Pont‐Lezica, L. , Rostaing, P. , Low, D. , Bessis, A. , Ginhoux, F. , & Garel, S. (2014). Microglia modulate wiring of the embryonic forebrain. Cell Reports, 8, 1271–1279. [DOI] [PubMed] [Google Scholar]
- Staszewski, O. , & Hagemeyer, N. (2019). Unique microglia expression profile in developing white matter. BMC Research Notes, 12, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani, J. , Tschesnokowa, O. , Parrilla, M. , Robaye, B. , Boeynaems, J.‐M. , Acker‐Palmer, A. , Zimmermann, H. , & Gampe, K. (2018). Disruption of the microglial ADP receptor P2Y13 enhances adult hippocampal neurogenesis. Frontiers in Cellular Neuroscience, 12, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, B. , Allen, N. J. , Vazquez, L. E. , Howell, G. R. , Christopherson, K. S. , Nouri, N. , Micheva, K. D. , Mehalow, A. K. , Huberman, A. D. , Stafford, B. , Sher, A. , Litke, A. M. , Lambris, J. D. , Smith, S. J. , John, S. W. M. , & Barres, B. A. (2007). The classical complement cascade mediates CNS synapse elimination. Cell, 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Stowell, R. D. , Wong, E. L. , Batchelor, H. N. , Mendes, M. S. , Lamantia, C. E. , Whitelaw, B. S. , & Majewska, A. K. (2018). Cerebellar microglia are dynamically unique and survey Purkinje neurons in vivo. Developmental Neurobiology, 78, 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, K. , Sugihara, G. , Ouchi, Y. , Nakamura, K. , Futatsubashi, M. , Takebayashi, K. , Yoshihara, Y. , Omata, K. , Matsumoto, K. , Tsuchiya, K. J. , Iwata, Y. , Tsujii, M. , Sugiyama, T. , & Mori, N. (2013). Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry, 70, 49–58. [DOI] [PubMed] [Google Scholar]
- Swinnen, N. , Smolders, S. , Avila, A. , Notelaers, K. , Paesen, R. , Ameloot, M. , Brône, B. , Legendre, P. , & Rigo, J. M. (2013). Complex invasion pattern of the cerebral cortex bymicroglial cells during development of the mouse embryo. Glia, 61, 150–163. [DOI] [PubMed] [Google Scholar]
- Tay, T. L. , Mai, D. , Dautzenberg, J. , Fernández‐Klett, F. , Lin, G. , Datta, M. , Drougard, A. , Stempfl, T. , Ardura‐Fabregat, A. , Staszewski, O. , Margineanu, A. , Spobert, A. , Steinmetz, L. M. , Pospisilik, J. A. , Jung, S. , Priller, J. , Grün, D. , Ronneberger, O. , & Prinz, M. (2017). A new fate mapping system reveals context‐dependent random or clonal expansion of microglia. Nature Neuroscience, 20, 793–803. [DOI] [PubMed] [Google Scholar]
- Thion, M. S. , Ginhoux, F. , & Garel, S. (2018). Microglia and early brain development: An intimate journey. Science, 362, 185–189. [DOI] [PubMed] [Google Scholar]
- Thion, M. S. , Low, D. , Silvin, A. , Chen, J. , Grisel, P. , Schulte‐Schrepping, J. , Blecher, R. , Ulas, T. , Squarzoni, P. , Hoeffel, G. , Coulpier, F. , Siopi, E. , David, F. S. , Scholz, C. , Shihui, F. , Lum, J. , Amoyo, A. A. , Larbi, A. , Poidinger, M. , … Garel, S. (2018). Microbiome influences prenatal and adult microglia in a sex‐specific manner. Cell, 172, 500–516.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, M.‐È. , Lowery, R. L. , & Majewska, A. K. (2010). Microglial interactions with synapses are modulated by visual experience. PLoS Biology, 8, e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno, M. , Fujita, Y. , Tanaka, T. , Nakamura, Y. , Kikuta, J. , Ishii, M. , & Yamashita, T. (2013). Layer V cortical neurons require microglial support for survival during postnatal development. Nature Neuroscience, 16, 543–551. [DOI] [PubMed] [Google Scholar]
- Utz, S. G. , See, P. , Mildenberger, W. , Thion, M. S. , Silvin, A. , Lutz, M. , Ingelfinger, F. , Rayan, N. A. , Lelios, I. , Buttgereit, A. , Asano, K. , Prabhakar, S. , Garel, S. , Becher, B. , Ginhoux, F. , & Greter, M. (2020). Early fate defines microglia and non‐parenchymal brain macrophage development. Cell, 181, 557–573.e18. [DOI] [PubMed] [Google Scholar]
- Vainchtein, I. D. , Chin, G. , Cho, F. S. , Kelley, K. W. , Miller, J. G. , Chien, E. C. , Liddelow, S. A. , Nguyen, P. T. , Nakao‐Inoue, H. , Dorman, L. C. , Akil, O. , Joshita, S. , Barres, B. A. , Paz, J. T. , Molofsky, A. B. , & Molofsky, A. V. (2018). Astrocyte‐derived interleukin‐33 promotes microglial synapse engulfment and neural circuit development. Science, 359, 1269–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRyzin, J. W. , Marquardt, A. E. , Argue, K. J. , Vecchiarelli, H. A. , Ashton, S. E. , Arambula, S. E. , Hill, M. N. , & McCarthy, M. M. (2019). Microglial phagocytosis of newborn cells is induced by endocannabinoids and sculpts sex differences in juvenile rat social play. Neuron, 102, 435–449.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas, D. L. , Nascimbene, C. , Krishnan, C. , Zimmerman, A. W. , & Pardo, C. A. (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurology, 57, 67–81. [DOI] [PubMed] [Google Scholar]
- Vasek, M. J. , Garber, C. , Dorsey, D. , Durrant, D. M. , Bollman, B. , Soung, A. , Yu, J. , Perez‐Torres, C. , Frouin, A. , Wilton, D. K. , Funk, K. , DeMasters, B. K. , Jiang, X. , Bowen, J. R. , Mennerick, S. , Robinson, J. K. , Garbow, J. R. , Tyler, K. L. , Suthar, M. S. , … Klein, R. S. (2016). A complement‐microglial axis drives synapse loss during virus‐induced memory impairment. Nature, 534, 538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake, H. , Moorhouse, A. J. , Jinno, S. , Kohsaka, S. , & Nabekura, J. (2009). Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. The Journal of Neuroscience, 29, 3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman, S. , Béchade, C. , Roumier, A. , Bernard, D. , Triller, A. , & Bessis, A. (2008). Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. The Journal of Neuroscience, 28, 8138–8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Yue, H. , Hu, Z. , Shen, Y. , Ma, J. , Li, J. , Wang, X.‐D. , Wang, L. , Sun, B. , Shi, P. , Wang, L. , & Gu, Y. (2020). Microglia mediate forgetting via complement‐dependent synaptic elimination. Science, 367, 688–694. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Szretter, K. J. , Vermi, W. , Gilfillan, S. , Rossini, C. , Cella, M. , Barrow, A. D. , Diamond, M. S. , & Colonna, M. (2012). IL‐34 is a tissue‐restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nature Immunology, 13, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, S. , Nandi, S. , Chitu, V. , Yeung, Y.‐G. , Yu, W. , Huang, M. , Williams, L. T. , Lin, H. , & Stanley, E. R. (2010). Functional overlap but differential expression of CSF‐1 and IL‐34 in their CSF‐1 receptor‐mediated regulation of myeloid cells. Journal of Leukocyte Biology, 88, 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard, L. , Bartolomei, G. , di Bolasco, G. , Machado, P. , Schieber, N. L. , Neniskyte, U. , Exiga, M. , Vadisiute, A. , Raggioli, A. , Schertel, A. , Schwab, Y. , & Gross, C. T. (2018). Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nature Communications, 9, 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor‐Jedrzejczak, W. W. , Ahmed, A. , Szczylik, C. , & Skelly, R. R. (1982). Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. The Journal of Experimental Medicine, 156, 1516–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk, A. , Holtman, I. R. , Krueger, M. , Yogev, N. , Bruttger, J. , Khorooshi, R. , Benmamar‐Badel, A. , Boer‐Bergsma, J. J. , Martin, N. A. , Karram, K. , Kramer, I. , Boddeke, E. W. G. M. , Waisman, A. , Eggen, B. J. L. , & Owens, T. (2017). A novel microglial subset plays a key role in myelinogenesis in developing brain. The EMBO Journal, 36, 3292–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. J. , & Zhuo, M. (2008). Resting microglial motility is independent of synaptic plasticity in mammalian brain. Journal of Neurophysiology, 99, 2026–2032. [DOI] [PubMed] [Google Scholar]
- Yamamoto, M. , Kim, M. , Imai, H. , Itakura, Y. , & Ohtsuki, G. (2019). Microglia‐triggered plasticity of intrinsic excitability modulates psychomotor behaviors in acute cerebellar inflammation. Cell Reports, 28, 2923–2938.e8. [DOI] [PubMed] [Google Scholar]
- Yilmaz, M. , Yalcin, E. , Presumey, J. , Aw, E. , Ma, M. , Whelan, C. W. , Stevens, B. , McCarroll, S. A. , & Carroll, M. C. (2021). Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nature Neuroscience, 24, 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, D. , Li, T. , Delpech, J.‐C. , Zhu, B. , Kishore, P. , Koshi, T. , Luo, R. , Pratt, K. J. B. , Popova, G. , Nowakowski, T. J. , Villeda, S. A. , & Piao, X. (2022). Microglial GPR56 is the molecular target of maternal immune activation‐induced parvalbumin‐positive interneuron deficits. Science Advances, 8, eabm2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, Y. , Paolicelli, R. C. , Sforazzini, F. , Weinhard, L. , Bolasco, G. , Pagani, F. , Vyssotski, A. L. , Bifone, A. , Gozzi, A. , Ragozzino, D. , & Gross, C. T. (2014). Deficient neuron‐microglia signaling results in impaired functional brain connectivity and social behavior. Nature Neuroscience, 17, 400–406. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Rong, P. , Zhang, L. , He, H. , Zhou, T. , Fan, Y. , Mo, L. , Zhao, Q. , Han, Y. , Li, S. , Wang, Y. , Yan, W. , Chen, H. , & You, Z. (2021). IL4‐driven microglia modulate stress resilience through BDNF‐dependent neurogenesis. Science Advances, 7, eabb9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Velmeshev, D. , Hashimoto, K. , Huang, Y.‐H. , Hofmann, J. W. , Shi, X. , Chen, J. , Leidal, A. M. , Dishart, J. G. , Cahill, M. K. , Kelley, K. W. , Liddelow, S. A. , Seeley, W. W. , Miller, B. L. , Walther, T. C. , Farese, R. V., Jr. , Taylor, J. P. , Ullian, E. M. , Huang, B. , … Huang, E. J. (2020). Neurotoxic microglia promote TDP‐43 proteinopathy in progranulin deficiency. Nature, 588, 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


