Abstract
Introduction
Multiple groups have reported on the usefulness of ablating in atrial regions exhibiting abnormal electrograms during atrial fibrillation (AF). Still, previous studies have suggested that ablation outcomes are highly operator‐ and center‐dependent. This study sought to evaluate a novel machine learning software algorithm named VX1 (Volta Medical), trained to adjudicate multipolar electrogram dispersion.
Methods
This study was a prospective, multicentric, nonrandomized study conducted to assess the feasibility of generating VX1 dispersion maps. In 85 patients, 8 centers, and 17 operators, we compared the acute and long‐term outcomes after ablation in regions exhibiting dispersion between primary and satellite centers. We also compared outcomes to a control group in which dispersion‐guided ablation was performed visually by trained operators.
Results
The study population included 29% of long‐standing persistent AF. AF termination occurred in 92% and 83% of the patients in primary and satellite centers, respectively, p = 0.31. The average rate of freedom from documented AF, with or without antiarrhythmic drugs (AADs), was 86% after a single procedure, and 89% after an average of 1.3 procedures per patient (p = 0.4). The rate of freedom from any documented atrial arrhythmia, with or without AADs, was 54% and 73% after a single or an average of 1.3 procedures per patient, respectively (p < 0.001). No statistically significant differences between outcomes of the primary versus satellite centers were observed for one (p = 0.8) or multiple procedures (p = 0.4), or between outcomes of the entire study population versus the control group (p > 0.2). Interestingly, intraprocedural AF termination and type of recurrent arrhythmia (i.e., AF vs. AT) appear to be predictors of the subsequent clinical course.
Conclusion
VX1, an expertise‐based artificial intelligence software solution, allowed for robust center‐to‐center standardization of acute and long‐term ablation outcomes after electrogram‐based ablation.
Keywords: artificial intelligence, atrial fibrillation, catheter ablation, dispersion, driver, mapping, sinus rhythm
Ablation guided by Volta Medical, an expertise‐based artificial intelligence software solution, led to promising outcomes in persistent atrial fibrillation patients. Acute and long‐term outcomes between our primary center and satellite centers are not statistically different, demonstrating the standardization and the reproducibility of the approach.
1. INTRODUCTION
Although an increasingly larger number of patients are eligible for catheter ablation, the optimal ablation strategy for persistent atrial fibrillation (AF) remains elusive. Some past studies, based on the visual selection of target electrograms, have suggested that non‐pulmonary veins isolation (PVI) lesions targeting complex fractionated atrial electrograms (CFAE) are beneficial to patients with persistent AF. 1 , 2 , 3 , 4 , 5 By contrast, STAR AF II concluded that performing additional ablation lesions beyond PVI does not lead to improved long‐term outcomes. 6 Other groups had instead performed advanced signal analysis and provided a mechanistic‐based display to guide operators toward AF drivers. 7 , 8 , 9 , 10 , 11 Also, our group presented a clinical investigation, which supported the application of radio‐frequency (RF) energy in atrial regions exhibiting the dispersion of multipolar electrograms. 12 In that study, we observed better acute and long‐term outcomes when compared to a control group of patients ablated according to the Stepwise approach. Albeit promising, the visual/nonautomated selection of electrograms represents a substantial limitation to the standardization of electrogram‐based approaches with large differences in experience and learning curve profiles between centers. 1 , 2 , 3 , 4 , 5 , 6 , 12 , 13 , 14 Here, we aimed to evaluate an artificial intelligence‐based, expert‐trained, real‐time dispersion adjudication tool named VX1 (Volta Medical). The preliminary evaluation of the AIFib Software Trial (Ev‐AIFib) was conducted to determine the feasibility and relevance of constructing VX1 dispersion maps for the ablation of persistent AF. We tested the hypothesis that the use of VX1 allows for a robust center‐to‐center standardization of ablation outcomes.
2. METHODS
2.1. Patient selection
Patients 18 years of age or older, admitted for persistent AF ablation and for whom PVI alone was not the strategy retained, were enrolled. The exclusion criteria were a contraindication to ablation, a major bleeding disorder, while a dilated left atrium (LA) and the presence of structural heart disease were not exclusion criteria. Patients were recruited at our primary center (St‐Joseph Hospital, Marseille) and in seven satellite centers, chosen as centers having an experience in persistent AF ablation but without any involvement in the training and development of VX1 (list in Supporting Information). All enrolled patients provided written informed consent and the study was approved by the French Central Ethics Committee of Ile De France 3.
2.2. VX1‐enabled algorithmic dispersion adjudications, construction of dispersion maps
The VX1 device consists of a computer connected to cardiac electrophysiology recording systems through a customized data cable. Analog data received by VX1 is then digitized by an integrated analog‐to‐digital converter. VX1 relies on the off‐line pretraining of multiparametric machine learning algorithms on a database of annotated AF intracardiac electrograms (Figure 1A,B—see Supporting Information for more details).
Figure 1.
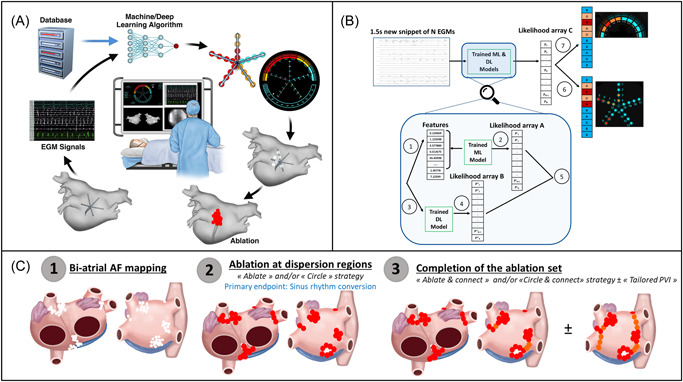
(A) The VX1 software is based on machine learning classification algorithms including a deep learning algorithm. VX1 is trained offline on a large proprietary database. Electrograms are digitized and processed in real‐time by VX1 which in turn provides operators with visual and audio cues representing areas of interest. A direct connection from the acquisition system to a computer installed with the VX1 software allows for data transmission. (B) Flowchart diagram explaining the main steps carried out when the trained algorithm is used on new data. Electrogram information provided by the electrophysiology recording system is processed by a feature extraction module extracting 65 features per single track (Step 1), these features are analyzed by a trained machine learning algorithm to produce an array of dispersion likelihood A (Step 2). In parallel, it is processed by a trained deep learning algorithm (Step 3) producing an array of dispersion likelihood B (Step 4). Both dispersion likelihood arrays are merged using a weighted average based on their agreement level to produce dispersion likelihood C (Step 5). Array C is being color‐coded and displayed on the catheter schematic (Step 6). To account for time‐wise stability, several iterations in time are used to build a different color‐coded list which is displayed on the upper frame of the software interface (Step 7). (C) Schematic description of the tailored ablation protocol implemented in Ev‐AIFib. After biatrial mapping of dispersion regions (Step 1), ablation at these regions was conducted (Step 2). Endpoint: Sinus rhythm conversion. The ablation set was completed with the connection of the regions ablated/isolated (“Ablate & Connect” or “Circle & Connect”) and in 42% of the patients with Tailored PVI (Step 3).
As shown in Figure 1A,B, VX1's interface presents operators with a simple color coding of multipolar catheters' dipole numbers: blue for no dispersion; orange for the high likelihood of dispersion; red for a very high likelihood of dispersion. Then, stable adjudications are sound‐coded and color‐coded on an upper hemicycle schematic of the dipoles under consideration (see Supporting Information: Video). Thus, VX1 provides operators with real‐time visual and sound cues that represent the corresponding algorithmic adjudications for the presence or absence of dispersion. This information may then be used by operators for VX1‐based anatomical tagging within commercially available 3D navigation systems.
Operators were requested to indicate whether they had noted regional discrepancies between their own visual analysis of dispersion and VX1 dispersion maps. To do so, they were asked to evaluate the extent of the differences between visual and VX1‐based adjudications from region to region, in annotating an atrial segmentation schematic shown in Supporting Information: Figure 1. Ultimately, the decision to treat/ablate a dispersion‐tagged area was left to the discretion of the operator.
2.3. Mapping and ablation protocol
A three‐dimensional cardiac mapping system (Carto® 3; Biosense Webster or EnSite PrecisionTM, Abbott or Rhythmia HDxTM, Boston Scientific) was used to guide the procedures. The specific ablation protocol is detailed in the Supporting Information.
Baseline mapping in both atria was performed during AF with one of the following mapping multipolar catheters: the PentaRay® catheter spacing 2‐6‐2 (Biosense Webster), the AdvisorTM HD Grid (Abbott), the ReflexionTM HD (Abbott), the AdvisorTM FL (Lasso 10‐pole; Abbott), the Lasso® Nav Eco catheter (Biosense Webster), the Intellamap OrionTM (Boston Scientific) (configurations in Supporting Information). The multielectrode catheter was sequentially positioned in various regions of the right atrium (RA) and LA. Attention was paid to iteratively positioning multipolar catheters in all right and left atrial regions. Importantly, operators had the opportunity to independently ensure that VX1's adjudications corroborated their own visual‐based impression. Figure 1C illustrates the “Tailored ablation protocol.” Operators were first requested to perform ablation in all dispersion regions. The endpoint of the procedure was sinus rhythm conversion by ablation. When operators decided to also perform PVI, recommendations were made that PV‐encircling is conducted along with the ablation of dispersion regions, a.k.a. “Tailored PVI.” When the ablation at all dispersion regions did not yield AF termination, at least one remapping/reablation was mandated (two were recommended). When a sustained atrial tachycardia (AT) resulted from the ablation, mapping and ablation of ATs were performed with the use of activation maps ± VX1 ± pacing maneuvers. If sinus rhythm was restored before ablation at all dispersion areas, the completion of the ablation set was mandatory, except for atrial regions nearby the esophagus, phrenic nerve, AV node, and/or sinus node.
To avoid isolated lesions, we adopted the so‐called “Ablate & Connect” strategy. When two ablation regions were nearly adjacent (<2 cm) or that one ablation area was adjacent to an electrically neutral structure (PV, valve), additional ablation was performed to connect regions, or regions and structures (see Figure 1C, steps 2 and 3).
For workflow optimization, operators also had the option to encircle dispersion regions. This approach was coined by some of the operators as the “Circle & Connect” approach, which represents an adaptation of the aforementioned “Ablate & Connect” strategy.
2.4. Follow‐up
The follow‐up consisted of 12‐lead ECGs and 24 h Holter ECGs at 3, 6, 9, and 12 months and in case of symptoms. Patients with implantable devices (n = 12, 14%) had regular follow‐up device interrogations over the course of the study. We recommended discontinuing antiarrhythmic drugs after the 3‐month blanking period. Recurrence was defined as any episode of atrial arrhythmia lasting longer than 30 s, in accordance with the current guidelines. 15 If AF/AT recurred, a repeat ablation guided by VX1 was recommended.
2.5. Comparison to a visual‐dispersion guided control group
We used the persistent AF patients of the Substrate‐HD study. 12 in whom dispersion‐guided ablation was performed visually by trained experts as a control group and compared acute and long‐term outcomes. Furthermore, a double‐blind comparison of VX1 and visual Dispersion maps was performed on 18 patients (see Supporting Information).
2.6. Statistical analysis
See Supporting Information.
3. RESULTS
3.1. Patients
A total of 85 patients were included between July 2018 and July 2019 in eight centers. Out of these 85 patients, most were in spontaneous AF at the outset of the procedure (n = 61). For the remaining patients, AF was induced by burst pacing and/or isoproterenol infusion. Table 1 presents patient characteristics. Importantly, 29% of the patients had long‐standing persistent AF. Nine patients did not discontinue antiarrhythmic drugs (AADs) before the procedure. No AADs were given during the procedures.
Table 1.
Patients baseline characteristics, including differences between primary and satellite centers cohorts
| Patients baseline characteristics | All patients (n = 85) | Primary center (n = 49) | Satellite centers (n = 36) | p |
|---|---|---|---|---|
| Male sex, no. (%) | 57 (67%) | 36 (73%) | 21 (58%) | 0.14 |
| Age, year | 70 [60–75] | 71 [65–76] | 66 [60–72] | 0.04 |
| Comorbidities, no. (%) | ||||
| Hypertension | 56 (66%) | 34 (69%) | 22 (61%) | 0.43 |
| Obesity – BMI > 30 | 30 (35%) | 20 (41%) | 10 (28%) | 0.21 |
| Sleep apnea | 18 (21%) | 11 (22%) | 7 (19%) | 0.74 |
| Diabetes | 15 (18%) | 10 (20%) | 5 (14%) | 0.44 |
| PM/ICD | 12 (14%) | 7 (14%) | 5 (14%) | 0.96 |
| Prior stroke/TIA | 4 (5%) | 4 (8%) | 0 (0%) | 0.13 |
| Structural heart disease, no. (%) | 33 (39%) | 23 (47%) | 10 (28%) | 0.07 |
| Ischemic cardiomyopathy | 18 (21%) | 14 (29%) | 4 (11%) | 0.05 |
| Dilated cardiomyopathy | 8 (9%) | 3 (6%) | 5 (14%) | 0.27 |
| Rhythmic cardiomyopathy | 4 (5%) | 3 (6%) | 1 (3%) | 0.63 |
| Hypertrophic cardiomyopathy | 2 (2%) | 2 (4%) | 0 (0%) | 0.51 |
| Valvular disease | 1 (1%) | 1 (2%) | 0 (0%) | 1 |
| CHA2DS2‐VASc score | 2.5 [1–4] | 3 [2–4] | 2 [1–3] | 0.08 |
| LVEF, % | 50 [41–60] | 52 [41–60] | 50 [45–57] | 0.74 |
| LA vol (ml) | 165 ± 38 | 171 ± 40 | 159 ± 35 | 0.16 |
| Atrial fibrillation type, no. (%) | ||||
| Persistent | 60 (71%) | 36 (73%) | 24 (67%) | 0.50 |
| Long‐standing persistent | 25 (29%) | 13 (27%) | 12 (33%) | |
| AF history, year | 2.5 [1–4.5] | 3 [1–6] | 1 [1–4] | 0.05 |
| Arrhythmia max duration, mo | ||||
| All patients | 6 [3–12] | 6 [2–12] | 8 [5–12] | 0.17 |
| Long‐standing persistent patients | 12 [12–30] | 22 [12–36] | 12 [12–12] | 0.01 |
Note: Values are mean ± standard deviation, median [interquartile range], or no. (%).
Continuous data were compared between primary and satellite centers using a nonparametric Wilcoxon test or Welch t‐test, according to their distribution. Categorical data were compared using χ 2 or Fisher test. All analyses were conducted using R version 4.0.0 (www.r-project.org). A p < 0.05 was considered statistically significant.
Abbreviations: BMI, body mass index; ICDs, implantable cardioverter defibrillators; LA, left atrium; LVEF, left ventricular ejection fraction; PMs, pacemakers; TIA, transient ischaemic attack.
3.2. Feasibility and usability of VX1 dispersion maps
Overall, VX1 enabled the construction of standardized dispersion maps in all patients by 17 operators, with two acquisition systems, three 3D navigation apparatuses, and six multipolar catheters (the PentaRay® catheter was predominantly used). Each operator's confirmatory visual analysis did not report major regional discordances with VX1. A more detailed investigation, including a double‐blind comparison of VX1 and visual dispersion maps in 18 patients, is presented in Supporting Information (see Figure 4 for examples). Analysis of the regional distribution of dispersion is presented in Supporting Information: Figures 5 and 6.
3.3. Comparison of VX1 maps with CFAE maps
We constructed CFAEs maps with the CARTO Carto® 3 system and compared the surface area and the regional distribution of locations harboring the highest levels of CFAEs with the ones of VX1‐tagged dispersion regions. In summary, (i) VX1‐map dispersion regions span a significantly smaller surface than CFAE regions. (ii) Albeit VX1‐map dispersion regions and CFAE regions may be found in similar atrial subregions, nonoverlapping CFAE/VX1 regions are significantly larger than overlapping ones (see Supporting Information for more details and Supporting Information: Figure 7 for examples).
3.4. Acute procedural outcomes
Ablation conducted in regions tagged with the help of VX1 led to AF termination in 88% of the patients after 27 ± 16 min of RF (Table 2). Importantly, we collected the locations of 100 crucial ablation tags (i.e., the location where ablation terminated the arrhythmia or increased the AF cycle length of ≥15%) and 100/100 were located within VX1‐detected regions (28/100 in the PV regions).
Table 2.
Acute procedural characteristics, including differences between primary and satellite centers cohorts
| Acute procedural characteristics | All patients (n = 85) | Primary center (n = 49) | Satellite centers (n = 36) | p |
|---|---|---|---|---|
| AF at baseline, no. (%) | 61 (72%) | 34 (69%) | 27 (75%) | 0.57 |
| AA drugs at the time of the procedure | 9 (11%) | 6 (12%) | 3 (8%) | 0.72 |
| Arrhythmia mean cycle length, ms | 175 [163–200] | 175 [162–205] | 175 [165–190] | 0.67 |
| Bi‐atrial mapping, no. (%) | 75 (88%) | 41 (84%) | 34 (94%) | 0.18 |
| AF mappings per pt, no. | 1 [1–2] | 1 [1–2] | 1 [1–2] | 0.13 |
| Ablation protocol, no. (%) | ||||
| Electrogram‐based | 85 (100%) | 49 (100%) | 36 (100%) | |
| Additional pulmonary veins isolation | 36 (42%) | 12 (24%) | 24 (67%) | <0.001 |
| Total radiofrequency time, min | 43 ± 13 | 48 ± 13 | 37 ± 10 | <0.001 |
| Radiofrequency time to AF termination, min | 27 ± 16 | 31 ± 17 | 21 ± 12 | 0.004 |
| Fluoroscopy time, min | 5 [2–9] | 4 [2–7] | 8 [4–13] | 0.001 |
| Dose, cGy/cm2 | 2014 [808–4300] | 1422 [690–2538] | 4028 [1705–6725] | <0.001 |
| Procedure time, min | 162 [130–205] | 170 [150–210] | 142 [120–191] | 0.005 |
| AF termination, no. (%) | 75 (88%) | 45 (92%) | 30 (83%) | 0.31 |
| Sinus rhythm conversion by ablation, no. (%) | 55 (65%) | 34 (69%) | 21 (58%) | 0.29 |
| Complications, no. (%) | 1 (1%) | 1 (2%) | 0 (0%) | 1 |
| Transient AV block | 1 | 1 | 0 |
Note: Values are mean ± standard deviation, median [interquartile range], or no. (%).
Continuous data were compared between primary and satellite centers using a non‐parametric Wilcoxon test or Welch t‐test, according to their distribution. Categorical data were compared using the χ 2 or Fisher test. All analyses were conducted using R version 4.0.0 (www.r-project.org). A p < 0.05 was considered statistically significant.
In addition to the ablation performed in the regions harboring dispersion, PVI was conducted in 36/85 patients (42%). Marshall alcoholization was conducted in two patients at the end of the procedure because of the presence of RF‐resistant peri‐mitral flutter. As shown in Supporting Information: Figure 8, about one‐third of the terminations corresponded to a transition AF‐to‐Sinus rhythm (SR), while about two‐third represented a transition AF‐to‐AT. Transient ATs were originating predominantly in the LA (1/3 from the RA) and manifest as macro‐reentry and focal episodes in 60% and 40% of the episodes, respectively. Thereafter, ablation of AT episodes allowed for an overall rate of SR conversion with an ablation of 65%. When considering those patients with AF termination by ablation (n = 75), RA ablation was performed in 48/75 patients (64%).
All patients in sinus rhythm at the beginning of the procedure (n = 24) had AF termination by ablation. Among the patients in AF at the beginning of the procedure (n = 56), 46/56 had AF termination (82%).
When comparing the acute outcomes between primary and satellite centers (Table 2), a nonsignificant increase in performance was observed for the primary center (92% vs. 83% of AF termination, p = 0.31, and 69% vs. 58% of SR conversion, p = 0.29).
3.5. Long‐term outcome
After a mean follow‐up of 13.5 ± 3.2 months, the average rate of freedom from documented AF—with or without AADs—was 86% after a single procedure, and 89% after an average of 1.3 procedures per patient (p = 0.4). The rate of freedom from any documented atrial arrhythmia, with or without AADs was 54% and 73% after a single or an average of 1.3 procedures per patient, respectively (p < 0.001) (Table 3, Figure 2).
Table 3.
Efficacy outcomes, including differences between primary and satellite centers cohorts
| Efficacy outcomes comparison | All patients | Primary center | Satellite centers | p |
|---|---|---|---|---|
| Mean follow‐up of 13.5 ± 3.2 months | (n = 80) | (n = 47) | (n = 33) | |
| Freedom from documented atrial fibrillation, after one procedure, with or without antiarrhythmic drugs, no. (%) | 69 (86%) | 43 (91%) | 26 (79%) | 0.19 |
| Freedom from documented atrial arrhythmia, after one procedure, with or without antiarrhythmic drugs, no. (%) | 43 (54%) | 25 (53%) | 18 (55%) | 0.90 |
| Freedom from documented atrial fibrillation, after one procedure or more,a with or without antiarrhythmic drugs, no. (%) | 71 (89%) | 44 (94%) | 27 (82%) | 0.15 |
| Freedom from documented atrial arrhythmia, after one procedure or more,a with or without antiarrhythmic drugs, no. (%) | 58 (73%) | 35 (74%) | 23 (70%) | 0.64 |
Note: Categorical data were compared between primary and satellite centers using χ 2 or Fisher test. All analyses were conducted using R version 4.0.0 (www.r-project.org). A p < 0.05 was considered statistically significant.
On average, 1.3 procedures/patient: 1.3 procedures/patient for reference center and 1.2 procedures/patient for satellite centers.
Figure 2.
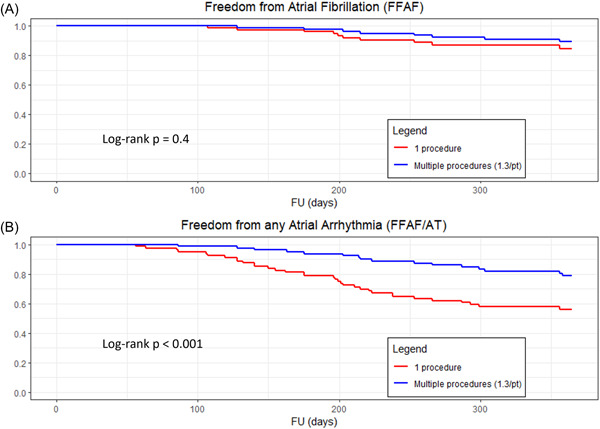
Kaplan–Meier estimates, for all patients, after a single versus multiple procedures, of (A) freedom from documented atrial fibrillation (p = 0.4), (B) freedom from any atrial arrhythmia (p < 0.001), with or without the use of antiarrhythmic medications. A p < 0.05 was considered statistically significant.
Unlike freedom from AF alone, the freedom of any atrial arrhythmia after a single procedure was significantly improved after the performance of a repeat procedure during the study (Figure 2B).
Importantly, among the patients free from any AF/AT and any symptoms, there were only nine patients for whom AADs were not discontinued by the investigators despite our recommendations. If we exclude these nine patients, the rate of freedom from any documented atrial arrhythmias without AADs was 48% (34/71 patients) after a single procedure and 69% (49/71 patients) after multiple procedures.
When comparing the long‐term rates of freedom from any atrial arrhythmia between primary and satellite centers (Figure 3), no statistically significant difference in outcome was observed for one or multiple procedures (p = 0.8, p = 0.4). The analysis of freedom from AF is shown in Supporting Information: Figure 9.
Figure 3.
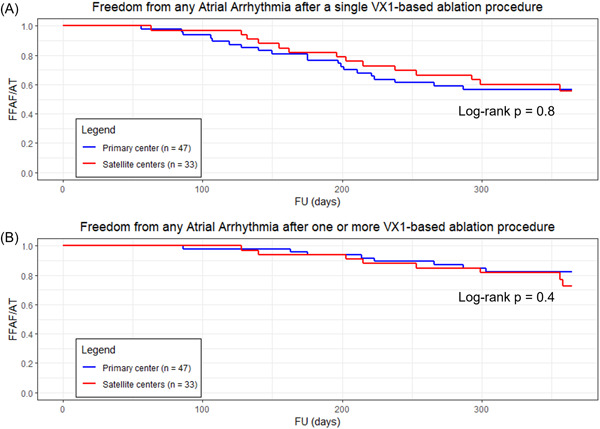
Kaplan–Meier estimates, for primary (blue) versus satellite centers (red), of freedom from any atrial arrhythmia (A) after a single procedure (p = 0.8), (B) after one or more procedures (p = 0.4), with or without the use of antiarrhythmic medications. A p < 0.05 was considered statistically significant.
Interestingly, patients who presented in SR at beginning of the procedure did not have a significantly better long‐term outcome than patients who presented in AF (Supporting Information: Figure 10). Furthermore, the freedom of any atrial arrhythmia after multiple procedures was significantly higher in patients who experienced AT recurrences versus patients who experienced AF recurrences after the index procedure (Supporting Information: Figure 11).
3.6. Influence of acute ablation outcomes on long‐term outcomes
There was significantly greater freedom from AF/AT when ablation led to acute AF termination versus when AF termination was not possible by ablation (Figure 4A).
Figure 4.
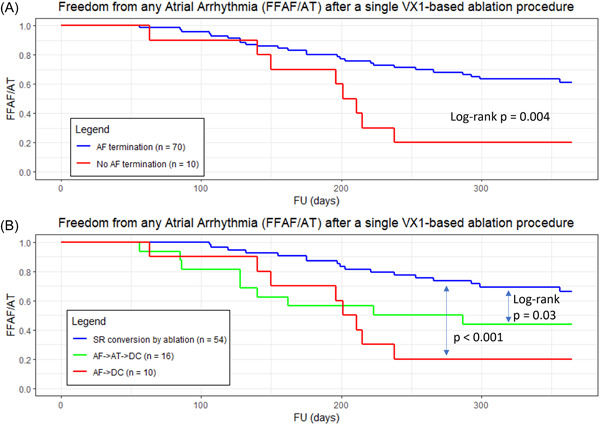
Kaplan–Meier estimates of freedom from any documented atrial arrhythmia after a single procedure, with or without the use of antiarrhythmic medications, for different groups of patients depending on acute outcomes during the index procedure: (A) patients for which AF was terminated by ablation have a statistically significant lower rate of recurrences than patients for which AF could not be terminated (p = 0.004). (B) Patients converted into sinus rhythm by ablation have a statistically significant lower rate of recurrences than patients converted into atrial tachycardia without further restoring sinus rhythm by ablation (p = 0.03) and patients for which AF could not be terminated (p < 0.001). A p < 0.05 was considered statistically significant. AF, atrial fibrillation.
Interestingly, there was also a significantly improved long‐term outcome when AF was converted into SR by ablation—that is, without cardioversion—either directly or after a transition through an AT, versus when the transition from AT to SR required cardioversion (Figure 4B).
3.7. Comparison with the Substrate‐HD control group
There was no statistical difference between this study (n = 85) and the persistent AF population of the Substrate‐HD study (n = 81) 12 in terms of acute and long‐term outcomes: AF termination (88% vs. 95%, p = 0.1), freedom from AF after a single procedure (p = 0.2, Figure 5A), freedom from AF/AT after a single procedure (p = 0.9, Figure 5B).
Figure 5.
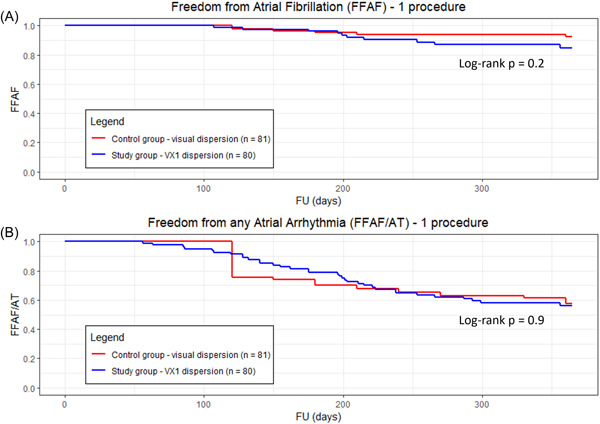
Kaplan–Meier estimates of (A) freedom from documented AF and (B) freedom from any atrial arrhythmia, after a single procedure for the study group (in blue) versus the control group from the Substrate‐HD study where dispersion was visual (in red). Both visual and VX1‐based dispersion groups are comparable (p = 0.2 and p = 0.9, respectively). A p < 0.05 was considered statistically significant. AF, atrial fibrillation; VX1, Volta Medical.
4. DISCUSSION
Here, we present an artificial intelligence‐based algorithm designed for real‐time adjudications of AF multipolar electrograms. To our knowledge, this study represents the first extensive validation that an artificial intelligence‐based software solution can reliably homogenize expert‐based electrogram analysis and lead to reproducible electrogram‐based ablation outcomes.
4.1. Standardization of electrogram‐based ablation outcomes and learning curve of the approach
Previously, artificial intelligence has notably been used to automate expert‐based tasks in ECG analysis, radiology, dermatology, and ophthalmology. 16 , 17 Thus far, its implementation in interventional cardiac electrophysiology has been limited. Previously, we and others have reported on visually analyzed dispersion to guide the ablation of persistent AF and its superiority to a control group ablated using the stepwise approach. 12 , 14 Despite a unifying definition, the heterogeneity of the presentation of dispersion in the cardiac electrophysiology laboratory represents a challenge for achieving real‐time and accurate visual recognition. VX1 allows for the building of standardized dispersion maps, that were used as a reference to conduct dispersion‐guided ablation. Our results show that across eight centers, with 17 operators and while implementing six distinct types of commercially available multipolar catheters, the building of standardized VX1 dispersion maps was achievable. We also show that the outcomes of dispersion‐guided ablation were not statistically different between our primary and satellite centers. Despite nonsignificant differences, our primary center tended to obtain superior AF termination and SR conversion rates. This tendency may reflect the more extensive experience in the use of VX1 and in this type of ablation approach in the primary center. Therefore, a learning curve phenomenon might underlie this tendency.
4.2. Automated approaches for real‐time AF electrograms analysis
In 2004, Nademanee et al. 1 suggested that the ablation at visually detected CFAEs locations can be beneficial to patients in AF. Subsequent studies, however, questioned the reproducibility of the approach and the accuracy of automated CFAEs detection. 6 , 13
Besides CFAEs, the use of dominant frequency analysis, phase transformation, AI‐guided pattern classification, or low voltage regions have been used to highlight so‐called “driver” or “substrate” regions during AF. 5 , 7 , 9 , 18 , 19 , 20 , 21 , 22 By contrast VX1's analysis is not bound to the preconceived importance of a single electrogram analysis parameter but is supported by the multiparametric mimicking of human expertise and adjudications are disconnected from any of the operator's mechanistic hunch.
4.3. Clinical outcomes
Our approach shows a high rate of freedom from AF after a single procedure and a more limited rate of freedom from AF/AT due to recurrent ATs. Our results indicate that performing an additional procedure after an index dispersion‐guided ablation in some patients significantly improves the population 1 year freedom from AF/AT (from 54% to 73% after 1.3 procedures, p < 0.001), corroborating previous findings. 12 Furthermore the arrhythmia type relapsing after the first procedure tended to predict ablation success after the repeat procedure (Supporting Information: Figure 11). As also observed by Ammar et al. 23 patients presenting for a repeat procedure after ablation of persistent AF in AT have a significantly better outcome compared with the ones presenting in persistent AF. These results suggest that AT might be considered a step toward stable sinus rhythm and that freedom from AF after a single procedure is predictive of freedom from AF/AT after multiple procedures.
Several randomized trials have been conducted in a similar population of AF patients, that is, persistent AF patients with a substantial proportion of patients with long‐standing persistent AF (>20%). Verma et al. 6 reported that the ablation of CFAEs in addition to PVI led to an acute AF termination rate of 45% and single procedure freedom from AF and AF/AT at 18 months of 49% and 41%, respectively (STAR AF II Trial). More recently, the ALSTER‐LOST AF study reported a rate of single procedure freedom from AF of 56%. 24 Also, Valderrabano et al. 25 recently reported on the vein of marshall ethanol for untreated persistent AF (VENUS) study, which enrolled about 50% of patients in long‐standing persistent AF. The 1 year single procedure freedom from AF/AT was 38% for the PVI‐only group, while the adjunction of Marshall vein ethanol infusion was associated with a rate of 49%.
Altogether, previous studies implementing extra‐PVI lesions in regions harboring CFAEs or performed following a purely “anatomical” blueprint have reported inconsistent and relatively low long‐term outcomes in persistent AF patients. Pure anatomical approaches have the benefit to be theoretically “simple” to standardize but can miss critical areas for AF perpetuation.Electrogram (EGM)‐based/patient‐tailored approaches can be very effective, but EGM detection is highly operator‐dependent. 1 , 13 Our results suggest that the standardization of the detection of abnormal EGMs allows for robust patient‐to‐patient and operator‐to‐operator performance. Should this observation be confirmed by subsequent clinical studies, algorithmic guidance for performing electrogram‐based ablation may become an indispensable tool for obtaining highly satisfactory outcomes in persistent AF patients and beyond.
5. LIMITATIONS
We did not report survival curves off‐drugs because of nine patients who did not discontinue AADs while they had no relapse or symptoms. We did not compare the study population to a control group ablated with a PVI‐based strategy. However, we and others have suggested the superiority of dispersion‐guided ablation over PVI‐based strategies. 12 , 14 The goal of this study was to establish the standardization and the reproducibility of this approach using the VX1 software. Importantly, this investigation does not allow for drawing conclusions on what is the best ablation strategy for persistent AF patients. Whether this ablation approach provides a long‐term benefit to AF patients, and whether AF termination and AT relapses are indicative of long‐term success are currently assessed in a randomized controlled clinical trial enrolling patients in the EU and the USA (the Tailored‐AF trial, ClinicalTrials. gov Identifier: NCT04702451).
6. CONCLUSIONS
Here, we present a multicenter, multicatheter, multioperator implementation of an artificial intelligence‐based software solution designed to assist operators in targeting of AF drivers. Overall, our acute and long‐term outcome results suggest that the artificial intelligence‐based, AF electrogram software allows for the delivery of simple peri‐operative cues, which, in turn, ensure standardization across multiple platforms, catheters, and operators.
Supporting information
Supplementary information.
Supplementary information.
ACKNOWLEDGMENT
This study was supported by St. Joseph Hospital Volta Medical.
Seitz J, Durdez TM, Albenque JP, et al. Artificial intelligence software standardizes electrogram‐based ablation outcome for persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2022;33:2250‐2260. 10.1111/jce.15657
Julien Seitz and Théophile Mohr Durdez contributed equally to this study.
Disclosure Dr. Seitz has received speaker fees from Biosense Webster, Abbott Laboratories, and Boston Scientific, and owns shares of Volta Medical; Mr. Mohr Durdez owns shares of Volta Medical; Dr. Albenque has received speaker fees from Biosense Webster, honoraria as a consultant from Abbott Laboratories, and from Volta Medical; Mrs. Siame, Mr. Appetiti, and Dr. Milpied own stock options of Volta Medical; Dr. Bars has received honoraria as a consultant from Abbott Laboratories and Biosense Webster and owns shares of Volta Medical; Dr. Kalifa has received honoraria as a consultant from Medtronic and Biosense Webster, grants from the American Heart Association, and owns shares of Volta Medical. Other authors: No disclosures.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044‐2053. [DOI] [PubMed] [Google Scholar]
- 2. O'Neill MD, Jaïs P, Takahashi Y, et al. The stepwise ablation approach for chronic atrial fibrillation—evidence for a cumulative effect. J Interv Card Electrophysiol. 2006;16:153‐167. [DOI] [PubMed] [Google Scholar]
- 3. Estner HL, Hessling G, Biegler R, et al. Complex fractionated atrial electrogram or linear ablation in patients with persistent atrial fibrillation—a prospective randomized study. Pacing Clin Electrophysiol. 2011;34:939‐948. [DOI] [PubMed] [Google Scholar]
- 4. Seitz J, Horvilleur J, Curel L, et al. Active or passive pulmonary vein in atrial fibrillation: is pulmonary vein isolation always essential? Heart Rhythm. 2014;11:579‐586. [DOI] [PubMed] [Google Scholar]
- 5. Jadidi AS, Lehrmann H, Keyl C, et al. Ablation of persistent atrial fibrillation targeting low‐voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol. 2016;9:e002962. 10.1161/CIRCEP.115.002962 [DOI] [PubMed] [Google Scholar]
- 6. Verma A, Jiang C, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812‐1822. [DOI] [PubMed] [Google Scholar]
- 7. Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W‐J, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. J Am Coll Cardiol. 2012;60:628‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haissaguerre M, Hocini M, Denis A, et al. Driver domains in persistent atrial fibrillation. Circulation. 2014;130:530‐538. [DOI] [PubMed] [Google Scholar]
- 9. Knecht S, Sohal M, Deisenhofer I, et al. Multicentre evaluation of non‐invasive biatrial mapping for persistent atrial fibrillation ablation: the AFACART study. Europace. 2017;19:1302‐1309. [DOI] [PubMed] [Google Scholar]
- 10. Willems S, Verma A, Betts TR, et al. Targeting nonpulmonary vein sources in persistent atrial fibrillation identified by noncontact charge density mapping. Circ Arrhythm Electrophysiol. 2019;12:e007233. [DOI] [PubMed] [Google Scholar]
- 11. Choudry S, Mansour M, Sundaram S, et al. RADAR: a multicenter food and drug administration investigational device exemption clinical trial of persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13:e007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seitz J, Bars C, Théodore G, et al. AF ablation guided by spatiotemporal electrogram dispersion without pulmonary vein isolation: a wholly patient‐tailored approach. J Am Coll Cardiol. 2017;69:303‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oral H, Chugh A, Yoshida K, et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long‐lasting persistent atrial fibrillation. J Am Coll Cardiol. 2009;53:782‐789. [DOI] [PubMed] [Google Scholar]
- 14. Lin R, Zeng C, Xu K, Wu S, Qin M, Liu X. Dispersion‐guided ablation in conjunction with circumferential pulmonary vein isolation is superior to stepwise ablation approach for persistent atrial fibrillation. Int J Cardiol. 2019;278:97‐103. [DOI] [PubMed] [Google Scholar]
- 15. Calkins H, Hindricks G, Cappato R, et al. Document reviewers: 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1‐e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Acharya UR, Oh SL, Hagiwara Y, et al. A deep convolutional neural network model to classify heartbeats. Comput Biol Med. 2017;89:389‐396. [DOI] [PubMed] [Google Scholar]
- 17. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25:30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high‐frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789‐797. [DOI] [PubMed] [Google Scholar]
- 19. Atienza F, Almendral J, Jalife J, et al. Real‐time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left‐to‐right frequency gradients predicts long‐term maintenance of sinus rhythm. Heart Rhythm. 2009;6:33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Honarbakhsh S, Hunter RJ, Ullah W, Keating E, Finlay M, Schilling RJ. Ablation in persistent atrial fibrillation using stochastic trajectory analysis of ranked signals (STAR) mapping method. JACC Clin Electrophysiol. 2019;5:817‐829. [DOI] [PubMed] [Google Scholar]
- 21. Alhusseini MI, Abuzaid F, Rogers AJ, et al. Machine learning to classify intracardiac electrical patterns during atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13:e008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Szili‐Torok T, Kis Z, Bhagwandien R, et al. Functional electrographic flow patterns in patients with persistent atrial fibrillation predict outcome of catheter ablation. J Cardiovasc Electrophysiol. 2021;32:2148‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ammar S, Hessling G, Reents T, et al. Arrhythmia type after persistent atrial fibrillation ablation predicts success of the repeat procedure. Circ Arrhythm Electrophysiol. 2011;4:609‐614. [DOI] [PubMed] [Google Scholar]
- 24. Fink T, Schlüter M, Heeger C‐H, et al. Stand‐alone pulmonary vein isolation versus pulmonary vein isolation with additional substrate modification as index ablation procedures in patients with persistent and long‐standing persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2017;10:e005114. [DOI] [PubMed] [Google Scholar]
- 25. Valderrábano M, Peterson LE, Swarup V, et al. Effect of catheter ablation with vein of marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: the Venus randomized clinical trial. JAMA. 2020;324:1620‐1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Supplementary information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


