Abstract
Introduction
Lower socioeconomic status is associated with significantly poorer outcomes in weight, lung function, and pulmonary exacerbation rates in people with cystic fibrosis (PwCF).
Global aim
We aim to reduce health disparities and inequities faced by PwCF by screening for and addressing unmet social needs.
Specific aims
We aimed to increase routine social determinants of health (SDoH) screening of eligible PwCF from 0% to 95% and follow‐up within 2 weeks for those PwCF who screened positive and requested assistance from 0% to 95% by December 31, 2021.
Methods
The Model for Improvement methodology was used. A process map and a simplified failure mode effects analysis chart were created for the screening and SDoH follow‐up process. For those who screened positive for SDoH and requested assistance, follow‐up contact was made to offer intervention.
Intervention
Adult PwCF who had at least one UVA Clinic encounter in 2021 were screened for SDoH. The SDoH screening tool included eight domains: housing, food, transportation, utilities, health‐care access, medication access, income/employment, and education. Follow‐up was completed with all PwCF who screened positive for SDoH.
Results
A total of 132 of 142 (93.0%) PwCF eligible for screening completed the SDoH screening. Of the PwCF who completed screening, 56 (42.4%) screened positive for SDoH. A follow‐up rate of 100% was achieved in June 2021 and maintained through December 2021.
Conclusion
Implementing screening for SDOH and follow‐up to mitigate social difficulties in adult PwCF at UVA was successful and could be reproduced at other CF care centers.
Keywords: cystic fibrosis, Model for Improvement, Plan‐Do‐Study‐Act, social determinants of health, social risk factors
1. INTRODUCTION
Cystic fibrosis (CF) is a systemic genetic disorder primarily characterized by recurrent respiratory infections and lung function decline, affecting approximately 31,000 people in the United States. 1 People with CF (PwCF) with similar CF genotypes can have significantly different outcomes due to socioeconomic factors. 2 , 3 Social determinants of health (SDoH) are “conditions in the places where people live, learn, work, and play that affect a wide range of health risks and outcomes”. 4
Lower socioeconomic status (SES) background is associated with significantly poorer outcomes in weight, lung function, and pulmonary exacerbation rates for children with CF. 3 Public health insurance status is associated with accelerated lung function decline, which is not explained by differences in outpatient care. 5 Lower SES is associated with disease severity, increased antibiotic requirements, greater health‐care utilization, and decreased survival of people with CF. 6 , 7 , 8 , 9 Additionally, minority status and lower SES may affect the health‐related quality of life for PwCF across their lifespan. 10
Social risk factors are defined as, “specific adverse social conditions that are associated with poor health, like social isolation or housing instability”. 11 Social risk factors can be conceptualized as a downstream effect of SDoH. 12 For example, SES is an SDoH that influences the neighborhoods where people can afford to live and therefore their housing conditions. 13 Where people live can also create challenges in accessing food. Studies that measure accessibility to food stores and healthy food in nearby stores have found disparities by race, income, and population density. 14 In this instance, SES could lead to social risk factors in the domains of housing and food insecurity.
Previous studies on food insecurity in the CF community indicate that between 26% and 33% of families of children with CF experience food insecurity and over 40% of adults with CF have food insecurity. 15 , 16 Food insecurity in PwCF is associated with higher weight loss, worse airway clearance adherence, and worse medication adherence compared to food‐secure PwCF. 17 Further research is needed to see how other social risk factors impact PwCF. One approach to addressing SDoH is identifying unmet social needs through a screening questionnaire. The screening questionnaire is then followed up by an intervention intended to mitigate unmet needs, often through referral(s) to appropriate resources. 18 , 19 , 20
1.1. Global aim
We aim to reduce health disparities and inequities faced by people with CF by screening for and addressing unmet social needs.
1.2. Specific aims
-
1.
We aimed to increase routine SDoH screening of eligible PwCF from 0% to 95% by December 31, 2021.
-
2.
We aimed to increase follow‐up within 2 weeks for those PwCF who screened positive and requested assistance from 0% to 95% by December 31, 2021.
Originally, Aim 2 was to increase follow‐up within 2 weeks for those PwCF who screened positive from 0% to 95% by December 31, 2021. During rapid testing, Aim 2 was changed. The original version (v1.0) of the screening tool did not give PwCF the opportunity to indicate whether they would like to receive assistance. Respondents could only screen positive or negative. At that time, follow‐up was attempted for all PwCF who screened positive. Aim 2 was changed when the screening tool was changed to include a final question that asked respondents if they would like to receive assistance with any social needs they reported during the screening. PwCF who answered “yes” to this final question were defined as having requested assistance. See follow‐up process Plan‐Do‐Study‐Act (PDSA) testing cycles for details.
2. METHODS
2.1. Context
In 2020 the UVA Health Adult Cystic Fibrosis Center conducted a study to determine the effect of the COVID‐19 pandemic on SDoH in adults with CF. Adult PwCF were screened for SDoH using a questionnaire developed by the UVA Adult CF care team. Screening results indicated that of the 76 PwCF who completed the screening, 22 (28.9%) answered “yes” to at least one question that indicated an undesired change in SDoH. 21 The results of this study prompted the conceptualization of a routine SDoH screening and intervention process. No IRB approval was required due to this being a quality improvement (QI) project.
The UVA Adult CF care team routinely utilizes QI tools to improve patient outcomes. Team members include pulmonologists, a respiratory therapist, registered dietitians, a social worker (SW), a psychologist, and a QI coordinator. The UVA QI team participates in the CF Learning Network (CFLN), a network of Cystic Fibrosis Foundation accredited centers committed to collaboration, innovation, and partnering to improve patient outcomes. CFLN uses the Model for Improvement as the QI methodology. 22
2.2. Interventions
Adult patients with a diagnosis of CF who had at least one UVA CF clinic encounter in 2021 were considered eligible for screening. The UVA adult CF care team used the SDoH screening tool used in the 2020 study 21 to develop a new screening tool intended for routine SDoH screening within the CF care model. The CF social needs screening tool screened for social risk factors in eight domains:
-
1.
Housing
-
2.
Food
-
3.
Transportation
-
4.
Utilities
-
5.
Health‐care access
-
6.
Medication access
-
7.
Income/employment
-
8.
Education
In addition to the eight domains above, questions about SDoH questions were included that were not associated with a specific domain. These questions helped gather patient data that could allow clinicians to determine potential eligibility for resources such as financial assistance or grants if an intervention was needed. The screening tool also included questions about race, ethnicity, age, and gender identity. Questions asking participants to identify race and ethnicity were based on US Census Bureau guidelines. A respondent was defined as having screened positive for SDoH if they answered “yes” to at least one question associated with one of the eight domains included in the survey.
A process map (Figure 1) was created for the screening and intervention process. Screening data were collected either via paper instrument, Health Insurance Portability and Accountability Act (HIPAA) secure online Qualtrics survey, or screen sharing during telehealth appointments. For PwCF who screened positive for SDoH and requested assistance, follow‐up contact was made to offer intervention. A simplified failure mode effects analysis was created to anticipate any potential problems in the process (Figure 2).
Figure 1.
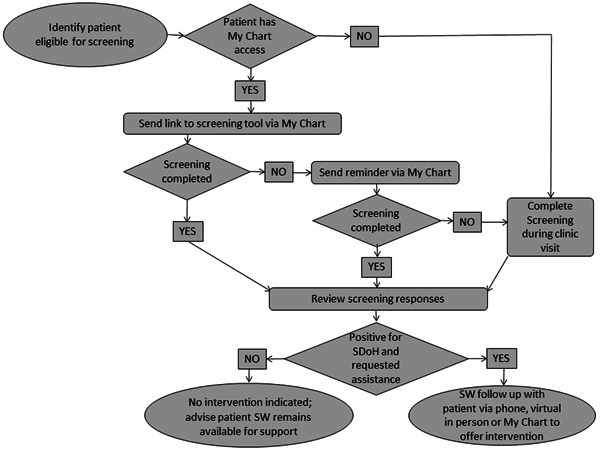
Process map for routine SDoH screening and intervention process. MyChart, Health Insurance Portability and Accountability Act (HIPAA) secure messaging system through patient electronic medical record; SDoH, social determinants of health; SW, social worker.
Figure 2.
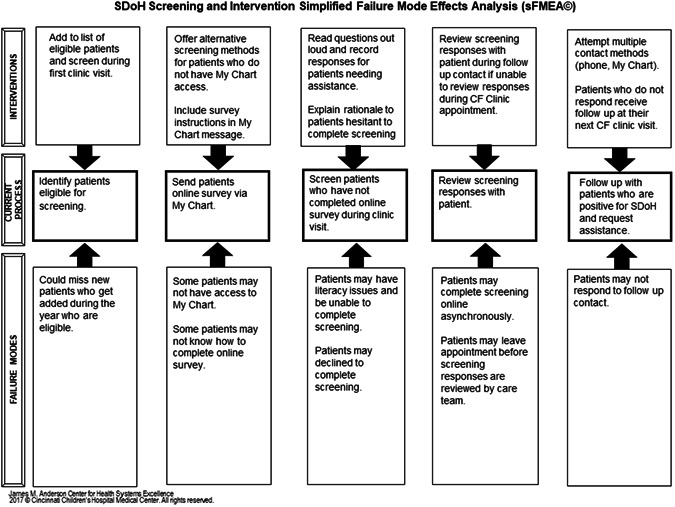
SDoH screening and intervention simplified failure mode effects analysis (sFMEA). CF, cystic fibrosis; MyChart, Health Insurance Portability and Accountability Act (HIPAA) secure messaging system through patient electronic medical record; SDoH, social determinants of health.
The project conceptualization, results, and PDSA testing cycles were reviewed during weekly QI team meetings. The screening and follow‐up process were tested with PDSA cycles. The screening process underwent a total of four PDSA testing cycles from January to March 2021 (Figure 3).
PDSA 1: Patients with access to Epic MyChart (HIPAA secure electronic message system) were sent a link to the HIPAA secure online survey
PDSA 2: Instructions for the online survey link were clarified based on feedback from PwCF. The remaining patients with MyChart access who had not yet completed the online survey were sent a link. A survey question soliciting the responders' name was also made mandatory to ensure all survey responses could be connected to the individual who completed the survey.
PDSA 3: Based on the low response rate during the second testing cycle, a reminder was sent via MyChart asking PwCF to complete the online survey. No further concerns were noted with the online screening. An online screening process was adopted.
PDSA 4: The process for screening PwCF during CF clinic visits was tested. The screening was administered via paper instrument for in‐person appointments and via screen sharing for telehealth appointments. No issues were noted with the in‐person or telehealth screening processes. All three screening processes were adopted.
Figure 3.
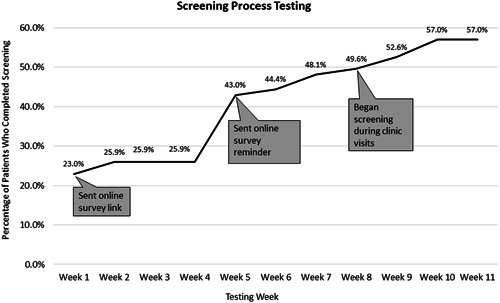
Annotated chart tracking the progress of percentage of PwCF screened during screening process testing. PwCF, people with cystic fibrosis.
The follow‐up process was tested from January to June 2021 and underwent six PDSA testing cycles.
PDSA 1: Attempted to contact patients via MyChart who screened positive for SDoH. Phone contact was attempted for those PwCF who did not respond to a MyChart message.
PDSA 2: The process map was adjusted to include follow‐up completed in person or during telehealth follow‐up for patients who had appointments or were hospitalized and had scored positive for SDoH. Despite attempting contact via four methods (MyChart, phone, telehealth, and in‐person), the follow‐up rate continued to be below aim.
PDSA 3: Began soliciting PwCF feedback about the follow‐up process, and inquired about the benefits of potential interventions offered during follow‐up contact. Feedback from PwCF about follow‐up indicated that they would be more receptive to follow‐up if the screening tool questions were more accurate. Respondents reported false positives as well as questions failing to capture needs. They also reported that because they did not trust the tool, they did not feel the need for follow‐up contact. It was hypothesized that a more accurate screening tool would correlate to sustained improvement in follow‐up rate. An original screening tool (v1.0) underwent revisions based on patient feedback.
PDSA 4: Began testing screening tool v2.3. The question on employment status was reported to be confusing and was revised. The patient partner, the CF care team, and an outside expert reviewed the tool and made revisions.
PDSA 5: V2.5 of the screening tool was tested. Results indicated that one question was not accurately capturing SDoH and needed to be eliminated from screening. Minor phrasing changes were made to questions based on feedback from PwCF with the aim of keeping all questions at or below the 5th grade reading level. In accordance with screening recommendations to incorporate patient perspective, a final question was added to the screening tool asking respondents if they would like to receive assistance. 12 Respondents who answered “yes” were defined as having requested assistance.
After this PDSA, Aim 2 was changed to increase follow‐up within 2 weeks for those PwCF who screened positive and requested assistance from 0% to 95% by December 31, 2021. The definition of follow‐up rate was therefore changed to the rate of PwCF who screened positive and requested assistance and received follow‐up within 2 weeks. See Figure 4.
PDSA 6: V2.6 was tested. Feedback from PwCFindicated that questions were easy to understand and accurately captured respondent information in all eight domains. The criteria for PwCF eligible for follow‐up was changed to those who both screened positive and answered “yes” to the final question requesting assistance. V2.6 and a follow‐up process, including four contact methods (telehealth, in‐person, phone, and MyChart), were adopted.
Figure 4.
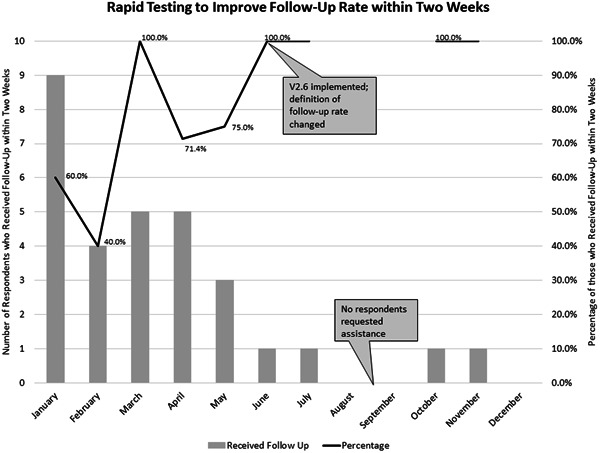
Annotated chart tracking the progress of testing percentage of patients who scored positive for SDoH and requested assistance who received follow‐up within 2 weeks. SDoH, social determinants of health.
2.3. Measures
-
1.Percentage of PwCF screened in 2021 for SDoH. Submeasures included:
-
a.Percentage of those who screened positive for SDoH.
-
b.Number of those screened via each screening method.
-
c.Percentage of those who screened positive for SDoH and requested assistance.
-
d.Needs as distributed by the domain of social risk factors.
-
a.
-
2.Percentage of follow‐up within 2 weeks for those PwCF who screened positive for SDoH and requested assistance. Submeasures included:
-
a.Those who received resources/referrals from SW.
-
b.Those who declined resources/referrals from SW.
-
a.
A screening was considered incomplete if more than four questions were unanswered or if more than two of the eight domains could not be assessed due to unanswered questions. In the online survey, participants were forced to respond to the question asking for their names before they could move on to the next questions. In both the paper instrument and online survey, patients could choose to skip all other questions or leave them blank. During screening via screen sharing, patients were advised to skip any question if they did not wish to answer.
3. RESULTS
A total of 132 of 142 PwCF eligible for screening (93.0%) completed the SDoH screening tool: 63 via an online survey, 48 via paper instrument in a clinic, and 21 via screen sharing. Of 142 eligible PwCF, 123 (86.6%) have access to MyChart. All of the 123 PwCF with MyChart access were sent a survey link via MyChart; 63 completed the screening via an online survey. Ten PwCF did not complete the screening (Table 1). The accuracy of screening data was assessed during the follow‐up process testing described in the interventions. Of all PwCF screened, only one responder's screening was considered incomplete. That screening was not included in the cumulative data. Of the PwCF who completed screening, 56 (42.4%) screened positive for SDoH cumulatively across all versions of the screening tool. Of those 132 who completed the screening, 27 responders were screened with the final version (v2.6). Of the PwCF screened with v2.6, 11 of 27 (40.7%) screened positive, but only 4 (14.8%) of those who screened positive for SDoH requested assistance. All four PwCF received follow‐up contact from SW. All four received resources/referrals from SW during that follow‐up contact; 0 declined resources/referrals.
Table 1.
Cumulative SDoH screening results
| Number of eligible PwCF | 142 |
| Total PwCF screened | 132 (93.0%) |
| Screened via online survey | 63 (47.7%) |
| Screened via paper instrument | 48 (36.4%) |
| Screened via screen sharing | 21 (15.9%) |
| Positive for SDoH | 56 (42.4%) |
Abbreviations: PwCF, people with cystic fibrosis; SDoH, social determinants of health.
All PwCF screened were identified as not of Hispanic, Latino, or Spanish origin. Two respondents were identified as Black or African American; two were identified as multiracial (selected at least two racial identities). All other respondents were identified as White or Caucasian.
A follow‐up rate of 100% (one out of one) was achieved in June 2021 and maintained in July, October, and November (Figure 4). (No patients were eligible for follow‐up in August, September, or December.)
The distribution of social needs by domain for those PwCF who screened positive for SDoH was examined. PwCF who screened positive indicated a need in 1.5 social risk factor domains on average. Health‐care and medication access together made up 33 (51.6%) of the 63 reported needs (Table 2). Identification of domains where PwCF most frequently reported needs allowed for the examination of other clinic processes regarding financial assistance options for healthcare and/or medication. Accordingly, new processes were established for making discussion of financial assistance options a routine part of annual social work assessment and social work assessment of new patients establishing care with UVA Adult CF Center.
Table 2.
Social risk factors and examples of resources distributed by domain
| Total positive needs (average per respondent) | 63 (1.5) |
| Housing (percentage of reported needs) | 7 (10.9%) |
| |
| Food | 6 (9.4%) |
| |
| Transportation | 6 (9.4%) |
| |
| Utilities | 7 (10.9%) |
| |
| Health‐care access | 22 (34.4%) |
| |
| Medication Access | 11 (17.2%) |
| |
| Income/employment | 4 (6.3%) |
| |
| Education | 1 (1.6%) |
|
Abbreviations: FMLA, family medical leave act; SNAP, supplemental nutrition assistance program; WIC, special supplemental nutrition program for women, infants, and children
4. DISCUSSION
The rapid testing of the screening and intervention was shown to be an effective method for implementing a routine SDoH process as part of CF care. At this time, there are no standardized SDoH screening or intervention guidelines for the CF community. This proposed screening would be done annually or more frequently when a change in a social situation (such as loss of housing or employment) is identified. Only one responder's screening was considered incomplete, indicating PwCF understood screening tool questions.
In 2020, 76 patients were screened for SDoH. This number increased to 132 in 2021 when the screening tool was changed from a one‐time questionnaire to a routine screening tool. While this process did not examine sustainability, results of screening patients in both 2020 and 2021 point to a high likelihood, and this will be examined in future studies.
A screening rate of 93.0% was achieved in 2021. Some PwCF attended only one clinic visit in 2021, which was before screening when appointments were implemented. Some PwCF attended only one clinic visit in 2021, which was prior to the implementation of screening during clinic appointments in March. There was no further opportunity to screen those PwCF who did not complete the survey via My Chart and did not have another clinic appointment after screening began. It is hypothesized that in 2022 when multiple screening methods are utilized for the entire year, a screening rate of 95% will be achieved.
The follow‐up process aim of a 100% follow‐up rate within 2 weeks was achieved in June 2021 and maintained in July, October, and November. (No patients were eligible for follow‐up in August, September, or December.) Only four respondents totally requested assistance from June to December of 2022, which limited the ability to test changes made to the follow‐up process. The same follow‐up process is planned for 2022, which will provide further data on its efficacy.
The 100% follow‐up rate corresponded with the change to v2.6 of the screening, which included the final question where respondents were able to either request or decline receiving assistance. A positive screening response alone may not always be reflective of respondent priorities or perceived need for support. Adding the final question is more reflective of respondent priorities. It is hypothesized that adding this question was a contributor to the sustained 100% follow‐up rate, because PwCF who both screened positive and requested assistance may be more likely to respond to attempts to contact the respondent for follow‐up.
Screening via multiple methods demonstrated the success of three screening types as well as improvement in screening rate. The online survey allowed for PwCF to be screened asynchronously outside a CF clinic appointment. Three PwCF who reported they could not come to the clinic due to social barriers (such as lack of healthcare coverage or transportation) were able to complete the asynchronous screening and receive an intervention that reduced barriers to care and enabled those respondents to return to the clinic. Screening via an online survey, therefore, created the potential to support PwCF who might otherwise have been missed.
The screening tool developed by the UVA adult CF team includes questions unique to CF care and the CF chronic care model. Existing literature on SDoH within the CF population is limited. By examining social risk factors by domain, the screening provided insight into areas of greatest need. This insight then led to further clinic processes being adjusted to provide increased support to patients to improve healthcare and medication access. The 2021 screening results reinforce the importance of SDoH screening and demonstrate how results can be used to implement interventions on both the level of individual PwCF and the center process level.
Screening data insights are limited by both single‐site testing and demographics. The overwhelming majority of PwCF screened were White and not of Hispanic, Latino, or Spanish origin. The final screening tool, v2.6, was used to screen 27 patients. Another year of screening with v2.6 will provide more data with this version for the screening tool. SDoH results were likely impacted by the ongoing pandemic. No screening results from a nonpandemic year are available for comparison. It cannot be determined to what extent the pandemic influenced the number of people who scored positive for SDoH.
Existing infrastructure from the UVA Adult CF Center QI team contributed to the success of this process. UVA CF care team members have dedicated time, space, and support from their QI team to work on the conceptualization and implementation of QI projects. Patient and family partner contributions along with responder feedback and buy‐in were critical to the success of the screening tool and process testing. Implementing an SDoH screening and intervention process may be more difficult at a CF care center without this infrastructure.
Some patients declined to complete SDoH screening even after they were provided education on the rationale and potential benefits of screening. The CF SW assessed those patients for social needs via an informal conversation about their social situation. The lack of screening data would make tracking changes in needs over time and reviewing previous SDoH data difficult.
5. CONCLUSION
Implementing screening for SDOH and interventions to mitigate social difficulties in adult PwCF at UVA was successful and could be reproduced by other CF care centers. Multiple methods of screening (online survey, paper instrument, and screen sharing) were shown to be effective.
AUTHOR CONTRIBUTIONS
Deirdre Jennings: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); project administration (lead); validation (lead); visualization (lead); writing–original draft (lead); writing–review and editing (lead). Rhonda List: Conceptualization (lead); data curation (lead); methodology (lead); project administration (lead); visualization (lead); writing–review and editing (lead). Heather Bruschwein: Conceptualization (supporting); methodology (supporting); project administration (supporting); writing–review and editing (supporting). Martina Compton: Conceptualization (supporting); methodology (supporting); project administration (supporting); writing–review and editing (supporting). Lindsay Somerville: Conceptualization (supporting); methodology (supporting); project administration (supporting); writing–review and editing (supporting). Lauren Williamson: Conceptualization (supporting); methodology (supporting); project administration (supporting); writing–review and editing (supporting). Rachel Murray: Conceptualization (supporting); methodology (supporting); project administration (supporting); writing–review and editing (supporting). Brielle Evangelista: Conceptualization (supporting); methodology (supporting); project administration (supporting); writing–review and editing (supporting). Dana Albon: Conceptualization (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); supervision (lead); validation (lead); writing–original draft (lead); writing–review and editing (lead).
CONFLICT OF INTERESTS
The authors have no conflicts of interest to disclose.
ACKNOWLEDGMENTS
The authors thank Rachel Turner for her assistance in this paper. Cystic Fibrosis Foundation Care Center Grant (CFF CC043‐AD) supported salaries for our Quality Improvement coordinators and principal investigator. Cystic Fibrosis Learning Network Grant (CFLN SEID16AB0) supported salaries for our Quality Improvement coordinators.
Jennings D, List R, Bruschwein H, et al. Social determinants of health screening and intervention: A cystic fibrosis quality improvement process. Pediatric Pulmonology. 2022;57:3035‐3043. 10.1002/ppul.26131
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supporting Information: Material of this article.
REFERENCES
- 1. Cystic Fibrosis Foundation. Patient Registry 2020 Annual Data Report. 2021. [Google Scholar]
- 2. Oates GR, Schechter MS. Socioeconomic status and health outcomes: cystic fibrosis as a model. Expert Rev Respir Med. 2016;10(9):967‐977. 10.1080/17476348.2016.1196140 [DOI] [PubMed] [Google Scholar]
- 3. Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331‐1337. 10.1164/ajrccm.163.6.991210010.1016/j.jpeds.2009.04.059 [DOI] [PubMed] [Google Scholar]
- 4. Center for Disease Control and Prevention. About Social Determinants of Health (SDOH). 2022. [Google Scholar]
- 5. Tumin D, Crowley EM, Li SS, Wooten W, Ren CL, Hayes D. Patterns of health insurance coverage and lung disease progression in adolescents and young adults with cystic fibrosis. Ann Am Thorac Soc. 2021;18(2):290‐299. 10.1513/AnnalsATS.201911-839OC [DOI] [PubMed] [Google Scholar]
- 6. O'Connor GT, Quinton HB, Kneeland T, et al. Median household income and mortality rate in cystic fibrosis. Pediatrics. 2003;111(4 pt 1):e333‐e339. 10.1542/peds.111.4.e333 [DOI] [PubMed] [Google Scholar]
- 7. Schechter MS, McColley SA, Silva S, Haselkorn T, Konstan MW, Wagener JS, Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis, North American Scientific Advisory Group for ESCF . Association of socioeconomic status with the use of chronic therapies and healthcare utilization in children with cystic fibrosis. J Pediatr. 2009;155(5):634‐639.e1. 10.1016/j.jpeds.2009.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schechter MS, Margolis PA. Relationship between socioeconomic status and disease severity in cystic fibrosis. J Pediatr. 1998;132(2):260‐264. 10.1016/s0022-3476(98)70442-1 [DOI] [PubMed] [Google Scholar]
- 9. Schechter MS. Non‐genetic influences on cystic fibrosis lung disease: the role of sociodemographic characteristics, environmental exposures, and healthcare interventions. Semin Respir Crit Care Med. 2003;24(6):639‐652. 10.1055/s-2004-815660 [DOI] [PubMed] [Google Scholar]
- 10. Quittner AL, Schechter MS, Rasouliyan L, Haselkorn T, Pasta DJ, Wagener JS. Impact of socioeconomic status, race, and ethnicity on quality of life in patients with cystic fibrosis in the United States. Chest. 2010;137(3):642‐650. 10.1378/chest.09-0345 [DOI] [PubMed] [Google Scholar]
- 11. Alderwick H, Gottlieb LM. Meanings and misunderstandings: a social determinants of health lexicon for health care systems. Milbank Q. 2019;97(2):407‐419. 10.1111/1468-0009.12390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magnan S. Social Determinants of Health 201 for Health Care: Plan, Do, Study, Act. NAM Perspectives. Discussion Paper. National Academy of Medicine; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braveman P, Cubbin C, Egerter S, Pedregon V. Neighborhood Effects on Health. Robert Wood Johnson Foundation; 2022. [Google Scholar]
- 14. PolicyLink, The Food Trust. The Grocery Gap: Who Has Access to Healthy Food and Why It Matters. 2010. [Google Scholar]
- 15. McDonald CM, Christensen NK, Lingard C, Peet KA, Walker S. Nutrition knowledge and confidence levels of parents of children with cystic fibrosis. Infant Child Adolesc Nutr. 2009;1(6):325‐331. 10.1177/1941406409355192 [DOI] [Google Scholar]
- 16. Brown PS, Durham D, Tivis RD, et al. Evaluation of food insecurity in adults and children with cystic fibrosis: community case study. Front Public Health. 2018;6:348. 10.3389/fpubh.2018.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lim JT, Ly NP, Willen SM, et al. Food insecurity and mental health during the COVID‐19 pandemic in cystic fibrosis households. Pediatr Pulmonol. 2022;57:1238‐1244. 10.1002/ppul.25850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. CMS Innovation Center. Accountable Health Communities Model. 2022. [Google Scholar]
- 19. Gurewich D, Garg A, Kressin NR. Addressing social determinants of health within healthcare delivery systems: a framework to ground and inform health outcomes. J Gen Intern Med. 2020;35(5):1571‐1575. 10.1007/s11606-020-05720-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guyer J, Boozang P, Nabet B. Addressing social factors that affect health: emerging trends and leading edge practices in Medicaid. Manatt Health. April 2019.Accessed March 17, 2022. https://www.manatt.com/Manatt/media/Documents/Articles/Social-Factors-That-Affect-Health_Final.pdf
- 21. Albon D, Bruschwein H, Soper M, et al. Impact of COVID‐19 on social determinants of health for adults with cystic fibrosis. Ther Adv Respir Dis. 2021;15:17534666211037460. 10.1177/17534666211037459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langley N, Norman P. The Improvement Guide: A Practical Approach To Enhancing Organizational Performance. Jossey‐Bass Inc; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information: Material of this article.


