Abstract
Temperature (T) and vapour pressure deficit (VPD) are important drivers of plant hydraulic conductivity, growth, mortality, and ecosystem productivity, independently of soil water availability. Our goal was to disentangle the effects of T and VPD on plant hydraulic responses. Young trees of Fagus sylvatica L., Quercus pubescens Willd. and Quercus ilex L. were exposed to a cross‐combination of a T and VPD manipulation under unlimited soil water availability. Stem hydraulic conductivity and leaf‐level hydraulic traits (e.g., gas exchange and osmotic adjustment) were tracked over a full growing season. Significant loss of xylem conductive area (PLA) was found in F. sylvatica and Q. pubescens due to rising VPD and T, but not in Q. ilex. Increasing T aggravated the effects of high VPD in F. sylvatica only. PLA was driven by maximum hydraulic conductivity and minimum leaf conductance, suggesting that high transpiration and water loss after stomatal closure contributed to plant hydraulic stress. This study shows for the first time that rising VPD and T lead to losses of stem conductivity even when soil water is not limiting, highlighting their rising importance in plant mortality mechanisms in the future.
Keywords: Fagus sylvatica, hydraulic conductivity, PLA, PLC, Quercus ilex, Quercus pubescens, X‐ray micro‐CT
1. INTRODUCTION
Rising temperatures (T) have caused exponential increases in atmospheric evaporative demand (i.e., vapour pressure deficit [VPD]) in many parts of the world (Dai, 2006; Grossiord, Buckley, et al., 2020), as the air humidity is not increasing at the same speed as the exponentially rising saturation vapour pressure of the atmosphere. As a result, T and VPD have been identified as increasingly important drivers of plant hydraulic conductivity losses (Olson et al., 2020), growth reduction (Trotsiuk et al., 2021), plant mortality (Adams et al., 2009; Allen et al., 2015) and reduced ecosystem productivity (Ciais et al., 2005). Many studies focus on plant responses to a combination of soil drought and either high T (‘hot droughts') (Allen et al., 2015; Cochard, 2019; Grossiord et al., 2018; Rehschuh et al., 2021), or high VPD (Anderegg & Meinzer, 2015; Eamus et al., 2013; Fontes et al., 2018). For example, high VPD combined with soil drought leads to extreme xylem tensions and embolisms (Tardieu & Simonneau, 1998). VPD and soil water dynamics are generally closely coupled on timescales from months to seasons (Liu et al., 2020; Novick et al., 2016), but their individual contributions to plant hydraulics on the timescale from days to weeks are not well established. Disentangling T and VPD under field conditions is challenging because higher T inherently increases VPD (Urban et al., 2017). As a result, few studies have isolated the physiological effects of rising VPD versus T on plants without soil moisture stress, limiting our ability to anticipate future impacts on terrestrial ecosystems.
Higher VPD enhances the driving force for water loss from the leaves. When the water demand exceeds the supply, the water potential in the leaves and stems becomes more negative, which below a given threshold, can lead to embolisms in the xylem vessels, in turn causing a loss of hydraulic conductivity (K). Due to species differences in vessel pit structure and width, some species are more vulnerable to embolisms than others (Lens et al., 2011; Tixier et al., 2014). To prevent expensive and sometimes irreparable damages, leaves regulate water loss under high evaporative demand and/or low soil moisture by controlling stomatal opening, thereby regulating leaf and stem water potentials (Martínez‐Vilalta et al., 2014). With increasing VPD, leaf stomata close gradually (Jarvis & McNaughton, 1986; Monteith, 1995). Although the exact sensing mechanism involved in stomatal closure to rising VPD is unclear, it is thought to involve changes in the water status in stomatal guard cells mediated by hormonal signals like abscisic acid (Buckley, 2005; McAdam & Brodribb, 2016). While it is generally thought that stomata close to prevent embolisms, the relationship between the two is still under discussion, and it is unknown whether and to what extent embolisms may occur before stomata are fully closed (Hochberg et al., 2017).
The rate at which stomatal closure occurs, that is, the stomatal sensitivity to VPD (m), differs per species and along climatic gradients, with plants adapted to more xeric biomes having lower stomatal sensitivity to changes in VPD (i.e., stomata close more slowly) than those adapted to mesic ones (Martínez‐Vilalta et al., 2014; Novick et al., 2016). Yet, how stomatal sensitivity variation between xeric and mesic species alters hydraulic damages without soil moisture limitation remains unclear. Moreover, stomatal sensitivity can be adjusted in response to enduring environmental stress. For instance, Cardoso et al. (2020) showed that stomatal closure in response to VPD was delayed in plants with lowered leaf osmotic potential. This reduction in osmotic potential is achieved, among others, by accumulating soluble sugars in the cells, which lowers the turgor loss point (ψ TLP), that is, the leaf water potential below which the cells lose turgor and start to wilt. Such a response would allow extended stomatal opening and higher water losses before risking hydraulic failure under high VPD, thereby benefiting carbon assimilation. However, while adjustment of osmotic potential has been documented in roots and leaves in response to soil drought (Schönbeck et al., 2018), it is unknown whether similar mechanisms occur in response to high VPD and/or T under ample water supply.
Even after stomatal closure, water loss continues through incompletely closed stomata and the cuticle (i.e., minimum leaf conductance, g min) (Duursma et al., 2019), representing a significant risk for plants, particularly in the context of rising VPD. The cuticle, meant to serve as a protective leaf shield against water loss, pathogens and UV damage (Kerstiens, 1996; Schuster et al., 2017), still provides a significant alternative pathway for water to exit the leaf, with its conductance even exceeding that of leaky stomata (Gardingen & Grace, 1992). The mechanisms behind g min and the role of the cuticle are still poorly understood, as are the responses of g min to environmental changes. A reduction in g min was observed in response to soil drought and increasing VPD (Bengtson et al., 1978; Drake et al., 2018; Gardingen & Grace, 1992). In response to high T, both steep increases of g min (Schuster et al., 2016) and reduction due to long‐term heat stress have been demonstrated (Duursma et al., 2019). Nevertheless, the possible prominent role of g min in total water loss indicates that the mechanism must be considered a final step to plant desiccation under plant stress conditions.
In addition to leaf hydraulic properties, leaf T control is essential to maintain photosynthetic capacity under high T because biochemical processes like photosynthesis and respiration have a certain T optimum, below and above which these enzymatic processes slow down (Berry & Bjorkman, 1980). Higher T can induce stomatal opening to provide leaf cooling by evaporation (Urban et al., 2017), and may thus induce opposite effects to high VPD. Thermal tolerance, that is, the ability to photosynthesise under a specific high T (Seemann et al., 1984), might be strongly connected to plant hydraulics and drought tolerance (Gimeno et al., 2009; Knight & Ackerly, 2002), with low thermal tolerance requiring more leaf cooling and resulting in a high water demand under warm conditions. Xeric species adapted to dry conditions may thus have the possibility for stronger leaf cooling without risking hydraulic failure compared to mesic species (Urban et al., 2017). Higher T also decreases water viscosity, allowing higher leaf transpiration rates and possibly exerting more substantial reductions in leaf and stem water potential in addition to high VPD (Cochard, Martin, et al., 2000; Yang et al., 2020).
In this study, our goal was to disentangle the effects of T and VPD on plant hydraulic responses. We exposed well‐watered young trees from Fagus sylvatica L., Quercus pubescens Willd. and Quercus ilex, three species differing in hydraulic safety strategies (Supporting Information: Table S1 and Figure S1), to a cross‐combination of a T and VPD manipulation under unlimited soil water availability. We tracked the response of stem hydraulic conductivity and the leaf‐level mechanisms that may drive the loss of conductivity (g s, m, g min, ψ leaf, ψ TLP, leaf sugar concentrations). Specifically, we investigated whether increasing T and VPD would induce hydraulic stress in the form of a higher percentage loss of conductive area (PLA, %) of the stem xylem. We used microcomputed tomography (µCT) to determine PLA and confirmed the method with pressure‐flow techniques to assess loss of hydraulic conductance (PLC, %) (Sperry et al., 1988). We compared PLA responses with a range of plant traits (g s, g min, K s leaf sugar concentrations, and ψ TLP) to find potential drivers of PLA among all three species. We hypothesised that (1) increasing VPD, independent of T changes and in the absence of soil drought, causes tension on the hydraulic transport system as long as stomata remain open by reducing leaf water potential and inducing loss of xylem conductivity (PLC and PLA) with mesic species being more strongly affected than xeric ones; (2) higher T alone leads to higher foliar transpiration (and little to no stomatal regulation) thereby supporting leaf cooling but causing an aggravating effect on the loss of conductivity in combination with increasing VPD, especially in mesic species with a lower T optimum.
2. MATERIALS AND METHODS
2.1. Species and experimental setup
Three ecologically and hydraulically contrasting tree species relevant to a wide range of European forest ecosystems were selected for the experiment. On a gradient from mesic to xeric species, these are: the maritime‐temperate European beech (Fagus sylvatica L., provenance Biberist, Switzerland, 440–490 m asl), the sub‐Mediterranean pubescent oak (Quercus pubescens Willd., provenance Leuk, Switzerland, 720–750 m asl), and the Mediterranean holm oak (Quercus ilex, provenance Veneto region, Italy, 0–50 m asl) (Supporting Information: Figure S1, Table S1 for ψ TLP, K max and ψ P50). In March 2020, 108 even‐sized 3‐year‐old trees per species were planted from quick‐pots into 3 L pots filled with water‐retaining soil (40% clay, 25% bark compost, 20% broken puffed clay, 15% peat replacement from wood fibres; Kübelpflanzenerde, RICOTER Erdaufbereitung AG, Aarberg, Switzerland). Quick‐pots are tree propagation trays (650 cm3) which allow the roots to stay connected to the soil and not to be disturbed during transplanting. This study used six climate chambers (PGV36, Conviron) at the Phytotron facility of ETH, Zürich, Switzerland, to manipulate air T and VPD using a factorial design, each housing 18 individuals per species. The light roofs of the climate chambers were adjusted in height so that light intensity at canopy height was in all chambers ~390 µmol m−2 s−1. At this light intensity, all three species are at, or approach their light saturation point (Čater & Kobler, 2017; Pena‐Rojas et al., 2004; Petersson et al., 2020; Staudt et al., 2003). All plants were regularly (i.e., every 2–3 days) watered by hand to ensure complete soil hydration, and soil volumetric water content (VWC) was manually measured bi‐weekly to ensure no soil drought occurred (Supporting Information: Figure S2).
Due to a lockdown during the global pandemic of 2020, the plants were kept in a cool climate chamber (4°C) with 6 h of daylength during March and April 2020 to delay bud break until access to the climate chambers was possible in May 2020. The plants were first exposed to an acclimation period of 5 weeks to recover from the transport and leaf flush inside the climate chambers. During this period, all chambers were set to 16 daylight hours, T of 25°C, and relative humidity (RH) of 50%. Nighttime was 6 h long with a T of 15°C and RH of 50%. One‐hour dawn and dusk occurred between day and night. Air T and humidity were continuously (10 min resolution) measured at canopy height in each chamber with Onset HOBO MX T and RH loggers (Onset computer corporation).
After the acclimation period, three chambers were set to daytime T of 25°C and three to 30°C. Nighttime T was set to 10°C lower than during the day in all chambers (i.e., 15°C and 20°C). Within every T group, chambers were given a low (1 kPa ± 0.3), medium (1.6 kPa ± 0.3), or high (2.2 kPa ± 0.3) daytime VPD treatment by setting RH to reach the desired VPD levels. The highest VPD level was selected based on the physical limitations of the climate chambers to reach a maximum temperature of 30°C and the minimum RH that could be reached with the addition of a dehumidifier. While a VPD of 2.2 kPa is not excessive compared to what the xeric species in this study experience during the dry season in their natural habitat (Tognetti, Longobucco, et al., 1998), we do believe the range of VPD was sufficient to induce plant hydraulic changes. Because of difficulties in regulating humidity levels in the chambers, RH was kept similar during day and night, even though such conditions are unlikely in real‐world conditions. The goal RH was calculated by solving the equation for VPD using the Tetens formula (Monteith & Unsworth 2013). VPD was calculated as the difference between saturated and actual VPD:
| (1) |
| (2) |
where VPsat is saturated VP at a given T in °C.
A humidifier was added to the chamber with 30°C + low VPD (to reach 78% RH), and dehumidifiers were used to increase VPD as high as possible in the 25°C and 30°C chambers + high VPD. While all chambers maintained stable T throughout the experiment, the difficulty in manipulating air humidity in the chambers led to slight VPD variation over time (Supporting Information: Figure S2). Despite this, VPD levels were consistently within the set range (0.7–1.3 kPa for low, 1.3–1.9 kPa for medium, and 1.9–2.5 kPa for high VPD) (Figure 1 and Supporting Information: Figure S2).
Figure 1.
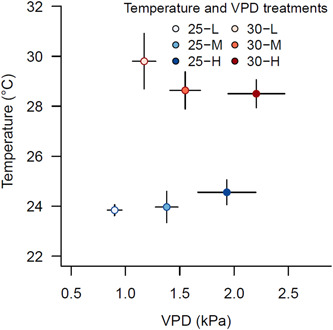
Average temperature and vapour pressure deficit (VPD) in the six climate chambers. Plants were exposed to two temperature treatments (25°C and 30°C) and three VPD levels that are defined by low (L), middle (M) and high (H) VPD. Symbols indicate the average (±SD) over the total treatment period (June 1st – November 8th, 2020). [Color figure can be viewed at wileyonlinelibrary.com]
Six plants per chamber and species were randomly selected for repeated physiological measurements. The physiological measurements were carried out during four campaigns that were held at a ~5‐week interval, with the first campaign just before the start of the treatments: 1–10 June (campaign 1, pretreatment); 13–23 July (campaign 2, +5 weeks); 26 August–4 September (campaign 3, +10 weeks); and 19–31 October (campaign 4, +15 weeks). Across all campaigns, physiological measurements were performed on the same leaf of each individual, unless the leaf wilted or dropped. During each campaign (apart from campaign 3), six randomly selected individuals per chamber and species were harvested for destructive measurements as described below (Supporting Information: Figure S2). The individuals used for physiological measurements were harvested during the last campaign.
2.2. Stomatal conductance and VPD response
Stomatal conductance (g s, mmol m−2 s−1) and transpiration (E, mmol m−2 s−1) were measured on each tree selected for repeated physiological measurements (six replicates per species) during each campaign using four LiCor LI‐6800 (LiCor Inc.). One leaf was clipped in the cuvette, set to ambient chamber T and RH, with a light intensity of 1500 µmol m−2 s−1 PAR and flow at 500 µmol s−1. While 1500 µmol m−2 s−1 is well above the ambient light conditions in the chambers, using this standard light value during gas exchange measurements ensures cross‐comparison with other studies and light‐saturation of the trees. The leaf was left acclimating for 20 min or longer if needed to reach stable g s. The g s at 400 ppm CO2 was extracted from photosynthesis over CO2 (A/Ci) measurements, including three log entries at 400 ppm CO2. The three measurements were then averaged.
Response curves of g s to VPD variation were measured on five replicates per species by measuring g s at 75, 60, 45, 30, 15 and 5% RH, with similar light, CO2, T, and flow as described above. RH was chosen to vary instead of VPD to ensure that the VPD would be solely controlled by RH in the LiCor instrument. Each step included a minimum waiting time of 15 min for F. sylvatica and 20 min for both Quercus species to allow for g s stabilisation between each RH step. F. sylvatica reached stable g s faster than the two Quercus species. In the chambers with the highest VPD (i.e., lowest humidity), the LiCor devices did not always reach 75% RH. Nonetheless, all g s to VPD curves started at VPD values <1.1 kPa. The reference g s at 1 kPa VPD (g s, ref, mmol m−2 s−1) and the stomatal sensitivity (m, mmol m−2 s−1 kPa−1) of each tree and each campaign was extracted by fitting logarithmic curves to the data (for detailed curve fitting methods, see Supporting Information: Methods S1, Figures S4 and S5):
| (3) |
The curve fits resulted in an m to g s, ref ratio of 0.46, which is slightly lower but close to the suggested ratio of 0.5–0.6 suggested by Oren et al. (1999) (Supporting Information: Figure S5). The g s to VPD response curves differ from the point measurements in the climate chambers at the ambient VPD levels. The VPD response curves represent the response to rapid changes in VPD (over 2 h), while the point measurements represent the long‐term acclimation of g s to different VPD levels. In addition, the VPD response curves were done over a more extensive range of VPD levels (0.8–3.5 kPa) than the chambers could reach (1–2.2 kPa) (see also Supporting Information: Methods S1).
2.3. Minimum leaf conductance (g min)
Minimum leaf conductance (Kerstiens, 1996) was measured as described in Pearcy and Zimmermann (2000). One leaf per individual was cut before dawn when stomata were assumed to be still closed. The cut petiole was immediately sealed with melted candle wax, and the leaf area was scanned using a flatbed scanner, followed by analysis using Pixstat (Schleppi, 2021). The leaves were stuck to a lab tape run between two lab stands, standing in a small dark climate chamber with stable T (26°C) and humidity (60%) and the ventilation on. Every 15–20 min, the leaves were taken from the climate chamber and weighed in a dark room using a fine‐precision scale (Mettler‐Toledo). This procedure was repeated eight times. g min (mmol m−2 s−1) was calculated as cuticular transpiration per mole fraction VPD, assuming the leaf internal air to be fully saturated (Pearcy & Zimmermann, 2000).
2.4. Pressure volume curves and leaf water potential at predawn and midday
Pressure‐volume curves were determined using the bench‐dehydration method (Koide et al., 2000). Before dawn, a leaf from the top of the crown was cut off and immediately sealed in a plastic bag (Whirlpak) that was previously exhaled. Predawn water potential (kPa) was measured directly using a Scholander‐type pressure chamber (PMS Instrument Company, Model 1505D). The same leaf was immediately weighed using a fine‐precision scale (Mettler‐Toledo), placed in a plastic bag, and allowed to dry progressively in the open plastic bag on a lab bench. The procedure of measuring water potential, weighing, and drying was repeated with increasing drying time intervals (from 10 s to 1 h) for the two Quercus species until achieving water potentials of about −4 MPa or until water potential reached a plateau. For Fagus sylvatica, the procedure was repeated continuously without letting the leaves dry on the bench due to the rapid water loss and a corresponding drop in leaf water potential. Subsequently, the leaves were individually put in a paper bag and dried in an oven at 60°C for 24 h to determine the dry mass. Leaf water potential at turgor loss point (Ψ TLP, MPa) was calculated after Koide et al. (2000). At midday, another leaf was cut off from the same individuals, and midday water potential (Ψ md) was measured.
2.5. Percent loss of conductive area (PLA)
On the three harvest dates (June, July and October), six trees per chamber and species were transported to the Interdisciplinary Platform for X‐ray microcomputed tomography (µCT) (PIXE, EPFL) and stored in a cool room in the absence of direct light (to avoid transpirational water loss), until they were scanned. For the µCT scanning, the tree was fixed in a custom‐built plant holder, and its branches were wrapped in cling film to prevent movements during the measurements that could alter the quality of the images. A 1 cm part of the stem to be scanned at approximately 40 cm height was marked with tape before starting the measurements. The tree was then moved onto the scanning platform and scanned at 80 keV and 87 µA in the RX‐Solutions Ultratom X‐ray scanner using a Hamamatsu 230 kV X‐ray tube in reflection mode. The sapling rotated in 0.22° increments during the scan, yielding between 1400 and 1600 two‐dimensional projections with a ~5–7 mm pixel resolution. The acquired longitudinal projections were reconstructed (Filtered backprojection) into a ‘stack’ of multiple transverses TIF images using Xact (RX‐Solutions, version 2.0 R9901). After scanning, the scanned part of the stem was cut and flushed with 1 bar air pressure for 1.5 min and subsequently scanned again to obtain a fully embolized stem cross‐section as a reference that allowed us to visualise all vessels in the sapwood (Figure 2).
Figure 2.
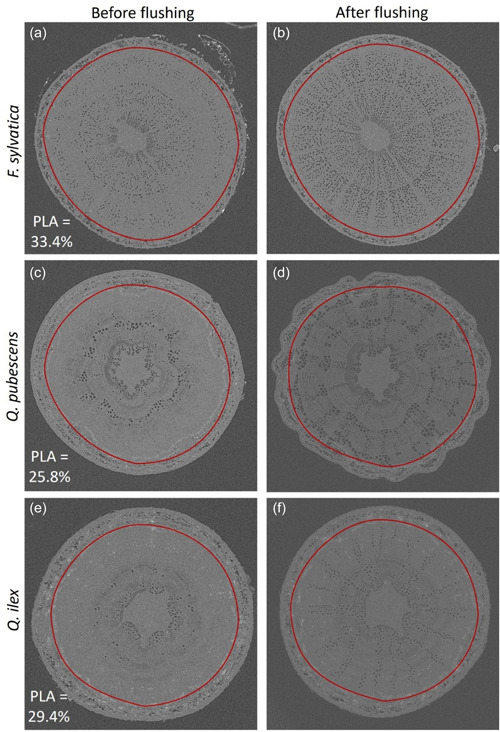
Microcomputed tomography images of stem sections of Fagus sylvatica (a, b), Quercus pubescens (c, d) and Quercus ilex (e, f) on the intact stems (a, c, e) and after flushing the stem segments with air at high pressure (b, d, f). Black areas indicate air‐filled vessels. Grey areas indicate wood and water‐filled sections. The red circles indicate the area of interest, including only the xylem and excluding bark and phloem. Percent loss of conductive area (PLA) was calculated as embolized vessel area/total vessel area × 100%. [Color figure can be viewed at wileyonlinelibrary.com]
Image analysis was done with the Avizo software (2019.4). The assessment was done on one image located in the middle of the scanned volume, as we found no significant differences between the bottom, top and middle of the 1 cm stem portion during preliminary tests. The area of interest was selected by excluding bark and phloem (Figure 2). Segmentation was performed by defining a selection threshold such that most of the air around the stem was chosen as a reference, without including any material on the bark and making sure that the concurrently selected void vessels did not merge due to a wide selection range. A visual assessment of each scan followed the automated threshold tool segmentation to assess scan quality, artifacts and white level. PLA (%) was calculated as the total embolized area in the intact stem divided by the total vessel area in the flushed stem (x100%). Due to flushing, some stem samples had shrunk. A correction factor was used to control the stem area of the shrunk sample. To estimate the impact of the treatments over time, we used the average PLA per species and chamber from the first harvest (i.e., to account for potential cavitation present before the treatments started) and deducted these values from the results of the second and third harvest (dPLA, %).
2.6. Percent loss of conductivity (PLC)
On the last harvest, after the trees were scanned by the µCT, the stem was cut immediately above the scanned part to measure the hydraulic conductivity. These measurements were done to confirm the methodology and results of the µCT scans. Hydraulic conductivity (K, kg m s−1 MPa−1) was measured using a commercial XYL'EM Plus apparatus (Bronkhorst) according to the method described by Sperry et al. (1988). The branch was recut underwater and left in the water for at least 30 min to relax xylem tension in the branch segment. The segment was then cut to its final size. Its proximal end was connected to the tubing system of the XYL'EM, which was filled with deionized filtered and degassed water with 10 mM KCl and 1 mM CaCl2, flowing from an elevated source. Initial hydraulic conductivity (Ki , kg m s−1 MPa−1) was recorded. The stem segment was then flushed with water at 1.5 bar for 1 min to remove emboli, and its maximum hydraulic conductivity (K max, kg m s−1 MPa−1) was measured. A second flush at 1.5 bar for 30 s followed by a measurement was done to confirm the maximum hydraulic conductivity value. Percentage loss of conductivity (PLC, %), a direct estimate of the percentage of embolized vessels (Cochard, Bodet, et al., 2000), was calculated as
| (4) |
2.7. Leaf sugar concentrations
At each destructive sampling campaign (i.e., first, second and last campaign), four leaves per individual were dried in an oven at 60°C until reaching stable weight. The leaf material was homogenised with a ball mill. Sugar concentrations were determined with an enzymatic extraction method described by Wong (1990) and adapted according to Hoch et al. (2002). The sugars measured using this method are defined as low molecular weight sugars (glucose, fructose and sucrose). 10–12 mg of ground material was boiled in 2 ml distilled water for 30 min. After centrifugation, an aliquot of 200 µl was treated with Invertase and Isomerase from baker's yeast (Sigma‐Aldrich) to degrade sucrose and convert fructose into glucose. The total amount of glucose (sugars) was determined photometrically at 340 nm in a 96‐well microplate photometer (HR 7000, Hamilton) after enzymatic conversion to gluconate‐6‐phosphate (hexokinase reaction, hexokinase from Sigma Diagnostics). Pure glucose‐, fructose‐ and sucrose‐ solutions were used as standards, and standard plant powder (Orchard leaves, Leco) was included to control the reproducibility of the extraction. Sugar concentrations are expressed on a percent dry matter basis. Because all samples were run in a single laboratory with no change in protocol during the processing, issues with comparing results across methods or labs were obviated (Quentin et al., 2015).
2.8. Statistical analysis
The similarities between PLA and PLC measurements were tested by fitting a linear model to the data with PLA explaining PLC. If the confidence interval of the slope includes 1, a 1:1 relationship between PLA and PLC is assumed.
2.9. Treatment differences
Data were analysed for each species separately. A mixed‐effect model was carried out for each parameter (excl. dPLA and PLC, see below) with T, VPD and campaign (only the three measurement campaigns after the start of treatment) as fixed factors, including all interactions while controlling for repeated measures on the tree individual (included as a random factor). The model was then analysed using a type‐3 analysis of variance (ANOVA) using Satterthwaite's estimation. The timepoint did not show any significant interactions with the treatments. Thus, it was decided to pool all data of the three campaigns. A two‐way ANOVA without mixed effects (no repeated measurements) was used for dPLA and PLC, with T and VPD, including their interaction, as explanatory variables.
2.10. Correlations between plant physiological parameters
To relate PLA to different leaf‐level hydraulic characteristics that may drive the loss of conductivity, correlation analyses were carried out for PLA paired with all other parameters: E, g s, g min, m, ψ TLP, ψ midday, K s, max and sugar concentration in the leaves. Data for all species were pooled. For significant correlations (p < 0.05), the parameters were plotted, and a regression line was added to illustrate the relationship between the two.
3. RESULTS
3.1. Correlation between PLA and PLC
The PLC (%) measured with the pressure‐flow technique and the PLA (%) measured using µCT were strongly correlated (Figure 3). The regression line did not significantly deviate from the 1:1 line, indicating the µCT method is reliable and comparable to the pressure‐flow technique (Nolf et al., 2017). We will focus mainly on the PLA results in the following sections because PLC was only measured in the last campaign, while PLA was measured during three campaigns.
Figure 3.
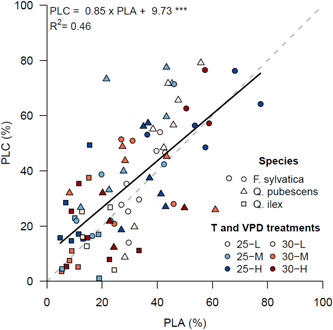
Relationship between the percentage loss of conductive area (PLA, %), measured using microcomputed tomography, and the percentage loss of conductivity (PLC, %), calculated using hydraulic conductivity measurements. Symbols indicate species and colours indicate temperature and vapour pressure deficit treatments. The dashed grey line indicates the 1:1 ratio. The black line indicates the fitted regression line. Confidence interval of the slope was 0.65–1.05, indicating no significant deviation from the 1:1 line. [Color figure can be viewed at wileyonlinelibrary.com]
3.2. VPD and T effects on plant hydraulics
3.2.1. F. sylvatica
Increased VPD and T significantly raised the loss of hydraulic conductance (dPLA, the difference between pretreatment and during‐treatment PLA, and PLC) in F. sylvatica (Figure 4, Supporting Information: Table S2). High VPD caused a decrease in ψ leaf, pd, ψ leaf, md and g min (Figures 4 and 5, Supporting Information: Tables S2 and S3), but the latter only in the 30°C chambers. Higher T reduced ψleaf, md, and interacted with VPD, causing even stronger reductions of ψ leaf, md with higher VPD. Transpiration (E) increased with rising VPD, but no effect of T was observed. T but not VPD was found to affect stomatal sensitivity (m), where m was higher at 30°C than at 25°C. No impact of T or VPD was seen on ψ TLP and g s, although a decreasing trend with higher VPD was visible for the latter.
Figure 4.
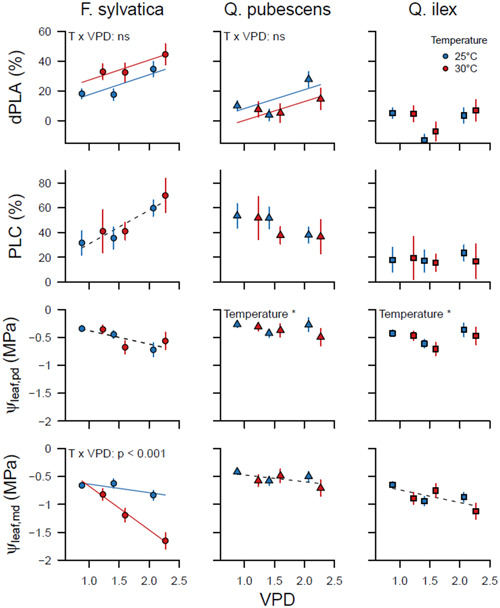
Percentage loss of conductive area, calculated as the change in percent loss of conductive area (PLA) from the start of the experiment (dPLA, %total 2dembolized−%embolized at campaign 1), percentage loss of conductivity (PLC) and predawn and midday leaf water potential (ψ leaf, pd & ψ leaf, md) in Fagus sylvatica, Quercus pubescens and Quercus ilex in the two temperature and three vapour pressure deficit (VPD) treatments. Data are shown in relation to the average VPD in the chambers during the treatment period. Symbols indicate the mean ± SE of the three measurement campaigns (n = 18), except for PLC which was measured once at the end of the experiment (n = 6). Dashed lines indicate significant VPD effects without temperature effects. Coloured lines—blue for 25°C and red for 30°C—indicate an additive (T x VPD: ns) or interacting (T x VPD: p < 0.05) temperature effect in addition to VPD. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 5.
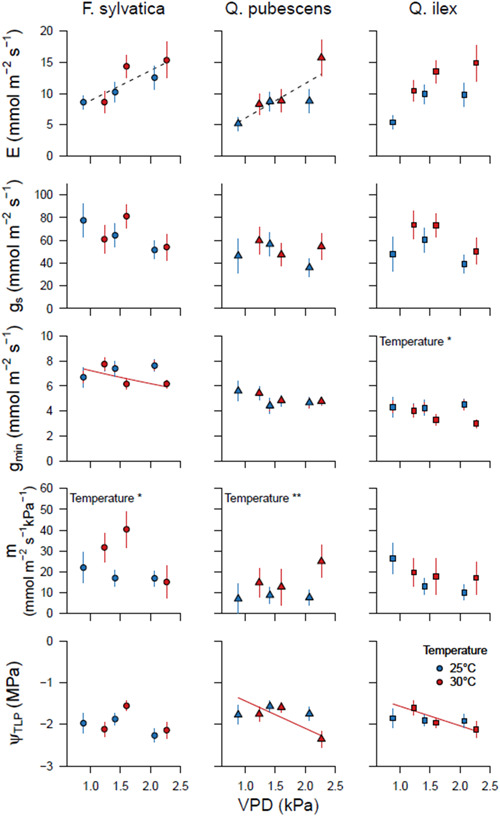
Transpiration (E), stomatal conductance (g s), minimum leaf conductance (g min), sensitivity of g s to vapour pressure deficit (VPD) (m) and turgor loss point (ψ TLP) in Fagus sylvatica, Quercus pubescens and Quercus Ilex in the two temperature and three VPD treatments. Data are shown in relation to the average VPD in the chambers during the treatment period. Symbols indicate the mean ± SE of three measurement campaigns (n = 18). Dashed lines indicate significant VPD effects without temperature effects. Coloured lines—blue for 25°C and red for 30°C—indicate the VPD effects in the separate temperature treatments in case of a T x VPD interaction. In case of absence of a VPD effect, temperature effects are indicated with asterisks (*p < 0.05; **p < 0.01; ***p < 0.001). [Color figure can be viewed at wileyonlinelibrary.com]
3.2.2. Q. pubescens
dPLA increased with rising VPD in Q. pubescens and was lower at 30°C than 25°C (Figure 4, Supporting Information: Table S2). No treatment effects were found for PLC. Higher VPD caused an increase in E and a reduction in ψ leaf, md, and ψ TLP. An interaction between T and VPD affected ψ TLP, where ψ TLP decreased with higher VPD only in the 30°C chambers (Figure 5, Supporting Information: Table S3). m was higher and ψ leaf, pd was lower at 30°C than at 25°C (Figures 4 and 5). No VPD or T effects were found for g s, or g min (Figure 5).
3.2.3. Q. ilex
VPD did not affect either dPLA or PLC (Figure 4, Supporting Information: Table S2), nor E, m, g s or g min (Figure 5, Supporting Information: Table S3). g min and ψ leaf, pd were slightly lower in the 30°C than the 25°C chambers. As for Q. pubescens, ψ TLP decreased with increasing VPD but only in the 30°C chambers (Figure 5, Supporting Information: Table S3).
3.3. Correlation between PLA and leaf traits
Across all species, PLA was positively correlated with g min, K max and sugar concentrations in the leaves, indicating that higher water transport rates, evaporative water loss, and osmotic potential were related to higher embolism rates (Figure 6). However, the correlations between PLA and K max or sugar concentrations were only found in the 30°C treatments, suggesting that enhanced water transport (potentially leading to higher E) and osmotic potential (potentially delaying stomatal closure) only drive increased PLA when the temperature is high. In addition, more negative ψ leaf, md were correlated to higher PLA, but only in the 30°C chambers, indicating that higher tension within the conductive leaf tissues (because of sustained stomatal opening) translated to higher levels of stem xylem embolism (Figure 6). No correlation was found between PLA and g s, m and ψ TLP.
Figure 6.
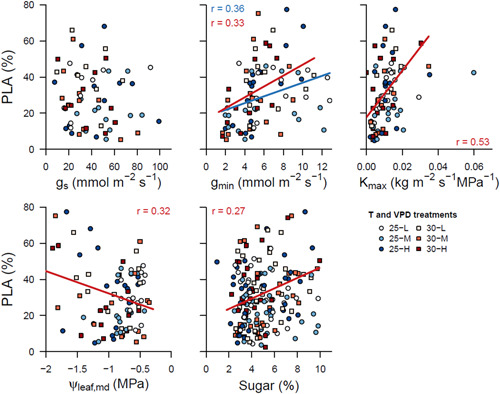
Correlation analysis of percent loss of conductive area with stomatal conductance (g s), minimum leaf conductance (g min), maximum hydraulic conductance of the stem (K max), water potential of the leaf at midday (ψ leaf, md) and sugar concentrations in the leaves. Coloured lines indicate significant correlations within the corresponding temperature treatment (blue for 25°C and red for 30°C). Analyses were done with all species pooled. [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
4.1. Effects of increasing VPD and temperature on the plant hydraulic system in the absence of soil drought
For the first time, we disentangled the effects of temperature (T), VPD, and their interactions in the absence of soil drought on plant hydraulic traits. Doing so is rare due to the tight relationship between T and VPD in nature. We show that rising T and VPD can cause major hydraulic dysfunctions in trees without soil drought. This was demonstrated by the significant loss of xylem conductive area and conductivity (PLA and PLC, respectively) in F. sylvatica and Q. pubescens and the increasingly negative leaf water potential (ψ leaf, md) in all species with increasing VPD and T (Figure 4). Considering that this study covered only one growing season and that VPD and T levels were moderate compared to the extreme conditions that occur in nature (e.g., the 2018 hot drought in Europe) (Fu et al., 2020; Senf & Seidl, 2021), these results highlight the severe threat that chronic VPD and T rise pose on mesic trees, even without any changes in precipitation. Given the high reliability of T predictions in climate models, compared to the uncertainties associated with precipitation (IPCC, 2021), these results are particularly relevant for modelling forest ecosystem functioning. While understanding how VPD and T affect plant function is fundamental, it is important to note that our experimental design limits our ability to extend these results to real‐world implications. In the field, elevated VPD for several weeks would most likely lead to reduced soil moisture.
We expected to see an increasing gradient in PLA from xeric towards mesic species in response to rising T and VPD, due to differing hydraulic strategies and adaptations (Meyer et al., 2020), with more extensive T and VPD effects on mesic F. sylvatica than the rather xeric Q. pubescens and Q. ilex. Indeed, 30°C and high VPD (2.2 kPa) exposed F. sylvatica to ψ leaf close to its turgor loss point (−2 MPa, Figures 4 and 5, Supporting Information: Table S1). Combined with barely declining g s and no change in sugar concentration, the absence of stomatal closure and osmotic adjustments increased PLA and PLC. Our results correspond to earlier findings where VPD levels as low as 1.4 kPa caused biomass and ψ leaf reduction in F. sylvatica (Lendzion & Leuschne,r 2008). Moreover, the lack of leaf‐level acclimation (e.g., stomatal closure or adjustment of turgor loss point) was previously observed in F. sylvatica during soil drought (Backes & Leuschner, 2000; Pflug et al., 2018; Schipka et al., 2005; Thomas, 2000). Our observations that transpiration (E) continues even at high levels of embolism (Figure 5) were confirmed in adult F. sylvatica trees in Switzerland (Walthert et al., 2021). In this study, the authors further suggested that F. sylvatica does not prevent water loss and embolism by leaf physiological acclimation or shedding but sheds its leaves only after embolism has occurred (Walthert et al., 2021). Recently, Zhu et al. (2022) showed how F. sylvatica leaf traits were driven by previous years' VPD over a record of 25 years, suggesting a strategy of leaf shedding and regrowth rather than acclimation during the current year. Overall, our work indicates that the strategy of F. sylvatica results in a high risk for hydraulic failure under moderate atmospheric stress (Burkhardt & Pariyar, 2016). Together with the slow recovery capability of this species after stress exposure (Hacke & Sauter, 1996), these findings highlight its high sensitivity to projected climate (Dittmar et al., 2003; Geßler et al., 2004).
For Q. pubescens, PLA increased with rising VPD, although it was generally lower than in F. sylvatica. ψ leaf, md did not reach values lower than −1 MPa, indicating a reduced T and VPD impact on the hydraulic system compared to F. sylvatica. In contrast to F. sylvatica, where no physiological adjustment to rising VPD and T was found, Q. pubescens lowered its ψ TLP to withstand more negative leaf water potentials and sustain high g s and E under rising T and VPD. These results indicate a more conservative water use strategy than F. sylvatica. Q. pubescens is one of the most widespread species in southern Europe and is known for its high thermal tolerance and drought resistance (Wellstein & Spada, 2015). Previous studies showed that this species is well protected against heat‐induced perturbations (Haldimann & Feller, 2004). Yet, our work suggests that rising T and VPD levels, even moderate ones that this species is frequently exposed to in nature, could, to some extent, negatively impact the efficiency of the hydraulic system. Here, we wanted to expose different tree species to comparable T and VPD levels to assess species sensitivities. Still, to better understand VPD and T effects in real‐world conditions, future work should focus on extreme conditions that southern tree populations are more likely to experience.
Variation between the two xeric Quercus species was expected due to their contrasting leaf habit (deciduous vs. evergreen) and xylem conduit size (ring‐porous vs. diffuse‐porous) (Tognetti, Longobucco & Raschi, 1998). PLA and PLC of Q. ilex were, in contrast to Q. pubescens, not affected by VPD and T, confirming the low sensitivity of this species to VPD and T, partially due to its smaller, diffuse‐porous vessels. This Mediterranean species is highly adapted to dry environments (Barbero et al., 1992), and the T and VPD levels it was exposed to are likely far from its thermal and hydraulic limits (Supporting Information: Figure S1, Table S1). Moreover, with its tough, evergreen leaves, it reaches photosynthetic efficiency both in cool winter T and dry summers, demonstrating adaptation of the species to a range of extreme conditions far from our experiment (García‐Plazaola et al., 1999). Its physiological plasticity was shown by reducing g min and ψ TLP in response to increasing T and VPD, respectively, even if these had no impact on PLA. The strong response to these relatively minor changes confirms the rather drought‐avoiding behaviour of the species (Gullo & Salleo, 1990).
4.2. Mechanisms driving PLA
We expected significant leaf‐level adjustments in response to VPD and T and a correlation between the leaf‐level responses and PLA. These relationships would help identify underlying drivers of hydraulic conductivity changes. Increasing VPD led to higher leaf‐level transpiration (E, Figure 5). Still, against our expectations, stomatal conductance and the stomatal sensitivity to VPD (m) showed the most negligible response to T and VPD, neither were they, nor E correlated with PLA (Figure 6). A reason for the absence of stomatal response (g s and m) to VPD and T in all species might be a combination of the choice of species and the level of evaporative demand in the climate chambers. In the case of F. sylvatica, a moderate increase in VPD in the absence of soil drought didn't lead to stomatal closure but enhanced E, thereby creating tensions within the xylem that sustained embolism formation. The strategies discussed for F. sylvatica point to a risk‐taking strategy where leaf shedding due to stress would be more likely than stomatal closure to prevent embolisms (Walthert et al., 2021). In contrast, Q. pubescens and Q. ilex kept their stomates open at the VPD levels in our chambers, but ψ leaf was not sufficiently low to induce embolism. These findings shed new insights into the sequence of hydraulic shutdown in plants. The sequence of stomatal closure, turgor loss, and loss of xylem conductivity have been studied thoroughly in relation to soil drought, where the ψ leaf is a leading indicator for the occurrence of leaf and wood hydraulic pathway failures (Bartlett et al., 2016). These findings suggest that 50% PLC approximately coincides with the point where g s decreases by 95% (ψ gs95), indicating a strong correlation between g s and PLC. In our study, we could not confirm the strong correlation between hydraulic conductance and g s, suggesting different pathways in response to atmospheric drought compared to soil drought.
Minimum leaf conductance (g min) was positively correlated with PLA across all species (Figure 6), indicating that plants or species with higher evaporative water loss would have a higher risk for embolisms under rising VPD and T. Interestingly, with increasing T, this correlation was even steeper. g min has long been considered an insignificant factor in crop drought resistance (Kerstiens 1996). However, recent studies provide evidence that g min may be the last hurdle before dehydration, thereby playing a much more important role than previously thought (Duursma et al., 2019). Here, we show that g min might be responsible for increased cavitation risk under high VPD, T and non‐limiting soil moisture conditions. The PLA effect of g min might have been exacerbated by the relatively high nighttime VPD levels in our experiment (Supporting Information: Figure S2), compared to natural conditions where relative humidity often approaches 100% during the night. The relatively high VPD and residual water loss from the leaves caused lowered predawn water potentials in F. sylvatica even though the soil was fully hydrated (Figure 4). In F. sylvatica and Q. pubescens, g min rates were approximately 10% of the g s values (Figure 5), indicating a significant water loss at night or when stomata close. The capability to adjust g min in response to a changing environment could be advantageous for protecting valuable xylem vessels. g min reduction was indeed observed in F. sylvatica and Q. ilex in response to increasing T and VPD, or T only, respectively (Figure 5), suggesting lower residual water loss in warmer and drier conditions. These results correspond with other studies that have shown a decrease in g min in response to higher evaporative demand (Fanourakis et al., 2013). It is unknown whether g min changes are caused by the altered chemical composition of the cuticle, increased cuticle deposition, or changing stomatal anatomy in the longer term (Duursma et al., 2019). The relationship between g min and T turns out to be even more complex: rapid increases of g min were observed in response to increasing T (Drake et al., 2018; Schuster et al., 2016), but a negative relationship was found between thermal tolerance and g min (Schuster, 2016), indicating that acclimation to increasing T leads to lower g min. However, g min adjustments in F. sylvatica were insufficient in our study to prevent plant dehydration and increase PLA under moderately rising VPD.
Higher PLA was also associated with higher maximum stem hydraulic conductance (K max) across species, supporting previous work that reported increased risk for embolisms with higher water transport capacity (Tognetti, Longobucco, Raschi, 1998). There was a gradient in K max between F. sylvatica, Q. pubescens and Q. ilex (0.014, 0.013 0.007 kg m s−1 MPa−1 resp.), in line with the degree of PLA over those three species (Table S1, Supporting Information: Figure S6). These results correspond to the safety‐efficiency trade‐off (Grossiord, Ulrich, et al., 2020), whereby high K max provides fast and efficient water transport but with an increased risk of embolism even in the absence of soil moisture stress. The strongest correlation between PLA and K max in the 30°C chambers could be explained by the lower water viscosity at warmer T, as higher water transport rates could lead to faster dehydration and increased PLA (Cochard, Martin, et al., 2000).
Interestingly, leaf sugar concentration was also positively correlated with PLA. VPD and T effects were only found on sugar concentrations of Q. pubescens (Supporting Information: Figure S6). Increasing T resulted in higher leaf sugar concentrations, probably due to rising assimilation rates as T optima for temperate European Quercus species can reach up to ~30°C–35°C (Daas et al., 2008). In contrast, higher VPD resulted in a minor but significant reduction of sugar concentration in the leaves of Q. pubescens, thereby reducing the osmotic potential. Trees tend to accumulate sugars in leaves and roots, lower the turgor loss point, and increase the water holding capacity in response to soil drought (Schönbeck et al., 2018). Although for Quercus species, an adjustment of ψ TLP was observed, the reduced sugar concentrations suggest that other chemical compounds might be responsible for the reduction in ψ TLP.
4.3. The individual role of T and VPD on plant hydraulics
The aggravating effect of T in interaction with VPD, mainly in F. sylvatica, suggests that T and VPD play independent roles in affecting plant hydraulics. However, VPD seems to be the stronger driver of plant hydraulics. PLA, PLC, ψ leaf, E, g min and ψ TLP were all affected by VPD in one or more species. On the other hand, T appears to aggravate VPD effects (for ψ leaf, g min, ψTLP) while only acting independently towards PLA and m (Figures 4 and 5). Earlier studies confirm that higher T can aggravate the adverse effects of increasing VPD (Barron‐Gafford et al., 2007), as physiological controls for water transport become less effective at higher T (Sermons et al., 2012). The relationship between T and plant hydraulics is complex and partly indirect: T increases E (Urban et al., 2017), thereby providing leaf cooling in warmer climates. However, against expectation, we did not find an individual role for T in affecting E (Figure 5). This finding indicates that 30°C was insufficient to induce active leaf cooling.
5. CONCLUSION
For the first time, we show that rising VPD and T can lead to stem conductivity losses even when soil water is not limiting. Although VPD and soil water are often correlated on a monthly to seasonal time scale, our results show the possible outcomes in the case of a heatwave occurring after or during a period of sustained precipitation. Disentangling the effects of VPD and T on plant hydraulics is of the utmost importance, as future T scenarios are well developed. Still, much more uncertainty exists on the air relative humidity. Therefore, predicting the effects of rising atmospheric evaporative demand on plants is challenging. Our findings highlight that VPD and T affect different hydraulic functions, hence having differential consequences that are species‐dependent. A prolonged but moderate increase in VPD and, to a certain extent, T led to hydraulic dysfunctions for F. sylvatica and Q. pubescens because of limited stomatal closure, higher transpiration, and more negative leaf water potentials. Whether these mechanisms are universal across a broad range of species remains to be tested as the relatively mild conditions used in this experiment were insufficient to induce significant xylem tensions for the xeric Q. ilex species. Although rising CO2 levels are thought to possibly compensate for the adverse rising VPD effects by increasing the water use efficiency (Eamus, 1991), uncertainties are significant, and further investigation into the interaction between VPD and CO2 is needed. Nevertheless, our work emphasises the importance of recognising VPD and T as dominant drivers of plant functioning, both independently from each other and in interaction, to anticipate future impacts on ecosystems.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
We thank Prof. Nina Buchmann and Hans‐Jürg Lendi for their support and use of the climate chambers. We are thankful to Gary Perrenoud for his help with the µCT measurements. Leonie C. Schönbeck, Charlotte Grossiord, Philipp Schuler and Marco M. Lehmann were supported by the Swiss National Science Foundation (P500PB_203127, PZ00P3_174068, and PZ00P2_179978). The Sandoz Family Foundation also supported Charlotte Grossiord. Open access funding provided by Ecole Polytechnique Federale de Lausanne.
Schönbeck, L. C. , Schuler, P. , Lehmann, M. M. , Mas, E. , Mekarni, L. , Pivovaroff, A. L. , et al. (2022) Increasing temperature and vapour pressure deficit lead to hydraulic damages in the absence of soil drought. Plant, Cell & Environment, 45, 3275–3289. 10.1111/pce.14425
Journal: Plant, Cell and Environment
REFERENCES
- Adams, H.D. , Guardiola‐Claramonte, M. , Barron‐Gafford, G.A. , Villegas, J.C. , Breshears, D.D. , Zou, C.B. et al. (2009) Temperature sensitivity of drought‐induced tree mortality portends increased regional die‐off under global‐change‐type drought. Proceedings of the National Academy of Sciences, 106, 7063–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, C.D. , Breshears, D.D. & McDowell, N.G. (2015) On underestimation of global vulnerability to tree mortality and forest die‐off from hotter drought in the anthropocene. Ecosphere , 6(8), art129. 10.1890/es15-00203.1 [DOI] [Google Scholar]
- Anderegg, W.R.L. & Meinzer, F.C. (2015) Wood anatomy and plant hydraulics in a changing climate. In: Hacke, U. (Ed.) In functional and ecological xylem anatomy . Springer International Publishing. pp. 235–253. [Google Scholar]
- Backes, K. & Leuschner, C. (2000) Leaf water relations of competitive Fagus sylvatica and Quercus petraea trees during 4 years differing in soil drought. Canadian Journal of Forest Research, 30, 335–346. [Google Scholar]
- Barbero, M. , Loisel, R. & Quézel, P. (1992) Biogeography, ecology and history of Mediterranean Quercus ilex ecosystems. In: Romane, F. & Terradas, J. (Eds.) In Quercus ilex L. ecosystems: function, dynamics and management . Springer Netherlands, Dordrecht. pp. 19–34. [Google Scholar]
- Barron‐Gafford, G.A. , Grieve, K.A. & Murthy, R. (2007) Leaf‐ and stand‐level responses of a forested mesocosm to independent manipulations of temperature and vapor pressure deficit. New Phytologist, 174, 614–625. [DOI] [PubMed] [Google Scholar]
- Bartlett, M.K. , Klein, T. , Jansen, S. , Choat, B. & Sack, L. (2016) The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proceedings of the National Academy of Sciences, 113, 13098–13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson, C. , Larsson, S. & Liljenberg, C. (1978) Effects of water stress on cuticular transpiration rate and amount and composition of epicuticular wax in seedlings of six oat varieties. Physiologia Plantarum, 44, 319–324. [Google Scholar]
- Berry, J. & Bjorkman, O. (1980) Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology, 31, 491–543. [Google Scholar]
- Buckley, T.N. (2005) The control of stomata by water balance. New Phytologist, 168, 275–292. [DOI] [PubMed] [Google Scholar]
- Burkhardt, J. & Pariyar, S. (2016) How does the VPD response of isohydric and anisohydric plants depend on leaf surface particles. Plant Biology, 18, 91–100. [DOI] [PubMed] [Google Scholar]
- Cardoso, A.A. , Brodribb, T.J. , Kane, C.N. , DaMatta, F.M. & McAdam, S.A.M. (2020) Osmotic adjustment and hormonal regulation of stomatal responses to vapour pressure deficit in sunflower. AoB Plants , 12(4). 10.1093/aobpla/plaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čater, M. & Kobler, A. (2017) Light response of Fagus sylvatica L. and Abies alba mill. in different categories of forest edge – vertical abundance in two silvicultural systems. Forest Ecology and Management, 391, 417–426. [Google Scholar]
- Ciais, P. , Reichstein, M. , Viovy, N. , Granier, A. , Ogée, J. , Allard, V. et al. (2005) Europe‐wide reduction in primary productivity caused by the heat and drought in 2003. Nature, 437, 529–533. [DOI] [PubMed] [Google Scholar]
- Cochard, H. (2019) A new mechanism for tree mortality due to drought and heatwaves. 10.1101/531632 [DOI]
- Cochard, H. , Bodet, C. , Améglio, T. & Cruiziat, P. (2000) Cryo‐scanning electron microscopy observations of vessel content during transpiration in walnut petioles. facts or artifacts. Plant Physiology, 124, 119––120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard, H. , Martin, R. , Gross, P. & Bogeat‐Triboulot, M.B. (2000) Temperature effects on hydraulic conductance and water relations of Quercus robur L. Journal of Experimental Botany, 51, 1255–1259. [PubMed] [Google Scholar]
- Daas, C. , Montpied, P. , Hanchi, B. & Dreyer, E. (2008) Responses of photosynthesis to high temperatures in oak saplings assessed by chlorophyll‐a fluorescence: inter‐specific diversity and temperature‐induced plasticity. Annals of Forest Science, 65, 305. [Google Scholar]
- Dai, A. (2006) Recent climatology, variability, and trends in global surface humidity. Journal of Climate, 19, 3589–3606. [Google Scholar]
- Dittmar, C. , Zech, W. & Elling, W. (2003) Growth variations of common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe—a dendroecological study. Forest Ecology and Management, 173, 63–78. [Google Scholar]
- Drake, J.E. , Tjoelker, M.G. , Vårhammar, A. , Medlyn, B.E. , Reich, P.B. , Leigh, A. et al. (2018) Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Global Change Biology, 24, 2390–2402. [DOI] [PubMed] [Google Scholar]
- Duursma, R.A. , Blackman, C.J. , Lopéz, R. , Martin‐StPaul, N.K. , Cochard, H. , Medlyn, B.E. et al. (2019) On the minimum leaf conductance: its role in models of plant water use, and ecological and environmental controls. New Phytologist, 221, 693–705. [DOI] [PubMed] [Google Scholar]
- Eamus, D. (1991) The interaction of rising CO2 and temperatures with water use efficiency. Plant, Cell & Environment, 14, 843–852. [Google Scholar]
- Eamus, D. , Boulain, N. , Cleverly, J. & Breshears, D.D. (2013) Global change‐type drought‐induced tree mortality: vapor pressure deficit is more important than temperature per se in causing decline in tree health. Ecology and Evolution, 3, 2711–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanourakis, D. , Heuvelink, E. & Carvalho, S.M.P. (2013) A comprehensive analysis of the physiological and anatomical components involved in higher water loss rates after leaf development at high humidity. Journal of Plant Physiology, 170, 890–898. [DOI] [PubMed] [Google Scholar]
- Fontes, C.G. , Dawson, T.E. , Jardine, K. , McDowell, N. , Gimenez, B.O. , Anderegg, L. et al. (2018) Dry and hot: the hydraulic consequences of a climate change‐type drought for Amazonian trees. Philosophical Transactions of the Royal Society, B: Biological Sciences, 373, 20180209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Z. , Ciais, P. , Bastos, A. , Stoy, P.C. , Yang, H. , Green, J.K. et al. (2020) Sensitivity of gross primary productivity to climatic drivers during the summer drought of 2018 in Europe. Philosophical Transactions of the Royal Society, B: Biological Sciences, 375, 20190747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Plazaola, J.I. , Artetxe, U. & Becerril, J.M. (1999) Diurnal changes in antioxidant and carotenoid composition in the Mediterranean schlerophyll tree Quercus ilex (L) during winter. Plant Science, 143, 125–133. [Google Scholar]
- Gardingen, P.R.V.A.N. & Grace, J. (1992) Vapour pressure deficit response of cuticular conductance in intact leaves of Fagus sylvatica L. Journal of Experimental Botany, 43, 1293–1299. [Google Scholar]
- Geßler, A. , Keitel, C. , Nahm, M. & Rennenberg, H. (2004) Water shortage affects the water and nitrogen balance in Central European beech forests. Plant Biology (Stuttg), 6, 289–298. [DOI] [PubMed] [Google Scholar]
- Gimeno, T.E. , Pías, B. , Lemos‐Filho, J.P. & Valladares, F. (2009) Plasticity and stress tolerance override local adaptation in the responses of Mediterranean holm oak seedlings to drought and cold. Tree Physiology, 29, 87–98. [DOI] [PubMed] [Google Scholar]
- Grossiord, C. , Buckley, T.N. , Cernusak, L.A. , Novick, K.A. , Poulter, B. , Siegwolf, R.T.W. et al. (2020) Plant responses to rising vapor pressure deficit. New Phytologist, 226, 1550–1566. [DOI] [PubMed] [Google Scholar]
- Grossiord, C. , Gessler, A. , Reed, S.C. , Borrego, I. , Collins, A.D. , Dickman, L.T. et al. (2018) Reductions in tree performance during hotter droughts are mitigated by shifts in nitrogen cycling. Plant, Cell & Environment, 41, 2627–2637. [DOI] [PubMed] [Google Scholar]
- Grossiord, C. , Ulrich, D.E.M. & Vilagrosa, A. (2020) Controls of the hydraulic safety–efficiency trade‐off. Tree Physiology, 40, 573–576. [DOI] [PubMed] [Google Scholar]
- Gullo, M.A.L.O. & Salleo, S. (1990) Wood anatomy of some trees with diffuse‐ and ring‐porous wood: some functional and ecological interpretations. Giornale Botanico Italiano, 124, 601–613. [Google Scholar]
- Hacke, U. & Sauter, J.J. (1996) Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse‐porous and ring‐porous trees. Oecologia, 105, 435–439. [DOI] [PubMed] [Google Scholar]
- Haldimann, P. & Feller, U. (2004) Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat‐dependent reduction of the activation state of ribulose‐1,5‐bisphosphate carboxylase/oxyg. Plant, Cell & Environment, 27, 1169–1183. [Google Scholar]
- Hoch, G. , Popp, M. & Körner, C. (2002) Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos, 3, 361–374. [Google Scholar]
- Hochberg, U. , Windt, C.W. , Ponomarenko, A. , Zhang, Y.J. , Gersony, J. , Rockwell, F.E. et al. (2017) Stomatal closure, basal leaf embolism, and shedding protect the hydraulic integrity of grape stems. Plant Physiology, 174, 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC . (2021) Climate change 2021: The physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge, UK and New York: Cambridge University Press.
- Jarvis, P.G. & McNaughton, K.G. (1986) Stomatal control of transpiration: scaling up from leaf to region. Advances in Ecological Research, 15, 1–49. [Google Scholar]
- Kerstiens, G. (1996) Cuticular water permeability and its physiological significance. Journal of Experimental Botany, 47, 1813–1832. [Google Scholar]
- Knight, C.A. & Ackerly, D.D. (2002) An ecological and evolutionary analysis of photosynthetic thermotolerance using the temperature‐dependent increase in fluorescence. Oecologia, 130, 505–514. [DOI] [PubMed] [Google Scholar]
- Koide, R.T. , Robichaux, R.H. , Morse, S.R. & Smith, C.M. (2000) Plant Physiological Ecology: Field Methods and Instrumentation . Springer Netherlands, Dordrecht. pp. 161–183. [Google Scholar]
- Lendzion, J. & Leuschner, C. (2008) Growth of European beech (Fagus sylvatica L.) saplings is limited by elevated atmospheric vapour pressure deficits. Forest Ecology and Management, 256, 648–655. [Google Scholar]
- Lens, F. , Sperry, J.S. , Christman, M.A. , Choat, B. , Rabaey, D. & Jansen, S. (2011) Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. The New Phytologist, 190, 709–723. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Gudmundsson, L. , Hauser, M. , Qin, D. , Li, S. & Seneviratne, S.I. (2020) Soil moisture dominates dryness stress on ecosystem production globally. Nature Communications, 11, 4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Vilalta, J. , Poyatos, R. , Aguadé, D. , Retana, J. & Mencuccini, M. (2014) A new look at water transport regulation in plants. New Phytologist, 204, 105–115. [DOI] [PubMed] [Google Scholar]
- McAdam, S.A.M. & Brodribb, T.J. (2016) Linking turgor with ABA biosynthesis: implications for stomatal responses to vapor pressure deficit across land plants. Plant Physiology, 171, 2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B.F. , Buras, A. , Rammig, A. & Zang, C.S. (2020) Higher susceptibility of beech to drought in comparison to oak. Dendrochronologia, 64, 125780. [Google Scholar]
- Monteith, J.L. (1995) A reinterpretation of stomatal responses to humidity. Plant, Cell and Environment, 18, 357–364. [Google Scholar]
- Monteith, J.L. & Unsworth, M.H. (2013) Principles of Environmental Physics. Elsevier. [Google Scholar]
- Nolf, M. , Lopez, R. , Peters, J.M.R. , Flavel, R.J. , Koloadin, L.S. , Young, I.M. et al. (2017) Visualization of xylem embolism by W‐ray microtomography: a direct test against hydraulic measurements. New Phytologist, 214, 890–898. [DOI] [PubMed] [Google Scholar]
- Novick, K.A. , Ficklin, D.L. , Stoy, P.C. , Williams, C.A. , Bohrer, G. , Oishi, A.C. et al. (2016) The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nature Climate Change, 6, 1023–1027. [Google Scholar]
- Olson, M.E. , Anfodillo, T. , Rosell, J.A. & Martínez‐Méndez, N. (2020) Across climates and species, higher vapour pressure deficit is associated with wider vessels for plants of the same height. Plant, Cell & Environment, 43, 3068–3080. [DOI] [PubMed] [Google Scholar]
- Oren, R. , Sperry, J.S. , Katul, G.G. , Pataki, D.E. , Ewers, B.E. , Phillips, N. et al. (1999) Survey and synthesis of intra‐ and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant, Cell & Environment, 22, 1515–1526. [Google Scholar]
- Pearcy, R.W. & Zimmermann, R. (2000) Measurement of transpiration and leaf conductanceIn: Pearcy, R.W. , Ehleringer, J.R. , Mooney, H.A. & Rundel, P. (Eds.) In plant physiological ecology: Field methods and instrumentation. Dordrecht, The Netherlands: Kluwer Academic, pp. 137–160. (Eds.) [Google Scholar]
- Pena‐Rojas, K. , Aranda, X. & Fleck, I. (2004) Stomatal limitation to CO2 assimilation and down‐regulation of photosynthesis in Quercus ilex resprouts in response to slowly imposed drought. Tree Physiology, 24, 813–822. [DOI] [PubMed] [Google Scholar]
- Petersson, L.K. , Löf, M. , Jensen, A.M. , Chastain, D.R. & Gardiner, E.S. (2020) Sprouts of shoot‐clipped oak (Quercus alba and Q. robur) germinants show morphological and photosynthetic acclimation to contrasting light environments. New Forests, 51, 817–834. [Google Scholar]
- Pflug, E.E. , Buchmann, N. , Siegwolf, R.T.W. , Schaub, M. , Rigling, A. & Arend, M. (2018) Resilient leaf physiological response of European beech (Fagus sylvatica L.) to summer drought and drought release. Frontiers in Plant Science, 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin, A.G. , Pinkard, E.A. , Ryan, M.G. , Tissue, D.T. , Baggett, L.S. , Adams, H.D. et al. (2015) Non‐structural carbohydrates in woody plants compared among laboratories. Tree Physiology, 35, 1146–1165. [DOI] [PubMed] [Google Scholar]
- Rehschuh, R. , Rehschuh, S. , Gast, A. , Jakab, A.‐L. , Lehmann, M.M. , Saurer, M. et al. (2021) Tree allocation dynamics beyond heat and hot drought stress reveal changes in carbon storage, belowground translocation and growth. New Phytologist, 233, 687–704. [DOI] [PubMed] [Google Scholar]
- Schipka, F. , Heimann, J. & Leuschner, C. (2005) Regional variation in canopy transpiration of Central European beech forests. Oecologia, 143, 260–270. [DOI] [PubMed] [Google Scholar]
- Schleppi, P. (2021) Pixstat. https://www.schleppi.ch/pixstat/.
- Schönbeck, L. , Gessler, A. , Hoch, G. , McDowell, N.G. , Rigling, A. , Schaub, M. et al. (2018) Homeostatic levels of nonstructural carbohydrates after 13 yr of drought and irrigation in Pinus sylvestris . New Phytologist, 219, 1314–1324. [DOI] [PubMed] [Google Scholar]
- Schuster, A.‐C. (2016) Chemical and functional analyses of the plant cuticle as leaf transpiration barrier. (Doctoral dissertation). Universität Würzburg.
- Schuster, A.‐C. , Burghardt, M. , Alfarhan, A. , Bueno, A. , Hedrich, R. , Leide, J. et al. (2016) Effectiveness of cuticular transpiration barriers in a desert plant at controlling water loss at high temperatures. AoB Plants , 8(1). 10.1093/aobpla/plw027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster, A.‐C. , Burghardt, M. & Riederer, M. (2017) The ecophysiology of leaf cuticular transpiration: are cuticular water permeabilities adapted to ecological conditions. Journal of Experimental Botany, 68, 5271–5279. [DOI] [PubMed] [Google Scholar]
- Seemann, J.R. , Berry, J.A. & Downton, W.J.S. (1984) Photosynthetic response and adaptation to high temperature in desert plants 1: a comparison of gas exchange and fluorescence methods for studies of thermal tolerance. Plant Physiology, 75, 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senf, C. & Seidl, R. (2021) Persistent impacts of the 2018 drought on forest disturbance regimes in Europe. Biogeosciences, 18, 5223–5230. [Google Scholar]
- Sermons, S.M. , Seversike, T.M. , Sinclair, T.R. , Fiscus, E.L. & Rufty, T.W. (2012) Temperature influences the ability of tall fescue to control transpiration in response to atmospheric vapour pressure deficit. Functional Plant Biology, 39, 979–986. [DOI] [PubMed] [Google Scholar]
- Sperry, J.S. , Donnelly, J.R. & Tyree, M.T. (1988) A method for measuring hydraulic conductivity and embolism in xylem. Plant, Cell and Environment, 11, 35–40. [Google Scholar]
- Staudt, M. , Joffre, R. & Rambal, S. (2003) How growth conditions affect the capacity of Quercus ilex leaves to emit monoterpenes. New Phytologist, 158, 61–73. [Google Scholar]
- Tardieu, F. & Simonneau, T. (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany, 49, 419–432. [Google Scholar]
- Thomas, F.M. (2000) Growth and water relations of four deciduous tree species (Fagus sylvatica L., Quercus petraea [MATT.] LIEBL., q‐pubescens WILLD., Sorbus aria [L.] CR.) occurring at Central‐European tree‐line sites on shallow calcareous soils: physiological reactions of. Flora, 195, 104–115. [Google Scholar]
- Tixier, A. , Herbette, S. , Jansen, S. , Capron, M. , Tordjeman, P. , Cochard, H. et al. (2014) Modelling the mechanical behaviour of pit membranes in bordered pits with respect to cavitation resistance in angiosperms. Annals of Botany, 114, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti, R. , Longobucco, A. , Miglietta, F. & Raschi, A. (1998) Transpiration and stomatal behaviour of Quercus ilex plants during the summer in a Mediterranean carbon dioxide spring. Plant, Cell and Environment, 21, 613–622. [Google Scholar]
- Tognetti, R. , Longobucco, A. & Raschi, A. (1998) Vulnerability of xylem to embolism in relation to plant Hhdraulic resistance in quercus pubescens and Quercus ilex co‐occurring in a Mediterranean coppice stand in central Italy. The New Phytologist, 139, 437–447. [Google Scholar]
- Trotsiuk, V. , Babst, F. , Grossiord, C. , Gessler, A. , Forrester, D.I. , Buchmann, N. et al. (2021) Tree growth in Switzerland is increasingly constrained by rising evaporative demand. Journal of Ecology, 109, 2981–2990. [Google Scholar]
- Urban, J. , Ingwers, M.W. , McGuire, M.A. & Teskey, R.O. (2017) Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. Journal of Experimental Botany, 68, 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walthert, L. , Ganthaler, A. , Mayr, S. , Saurer, M. , Waldner, P. , Walser, M. et al. (2021) From the comfort zone to crown dieback: sequence of physiological stress thresholds in mature European beech trees across progressive drought. Science of the Total Environment, 753, 141792. [DOI] [PubMed] [Google Scholar]
- Wellstein, C. & Spada, F. (2015) The status of quercus pubescens willd in Europe. In: Box, E.O. & Fujiwara, K. , (Eds.) In warm‐temperate deciduous forests around the Northern Hemisphere . Springer International Publishing, Cham. pp. 153–163. [Google Scholar]
- Wong, S.C. (1990) Elevated atmospheric partial pressure of CO2 and plant growth ‐ II. Non‐structural carbohydrate content in cotton plants and its effect on growth parameters. Photosynthesis Research, 23, 171–180. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Zhang, Q. , Huang, G. , Peng, S. & Li, Y. (2020) Temperature responses of photosynthesis and leaf hydraulic conductance in rice and wheat. Plant, Cell & Environment, 43, 13743. [DOI] [PubMed] [Google Scholar]
- Zhu, J. , Thimonier, A. , Etzold, S. , Meusburger, K. , Waldner, P. , Schmitt, M. et al. (2022) Variation in leaf morphological traits of European beech and Norway spruce over two decades in Switzerland. Frontiers in Forests and Global Change , 4. 10.3389/ffgc.2021.778351 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


