Abstract
Background and purpose
Disorders of the autonomic nervous system (ANS) are common conditions, but it is unclear whether access to ANS healthcare provision is homogeneous across European countries. The aim of this study was to identify neurology‐driven or interdisciplinary clinical ANS laboratories in Europe, describe their characteristics and explore regional differences.
Methods
We contacted the European national ANS and neurological societies, as well as members of our professional network, to identify clinical ANS laboratories in each country and invite them to answer a web‐based survey.
Results
We identified 84 laboratories in 22 countries and 46 (55%) answered the survey. All laboratories perform cardiovascular autonomic function tests, and 83% also perform sweat tests. Testing for catecholamines and autoantibodies are performed in 63% and 56% of laboratories, and epidermal nerve fiber density analysis in 63%. Each laboratory is staffed by a median of two consultants, one resident, one technician and one nurse. The median (interquartile range [IQR]) number of head‐up tilt tests/laboratory/year is 105 (49–251). Reflex syncope and neurogenic orthostatic hypotension are the most frequently diagnosed cardiovascular ANS disorders. Thirty‐five centers (76%) have an ANS outpatient clinic, with a median (IQR) of 200 (100–360) outpatient visits/year; 42 centers (91%) also offer inpatient care (median 20 [IQR 4–110] inpatient stays/year). Forty‐one laboratories (89%) are involved in research activities. We observed a significant difference in the geographical distribution of ANS services among European regions: 11 out of 12 countries from North/West Europe have at least one ANS laboratory versus 11 out of 21 from South/East/Greater Europe (p = 0.021).
Conclusions
This survey highlights disparities in the availability of healthcare services for people with ANS disorders across European countries, stressing the need for improved access to specialized care in South, East and Greater Europe.
Keywords: Composite Autonomic Severity Score, cardiovascular autonomic function tests, (disorders of) autonomic nervous system, disorders of consciousness (other than epilepsy), neurological disorders, neurodisparity, orthostatic hypotension, Survey, syncope, sweat tests
The survey aimed to identify neurology‐driven or interdisciplinary clinical autonomic nervous system laboratories in Europe, describe their characteristics and explore regional differences. We found significant differences in the availability of healthcare services for people with ANS disorders across European countries, stressing the need for improved access to specialized care in South, East and Greater Europe.
INTRODUCTION
Disorders of the autonomic nervous system (ANS) are common conditions estimated to affect 70 million people worldwide [1]. The cardiovascular ANS is the most frequently affected domain, with an incidence of a first‐in‐life syncope episode of 6.2 per 1000 person‐years [2]. In contrast to other neurological disciplines, the evaluation of the ANS is associated with special caveats. Since on most occasions the ANS cannot be tested directly, its assessment relies on examining noninvasive physiological variables that express not only the activity of the autonomic reflex arch, but also the function of the effector organ and interaction of the body with external physical stimuli [3]. The evaluation is extensive and time consuming and test batteries typically include the head‐up tilt test, the Valsalva maneuver and deep breathing for the evaluation of sympathetic adrenergic and cardiovagal function, and the quantitative sudomotor axon reflex test (QSART), thermoregulatory sweat test, sympathetic skin response, and electrochemical skin conductance measurement for the evaluation of sudomotor function.
In 2005, the ANS panel of the European Federation of Neurological Societies conducted a survey on the distribution of ANS laboratories in Europe, asking for information on applied methods and equipment, existence of own normative data, commercial versus self‐developed systems, and research and educational activities [4]. Although this survey included all types of ANS laboratories (i.e., cardiological, endocrinological, etc.) and not only neurologically oriented ANS laboratories, it pinpointed a high heterogeneity in ANS services across Europe and, most importantly, large diversities in the techniques used to investigate ANS disorders, altogether highlighting an unmet need for ANS testing standards across European countries.
Recently, many efforts have been made to standardize clinical assessment of the ANS. Consensus definitions have been published to diagnose key aspects of cardiovascular autonomic failure, such as orthostatic hypotension and supine hypertension [5, 6]. The European Federation of Autonomic Societies (EFAS), endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN), provided recommendations on the use of tilt table testing in the diagnosis of disorders that may cause transient loss of consciousness [7]. The AAS and the International Federation of Clinical Neurophysiology (IFCN) released definitions of autonomic disorders and methodological guidance for appropriate autonomic function testing [3]. The latter consensus statement emphasized the importance of evaluating the ANS with screening batteries encompassing tests of sympathetic adrenergic, cardiovagal and sudomotor function. In case of pathological findings, the severity of autonomic impairment in the different domains can be graded with a 10‐point Composite Autonomic Severity Score (CASS) after normalizing each component for the confounding effects of age and gender [3, 8].
All the above international scientific efforts, together with the improvement of signal processing methodologies, increased availability of devices for autonomic function testing, and new treatment approaches have recently led to significant advances in the diagnosis and management of autonomic disorders. However, it is still unclear whether access to ANS healthcare facilities and the characteristics thereof are homogeneous across European regions.
In this joint effort between the Scientific Panel for ANS Disorders of the EAN and the EFAS, we aimed to identify neurology‐driven or interdisciplinary (with at least one neurologist in the core team) clinical ANS laboratories in Europe, to understand the laboratory characteristics in terms of equipment, personnel, metrics, case mixture of the patients and research focus, and to investigate the differences in each of the studied variables among different European regions. Finally, we aimed to identify laboratories that have the necessary equipment for calculation of the CASS.
METHODS
After a series of online preparatory meetings run in spring 2021, a panel of EAN and EFAS representatives, selected for their project‐relevant expertise among the members of the EAN and EFAS professional network (the authors of this publication, n = 27) prepared a web‐based survey (Appendix S1). The study protocol and survey were drafted in English by A.F., M.H., D.R.C., W.S., R.H., and J.S., and reviewed and approved by all panel members, the EFAS Council and the EAN Scientific Committee on 28 May 2021.
Prior to launching the survey, the coordinating authors contacted eight autonomic and 29 neurological European national societies with an introductory letter about the nature and purposes of the survey. The representatives of each country were asked about the number and localization of neurology‐driven or interdisciplinary laboratories in the respective countries. Whenever possible, we asked to be in direct contact with the directors of the abovementioned laboratories. Otherwise, the respective national secretaries forwarded our communication to the members of their societies. In a following step, we used a snowball sampling technique, in which we asked the recruited survey participants to identify other directors of neurology‐driven or interdisciplinary ANS laboratories in their country in order to invite them to participate in the survey as well [9]. Finally, each project team member was asked to identify any laboratory missed after the initial steps from his or her own professional network (see also Figure 1 and the Consensus‐Based Checklist for Reporting of Survey Studies [CROSS] in Appendix S2).
FIGURE 1.
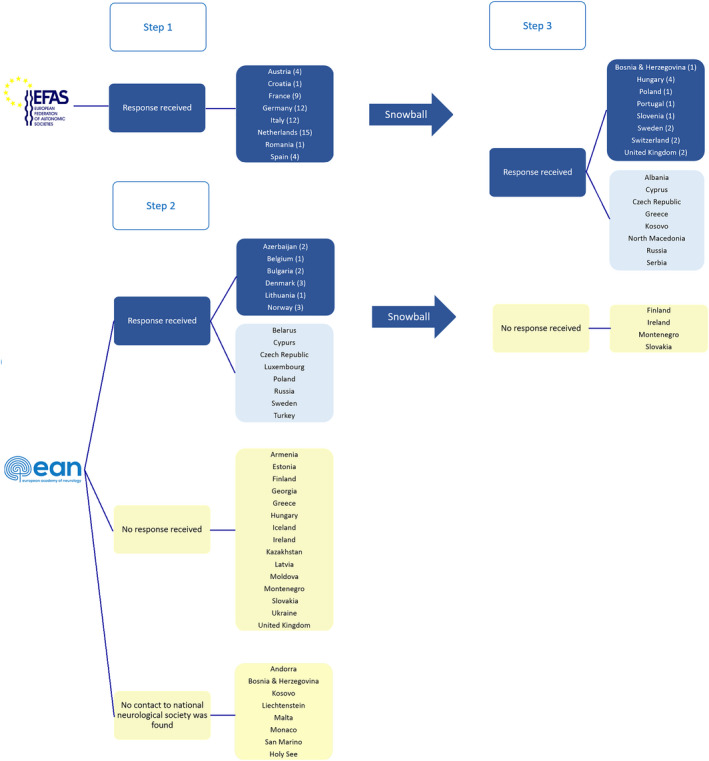
Methodological approach to identify the neurology‐driven or interdisciplinary clinical autonomic nervous system (ANS) laboratories in Europe. In Step 1, we contacted all eight national ANS societies by e‐mail (first e‐mail in June 2021, up to three reminders until all information was collected) and asked for the number of neurology‐driven or interdisciplinary clinical ANS laboratories in their countries. In this step we identified 58 laboratories. In Step 2, we consulted the European Academy of Neurology (EAN) Website in June 2021 for contact information of the European National Neurological Societies and, whenever possible, we compared this information with each National Neurology Society webpage. We found no National Neurology Society or no e‐mail contact thereof in eight countries: Andorra, Bosnia & Herzegovina, Kosovo, Liechtenstein, Malta, Monaco, San Marino, Holy See. For 29 countries we sent an introductory mail to the institutional representatives listed on the EAN website in June 2021 explaining the nature and scope of the project and asking to be put in contact with neurology‐driven or interdisciplinary clinical autonomic laboratories in the respective countries. Up to two reminders were sent to non‐responders until the end of July 2021. Whenever survey participants were recruited, we asked them to identify other directors of neurology‐driven or interdisciplinary laboratories in their country, in order to invite them to participate in the survey as well. This second step identified 12 more laboratories. In Step 3, the project team members reached out to colleagues of their professional network to gain (more detailed) information from those countries, for which no conclusive information was obtained in the former steps. This final step identified 14 more laboratories. Blue boxes indicate countries with at least one identified ANS laboratory, light blue boxes countries without identified ANS laboratories and yellow boxes countries from which no response was obtained. [Colour figure can be viewed at wileyonlinelibrary.com
On 16 September 2021 the survey was launched on a web‐based platform (Survey Monkey Momentive Europe UC – Dublin, Ireland) and all identified laboratory directors were invited to complete it by 26 November 2021.
The survey censored the following information:
Equipment: questions related to devices for blood pressure and heart rate monitoring, sudomotor testing, other autonomic function tests, blood examinations and histological analysis.
Personnel: age, gender and years into practice of the head of the laboratory and number and rank of medical personnel and allied healthcare professionals.
Metrics and case mixture of the patients: number of head‐up tilt tests, inpatient and outpatient visits per year and case mix in the respective laboratories (including percentages of people with reflex syncope, cardiac syncope, postural orthostatic tachycardia syndrome, classic, initial and delayed orthostatic hypotension, psychogenic pseudosyncope and other causes of transient loss of consciousness).
Research activities: whether the laboratory is actively involved in research activities and, if so, what is the focus of the research.
To analyze geographical differences, we applied the United Nations' geoscheme for Europe in the following way [10]:
Eastern Europe: Belarus, Bulgaria, Czech Republic, Hungary, Poland, Moldova, Romania, Russia, Slovakia, Ukraine;
Western Europe: Austria, Belgium, France, Germany, Liechtenstein, Luxembourg, Monaco, Netherlands, Switzerland;
Northern Europe: Denmark, Estonia, Finland, Iceland, Ireland, Latvia, Lithuania, Norway, Sweden, United Kingdom;
Southern Europe: Albania, Andorra, Bosnia and Herzegovina, Croatia, Greece, Holy See, Italy, Kosovo, Malta, Montenegro, North Macedonia, Portugal, San Marino, Serbia, Slovenia, Spain;
Greater Europe: Turkey, Cyprus, Armenia, Georgia, Kazakhstan, Azerbaijan.
We evaluated the differences in the studied variables between the North/West versus South/East/Greater European regions. The assignment of countries or areas to a specific group of the United Nations' geoscheme for Europe was chosen for statistical convenience and does not imply any assumption regarding political or other affiliation of countries or territories.
Laboratories were deemed equipped for CASS testing if both QSART and invasive or noninvasive continuous blood pressure and heart rate monitoring devices were available.
Statistical analysis
Statistical analysis was performed with the IBM SPSS v25 software (IBM Corporation, United States). We tested the data distribution using the Kolmogorov–Smirnov test. Qualitative variables were summarized in numbers (percentage), and quantitative variables by median (interquartile range [IQR]) for non‐normally or mean ± standard deviation for normally distributed data. Outliers were assessed for all variables and analyzed in their national and geographical context. The differences in qualitative variables between two European regions and according to gender distribution of the head of the laboratories were tested with the chi‐squared test. Differences in quantitative variables were tested with the parametric independent samples t‐test or non‐parametric Mann–Whitney test, depending on the data distribution. Two‐tailed p values <0.05 were taken to indicate statistical significance. Due to the large number of comparisons, a Bonferroni correction was applied. We tested four hypotheses: whether there is a difference in (i) equipment, (ii) personnel, (iii) metrics and case mix, and (iv) research focus of the ANS laboratories between the two European regions. For each hypothesis, the denominator of the Bonferroni formula was adapted to the number of comparisons run.
Ethical Standards
During the study purposing phase, the coordinating authors were counseled by the Innsbruck Ethical Committee and the Internal Data Protection Coordinator of the Medical University of Innsbruck. Given the observational nature of this study, which did not include any direct patient‐related data but was instead focused on collecting general information from the neurologists running the European laboratories, no ethics approval was needed by Austrian law. The participants gave electronic informed consent to participate in the survey and to have their name and affiliations listed among the collaborators of the study in the resulting publications. The study was performed in accordance with the Declaration of Helsinki and followed the current European regulations for data protection.
RESULTS
Participating societies
We collected information on the existence of neurology‐driven and interdisciplinary ANS laboratories from 33 out of 51 European countries (65%). There was no statistically significant difference in the response rate between North/West European (12 of 19) versus South/East/Greater European countries (21 of 32; p = 0.859). We identified 84 laboratories in 22 countries and 46 (55%) responded to the survey (Figure 2).
FIGURE 2.
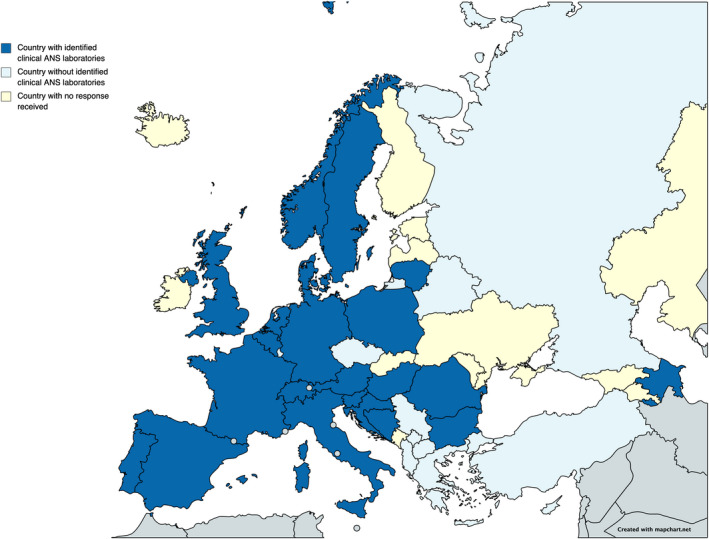
Distribution of clinical autonomic nervous system (ANS) laboratories across Europe. Blue indicates countries with at least one identified clinical ANS laboratory (four from Eastern Europe, six from Western Europe, five from Northern Europe, six from Southern Europe and one from Greater Europe). Light blue indicates countries without identified clinical ANS laboratories and yellow indicates countries from which no response was obtained. Created using https://mapchart.net/europe.htmlwileyonlinelibrary.com]. This work is licensed under a Creative Commons Attribution‐ShareAlike 4.0 International License. [Colour figure can be viewed at wileyonlinelibrary.com]
The numbers of identified neurology‐driven ANS laboratories per country are shown in Figure 3. The number of inhabitants per laboratory ranged from 1,180,527 inhabitants/laboratory in Netherlands to 38,179,800 inhabitants/laboratory in Poland.
FIGURE 3.
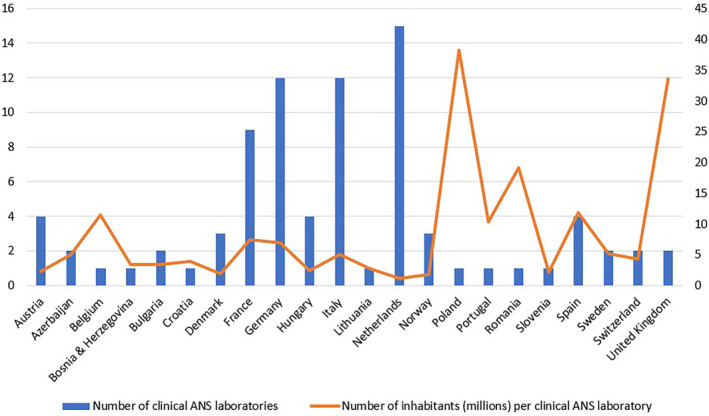
Number of clinical autonomic nervous system (ANS) laboratories in each European country from which information on the availability of clinical ANS laboratories was obtained. [Colour figure can be viewed at wileyonlinelibrary.com
Equipment and personnel
Table 1 provides an overview of the equipment and personnel characteristics of the laboratories. All laboratories perform cardiovascular autonomic function testing, while 83% also perform sweat testing. Sixty‐three percent of the laboratories perform epidermal nerve fiber density and other histological analyses. We identified 13 laboratories (29%) that have the required equipment for performing the CASS assessment. The majority of the directors of the laboratories are male (61%), are aged between 40 and 49 years, and have 10–19 years of clinical experience. There was no statistically significant difference in characteristics of the laboratories based on the gender of the head of the laboratory, except that female directors reported a higher number of inpatient admissions per year [90 (IQR 13–148) vs. 8 [IQR 1–36]; p = 0.007]. The laboratories are staffed by a median (IQR) of two consultants (1–3), one resident (0–2), one technician (1–2), and one nurse (0–2).
TABLE 1.
Equipment and personnel of the European clinical autonomic nervous system laboratories
| Equipment (N = 46) | |
| Blood pressure monitoring, n (%) | 46 (100) |
| Riva‐Rocci | 19 (41.3) |
| Invasive continuous | 4 (8.7) |
| Noninvasive continuous | 41 (89.1) |
| Volume clamp | 27 (58.7) |
| Pulse wave | 19 (41.3) |
| Other (Photoplethysmography) | 1 (2.2) |
| Heart rate monitoring, n (%) | 46 (100) |
| Other a | 8 (17.4) |
| Sweat testing, n (%) | 38 (82.6) |
| Sympathetic skin response | 29 (63) |
| Quantitative sudomotor axon reflex test | 14 (30.4) |
| Eectrochemical skin conductance | 10 (21.7) |
| Thermoregulatory sweat test | 6 (13) |
| Other b | 3 (6.5) |
| Blood tests, n (%) | |
| Catecholamines | 29 (63) |
| Antibodies | 26 (56.5) |
| Others c | 4 (8.9) |
| Histology, n (%) | |
| Dermal nerve fiber density | 29 (63) |
| Others d | 5 (10.9) |
| Personnel | |
| Head of the laboratory, n (%) | |
| Female | 18 (39.1) |
| Age group, n (%) | |
| 30–39 years | 8 (17.4) |
| 40–49 years | 21 (45.7) |
| 50–59 years | 14 (30.4) |
| 60–69 years | 3 (6.5) |
| Years into practice, n (%) | |
| Resident | 2 (4.3) |
| Junior consultant (0–4 years) | 1 (2.2) |
| Consultant (5–9 years) | 4 (8.7) |
| Senior consultant (10–19 years) | 16 (34.8) |
| >20 years | 23 (50) |
| Consultants, median (IQR) | 2 (1–3) |
| Residents, median (IQR) | 1 (0–2) |
| PhD students, median (IQR) | 1 (0–2) |
| Postdoctoral fellows, median (IQR) | 0 (0–0) |
| Medical students, median (IQR) | 1 (0–2) |
| Technicians, median (IQR) | 1 (1–2) |
| Nurses, median (IQR) | 1 (0–2) |
| Biomedical engineer, median (IQR) | 0 (0–1) |
24‐h electrocardiogram (ECG), 24‐h ambulatory blood pressure monitoring, ergometry, implantable loop recorder; variability studies; photopletysmography, long‐term noninvasive ECG monitoring.
Two laboratories use dynamic sweat test, and one has thermal sensory testing.
Copeptin, synacthen test, cryoglobulin.
Abdominal fat biopsy for amyloidosis, skin biopsy with a‐syn staining for research purposes, skin biopsy with assessment of sweat glands, hairs and vessels innervation; three laboratories referred their patients elsewhere for biopsies.
Metrics and case mixture of the patients
The metrics and case mixture of the patients referred to the laboratories are shown in Table 2. The median (IQR) number of head‐up tilt tests/laboratory/year is 105 (49–251), with two laboratories performing more than 1000 examinations per year. An ANS outpatient clinic is available in 35 centers (76%) with a median of 200 (IQR 100–360) outpatient visits/year. Nine centers report more than 300 outpatient visits/year. Inpatient admissions are available in 42 centers (91%) with a median (IQR) of 20 (4–110) inpatient stays/year. Eleven centers care for more than 100 patients with autonomic disturbances per year in inpatient settings.
TABLE 2.
Metrics and case mix of patients referred to European clinical autonomic nervous system laboratories
| Laboratory metrics | |
| Number of tilt table tests per year | 105 (48.5–251) |
| Number of outpatient visits per year | 200 (100–360) |
| Number inpatient visits per year | 20 (4.25–110) |
| Case mixture in the respective laboratories | |
| Reflex syncope, % | 20.5 ± 18.6 |
| Neurogenic orthostatic hypotension, % | 20.3 ± 18.2 |
| Non‐neurogenic orthostatic hypotension, % | 6.7 ± 7.0 |
| Postural orthostatic tachycardia syndrome, % | 5 (2–15) |
| Initial orthostatic hypotension, % | 5 (0.75–10) |
| Delayed orthostatic hypotension, % | 3.7 ± 3.9 |
| Psychogenic pseudosyncope, % | 3 (1–7.75) |
| Cardiac syncope, % | 1 (0–4) |
| Other transient loss of consciousness, % | 1 (0–5) |
Note: Data are presented as mean ± standard deviation or median (interquartile range).
Research activities
Forty‐one laboratories (89.1%) are currently involved in research activities. Table 3 summarizes the censored research focuses. The five most frequent research areas are orthostatic hypotension, movement disorders, postural orthostatic tachycardia syndrome, reflex syncope and rare diseases.
TABLE 3.
Research focus of the European clinical autonomic nervous system laboratories
| Involved in research activities, N (%) | 41 (89.1) |
| Research focus, n (%) | |
| Orthostatic hypotension | 29 (63) |
| Movement disorders | 23 (50) |
| Postural orthostatic tachycardia syndrome | 17 (37) |
| Reflex syncope | 15 (32.6) |
| Rare diseases | 15 (32.6) |
| Stroke | 13 (28.3) |
| Other | 12 (26.1) |
| Sweating disorders | 11 (23.9) |
| Multiple sclerosis | 10 (21.7) |
| Epilepsy | 9 (19.6) |
| Autoimmune autonomic ganglionopathy | 9 (19.6) |
| Sleep disorders | 9 (19.6) |
| Cardiac syncope | 7 (15.2) |
| Other transient loss of consciousness | 6 (13) |
| Urinary/bowel dysfunction | 6 (13) |
| Headache | 6 (13) |
| Psychogenic pseudosyncope | 4 (8.7) |
Differences among European regions
We observed a significant difference in the availability of ANS healthcare services among different European regions (11/21 countries from South/East/Greater Europe vs. 11/12 countries from North/West Europe; p = 0.021). However, there was no statistically significant difference in the number of inhabitants per laboratory between the North/West Europe and South/East/Greater Europe (7,174,395 [1,180,527–33,540,500] vs. 9,547,146 [2,108,708–38,179,800]; p = 0.587). There was also no difference between the two European regions in terms of equipment, personnel, metrics, case mixture of the patients, and research activities of the laboratories (Tables 4 and 5).
TABLE 4.
Differences in equipment, personnel, metrics, case mix of the patients referred to the clinical autonomic nervous system laboratories between macro‐European regions
| South/East/Greater Europe (16 laboratories) | North/West Europe (30 laboratories) | p value | |
|---|---|---|---|
| Equipment | |||
| Blood pressure monitoring, n (%) | |||
| Riva‐Rocci | 5 (31.3) | 14 (46.7) | 0.362 |
| Invasive continuous | 0 (0) | 4 (13.3) | 0.282 |
| Noninvasive continuous | 13 (81.3) | 28 (93.3) | 0.325 |
| Volume clamp | 7 (43.8) | 20 (66.7) | 0.209 |
| Pulse wave | 8 (50) | 11 (36.7) | 0.531 |
| Other (photopletysmography) | 1 (6.3) | 0 (0) | 0.348 |
| Heart rate monitoring, n (%) | |||
| ECG | 16 (100) | 30 (100) | ‐ |
| Other | 4 (25) | 4 (13.3) | 0.421 |
| Sweat testing, n (%) | 15 (93.8) | 23 (76.7) | 0.230 |
| Sympathetic skin response | 12 (75) | 17 (56.7) | 0.338 |
| Quantitative sudomotor axon reflex test | 4 (25) | 10 (33.3) | 0.739 |
| Electrochemical skin conductance | 3 (18.8) | 7 (23.3) | 1.000 |
| Thermoregulatory sweat test | 2 (12.5) | 4 (13.3) | 1.000 |
| Other | 2 (12.5) | 1 (3.3) | 0.542 |
| Blood testing, n (%) | |||
| Catecholamines | 9 (56.3) | 20 (66.7) | 0.534 |
| Antibodies | 8 (50) | 18 (60) | 0.548 |
| Other | 0 (0) | 4 (13.3) | 0.282 |
| Histology, n (%) | |||
| Dermal fiber density | 8 (50) | 21 (70) | 0.213 |
| Other | 1 (6.3) | 4 (13.3) | 0.645 |
| CASS a | 3 (18.8) | 10 (33.3) | 0.333 |
| Personnel*, n (%) | |||
| Head of the laboratory | |||
| Female | 9 (56.3) | 9 (30) | 0.116 |
| Age group | |||
| 30–39 years | 2 (12.5) | 6 (20) | 0.742 |
| 40–49 years | 7 (43.8) | 14 (46.7) | |
| 50–59 years | 5 (31.3) | 9 (30) | |
| 60–69 years | 2 (12.5) | 1 (3.3) | |
| Years into practice, n (%) | |||
| Resident | 0 | 2 (6.7) | 0.530 |
| Junior consultant (0–4 years) | 0 | 1 (3.3) | |
| Consultant (5–9 years) | 2 (12.5) | 2 (6.7) | |
| Senior consultant (10–19 years) | 4 (25) | 12 (40) | |
| >20 years | 10 (62.5) | 13 (43.3) | |
| Number of consultants, median (IQR) | 2 (1–2.75) | 2 (1–4) | 0.649 |
| Number of residents, mean ± SD | 0.75 ± 0.775 | 1.97 ± 2.189 | 0.009 |
| Number of PhD students, median (IQR) | 1 (0–1.75) | 0.5 (0–2) | 0.911 |
| Number of postdoctoral fellows, median (IQR) | 0 (0–0) | 0 (0–0) | 0.937 |
| Number of medical students, median (IQR) | 1 (0–1.75) | 1 (0–2) | 0.807 |
| Number of technicians, median (IQR) | 1 (1–2) | 1 (0–2.25) | 0.772 |
| Number of nurses, median (IQR) | 1 (0–1) | 1 (0–2.25) | 0.530 |
| Number of biomedical engineers, median (IQR)s | 0 (0–1) | 0 (0–1) | 0.802 |
| Laboratory metrics**, median (IQR) | |||
| Number of tilt table tests per year | 130 (41–250) | 100 (50–265.5) | 0.917 |
| Number of outpatient visits per year | 175 (105–380) | 212 (100–350) | 1.000 |
| Number of inpatient visits per year | 75 (32.5–129.75) | 10 (2.75–25) | 0.036 |
| Case mix in the respective laboratories*** | |||
| Reflex syncope, %, mean ± SD | 22.0 ± 19.0 | 19.8 ± 18.6 | 0.702 |
| Neurogenic orthostatic hypotension, %, mean ± SD | 21 ± 15 | 20 ± 19.9 | 0.857 |
| Postural orthostatic tachycardia syndrome, %, mean ± SD | 6.6 ± 9.9 | 13.8 ± 16.9 | 0.125 |
| Initial orthostatic hypotension, %, mean ± SD | 7.6 ± 9.6 | 4.8 ± 4.7 | 0.188 |
| Non‐neurogenic orthostatic hypotension, %, mean ± SD | 7.2 ± 7.7 | 6.5 ± 6.8 | 0.757 |
| Delayed orthostatic hypotension, %, mean ± SD | 4 ± 4.7 | 3.6 ± 3.6 | 0.727 |
| Psychogenic pseudosyncope, %, mean ± SD | 3.3 ± 3.0 | 4.9 ± 4.2 | 0.186 |
| Cardiac syncope, %, median (IQR) | 1 (0–10) | 0 (0–3.5) | 0.571 |
| Other transient loss of consciousness, %, median (IQR) | 2 (0–8.75) | 0 (0–5) | 0.152 |
Note: Due to corrections for multiple comparisons, every section has corrected p value for statistical significance, calculated according to number of comparisons.
Abbreviations: CASS, Composite Autonomic Severity Score; ECG, electrocardiogram; IQR, interquartile range; SD, standard deviation.
Quantitative sudomotor axon reflex test and (invasive or noninvasive continuous blood pressure) and heart rate monitoring devices.
*p < 0.006; **p < 0.017; ***p < 0.006.
TABLE 5.
Comparison of the research focus of the clinical autonomic nervous system laboratories between macro‐European regions
|
South/East/Greater Europe (16 laboratories) n (%) |
North/West Europe (30 laboratories) n (%) |
p value | |
|---|---|---|---|
| Research | |||
| Research activity | 16 (100) | 25 (83.3) | 0.147 |
| Research focus | |||
| Orthostatic hypotension | 12 (75) | 17 (56.7) | 0.338 |
| Movement disorders | 11 (68.8) | 12 (40) | 0.120 |
| Postural orthostatic tachycardia syndrome | 7 (43.8) | 10 (33.3) | 0.534 |
| Reflex syncope | 5 (31.3) | 10 (33.3) | 1.000 |
| Rare diseases | 6 (37.5) | 9 (30) | 0.744 |
| Stroke | 5 (31.3) | 8 (26.7) | 1.000 |
| Other | 5 (31.3) | 7 (23.3) | 0.726 |
| Sweating disorders | 4 (25) | 7 (23.3) | 1.000 |
| Multiple sclerosis | 5 (31.3) | 5 (16.7) | 0.283 |
| Epilepsy | 4 (25) | 5 (16.7) | 0.698 |
| Autoimmune autonomic ganglionopathy | 5 (31.3) | 4 (13.3) | 0.241 |
| Sleep disorders | 6 (37.5) | 3 (10) | 0.047 |
| Cardiac syncope | 2 (12.5) | 5 (16.7) | 1.000 |
| Other transient loss of consciousness | 4 (25) | 2 (6.7) | 0.163 |
| Urinary/bowel dysfunction | 3 (18.8) | 3 (10) | 0.649 |
| Headache | 1 (6.3) | 5 (16.7) | 0.406 |
| Psychogenic pseudosyncope | 1 (6.3) | 3 (10) | 1.000 |
Note: Due to corrections for multiple comparisons, p value is statistically significant at p < 0.003.
DISCUSSION
The EAN and the EFAS play a key role in defining the standards of care and research for European people with autonomic disorders through guidelines, consensus statements and interactions with health policy makers [11]. In order to make applicable and realistic recommendations, these need to be tailored to the available resources across European countries and take regional differences into account. Both aspects were not well known to date. The main goal of the present EAN‐EFAS joint effort was to fill this gap in knowledge by collecting information on ANS laboratories, ultimately facilitating benchmarking of autonomic healthcare provision across European countries.
The survey provides several important insights. First, the 2005 survey of the EFNS Scientist Panel on Autonomic Nervous System Disorders [4] identified 48 neurology‐driven or interdisciplinary ANS laboratories, and the current survey 84. Even though a direct comparison between the two surveys is not entirely possible due to design differences, both surveys focused on European countries, adopted comparable laboratory identification strategies, and clearly distinguished between neurology and non‐neurology‐driven ANS laboratories. It is therefore conceivable that the 75% increase in the number of censored neurology‐driven or interdisciplinary ANS laboratories reflects an incremental trend in the availability of specialized healthcare services for people with ANS disorders in Europe. This is particularly relevant in light of the results of the Global Burden of Disease 2015 Neurological Disorders Collaborator Group, which showed that neurological disorders are the leading global cause of disability‐adjusted life years and the second cause of death after cardiovascular disease [12]. In line with this, the Intersectoral Global Action Plan on Epilepsy and Other Neurological Disorders (GAP), which addresses the worldwide and European challenges and gaps in providing care and services for people with neurological disorders, has recently identified a unique window of opportunity to provide an integrated and cross‐sectorial response to improve neurological health in the general population [13].
The second objective of our survey was to understand the equipment and personnel composition of each ANS laboratory. Cardiovascular autonomic function testing represents the cornerstone of every identified laboratory. More than 80% of laboratories also have at least one test for sudomotor function. This again represents a significant improvement compared to the 2005 survey, in which only 43% of the identified laboratories performed sweat testing [6]. A substantial number of laboratories also has access to additional tests. This is of particular importance since combining ANS tests often enables a more accurate assessment of the severity degree and neuroanatomical localization of autonomic dysfunction, in turn, facilitating an earlier diagnosis and personalized interventions [14].
The recent AAS/IFCN consensus statement emphasized the CASS as an important tool for the evaluation and follow‐up of people with ANS disorders. We found that 28% of all censored laboratories have the necessary equipment for completing the CASS battery. This represents good potential for running future multicenter clinical studies, both observational (i.e., natural history studies and registries for rare autonomic disorders) and interventional. Using the CASS has the advantage of standardizing findings, thus facilitating comparisons among laboratories for both routine and research purposes. Its applicability is, however, limited to centers with a QSART facility, while not considering other, equally adequate, sudomotor function tests.
In the personnel part of the survey, we found that most of the laboratories have at least one consultant, resident, technician and nurse. We also found that the head of the laboratories was females in 18 (39%) centers only, emphasizing gender gaps in leadership positions. Gender disparities also affect autonomic research; it has been reported that the percentage of publications in autonomic medicine by a female first author is consistently below 50% over the last 30 years [15]. The percentage of publications with at least one female author, however, increased from 49% in 1994 to 69% in 2019, indicating a positive trend.
The availability of postdoctoral fellows and biomedical engineers is limited. The discipline of biomedical engineering has emerged as a connection element between medicine and engineering [16]. The results of our survey indicate that efforts should be made in the future to further implement bioengineer positions in clinical autonomic practice and research. Despite a presumably similar spectrum of patients, there is also substantial variation among European countries in the duration of residency training programs, and especially in the choice of obligatory rotations to external medical disciplines [17]. It is important for the laboratories to train an adequate number of residents, clinical fellows and postdoctorate students in clinical autonomic practice and research to secure enough personnel resources in the future, particularly in view of the aging population and increasing demand resulting from neurological disease burden [14].
In the third part of the survey, we evaluated the metrics and case mixture of the patients referred to the laboratories. We observed high variability in the numbers of head‐up tilt examinations, and inpatient and outpatient visits per laboratory. The fact that reflex syncope and neurogenic orthostatic hypotension represented the most frequently diagnosed cardiovascular autonomic disorders is in line with the respective prevalence of such autonomic disorders in the general population [1].
Another positive aspect highlighted by the survey is that most of the interviewed laboratories are involved in research activities at a similar rate to that found in the 2005 survey [6]. The observation that orthostatic hypotension is the most frequent area of research of neurology‐driven and interdisciplinary ANS laboratories may be based on the fact that it develops in several common neurological conditions such as Parkinson's disease, neuropathies and lesions to the central ANS system of diverse etiologies, thus introducing a referral bias in comparison to cardiology‐driven laboratories, which may, in turn, be more biased towards other causes of syncope.
Health disparities, defined as differences in health and healthcare among different groups of people, can affect people with any disease, including neurological disorders [18]. The term neurodisparity has been used to highlight this inequity, which has existed across a range of neurological conditions for decades, both in the United States and Europe [18, 19, 20]. In order to minimize neurodisparities, neurology stakeholders first need to recognize differences in the availability of neurological care and then act accordingly [20]. Maybe one of the best examples of neurodisparity in Europe is seen in access to treatment and physical rehabilitation among people with multiple sclerosis, where geographical location determines access to and the type of treatment provided [21, 22]. Similarly, our survey identified neurodisparity in the availability of ANS services across Europe. Laboratories were more frequent in North/West Europe compared to South/East/Greater Europe. This indicates that geographical factors are particularly relevant in producing neurodisparity in Europe. However, additional factors have to be taken into account when looking at a wider European context, such as the differences in population density among European regions, the reimbursement policies for ANS services in each country, and the historical autonomic background of some countries. We also observed interesting organizational differences among countries. Some, such as the United Kingdom or Croatia, have a centralized model with one or two high‐volume centers per country; others, such as the Netherlands, Italy, Austria, France and Germany have a higher number of centers across the country, but these are medium sized. Reimbursement policies and an “all‐in‐one test battery” versus a “stepwise examination approach” may contribute to determining such organizational structures, and both pros and cons for a centralized versus decentralized ANS care provision can be postulated. The all‐in‐one test battery likely leads to better opportunities to build adequately sized case series to study rare autonomic disorders and educate a higher number of healthcare professionals, while a stepwise approach may warrant better access to ANS healthcare services for people living in remote areas or with reduced mobility due to age or disease‐related causes.
Some limitations should be taken into account when interpreting the results reported in this study. First, we had a responder rate of 55%, which is relatively low. The multiple networking approaches described in the methods were developed on purpose to counteract this anticipated difficulty of the chosen study design. Moreover, the results of the survey are based on the personal opinion and view of responders, who, however, proved to be physicians with 10 to 19 years' professional experience, and thus were likely well informed in clinical autonomic practice and research matters. Finally, our approach might have missed smaller laboratories in some countries, especially those with no national autonomic society, emphasizing an important need for more capillary professional networking activities to reach out to ANS experts practicing outside of larger academic centers.
In conclusion, our data provide a good basis for understanding the challenges of clinical autonomic practice in Europe and for planning concerted actions for better disease management and collaborative research. It also highlights significant differences in the availability of healthcare services for people with ANS disorders across European countries, stressing the need for improved access to diagnostic and treatment facilities across Europe, and thereby investment in health service improvement and spending by national governments. Both the EFAS and the EAN are called to intensify their educational and health policy activities to increase the expertise in ANS disturbances in the neurological community and to promote the establishment of new ANS laboratories in underserved countries.
AUTHOR CONTRIBUTIONS
Study conception: Alessandra Fanciulli, Mario Habek, Jennifer Camaradou, Pietro Cortelli, Cristian Falup‐Pecurariu, Pietro Guaraldi, Raimund Helbok, Max J. Hilz, Valeria Iodice, Jens Jordan, Anne Pavy Le Traon, Isabel Rocha, Johann Sellner, Jean Michel Senard, Astrid Terkelsen, Gregor K. Wenning, Thomas Berger, Roland Thijs, Walter Struhal. Study design: Alessandra Fanciulli, Mario Habek, Diogo Reis Carneiro, Raimund Helbok, Anne Pavy Le Traon, Johann Sellner, Thomas Berger, Roland Thijs, Walter Struhal. Study coordination: Alessandra Fanciulli, Mario Habek. Data collection: Alessandra Fanciulli, Mario Habek, Fabian Leys, Giovanna Calandra‐Buonaura, Giacomo Chiaro, Cristian Falup‐Pecurariu, Roberta Granata, Pietro Guaraldi, Valeria Iodice, Evert C. A. Kaal, Anita Kamondi, Anne Pavy Le Traon, Astrid Terkelsen, Gregor K. Wenning, Roland Thijs, Walter Struhal. Data analysis: Mario Habek, Magdalena Krbot Skorić, Alessandra Fanciulli, Fabian Leys. Writing of the first draft: Mario Habek . Writing – review and editing: Alessandra Fanciulli, Diogo Reis Carneiro, Fabian Leys, Giovanna Calandra‐Buonaura, Jennifer Camaradou, Giacomo Chiaro, Pietro Cortelli, Cristian Falup‐Pecurariu, Roberta Granata, Pietro Guaraldi, Raimund Helbok, Max J. Hilz, Valeria Iodice, Jens Jordan, Evert C. A. Kaal, Anita Kamondi, Anne Pavy Le Traon, Isabel Rocha, Johann Sellner, Jean Michel Senard, Astrid Terkelsen, Gregor K. Wenning, Magdalena Krbot Skorić, Thomas Berger, Roland Thijs, Walter Struhal.
FUNDING INFORMATION
Academic study without external financial support. We received administrative support from the EAN Head Office.
CONFLICT OF INTEREST
Mario Habek: participated as a clinical investigator and/or received consultation and/or speaker fees from Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals and TG Pharmaceuticals, outside of the present work. Fabian Leys: none. Dr Leys is supported by grants from the US MSA Coalition and Dr Johannes and Herta Tuba Foundation. Magdalena Krbot Skorić: received consultation and/or speaker fees from Sanofi Genzyme and Roche, outside of the present work. Diogo Reis Carneiro: received speaker fees from Almirall, outside of the present work. Giovanna Calandra‐Buonaura: received honoraria for speaking engagements or consulting activities from Abbvie, Bial and Zambon, outside the present work. Jennifer Camaradou: participated as a costed PPI Patient Public Involvement lead in a study at University College London funded by the UK National Institute for Health Research (NIHR)132914 and UK NIHR20334 at Bristol UWE, received consultation fees from Roche Canada, Roche Global, Medable Inc, GSK Glaxco Smith Klein, outside of the present work, and received small honoraria as a lay member of the UK NICE Covid expert panel, as a Citizen Partner COVID END, Evidence Synthesis network (WHO COVID‐19 evidence collaborative partner) and as a lay member on the NIHR AI AWARD panel, and is a Cochrane Convenes citizen partner, Patient Editor Advances in Therapy Journal Springer Health, a patient representative on UK MRC ADPD Advanced Pain Discovery Platform and an ambassador for the OneNeurology Global Partnership for EMEA. Giacomo Chiaro: none. Pietro Cortelli: reports no financial disclosure. Cristian Falup‐Pecurariu: received royalties from Elsevier, Springer Verlag, honoraria from Abbvie, International Parkinson Disease and Movement Disorders Society, outside of the present work. Roberta Granata: none. Pietro Guaraldi: has been an advisory board member of Alnylam and Sobi; received speaker fees and honoraria from Theravance Biopharma, Akcea Therapeutics; Alnylam and Chiesi, received congress and travel accommodation expense compensations from Alnylam, Bial, Zambon, Sobi and Abbvie, all outside of the present work. Raimund Helbok: none. Max J. Hilz: received consultancy and speaker fees from Sanofi Genzyme, consultancy fees from Pfizer, and editor honoraria from Elsevier BV, outside of the present work. Valeria Iodice: reports speaker fees and honoraria from Theravance Biopharma, Janssen, outside of the present work. Jens Jordan: served as advisor for Novo‐Nordisk, received research support from Boehringer‐Ingelheim and Novo‐Nordisk, and is co‐founder of Eternygen GmbH. Evert C. A. Kaal: none. Anita Kamondi: none. Anne Pavy Le Traon: reports honoraria from Biohaven outside of the present work. Isabel Rocha: nothing to disclose. Johann Sellner: none related to this work. Jean Michel Senard: none. Astrid Terkelsen: received consultation and/or speaker fees from Alnylam Pharmaceuticals, Akcea Therapeutics, Pfizer and Allergan, however, without relation to the present work. Gregor K. Wenning: reports consultancy and lecture fees from AbbVie, AFFiRiS AG, AstraZeneca, Biogen, Biohaven, Inhibicase, Lundbeck, Merz, Ono, Teva and Theravance, and research grants from the Austrian Science Fund (FWF), the Austrian National Bank, the US MSA Coalition, Parkinson Fonds Austria, the Dr Johannes und Hertha Tuba Foundation, and the International Parkinson and Movement Disorder Society, outside of the submitted work. Thomas Berger: has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, BMS/Celgene, GSK, GWD/Jazz Pharma, Horizon, Janssen‐Cilag, MedDay, Merck, Novartis, Octapharma, Roche, Sandoz, Sanofi‐Genzyme, Teva. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, BMS/Celgene, Merck, Novartis, Sanofi Aventis, Teva) and for participation in clinical trials in multiple sclerosis and related disorders sponsored by Alexion, Bayer, Biogen, BMS/Celgene, Merck, Novartis, Octapharma, Roche, Sanofi‐Genzyme, Teva. Roland Thijs: reports research support from the ‘Christelijke Vereniging voor de Verpleging van Lijders aan Epilepsie’, Michael J Fox Foundation, Human Measurement Models Programme co‐funded by Health~Holland, Top Sector Life Sciences & Health and ZonMw under grant agreement 114,025,101 (Brain@Home) and speaker or consultant fees from Theravance Biopharma, Arvelle, Medtronic, Zogenix, UCB and NewLife Wearables. Walter Struhal: none. Alessandra Fanciulli: reports royalties from Springer Verlag, speaker fees and honoraria from Theravance Biopharma, Abbvie, Healthware, the International Parkinson Disease and Movement Disorders Society, the Austrian Neurology Society, the Austrian Autonomic Society and research grants from the Parkinson Fond, US MSA Coalition, Dr Johannes and Hertha Tuba Foundation and Austrian Exchange Program, outside of the present work.
Supporting information
Appendix S1
Appendix S2
ACKNOWLEDGMENTS
We would like to thank Ms Lucia Pavlakova and Ms Abigail Magno (Scientific Department, European Academy of Neurology) for the excellent administrative support and the following collaborators of the European Network of Clinical ANS laboratories for their thoughtful completion of the survey:
Adamec Ivan – Department of Neurology, University Hospital Centre Zagreb, Zagreb, Croatia; Aerts Arnaud – Department of Cardiology, Zuyderland Medical Centre, Heerlen, Netherlands; Canta LR (Leo) – Department of Neurology, Catharina Ziekenhuis, Eindhoven, Netherlands; Delamont Robert Shane – Department of Neurology and Clinical Neurophysiology, King's College Hospital, London, UK; de Lange Frederik – Syncope Unit, Amsterdam University Medical Center, Amsterdam, Netherlands; Del Sorbo Francesca – Parkinson and Movement Disorders Unit, ASST Gaetano Pini‐CTO, Milano, Italy; Devigili Grazia – Parkinson and Movement Disorder Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano, Italy; Di Leo Rita – Department of Neurology, Ospedale San Giovanni e Paolo, Venezia, Italy; Dinh Trang – Department of Cardiology, Maastricht University Medical Center, Maastricht, Netherlands; Fortrat Jacques‐Olivier – Explorations Fonctionnelles Vasculaires, Centre Hospitalier Universitaire, Angers, France; Gierthmühlen Janne – Department of Neurology, University Hospital of Schleswig‐Holstein, Campus Kiel, Kiel, Germany; Hemels Martin – Department of Cardiology, Rijnstate Hospital and Radboud University Medical Center, Arnhem ‐ Nijmegen, Netherlands; Köhn Julia – Department of Neurology, University of Erlangen‐Nuremberg, Erlangen, Germany; Krøigård Thomas – Department of Neurology, Odense University Hospital, Odense, Denmark; Lipp Axel – Department of Neurology, Park‐Klinik Weißensee, Berlin, Germany; Maier Andrea – Department of Neurology, Outpatient Service for ANS Disorders, University Clinic RWTH Aachen, Aachen, Germany; Marinelli Lucio – Department of Neuroscience, IRCCS Ospedale Policlinico San Martino, Genova, Italy; Mazzeo Anna – Department of Clinical and Experimental Medicine, Neurology and Neuromuscular Disease Unit, Messina, Italy; Milenkovic Ivan – Department of Neurology, Medical University of Vienna, Vienna, Austria; Motyl Maciej – Department of Neurology, Jagiellonian University Medical College, Krakow, Poland; Natali Sora Maria Grazia – Department of Neurology, Ospedale San Raffaele, Milano, Italy; Navarro‐Otano Judith – Department of Neurology, Hospital Clinic, Barcelona, Spain; Nilsen Kristian Bernhard – Department of Neurology, Oslo University Hospital, Oslo University Hospital, Norway; Oliveira Mario – Department of Cardiology, Santa Marta Hospital, Lisbon, Portugal; Omland Petter Moe – Department of Neurology, Section of Clinical Neurophysiology, St. Olavs University Hospital, Trondheim, Norway; Pelliccioni Giuseppe – Department of Neurology, Istituto Nazionale di Ricovero e Cura per Anziani IRCCS Ancona, Ancona, Italy; Pereon Yann – Reference Centre from Neuromuscular Disorders, Department of Neurology, University Hospital, Nantes, France; Resch Roland Josef – Department of Neurology, Kepler University, Linz, Austria; Rocchi Camilla – Department of Neurology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Rome, Italy; Roche Frederic – Department of Clinical Physiology, CHU And INSERM U 1059 Sainbiose, Saint Étienne, France; Rutten Joost – Department of Internal Medicine, Radboud University Medical Center, Nijmegen, Netherlands; Tijero Merino Beatriz – Movement Disorders and Autonomic Disorders Unit, Cruces Hospital, Barakaldo, Spain; Tutaj Marcin – Department of Neurology, Jagiellonian University Medical College, Krakow, Poland; van der Heijden‐Montfroy AMHG – Department of Neurology, VieCuri, Venlo, Netherlands; van Hoeve Bas JA – Department of Neurology, ZorgSaam Hospital, Terneuzen, Netherlands; van Orshoven Narender – Department of Neurology, Zuyderland Medical Centre, Heerlen, Netherlands; Wang Ruihao – Department of Neurology, University of Erlangen‐Nuremberg, Erlangen, Germany; Z'Graggen Werner J – Department of Neurology, University Hospital Bern, Bern, Switzerland.
Habek M, Leys F, Krbot Skorić M, et al. Clinical autonomic nervous system laboratories in Europe. Eur J Neurol. 2022;29:3633‐3646. doi: 10.1111/ene.15538
European Network of Clinical ANS Laboratory (members are listed in Acknowledgments section).
Contributor Information
Alessandra Fanciulli, Email: alessandra.fanciulli@i-med.ac.at.
the Collaborators of the European Network of Clinical ANS laboratories:
Ivan Adamec, Arnaud Aerts, Leo L.R. Canta, Robert Shane Delamont, Frederik de Lange, Francesca Del Sorbo, Grazia Devigili, Rita Di Leo, Trang Dinh, Jacques‐Olivier Fortrat, Janne Gierthmühlen, Martin Hemels, Julia Köhn, Thomas Krøigård, Axel Lipp, Andrea Maier, Lucio Marinelli, Anna Mazzeo, Ivan Milenkovic, Maciej Motyl, Maria Grazia Natali Sora, Judith Navarro‐Otano, Kristian Bernhard Nilsen, Mario Oliveira, Petter Moe Omland, Giuseppe Pelliccioni, Yann Pereon, Roland Josef Resch, Camilla Rocchi, Frederic Roche, Joost Rutten, Beatriz Tijero Merino, Marcin Tutaj, A.M.H.G. van der Heijden‐Montfroy, Bas J.A. van Hoeve, Narender van Orshoven, Ruihao Wang, and Werner J. Z’Graggen
DATA AVAILABILITY STATEMENT
The first and the last authors take full responsibility for the integrity of data and agree to share any data not published within this article in a de‐identified form upon reasonable request from any qualified investigator.
REFERENCES
- 1. Kaufmann H, Norcliffe‐Kaufmann L, Palma JA. Baroreflex dysfunction. N Engl J Med. 2020;382(2):163‐178. [DOI] [PubMed] [Google Scholar]
- 2. Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878‐885. [DOI] [PubMed] [Google Scholar]
- 3. Cheshire WP, Freeman R, Gibbons CH, et al. Electrodiagnostic assessment of the autonomic nervous system: a consensus statement endorsed by the American autonomic society, American Academy of Neurology, and the International Federation of Clinical Neurophysiology. Clin Neurophysiol. 2021;132(2):666‐682. [DOI] [PubMed] [Google Scholar]
- 4. Lahrmann H, Magnifico F, Haensch CA, Cortelli P. Autonomic nervous system laboratories: a European survey. Eur J Neurol. 2005;12(5):375‐379. [DOI] [PubMed] [Google Scholar]
- 5. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69‐72. [DOI] [PubMed] [Google Scholar]
- 6. Fanciulli A, Jordan J, Biaggioni I, et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American autonomic society (AAS) and the European Federation of Autonomic Societies (EFAS): endorsed by the European academy of neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res. 2018;28(4):355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thijs RD, Brignole M, Falup‐Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American autonomic society (AAS) and the European academy of neurology (EAN). Clin Auton Res. 2021;31:369‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;68:748‐752. [DOI] [PubMed] [Google Scholar]
- 9. Boynton PM, Greenhalgh T. Selecting, designing, and developing your questionnaire. BMJ. 2004;328(7451):1312‐1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. https://unstats.un.org/unsd/methodology/m49/. Accessed April 4, 2022
- 11. Deuschl G. The future of neurology in Europe. Clin Trans Neurosci. 2017;1(1). doi: 10.1177/2514183X17714096 [DOI] [Google Scholar]
- 12. GBD 2015 Neurological Disorders Collaborator Group . Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Neurol. 2017;16:877‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. https://www.who.int/news/item/28‐04‐2022‐draft‐intersectoral‐global‐action‐plan‐on‐epilepsy‐and‐other‐neurological‐disorders‐2022‐2031. Accessed May 31, 2022
- 14. Padovani A, Pilotto A. Looking at the burden of neurological disorders in Europe. Lancet Public Health. 2020;5:e523. [DOI] [PubMed] [Google Scholar]
- 15. Taylor CE, Arnold AC, Fanciulli A, et al. Women in clinical autonomic research and the autonomic societies: how far have we come in thirty years? Clin Auton Res. 2021;31:23‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bronzino J. Biomedical engineering: a historical perspective. In: Enderle JD, Blanchard SM, Bronzino JD, eds. Biomedical Engineering, Introduction to Biomedical Engineering. Second ed. Academic Press; 2005:1‐29. [Google Scholar]
- 17. Kleineberg NN, van der Meulen M, Franke C, et al. Differences in neurology residency training programmes across Europe ‐ a survey among the residents and research fellow section of the European academy of neurology national representatives. Eur J Neurol. 2020;27(8):1356‐1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Lancet Neurology . Disparities in neurological care: time to act on inequalities. Lancet Neurol. 2020;19(8):635. [DOI] [PubMed] [Google Scholar]
- 19. Marulanda‐Londoño ET, Bell MW, Hope OA, et al. Reducing neurodisparity: recommendations of the 2017 AAN diversity leadership program. Neurology. 2019;92(6):274‐280. [DOI] [PubMed] [Google Scholar]
- 20. McCarron MO, Clarke M, Burns P, et al. A Neurodisparity index of Nationwide access to neurological health care in Northern Ireland. Front Neurol. 2021;12(12):608070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Filippi M, Danesi R, Derfuss T, et al. Early and unrestricted access to high‐efficacy disease‐modifying therapies: a consensus to optimize benefits for people living with multiple sclerosis. J Neurol. 2022;269(3):1670‐1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Řasová K, Freeman J, Cattaneo D, et al. Content and delivery of physical therapy in multiple sclerosis across Europe: a survey. Int J Environ Res Public Health. 2020;17(3):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Data Availability Statement
The first and the last authors take full responsibility for the integrity of data and agree to share any data not published within this article in a de‐identified form upon reasonable request from any qualified investigator.


