Abstract
Background and purpose
Mos scales currently used to evaluate spinal muscular atrophy (SMA) patients have only been validated in children. The aim of this study was to assess the construct validity and responsiveness of several outcome measures in adult SMA patients.
Methods
Patients older than 15 years and followed up in five referral centres for at least 6 months, between October 2015 and August 2020, with a motor function scale score (Hammersmith Functional Motor Scale Expanded [HFMSE], Revised Upper Limb module [RULM]) were included. Bedside functional scales (Egen Klassification [EK2], Revised Amyotrophic Lateral Sclerosis Functional Rating Scale [ALSFRS‐R]) were also collected when available. Spearman's rho correlations (rs) and Bangdiwala's concordance test (B) were used to evaluate the scales' construct validity. Monthly slopes of change were used to calculate their responsiveness of the scales.
Results
The study included 79 SMA patients, followed up for a mean of 16 months. All scales showed strong correlations with each other (rs > 0.70). A floor effect in motor function scales was found in the weakest patients (HFMSE < 5 and RULM < 10), and a ceiling effect was found in stronger patients (HFMSE > 60 and RULM > 35). The ALSFRS‐R (B = 0.72) showed a strong ability to discriminate between walkers, sitters and non‐sitters, and the HFMSE (B = 0.86) between walkers and sitters. The responsiveness was low overall, although in treated patients a moderate responsiveness was found for the ALSFRS‐R and HFMSE in walkers (0.69 and 0.61, respectively) and for EK2 in sitters (0.65) and non‐sitters (0.60).
Conclusions
This study shows the validity and limitations of the scales most frequently used to assess adult SMA patients. Overall, bedside functional scales showed some advantages over motor scales, although all showed limited responsiveness.
Keywords: adults, cohort study, nusinersen, spinal muscular atrophy, treatment
INTRODUCTION
5q spinal muscular atrophy (SMA) is a genetic neurodegenerative disease, causing progressive muscular weakness and atrophy, followed by respiratory insufficiency, dysarthria and dysphagia [1]. Adult SMA patients are classified, according to their current functionality, into non‐sitters, sitters and walkers [1, 2].
In the last few years, three gene‐based therapies (nusinersen, onasemnogene abeparvovec and risdiplam) have been approved for the treatment of SMA patients. However, high‐quality evidence of their efficacy in adult patients is scarce. One major challenge is how to measure changes in adult SMA patients.
One such method is the use of motor function scales. These assess the ability of a patient to perform certain tasks in the clinic, which are used as proxies of day‐to‐day patient functionality. The Hammersmith Functional Motor Scale Expanded (HFMSE) and the Revised Upper Limb Module (RULM) are probably the most widely used motor scales in late‐onset SMA patients [2, 3]. However, they require qualified staff, appropriate facilities and are time‐consuming to administer [2, 3]. Moreover, they have been developed and validated in paediatric populations and their construct validity or sensitivity in adult patients have not been formally assessed [2, 3]. Other motor scales frequently used in adult SMA patients are muscle strength measurement and the 6‐Minute Walk Test (6MWT) [2, 3, 4, 5, 6]. Nevertheless, there is no consensus on how to measure muscle strength in motor neuron diseases [7] and the 6MWT can only be used in ambulant SMA patients.
Another method of assessing change in SMA patients is the use of bedside functional scales, which measure patients' disability based on a rater's scoring of certain signs or symptoms. Compared with motor function scales, they are usually faster and easier to administer. Consequently, they are frequently used as outcome measures in both clinical practice and research in adult patients with neurodegenerative diseases. The Egen classification 2 (EK2) scale, the revised version of the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS‐R) and the SMA Functional Rating Scale are the most frequently used bedside functional scales in adult SMA patients [2]. They are reliable, fast, and easy to use, but data on their construct validity and responsiveness (i.e. sensitivity to change) in adult SMA patients are lacking or scarce [4, 5, 8].
The aim of the present study therefore was to assess the construct validity and responsiveness of a set of motor function (HFMSE, RULM, 6MWT) and bedside functional scales (EK2 and ALSFRS) in adult SMA patients. These properties could help to define their usefulness for both clinical trials and clinical practice.
METHODS
Study design and participants
This prospective observational study included SMA patients from five centres in Spain. The inclusion criteria were: (i) genetically confirmed SMA (biallelic mutation in SMN1); (ii) age >15 years at baseline visit; and (iii) follow‐up data on at least one motor scale at the time of study closure (10 August 2020). Some patients were treated with nusinersen according to routine clinical practice. When available, retrospective data on untreated patients were also collected from October 2015.
Procedures
Motor and functional scales were administered every 6–12 months by experienced and/or trained neurologists and physiotherapists. Although all centres shared the same protocol, some tests were missing for some visits. Moreover, not all scales were applicable to all patients (see below). Consequently, the number of subjects varies according to scale and visit.
Clinical variables and outcomes
Age, gender, age at symptom onset, and presence of severe scoliosis (>45° Cobb angle) and/or scoliosis surgery were recorded for all patients upon recruitment. Patients were classified into type 1 to 4 SMA as defined elsewhere [9], as well as into functional subgroups [2]: walkers (able to walk at least five steps without assistance), sitters (able to sit without assistance or head support for more than 10 s) and non‐sitters.
For this study, the following scales were collected:
The HFMSE, which consists of 33 items, with a maximum of 66 points (higher scores indicating better function). This scale was originally designed for the assessment of high‐functioning type 2 and 3 SMA patients, that is, sitters and walkers [10]. It has been validated in children with SMA [11]. Although its content validity and clinical meaningfulness has also been explored in adults [12], a significant floor effect has been found in these patients [6].
The RULM, which includes 20 items with a maximum score of 37 (higher scores indicating better function) [13]. Although it has been validated in both ambulant and non‐ambulant patients, it shows a ceiling effect in up to a third of ambulant patients with SMA type 3 (without upper limb weakness) [14] and a floor effect at least in a proportion of non‐sitters [15].
The 6MWT, which measures the distance a patient is able to walk within 6 min, and has been validated in ambulant adult SMA patients [5].
The EK2, a functional scale that includes 17 items for eight daily‐life categories (wheelchair use, wheelchair transfers, trunk mobility, eating, swallowing, breathing, coughing, fatigue). Each item is scored from 0 to 3 for a maximum of 51 points (higher scores indicating worse function). The EK2 was designed for non‐ambulatory SMA patients, and its convergent validity has been shown in SMA patients with different age ranges, including older adults [16, 17, 18].
The ALSFRS‐R, which is a functional scale that includes 12 items covering four domains (bulbar, upper limbs, lower limbs, respiratory). Each item is scored from 0 to 4 for a maximum of 48 points (higher scores indicating better function). It was designed for ALS patients, but has also been used to assess disability in SMA patients [19, 20], although a formal validation is lacking.
Percent‐predicted forced vital capacity (FVC%).
All evaluators were appropriately trained in using the scales and had experience in the disease. All efforts were made to keep the same evaluator for every patient throughout the study.
Statistical analysis
Data were summarized as means, standard deviations, medians, and first and third quartiles for the continuous variables, and as relative and absolute frequencies for the categorical variables. Exploratory descriptive analyses were used to assure the quality of the data.
Convergent validity of the different scales was assessed by means of a correlation matrix, using Spearman's rho correlations (rs). The strength of correlation was quantified as moderate when rs was 0.50 to 0.69, strong when rs was 0.70 to 0.89 and very strong when rs was >0.90. Scatter plots with trend lines were estimated using local regression analysis to assess possible floor and ceiling effects of the different scales.
For the discriminant validity assessment either logistic (EK2, HFMSE) or ordinal (ALSFRS‐R, RULM, FVC%) regression models were performed for each scale, using as response variable the functional classification (walker, sitter, non‐sitter). We then assessed the concordance between the predictions made by these models and the actual classification, using Bangdiwala's observer agreement card for ordinal variables [21]. The agreement was quantified as moderate when B was 0.50 to 0.69, strong when B was 0.70 to 0.89 and very strong when B was > 0.90 [21].
The internal responsiveness of each scale was studied by analysing their monthly slopes of change between baseline and the last available follow‐up using linear regression [22]. These slopes were then expressed as standardized response means by calculating the ratios of the mean slopes to their SDs [22]. Responsiveness was considered low if <0.50, moderate if ranging from 0.50 to 0.79, and large if >0.80 [22].
All analyses were prespecified before looking at the data. p values <0.05 were taken to indicate statistical significance. All the statistical analyses and graphs were performed with R software (version 4.0.3).
Ethics Statement
The study was approved by the Ethics Committee for Biomedical Research of the Instituto de Investigación Sanitaria la Fe and Fundació Sant Joan de Déu. All participants provided written informed consent.
RESULTS
Patient characteristics
The study included 79 SMA patients: 38 from the Hospital la Fe, 21 from the Hospital de Bellvitge, 12 from the Hospital Sant Joan de Deu, four from the Hospital Virgen de la Arrixaca, and four from the Hospital de Basurto. Their demographic and clinical characteristics are summarized in Table 1. As expected, the patient functional subgroups differed in their clinical characteristics, although there was considerable overlap in SMA type and SMN2 copy number.
TABLE 1.
Demographic and baseline clinical characteristics of SMA patients included in the study
| Variable |
Non‐sitters (n = 30) |
Sitters (n = 25) |
Walkers (n = 24) |
|
|---|---|---|---|---|
| Age, years | Mean (SD) | 26.66 (12.77) | 34.16 (12.71) | 35.84 (14.34) |
| Median (IQR) | 21.55 (16.83, 34.38) | 33.48 (25.14, 43.59) | 33.51 (22.23, 48.55) | |
| Male sex | N (%) | 15 (50) | 8 (32) | 14 (58.33) |
| SMA type | ||||
| 2a | N (%) | 21 (70) | 1 (4) | 0 (0) |
| 2b | 3 (10) | 5 (20) | 0 (0) | |
| 3a | 4 (13.33) | 13 (52) | 6 (25) | |
| 3b | 2 (6.67) | 6 (24) | 15 (62.5) | |
| 4 | 0 (0) | 0 (0) | 3 (12.5) | |
| SMN2 copies | ||||
| 1 | N (%) | 1 (3.33) | 0 (0) | 0 (0) |
| 2 | 5 (16.67) | 1 (4) | 1 (4.17) | |
| 3 | 22 (73.33) | 20 (80) | 8 (33.33) | |
| 4 | 2 (6.67) | 4 (16) | 15 (62.5) | |
| Disease duration, years | Mean (SD) | 25.45 (11.88) | 30.21 (10.64) | 25.19 (16) |
| Median (IQR) | 20.88 (16.22, 33.1) | 27.8 (22.47, 39.1) | 23.44 (8.83, 37.61) | |
| NIV use | ||||
| No | N (%) | 10 (34.48) | 20 (80) | 24 (100) |
| 8 h | 18 (62.07) | 5 (20) | 0 (0) | |
| 24 h | 1 (3.45) | 0 (0) | 0 (0) | |
| Gastrostomy | N (%) | 1 (3.33) | 0 (0) | 0 (0) |
| Severe scoliosis | N (%) | 30 (100) | 16 (64) | 1 (4.17) |
| Nusinersen | N (%) | 10 (33.33) | 16 (64) | 13 (54.17) |
| Salbutamol | N (%) | 20 (66.66) | 12 (48) | 9 (37.5) |
| HFMSE (0–66), n = 50 | Mean (SD) | NA (NA) | 10.04 (8.84) | 49.95 (11.54) |
| Median (IQR) | NA (NA, NA) | 6.5 (3.75, 16.5) | 53.5 (44.75, 57) | |
| RULM (0–37), n = 75 | Mean (SD) | 6.69 (6.35) | 19.03 (9.17) | 33.35 (4.95) |
| Median (IQR) | 5 (1, 10.12) | 19 (12.5, 25.5) | 36 (30.75, 37) | |
|
6MWT, m n = 22 |
Mean (SD) | NA (N) | NA (NA) | 342.06 (147.27) |
| Median (IQR) | NA (NA, NA) | NA (NA, NA) | 362.75 (221.25, 454.5) | |
| ALSFRS‐R (0–48), n = 52 | Mean (SD) | 18 (5.42) | 30.58 (4.14) | 42.25 (2.87) |
| Median (IQR) | 19 (16, 21) | 31 (28, 32.5) | 42.5 (40, 43.75) | |
| EK2 (0–51), n = 38 | Mean (SD) | 26.88 (8.75) | 14.31 (6.92) | NA (NA) |
| Median (IQR) | 26.5 (21.25, 32.5) | 14 (8.75, 19.25) | NA (NA, NA) | |
| FVC%, n = 48 | Mean (SD) | 32.42 (17.06) | 76.55 (37.62) | 101.11 (17.23) |
| Median (IQR) | 31.6 (18.5, 40.5) | 70.2 (49, 105) | 102.5 (88.78, 110.75) | |
| Follow‐up, months | Mean (SD) | 16.19 (9.66) | 14.95 (5.45) | 16.64 (7.67) |
| Median (IQR) | 15.17 (9.8, 22.1) | 14.47 (11.43, 17.73) | 15.3 (12.07, 21.68) | |
Abbreviations: ALSFRS‐R, Revised version of the Amyotrophic Lateral Sclerosis Functional Scale; EK2, Egen Klassifikation 2; FVC%, percent‐predicted forced vital capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; IQR, interquartile range; RULM, Revised Upper Limb Module; 6MWT, 6‐Minute Walk Test.
Convergent validity
All motor function and bedside functional scales showed either strong or very strong correlation with each other (Figure 1). The greatest correlations were between the EK2 and ALSFRS‐R (rs = −0.96) and between the HFMSE and ALSFRS‐R (rs = 0.89). The weakest correlations were between FVC and the HFMSE (rs = 0.5) and between FVC and the 6MWT (rs = −0.04).
FIGURE 1.
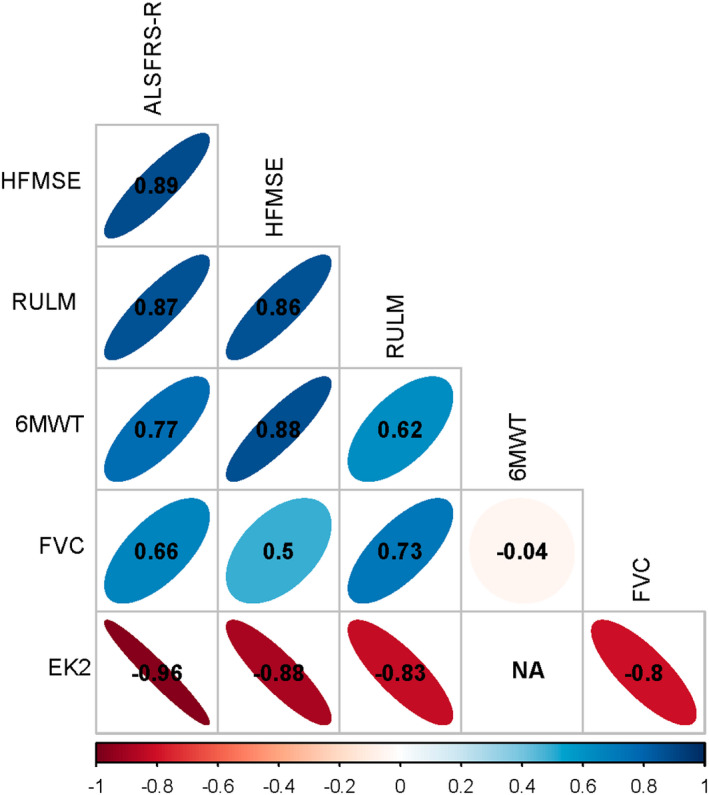
Graphical representation of correlations between outcome measures. Colours represent the strength of correlations and numbers correspond to Spearman's rho correlations. 6MWT, 6‐Minute Walk Test; EK2, Egen Klassifikation 2; FVC, forced vital capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; RULM, Revised Upper Limb Module. [Colour figure can be viewed at wileyonlinelibrary.com
Compared with the ALSFRS‐R, EK2 and RULM scales, a floor effect of the HFMSE was found in the weakest sitters (HFMSE < 5; Figure 2a,b,g). Moreover, a ceiling effect was apparent for walkers with an HFMSE score >60, when compared with the 6MWT (Figure 2c). Regarding the RULM scale, a floor effect was found in patients with RULM score <10, when compared with the ALSFRS‐R and EK2 (Figure 2d,e), and a ceiling effect in patients with RULM score > 35 when compared with the ALSFRS‐R, 6MWT and HFMSE (Figure 2d,f,g). The ALSFRS‐R and EK2 showed no apparent floor or ceiling effect, either when compared with each other (Figure 2h) or with motor scales (Figure 2a,b,d,e,i).
FIGURE 2.
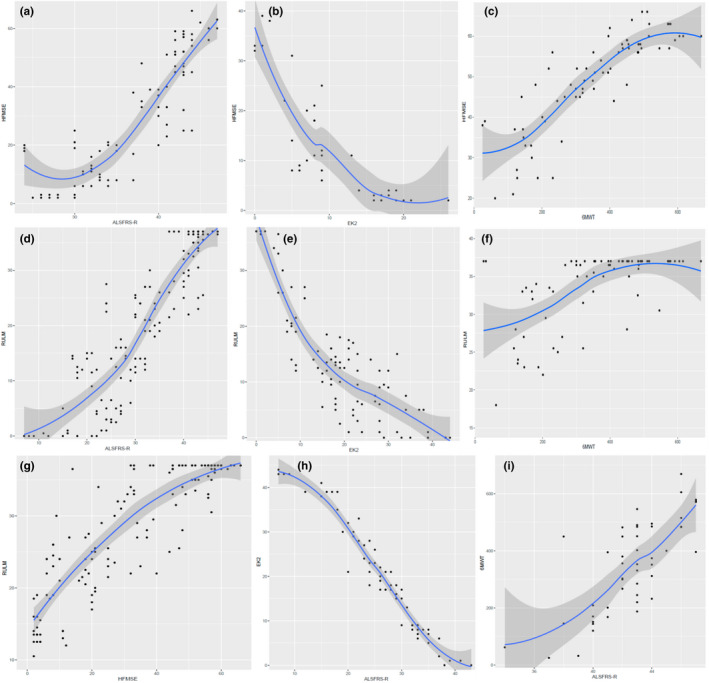
Correlations of (a) the Hammersmith Functional Motor Scale Expanded (HFMSE) and the Revised version of the Amyotrophic Lateral Sclerosis Functional Scale (ALSFRS‐R), (b) the HFMSE and the Egen Klassifikation 2 (EK2); (c) the HFMSE and the 6‐Minute Walk Test (6MWT), (d) the Revised Upper Limb Module (RULM) and the ALSFRS‐R, (e) the RULM and the EK2, (f) the RULM and the 6MWT, (g) the RULM and the HFMSE, (h) the EK2 and the ALSFRS‐R and (i) the 6MWT and the ALSFRS‐R. Scatter plots with trend lines were estimated by local regression to analyse possible floor and ceiling effects of the different scales. [Colour figure can be viewed at wileyonlinelibrary.com
Discriminant validity
All scales discriminated between functional subgroups, although with considerable overlap in most of them. Among those tests applicable to all subgroups of patients, only the ALSFRS‐R showed a strong discriminative ability (B = 0.72, Figure 3a). The RULM showed moderate discriminative ability (B = 0.62; Figure 3b) and FVC showed low discriminative ability (B = 0.35; Figure 3c). Among those scales applicable only to two subgroups of patients, the HFMSE showed strong discriminative ability between walkers and sitters (B = 0.86, Figure 3d) and the EK2 moderate discriminative ability between sitters and non‐sitters (B = 0.68, Figure 3e).
FIGURE 3.

Boxplots of (a) the Revised version of the Amyotrophic Lateral Sclerosis Functional Scale (ALSFRS‐R), (b) the Revised Upper Limb Module (RULM), (c) percent‐predicted forced vital capacity (FVC%), (d) the Hammersmith Functional Motor Scale Expanded (HFMSE) and (e) and the Egen Klassifikation 2 (EK2), according to functional subgroups to represent the discriminative ability. [Colour figure can be viewed at wileyonlinelibrary.com
Responsiveness
Both treated and untreated patients were followed up for a mean of 16 months. In untreated patients the responsiveness of the scales overall was low (Table 2): in walkers no measure appeared to adequately capture worsening during follow‐up, while the ALSFRS‐R was the most responsive measure in sitters (−0.43) and FVC in non‐sitters (−0.37). In treated patients, the responsiveness was also low overall, with some exceptions (Table 3). Moderate responsiveness was found for the ALSFRS‐R and HFMSE in walkers (0.69 and 0.61 respectively), and for the EK2 in sitters (0.65) and non‐sitters (0.60).
TABLE 2.
Standardized response means of each scale in untreated patients, globally and by functional subgroup
| Untreated patients |
RULM (n = 36) |
HFMSE (n = 21) |
6MWT (n = 10) |
EK2 (n = 24) |
ALSFRS‐ R (n = 21) |
FVC% (n = 14) |
|---|---|---|---|---|---|---|
| Global | −0.13 | 0.15 | 0.25 | 0.24 | −0.19 | −0.39 |
| Walkers | −0.03 | 0.27 | 0.25 | NA | 0.37 | NA |
| Sitters | −0.13 | 0.04 | NA | 0.21 | −0.43 | −0.16 |
| Non‐sitters | −0.18 | NA | NA | 0.25 | −0.30 | −0.37 |
Abbreviations: ALSFRS‐R, Revised version of the Amyotrophic Lateral Sclerosis Functional Scale; EK2, Egen Klassifikation 2; FVC%, percent‐predicted forced vital capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; RULM, Revised Upper Limb Module; 6MWT, 6‐Minute Walk Test.
TABLE 3.
Standardized response means of each scale in treated patients, globally and by functional subgroup
| Treated patients |
RULM (n = 39) |
HFMSE (n = 29) |
6MWT (n = 12) |
EK2 (n = 14) |
ALSFRS‐R (n = 31) |
FVC% (n = 34) |
|---|---|---|---|---|---|---|
| Global | 0.37 | 0.50 | 0.37 | −0.69 | 0.38 | 0.18 |
| Walker | 0.45 | 0.61 | 0.37 | NA | 0.69 | 0.18 |
| Sitter | 0.36 | 0.37 | NA | −0.65 | 0.30 | 0.21 |
| Non‐sitter | 0.17 | NA | NA | −0.60 | −0.06 | 0.41 |
Note: Bold text indicates those scales showing moderate responsiveness.
Abbreviations: ALSFRS‐R, Revised version of the Amyotrophic Lateral Sclerosis Functional Scale; EK2, Egen Klassifikation 2; FVC%, percent‐predicted forced vital capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; RULM, Revised Upper Limb Module; 6MWT, 6‐Minute Walk Test.
DISCUSSION
In this longitudinal study, we addressed for the first time the validation of a set of motor and functional scales frequently used in clinical practice to evaluate adult SMA patients. For the purposes of this study, adult patients were those older than 15 years, who were routinely followed up in adult clinics, while children were those younger than 15 years, who were routinely followed up by paediatricians. We avoided the term ‘adolescent’ because there is no uniform definition of the ages that comprise adolescence and this term could mislead the reader. The results of this study are applicable to patients older than 15 years.
Scales that are both valid and responsive are warranted to improve research and decision‐making in adult SMA patients. To date, motor function scales (such as the 6MWT, HFMSE and RULM) are those most frequently used in adult SMA patients to assess the efficacy of nusinersen [22]. However, most of these were designed and validated in children [9, 11, 13, 14], and children present considerable differences (e.g., in disease progression rate, contractures and scoliosis, comorbidities) compared with adults that could affect the validity and responsiveness of the scales. This is crucial considering that decisions on the continuation or reimbursement of disease‐modifying treatments are frequently made based on the results of motor scales [23]. This study suggests the validity of the motor scales in many adult patients, but at the same time underscores some important limitations that should be considered when interpreting their results.
Motor scales
In this study, we showed the convergent and discriminative validity of the HFMSE in most adult patients. However, HFMSE showed a floor effect in weakest type 2 and 3a sitters (HFMSE score < 5) when compared with the other scales (especially the EK2), and a ceiling effect in highly functioning walkers (HFMSE score > 60) when compared with the 6MWT. The floor effect has also been recently described in adult patients, when compared with measurement of muscle strength [6]. Moreover, we found moderate responsiveness in walkers treated with nusinersen, but low responsiveness in sitters. Altogether, this suggests that the HFMSE might be useful for measuring the effect of treatments in a subgroup of sitters and walkers with intermediate disability (HFMSE score 5–60), but not in more severely or mildly affected patients.
This study confirms the convergent validity of the RULM in adult patients too, but again shows a ceiling effect in patients with RULM score > 35 and a mild floor effect in weakest patients scoring <10 on the RULM scale. Moreover, the RULM showed only moderate discriminant validity, since sitters scores were very variable, and its responsiveness in treated patients was low overall, especially in non‐sitters. Similar limitations have already been reported in adult patients [24], probably limiting the utility of this scale in more severely or mildly affected patients.
The convergent validity of the 6MWT in ambulant adult SMA patients has been described previously [24] and is confirmed in this study. However, its responsiveness and reliability were lower than those of other outcome measures in this and previous studies [4, 24, 25], probably limiting its utility. Nevertheless, our results on responsiveness must be interpreted with caution because they included only 10–12 patients.
Bedside functional scales
Few data on functional scales are available in SMA, despite being frequent outcome measures in most neurodegenerative diseases [26].
This study confirms the validity of the EK2 for non‐ambulant adult SMA patients, which had been already suggested [17], without the presence of floor or ceiling effects. Remarkably, it showed moderate responsiveness in both sitters and non‐sitters treated with nusinersen. This agrees with a previous study, which showed the ability of the EK2 to detect improvements in non‐ambulant patients after treatment with salbutamol [8].
The ALSFRS‐R has been widely used to assess disability in adult SMA patients [19, 20, 27], but had not been formally validated. Here, we demonstrate its validity, without floor or ceiling effects. Furthermore, it showed moderate responsiveness only in treated walkers.
Percent‐predicted forced vital capacity
This study showed moderate to strong correlations of FVC% with motor and functional scales but higher correlations overall with the latter. Its discriminative ability and responsiveness was low overall, but it might still be useful in non‐sitters, where other outcome measures are lacking.
Selecting the best outcome measures
Selecting the best outcome measures in adult SMA patients is key to assessing the efficacy of new treatments in both clinical practice and clinical trials. However, the huge heterogeneity of adult patients (in both age and function), the slow decline rate, and the scarcity of natural history studies are major challenges to be considered. All scales analysed here have their strengths and limitations. However, the bedside functional scales assessed in this study showed better convergent validity in patients in the extremes of the disease spectrum and have some important advantages over motor function scales. Firstly, they are usually faster, cheaper and easier to administer. Secondly, they can easily encompass and distinguish a great range of functional states, reducing the floor and ceiling effects of motor scales. Thirdly, they include non‐motor items (e.g., fatigue and bulbar or respiratory problems), which are important for patients (especially non‐sitters). Fourthly, self‐ and telematic administration of functional scales such as the ALSFRS‐R have been shown to be reliable and reproducible [28], facilitating patient follow‐up. Finally, bedside functional scales provide a unique insight into the clinical relevance of a score change at an individual level, which could be only inferred with motor scales. Pitfalls in the ALSFRS‐R and EK2, such as limited reliability and multidimensionality have been described in previous Rasch analyses [29, 30], although different approaches can mitigate them [30, 31].
Regarding responsiveness, both motor and functional scales showed low to moderate internal responsiveness in patients treated with nusinersen. Moreover, the responsiveness of each scale varied in ambulant and non‐ambulant patients but was higher overall for functional scales (ALSFRS‐R and EK2, respectively). The lower responsiveness of motor scales compared with some functional scales or quantitative strength measurements had been described before [4]. However, the responsiveness results should be interpreted with caution because of the small sample size in both studies.
New outcome measures, applicable and responsive to all functional subgroups, should probably be developed. Until then, the combined use of several outcome measures will be needed. Thus, the use of composite multimodal scores is an interesting approach that has been already tested [4].
Strengths and limitations
The main strength of this study is the thorough evaluation of adult SMA in a relatively large cohort of SMA patients older than 15 years. Further studies should assess whether the results of this study are applicable to all adolescents or only to those older than 15 years.
The study also has several limitations, which are common in real‐world studies in rare diseases. A greater sample size would have been desirable for the stratified results of responsiveness, especially for the 6MWT. Moreover, not all patients were visited at the same intervals and patients' baseline characteristics were somewhat different in the treated and untreated groups. These factors could have affected the responsiveness of the scales and the results should therefore be interpreted with caution. Nevertheless, the statistical analysis was designed to minimize these limitations, for example, by calculating responsiveness based on the slopes of change.
Future studies should replicate these analyses in larger cohorts and assess the minimal detectable change and the minimal clinically important change in the different scales [32]. These two variables could help to quantify individual responses to treatment, guiding decisions about treatment discontinuation in clinical practice or serving as endpoints in clinical trials.
In conclusion, this multicentre study shows the validity and limitations of the scales most frequently used to assess adult SMA patients. Overall, bedside functional scales showed some advantages over motor scales, although all showed limited responsiveness. Therefore, new outcome measures are warranted in adult SMA patients. Meanwhile, this study provides a framework for the selection of the most relevant scales for use in the evaluation of adult SMA patients.
AUTHOR CONTRIBUTIONS
Juan F. Vázquez‐Costa, Hospital Universitario y Politécnico la Fe, Valencia: designed the study, participated in clinical data acquisition and interpretation, and wrote and edited the manuscript. Mónica Povedano, Bellvitge Hospital‐IDIBELL: acquired and interpreted the clinical data, and critically revised the manuscript. Andrés Nascimento‐Osorio, Hospital Sant Joan de Déu, Barcelona: acquired and interpreted the clinical data, and critically revised the manuscript. Antonio Moreno Escribano, Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain: acquired and interpreted the clinical data, and critically revised the manuscript. Solange Kapetanovic Garcia, Hospital Universitario Basurto ‐ OSI Bilbao, Spain: acquired and interpreted the clinical data, and critically revised the manuscript. Raul Dominguez, Bellvitge Hospital‐IDIBELL: acquired the clinical data. Jessica M. Exposito, Hospital Sant Joan de Déu, Barcelona: acquired and interpreted the clinical data, and critically revised the manuscript. Laura González, Bellvitge Hospital‐IDIBELL: acquired the clinical data. Carla Marco, Bellvitge Hospital‐IDIBELL: acquired the clinical data. Julita Medina Castillo, Hospital Sant Joan de Déu, Barcelona: acquired and interpreted the clinical data, and critically revised the manuscript. Nuria Muelas, Hospital Universitario y Politécnico la Fe, Valencia: critically revised the manuscript. Daniel Natera, Hospital Sant Joan de Déu, Barcelona: acquired and interpreted the clinical data, and critically revised the manuscript. Nancy Carolina Ñungo Garzón, Hospital Universitario y Politécnico la Fe, Valencia: acquired and interpreted the clinical data, and critically revised the manuscript. Inmaculada Pitarch‐Castellano, Hospital Universitario y Politécnico la Fe, Valencia: acquired and interpreted the clinical data, and critically revised the manuscript. Teresa Sevilla, Hospital Universitario y Politécnico la Fe, Valencia: acquired and interpreted the clinical data, and critically revised the manuscript. David Hervás, Universitat Politècnica de València, Valencia: planned and performed statistical analysis and interpretation, and critically revised the manuscript.
FUNDING INFORMATION
This study has received funding from FUNDAME (FUN‐000‐2017‐01), from CUIDAME (PIC188‐18), from Instituto de Salud Carlos III (JR19/00030 PI JFVC, 21/00737 PI JFVC, 19/01178 PI TS), and from Generalitat Valenciana (PROMETEO/ 2018/135, PI TS). The Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER) is initiative from the ISCIII. Teresa Sevilla and Juan F. Vázquez‐Costa are members of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO‐NMD). Sponsors did not participate in the study design, data acquisition and analysis, data interpretation or writing the article.
CONFLICT OF INTEREST
This study has received funding from FUNDAME (FUN‐000‐2017‐01) and CUIDAME (PIC188‐18). Dr Vázquez‐Costa is funded by grants of the Instituto de Salud Carlos III (JR19/00030, PI Vázquez) and received personal fees from Biogen and Roche outside the submitted work. Dr Nascimento‐Osorio received personal fees from Avexis, Biogen and Roche outside the submitted work, and is principal investigator for ongoing Biogen and Roche clinical trials. Dr N. Muelas and Dr A. Moreno received personal fees from Biogen outside the submitted work. Dr M Povedano received personal fees from Biogen and Roche outside the submitted work. Dr Solange Kapetanovic Garcia, Dr Raul Dominguez, Dr Jessica M Exposito, Dr Laura González, Dr Carla Marco, Dr Julita Medina Castillo, Dr Daniel Natera de Benito Dr Nancy Carolina Ñungo Garzón and Dr David Hervás have nothing to disclose. Dr Pitarch‐Castellano received personal fees from Avexis, Biogen and Roche outside the submitted work, and is principal investigator for an ongoing Biogen clinical trial.
Vázquez‐Costa JF, Povedano M, Nascimiento‐Osorio AE, et al. Validation of motor and functional scales for the evaluation of adult patients with 5q spinal muscular atrophy. Eur J Neurol. 2022;29:3666‐3675. doi: 10.1111/ene.15542
DATA AVAILABILITY STATEMENT
Juan F. Vázquez‐Costa and David Hervás had full access to the database population used to create the study population. All data supporting our findings are available on reasonable request.
REFERENCES
- 1. Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103‐115. [DOI] [PubMed] [Google Scholar]
- 2. Sansone VA, Walter MC, Attarian S, et al. Measuring outcomes in adults with spinal muscular atrophy ‐ challenges and future directions ‐ meeting report. J Neuromuscul Dis. 2020;7(4):523‐534. [DOI] [PubMed] [Google Scholar]
- 3. Finkel R, Bertini E, Muntoni F, Mercuri E. 209th ENMC international workshop: outcome measures and clinical trial readiness in spinal muscular atrophy 7‐9 November 2014, Heemskerk, The Netherlands. Neuromuscular Disord. 2015;25(7):593‐602. [DOI] [PubMed] [Google Scholar]
- 4. Querin G, Lenglet T, Debs R, et al. Development of new outcome measures for adult SMA type III and IV: a multimodal longitudinal study. J Neurol. 2021;268:1792‐1802. [DOI] [PubMed] [Google Scholar]
- 5. Elsheikh B, King W, Peng J, et al. Outcome measures in a cohort of ambulatory adults with spinal muscular atrophy. Muscle Nerve. 2020;61(2):187‐191. [DOI] [PubMed] [Google Scholar]
- 6. Wijngaarde CA, Stam M, Otto LAM, et al. Muscle strength and motor function in adolescents and adults with spinal muscular atrophy. Neurology. 2020;95(14):e1988‐e1998. [DOI] [PubMed] [Google Scholar]
- 7. Shefner JM. Strength testing in motor neuron diseasesNeurotherapeutics Vol. 14.: LLC Springer; 2017:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frongia AL, Natera‐De Benito D, Ortez C, et al. Salbutamol tolerability and efficacy in patients with spinal muscular atrophy type II. Neuromuscul Dis. 2019;29:517‐524. [DOI] [PubMed] [Google Scholar]
- 9. Finkel R, Bertini E, Muntoni F, Mercuri E. 209th ENMC international workshop: outcome measures and clinical trial readiness in spinal muscular atrophy 7‐9 November 2014, Heemskerk, The Netherlands. Neuromuscul Dis. 2015;25(7):593‐602. [DOI] [PubMed] [Google Scholar]
- 10. O'Hagen JM, Glanzman AM, McDermott MP, et al. An expanded version of the hammersmith functional motor scale for SMA II and III patients. Neuromuscul Dis. 2007;17(9–10):693‐697. [DOI] [PubMed] [Google Scholar]
- 11. Glanzman AM, O'Hagen JM, McDermott MP, et al. Validation of the expanded hammersmith functional motor scale in spinal muscular atrophy type II and III. J Child Neurol. 2011;26(12):1499‐1507. [DOI] [PubMed] [Google Scholar]
- 12. Pera MC, Coratti G, Forcina N, et al. Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy. BMC Neurol. 2017;17(1):1‐10. doi: 10.1186/s12883-017-0790-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mazzone ES, Mayhew A, Montes J, et al. Revised upper limb module for spinal muscular atrophy: development of a new module. Muscle Nerve. 2017;55(6):869‐874. doi: 10.1002/mus.25430 [DOI] [PubMed] [Google Scholar]
- 14. Pera MC, Coratti G, Mazzone ES, et al. Revised upper limb module for spinal muscular atrophy: 12 month changes. Muscle Nerve. 2019;59(4):426‐430. [DOI] [PubMed] [Google Scholar]
- 15. Maggi L, Bello L, Bonanno S, et al. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J NeurolNeurosurg Psychiatry. 2020;91(11):1166‐1174. [DOI] [PubMed] [Google Scholar]
- 16. Fagoaga J, Girabent‐Farrés M, Bagur‐Calafat C, Febrer A, Steffensen BF. Evaluación funcional para personas no ambulantes afectas de atrofia muscular espinal y distrofia muscular de Duchenne. Traducción y validación de la escala Egen Klassifikation 2 para la población española. Rev Neurol. 2015;60(10):439‐446. [PubMed] [Google Scholar]
- 17. Werlauff U, Steffensen BF. The applicability of four clinical methods to evaluate arm and hand function in all stages of spinal muscular atrophy type II. Disabil Rehabil. 2014;36(25):2120‐2126. [DOI] [PubMed] [Google Scholar]
- 18. Steffensen BF, Mayhew A, Aloysius A, et al. Egen classification revisited in SMA. Neromuscul Disord. 2008;18(84):740‐741. [Google Scholar]
- 19. Walter MC, Wenninger S, Thiele S, et al. Safety and treatment effects of Nusinersen in longstanding adult 5q‐SMA type 3 ‐ a prospective observational study. J Neuromuscul Dis. 2019;6(4):453‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wurster CD, Steinacker P, Günther R, et al. Neurofilament light chain in serum of adolescent and adult SMA patients under treatment with nusinersen. J Neurol. 2020;267(1):36‐44. doi: 10.1007/s00415-019-09547-y [DOI] [PubMed] [Google Scholar]
- 21. Muñoz SR, Bangdiwala SI. Interpretation of kappa and B statistics measures of agreement. J Appl Stat. 1997;24(1):105‐112. [Google Scholar]
- 22. Coratti G, Cutrona C, Pera MC, et al. Motor function in type 2 and 3 SMA patients treated with Nusinersen: a critical review and meta‐analysis. Orphanet J Rare Dis. 2021;16(1):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pitarch Castellano I, Cabrera‐Serrano M, Calvo Medina R, et al. Delphi consensus on recommendations for the treatment of spinal muscular atrophy in Spain (RET‐AME consensus). Neurologia. 2022;37(3):216‐228. [DOI] [PubMed] [Google Scholar]
- 24. Elsheikh B, King W, Peng J, et al. Outcome measures in a cohort of ambulatory adults with spinal muscular atrophy. Muscle Nerve. 2020;61(2):187‐191. [DOI] [PubMed] [Google Scholar]
- 25. Yeo CJJ, Simeone SD, Townsend EL, Zhang RZ, Swoboda KJ. Prospective cohort study of Nusinersen treatment in adults with spinal muscular atrophy. J Neuromuscul Dis. 2020;7(3):257‐268. [DOI] [PubMed] [Google Scholar]
- 26. Vázquez‐Costa JF. Natural history data in adults with SMA. Lancet Neurol. 2020;19(7):564‐565. doi: 10.1016/S1474-4422(20)30183-6 [DOI] [PubMed] [Google Scholar]
- 27. Wan HWY, Carey KA, D'Silva A, Kasparian NA, Farrar MA. “Getting ready for the adult world”: how adults with spinal muscular atrophy perceive and experience healthcare, transition and well‐being. Orphanet J Rare Dis. 2019;14:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bakker LA, Schröder CD, Tan HHG, et al. Development and assessment of the inter‐rater and intra‐rater reproducibility of a self‐administration version of the ALSFRS‐R. J Neurol Neurosurg Psychiatry. 2020;91(1):75‐81. [DOI] [PubMed] [Google Scholar]
- 29. Cano SJ, Mayhew A, Glanzman AM, et al. Rasch analysis of clinical outcome measures in spinal muscular atrophy. Muscle Nerve. 2014;49(3):422‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fournier CN, Bedlack R, Quinn C, et al. Development and validation of the Rasch‐built overall amyotrophic lateral sclerosis disability scale (ROADS). JAMA Neurol. 2020;77(4):480‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Eijk RPA, de Jongh AD, Nikolakopoulos S, et al. An old friend who has overstayed their welcome: the ALSFRS‐R total score as primary endpoint for ALS clinical trials. Amyotrophic Lateral Scler Frontotemporal Degener. 2021b;22(3‐4):1‐8. doi: 10.1080/21678421.2021.1879865 [DOI] [PubMed] [Google Scholar]
- 32. Vázquez‐Costa JF, Hervás D. Minimal detectable change and minimal clinically important difference in spinal muscular atrophy patients. Eur J Neurol. 2021;28:e40‐e41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Juan F. Vázquez‐Costa and David Hervás had full access to the database population used to create the study population. All data supporting our findings are available on reasonable request.


